Abstract
Research suggests that inflammation is important in the pathophysiology of mental disorders. In addition, a growing body of evidence has led to the concept of the microbiota-gut-brain axis. To understand the potential interactions, we begin by exploring the liaison between the immune system and mental disorders, then we describe the evidence that the microbiota impact the immune response in the developing brain. Next, we review the literature that has documented microbiome alterations in major mental disorders. We end with a summary of therapeutic applications, ranging from psycho-biotics to immunomodulatory drugs that could affect the microbiota-gut-brain axis, and potential treatments to alleviate the adverse effects of antipsychotics. We conclude that there is promising evidence to support the position that the microbiota plays an important role in the immunological pathophysiology of mental disorders with an emphasis on psychotic disorders and mood disorders. However, more research is needed to elucidate the mechanisms.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00535-1) contains supplementary material, which is available to authorized users.
Keywords: Mental disorder, Microbiota, Immunology, Neurodevelopment
Introduction
Mental disorders, among which we mainly focus on schizophrenia (SCZ), autism spectrum disorder, mood disorders, and anxiety, rank among the top causes of years lived with disability worldwide [1]. For SCZ alone, the total cost estimates vary between countries, but are estimated to be ~$102 billion in the USA [2]. And “psychosis” is a common manifestation of several psychiatric disorders that range from major depressive disorder with psychosis to bipolar disorder type I with psychosis and SCZ [3]. Psychosis is defined by the presence of delusions, hallucinations, disorganized thinking, grossly disorganized or abnormal motor behavior, and negative symptoms such as diminished emotional expression, avolition, and social withdrawal [4].
Efforts toward defining clear mechanisms that explain the pathophysiology of mental disorders, biologically-based diagnoses, and novel treatments are needed [5]. In this context, much has been documented regarding the possible role of inflammation in mental disorders [6]. In parallel, many studies on the human microbiota have accumulated to the point that the microbiota-gut-brain axis is thought to play a role in neuropsychiatric illness [7–9]. As reviewed elsewhere [10], there are several pathways through which the microbiota can modulate the microbiota-gut-brain axis. These include endocrine pathways mediated via cortisol, neural pathways where the vagus nerve and the enteric nervous system are the main routes, metabolic pathways whereby the microbiota produce neurotransmitter precursors like tryptophan but also active substances like short-chain fatty acids (SCFAs), and finally the immune pathway. However, the immune response/inflammation overlaps most with the above pathways, which makes it an almost unavoidable pathway. In the present article, we comprehensively review the reported influence of both the immune system and the microbiota on mental disorders with an emphasis on psychosis. We focus on the pathogenesis of psychosis from a neurodevelopmental perspective, and finally provide perspectives on potential therapeutic applications (Fig. 1).
Fig. 1.
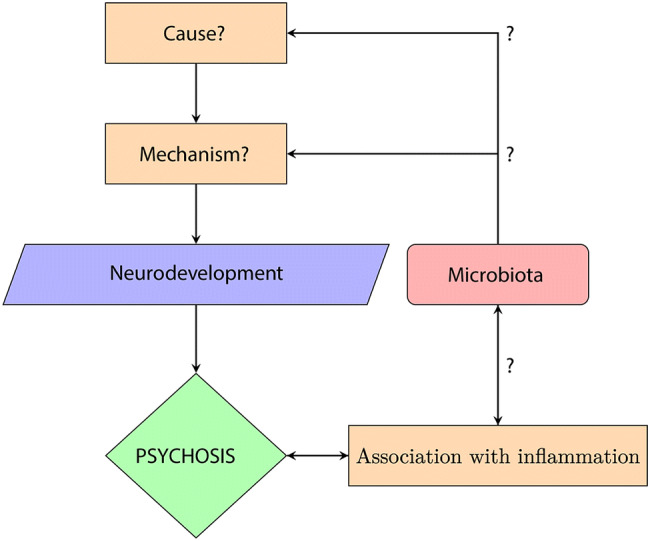
Summary flowchart. The exact cause and mechanism that explain psychosis remain unknown; however a disruption in neurodevelopment has been documented and is regarded as a pre-stage of psychosis. In parallel, a strong association between psychosis and inflammation has been documented through many sources of evidence. The hypothesis is that the microbiota, which is closely associated with the immune system, may be a piece of the puzzle that explains part of the cause and mechanism of psychosis.
Inflammation in Mental Disorders and Potential Role of the Microbiota
In this section, we review the evidence, both clinical and from basic science, supporting the existence of a link between mental disorders and inflammation. To date, this link has been explored more than the potential role of the microbiota. We begin by highlighting the epidemiologic data on associations between psychotic and immunologic disorders as well as evidence from the effects of immunomodulatory drugs. Subsequently, we address the mechanisms driven by microorganisms that could alter the immune response. This is followed by theories of the effects of the immune response on neurodevelopment.
Evidence Linking Mental Disorders to Immunological Disorders
Epidemiological Evidence. Epidemiological evidence links autoimmune and atopic disorders with mental disorders (Table 1). First, a nationwide Swedish study reported an increased risk of affective, personality, and neurotic disorders among individuals hospitalized for systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and ankylosing spondylitis (AS) [11]. They also found an increased risk of psychosis among women with SLE, RA, and AS but not among men [11]. Subsequently, a Danish study based on the records of 7,704 people, found that individuals with SCZ have a 50% lifetime prevalence of autoimmune disorders and that conversely, given a history of autoimmune disorders, the relative risk for SCZ increases by 45% [12]. Associations have been found for celiac disease, RA, autoimmune thyroiditis, type 1 diabetes mellitus (T1DM), SLE, Guillain-Barré syndrome, psoriasis, multiple sclerosis (MS) and autoimmune hepatitis, among others [13]. The case of celiac disease has been studied more extensively. A higher prevalence among patients with SCZ has been documented and immunological markers for celiac disease or gluten intolerance are present in SCZ patients [14]. Anti-gliadin, transglutaminase, and endomysium antibodies, which all participate in gluten sensitivity, are increased in SCZ [14]. Beyond autoimmune disorders, there is also evidence linking atopic disorders in childhood to the development of psychosis in adulthood [15].
Table 1.
Summary of studies that explore an association between immunological disorders and psychosis.
| Reference | Country | Sample characteristics | Associations found |
|---|---|---|---|
| Tiosano et al. 2017 [16] | Israel | 5,018 SLE patients and 25,090 matched controls | Independent association between SLE and BD |
| Tiosano et al. 2017 [17] | Israel | 5,018 SLE patients and 25,090 matched controls | SCZ and SLE |
| Jackson et al. 2014 [18] | USA | 100 people with SCZ and 100 matched controls | SCZ and gluten antibodies |
| Khandaker et al. 2014 [15] | UK | 6,785 adolescents with psychotic experiences | Atopic disorders prior to psychosis |
| Benros et al. 2014 [13] | Denmark | 3.83 million people; 39,364 with SCZ-like psychosis and 142,328 with autoimmune disease | Autoimmune disorders and psychosis |
| Kumar et al. 2013 [19] | India | 50 patients with pemphigus, 30 with psoriasis, and 30 matched controls | Psychosis with pemphigus and psoriasis |
| Kota et al. 2012 [20] | India | 260 patients with T1DM | Psychosis and T1DM |
| Sundquist et al. 2008 [11] | Sweden | Entire Swedish population | Psychosis and SLE or RA among women |
| Sturdy et al. 2002 [21] | UK | 533 cases and 533 controls | Psychosis as a risk factor for death certified as caused by asthma |
| Gilvarry et al. 1996 [22] | UK | 101 psychotic and 116 control patients | Family history of psychosis and thyrotoxicosis and T1DM |
| Nasr et al. 1981 [23] | USA | 82 psychiatric patients | Atopic disorders and affective disorders |
| Osterberg, 1978 [24] | Sweden | 58 psychiatric cases | SCZ and either RA or AS |
SLE, systemic lupus erythematosus; BD, bipolar disorder; SCZ, schizophrenia; T1DM, type 1 diabetes mellitus; RA, rheumatoid arthritis; AS, ankylosing spondylitis.
