Abstract
Pierre Robin sequence (PRS) is a craniofacial abnormality comprising micrognathia, glossoptosis and airway obstruction, which can impair the newborn’s feeding and breathing. While there has been much research around the cause of PRS and most appropriate methods of care, understanding the psychosocial aspects of a PRS diagnosis from the parents’ perspective is lacking. The aim of this study is to understand parental experiences of having a child diagnosed with PRS, as well as the role of genetic counselling in PRS. Fourteen semi-structured interviews were conducted with parents of children diagnosed with isolated PRS between 2 and 5 years prior. From these 14 interviews, eleven transcripts were analysed to find common themes and experiences. The diagnosis was confusing and overwhelming for participants during emotionally sensitive periods and little was understood about the cause of their child’s PRS. Those participants who did recall experiences with genetic services reported that they were minimal and uninformative. According to participant recollection, genetic counselling was rarely offered, despite there being a potential for this service in PRS. Genetic counselling would be a valuable source of information and support for parents both at the time of antenatal diagnosis, and potentially 6 to 12 months later in the outpatient environment when these children are all routinely reviewed by their clinical care team.
Keywords: Pierre Robin sequence, Genetic counselling, Parents, Experience, Diagnosis
Introduction
Pierre robin sequence (PRS) is characterized by a triad of micrognathia, glossoptosis, and airway obstruction, with or without a cleft palate (Tan et al. 2013). For infants with PRS, the severity of micrognathia and glossoptosis varies greatly, as too does the degree of respiratory and feeding difficulties (Morice et al. 2018). PRS is most commonly diagnosed within hours or days after birth, although antenatal detection of micrognathia accounts for around 7% of cases of PRS (Lind et al. 2015). An antenatal diagnosis allows for anticipation of breathing and feeding difficulties at birth and facilitates prompt intervention (Soulier et al. 2002).
There are three diagnostic categories used to classify PRS: syndromic or non-syndromic PRS, and PRS plus. Syndromic PRS is when the triad is present as a part of a genetic syndrome, such as, Stickler syndrome, velocardiofacial syndrome (22q11.2 deletion), or Treacher Collins syndrome (Izumi et al. 2012; Marcellus 2001). Non-syndromic PRS is ‘isolated’ where the triad comprises the only clinical feature in an otherwise healthy infant, and ‘PRS plus’ is characterized by the presence of other congenital abnormalities alongside the triad that do not indicate a known syndrome (Izumi et al. 2012; Van den Elzen et al. 2001).
Although there are some similar feeding and surgical requirements between PRS and other craniofacial abnormalities, for example, cleft palate, PRS has the additional and urgent challenge of airway obstruction and breathing difficulty which takes priority (Clarren et al. 1987; Denny et al. 1990). Therefore, it is possible that the experience of a PRS diagnosis and management may be quite different to other craniofacial abnormalities.
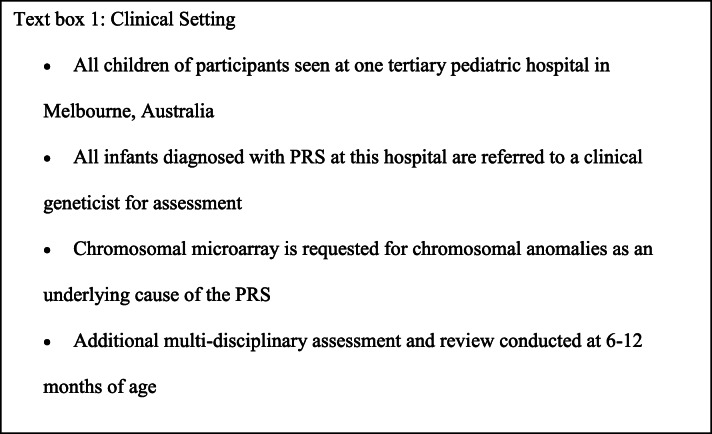
Determining the cause of PRS in a child can be challenging, especially when there is no known family history to indicate a possible genetic basis (Prows and Bender 1999). It is important to determine if there is a syndromic form of PRS because this will impact care, allow for anticipation of other clinical features, and inform recurrence risk information for the parents and other family members (Schreiner et al. 1973; Snead and Yates 1999; Tan et al. 2013). A clinical geneticist is usually recommended to assess whether the PRS is isolated or syndromic, and investigations may include molecular karyotyping and a detailed family pedigree (Gangopadhyay et al. 2012; Tan et al. 2013). Since many of the additional syndromic features do not present until later in childhood, a family with Stickler syndrome, for example, can go undetected for a long period of time, and possibly see recurrence in multiple children (Izumi et al. 2012; Snead and Yates 1999). Little is known about parents’ experiences of having a child with PRS. Only two studies were identified including an exploration of how to teach parents about PRS (Agrafiotis 1972) and an investigation of parents’ experiences of their child’s tracheostomy to relieve airway obstruction (Demke et al. 2008). The need for intervention and critical care for newborns with PRS has significant psychosocial ramifications for parents. However, without empirical evidence of parents’ experiences, any unmet needs generated by these implications remain undocumented. Further, the intersection during this period with genetic health professionals is also important as genetic counsellors possess skills that may be useful in providing support to parents. Yet even less is known about how parents perceive and experience these healthcare professionals’ involvement within their child’s care pathway. Therefore, this study aims to examine parents’ lived experience of their child’s diagnosis of PRS. This study explores the period at and after the time of diagnosis, the subsequent care and management required, and the supports accessed by parents. For information about the clinical setting for this study cohort, see Text Box 1.
