Abstract
Hypereosinophilic cardiomyopathy is a rare restrictive cardiomyopathy which often presents with left-sided heart failure. We present an interesting case of a 58-year-old male patient with known hypereosinophillic syndrome who had presented with congestive cardiac failure with predominant features of right-sided volume overload. Cardiac magnetic resonance imaging confirmed the diagnosis by demonstrating obliteration of the right ventricular apex and endocardial-delayed gadolinium enhancement at the mid-septal and inferior segment, at both the right ventricular and left ventricular apical region, consistent with fibrosis. He was successfully treated with intravenous diuretics to good effect.
This case report demonstrates a rare clinical presentation of right ventricle involvement in hypereosinophilic cardiomyopathy and the current pathway for diagnosis with the favoured emergence of cardiac magnetic resonance imaging. All patients with hypereosinophilic syndrome should be closely monitored for signs of cardiac involvement, as early treatment carries a better prognosis.
Keywords: Hypereosinophillic cardiomyopathy, Restrictive cardiomyopathy, Hypereosinophillic syndrome, Loeffler endocarditis, Cardiac magnetic resonance imaging, Heart failure
Introduction
Hypereosinophillic myocarditis is a rare multistage disorder characterized by eosinophil infiltration and degranulation in the myocardium, leading to a spectrum of inflammation, tissue damage, thrombosis, and eventually fibrosis [1]. The final stage was initially described by Löffler in 1936 as Loeffler's endocarditis, and is characterized by endomyocardial fibrosis causing restrictive cardiomyopathy [2]. This often presents with symptoms of left-sided heart failure such as dyspnea and pulmonary edema.
Any persistent hypereosinophilic state can predispose to the development of hypereosinophillic restrictive cardiomyopathy. The most common cause is hypereosinophilic syndrome (HES), which is a heterogenous group of hematological conditions characterised by persistent idiopathic peripheral eosinophilia (>1.5 × 109/L) for longer than 6 months, lack of evidence for other causes of eosinophilia, and end-organ damage [1,3]. HES is a rare disorder with an incidence of approximately 0.035 per 100,000, and heart involvement is found in 20%-75% of patients with HES [3], [4], [5]. Other causes include parasitic infections, hypersensitivity drug reactions, clonal myeloid disorders, neoplastic disorders, and autoimmune conditions [1].
Echocardiogram should be performed first as it is cheap and can lead to diagnosis if characteristic features are seen. However, electrocardiogram (ECG) and transthoracic echocardiogram (TTE) findings can be nonspecific in hypereosinophilic cardiomyopathy, and cardiac magnetic resonance imaging (CMRI) has emerged as a newer modality for the diagnosis of cardiac disease in HES [1,4,6]. CMRI can detect ventricular thrombi with a higher degree of sensitivity and specificity than echocardiography and in addition contrast-enhanced CMRI can identify inflammation and fibrosis [7]. Thus, CMRI may be able to detect myocardial abnormalities in the early stages of the cardiac involvement in HES (the acute myocarditis/necrotic stage) prior to echocardiographic abnormalities. Endomyocardial biopsy is the only definite diagnostic modality to confirm hypereosinophilic cardiomyopathy by detecting eosinophilic infiltration of the myocardium, though this is associated with a degree of morbidity [1,8,9].
Here, we report a rare case of right-sided heart failure secondary to restrictive cardiomyopathy on a background of HES. This case demonstrates the clinical presentation, recent developments in the diagnostic pathway, and treatment options available for this rare condition.
Case report
A 58-year-old man presented to the emergency department with the complaint of bilateral swollen scrotum. On examination, he appeared to be in profound fluid overload, with gross pitting edema up to his abdomen, raised jugular venous pressure, edematous scrotum, bibasal chest crackles, and was short of breath at rest. He also had an ejection systolic murmur. This was on a background of asthma and HES for which he was followed up by hematology and taking imatinib. His past medical history included a recent venous thromboembolism, for which he was taking apixaban.
Initial chest X-ray showed a right lower zone pleural effusion, and ECG showed nonspecific changes. Computerized tomography pulmonary angiogram confirmed moderate volume right pleural effusion with associated atelectasis of underlying lower lung, with no obvious pulmonary embolism. Ultrasound of the scrotum revealed only edematous tissue, with no evidence of inflammatory testicular pathology. His fluid overload was, therefore, treated with furosemide infusion with good effect.