Genetic Evidence. The co-occurrence of immunological disorders and mental disorders might potentially be attributed to common etiological factors. These may be genetic or environmental. Genetic evidence supporting a common link between mental disorders and the immune system has been provided by genome-wide association studies that have identified single-nucleotide polymorphisms associated with SCZ in the major histocompatibility complex on chromosome 6 [25]. Innate immunity has also been mechanistically implicated in the appearance of mental disorders [26]. The environmental evidence may be more complicated, but among others, the microbiota can be influenced by variations in both genetic and environmental conditions [27, 28]. This is one of the reasons why we consider that understanding its role in psychotic disorders is pertinent.
Cross-sectional Evidence. Further evidence of inflammation in mental disorders comes from studies during first-episode psychosis. In these patients, an upregulated inflammatory status has been documented by measuring cytokines such as interleukins 1β and 6, and tumor necrosis factor alpha. Also, adiponectin may play a unique pro-inflammatory role in this patient population [29–32]. Variations seem to exist according to the stage of illness. Specific inflammatory cytokines differ between first episodes, psychotic states, and remission states [33]. In the case of bipolar disorder, possible mechanisms have been reviewed elegantly elsewhere [34].
Evidence from Immunomodulatory Drugs. Another source of evidence is the efficacy of immunomodulatory drugs such as minocycline, non-steroidal anti-inflammatory drugs (NSAIDs), dehydroepiandrosterone, dehydroepiandrosterone sulfate, pregnenolone, polyunsaturated fatty acids, N-acetylcysteine, or L-theanine in the treatment of psychosis, which also suggests an underlying inflammatory process [35]. In addition, there is evidence of an anti-inflammatory effect of antipsychotics in inhibiting microglial activation [36].
Potential Role of the Microbiota
Evidence Linking Immunological Disorders and the Microbiota. In this section, we review evidence linking both atopic and autoimmune disorders to changes in either the normal microbiota or with exposure to infectious agents. According to Okada et al. (2010) the “hygiene hypothesis” can be extended from atopic to autoimmune diseases. First, there is epidemiological evidence such as the rise in incidence in places where the sanitation is better, and through migration and geographical distribution studies. Also, a causal relationship has been demonstrated mostly in animal models [37]. Apart from the explanation of T helper 1 (Th1) and Th2 deviation, Okada et al. (2010) proposed other explanations such as antigenic competition and bystander suppression by CD4+/CD5+ forkhead box P3 regulatory T cells and mechanisms independent of antigenic stimulation such as the stimulation of Toll-like receptors [37]. Alterations in the microbiota have been documented in autoimmune disorders such as T1DM [38, 39], MS [40], inflammatory bowel disease [41], primary biliary cirrhosis [42], and connective tissue diseases [43, 44] (Table 2).
Table 2.
The hygiene hypothesis: articles on associations between autoimmune disorders and changes in the composition of microbiota.
| Reference | Title | Sample characteristics | Associations found |
|---|---|---|---|
| De Groot et al. 2017 [38] | Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study | 53 patients with T1DM and 50 matched controls | Decreased Christensenella and Subdoligranulum are correlated with glycemic control, inflammatory parameters, and SCFAs |
| Knip et al. 2017 [39] | Modulation of type 1 diabetes risk by the intestinal microbiome | Review | Microbiome protects humans against T1DM |
| Wekerle, 2017 [40] | Nature, nurture, and microbes: The development of multiple sclerosis | Review | Microbiota may contribute to MS pathogenesis |
| Kim et al. 2017 [41] | The interplay between host immune cells and gut microbiota in chronic inflammatory diseases | Review | Role of the microbiota in IBD, MS, allergic asthma, and RA |
| Quigley, 2016 [42] | Primary biliary cirrhosis and the microbiome | Review | Role of a bacterium in the initiation of the autoimmune process that leads to the development of primary biliary cirrhosis |
| Talotta et al. 2017 [43] | The microbiome in connective tissue diseases and vasculitides: An updated narrative review | Review | The dysbiotic microbiome plays a role in the pathogenesis of SLE, systemic sclerosis, Sjögren’s syndrome, and Behçet’s disease |
| Yacoub et al. 2018 [44] | Lupus: the microbiome angle | Review | Mechanisms by which the microbiota affects SLE |
| Lowry et al. 2016 [45] | The microbiota, immunoregulation, and mental health: Implications for public health | Review | Environmental microbes modify risk for inflammatory disease, with a focus on neurodevelopmental and psychiatric conditions |
SCFAs, short-chain fatty acids; T1DM, type 1 diabetes mellitus; MS, multiple sclerosis; IBD, inflammatory bowel disease; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Signals Driven by Microorganisms. One of the reasons why we consider that the microbiota may play a significant role in mental disorders through modulation of the immune system is because microbial signals drive the balance between T helper and T regulatory cells [46, 47]. The signals that drive this balance can be metabolites such as tryptophan or SCFAs, microbial molecules that enhance immune regulatory circuits through stimulation of DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin) and the Lewis lipopolysaccharide that also binds DC-SIGN, or helminthic molecules such as fucose. These can originate (1) from commensal microbiota, mainly Firmicutes, (2) from old infective pathogens such as hepatitis A virus, Toxoplasma gondii, Salmonella spp., helminths, nematodes, Mycobacterium tuberculosis or Helicobacter pylori, and (3) from organisms from the natural environment such as non-tuberculous mycobacteria [45].
Leaky Gut. An altered, more permeable gut barrier has been reported in several disorders such as irritable bowel syndrome [48]. A possible explanation for this increased permeability is a dysbiosis favoring a pro-inflammatory state in the bowel. This allows the passage of inflammatory molecules such as lipooligosaccharide and amino-acids into the bloodstream, causing dysregulation of the immune response and antigen recognition [49]. Thus, we consider that alterations in the microbiota might be both a cause of leaky gut and the leaky gut in turn may be a mechanism through which dysbiosis exerts its effect on the immune and nervous systems. Gut permeability is thus interesting as a parameter to measure, such as by the presence of Saccharomyces cerevisiae or Candida albicans antibodies. Also, increased levels of these antibodies have been documented in SCZ [50–52].