Methods
Participants
Parents were purposively sampled from a list of children from the Melbourne Cleft Registry based at The Royal Children’s Hospital, Victoria, Australia, to identify those whose child was diagnosed with isolated PRS between 2 and 5 years prior. These parents were invited to participate in a qualitative, semi-structured interview with invitations to participate sent via mail to the address listed in the child’s medical records. The presence of a cleft palate was not a requirement for inclusion in the study. Ethics approval for this study was granted by the Human Research Ethics Committee at the Royal Children’s Hospital, Victoria Australia (12th of December 2014, HREC 34245 A).
Data collection and analysis
Interviews were conducted from April 2015 to July 2015 either in person or via telephone, depending on participant preference. All interviews were conducted by researcher RS who at the time of the study was a post-graduate student at The University of Melbourne. RS did not have any formal experience or training in conducting interviews, and all participants met her for the first time during the single interview. Participants were aware that the interviews were being conducted as part of a requirement for the degree of Master of Genetic Counselling. An interview schedule was used to guide the interviews and examined the following topics: (1) perceptions of antenatal detection and diagnosis; (2) timing and delivery of genetic information; and (3) participants’ recall of genetic service involvement and supports offered. The interviews were digitally audio-recorded and transcribed verbatim, transcripts were de-identified and pseudonyms were assigned to participants and their family members. These pseudonyms have been used in the results section of this publication. Field notes were not taken during the interviews, and interview transcripts were not returned to participants for review.
Transcripts were independently coded by members of the research team (RS, LF, NK and SR), and coding was compared for consistency. All transcripts were reviewed and analysed using a thematic analysis method until no additional themes could be drawn from the information. Both broad and more discrete codes were assigned to participant statements using NVivo qualitative data analysis software (QSR International Pty Ltd 2012).
Results
Of the 34 families invited to participate in the study, one family declined to participate, 14 families consented to being contacted for an interview, and 19 families did not respond. No further attempts were made to contact families who did not respond. Fourteen interviews were conducted with parents who had a child diagnosed with PRS. Participants who elected face-to-face interviews were given the option of conducting the interview at their home or at The Royal Children’s Hospital. The mean interview time was 45 min (range, 18–63 min). However, three interviews were excluded after information was revealed during the interviews indicating that the child could not be classified as having isolated PRS (see Table 1). In addition, another child had recently received a diagnosis of Stickler syndrome in the months prior to the interview. The authors chose to retain this interview because the diagnosis was made after the neonatal period and, therefore, would not have affected their experiences of their daughter’s initial diagnosis of PRS and subsequent management. All children in the study were found to have a cleft palate.
Table 1.