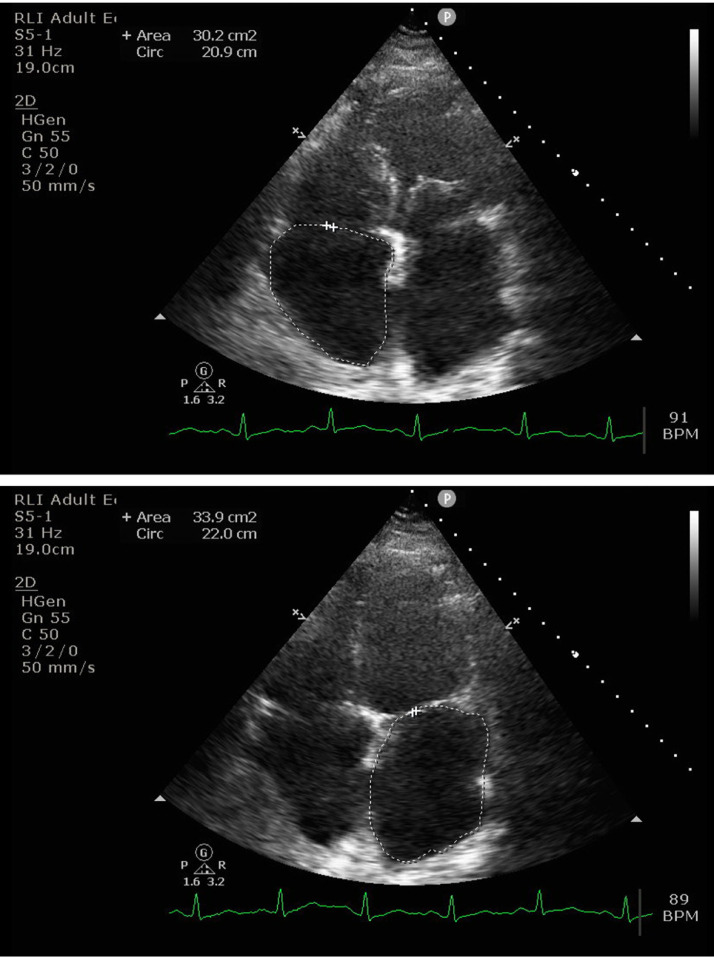
A TTE conducted 2 years previously had shown good left ventricular (LV) function. TTE this admission showed LV systolic function was still preserved, with ejection fraction >55%. LV cavity size was within normal limits, though the mid-anterior/septal and basal inferior wall regions appeared hypokinetic, and there was mildly uncoordinated septal motion. TTE also showed normal right ventricle (RV) size, and RV function appeared to be well preserved. There was moderate mitral regurgitation, mild-to-moderate tricuspid regurgitation, and biatrial enlargement (Fig. 1, Supplementary material 1-3). The anterior mitral valve leaflet was thin and mobile, and the posterior leaflet was thickened with reduced mobility. He was optimized on diuretics and was discharged home with outpatient CMRI to investigate restrictive cardiomyopathy. He had a total weight reduction from 110.6 kg to 91.4 kg after intravenous diuretic therapy and was asymptomatic on discharge.
Fig 1.
Transthoracic echocardiogram demonstrating biatrial enlargement.