Inflammation during Prenatal Life and Neurodevelopment. Finally, another perspective from which to view the impact of the immune system on the brain is neurodevelopmental. There is evidence that pro-inflammatory states during prenatal life, especially in the second trimester, are associated with the development of SCZ [35]. These states can be due to maternal exposure to infection or stressful situations such as the loss of a partner, war, obstetric complications, or starvation [35]. In their review, Suvisaari et al. (2013) considered three theories: First, that cytokines play a role in brain development in processes like neurogenesis, gliogenesis, proliferation, axon pathfinding, and microglial development. The second theory hypothesizes that microglia are hyperactive in SCZ. The third theory is based on the finding that auto-antibodies, such as anti-brain and antinuclear antibodies, are elevated in SCZ, and proposes that brain-reactive auto-antibodies participate in the pathophysiology of SCZ [35].
Direct Evidence of a Role of the Microbiota in Immunomodulation and Neuroimmunity
We have discussed evidence for a link between the immune system and mental disorders and in parallel between the microbiota and the immune system. The evidence that links these three elements comes mostly from observational studies which we treat in "Microbiota and Abnormal Neurodevelopment" section. Jang et al. in 2018 reported that exposure of mice to ampicillin causes anxiety and colitis and changes in the microbiota composition [53]. Then they showed that these changes are accompanied by increased blood corticosterone, interleukin-6, and lipopolysaccharide levels. Also, inflammatory cells such as monocytes and dendritic cells were recruited to the hippocampus. Finally, they demonstrated reversal of the changes and the symptoms following administration of Lactobacillus reuteri [53]. In a mouse model, it has been shown that altering the microbiome by means of antibiotics diminishes plaque deposition in the brain [54]. Another experiment, by Wilck et al., also demonstrated a link between the microbiota, T-h17 lymphocytes, and neurological health by treating mice with salt, showing compositional changes in the microbiota and reduced aggravation of induced encephalomyelitis, thus demonstrating the existence of what they call a “gut-immune” axis [55]. The presence of this axis is further supported by experiments in animal models of other brain conditions such as stroke [56]. There are even animal models of depression that are constructed by inducing inflammation [57]. It has also been reported that the signaling of the inflammasome associated with anxiety and depression affects the gut microbiota, suggesting that the communication is bidirectional [58].
Microbiota and Abnormal Neurodevelopment
Microbiota and Early Brain Development
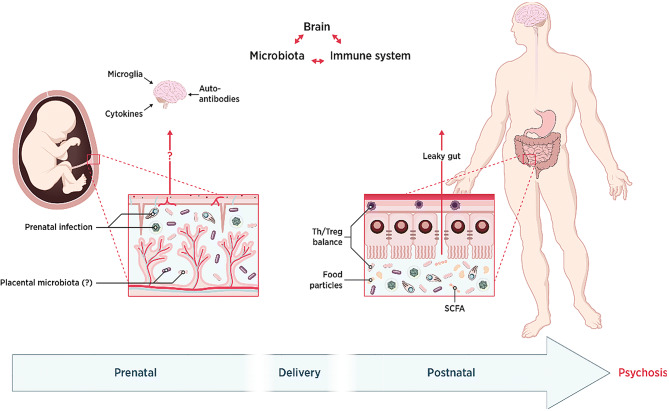
The prenatal and postnatal periods are critical neurodevelopmental windows in mammals, and they overlap with the original microbial colonization [59]. Several disruptions of normal development have been described that contribute to the pathogenesis of psychosis and could be mediated by changes in the microbiota (Fig. 2).
Fig. 2.
Role of the microbiota-gut-brain axis during neurodevelopment. We focus on three main critical periods. First, in the postnatal period, although there is consensus about the absence of placental microbiota, microbial disturbance caused by prenatal infection or the administration of antibiotics could impact brain development through diverse pathways. Second, microbial colonization of the newborn takes place differently depending on the mode and time of delivery and this impacts general health. Finally, during the postnatal period or even adulthood, the microbiota-gut-brain axis still functions through neural, endocrine, and immunological pathways, a particular one being the leaky gut. In the end, abnormal development of the brain might lead to psychosis.
Prenatal Period. Though several studies have supported the notion that the mammalian fetus is not germ-free, as once believed [60], the existence of a placental microbiome is still a matter of debate as the positive findings are thought to issue from contamination [61]. However, there is abundant evidence supporting the influence of the microbiota on neurodevelopment in humans and mice [62].
The most direct evidence comes from prenatal infection and antibiotics studies, which include research on both humans and rodents. Reported results from these assays suggest that infections with Toxoplasma gondii, human herpesvirus 2, and Chlamydophila have robust links to psychosis in humans [63, 64]. Drawing conclusions about the effects of antibiotics is, in contrast, more complicated and controversial due to the frequent coexistence of infection and the numerous types of antibiotic. Few studies have addressed this in the particular case of psychosis. However, a randomized controlled trial found that macrolide use in pregnant women is associated with an increased risk of childhood cerebral palsy and epilepsy [65], whereas another cohort study did not confirm this result [66].
In rodent studies, maternal exposure to antibiotics increases behavioral abnormalities such as anxiety-like and dissocial behavior in the offspring through perturbations in the microbiota [67, 68].
As well as the above, other factors that have been associated with dysbiotic microbiota and abnormal behavior in the offspring include a high-fat diet [69], maternal immune activation [70], prenatal stress [71, 72], peptidoglycan [73], and propionic acid [74].
Time and Mode of Delivery. The microbiota composition has been shown to differ between preterm and term infants [75]. Preterm infants have an increased risk of psychiatric or behavioral problems later in life [76, 77]. Another aspect that has been studied is the mode of delivery and the results are conflicting. One study reported that cesarean delivery is associated with an increase in psychosis among offspring [78] while another found no significant difference [79]. However, it is known that cesarean delivery is associated with colonization by microbes from the skin instead of the vagina [80]. The mechanisms by which the microbiota is involved in the development of psychosis later in life are not yet fully understood, however its potential involvement makes it worthy of further study.
Postnatal Period. Under normal conditions, infants are exposed to environmental and maternal microbes immediately after birth. As previously stated, the mechanisms by which the microbiota interacts with neurodevelopment remain elusive, the results of potential association studies being very varied. For example, several studies on germ-free animals have shown various abnormalities in behavior, including reduced social behavior and memory deficits [81, 82]; conversely, several studies have indicated that germ-free mice have less anxiety-like behavior and more motor activity than specific pathogen-free mice [9, 83]. In addition, probiotic and antibiotic interventions have been reported to alter neural responses in germ-free mice by mediating the hypothalamic-pituitary-adrenal axis or brain-derived neurotrophic factor [84–86]. Overall, a significant body of documentation supports the idea that the gut microbiota affects neurodevelopment during the postnatal period.
Specific Evidence of an Association between the Microbiota and Mental Disorders
Ultra-high Risk for Psychosis. Our recent study found that the levels of Clostridiales, Lactobacillales, and Bacteroidales are higher in fecal samples from ultra-high risk individuals than genetic high-risk subjects and healthy controls [87]. Combining this with magnetic resonance spectroscopy brain scans we hypothesized that compositional changes of gut microbiota might activate microglia in the brain through the elevation of SCFAs.