Summary of participant and interview characteristics
| Interview | Participant name* | Child’s gender | Child’s age | Interview mode | Siblings | Time when PRS suspected | Family history |
|---|---|---|---|---|---|---|---|
| 1 | (excluded) | Female | 3 | Face-to-face | |||
| 2 | Natalie and David | Male | 6 | Face-to-face | 1 older brother | Antenatal | Nil relevant |
| 3 | Claire | Male | 4 | Phone | None | Postnatal | Nil relevant |
| 4 | Megan | Female | 4 | Face-to-face | 1 older sister | Postnatal | Nil relevant |
| 5 | Stacey | Male | 3 | Phone | 1 older brother | Antenatal | Mother dx with PRS following son’s dx |
| 6 | (excluded) | Female | 4 | Phone | |||
| 7 | Donna | Male | 4 | Phone | Pregnant with 2nd child at time of interview | Postnatal | Nil relevant |
| 8 | (excluded) | Female | 3 | Face-to-face | |||
| 9 | Louise | Male | 5 | Phone | MCDA twin brother without PRS | Postnatal | Nil relevant |
| 10 | Naomi | Female | 6 | Face-to-face | 1 older brother and 1 younger sister | Postnatal | Nil relevant |
| 11 | James and Lauren | Female | 3 | Face-to-face | None | Postnatal | Mother dx with Stickler syndrome following daughter’s dx |
| 12 | Kim | Female | 3 | Face-to-face | None | Antenatal | Nil relevant |
| 13 | Grace | Female | 4 | Face-to-face | 1 younger brother | Postnatal | Nil relevant |
| 14 | Michelle | Male | 3 | Phone | 1 younger sister | Antenatal | Nil relevant |
Dx, diagnosis, diagnosed; MCDA, mono-chorionic di-amniotic; *Pseudonyms used to maintain anonymity
Across all the interviews, the time of their child’s diagnosis and hospital inpatient stay stood out as the most significant and challenging periods for participants. Confusion, uncertainty, and helplessness tied participant experiences together across what were very theme rich interviews. However just three themes have been selected for this paper to illustrate the role of genetic health professionals in providing information and support to parents during this crucial time. The first key theme describes participants’ experiences and perceptions of antenatal diagnosis for PRS, the second examines the timing and delivery of genetic information about PRS, and the third describes participant’s recollection of the involvement of genetic services during the neonatal period.
All the participants’ children received a formal diagnosis of PRS in the neonatal period; however, for four of the children, micrognathia was detected on antenatal ultrasound. This resulted in discussions of other possible abnormalities and the potential future clinical implications for the child. Participants who did not have any PRS-related anomalies detected on prenatal ultrasound hypothesized about the positives and negatives of receiving an antenatal diagnosis of PRS. Regardless of when the participants first learnt of PRS, many had little understanding about the cause of their child’s PRS. Nevertheless, it was the immediate healthcare needs of their child which participants were most concerned about during the first months of their child’s life. These health-related concerns initially relegated information about the cause of their child’s PRS to a lower priority. While most participants described a brief encounter with a clinical geneticist during the time their child was an inpatient, only one participant recalled meeting a genetic counsellor to discuss her child’s PRS.
Perceptions of antenatal detection and diagnosis
For the participants, four of whom had antenatal detection of micrognathia, there were both positives and negatives surrounding antenatal detection. Those who did not have any antenatal indications (seven families) also reflected how they thought an antenatal diagnosis might have changed their experience.
Perceived benefits of antenatal detection
Most participants, regardless of the timing of their child’s PRS diagnosis, could appreciate that an antenatal detection might make the neonatal period for a baby with PRS less confronting by allowing time to process information and prepare emotionally and practically by meeting with hospital staff.
I think, forewarned is forearmed so I think that, potentially knowing beforehand might have made the emotional rollercoaster of it, a little bit easier […] having the opportunity to meet with doctors or come and see the hospital and understand what this was [going to] look like. (Grace; postnatal detection)
It’s really great that we were diagnosed at twelve weeks like that […] apparently usually doesn’t happen that early […] I think yeah it was it was really good to prepare us. (Kim; antenatal detection)
While some said they would prefer one experience over another, Michelle could see the positives and negatives of both. Micrognathia was detected on foetal ultrasound during her pregnancy with her son, and she reflected:
I suppose in terms of practicality and in terms of adjustment and preparation, there might be something good about knowing when you’re pregnant because you can, begin that journey of adjustment and make sure you have all your supports around you and get things in place. (Michelle; antenatal detection)
Perceived harms or limitations of antenatal detection
Antenatal detection of micrognathia also came with challenges for some participants with increased anxiety during the pregnancy and uncertainty about what this finding means for their baby. In contrast to the quote above, Michelle also described:
The pregnancy was a bit, interrupted, and I couldn’t just enjoy it and relax because I had this cloud hanging over my head that something might be wrong […] I think women lose the joy of the pregnancy, and there is something about actually not knowing that […] is liberating. (Michelle; antenatal detection)
Other participants reflected feeling glad that there had not been any antenatal detection during their pregnancy, in part because the spectrum of severity of PRS meant an antenatal detection could have been unhelpful when “there’s so much they can’t tell you” (Michelle; antenatal detection):
I’m quite glad that we didn’t know about her small jaw when we were pregnant because I guess the stories I’ve heard about what a small jaw means, and all the implications […] I think if we had known that in advance there would have been a lot more anxiety so I think it was best that we didn’t know, had to deal with it when she was born. (Megan; postnatal detection)
I guess we’d prepared ourselves for the worst case scenario, I think the whole process of the pregnancy was such an anxious wait and we kind of didn’t know what to expect and, and I don’t think any doctors could really prepare you for what […] could possibly happen. (Kim; antenatal detection)
One participant, whose son was diagnosed with PRS postnatally, felt that antenatal detection of micrognathia or PRS would have altered her reproductive decision-making:
If I was told, this sounds awful I know, but if I was told at my twelve week scan that this baby is going to have all these problems, I wouldn’t have been able to do it I would have gone “I can’t do that and that’s not fair” and I probably would have terminated my pregnancy. (Claire; postnatal detection)
Timing and delivery of genetic information
The participants described feeling confronted with a lot of foreign and confusing information, often at a time when they were not able to completely absorb it. This was something expressed consistently across all interviews.