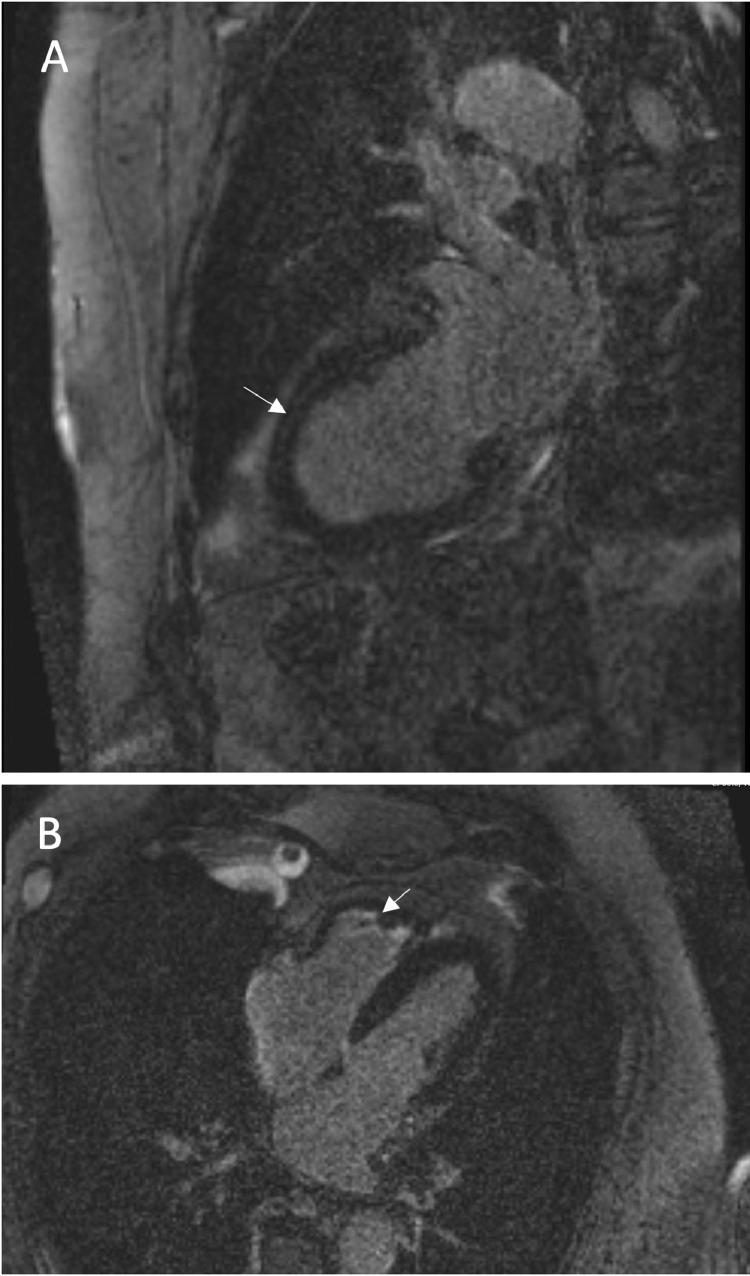
CMRI confirmed dilation of both atria, with the right atrium moderately dilated (29 cm2) and the left atrium severely dilated (33cm2). Unlike the TTE findings, on CMRI the RV had low diastolic volume (RV end-diastolic volume index 49 mL/m2) and was hypertrophic particularly at the apical region with obliteration of the RV apex. RV ejection fraction was low to normal. There was very mildly impaired LV ejection fraction (52.2%), and hypokinesia at the same regions identified on TTE with normal LV volume and wall thickness. There was also endocardial delayed gadolinium enhancement at the mid-septal and inferior segment, at both RV and LV apical region, consistent with fibrosis (Fig. 2). These findings were in keeping with cardiomyopathy associated with HES. The patient was subsequently followed up by hematology, who continued with imatinib treatment for HES.
Fig 2.
Cardiac MRI showing endocardial delayed gadolinium enhancement annotated with arrows at (A) the mid-septal segment and (B) RV and LV apical regions, consistent with fibrosis.
Discussion
Our patient presented with congestive cardiac failure and like many previously reported cases, he was in his 6th to 7th decade of life [10], [11], [12], [13], [14], [15], [16]. He was unusual in having greater right-sided myocardial involvement, as most cases of hypereosinophillic cardiomyopathy have reportedly greater involvement of the LV apical endocardium, leading to diastolic impairment [11,15,16]. However, cases of RV involvement affecting the apex and conducting system have been reported in literature, but there is no specific figure for incidence [12], [13], [14].
Hypereosinophillic cardiomyopathy is a subset of restrictive cardiomyopathy, and it is important to distinguish this from other causes. The common ECG changes associated with HES are nonspecific and include left atrial enlargement, LV hypertrophy, left axis deviation, T wave inversion, incomplete right bundle branch block, poor R wave progression, and first-degree atrioventricular block [4]. TTE can indicate a restrictive cardiomyopathy with ventricular wall thickening, often with apical thrombus and posterior mitral valve leaflet involvement [4,17]. In our case, TTE indicated hypokinetic regions with atrial enlargement, moderate mitral regurgitation with thickened posterior mitral leaflet with restricted mobility.