Schizophrenia. Unlike the ultra-high risk situation, which only has one related study, there have been many studies investigating the gut microbial composition in SCZ cohorts. Among these are 3 longitudinal studies, with treatments ranging from 6 weeks to 12 months. Two studies found significant changes in the gut microbiota after treatment [88, 89]. However, the other study showed non-significant results, possibly because of the non-first-episode subjects and the shortest intervention period – only 6 weeks [90]. Also, case-control studies have compared the gut microbiome diversity between SCZ patients and healthy controls [91–94]. Two studies found that SCZ is associated with reduced richness of the gut microbial composition [92, 93], but the other two failed to show consistent results [91, 94]. What is more, the differences in taxonomic composition between SCZ patients and healthy controls are even more complicated and heterogeneous. For example, the abundance of Clostridium was found to be increased in SCZ patients from two studies [91, 93], but the situation was just the opposite in another study [94]. All the above cases reflect the dilemma of microbiome studies of mental illness, which display high heterogeneity and difficulties with replicability.
Separately, two oropharyngeal microbiome studies have been conducted on the same population. One found that the level of Lactobacillus phage phiadh was significantly higher in SCZ patients than controls [95], and the other showed that Ascomycota, Lactobacilli, and Bifidobacterium, which have been associated with chronic inflammation, are more abundant in SCZ patients than controls [96].
In addition, several serological studies indirectly support the existence of differences in the microbiome of SCZ patients [97–99]. Torrey et al. (2007) conducted a meta-analysis and showed that antibodies to T. gondii are increased in individuals with SCZ [97]. Severance et al. (2012) detected an elevation of IgG antibodies to S. cerevisiae in SCZ compared to controls in 2012, and in 2016 found that C. albicans seropositivity increases the odds for SCZ in males [97, 98]. Houenou et al. found that higher Cytomegalovirus serointensity is related to right hippocampal volume in both SCZ and bipolar disorder patients [99]. A recent systematic review by Nguyen et al. (2018) found five microbiome studies and five translocation studies on SCZ, bipolar disorder, or other severe mental illness. Although the authors pointed out limitations in the literature reviewed, they found an association between reduced microbial diversity and other global community differences in patients with SCZ and bipolar disorder [100].
Beyond human studies, Zhu et al. and Zheng et al. transplanted fecal microbiota from SCZ patients into specific pathogen-free mice and caused SCZ-like behaviors [92, 101]. After that, they suggested that the abnormal behaviors might be induced by subsequently dysregulated kynurenine metabolism or a disrupted glutamate-glutamine-GABA cycle from the morbid gut microbiota.
Autism Spectrum Disorders. At the genus level, Clostridium [102–104], Lactobacillus [105–107], Sutterella [108–110], and Desulfovibrio [106, 111] have often been identified in increased proportions in fecal samples from autistic children. And Prevotella, Coprococcus, and unclassified Veillonellaceae have been reported to occur in low abundance in fecal samples from autistic individuals [112]. However, some research data did not show differences in gut microbiota between autistic children and their neurotypical siblings [113]. Like in human samples, the mouse model of autism also showed consensus results that the phyla Bacteroidetes and Firmicutes and the order Desulfovibrionales are associated with autistic behaviors [74, 114, 115]. And interestingly, treatment with L. reuteri [116] and B. fragilis [114] can reverse some of the core symptoms of autism, such as social deficits and stereotyped behaviors. Also, Chen et al. found that deficiency of KDM5 demethylase causes autistic behaviors in flies through gut dysbiosis, and the administration of Lactobacillus plantarum restores the behavioral impairments [117]. Compared to adult psychosis, autism usually develops in early life and seems to have fewer psychological factors. Hence, the gut-brain axis is expected to play a role in the etiology and cure of this disabling disease.
Mood Disorders. The topic of gut microbiota and mood disorders has been widely studied. The phylum Actinobacteria, the order Bacteroidales, and the genus Oscillibacter have been consistently reported to be over-represented in association with depression, in both patients and rodent models [118–121]. Perhaps due to the difficulty of establishing animal models of bipolar disorder (especially mania), almost all evidence is from human studies. And increased Bacteroidetes and Clostridiales and decreased Faecalibacterium have been repeatedly reported in individuals with bipolar disorder [122–126]. Furthermore, Hu et al. considered that the decreased Faecalibacterium and other butyrate-producing bacteria might contribute to bipolar depression, and treatment with Quetiapine could change the microbial composition [123].
Anxiety and Stress-related Disorders. That stressor exposure alters the gut microbiota in rodents and humans has been well studied. The results have shown a decrease of Lactobacillus and an increase of Lachnospiraceae after stress [81, 127–129]. The fact that both parasite-infected mice and those on an altered diet show anxiety-like behavior strengthens the hypothesis that the microbiota plays a role in anxiety and stress-related disorders [84, 130]. Also, an exploratory study demonstrated that decreased total abundance of Actinobacteria, Lentisphaerae, and Verrucomicrobia is associated with more severe symptoms of post-traumatic stress disorder [131]. Whether germ-free rodents show increased or reduced anxiety-like behavior has not yet been consistently established [9, 132]. The influence of the gut microbiota on anxiety and stress-related disorders warrants further investigation.
Overall, most of the specific evidence either points to the regulation of the immune system or neurotransmitters. And because of all the limitations of animal studies and the conclusions drawn from them, translational studies are critically needed in this field. A summary of studies that explored microbial influence during neurodevelopmental windows and subsequent mental disorders is detailed in the supplementary material (Table S1).
Prospective Therapeutic Applications in Mental Disorders
Probiotic Studies
A probiotic is a live organism that, when ingested in adequate amounts, exerts a health benefit. Their use in mental illness has been reviewed more extensively elsewhere [133]. Several members of the microbiota are known to produce neurotransmitters such as dopamine, gamma-aminobutyric acid (GABA), norepinephrine, serotonin (5-HT), acetylcholine, and endocannabinoids [134]. The fact that the intestinal microbiota produces neuroactive compounds is one of the reasons why it is pertinent to study and test its therapeutic potential. Dinan et al. (2013) define a psychobiotic as such a substance that produces a health benefit in patients suffering from psychiatric illness [134]. It has been reported that 5-HT plasma levels are significantly higher in normal mice than in germ-free mice [135]. This, together with the finding that ingestion of Bifidobacterium infantis in rats increases the levels of tryptophan [136], supports the hypothesis that the microbiota plays a role in the modulation of neurotransmitter levels and possibly also mood. Furthermore, the gut microbiota has been shown to affect the levels of brain-derived neurotrophic factor in the brain [84]. It has even been postulated that the microbial colonization of the newborn and infant participates in modulating the development of the hypothalamic-pituitary-adrenal axis [86]. It has also been demonstrated that the regulation of mood by the gut microbiota is mediated by the vagus nerve as it disappears when the vagus is sectioned [137]. The gut microbiota has also been implicated in anxiety regulation in animal models [83, 138].
The use of psychobiotics has been studied in humans in terms of stress [128, 133, 139–142], anxiety [133, 142–145], and mood [133, 142–145]. However, there is little evidence to support the clinical use of psychobiotics and their efficacy. In a systematic review, Romijin et al. [146] studied the evidence behind the use of psychobiotics in humans. Their search led to a preselection of ten studies of various mental disorders, none of which showed a statistically significant difference after the administration of probiotics. They selected only one study concerning SCZ in which Dickerson et al. (2014) found that repeated-measures analysis of variance showed no significant differences in the total score on the Positive and Negative Symptom Scale (PANSS) between probiotic and placebo supplementation [147]. Romijin et al. (2015) concluded that there is little supporting evidence for the use of psychobiotics in humans, and recommended that further research be conducted in affected populations while taking into consideration the duration of the intervention period and the probiotic strain [146]. Nonetheless, a more recent study by Dickerson et al. has shown fewer re-hospitalizations after mania with psychobiotic administration [148].