The shocking nature of a PRS diagnosis
When information was given to participants at the time of diagnosis, sometimes the manner in which it was done is the thing they remember most strongly. Louise recalled the diagnosis of PRS in her son the morning after his delivery:
[Doctor] handed me a photocopy probably Wikipedia or something like that of Pierre Robin […] a two page read […] I was on my own […] then they sort of just walked out […] I was quite distressed. (Louise; postnatal detection)
Other participants were simply not ready to have a discussion about PRS so soon after delivery of their baby, and the timing of the diagnosis became very important:
When she was born and the pediatrician came in and he was talking about Stickler syndrome and PRS and all this sort of stuff, and I just hated this man’s guts I was just like “get out of here!” like you know “leave us alone!” […] I mean he was an incredible guy but at the time it was ‘A’ it was shock, ‘B’ it was way too much information too quickly, and you know ‘C’ it was they were taking my baby away so I had a very negative reaction. (Lauren; postnatal detection)
For one participant in particular, the first indication she was given about any health problems for her baby did not even include being informed of the condition suspected. Instead the healthcare professionals approached this discussion from very different angle:
Four or five people just suddenly standing round us and I remember the nurse in charge […] the first thing she said to me was “we’ve sorted out special parking vouchers” (laughing) “for you” and I was thinking “why do I need parking vouchers?” and then we got given the diagnosis of Pierre Robin sequence by the pediatrician […] you’re really told worst case scenario at that stage […] as it turns out with us none of those scenarios have been relevant to Poppy’s life […] I don’t know whether there’s a better way that you can tell people really. (Megan; postnatal detection)
When genetic information was not retained
For many participants, their first priority was their baby’s health and wellbeing during the newborn period after the diagnosis of PRS. They described themselves as not being particularly focused on genetic information and testing or the cause of PRS, especially during the time that their baby was in hospital:
[Genetics] certainly wasn’t the focus, I mean we were, I guess we were interested at the start as to whether, there was any connection […] I think we were just sort of focusing on [maxillofacial] for, you know for dealing with the craniofacial issues. (David; antenatal detection)
There was only so much I could take in at a time so I think in, particularly in those first couple of months I was probably just processing the information that was essential at the time […] it was probably prioritizing other medical specialists and information. (Michelle; antenatal detection)
One participant did not even recall any diagnosis of PRS, despite confirmation of the diagnosis in the hospital records. She reported she first heard about PRS when she was approached by another researcher the year prior to this study:
He was about eleven days old I think before they discovered he had a cleft palate […] he was eighteen months when that got operated on and fixed, but no one had ever said anything about him having Pierre Robin [sequence]. (Donna; postnatal detection)
When genetic information would have been appreciated
Despite the initial lack of interest in genetic or causal information, on reflection, many participants felt that contact with genetic services after the initial period would be useful. They did want to learn about recurrence and reproductive risks for themselves as well as for their child in the future:
I don’t think we were really switched on at the time to be sort of thinking about, you know what if one day he grows up and he has kids, what does that mean? (David; antenatal detection)
We were quite interested in, more around Olivia, so if she would have children what was the likelihood […] because that’s something we want her to know about when she makes decisions about her life, and likewise if we were to have another baby […] I always remember all the doctors we’ve ever seen kind of just brushed the genetics side of it off […] maybe I should’ve pushed it further, I don’t know. (Grace; postnatal detection)
Participants were sometimes left feeling lost to follow up and wanted more information about genetics and causes of PRS:
I did see my surgeon probably about three weeks ago and I did mention that to him that we hadn’t had any follow up […] it obviously wouldn’t deter us from having another baby but it would be nice to know I guess, you know what the chances of having another baby with this condition are […] I think that a bit of education around that and […] the whole genetics thing as to why this might’ve occurred. (Kim; antenatal detection)
Some participants were able to pinpoint the time in which they then felt ready to discuss and ask questions not only about their child’s diagnosis but also the experience as a whole:
I would have liked the opportunity to sit down with someone who was a specialist in Pierre Robin, six months after it happened and just fire questions at them. (Grace; postnatal detection)
My doctor one day said to me “do you want to see this psychologist or do you want to chat to her?” and it was sort of it was right at the time where I could probably like a year or so later where I could actually, process some things. (Naomi; postnatal detection)
Participants’ recall of genetic service involvement and supports offered
Many participants did not recall seeing a genetic health professional during their child’s admission to the neonatal intensive care unit, and others reported what interactions they did have as being brief. As evident in the previous section, the overwhelming amount of information and attempts to prioritize craniofacial information may have contributed to poor recall of the involvement of the genetics service. We know from hospital protocol that every child in this cohort diagnosed with PRS was referred to a clinical geneticist, and then would have been seen again 6 to 12 months later for a review.
