Microglia are the main immune cells of the central nervous system that maintain normal neuronal functions. Microglial cells are also linked with major diseases in humans including neurodegenerative disorders such as Alzheimer’s disease, stroke, epilepsy, and psychiatric diseases such as schizophrenia [1]. Microglia perform surveillance of the brain microenvironment through their motile processes. Currently, there has been increased attention on the interaction between microglial processes and synaptic elements. At least part of the dynamic motility of resting microglial processes in vivo is directed toward neuronal synapses. Resting microglial processes have brief and direct contact with synapses lasting ~5 min and this occurs at a frequency of about once per hour in a neuronal activity-dependent manner. Under the conditions of cerebral ischemia, the duration of these microglia-synapse contacts is significantly prolonged to about an hour, and this is followed by the disappearance of the presynaptic bouton, suggesting that microglia contribute to the subsequent increased turnover of synaptic connections [2]. However, the molecular mechanisms of microglia-neuron communication are not well understood.
Microglial cells are involved in the formation and maintenance of synapses in the brain [3] during development and synaptic plasticity. Researchers have demonstrated that microglial processes interact with axonal terminals and dendritic spines in the visual cortex in a neuronal activity-dependent manner, and for a long time, these were believed to be the main forms of interaction between microglia and neurons [2, 4, 5]. Moreover, the interactions between microglia and synaptic elements, including both axonal boutons and dendritic spines, have also received increased attention. Neuronal cell bodies are relatively stable in most conditions, while the synaptic structures are highly dynamic. The microglial processes actively monitor the surrounding neural parenchyma and respond promptly to brain injury [6]. The interactions between microglia and synapses do not elucidate how microglia monitor and affect neuronal activity spatiotemporally. Therefore, the mechanisms of effective communication between microglia and neuronal somata require investigation. Recently, Cserép and colleagues identified a novel communication site between microglial processes and neuronal cell bodies in both mice and humans. This was based on in vivo two-photon imaging, high-resolution light and electron microscopy combined with advanced 3D-analysis. Interestingly, they found that microglia form junctions with most neuronal somata regardless of their cell type in a P2Y12 receptor (P2Y12R)- and neuronal mitochondrial activity-dependent manner. In addition, the study highlighted that microglial junctions are essential for microglia-neuron communication, and for the neuroprotective effects of microglia after acute brain injury [7] (Fig. 1).
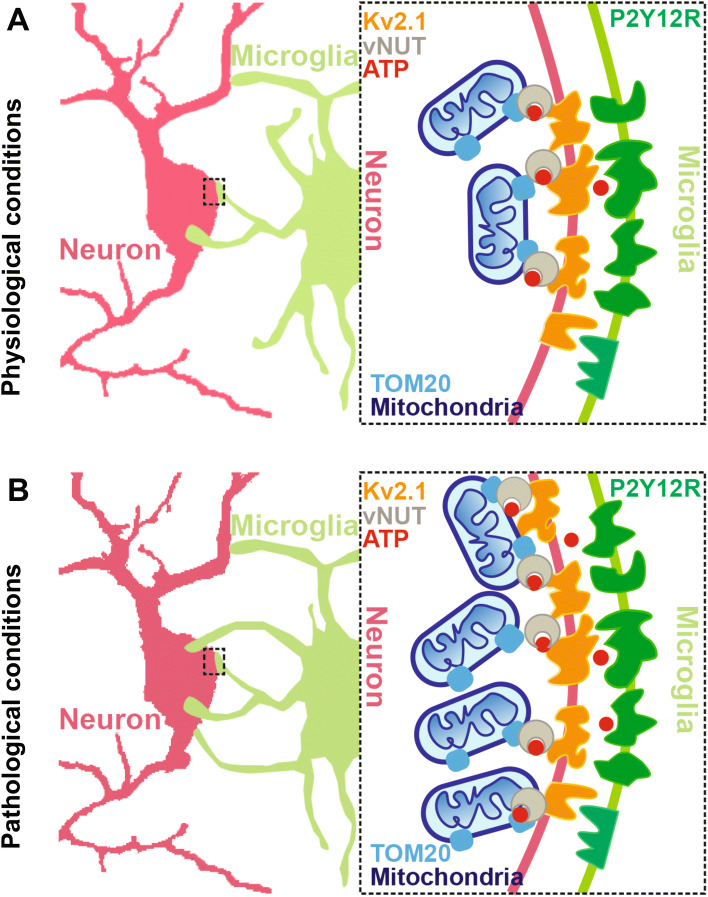
Fig. 1.
