Graphical abstract
Keywords: Antifungal, Copper nanoparticles, Green synthesis, Methylene blue, Photocatalytic
Highlights
-
•
Green synthesis of CuNPs by leaf extract of C. paniculatus.
-
•
CuNPs showed characteristic peak at 269 nm with particle size of 2−10 nm.
-
•
CuNPs Characterized via UV–vis spectroscopy, FT-IR, SEM-EDS, TEM, and DLS analysis.
-
•
Antifungal activity tested against F. oxysporum.
-
•
Photocatalytic degradation of organic dye methylene blue using CuNPs.
Abstract
This research aimed to explore the eco-friendly green synthesis of copper nanoparticles (CuNPs) using Celastrus paniculatus leaves extract. Primarily, the biosynthesized CuNPs characterized by UV–vis spectroscopy showed an absorption peak at 269 nm. Further, The SEM and TEM studies revealed the spherical shape of particles with size ranged between 2−10 nm with an average particle diameter of 5 nm. FT-IR analysis confirmed the presence of functional groups —OH, C C and C—H triggers the synthesis of CuNPs. The negative zeta potential -22.2 mV indicated the stability of CuNPs was confirmed by DLS and the composition and purity by EDS studies. Further, the photocatalytic property of the CuNPs was divulged by their methylene blue dye degradation potential. The reaction kinetics followed pseudo-first-order with k-values (rate constant) 0.0172 min−1. In addition, this material was found to be a good antifungal agent against plant pathogenic fungi Fusarium oxysporum showed 76.29 ± 1.52 maximum mycelial inhibition.
1. Introduction
Modernization and industrialization discharged a bulk amount of industrial effluents along with organic dyes into the water bodies. Organic dyes are widely used as a colorant in various industries such as textile, leather tanning, paper, cosmetics, pharmaceutical, and plastic [1]. These organic dyes highly toxic, carcinogenic, and non-degradable, can cause serious health problems such as skin diseases, cancer, allergic reactions, and mutation for people [2,3]. For such purposes, numerous water treatment approaches have been explored for the treatment of industrial wastewater effluents such as precipitation, coagulation, electrolysis, activated carbon, oxidation, and reduction reactions [4]. However, these techniques are costly and often transfer toxic pollutants to water bodies. Therefore, need to develop an eco-friendly and cost-effective method for the degradation of an organic pollutant from wastewater [5]. Recently, biosynthesized nanoparticles (NPs) attracted much attention due to their photocatalytic application in the degradation of organic dyes [6]. Different types of plants and their derived products have been used successfully in the synthesis of different green nanoparticles of zinc oxide [7,8], platinum [9], palladium [10], silver [11,12], cobalt [13], magnetic [14], and gold [15].
However, there are several studies on CuNPs synthesis using different plants extract [[16], [17], [18], [19], [20], [21], [22], [23]] have been reported but the study of application of CuNPs on the treatment of dye effluent is limited. The agriculture sector exploits different kind of pesticides, herbicides, and antimicrobial [24,25] substances to control plant diseases. These substances are responsible for soil pollution as well as biomagnification in living organisms [26,27]. Despite photocatalytic activity, CuNPs attracted more attention due to its nontoxic, antimicrobial efficacy in controlling plant diseases. An extensive literature survey revealed that the antifungal activity of CuNPs mostly tested against human pathogenic fungi [28]. The least study conducted on CuNPs antifungal activity on plant pathogenic fungi, so, there is a crucial need for more assessment and evaluation in this field [29].
Nowadays nanomaterials are of huge interest due to a wide range of applications in chemical, biological, and environmental sciences [30,31]. The NPs exhibited a variety of applications, including optical, electrical, thermal conductivity, catalysts, antioxidant, antimicrobial, and anticancer activity. Among the NPs, CuNPs have great attention due to its catalytic, high electrical conductivity, optical, antifungal, and antibacterial properties [6,[32], [33], [34]]. The unique physical and chemical properties of NPs which are not exhibited by the bulk materials, received much attention to synthesis of NPs. In the last few years ago several methods such as physical, chemical, and biological used for the NPs synthesis. The physical methods for NPs synthesis such as pulse laser ablation, mechanical/ball milling, pulsed wire discharge, sputtering [[35], [36], [37], [38], [39]], etc. have been reported. The chemical synthesis includes colloidal [40,41], electrochemical [42,43], Chemical reduction [44], and photochemical [45] methods. The toxicity and relatively high material cost of these methods restricted their use in a better way. Biological method for NPs synthesis attracted researchers due to its simple, direct, non-toxicity, and ecofriendly characteristics upon chemical and physical methods. The biological method of NPs synthesis carried out by various sources bacterial, fungal, actinomycetes, yeast, algal, viruses [[46], [47], [48], [49], [50], [51]], and plant extracts. Plants are reservoir of phytochemicals such as flavonoids, polyphenols, alkaloids, terpenoids, saponins, vitamins, polysaccharides, and proteins which act as reducing, capping and stabilizing agents for the biosynthesis of NPs [52]. Celastrus paniculatus (C. paniculatus) commonly known as black oil plant, Malkangani, and Jyotishmati is a traditional ayurvedic medicinal plant of family Celastraceae. The phytochemicals in crude extracts of C. paniculatus found alkaloids, flavonoids, phenylpropanoids, diterpenoids, triterpenoids, tetraterpenes, β-dihydroagarofuranoids, lignans, etc. [53].
This study reports a green route for the synthesis of CuNPs using C. paniculatus leaf extract, evaluation of its antifungal activity against phytopathogenic fungi Fusarium oxysporum (F. oxysporum), and its photocatalytic efficiency in the decomposition of organic dye. There is no report of C. paniculatus leaf extract mediated green synthesis of CuNPs and application in antifungal and photocatalytic activity to date.
2. Materials and methods
2.1. Materials
Copper (II) Sulfate pentahydrate (CuSO4.5H2O, CAS-No: 7758-99-8), was purchased from Sigma Aldrich. Methylene blue AR (RM116) was obtained from Himedia and F. oxysporum (ITCC No. 4998) procured from IAARI, New Delhi. The leaf samples of C. paniculatus were collected from Madan Mohan Malviya Government Ayurvedic College, Udaipur (Raj.) India. Collected plant material was authenticated by Herbarium, Botany Department, University of Rajasthan, Jaipur, India (No. RUBL211672). Deionized water was used to prepare plant extract and copper sulfate solution.
2.2. Methodology
2.2.1. Preparation of plant extract
Collected leaves were rinsed with tap water to remove dust particles. Further, leaves were rinsed with double distilled water (DDW) and shade dried for 1 week to remove the moisture content. The dried leaves were powdered in grinder mixer and powder stored in dark at ambient temperature. To prepare the plant extract, 2 gm of dried leaf powder was added in 200 mL deionized water in 500 mL flask, mixed well on a magnetic stirrer with hot plate at 60 °C for 20 min. The prepared extract was filtered using Whatman filter paper with size 11 μm followed by vacuum filtration using cellulose nitrate membrane. The filtrate was used immediately or stored at 4 °C for further use.
