Abstract
This study aimed to obtain the first national estimate of the prevalence of autism spectrum disorder (ASD) in Chinese children. We targeted the population of 6 to 12-year-old children for this prevalence study by multistage convenient cluster sampling. The Modified Chinese Autism Spectrum Rating Scale was used for the screening process. Of the target population of 142,086 children, 88.5% (n = 125,806) participated in the study. A total of 363 children were confirmed as having ASD. The observed ASD prevalence rate was 0.29% (95% CI: 0.26%–0.32%) for the overall population. After adjustment for response rates, the estimated number of ASD cases was 867 in the target population sample, thereby achieving an estimated prevalence of 0.70% (95% CI: 0.64%–0.74%). The prevalence was significantly higher in boys than in girls (0.95%; 95% CI: 0.87%–1.02% versus 0.30%; 95% CI: 0.26%–0.34%; P < 0.001). Of the 363 confirmed ASD cases, 43.3% were newly diagnosed, and most of those (90.4%) were attending regular schools, and 68.8% of the children with ASD had at least one neuropsychiatric comorbidity. Our findings provide reliable data on the estimated ASD prevalence and comorbidities in Chinese children.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00530-6) contains supplementary material, which is available to authorized users.
Keywords: Autism spectrum disorder, Prevalence, Co-morbidity, Autism Spectrum Rating Scale, China
Introduction
Autism spectrum disorder (ASD) is a group of neurodevelopmental dysfunctions characterized by impaired social communication and interaction as well as repetitive and stereotypical behaviors [1]. The World Health Organization (WHO) estimated that 0.76% of the world’s children have ASD, based on studies in countries accounting for < 16% of the population [2]. The alarming increase in the prevalence of ASD reported over the last two decades also poses an important public health concern [3–8].
Various methods of case ascertainment and determination have been employed in ASD prevalence studies in different countries. In the USA, the estimated prevalence among 8-year-old children was 0.66% in 2002, 1.46% in 2012, and 1.68% in 2014 based on active surveillance and expert record review by the Centers for Disease Control and Prevention (CDC) [3, 9, 10]. Most recently, a prevalence of 2.47% was estimated for children aged 3–17 years based on parental reports of physicians’ diagnoses from a representative sample of households included in the National Health Interview Survey [4, 11]. The estimated prevalence and the trends in prevalence over time have been reported for Finland and Denmark, where national registries are available [12]. A prevalence of 1.57% was reported based on a school-based survey in the UK in 2009 [13]. A total population survey (55,266) was conducted in South Korea in 2011, and an estimated prevalence of 2.64% for children aged 7–12 years was reported [14, 15]. An epidemiological survey with a large sample size administered through questionnaires and home visits was conducted in India in 2016, and the estimated prevalence of ASD was 0.23% among those aged 1–30 years [16]. The association between indicators of socioeconomic status, ethnic background, and the prevalence of ASD in the USA in 2002 has also been reported [17]. Studies of ASD prevalence in developing countries are rare or generally of low quality due to a small sample size or the use of non-standard methods for case determination [5, 18].
China has ~ 22% of the world’s population. Over the last decade, the medical and educational communities have witnessed and experienced a demand for services and concerns for children with ASD across the country [19]. A few small-scale studies have reported the prevalence of ASD in mainland China since 2003 using various methods of case ascertainment [20, 21]. A recent meta-analysis of 25 studies of ASD in Chinese children, mostly in China, found an estimated prevalence of 0.12% (95% CI: 0.08%–0.15%) in mainland China and 0.27% (95% CI: 0.19%–0.35%) in mainland China, Hong Kong, and Taiwan region [22]. These prevalence estimates are much lower than those reported in most population-based studies in other countries and are believed to be underestimates because of the methodologies used. These epidemiological surveys were conducted using non-representative samples and regions with non-standard methods of case ascertainment and diagnostic confirmation. Thus, reliable data on the national prevalence of ASD in China is not yet available.
To assess the medical and educational service needs of the growing number of children with ASD in China, medical professionals and public policy-makers count on reliable prevalence data. Thus, with support from the National Health Commission of the central government in China, we conducted the first nationwide population-based study with a large representative sample to investigate the prevalence of ASD and describe its comorbidities among children in China.
Methods
Study Sites and Recruitment Procedures
A pilot study was conducted from January to July 2014 at four sites (Shanghai, Guangzhou, Changsha, and Harbin) to develop a modified Chinese version of the Autism Spectrum Rating Scale (MC-ASRS) [23–25] (Supplementary Method A1). The main study was conducted from July 2014 to December 2016. We used a multi-stage convenience cluster sampling strategy and selected eight cities (Shanghai, Guangzhou, Changsha, Chongqing, Chengdu, Wenzhou, Beijing, and Harbin) from five provinces (Zhejiang, Hunan, Sichuan, Guangdong, and Heilongjiang) and three municipalities (Shanghai, Beijing, and Chongqing) as the study sites (Table 1 and Supplementary Method A2). Multistage convenience cluster sampling was applied at each site (Table 2). We selected one to three urban districts based on the size of the population and the proportion of the migrant population, information that was obtained from the local Public Security Bureau Household Registration System (PSBHSS) (Supplementary Method A2).