Schematic of somatic microglial junctions under physiological and pathological conditions. A Under physiological conditions, microglia form junctions with most neuronal somata, but not with synaptic elements of neurons, regardless of cell type, in a P2Y12R-dependent manner. Somatic microglial junctions are the main communication sites between neurons and microglia in both mice and humans. They possess a unique nano-architecture within the neuronal somata consisting of Kv2.1 clusters (orange), which are mainly expressed in neurons and are the contact sites of microglia and neurons, TOM20 (sky blue), the main element of the transport protein complex on the outer mitochondrial membrane, and vNUT (brown), an important molecule for the vesicular release of mitochondria-derived ATP from neurons. B Under stroke conditions, potentially viable neurons are activated, inducing the release of ATP (red) from the mitochondria (dark blue), and with the help of vNUT, ATP is released from the neurons. The released extracellular ATP regulates microglial branch dynamics via the purinoceptor P2Y12R (green), expressed specifically in microglia. The microglial processes are recruited to form new microglial-somatic junctions and to protect viable neurons. Somatic microglial junctions are robustly increased in both mice and human post-mortem brain after stroke. In addition, the disintegration of somatic microglial junctions after stroke induces an increase of microglial process coverage in the cell bodies of viable neurons in a P2Y12R- and mitochondrial signaling-dependent manner. This leads to the initiation of protective microglial responses that minimize brain injury. P2Y12R, purinergic receptor P2Y12; TOM20, translocase of outer mitochondrial membrane 20; vNUT, vesicular nucleotide transporter.
Unlike the previous studies showing that the interactions between microglial processes and synaptic elements of neurons are the main configuration [4], this study reported that only a small proportion of glutamatergic or GABAergic synapses are associated with microglial processes. In addition, >90% of cortical pyramidal cells, vGluT3+ cells, and >80% of PV+ interneurons are involved in the formation of somatic microglial junctions in mice. Similar results were reported in the human neocortex as well. Furthermore, microglia contact neuronal somatic membranes mainly at sites of Kv2.1 clustering, which have been reported to be mainly expressed in neurons [8], in both mice and humans. Extracellular ATP released by neurons regulates microglial branch dynamics via the purinoceptor P2Y12R. P2Y12Rs are expressed specifically in microglia and their activation is required in response to neuronal injury in the brain [9]. Indeed, dense P2Y12R clusters on microglial processes at somatic junction sites were found to directly face neuronal Kv2.1 clusters in pyramidal cells and interneurons (Fig. 1A). Consistent with these results, an in vivo imaging study using zebrafish reported that microglia preferentially contact the cell bodies of neurons with higher spontaneous activity, and this results in the reduction of visually-evoked neuronal activity [10]. Given that most studies have shown that the junctions mainly form between microglia and synaptic elements, the underlying mechanisms can be more complex and involve a number of molecules in addition to P2Y12Rs, which requires further investigation. Therefore, understanding the microglial P2Y12R signaling pathway will provide novel candidates for therapeutic interventions in pathologies involving microglial P2Y12Rs such as stroke.
What are the components of somatic microglia-neuron junctions? By using transmission electron microscopy and high-resolution electron tomography with 3D reconstruction, somatic microglial junctions were found to possess unique nano-architecture within the neuronal somata, and the junctions are composed of closely apposed mitochondria, reticular membrane structures, intracellular tethers, and associated vesicle-like membrane structures. However, these features were not observed in perisomatic boutons contacted by microglia. The Kv2.1 clusters were closely associated with the neuronal structures within the junctions. Furthermore, TOM20 (the main element of the transport protein complex in the outer mitochondrial membrane) and vesicular nucleotide transporter (vNUT, an important molecule for vesicular release of mitochondria-derived ATP from neurons) were also reported to be remarkably higher at somatic junctions than adjacent areas (Fig. 1A).
As noted above, mitochondria were close to the junctions, which has been reported to be essential for neuroglial crosstalk [11], so it is important to investigate whether microglial process recruitment to somatic junctions is functionally linked with the activity of mitochondria in neurons. In vivo two-photon imaging revealed that the number of mitochondria significantly increases and is accompanied by the formation of somatic microglial junctions in wild-type but not in P2Y12R−/− tissue. This suggests that microglial process recruitment to somatic junctions is linked to neuronal mitochondrial activity and occurs in a P2Y12R-dependent manner. Moreover, neuronal activation induced the release of ATP from the mitochondria, which was inhibited by a vNUT blocker but not a synaptic calcium channel blocker (Fig. 1A). The findings demonstrated that microglia dynamically monitor neuronal activity at somatic microglia-neuron junctions in a P2Y12R-dependent manner, leading to a rapid increase of somatic coverage by microglial processes. This suggests an interesting possibility that microglia and neurons engage in dynamic communication essential for nervous system health and homeostasis.