2.2.2. Synthesis of nanoparticles
For the synthesis of C. paniculatus copper nanoparticles, 50 mL (5 mM) copper sulfate solution was mixed with 5 mL of aqueous plant extract [54]. The pH value 7.0 adjusted for the mixture by the addition of NaOH (1 N) solution. Further, the green color mixture was obtained. The mixture centrifuged, pellets collected and dried overnight in a hot air oven at 60 °C. A dark green color powder obtained was stored at room temperature for further use.
2.2.3. Photocatalytic activity
The Photocatalytic activity of the CuNPs was evaluated by the degradation of MB in an aqueous solution under sunlight irradiation. Stock solution (10 mg/l) of MB was prepared. In the experiment, 10 mg CuNPs mixed with 100 mL of 10 mg/l MB solution and pH adjusted to 9.0 in the dark at ambient temperature [55]. Afterward, the resulting solution was kept under direct sunlight with a solar flux of 1100 lx measured by lux meter. About 3 mL aliquot of the suspension was taken and centrifuged at selected time intervals (every 15 min) to remove suspended CuNPs. The rate of dye degradation was determined by measuring the absorption spectrum using a UV–vis spectrophotometer at 664 nm. The photocatalytic degradation efficiency was assessed based on the formula.
Where, C0 is the initial MB concentration, C is residual MB concentration after time t.
2.2.4. Antifungal activity
Antifungal activity of CuNPs was accessed using poison food technique against F. oxysporum. In this study, seven treatments (one control with water and three CuNPs at 0.12, 0.18 and 0.24 %, w/v in water along with 0.1 %, 1 % CuSO4, and plant extract) were performed to evaluate antifungal activity. These treatments carried out in triplicate and the experiment was repeated thrice. The treated plated compared with control (without CuNPs) to calculate the % mycelial inhibition rate by using the formula given by Vincent [56].
Where Mc is the mycelial growth in control, Mt is the mycelial growth in treatment.
3. Characterization of CuNPs
The absorbance spectrum of green synthesized CuNPs was analyzed using UV–vis spectroscopy (ELICO SL-159 UV–vis spectrophotometer) in the range of 220−540 nm. The morphological features of CuNPs were studied by using the transmission electron microscopy (TEM) (FEI Tecnai G2 20) and scanning electron microscopy (SEM). The elemental composition of the particles was examined by Energy-Dispersive X-ray spectroscopy (EDS) using JEOL SM-7600 F, Japan model. Fourier-transform infrared spectroscopy (FT-IR) analysis was employed to find out the role of biomolecules in leaf extract for metal reduction in the range of 500-4000 cm−1. The charge and size distribution of CuNPs was measured using Malvern Zetasizer (Malvern Instrument Inc., London, U.K). Dynamic light scattering (DLS) measurements were performed by dispersing 20 mg CuNPs powder in 40 mL deionized water. The solution was stirred in a vortex mixer for 5 min to break up any aggregates and then 1−2 ml was transferred to the zeta-disposable cell.
4. Results and discussion
4.1. UV–vis spectra of CuNPs
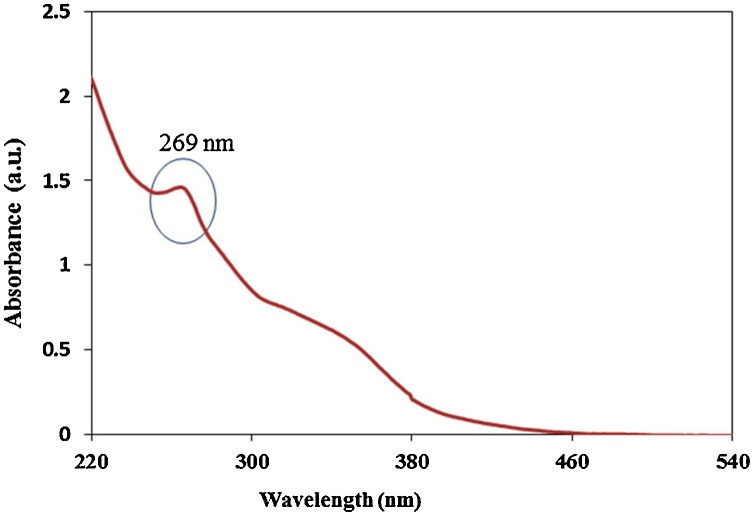
Primarily, the formation of CuNPs confirmed by the change in color from yellow to green upon the addition of plant extract into aqueous CuSO4 solution. The interaction between conduction electrons of metal NPs and incident photons was responsible for color change [57]. Further, CuNPs synthesis confirmed by a characteristic peak obtained at 269 nm (Fig. 1) [58,16]. In this experiment, effect of pH 7 on reduction of CuSO4 into CuNPs was assessed by UV–vis spectroscopy. The neutral pH sharp absorbance peak was observed which may be due to the ionization of the phenolic groups present in plant extract [59]. The peak value was found to be gradually decreased with an increase in particle size (Fig. 1). This experiment concluded that the pH 7 is optimum for reduction of Cu2+ ions into CuNPs.
Fig. 1.
UV–vis absorption spectrum.
4.2. FT-IR characterization of CuNPs
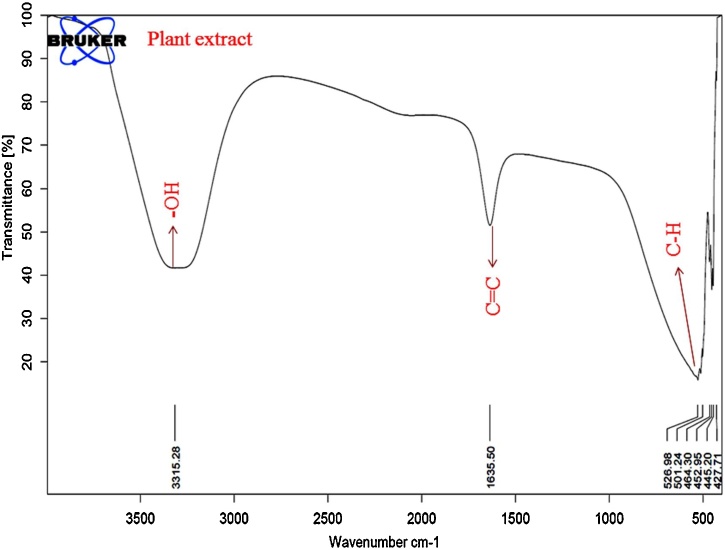
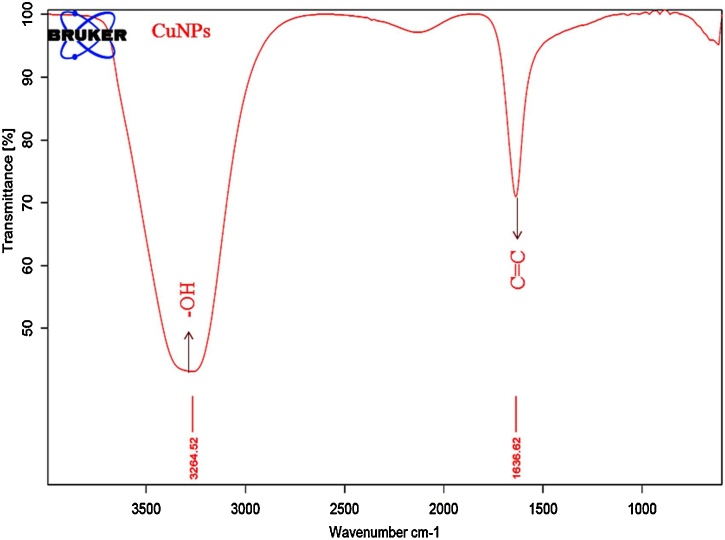
FT-IR studies find out the possible biomolecules in plant extract which are responsible for the reduction and stabilization of CuNPs. FTIR spectra of C. paniculatus leaf extract have shown in Fig. 2, where the spectra of C. paniculatus leaf extract depicted broad peaks at 3315.28 cm−1 representing the hydroxyl (—OH) functional group in alcohols and phenolic compounds and 1635.50 cm−1 can be assigned to the aromatic bending of alkene group (C C), while smaller peaks at 526.98–452.95 cm−1 are also assigned to the aromatic bending vibration of alkane groups (C—H) (Fig. 2). The FTIR spectrum of CuNPs depicts the distinctive characteristic bands at 3264.52 and 1636.62 cm−1 corresponds to the C. paniculatus leaf extract bands (Fig. 3). These peaks indicate the presence of flavonoid and other phenolic compounds in the plant leaf extract [60,61]. The flavonoid biomolecules transformed enol-form to the keto-form by releasing a reactive hydrogen atom and that can reduce Cu2+ ions to form CuNPs. These biomolecules stabilizes NPs by chelating with metal ions with their carbonyl groups or π-electrons [62]. Thus, results conclude that the surface of synthesized CuNPs was capped and stabilized by flavonoid and other phenolic compounds in the C. paniculatus leaf extract.