CMRI is an effective modality for accurately diagnosing eosinophilic cardiomyopathy, as it is able to characterize endocardial tissue in greater detail compared to TTE and can detect myocardial fibrosis and inflammation [17,18]. Findings which suggest hypereosinophillic cardiomyopathy include diffuse subendocardial delayed enhancement after intravenous administration of gadolinium and thrombus formation [18]. The first is reflective of fibrosis of the endocardium, and the second is reflective of the thrombogenic state associated with eosinophilia [3,18]. CMRI is also more accurate than TTE at visualizing RV structure and function, and in our case CMRI was able to identify hypereosinophilic cardiomyopathy involvement in the RV.
Endomyocardial biopsy still remains the gold standard to confirm the infiltration of eosinophils on histology [14,19]. However, this is associated with a complication rate of around 6% and cannot be carried out in patients with clinical factors such as thrombocytopenia [19]. In this case biopsy was not performed as this was a confirmed case of HES and CMRI showed typical features consistent with hypereosinophilic cardiomyopathy. Biopsy also comes with inherent sampling error which affects diagnostic sensitivity. Diagnosis purely on the basis of CMRI findings without endomyocardial biopsy has been reported in patients, and CMRI was able to exclude myocarditis and coronary artery disease [10,15,20]. CMRI is also a promising modality to follow-up patients to assess response to therapy, as it enables the comparison of serial images [16,18]. It is also more practical than endomyocardial biopsy as a screening tool to potentially diagnose hypereosinophilic cardiomyopathy in high risk patients [18].
Echocardiography should be used to follow documented cardiac disease at relatively frequent intervals (<6 months) [7]. If no cardiac abnormalities are seen initially in the evaluation of HES, repeat evaluation and imaging should take place at least every 6 months [7].
The management of such a patient initially presenting in secondary care first involves treatment of acute heart failure symptoms, then elucidation of whether the eosinophilia can be correctable. If diagnosed early, treatment with corticosteroids and cytotoxic medications such as imatinib can be beneficial [14]. Congestive heart failure should be managed by current heart failure guidelines, which include diuretic therapy, β -blockade, and ACE inhibitors. LV or RV thrombi or embolic phenomena would indicate the need for anticoagulation therapy. The duration of the therapy is best determined by the ongoing presence of ventricular thrombi along with the status of the endomyocardial disease activity. Valvular disease that is significant and contributing to ongoing heart failure symptoms may require surgical intervention [12]. Some patients may benefit from surgery to strip the endocardium and decorticate fibrosed tissues to decrease ventricular filling pressures [12]. Endocardectomy is not without risks, as this may lead to damage of the conduction system and the subvalvular area [12].
This case report demonstrates the clinical presentation of a rare restrictive cardiomyopathy causing heart failure, and the process of diagnosis and treatment. It also highlights the importance of regular monitoring in patients with persistent eosinophilia to allow for the detection of cardiac involvement early. This patient had known HES but was not regularly assessed for hypereosinophilic myocarditis. As 20%-75% of HES patients will have cardiac involvement, a regular screening program with CMRI will prevent patients presenting late in secondary care with profuse heart failure symptoms.
Lessons
-
•
Suspect a diagnosis of hypereosinophilic cardiomyopathy in patients with persistent eosinophilia and new heart failure symptoms.
-
•
Serial imaging with CMRI can be useful in patients with persistent eosinophilia to monitor cardiac involvement, allowing early treatment with steroids and cytotoxic medications to be initiated.
-
•
CMRI is the gold standard noninvasive imaging modality for diagnosis.
-
•
Endomyocardial biopsy remains gold standard for diagnosis, but is associated with considerable morbidity.
-
•
Surgery including endocardectomy and valve replacement to remove fibrosed tissue may be necessary in some patients.
Patient consent
The patient gave informed consent for the writing and publication of this case report. All identifyingpersonal information has been removed in order to anonymise the case report.
Footnotes
Declarations:
This paper is not under consideration elsewhere.
None of the paper's contents have been previously published.
All authors have read and approved the manuscript.
Declaration of Competing Interest: The authors declare that there is no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2020.08.007.