Cause of their child’s PRS was unclear
Participants reported that they “never saw [the genetics service] again” (Natalie; antenatal, and Claire; postnatal). Sometimes the information that was given to participants after diagnosis was not completely understood. When asked what she understood about the genetics of PRS, Stacey who was herself diagnosed with PRS following her son’s diagnosis said:
I think there’s something to do with [Stickler syndrome] but Ben doesn’t have that […] I did see [a genetic counsellor] myself when I was pregnant but not when Ben was born. (Stacey; antenatal detection)
I don’t know much about, and maybe I was told and I’ve forgotten, but I don’t remember learning much about if it’s in your DNA or if it’s a different type what it looks like. (Grace; postnatal detection)
Many participants appeared to have accepted that they may never know why their child has PRS and did not voice any desire to pursue further investigation. For Louise (postnatal detection), the possible reasons why one of her mono-chorionic di-amniotic twin boys developed PRS but the other did not “never was discussed previously…it was always just a mystery to us”.
I do get a lot of friends asking and wondering why it happened I guess and yeah it would be nice to know I guess but yeah, not sure if even that there are answers out there. (Kim; antenatal detection)
“We never really did find out…I guess unless you genetically test everybody it’s probably hard to know […] where Pierre Robin comes from. (Natalie; antenatal detection)
Genetics service involvement was rarely recalled
Most participants did not recall seeing any genetic counsellors or geneticists, or at the very least it did not stand out to them as an important part of their child’s diagnosis and management:
Look it’s quite possible someone did speak to us but it doesn’t stand out to me as a major consultation. I think that the, yeah like the doctors I was probably really focused on hearing it from were the neonatologist and the surgeons who were checking him out for surgery. (Michelle; antenatal detection)
I don’t remember a lot about [the genetics consultation], I know we had to you know, they gave us some questions to go away and research and we answered them […] I don’t remember anything about it. (David; antenatal detection)
The main exception being Lauren and James, as their daughter was eventually diagnosed with Stickler syndrome, and then Lauren was subsequently diagnosed too:
She had normal screening done very, very early on we didn’t really have too much contact with genetics but, we met [geneticist] fairly early on and he had suspected Sticklers quite early on so he was in sort of constant dialogue maybe every six months. (Lauren; postnatal detection)
Additional support needed around the PRS diagnosis
Participants mostly felt well supported by the medical team and nurses in regard to feeding, craniofacial surgeries, and the clinical features of PRS. However, some described needing extra support around how to explain PRS to their friends and family, and the need for professional psychosocial or counselling support while their child was an inpatient, especially to address feelings of guilt:
Trying to tell our parents what was wrong with Tyler was really difficult because we didn’t even know what was wrong with him really, we could hardly even remember what it was called let alone try to explain what it was. (Claire; postnatal detection)
I think I spent the first four weeks thinking that my child was here because I had done something, I’d eaten something wrong […] and that’s probably not the case […] huge amount of guilt, huge amount of guilt…no one ever talks to you about that and maybe the genetics person should’ve […] I think that could go a long way not just for children with Pierre Robin. (Grace; postnatal detection)
Discussion
This study aimed to provide evidence of parents’ experiences of a diagnosis of PRS and the role of genetic health professionals in supporting these families. For the participants, the diagnosis and information given at that time was foreign and confusing, irrespective of the timing of detection of PRS. These findings are consistent with other literature describing the experience of parents whose children have been diagnosed with cleft lip and/or palate (Berggren et al. 2012; Johansson and Ringsberg 2004; Kuttenberger et al. 2010). Information was often presented at what was a very emotionally turbulent time, when their newborn needed urgent medical assistance.
Despite an established PRS care pathway at the hospital which includes an early genetic consultation, participants had little recollection of any interaction with genetics services with only one participant remembering meeting a genetic counsellor. What they did recall seemed largely uninformative and most often of low priority for the participants at the time. Consequently, it is difficult to know exactly what information participants did receive about the cause and reproductive implications of PRS. It is possible they were not given this information, or it is possible that they were but it was not retained. We did not examine genetic files for consultation notes to clarify this. It is not uncommon to find variation in client recollection of recurrence risk (Austin 2010). Although not a priority at the time of diagnosis, many participants suggested that they would now like more clarity about the cause of their child’s PRS, and reproductive risks. This is information which genetic counsellors may be able to provide in conjunction with clinical geneticists.