Fig. 2.
FT-IR spectra of aqueous C. paniculatus leaves extract.
Fig. 3.
FT-IR spectra of synthesized CuNPs.
4.3. Morphological characterization of CuNPs
4.3.1. SEM, TEM and EDS analysis
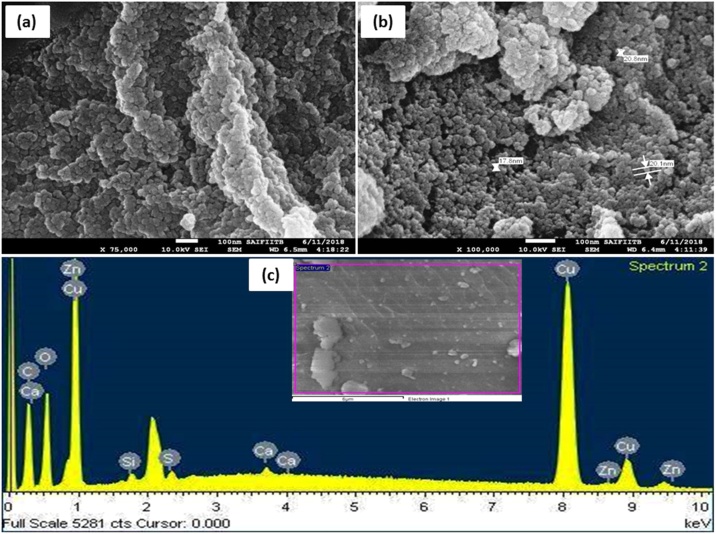
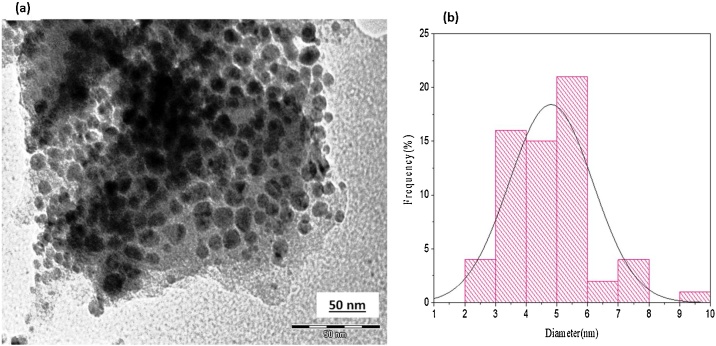
The morphological characterization of CuNPs was carried out using SEM-EDS and TEM analyses. SEM analysis revealed the presence of spherical particles with some agglomeration due to sampling preparation (Fig. 4a-b). The size of the particles was calculated by the TEM and SEM analysis was found to be in the range of 2−10 nm with an average particle diameter of 5 nm as displayed in size distribution histogram (Fig. 5b).
Fig. 4.
(a-b) SEM micrographs of CuNPs, (c) EDS spectrum.
Fig. 5.
(a) TEM micrograph of CuNPs, (b) Size distribution histogram of CuNPs.
The EDS analyses confirmed the composition and stability of synthesized CuNPs (Fig. 4c). The purity levels of the particles were examined, which indicated that C. paniculatus mediated CuNPs had 79.87 % of Cu and some weak signals of C, O, Si, S, Ca and Zn elements (Table 1). These weak signals may be due to the X-ray emission from the macromolecules like flavonoids, phenolic compounds, carbohydrates, glycosides, steroids and tannins present in the extracts [63].
Table 1.
Compositional and particle stability analysis of CuNPs.
| Element | Weight% | Atomic% |
|---|---|---|
| C K | 13.02 | 39.92 |
| O K | 5.32 | 12.25 |
| Si K | 0.27 | 0.35 |
| S K | 0.40 | 0.46 |
| Ca K | 0.28 | 0.26 |
| Cu K | 79.87 | 46.29 |
| Zn K | 0.84 | 0.47 |
| Totals | 100.00 |
4.4. Dynamic light scattering (DLS) studies
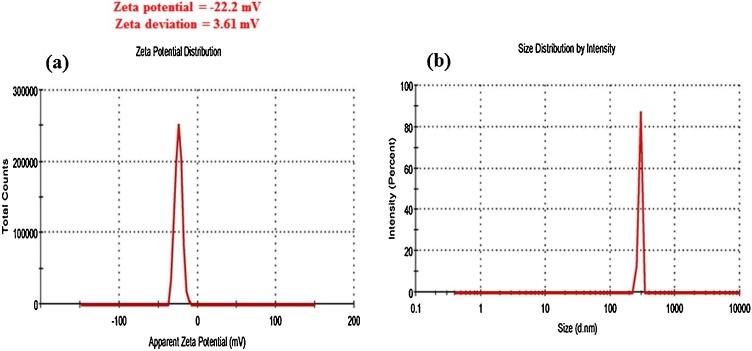
DLS analysis was used to find out the size and surface charge of NPs through the colloidal solutions. In the present study, the negative zeta potential was found at -22.2 mV and zeta deviation was 3.61 mV (Fig. 6a). The high negative value of zeta potential specifies a strong repellent force among the particles and prevents agglomeration [64,65]. The polydispersity index value of CuNPs was 1.000. Fig. 6b shows green synthesized CuNPs average particle size distribution was 290 nm. The larger size of CuNPs due to the measured size included biomolecule and water layer covering the surface of NPs [66]. It suggested that the size and charge distribution of the synthesized NPs promoted or enhanced the biological property of CuNPs [67].