Anti-inflammatory or Immunomodulatory Drugs and Dietary Modifications as Add-on Therapy in Mental Disorders
NSAIDs, aspirin, omega-3 fatty acids, and minocycline have been tested on the symptoms of SCZ, and there is evidence that they are modestly effective [149, 150]. The second-generation tetracycline minocycline has been tested in humans for the treatment of SCZ. In a systematic review and meta-analysis of six randomized controlled trials by Solmi et al. (2017), it was found that minocycline has significant beneficial effects, particularly on improving the negative symptoms. In the PANSS the standardized median difference (SMD) was – 0.59 with a confidence interval of [–0.88, –0.00] and a P = 0.04. A difference in negative symptoms was also documented (SMD = –0.76, CI = [–1.21, –0.31], P = 0.001). It also has positive effects on cognitive symptoms such as attention and vigilance as well as executive functioning. More trials are necessary to study the effect on positive symptoms [151]. Whether it acts through modification of the microbiota, immunomodulation, or another mechanism remains to be determined [152].
Finally, according to a revision by Kalaydjian et al. (2006) there is evidence from ecological studies, prevalence studies, clinical trials that include dietary recommendations, and immunological and genetic findings, that supports the view that SCZ and celiac disease may be heterogeneous presentations of a similar cause and that individuals with SCZ could benefit from dietary modifications [153].
Adjuvant Therapy to Counteract Antipsychotic Side-effects
Antipsychotic medication is known to cause long-term metabolic side-effects such as metabolic syndrome, dyslipidemia, weight gain, insulin resistance, T2MD, and cardiovascular disease [154–160]. In an attempt to assess the mechanisms underlying these adverse effects, Davey et al. (2012) studied the consequences of olanzapine administration in rats. Olanzapine-treated rats had an increase in Firmicutes and a decrease in Bacteroidetes [161] which coincided with the microbiome changes already documented for obesity in humans [162]. To investigate whether the microbiota is directly involved in the metabolic effects of olanzapine, in a second study, Davey et al. [163] administered broad-spectrum antibiotics to rats and found that they attenuated the side-effects of olanzapine. Moreover, they found that when antibiotics were administered along with olanzapine the increase in Firmicutes and decrease in Bacteroidetes no longer occurred. This was later confirmed with risperidone when Bahr et al. [164] demonstrated that one of the mechanisms by which risperidone causes weight gain is a decrease in energy expenditure. Interestingly, when they transplanted into naïve mice feces from mice that had suffered weight gain after risperidone administration, these naïve mice also experienced weight gain, demonstrating that changes in the microbiota alone are sufficient to cause weight gain. The same group also documented changes in the microbiota coherent with those described in obesity among children taking risperidone chronically [165].
It has recently been reported that ~24% of prescription drugs inhibit bacterial growth in vitro, and, despite their chemical variability, antipsychotics are one of the groups most notorious for their inhibition of bacterial growth, and it has been proposed that this may be involved in the mechanism of action of these drugs [166].
Although causality and mechanism cannot yet be established, the manipulation of the microbiota by the use of prebiotics, probiotics, dietary modifications, or even antibiotics is a promising tool when it comes to palliating the adverse effects of antipsychotics on energy metabolism. Furthermore, changes in the microbiota may be involved in the mechanism of action of antipsychotics.
Conclusions
Psychotic disorders are highly disabling, hence efforts to understand their pathophysiology are of the utmost importance. It has been extensively documented that inflammation plays a role in these phenomena, as shown by the association between mental disorders and immunological alterations in epidemiological studies.
We have reviewed studies that show an association between prenatal infection and antibiotic use during the windows of development (prenatal, during delivery, and during the postnatal period) that affect the maternal microbiota and might impact brain development. The major drawback of the included studies is that most of them are descriptive of associations and fail to provide insight into the causality and mechanisms of the observed phenomena. Other limitations included small sample sizes and the diversity of differences in the microbiotas of cases and controls. Therefore, there is a clear need for more reports that elucidate the mechanisms by which disturbances in the microbiota cause changes in the gut and the immune system and how these translate into brain pathology. We can still conclude that there is enough evidence to suggest a role for the microbiota and the immune system in the pathophysiology of mental disorders. And further exploration of how suspect microbiota affect the existing psycho-immunology pathway might be a short-cut in this field. In short, investigation of the microbiota-immune-brain axis is a promising field for future study as it may both shed light on one of the mechanisms underlying mental disorders as well as be a source of therapeutic interventions and diagnostic tools such as potential biomarkers. We hope that soon the collective effort in microbiome research will translate into bench interventions and public health recommendations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This review was supported by the National Natural Science Foundation of China (81871056). We would like to thank Okko Alitalo, MSc, from the Laboratory of Neurotherapeutics of the Faculty of Pharmacy at the University of Helsinki for designing figure 2. We would also like to thank Dr. Henriette Raventós and her team at the Cellular and Molecular Biology Research Centre of the University of Costa Rica for their corrections and comments on this paper.