The third and final research question asked what types of support genetic counsellors could provide to parents whose child has been diagnosed with PRS. This study has identified two potential points in time throughout the diagnosis and management experience where genetic counselling may add value. The first important time point is antenatally, when foetal ultrasound detects abnormalities suggesting PRS. Parents may be given little information about what micrognathia or PRS means and may be considering termination of the pregnancy due to either uncertainty or lack of accurate information. While uncommon, it is possible to detect signs of PRS on antenatal ultrasound (Lind et al. 2015). At this point, a genetic counsellor could not only inform but also provide emotional and psychosocial support while helping the client understand possible implications of the condition (Kessler 1997; Resta 2006). This would include facilitating decision-making in regard to invasive testing procedures or the continuation or termination of the pregnancy particularly given the potential for a range of PRS associated co-morbidities. Berggren et al. (2012) also found that suitable counselling following an antenatal diagnosis of cleft lip and/or palate was important for parental experience and satisfaction. Even in the absence of medical or genetic diagnostic information, genetic counsellors still maintain an important role as emotional and psychological support. Studies have demonstrated the benefits of genetic counsellors being able to anticipate and discuss the uncertainty, acknowledge lack of information, and reassure clients struggling with uncertainty (Berkenstadt et al. 1999; Lipinski et al. 2006). Attempts to help parents regain feelings of control in whatever aspect possible can increase their acceptance of a lack of prognostic information as well as increase satisfaction with genetic counselling (Berkenstadt et al. 1999). For example, those who have an antenatal diagnosis of micrognathia or PRS may find comfort in being able to plan other aspects of the pregnancy. Although it is not possible to change how and when such diagnoses are made, it is important to understand how the differences in timing of diagnosis could affect families.
The initial lack of interest in genetic and causal information described by participants shortly after their baby’s diagnosis may also be true for other parents of children with PRS. Since all families attend a 6- to 12-month outpatient review, this presents an opportunistic and perhaps more appropriate second point for genetic counselling to be offered. By 6 months of age, the health and general development of infants with isolated PRS has largely stabilized, and most have been living at home within the family setting for some months (Lee et al. 2019). Genetic information was not a focus for participants prior to this but they may now be in a better place to hear this information and have questions of their own. In the case of a multifactorial condition such as PRS, genetic counsellors can assist with the interpretation of information and risk through non-directive discussion, and facilitating client reflection (Austin 2010; Austin et al. 2006). At this point, genetic counselling could also enhance psychosocial support by informing parents about PRS support groups. It may be best to allow parents to contact a genetic counsellor when they desire; however, this relies on a member of the multidisciplinary health team ensuring parents are aware of the role of a genetic counsellor. A genetic counsellor could instead follow-up families with a phone call to offer a genetic counselling appointment or send a letter with information about what genetic counselling offers and contact details if they wish to pursue this. The high participant recruitment from such a narrow target group could be an indicator that many parents want the opportunity to talk about their experiences, yet this was not being provided.
Strengths, limitations, and further research
The strength in using semi-structured interviews is that it allows participants to direct the conversation to the experiences which they felt were most important. This prevents researcher bias from controlling questions and therefore the types of answers. In this study, the sample size and possible ascertainment bias represent some limitations. The final data set included eleven interviews in which data saturation was not reached. A larger sample size would ensure a greater variation in experiences was captured. Ascertainment bias appreciates that the participant group may comprise of families from either end of the spectrum of experience (high satisfaction or low satisfaction), and not those who feel their experience was not noteworthy enough for research. This is however speculation, and there could be many factors influencing participant response. The upper age limit of 5 years for children of participants may contribute to reduced recall of events compared to participants with younger children. However, the sample size was not large enough to compare participant recall with child’s age and lowering the upper age limit would have further reduced the sample size. Irrespective of age or time since diagnosis, participant recall of events is subjective and may not align with objective facts.
Considering this research restricted participation to parents of a child diagnosed with ‘isolated PRS’, future research may expand to diagnoses of ‘PRS plus’ or syndromic causes of PRS. This would likely give a different perspective of the involvement of the genetics service and genetic counselling, especially in the case of ‘syndromic PRS’ where genetic investigations and therefore genetic information may be more pronounced and better recalled by parents.