Fig. 6.
DLS analysis of Cu NPs (a) zeta potential, (b) Size distribution.
4.5. Photocatalytic degradation of MB
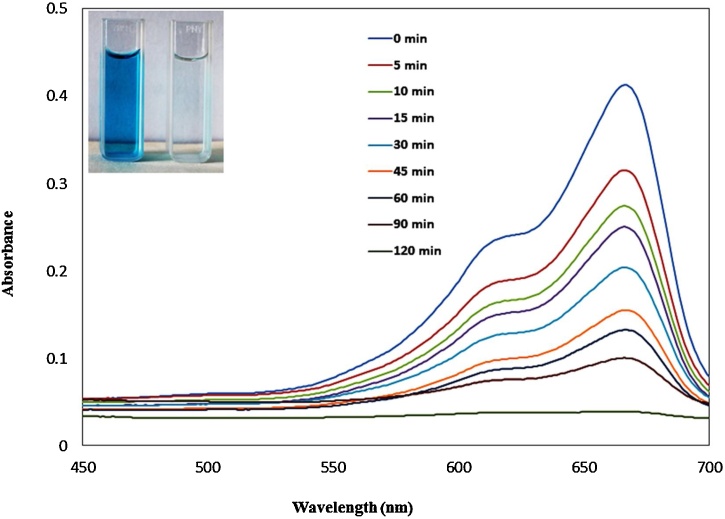
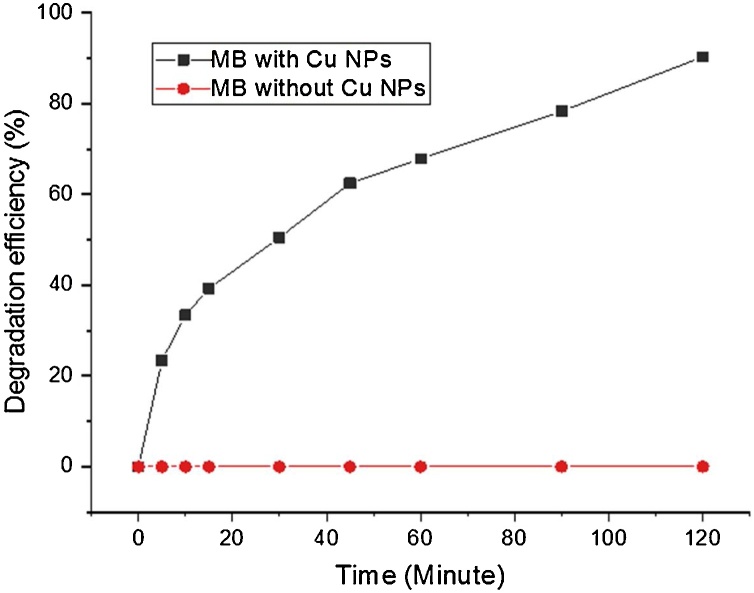
The potential of synthesized CuNPs for photocatalytic degradation of MB was examined under direct sunlight. The time dependent decrease in the absorption band intensity of MB dye was observed after the addition of CuNPs under solar light exposure. The photocatalytic degradation efficiency measured using spectrophotometer at 664 nm. In the experiment 10 mg L−1 concentration of MB mixed with 10 mg dosages of photocatalyst. Almost complete degradation of MB seen in 120 min (Fig. 7). In the presence of CuNPs the photodegradation was significantly enhanced at basic pH (pH = 9). The basic pH influences the surface charge properties of photocatalyst, the anionic dye molecule is negatively charged adsorbed on the photocatalyst surface [68]. The high pH favors adsorption of dye on the photocatalyst surface. The calculated degradation efficiency for MB was 90 % plotted in Fig. 8. The degradation experiments were performed with control (both in presence and absence of catalyst) were carried out in the dark to nullify any possibility of dye self-degradation, dye adsorption, and catalytic activity of CuNPs in dark. Under dark conditions, CuNPs have not exhibited any insignificant effect on degradation of dye. Thus, experiments concluded that the dyes were not significantly degraded in dark conditions. Besides, dye degradation experiments performed under direct sunlight in the absence of catalyst showed negligible dye degradation while with catalyst dye almost completely degraded (Fig. 8). These experiments depicted that dye degradation was driven by a photocatalytic process.
Fig. 7.
Photocatalytic degradation of MB using CuNPs.
Fig. 8.
Photocatalytic degradation efficiency of MB under sunlight irradiation.
In general, there were following steps in the photocatalytic degradation which is summarized below.
| Cu + hν → Cu (e− + h+) | (i) |
Firstly, the CuNPs absorbed the solar irradiation get photo excited due to SPR influence (Eq. (ii)). Secondly, the electron and holes produced can react with O2 (Eq. (iii)) and H2O (Eq. (iv)) particles to provide active hydroxyl radical (OH−), and anionic superoxide radical (O2−), respectively (Eq. (v)).
| Cu + hν → Cu (e−) + Cu (h+) | (ii) |
| Cu (e−) + O2 → O2− | (iii) |
| Cu (h+) + H2O → OH− | (iv) |
| O2− or OH− + Dye → degraded product | (v) |
Finally, both oxidation as well as reduction proceeds on the photocatalyst surface. These highly reactive •OH and •O2 radicals can interface with the MB aromatic ring and possibly break the bond producing CO2, H2O, and numerous ions as by-products. The literature sharma and dutta [69] described that hydroxyl radical were dominant reactive oxygen species that contributed to degradation using NPs. Thus, their study provided the suitable justification for active species based photocatalytic degradation of dyes when using CuNPs, as discussed in our work.
The kinetics of the photocatalyzed decolorization process described by a pseudo first-order reaction for the concentration of MB [70].