Conflict of interest
The authors claim that there are no conflicts of interest.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390: 1211–1259. [DOI] [PMC free article] [PubMed]
- 2.Chong HY, Teoh SL, Wu DB-C, Kotirum S, Chiou C-F, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat 2016, 12: 357–373. [DOI] [PMC free article] [PubMed]
- 3.Arciniegas DB. Psychosis. Continuum (Minneap Minn) 2015, 21: 715–736. [DOI] [PMC free article] [PubMed]
- 4.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 5.Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- 6.García-Bueno B, Bioque M, MacDowell KS, Santabárbara J, Martínez-Cengotitabengoa M, Moreno C, et al. Pro-/antiinflammatory dysregulation in early psychosis: results from a 1-year follow-up study. Int J Neuropsychopharmacol 2014, 18. 10.1093/ijnp/pyu037. [DOI] [PMC free article] [PubMed]
- 7.Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front Neuroendocrinol. 2018;51:80–101. doi: 10.1016/j.yfrne.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Hong J, Reed C, Novick D, Haro JM, Aguado J. Clinical and economic consequences of medication non-adherence in the treatment of patients with a manic/mixed episode of bipolar disorder: results from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM) study. Psychiatry Res. 2011;190:110–114. doi: 10.1016/j.psychres.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 11.Sundquist K, Li X, Hemminki K, Sundquist J. Subsequent risk of hospitalization for neuropsychiatric disorders in patients with rheumatic diseases: a nationwide study from Sweden. Arch Gen Psychiatry. 2008;65:501–507. doi: 10.1001/archpsyc.65.5.501. [DOI] [PubMed] [Google Scholar]
- 12.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–1310. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 13.Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune diseases and psychosis. Biol Psychiatry. 2014;75:300–306. doi: 10.1016/j.biopsych.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, et al. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011;37:94–100. doi: 10.1093/schbul/sbp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khandaker GM, Zammit S, Lewis G, Jones PB. A population-based study of atopic disorders and inflammatory markers in childhood before psychotic experiences in adolescence. Schizophr Res. 2014;152:139–145. doi: 10.1016/j.schres.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiosano S, Nir Z, Gendelman O, Comaneshter D, Amital H, Cohen AD, et al. The association between systemic lupus erythematosus and bipolar disorder - a big data analysis. Eur Psychiatry. 2017;43:116–119. doi: 10.1016/j.eurpsy.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Tiosano S, Farhi A, Watad A, Grysman N, Stryjer R, Amital H, et al. Schizophrenia among patients with systemic lupus erythematosus: population-based cross-sectional study. Epidemiol Psychiatr Sci. 2017;26:424–429. doi: 10.1017/S2045796016000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson J, Eaton W, Cascella N, Fasano A, Santora D, Sullivan K, et al. Gluten sensitivity and relationship to psychiatric symptoms in people with schizophrenia. Schizophr Res. 2014;159:539–542. doi: 10.1016/j.schres.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151–156. doi: 10.1016/j.ajp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Kota SK, Meher LK, Jammula S, Kota SK, Modi KD. Clinical profile of coexisting conditions in type 1 diabetes mellitus patients. Diabetes Metab Syndr. 2012;6:70–76. doi: 10.1016/j.dsx.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Sturdy PM, Victor CR, Anderson HR, Bland JM, Butland BK, Harrison BDW, et al. Psychological, social and health behaviour risk factors for deaths certified as asthma: a national case-control study. Thorax. 2002;57:1034–1039. doi: 10.1136/thorax.57.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilvarry CM, Sham PC, Jones PB, Cannon M, Wright P, Lewis SW, et al. Family history of autoimmune diseases in psychosis. Schizophr Res. 1996;19:33–40. doi: 10.1016/0920-9964(95)00045-3. [DOI] [PubMed] [Google Scholar]
- 23.Nasr S, Altman EG, Meltzer HY. Concordance of atopic and affective disorders. J Affect Disord. 1981;3:291–296. doi: 10.1016/0165-0327(81)90030-6. [DOI] [PubMed] [Google Scholar]
- 24.Osterberg E. Schizophrenia and rheumatic disease. A study on the concurrence of inflammatory joint diseases and a review of 58 case-records. Acta Psychiatr Scand. 1978;58:339–359. doi: 10.1111/j.1600-0447.1978.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 25.Corvin A, Morris DW. Genome-wide association studies: findings at the major histocompatibility complex locus in psychosis. Biol Psychiatry. 2014;75:276–283. doi: 10.1016/j.biopsych.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Liu JF, Wu R, Li JX. Toll of mental disorders: TLR-mediated function of the innate immune system. Neurosci Bull. 2019;35:771–774. doi: 10.1007/s12264-018-00335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, et al. American Gut: an open platform for citizen science microbiome research. mSystems. 2018;3:e00031-18. doi: 10.1128/mSystems.00031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song X, Fan X, Song X, Zhang J, Zhang W, Li X, et al. Elevated levels of adiponectin and other cytokines in drug naïve, first episode schizophrenia patients with normal weight. Schizophr Res. 2013;150:269–273. doi: 10.1016/j.schres.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 30.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Tourjman V, Kouassi É, Koué M-È, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res. 2013;151:43–47. doi: 10.1016/j.schres.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Wood SJ, Yung AR, McGorry PD, Pantelis C. Neuroimaging and treatment evidence for clinical staging in psychotic disorders: from the at-risk mental state to chronic schizophrenia. Biol Psychiatry. 2011;70:619–625. doi: 10.1016/j.biopsych.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 34.Niu Z, Yang L, Wu X, Zhu Y, Chen J, Fang Y. The relationship between neuroimmunity and bipolar disorder: mechanism and translational application. Neurosci Bull. 2019;35:595–607. doi: 10.1007/s12264-019-00403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suvisaari J, Mantere O. Inflammation theories in psychotic disorders: a critical review. Infect Disord Drug Targets. 2013;13:59–70. doi: 10.2174/18715265112129990032. [DOI] [PubMed] [Google Scholar]
- 36.Bian Q, Kato T, Monji A, Hashioka S, Mizoguchi Y, Horikawa H, et al. The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:42–48. doi: 10.1016/j.pnpbp.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 37.Okada H, Kuhn C, Feillet H, Bach JF. The, “hygiene hypothesis” for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Groot PF, Belzer C, Aydin Ö, Levin E, Levels JH, Aalvink S, et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS One. 2017;12:e0188475. doi: 10.1371/journal.pone.0188475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knip M, Honkanen J. Modulation of type 1 diabetes risk by the intestinal microbiome. Curr Diab Rep. 2017;17:105. doi: 10.1007/s11892-017-0933-9. [DOI] [PubMed] [Google Scholar]
- 40.Wekerle H. Nature, nurture, and microbes: The development of multiple sclerosis. Acta Neurol Scand. 2017;136(Suppl 201):22–25. doi: 10.1111/ane.12843. [DOI] [PubMed] [Google Scholar]
- 41.Kim D, Zeng MY, Núñez G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp Mol Med. 2017;49:e339. doi: 10.1038/emm.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quigley EMM. Primary biliary cirrhosis and the microbiome. Semin Liver Dis. 2016;36:349–353. doi: 10.1055/s-0036-1594006. [DOI] [PubMed] [Google Scholar]
- 43.Talotta R, Atzeni F, Ditto MC, Gerardi MC, Sarzi-Puttini P. The microbiome in connective tissue diseases and vasculitides: an updated narrative review. J Immunol Res. 2017;2017:6836498. doi: 10.1155/2017/6836498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yacoub R, Jacob A, Wlaschin J, McGregor M, Quigg RJ, Alexander JJ. Lupus: The microbiome angle. Immunobiology. 2018;223:460–465. doi: 10.1016/j.imbio.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Lowry CA, Smith DG, Siebler PH, Schmidt D, Stamper CE, Hassell JE, et al. The microbiota, immunoregulation, and mental health: implications for public health. Curr Environ Health Rep. 2016;3:270–286. doi: 10.1007/s40572-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJM, et al. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol. 2011;14:746–755. doi: 10.1017/S1461145710001653. [DOI] [PubMed] [Google Scholar]
- 47.de Araujo EG, da Silva GM, Dos Santos AA. Neuronal cell survival: the role of interleukins. Ann N Y Acad Sci. 2009;1153:57–64. doi: 10.1111/j.1749-6632.2008.03974.x. [DOI] [PubMed] [Google Scholar]
- 48.Gecse K, Róka R, Séra T, Rosztóczy A, Annaházi A, Izbéki F, et al. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion. 2012;85:40–46. doi: 10.1159/000333083. [DOI] [PubMed] [Google Scholar]