Table 1.
Geographic characteristics of the eight study sites.
| Site | Location | Area (km2) | Total Population (million) | 2016 PCI (Yuan) | Site Ranking | National Ranking (100 cities) |
|---|---|---|---|---|---|---|
| Shanghai | East | 6,340 | 24 | 52,962 | Top 1 | 1 |
| Guangzhou | South | 7,434 | 13.5 | 52,829 | Top 3 | 7 |
| Changsha | Middle | 11,819 | 7.4 | 46,735 | Top 5 | 20 |
| Chengdu | Southwest | 14,312 | 15.7 | 27,239 | Top 6 | 47 |
| Chongqing | Southwest | 82,400 | 33.7 | 33,476 | Top 8 | 100 |
| Wenzhou | Southeast | 12,061 | 8.1 | 39,961 | Top 4 | 13 |
| Beijing | North | 16,410 | 21.7 | 30,978 | Top 2 | 2 |
| Harbin | Northeast | 53,100 | 9.6 | 44,026 | Top 7 | 64 |
| Total | / | 203,876 (2.1) | 133.7 (10) | / | / | / |
PCI, average per capita income in yuan (1 US dollar equals ~ 6.4 yuan); data from the National Bureau of Statistics of China 2016 (http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm).
Table 2.
Sampling strategy for the study population.
| Study site | Number of selected districts | Names of selected districts | Number of selected streets | Names of selected streets | Number of regular schools |
|---|---|---|---|---|---|
| Shanghai | 3 out of 16 | Xuhui | 2 out of 12 | Tianping, Fenglin | 11 |
| Minhang | 1 out of 13 | Qibao | 4 | ||
| Qingpu | 2 out of 11 | Yingpu, Xiayang | 8 | ||
| Guangzhou | 1 out of 11 | Huangpu | 9 out of 9 | Huangpu, Hongshan, Yuzhu, Taisha, Wenchong, Nilian, Nangang, Huidong, Changzhou | 21 |
| Changsha | 1 out of 8 | Liuyang | 4 out of 4 | Huaichuan, Jili, Hehua, Guankou | 16 |
| Harbin | 3 out of 9 | Pingfang | 6 out of 6 | Xingjian, Baoguo, Lianmeng, Youxie, Xinjiang, Xinwei | 20 |
| Nangang | 2 out of 18 | Xinchun, Baojilu | 72 | ||
| Daowai | 4 out of 4 | Nongchun, Juyuan, Yongyuan, Mingzhu | 52 | ||
| Beijing | 2 out of 16 | Dongchen | 5 out of 17 | Hepingli, Andingmen, Jiaodaokou, Tiyuguanlu, Longtan | 38 |
| Daxing | 6 out of 22 | Huangxing, Tuanhe, Huangchun, Sunchun, Tiangongyuan, Guanyingshi | 32 | ||
| Chongqing | 3 out of 26 | Jiulongpo | 3 out of 8 | Jiulongpo, Yangjiaping, Shiping | 5 |
| Changshou | 1 out of 7 | Fengcheng | 5 | ||
| Fengdu | 1 out of 2 | Sanhe | 2 | ||
| Chengdu | 2 out of 20 | Pixian | 3 out of 3 | Pitong, Hezuo, Xiyuan | 16 |
| Tianfu | 31 out of 31 | Huayang, Wanan, Xingnong, Zhengxing, Baisha, and others | 31 | ||
| Wenzhou | 2 out of 11 | Pingyang | 2 out of 16 | Xiaojiang, Shuitou | 31 |
| Total | 17 | 82 | 364 |
We used the local PSBHSS as a sampling frame; this covered all children in the targeted districts because every child born in China must be registered in the PSBHSS by law. The information documented by the PSBHSS includes each child’s full name, identity number, nationality, date of birth, sex, home address, and home phone number. Thus, the use of this system as a sampling frame was considered the best approach; it is superior to the use of other registration systems such as the school registration system, the Disabled Persons’ Federation (DPF) registry system, and hospital information systems, which have frequently been used for other prevalence studies in China [22]. Studies using the other registration systems for sampling might have missed children with ASD who do not attend special or regular schools and instead stay at home.