If a larger scale study were possible, the inclusion of participants from other states or territories within Australia, with experiences from other specialist hospitals, could make for an interesting comparison with how different care pathways affect parental experiences. All children of participants in this study were seen within the same clinical service. A larger scale multicentre study could also capture a wider age range to explore how experiences with genetics services, genetic counselling, and overall care outcomes for these babies has changed over time.
Future research may employ the use of structured interviews or semi-structured interviews with a different focus and research aim. This may allow for further exploration of themes such as experiences with genetics services, supports offered, or understanding of genetic information and the cause of PRS in their child, to gain further understanding of specific parental experiences.
Conclusion
In the context of a child’s diagnosis of PRS, the aims of this study were to examine parents’ lived experience and examine the role of genetic counselling in PRS. For participants of this study, a diagnosis of PRS for their child was often shocking and confusing. It was clear that the information given at the time of diagnosis was not suitable to the immediate needs and understanding of the parents, and adjusting this could make a significant difference to the overall experience. In addition to this, antenatal detection of PRS stands out as a critical point where parents could benefit most from genetic counselling. Whereas later in the child’s first year of life, the role of the genetic counsellor would then be to ensure parents are aware of the services available, be it PRS support groups or accessing genetic counselling as a source of psychosocial support coupled with PRS and reproductive information. This study has demonstrated that there is indeed an important role for genetic counsellors in PRS which thus far does not appear to be being utilized.
Acknowledgements
The work conducted in this study was done so to fulfil a requirement for the degree of Master of Genetic Counselling.
Authors’ contributions
Rhiannon Sandow:
• Substantial contributions to the conception or design of the work; acquisition, analysis, and interpretation of data for the work
• Drafting the work and revising it critically for important intellectual content
• Final approval of the version to be published
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Nicky Kilpatrick:
• Substantial contributions to the conception or design of the work; acquisition, analysis, and interpretation of data for the work
• Revising the work critically for important intellectual content
Tiong Yang Tan:
• Substantial contributions to the conception or design of the work; acquisition, analysis, and interpretation of data for the work
• Revising the work critically for important intellectual content
Supriya Raj:
• Substantial contributions to the conception or design of the work; acquisition, analysis, and interpretation of data for the work
• Revising the work critically for important intellectual content
Laura Forrest:
• Substantial contributions to the conception or design of the work; acquisition, analysis, and interpretation of data for the work
• Drafting and revising the work critically for important intellectual content
• Final approval of the version to be published
Funding information
No funding was sought or obtained for this study.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors of this article declare that they have no conflicts of interest in the undertaking of this research study.
Human studies and informed consent
All procedures followed were in accordance with the ethical standards of the Royal Children’s Hospital Human Research Ethics Committee. Informed consent was obtained from each participant before any interviews were conducted.
Animal studies
No animal studies were carried out by the authors for this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agrafiotis PC. Teaching parents about Pierre Robin syndrome. Am J Nurs. 1972;72:2040–2041. [PubMed] [Google Scholar]
- Austin JC. Re-conceptualizing risk in genetic counseling: implications for clinical practice. J Genet Couns. 2010;19(3):228–234. doi: 10.1007/s10897-010-9279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JC, Smith GN, Honer WG. The genomic era and perceptions of psychotic disorders: genetic risk estimation, associations with reproductive decisions and views about predictive testing. Am J Med Genet B Neuropsychiatr Genet. 2006;141(8):926–928. doi: 10.1002/ajmg.b.30372. [DOI] [PubMed] [Google Scholar]
- Berggren H, Hansson E, Uvemark A, Svensson H, Becker M. Prenatal compared with postnatal cleft diagnosis: what do the parents think? J Plast Surg Hand Surg. 2012;46(3–4):235–241. doi: 10.3109/2000656X.2012.698416. [DOI] [PubMed] [Google Scholar]