- 49.Dinan TG, Cryan JF. The impact of gut microbiota on brain and behaviour: Implications for psychiatry. Curr Opin Clin Nutr Metabol Care. 2015;18:552–558. doi: 10.1097/MCO.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 50.Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012;138:48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, et al. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophrenia. 2016;2:16018. doi: 10.1038/npjschz.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013;148:130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang HM, Lee HJ, Jang SE, Han MJ, Kim DH. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018;11:1386–1397. doi: 10.1038/s41385-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 54.Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, et al. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci. 2016;36:7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma L, Demin KA, Kolesnikova TO, Khatsko SL, Zhu X, Yuan X, et al. Animal inflammation-based models of depression and their application to drug discovery. Expert Opin Drug Discov. 2017;12:995–1009. doi: 10.1080/17460441.2017.1362385. [DOI] [PubMed] [Google Scholar]
- 58.Wong M-L, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Kuperman AA, Koren O. Antibiotic use during pregnancy: how bad is it? BMC Med. 2016;14:91. doi: 10.1186/s12916-016-0636-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leiby JS, McCormick K, Sherrill-Mix S, Clarke EL, Kessler LR, Taylor LJ, et al. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome. 2018;6:196. doi: 10.1186/s40168-018-0575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 63.Monroe JM, Buckley PF, Miller BJ. Meta-analysis of anti-Toxoplasma gondii IgM antibodies in acute psychosis. Schizophr Bull. 2015;41:989–998. doi: 10.1093/schbul/sbu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arias I, Sorlozano A, Villegas E, de Dios Luna J, McKenney K, Cervilla J, et al. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res. 2012;136:128–136. doi: 10.1016/j.schres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 65.Meeraus WH, Petersen I, Gilbert R. Association between antibiotic prescribing in pregnancy and cerebral palsy or epilepsy in children born at term: a cohort study using the health improvement network. PLoS One. 2015;10:e0122034. doi: 10.1371/journal.pone.0122034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with preterm rupture of the membranes: 7-year follow-up of the ORACLE I trial. Lancet. 2008;372:1310–1318. doi: 10.1016/S0140-6736(08)61202-7. [DOI] [PubMed] [Google Scholar]
- 67.Tochitani S, Ikeno T, Ito T, Sakurai A, Yamauchi T, Matsuzaki H. Administration of non-absorbable antibiotics to pregnant mice to perturb the maternal gut microbiota is associated with alterations in offspring behavior. PLoS One. 2016;11:e0138293. doi: 10.1371/journal.pone.0138293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Degroote S, Hunting DJ, Baccarelli AA, Takser L. Maternal gut and fetal brain connection: Increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:76–82. doi: 10.1016/j.pnpbp.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165:1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pendyala G, Chou S, Jung Y, Coiro P, Spartz E, Padmashri R, et al. Maternal immune activation causes behavioral impairments and altered cerebellar cytokine and synaptic protein expression. Neuropsychopharmacology. 2017;42:1435–1446. doi: 10.1038/npp.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 2015;156:3265–3276. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gur TL, Palkar AV, Rajasekera T, Allen J, Niraula A, Godbout J, et al. Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring. Behav Brain Res. 2019;359:886–894. doi: 10.1016/j.bbr.2018.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Humann J, Mann B, Gao G, Moresco P, Ramahi J, Loh LN, et al. Bacterial peptidoglycan traverses the placenta to induce fetal neuroproliferation and aberrant postnatal behavior. Cell Host Microbe. 2016;19:388–399. doi: 10.1016/j.chom.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foley KA, Ossenkopp KP, Kavaliers M, Macfabe DF. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS One. 2014;9:e87072. doi: 10.1371/journal.pone.0087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barrett E, Guinane CM, Ryan CA, Dempsey EM, Murphy BP, O’Toole PW, et al. Microbiota diversity and stability of the preterm neonatal ileum and colon of two infants. Microbiologyopen. 2013;2:215–225. doi: 10.1002/mbo3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 2012;69:E1–E8. doi: 10.1001/archgenpsychiatry.2011.1374. [DOI] [PubMed] [Google Scholar]
- 77.Quigley MA, Hockley C, Carson C, Kelly Y, Renfrew MJ, Sacker A. Breastfeeding is associated with improved child cognitive development: a population-based cohort study. J Pediatr. 2012;160:25–32. doi: 10.1016/j.jpeds.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 78.O’Neill SM, Curran EA, Dalman C, Kenny LC, Kearney PM, Clarke G, et al. Birth by caesarean section and the risk of adult psychosis: a population-based cohort study. Schizophr Bull. 2016;42:633–641. doi: 10.1093/schbul/sbv152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fond G, Bulzacka E, Boyer L, Llorca PM, Godin O, Brunel L, et al. Birth by cesarean section and schizophrenia: results from the multicenter FACE-SZ data-set. Eur Arch Psychiatry Clin Neurosci. 2017;267:587–594. doi: 10.1007/s00406-016-0708-3. [DOI] [PubMed] [Google Scholar]
- 80.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 82.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(255–264):e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 84.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(599–609):609.e1–609.e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 85.Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol (Lond) 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He Y, Kosciolek T, Tang J, Zhou Y, Li Z, Ma X, et al. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur Psychiatry. 2018;53:37–45. doi: 10.1016/j.eurpsy.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 88.Schwarz E, Maukonen J, Hyytiäinen T, Kieseppä T, Orešič M, Sabunciyan S, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398–403. doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 89.Yuan X, Zhang P, Wang Y, Liu Y, Li X, Kumar BU, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299–306. doi: 10.1016/j.schres.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 90.Pełka-Wysiecka J, Kaczmarczyk M, Bąba-Kubiś A, Liśkiewicz P, Wroński M, Skonieczna-Żydecka K, et al. Analysis of gut microbiota and their metabolic potential in patients with schizophrenia treated with olanzapine: results from a six-week observational prospective cohort study. J Clin Med. 2019;8:1605. doi: 10.3390/jcm8101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen Y, Xu J, Li Z, Huang Y, Yuan Y, Wang J, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophr Res. 2018;197:470–477. doi: 10.1016/j.schres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 92.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv 2019, 5: eaau8317. [DOI] [PMC free article] [PubMed]
- 93.Xu R, Wu B, Liang J, He F, Gu W, Li K, et al. Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav Immun. 2020;85:120–127. doi: 10.1016/j.bbi.2019.06.039. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23–29. doi: 10.1016/j.schres.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, et al. Metagenomic sequencing indicates that the oropharyngeal phageome of individuals with schizophrenia differs from that of controls. Schizophr Bull. 2015;41:1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castro-Nallar E, Bendall ML, Pérez-Losada M. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Torrey EF, Bartko JJ, Lun Z-R, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Severance EG, Kannan G, Gressitt KL, Xiao J, Alaedini A, Pletnikov MV, et al. Anti-gluten immune response following Toxoplasma gondii infection in mice. PLoS ONE. 2012;7:e50991. doi: 10.1371/journal.pone.0050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Houenou J, d’Albis M-A, Daban C, Hamdani N, Delavest M, Lepine JP, et al. Cytomegalovirus seropositivity and serointensity are associated with hippocampal volume and verbal memory in schizophrenia and bipolar disorder. Prog Neuro-Psychopharmaco Biol Psychiatry. 2014;48:142–148. doi: 10.1016/j.pnpbp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 100.Nguyen TT, Kosciolek T, Eyler LT, Knight R, Jeste DV. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J Psychiatr Res. 2018;99:50–61. doi: 10.1016/j.jpsychires.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0475-4. [DOI] [PubMed] [Google Scholar]
- 102.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen M, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 103.Parracho HMRT, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 104.Angelis M, Piccolo M, Vannini L, Siragusa S, Giacomo A, Serrazzanetti D, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 2013, 8. 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed]