The sample-size calculation suggested that 15,000 participants were required for each site, with the assumption of a prevalence of 1%, an alpha of 0.05, and a power of at least 0.8 and allowing for 5/1000 error (Supplementary Method A3). All children born between January 1, 2002 and December 31, 2008 (aged 6–12 years) with local residency registration in the PSBHSS were eligible for the study (Supplementary Method A2). These children attended regular schools, special education schools or rehabilitation centers, or remained at home. After comparing the list from the PSBHSS with the school registration system and DPF registry system for each study site, we assigned each eligible child to one of the following: Source 1 indicated children studying in regular schools located within the sampled districts; Source 2 indicated children studying in regular schools located outside the sampled districts; Source 3 indicated children registered at special education schools, rehabilitation centers, or a DPF anywhere in the local city or children staying at home; and Source 4 indicated children who could not be located or assigned to one of the above sources. Comparison of the sources allowed us to identify children who were born in the sampled districts but had never lived in the area or had moved; these children were subsequently removed from the sample used for prevalence calculations.
This study was approved by the Institutional Ethics Committee at the Children’s Hospital of Fudan University. Written informed consent was given by the parents of the participants.
ASD Screening and Diagnosis
The protocols for screening and diagnosing ASD were different for children from each of the four sources. For children attending regular schools (sources 1 and 2), we used a two-step screening protocol (Supplementary Method A4) because of the large sample size and the expected large number of children screened as positive by the MC-ASRS. Children registered at special education schools, rehabilitation centers, or the DPF registry or children staying at home (Source 3) were relatively few in number and were expected to be at high risk for ASD (Supplementary Method A5). All children in this category underwent direct diagnostic testing.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) was used by a trained team of clinicians to diagnose ASD (Supplementary Method A6). Additional clinical assessment procedures, including the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview-Revised (ADI-R) [26, 27], a cognitive assessment using the Wechsler Intelligence Scale for Children in Chinese (WISC-C) [28], and neuropsychiatric comorbidity assessment using the Chinese Mini International Neuropsychiatric Interview for Children and Adolescents–Parent Version (MINI kid) [29] were administered in a subset of ASD cases. A caregiver interview, growth and development measurements, family history data, and comprehensive physical examination were also obtained. All members of the clinical assessment team were trained in using standard protocols to ensure high quality in the administration of diagnostic evaluations. They were blinded to the MC-ASRS scores during assessments.
The diagnosis of confirmed ASD cases fell into the following four categories: (1) the diagnosis based on the DSM-5 was also supported by the ADI-R, the ADOS, or both; (2) the diagnosis based on the DSM-5 was not supported by either the ADI-R or the ADOS; (3) the diagnosis of ASD was made based on the DSM-5, but neither the ADI-R nor the ADOS were administered; and (4) in a small number of cases from Source 3, participants were not clinically assessed by the research team, but the diagnosis or description of ASD was documented in their medical records or reported by others, such as the DPF and the special education database. In this situation, the available medical records were reviewed, and the ASD diagnosis was made by more than two experienced clinicians on the study team.
Statistical Analysis and the Methods Used to Estimate Prevalence
We used the following two prevalence calculations for ASD: (1) the observed prevalence, i.e., a proportion based on the observed number of ASD cases; and (2) an estimated prevalence that took into account non-responses in different phases of the investigation (Supplementary Method A7). The number of ASD cases among the non-responders was estimated based on the assumption of a rate equal to that observed in the responders from the same survey phase. For students in regular schools outside the sampled districts (Source 2), the response to our mailed invitations to participate in the screening was poor. Thus, we estimated the number of ASD cases based on Source 1 data with an assumption that there was no significant difference in prevalence between the two sources.
The denominator used for prevalence calculations (125,806) was determined by the total number of eligible participants. The observed prevalence was calculated based on the exact number of confirmed ASD cases from all study sites divided by the denominator. For the estimation of overall and sex-specific prevalence, we applied weights to adjust for varying participation rates by site during different survey phases. In addition, the 95% CI for the overall prevalence and site prevalence were determined based on the Poisson distribution.
The prevalence across study sites was compared using Mantel–Haenszel tests. Prior to these comparisons, standardization was performed to adjust for differences in sex stratification and the contributions of sites to the overall sample size of the study by using the whole study sample as a reference. The overall sex and age stratification of the study population across sites or in the included versus excluded populations were compared using the χ2 and Mantel–Haenszel tests to evaluate possible selection bias due to the exclusion of untraceable participants. The clinical assessment scores were compared between previously-diagnosed and newly-diagnosed cases and between the sexes using Student’s t test. We used Cohen’s d to measure between-group differences, using thresholds for small (d = 0.20), medium (d = 0.50), or large (d = 0.80) effect sizes as a guide to make inferences [30].