- Berkenstadt M, Shiloh S, Barkai G, Katznelson MBM, Goldman B. Perceived personal control (PPC): a new concept in measuring outcome of genetic counseling. Am J Med Genet. 1999;82(1):53–59. doi: 10.1002/(SICI)1096-8628(19990101)82:1<53::AID-AJMG11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Anderson B, Wolf LS. Feeding infants with cleft lip, cleft palate, or cleft lip and palate. Cleft Palate J. 1987;24(3):244–249. [PubMed] [Google Scholar]
- Demke J, Bassim M, Patel MR, Dean S, Rahbar R, van Aalst JA, Drake A. Parental perceptions and morbidity: tracheostomy and Pierre Robin sequence. Int J Pediatr Otorhinolaryngol. 2008;72(10):1509–1516. doi: 10.1016/j.ijporl.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Denny N, Desilva K, Webber P. Laryngeal mask airway for emergency tracheostomy in a neonate. Anaesthesia. 1990;45(10):895–895. doi: 10.1111/j.1365-2044.1990.tb14605.x. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay N, Mendonca DA, Woo AS. Pierre Robin Sequence. Semin Plast Surg. 2012;26(2):76–82. doi: 10.1055/s-0032-1320065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K, Konczal LL, Mitchell AL, Jones MC. Underlying genetic diagnosis of Pierre Robin sequence: retrospective chart review at two children’s hospitals and a systematic literature review. J Pediatrics. 2012;160(4):645–650. e642. doi: 10.1016/j.jpeds.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Johansson B, Ringsberg KC. Parents’ experiences of having a child with cleft lip and palate. J Adv Nurs. 2004;47(2):165–173. doi: 10.1111/j.1365-2648.2004.03075.x. [DOI] [PubMed] [Google Scholar]
- Kessler S. Psychological aspects of genetic counseling. IX. Teaching and counseling. J Genet Counsel. 1997;6(3):287–295. doi: 10.1023/A:1025676205440. [DOI] [PubMed] [Google Scholar]
- Kuttenberger J, Ohmer JN, Polska E. Initial counselling for cleft lip and palate: parents’ evaluation, needs and expectations. Int J Oral Maxillofac Surg. 2010;39:214–220. doi: 10.1016/j.ijom.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Lee M, Ho ES, Forrest CR. Pierre Robin sequence: cost-analysis and qualitative assessment of 89 patients at the hospital for sick children. Plastic Surg. 2019;27(1):14–21. doi: 10.1177/2292550318767922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind K, Aubry MC, Belarbi N, Chalouhi C, Couly G, Benachi A, Lyonnet S, Abadie V. Prenatal diagnosis of Pierre Robin sequence: accuracy and ability to predict phenotype and functional severity. Prenat Diagn. 2015;35:853–858. doi: 10.1002/pd.4619. [DOI] [PubMed] [Google Scholar]
- Lipinski SE, Lipinski MJ, Biesecker LG, Biesecker BB (2006) Uncertainty and perceived personal control among parents of children with rare chromosome conditions: The role of genetic counseling. Paper presented at the American Journal of Medical Genetics Part C: Seminars in Medical Genetics [DOI] [PubMed]
- Marcellus L. The infant with Pierre Robin sequence: review and implications for nursing practice. J Pediatr Nurs. 2001;16(1):23–34. doi: 10.1053/jpdn.2001.20550. [DOI] [PubMed] [Google Scholar]
- Morice A, Soupre V, Mitanchez D, Renault F, Fauroux B, Marlin S, et al (2018) Severity of retrognathia and glossoptosis does not predict respiratory and feeding disorders in pierre robin sequence. Front Pediatr 6:351 [DOI] [PMC free article] [PubMed]
- Prows CA, Bender PL. Beyond Pierre Robin sequence. Neonatal Netw J Neonatal Nurs. 1999;18(5):13–19. doi: 10.1891/0730-0832.18.5.13. [DOI] [PubMed] [Google Scholar]
- QSR International Pty Ltd (2012) NVivo qualitative data analysis Software (Version 10). Retrieved from http://www.qsrinternational.com/
- Resta R (2006) Defining and redefining the scope and goals of genetic counseling. Am J Med Genet C Semin (142 C):269–275 [DOI] [PubMed]
- Schreiner RL, McAlister WH, Marshall RE, Shearer WT. Stickler syndrome in a pedigree of Pierre Robin syndrome. Am J Dis Child. 1973;126(1):86–90. doi: 10.1001/archpedi.1973.02110190074016. [DOI] [PubMed] [Google Scholar]
- Snead MP, Yates JR. Clinical and molecular genetics of stickler syndrome. J Med Genet. 1999;36(5):353–359. [PMC free article] [PubMed] [Google Scholar]
- Soulier M, Sigaudy S, Chau C, Philip N. Prenatal diagnosis of Pierre–Robin sequence as part of stickler syndrome. Prenat Diagn. 2002;22(7):567–568. doi: 10.1002/pd.369. [DOI] [PubMed] [Google Scholar]
- Tan TY, Kilpatrick N, Farlie PG (2013) Developmental and genetic perspectives on Pierre Robin sequence. Paper presented at the American Journal of Medical Genetics Part C: Seminars in Medical Genetics [DOI] [PubMed]
- Van den Elzen AP, Semmekrot BA, Bongers EM, Huygen PL, Marres HA. Diagnosis and treatment of the Pierre Robin sequence: results of a retrospective clinical study and review of the literature. Eur J Pediatr. 2001;160(1):47–53. doi: 10.1007/s004310000646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.