- 105.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism - comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 107.Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5:24. doi: 10.1186/s40168-017-0242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio 2012, 3. 10.1128/mbio.00261-11. [DOI] [PMC free article] [PubMed]
- 109.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism 2013, 4: 42. [DOI] [PMC free article] [PubMed]
- 110.Zhai Q, Cen S, Jiang J, Zhao J, Zhang H, Chen W. Disturbance of trace element and gut microbiota profiles as indicators of autism spectrum disorder: A pilot study of Chinese children. Environ Res. 2019;171:501–509. doi: 10.1016/j.envres.2019.01.060. [DOI] [PubMed] [Google Scholar]
- 111.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 112.Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gondalia SV, Palombo EA, Knowles SR, Cox SB, Meyer D, Austin DW. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res. 2012;5:419–427. doi: 10.1002/aur.1253. [DOI] [PubMed] [Google Scholar]
- 114.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Theije CGM, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:197–206. doi: 10.1016/j.bbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 116.Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101(246–259):e6. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen K, Luan X, Liu Q, Wang J, Chang X, Snijders AM, et al. Drosophila histone demethylase KDM5 regulates social behavior through immune control and gut microbiota maintenance. Cell Host Microbe. 2019;25:537–552e8. doi: 10.1016/j.chom.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 119.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 120.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 121.Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, et al. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal. 2017;138:231–239. doi: 10.1016/j.jpba.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 122.Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, et al. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res. 2017;87:23–29. doi: 10.1016/j.jpsychires.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu S, Li A, Huang T, Lai J, Li J, Sublette ME, et al. Gut microbiota changes in patients with bipolar depression. Adv Sci (Weinh) 2019;6:1900752. doi: 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McIntyre RS, Subramaniapillai M, Shekotikhina M, Carmona NE, Lee Y, Mansur RB, et al. Characterizing the gut microbiota in adults with bipolar disorder: a pilot study. Nutr Neurosci. 2019 doi: 10.1080/1028415X.2019.1612555. [DOI] [PubMed] [Google Scholar]
- 125.Painold A, Mörkl S, Kashofer K, Halwachs B, Dalkner N, Bengesser S, et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019;21:40–49. doi: 10.1111/bdi.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rong H, Xie XH, Zhao J, Lai WT, Wang MB, Xu D, et al. Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J Psychiatr Res. 2019;113:90–99. doi: 10.1016/j.jpsychires.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 127.Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245. doi: 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 128.Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;35:146–155. [PubMed] [Google Scholar]
- 129.De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun. 2015;6:7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 130.Pyndt Jørgensen B, Winther G, Kihl P, Nielsen DS, Wegener G, Hansen AK, et al. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015;27:307–311. doi: 10.1017/neu.2015.10. [DOI] [PubMed] [Google Scholar]
- 131.Hemmings SMJ, Malan-Müller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: An exploratory study. Psychosom Med. 2017;79:936–946. doi: 10.1097/PSY.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 133.Bambury A, Sandhu K, Cryan JF, Dinan TG. Finding the needle in the haystack: systematic identification of psychobiotics. Br J Pharmacol. 2018;175:4430–4438. doi: 10.1111/bph.14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 135.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 136.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 137.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol Behav. 1998;65:63–68. doi: 10.1016/s0031-9384(98)00145-0. [DOI] [PubMed] [Google Scholar]
- 139.Bangsgaard Bendtsen KM, Krych L, Sørensen DB, Pang W, Nielsen DS, Josefsen K, et al. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One. 2012;7:e46231. doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 141.McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol Motil. 2010;22(1029–1035):e268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 142.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 143.Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot J-M, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol. 2012;18:2067–2075. doi: 10.3748/wjg.v18.i17.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61:355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 146.Romijn AR, Rucklidge JJ. Systematic review of evidence to support the theory of psychobiotics. Nutr Rev. 2015;73:675–693. doi: 10.1093/nutrit/nuv025. [DOI] [PubMed] [Google Scholar]
- 147.Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CLG, Schweinfurth LAB, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord 2014, 16. 10.4088/pcc.13m01579. [DOI] [PMC free article] [PubMed]
- 148.Dickerson F, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: A randomized controlled trial. Bipolar Disord. 2018;20:614–621. [Google Scholar]
- 149.Keller WR, Kum LM, Wehring HJ, Koola MM, Buchanan RW, Kelly DL. A review of anti-inflammatory agents for symptoms of schizophrenia. J Psychopharmacol (Oxford) 2013;27:337–342. doi: 10.1177/0269881112467089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73:414–419. doi: 10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- 151.Solmi M, Veronese N, Thapa N, Facchini S, Stubbs B, Fornaro M, et al. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectr. 2017;22:415–426. doi: 10.1017/S1092852916000638. [DOI] [PubMed] [Google Scholar]
- 152.Nemani K, Hosseini Ghomi R, McCormick B, Fan X. Schizophrenia and the gut-brain axis. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:155–160. doi: 10.1016/j.pnpbp.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 153.Kalaydjian AE, Eaton W, Cascella N, Fasano A. The gluten connection: the association between schizophrenia and celiac disease. Acta Psychiatr Scand. 2006;113:82–90. doi: 10.1111/j.1600-0447.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 154.Citrome L, Holt RIG, Walker DJ, Hoffmann VP. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31:455–482. doi: 10.2165/11589060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 155.Birkenaes AB, Birkeland KI, Engh JA, Faerden A, Jonsdottir H, Ringen PA, et al. Dyslipidemia independent of body mass in antipsychotic-treated patients under real-life conditions. J Clin Psychopharmacol. 2008;28:132–137. doi: 10.1097/JCP.0b013e318166c4f7. [DOI] [PubMed] [Google Scholar]
- 156.Chintoh AF, Mann SW, Lam TKT, Giacca A, Remington G. Insulin resistance following continuous, chronic olanzapine treatment: an animal model. Schizophr Res. 2008;104:23–30. doi: 10.1016/j.schres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 157.Perez-Iglesias R, Mata I, Pelayo-Teran JM, Amado JA, Garcia-Unzueta MT, Berja A, et al. Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naïve population. Schizophr Res. 2009;107:115–121. doi: 10.1016/j.schres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 158.Patel JK, Buckley PF, Woolson S, Hamer RM, McEvoy JP, Perkins DO, et al. Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res. 2009;111:9–16. doi: 10.1016/j.schres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 159.Cohen D, Correll CU. Second-generation antipsychotic-associated diabetes mellitus and diabetic ketoacidosis: mechanisms, predictors, and screening need. J Clin Psychiatry. 2009;70:765–766. doi: 10.4088/jcp.09ac05255. [DOI] [PubMed] [Google Scholar]
- 160.Farwell WR, Stump TE, Wang J, Tafesse E, L’Italien G, Tierney WM. Weight gain and new onset diabetes associated with olanzapine and risperidone. J Gen Intern Med. 2004;19:1200–1205. doi: 10.1111/j.1525-1497.2004.40126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl) 2012;221:155–169. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- 162.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 163.Davey KJ, Cotter PD, O’Sullivan O, Crispie F, Dinan TG, Cryan JF, et al. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry. 2013;3:e309. doi: 10.1038/tp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bahr SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CML, et al. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine. 2015;2:1725–1734. doi: 10.1016/j.ebiom.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. 2015;5:e652. doi: 10.1038/tp.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.