The authors examined the interaction between microglia and neurons in the healthy brain. Currently, findings on the roles of microglia in pathological conditions such as stroke are inconsistent across studies [12]. Several studies have explored the contribution of microglia in synaptic functions. In this paper, the somatic microglial junctions were reported to have robustly increased after stroke in both mice and human post-mortem brain tissue (Fig. 1B). Microglial process coverage around the somatic junctions was completely abolished after the administration of a P2Y12R inhibitor or a mitochondrial ATP-sensitive potassium (KATP) channel opener (KATP can prevent mitochondrial injury), which also decreased neuron viability. Stroke-induced disintegration of somatic microglial junctions increased the microglial process coverage of cell bodies in a P2Y12R- and mitochondrial signaling-dependent manner. This led to protective microglial responses hence minimizing brain injury. However, given the limitations of the imaging approach, other methods such as pharmacological and genetic approaches to inactivating or eliminating microglia are needed to further investigate the role of microglia after stroke.
In summary, Cserép and colleagues reported a novel form of interaction between microglia and neurons. Unlike previous results [4], they found that somatic microglial junctions are the main communication sites in both mice and humans under physiological conditions and can respond to brain injury rapidly to protect viable neurons. The injured neurons release ATP which recruits microglial processes to the neuronal somata and protects them in a P2Y12R-dependent manner. However, a number of unanswered questions require further investigations into the mechanism of neuronal protection by microglia. In addition, it is important to investigate whether microglia possess the ability to release certain molecular signals that can alter neuronal behavior following the formation of somatic microglial junctions. Another question to investigate is whether other cell types are involved in neuronal protection such as astrocytes activated through microglia and ATP release in injured tissue. Are there other related molecules important in the formation of microglial somata junctions? Using an epilepsy model, Eyo and colleagues performed in vitro and in vivo studies and reported that global glutamate sharply increases the numbers of microglial processes. These are involved in the activation of neuronal NMDA receptors, calcium influx, and subsequent ATP release in both a microglial P2Y12R-dependent and an NMDA receptor activation-dependent manner. P2Y12R-KO mice exhibit reduced seizure-induced increases in microglial process numbers and worsen seizure behaviors [13]. This suggests that both NMDA receptors and P2Y12Rs are key components for junction formation, and P2Y12Rs play an important role in disease. Indeed, a more recent study has shown that microglial P2Y12Rs modulate neuronal excitability and innate fear behaviors in both developing and adult mice [14].
Brain microglial cells display complex phenotypes and roles, and their functions vary based on diverse sites on neurons. Further, they play a vital role in development, learning, and memory in synaptic sites while they play a protective role in stroke. In addition, microglial processes can form junctions with both neuronal somata and synapses, for example in Alzheimer’s disease and Parkinson’s disease. The communication between microglia and neurons is bidirectional, involving several important factors and signaling axes including P2Y12Rs, ATP, vNUT, and other molecules. Therefore, it is essential to determine the roles of microglia in various neuronal sites. These findings provide novel insights for deep exploration of the crosstalk between microglia and neurons, further advancing knowledge of the mechanisms of brain function.
Acknowledgements
This highlight was supported by the Natural Science Foundation of Zhejiang Province (LR18C090001), the National Natural Science Foundation of China (31671071 and 81801102), the National Postdoctoral Science Foundation of China (2018M642413 and 2019T120507), and the Research Start-up Project by Hangzhou Normal University (4125C5021920453).
References
- 1.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nature medicine. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 2.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. Journal of Neuroscience. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang H-Y, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinhard L, Di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature communications. 2018;9:1228. doi: 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremblay M-È, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:1–16. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nature neuroscience. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 7.Cserép C, Pósfai B, Lénárt N, Fekete R, László ZI, Lele Z, et al. Microglia monitor and protect neuronal function via specialized somatic purinergic junctions. Science. 2019;367:528–537. doi: 10.1126/science.aax6752. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher EV, Simon CM, Pagiazitis JG, Chalif JI, Vukojicic A, Drobac E, et al. Reduced sensory synaptic excitation impairs motor neuron function via Kv2. 1 in spinal muscular atrophy. Nature neuroscience. 2017;20:905–916. doi: 10.1038/nn.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, et al. The P2Y 12 receptor regulates microglial activation by extracellular nucleotides. Nature neuroscience. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Developmental cell. 2012;23:1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Ho MS. Neuroglial crosstalk by mitochondria. Neuroscience bulletin. 2017;33:111–112. doi: 10.1007/s12264-016-0072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, et al. Dual Functions of Microglia in Ischemic Stroke. Neuroscience bulletin. 2019;34:1–13. doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. Journal of Neuroscience. 2014;34:10528–10540. doi: 10.1523/JNEUROSCI.0416-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng J, Liu Y, Umpierre AD, Xie M, Tian DS, Richardson JR, et al. Microglial P2Y12 receptor regulates ventral hippocampal CA1 neuronal excitability and innate fear in mice. Molecular brain. 2019;12:1–10. doi: 10.1186/s13041-019-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]