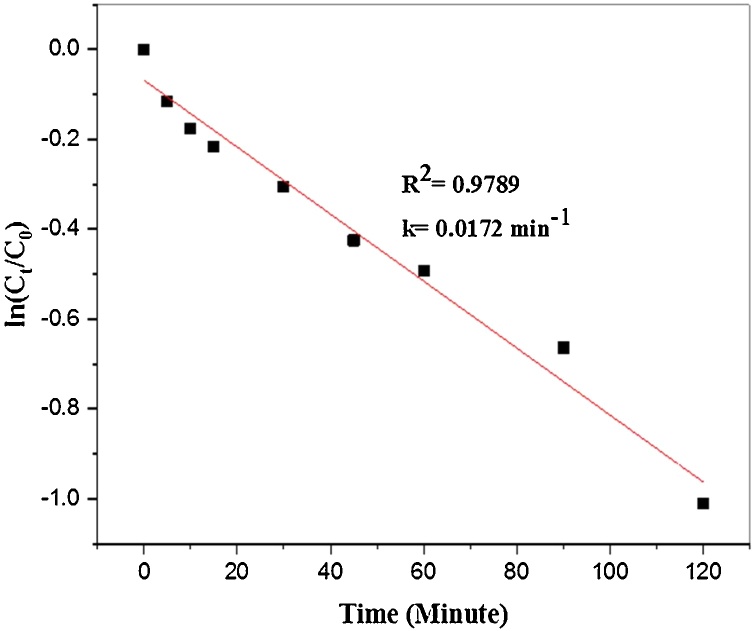
Where, C0 is the initial MB concentration and Ct is the MB concentration at the irradiation time (t) and k is the rate constant (min−1). The linear relationship between ln(Ct/C0) vs irradiation time (t) described in Fig. 9 showed good linear correlation with the values of correlation coefficient (R2>95). The slope of the linear fitting line as shown in Fig. 9 concluded the rate constant (k) of the reaction was found 0.0172 min−1. From this study we have concluded that the time duration for degradation of MB dye was 120 min. pseudo first-order kinetics resulted that obtained value of rate constant was found to be 0.0172 min-1. A comparative study of photocatalytic reduction of MB using different types of photocatalyst described in Table 2.
Fig. 9.
Kinetic data for the degradation of aqueous MB under sunlight irradiation.
Table 2.
Comparison of various photocatalysts in the reduction of MB.
Degradation mechanism of MB
5. Antifungal assay of CuNPs
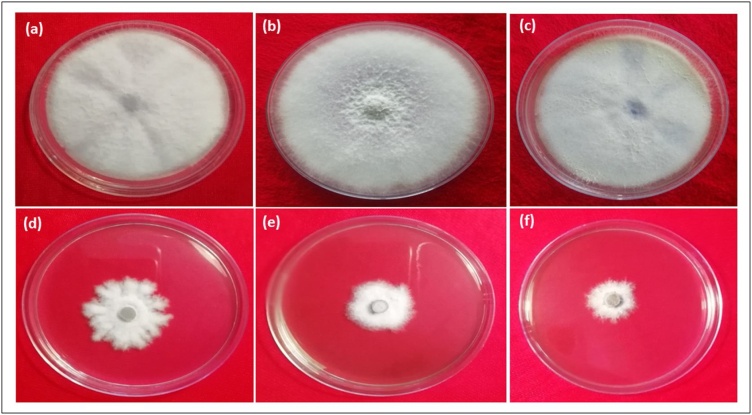
The antifungal activity of the synthesized CuNPs was assessed against F. oxysporum by measuring the mycelial radial growth. Study results showed that F. oxysporum exhibited mycelial growth inhibition at 0.24, 0.18, and 0.12 % CuNPs concentration (Fig. 10). CuNPs showed 76.29 ± 1.52, 73.70 ± 1.52 and 59.25 ± 0.57 mycelial growth inhibition at 0.24 and 0.18 and 0.12 %, respectively (Table 3). Maximum mycelial growth inhibition observed at 0.24 % CuNPs. The experiment, confirmed that mycelial growth inhibition depends on NPs concentrations. Commercial fungicide bavistin (0.1 %) was used as a positive control showing 100 % inhibition of fungal mycelial growth (Fig. 10). Whereas CuSO4 (1.0 %) showed 20.74 ± 1.52 inhibition and plant extract was found ineffective in inhibiting mycelial growth and spore germination. Possible mechanisms of action of CuNPs are based on changes in the structure and function of fungi cell. Furthermore, these particles can affect macromolecule DNA, its replication and protein synthesis which ultimately lead to death of fungi. Similar studies have been reported for the antifungal activity of CuNPs against different fungi [76,77].
Fig. 10.
Antifungal activity (a) control, (b) plant extract, (c) 1 % CuSO4, and CuNPs (d, e, f) 0.12, 0.18 and 0.24 % respectively.
Table 3.
Effect of CuNPs on in vitro mycelial growth of F. oxysporum.
| Treatment (%) | % Inhibition (mycelial growth) F. oxysporum |
|---|---|
| Control | 0.0 ± 0.0 |
| CuNPs | |
| 0.12 | 59.25 ± 0.57 |
| 0.18 | 73.70 ± 1.52 |
| 0.24 | 76.29 ± 1.52 |
| CuSO4 (1%) | 20.74 ± 1.52 |
| Plant extract | 0.0 ± 0.0 |
| Bavistin (0.1 %) | 100 ± 0.0 |
The mycelial growth inhibition of CuNP was performed in triplicate. Standard deviation values are given in the above mentioned table.
6. Conclusion
In the present study, CuNPs synthesized by a simple and benign method from leaf extract of C. paniculatus. The characterization studies revealed the morphological parameters and role of stabilizing agents during CuNPs synthesis. The TEM and SEM results concluded that the particles were spherical shaped and monodispersed with size ranging from 2 to 10 nm. The purity of green synthesized examined by EDS studies. The flavonoid and other phenolic compounds present in the C. paniculatus leaf extract reduce Cu2+ ions into CuNPs confirmed by FT-IR studies. The DLS studies revealed that biological property of CuNPs enhanced by the size and charge distribution of the NPs. The synthesized CuNPs exploited as photocatalyst exhibited excellent degradation efficiency on organic dye MB under sunlight. The dye adsorption results were compared with previously reported literature. The synthesized CuNPs showed significant antifungal activity against F. oxysporum as demonstrated using the poison food technique. The overall study revealed that CuNPs successfully synthesized by green route and used as photocatalyst and antifungal agents.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The author gratefully acknowledges DBT-JRF fellowship F. No. DBT/JRF/BET 16/I/2016/AL/63 New Delhi, India for financial support to Suresh Chand Mali. The authors also thank UGC-DAE, Indore for TEM, and IIT SAIF, Bombay for SEM-EDS Analysis.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00518.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Naseem K., Begum R., Wu W., Irfan A., Al-Sehemi A.G., Farooqi Z.H. Catalytic reduction of toxic dyes in the presence of silver nanoparticles impregnated core-shell composite microgels. J. Clean. Prod. 2019;211:855–864. doi: 10.1016/j.jclepro.2018.11.164. [DOI] [Google Scholar]
- 2.Daniel S., Syed P.S. Shabudeen Sequestration of carcinogenic dye in waste water by utilizing an encapsulated activated carbon with nano MgO. Int. J. Chemtech Res. 2014;7:2235–2243. [Google Scholar]
- 3.Vasantharaj S., Sathiyavimal S., Saravanan M., Senthilkumar P., Gnanasekaran K., Shanmugavel M., Pugazhendhi A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol. B Biol. 2019;191:143–149. doi: 10.1016/j.jphotobiol.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Latif A., Noor S., Sharif Q.M., Najeebullah M. Different techniques recently used for the treatment of textile dyeing effluents: a review. J. Chem. Soc. Pak. 2010;32:115–116. [Google Scholar]
- 5.Pakzad K., Alinezhad H., Nasrollahzadeh M. Green synthesis of Ni@Fe3O4 and CuO nanoparticles using Euphorbia maculata extract as photocatalysts for the degradation of organic pollutants under UV-irradiation. Ceram. Int. 2019;45:17173–17182. doi: 10.1016/j.ceramint.2019.05.272. [DOI] [Google Scholar]
- 6.Issaabadi Z., Nasrollahzadeh M., Sajadi S.M. Green synthesis of the copper nanoparticles supported on bentonite and investigation of its catalytic activity. J. Clean. Prod. 2017;142:3584–3591. doi: 10.1016/j.jclepro.2016.10.109. [DOI] [Google Scholar]