Appendix. Supplementary materials
References
- 1.Kuchynka P, Palecek T, Masek M, Cerny V, Lambert L, Vitkova I. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. Biomed Res Int. 2016;2016 doi: 10.1155/2016/2829583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome. Analysis of fourteen cases with review of the literatur. Medicine (Baltimore). 1975;54:1–27. [PubMed] [Google Scholar]
- 3.Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–1325. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrillo JE, Borer JS, Henry WL, Wolff SM, Fauci AS. The cardiovascular manifestations of the hypereosinophilic syndrome. Prospective study of 26 patients, with review of the literature. Am J Med. 1979;67(4):572–582. doi: 10.1016/0002-9343(79)90227-4. [DOI] [PubMed] [Google Scholar]
- 5.Crane MM, Chang CM, Kobayashi MG, Weller PF. Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence. J Allergy Clin Immunol. 2010;126:179–181. doi: 10.1016/j.jaci.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mankad R, Bonnichsen C, Mankad S. Hypereosinophilic syndrome: cardiac diagnosis and management. Heart. 2016;102(2):100–106. doi: 10.1136/heartjnl-2015-307959. [DOI] [PubMed] [Google Scholar]
- 8.Rammos A, Meladinis V, Vovas G, Patsouras D. Restrictive cardiomyopathies: the importance of noninvasive cardiac imaging modalities in diagnosis and treatment-a systematic review. Radiol Res Pract. 2017;2017 doi: 10.1155/2017/2874902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mankad R, Bonnichsen C, Mankad S. Hypereosinophilic syndrome: cardiac diagnosis and management. Heart. 2016;102:100–106. doi: 10.1136/heartjnl-2015-307959. [DOI] [PubMed] [Google Scholar]
- 10.van Kessel D, Jerzewski A, Kardux J, Habets J. Loeffler's endocarditis. Eur Heart J. 2014;16(3):343. doi: 10.1093/ehjci/jeu208. [DOI] [PubMed] [Google Scholar]
- 11.Park J, Hemu M, Kalra D. Loeffler's endocarditis: a diagnosis made with cardiac magnetic resonance (CMR) imaging. J Am Coll Cardiol. 2019;73(9 Supplement 1):2267. doi: 10.4250/jcvi.2019.27.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alam A, Thampi S, Saba SG, Jermyn R. Loeffler endocarditis: a unique presentation of right-sided heart failure due to eosinophil-induced endomyocardial fibrosis. Clin Med Insights Case Rep. 2017;10 doi: 10.1177/1179547617723643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Çetin S, Heper G, Gökhan Vural M, Hazirolan T. Loeffler endocarditis: silent right ventricular myocardium! Wien Klin Wochenschr. 2016;128(13-14):513–515. doi: 10.1007/s00508-016-0981-1. [DOI] [PubMed] [Google Scholar]
- 14.Beedupalli J, Modi K. Early-stage Loeffler's endocarditis with isolated right ventricular involvement: management, long-term follow-up, and review of literature. Echocardiography. 2016;33(9):1422–1427. doi: 10.1111/echo.13264. [DOI] [PubMed] [Google Scholar]
- 15.ten Oever J, Theunissen LJ, Tick LW, Verbunt RJ. Cardiac involvement in hypereosinophilic syndrome. Neth J Med. 2011;69(5):240–244. [PubMed] [Google Scholar]
- 16.Debl K, Djavidani B, Buchner S, Poschenrieder F, Heinicke N, Feuerbach S. Time course of eosinophilic myocarditis visualized by CMR. J Cardiovasc Magn Reson. 2008;10:21. doi: 10.1186/1532-429X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ommen SR, Seward JB, Tajik AJ. Clinical and echocardiographic features of hypereosinophilic syndromes. Am J Cardiol. 2000;86(1):110–113. doi: 10.1016/s0002-9149(00)00841-9. [DOI] [PubMed] [Google Scholar]
- 18.Syed IS, Martinez MW, Feng DL, Glockner JF. Cardiac magnetic resonance imaging of eosinophilic endomyocardial disease. Int J Cardiol. 2008;126(3):e50–e52. doi: 10.1016/j.ijcard.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 19.From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc. 2011;86(11):1095–1102. doi: 10.4065/mcp.2011.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharabish A, Haroun D. Cardiac MRI findings of endomyocardial fibrosis (Loeffler's endocarditis) in a patient with rheumatoid arthritis. J Saudi Heart Assoc. 2015;27(2):127–131. doi: 10.1016/j.jsha.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.