- 7.Selim Y.A., Azb M.A., Ragab I., Abd El-Azim M.H.M. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-60541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luque P.A., Nava O., Soto-Robles C.A., Vilchis-Nestor A.R., Garrafa-Galvez H.E., Castro Beltran A. Effects of Daucus carota extract used in green synthesis of zinc oxide nanoparticles. J. Mater. Sci. Mater. Electron. 2018;29:17638–17643. doi: 10.1007/s10854-018-9867-5. [DOI] [Google Scholar]
- 9.Kumar P.V., Jelastin Kala S.M., Prakash K.S. Green synthesis derived Pt-nanoparticles using Xanthium strumarium leaf extract and their biological studies. J. Environ. Chem. Eng. 2019;7:103146. doi: 10.1016/j.jece.2019.103146. [DOI] [Google Scholar]
- 10.Olajire A.A., Mohammed A.A. Green synthesis of palladium nanoparticles using Ananas comosus leaf extract for solid-phase photocatalytic degradation of low density polyethylene film. J. Environ. Chem. Eng. 2019;7:103270. doi: 10.1016/j.jece.2019.103270. [DOI] [Google Scholar]
- 11.Khodadadi B., Bordbar M., Nasrollahzadeh M. Achillea millefolium L. extract mediated green synthesis of waste peach kernel shell supported silver nanoparticles: application of the nanoparticles for catalytic reduction of a variety of dyes in water. J. Colloid Interface Sci. 2017;493:85–93. doi: 10.1016/j.jcis.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Behravan M., Panahi A.H., Naghizadeh A., Ziaee M., Mahdavi R., Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019;124:148–154. doi: 10.1016/j.ijbiomac.2018.11.101. [DOI] [PubMed] [Google Scholar]
- 13.Bibi I., Nazar N., Iqbal M., Kamal S., Nawaz H., Nouren S., Rehman F. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: characterization and photo-catalytic activity. Adv. Powder Technol. 2017;28:2035–2043. doi: 10.1016/j.apt.2017.05.008. [DOI] [Google Scholar]
- 14.Sajadi S.M., Nasrollahzadeh M., Maham M. Aqueous extract from seeds of Silybum marianum L. as a green material for preparation of the Cu/Fe3O4 nanoparticles: a magnetically recoverable and reusable catalyst for the reduction of nitroarenes. J. Colloid Interface Sci. 2016;469:93–98. doi: 10.1016/j.jcis.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Nasrollahzadeh M., Sajadi S.M. Preparation of Au nanoparticles by Anthemis xylopoda flowers aqueous extract and their application for alkyne/aldehyde/amine A3-type coupling reactions. RSC Adv. 2015;5:46240–46246. doi: 10.1039/c5ra08927a. [DOI] [Google Scholar]
- 16.Chand Mali S., Raj S., Trivedi R. Biosynthesis of copper oxide nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Rep. 2019;20:100699. doi: 10.1016/j.bbrep.2019.100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajeshkumar S., Menon S., Kumar S.V., Tambuwala M.M., Bakshi H.A., Mehta M., Dua K. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. B Biol. 2019;197:111531. doi: 10.1016/j.jphotobiol.2019.111531. [DOI] [PubMed] [Google Scholar]
- 18.Bordbar M., Negahdar N., Nasrollahzadeh M. Melissa Officinalis L. leaf extract assisted green synthesis of CuO/ZnO nanocomposite for the reduction of 4-nitrophenol and Rhodamine B. Sep. Purif. Technol. 2018;191:295–300. doi: 10.1016/j.seppur.2017.09.044. [DOI] [Google Scholar]
- 19.Nasrollahzadeh M., Sajjadi M., Mohammad Sajadi S. Biosynthesis of copper nanoparticles supported on manganese dioxide nanoparticles using Centella asiatica L. leaf extract for the efficient catalytic reduction of organic dyes and nitroarenes Cuihua Xuebao/Chinese. J. Catal. 2018;39:109–117. doi: 10.1016/S1872-2067(17)62915-2. [DOI] [Google Scholar]
- 20.Nagar N., Devra V. Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater. Chem. Phys. 2018;213:44–51. doi: 10.1016/j.matchemphys.2018.04.007. [DOI] [Google Scholar]
- 21.Nasrollahzadeh M., Mohammad Sajadi S., Rostami-Vartooni A. Green synthesis of CuO nanoparticles by aqueous extract of Anthemis nobilis flowers and their catalytic activity for the A3 coupling reaction. J. Colloid Interface Sci. 2015;459:183–188. doi: 10.1016/j.jcis.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Lee H.J., Song J.Y., Kim B.S. Biological synthesis of copper nanoparticles using Magnolia kobus leaf extract and their antibacterial activity. J. Chem. Technol. Biotechnol. 2013;88:1971–1977. doi: 10.1002/jctb.4052. [DOI] [Google Scholar]
- 23.Hafeez M., Arshad R., Khan J., Akram B., Ahmad M.N., Hameed M.U., Haq S. Populus ciliata mediated synthesis of copper oxide nanoparticles for potential biological applications. Mater. Res. Express. 2019;6:055043. doi: 10.1088/2053-1591/ab0601. [DOI] [Google Scholar]
- 24.Campos E.V.R., Proença P.L.F., Oliveira J.L., Bakshi M., Abhilash P.C., Fraceto L.F. Use of botanical insecticides for sustainable agriculture: future perspectives. Ecol. Indic. 2019;105:483–495. doi: 10.1016/j.ecolind.2018.04.038. [DOI] [Google Scholar]
- 25.Lamichhane J.R., Osdaghi E., Behlau F., Köhl J., Jones J.B., Aubertot J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018;38:28. doi: 10.1007/s13593-018-0503-9. [DOI] [Google Scholar]
- 26.Rashid B., Husnain T., Riazuddin S. Herbicides and pesticides as potential pollutants: a global problem in Plant. Adapt. Phytorem. 2010:427–447. [Google Scholar]
- 27.Rohr J.R., Crumrine P.W. Effects of an herbicide and an insecticide on pond community structure and processes. Ecol. Appl. 2005;15:1135–1147. doi: 10.1890/03-5353. [DOI] [Google Scholar]
- 28.Joshi A., Sharma A., Bachheti R.K., Husen A., Mishra V.K. 2019. Plant-Mediated Synthesis of Copper Oxide Nanoparticles and Their Biological Applications in Nanomaterials and Plant Potential; pp. 221–237. [Google Scholar]
- 29.Kalatehjari P., Yousefian M., Khalilzadeh M.A. Assessment of antifungal effects of copper nanoparticles on the growth of the fungus Saprolegnia sp. on white fish (Rutilus frisii kutum) eggs Egypt. Int. J. Aquat. Res. 2015;41:303–306. doi: 10.1016/j.ejar.2015.07.004. [DOI] [Google Scholar]
- 30.Katwal R., Kaur H., Sharma G., Naushad M., Pathania D. Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity. J. Ind. Eng. Chem. 2015;31:173–184. doi: 10.1016/j.jiec.2015.06.021. [DOI] [Google Scholar]
- 31.Kumar A., Sharma G., Naushad M., Singh P., Kalia S. Polyacrylamide/Ni0.02Zn0.98O nanocomposite with high solar light photocatalytic activity and efficient adsorption capacity for toxic dye removal. Ind. Eng. Chem. Res. 2014;53:15549–15560. doi: 10.1021/ie5018173. [DOI] [Google Scholar]
- 32.Yabuki A., Arriffin N. Electrical conductivity AR of copper nanoparticle thin films annealed at low temperature. Thin Solid Films. 2010;518:7033–7037. doi: 10.1016/j.tsf.2010.07.023. [DOI] [Google Scholar]
- 33.Dang T.M.D., Le T.T.T., Fribourg-Blanc E., Dang M.C. Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011;2:015009. doi: 10.1088/2043-6262/2/1/015009. [DOI] [Google Scholar]
- 34.Lv Q., Zhang B., Xing X., Zhao Y., Cai R., Wang W., Gu Q. Biosynthesis of copper nanoparticles usingShewanella loihica PV-4 with antibacterial activity: Novel approach and mechanisms investigation. J. Hazard. Mater. 2018;347:141–149. doi: 10.1016/j.jhazmat.2017.12.070. [DOI] [PubMed] [Google Scholar]
- 35.Fernández-Arias M., Boutinguiza M., Del Val J., Covarrubias C., Bastias F., Gómez L., Maureira M., Arias-González F., Riveiro A., Pou J. Copper nanoparticles obtained by laser ablation in liquids as bactericidal agent for dental applications. Appl. Surf. Sci. 2020;507:145032. doi: 10.1016/j.apsusc.2019.145032. [DOI] [Google Scholar]
- 36.Han T., Li J., Zhao N., He C. Microstructure and properties of copper coated graphene nanoplates reinforced Al matrix composites developed by low temperature ball milling. Carbon N. Y. 2020;159:311–323. doi: 10.1016/j.carbon.2019.12.029. [DOI] [Google Scholar]
- 37.Abu-Oqail A., Wagih A., Fathy A., Elkady O., Kabeel A.M. Effect of high energy ball milling on strengthening of Cu-ZrO2 nanocomposites. Ceram. Int. 2019;45:5866–5875. doi: 10.1016/j.ceramint.2018.12.053. [DOI] [Google Scholar]
- 38.Sugashima K., Suzuki K., Suzuki T., Nakayama T., Suematsu H., Niihara K. Synthesis of zirconium carbide nanosized powders by pursed wire discharge in oleic acid. J. Korean Phys. Soc. 2016;68:345–350. doi: 10.3938/jkps.68.345. [DOI] [Google Scholar]
- 39.Verma M., Tyagi I., Chandra R., Gupta V.K. Adsorptive removal of Pb (II) ions from aqueous solution using CuO nanoparticles synthesized by sputtering method. J. Mol. Liq. 2017;225:936–944. doi: 10.1016/j.molliq.2016.04.045. [DOI] [Google Scholar]
- 40.Destro P. 2019. Synthesis of Gold-copper Nanoparticles by Colloidal Method Varying the Compositions As a Function of the Synthesis Final Temperature in Colloidal Nanoparticles for Heterogeneous Catalysis; pp. 17–40. [Google Scholar]
- 41.Sabbah I.A., Zaky M.F., Hendawy M.E., Negm N.A. Synthesis, characterization and antimicrobial activity of colloidal copper nanoparticles stabilized by cationic thiol polyurethane surfactants. J. Polym. Res. 2018;25:252. doi: 10.1007/s10965-018-1649-5. [DOI] [Google Scholar]
- 42.Das S., Srivastava V.C. Synthesis and characterization of ZnO/CuO nanocomposite by electrochemical method. Mater. Sci. Semicond. Process. 2017;57:173–177. doi: 10.1016/j.mssp.2016.10.031. [DOI] [Google Scholar]
- 43.Dighore N., Jadhav S., Gaikwad S., Rajbhoj A. Copper oxide nanoparticles synthesis by electrochemical method Medziagotyra. Biomater. Sci. 2016;22:170–173. doi: 10.5755/j01.ms.22.2.7501. [DOI] [Google Scholar]
- 44.Van Viet P., Nguyen H.T., Cao T.M., Van Hieu L. Fusarium antifungal activities of copper nanoparticles synthesized by a chemical reduction method. J. Nanomater. 2016;2016 doi: 10.1155/2016/1957612. [DOI] [Google Scholar]
- 45.Pori M.B., Likozar B., Marinšek M., Crnjak Z. Orel Preparation of Cu/ZnO-based heterogeneous catalysts by photochemical deposition, their characterisation and application for methanol synthesis from carbon dioxide and hydrogen. Fuel Process. Technol. 2016;146:39–47. doi: 10.1016/j.fuproc.2016.02.021. [DOI] [Google Scholar]
- 46.Prema P., Iniya P.A., Immanuel G. Microbial mediated synthesis, characterization, antibacterial and synergistic effect of gold nanoparticles using Klebsiella pneumoniae (MTCC-4030) RSC Adv. 2016;6:4601–4607. doi: 10.1039/c5ra23982f. [DOI] [Google Scholar]
- 47.El-Batal A.I., El-Sayyad G.S., Mosallam F.M., Fathy R.M. Penicillium chrysogenum-mediated mycogenic synthesis of copper oxide nanoparticles using gamma rays for in vitro antimicrobial activity against some plant pathogens. J. Clust. Sci. 2020;31:79–90. doi: 10.1007/s10876-019-01619-3. [DOI] [Google Scholar]
- 48.Hassan S.E.D., Fouda A., Radwan A.A., Salem S.S., Barghoth M.G., Awad M.A., Abdo A.M., El-Gamal M.S. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. 2019;24:377–393. doi: 10.1007/s00775-019-01654-5. [DOI] [PubMed] [Google Scholar]
- 49.Sivaraj A., Kumar V., Sunder R., Parthasarathy K., Kasivelu G. Commercial yeast extracts mediated green synthesis of silver chloride nanoparticles and their anti-mycobacterial activity. J. Clust. Sci. 2020;31:287–291. doi: 10.1007/s10876-019-01626-4. [DOI] [Google Scholar]
- 50.Rahman A., Kumar S., Nawaz T. 2020. Biosynthesis of Nanomaterials Using Algae in Microalgae Cultivation for Biofuels Production; pp. 265–279. [Google Scholar]
- 51.Wen A.M., Steinmetz N.F. Design of virus-based nanomaterials for medicine, biotechnology, and energy. Chem. Soc. Rev. 2016;45:4074–4126. doi: 10.1039/c5cs00287g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pugazhendhi A., Prabhu R., Muruganantham K., Shanmuganathan R., Natarajan S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019;190:86–97. doi: 10.1016/j.jphotobiol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Shen Y., lian Chen B., xiu Zhang Q., zhong Zheng Y., Fu Q. Traditional uses, secondary metabolites, and pharmacology of Celastrus species-a review. J. Ethnopharmacol. 2019;241:111934. doi: 10.1016/j.jep.2019.111934. [DOI] [PubMed] [Google Scholar]
- 54.Sankar R., Manikandan P., Malarvizhi V., Fathima T., Shivashangari K.S., Ravikumar V. Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2014;121:746–750. doi: 10.1016/j.saa.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Sinha T., Ahmaruzzaman M. Green synthesis of copper nanoparticles for the efficient removal (degradation) of dye from aqueous phase. Environ. Sci. Pollut. Res. 2015;22:20092–20100. doi: 10.1007/s11356-015-5223-y. [DOI] [PubMed] [Google Scholar]
- 56.Vincent J.M. Distortion of fungal hyphæ in the presence of certain inhibitors. Nature. 1947;159:850. doi: 10.1038/159850b0. [DOI] [PubMed] [Google Scholar]
- 57.Jana J., Ganguly M., Pal T. Enlightening surface plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv. 2016;6:86174–86211. doi: 10.1039/c6ra14173k. [DOI] [Google Scholar]
- 58.Turakhia B., Divakara M.B., Santosh M.S., Shah S. Green synthesis of copper oxide nanoparticles: a promising approach in the development of antibacterial textiles. J. Coat. Technol. Res. 2020;17:531–540. doi: 10.1007/s11998-019-00303-5. [DOI] [Google Scholar]
- 59.Martínez-Castañón G.A., Niño-Martínez N., Martínez-Gutierrez F., Martínez-Mendoza J.R., Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008;10:1343–1348. doi: 10.1007/s11051-008-9428-6. [DOI] [Google Scholar]
- 60.Długosz O., Chwastowski J., Banach M. Hawthorn berries extract for the green synthesis of copper and silver nanoparticles. Chem. Pap. 2020;74:239–252. doi: 10.1007/s11696-019-00873-z. [DOI] [Google Scholar]
- 61.Al-Hakkani M.F. Biogenic copper nanoparticles and their applications: a review. SN Appl. Sci. 2020;2:1–20. doi: 10.1007/s42452-020-2279-1. [DOI] [Google Scholar]
- 62.Akturk A., Güler F.K., Taygun M.E., Goller G., Küçükbayrak S. Synthesis and antifungal activity of soluble starch and sodium alginate capped copper nanoparticles. Mater. Res. Express. 2019;6:1250g3. doi: 10.1088/2053-1591/ab677e. [DOI] [Google Scholar]
- 63.Zhang H., Lv X., Li Y., Wang Y., Li J. P25-graphene composite as a high performance photocatalyst. ACS Nano. 2010;4:380–386. doi: 10.1021/nn901221k. [DOI] [PubMed] [Google Scholar]
- 64.Lin L., Qiu P., Cao X., Jin L. Colloidal silver nanoparticles modified electrode and its application to the electroanalysis of Cytochrome c. Electrochim. Acta. 2008;53:5368–5372. doi: 10.1016/j.electacta.2008.02.080. [DOI] [Google Scholar]
- 65.Sankar R., Karthik A., Prabu A., Karthik S., Shivashangari K.S., Ravikumar V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces. 2013;108:80–84. doi: 10.1016/j.colsurfb.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 66.Bhakya S., Muthukrishnan S., Sukumaran M., Muthukumar M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016;6:755–766. doi: 10.1007/s13204-015-0473-z. [DOI] [Google Scholar]
- 67.Sankar R., Maheswari R., Karthik S., Shivashangari K.S., Ravikumar V. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C. 2014;44:234–239. doi: 10.1016/j.msec.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 68.Alkaim A.F., Aljeboree A.M., Alrazaq N.A., Baqir S.J., Hussein F.H., Lilo A.J. Effect of pH on adsorption and photocatalytic degradation efficiency of different catalysts on removal of methylene blue. Asian J. Chem. 2014;26:8445. doi: 10.14233/ajchem.2014.17908. [DOI] [Google Scholar]
- 69.Sharma A., Dutta R.K. Studies on the drastic improvement of photocatalytic degradation of acid orange-74 dye by TPPO capped CuO nanoparticles in tandem with suitable electron capturing agents. RSC Adv. 2015;5:43815–43823. doi: 10.1039/c5ra04179a. [DOI] [Google Scholar]
- 70.Singh R., Dutta S. Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties. Adv. Powder Technol. 2018;29:211–219. doi: 10.1016/j.apt.2017.11.005. [DOI] [Google Scholar]
- 71.Paul B., Bhuyan B., Dhar Purkayastha D., Dey M., Dhar S.S. Green synthesis of gold nanoparticles using Pogestemon benghalensis (B) O. Ktz. Leaf extract and studies of their photocatalytic activity in degradation of methylene blue. Mater. Lett. 2015;148:37–40. doi: 10.1016/j.matlet.2015.02.054. [DOI] [Google Scholar]
- 72.Cittrarasu V., Balasubramanian B., Kaliannan D., Park S., Maluventhan V., Kaul T., Liu W.C., Arumugam M. Biological mediated Ag nanoparticles from Barleria longiflora for antimicrobial activity and photocatalytic degradation using methylene blue Artif. Cells. Nanomedicine Biotechnol. 2019;47:2424–2430. doi: 10.1080/21691401.2019.1626407. [DOI] [PubMed] [Google Scholar]
- 73.Elango G., Roopan S.M. Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue Dye. J. Photochem. Photobiol. B Biol. 2016;155:34–38. doi: 10.1016/j.jphotobiol.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Ranjith R., Renganathan V., Chen S.M., Selvan N.S., Rajam P.S. Green synthesis of reduced graphene oxide supported TiO2/Co3O4 nanocomposite for photocatalytic degradation of methylene blue and crystal violet. Ceram. Int. 2019;45:12926–12933. doi: 10.1016/j.ceramint.2019.03.219. [DOI] [Google Scholar]
- 75.Chen L., Batjikh I., Hurh J., Han Y., Huo Y., Ali H., Li J.F., Rupa E.J., Ahn J.C., Mathiyalagan R., Yang D.C. Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik (Stuttg) 2019;184:324–329. doi: 10.1016/j.ijleo.2019.03.051. [DOI] [Google Scholar]
- 76.Gomaa E.Z., Housseiny M.M., Omran A.A.A.K. Fungicidal efficiency of silver and copper nanoparticles produced by Pseudomonas fluorescens ATCC 17397 against four Aspergillus species: a molecular study. J. Clust. Sci. 2019;30:181–196. doi: 10.1007/s10876-018-1474-3. [DOI] [Google Scholar]
- 77.Pariona N., Mtz-Enriquez A.I., Sánchez-Rangel D., Carrión G., Paraguay-Delgado F., Rosas-Saito G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019;9:18835–18843. doi: 10.1039/c9ra03110c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.