Results
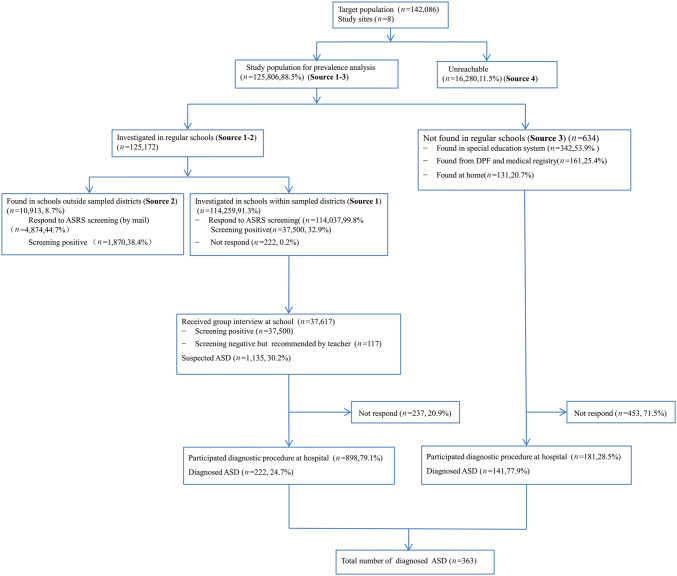
The flow chart of the study population at different sampling and assessment stages is shown in Figure 1. After exclusion of the 16,280 non-participants, 125,806 participants (88.5%) were included in the final prevalence analysis. The age and sex distribution of the entire study population and for each study site are listed in Table 3. The study population included 66,687 (53.0%) males (Table S1). The age (from 6 to 12 years) was relatively evenly distributed throughout the entire study population from the 8 sites (Table S2). The sex distribution of the included sample did not differ from that of the excluded sample (53.0% vs 53.7% for males, respectively); however, the age distributions did differ (Sources 1–3 vs Source 4; P = 0.097 for sex; P < 0.001 for age) (Tables S1 and S3).
Fig. 1.
Flowchart of ASD case screening and determination. ASRS, Autism Spectrum Rating Scale; DPF, Disabled Persons’ Federation; ASD, Autism Spectrum Disorder.
Table 3.
Age, sex, and site distributions of the study population by sampling source.
| Participants | Non-participants | Total n (%) | |||
|---|---|---|---|---|---|
| Source 1 n (%) | Source 2 n (%) | Source 3 n (%) | Source 4 n (%) | ||
| Sex | |||||
| Male | 60,499 (52.9) | 5,768 (52.9) | 420 (66.3) | 8,742 (53.7) | 75,429 (53.1) |
| Female | 53,760 (47.1) | 5,145 (47.1) | 214 (33.7) | 7,538 (46.3) | 66,657 (46.9) |
| Age (years) | |||||
| 6 | 13,223 (11.6) | 1,750 (16.0) | 97 (15.3) | 2,736 (16.8) | 17,806 (12.5) |
| 7 | 20,370 (17.8) | 1,105 (10.1) | 99 (15.6) | 2,680 (16.5) | 24,254 (17.1) |
| 8 | 16,669 (14.6) | 1,044 (9.6) | 83 (13.1) | 2,139 (13.1) | 19,935 (14.0) |
| 9 | 16,527 (14.5) | 1,139 (10.4) | 93 (14.7) | 1,952 (12.1) | 19,711 (13.9) |
| 10 | 18,155 (15.9) | 1,218 (11.2) | 102 (16.1) | 2,138 (13.1) | 21,613 (15.2) |
| 11 | 14,402 (12.6) | 2,410 (22.1) | 79 (12.4) | 1,650 (10.1) | 18,541 (13.1) |
| 12 | 14,913 (13.0) | 2,247 (20.6) | 81 (12.8) | 2,985 (18.3) | 20,226 (14.2) |
| Site | |||||
| Shanghai | 14,490 (12.7) | 2,731 (25.0) | 200 (31.6) | 2,744 (16.9) | 20,165 (14.2) |
| Guangzhou | 12,398 (10.9) | 403 (3.7) | 186 (29.3) | 1,707 (10.5) | 14,694 (10.3) |
| Changsha | 9,744 (8.5) | 1,425 (13.1) | 15 (2.4) | 298 (1.8) | 11,482 (8.1) |
| Harbin | 22,376 (19.6) | 811 (7.4) | 51 (8.0) | 1,245 (7.6) | 24,483 (17.2) |
| Beijing | 7,112 (6.2) | 783 (7.2) | 25 (3.9) | 3,355 (20.6) | 11,275 (7.9) |
| Chongqing | 20,010 (17.5) | 2,167 (19.8) | 98 (15.5) | 3,012 (18.5) | 25,287 (17.8) |
| Chengdu | 15,021 (13.1) | 1,035 (9.5) | 35 (5.5) | 628 (3.9) | 16,719 (11.8) |
| Wenzhou | 13,108 (11.5) | 1,558 (14.3) | 24 (3.8) | 3,291 (20.2) | 17,981 (12.7) |
| Total | 114,259 (100.0) | 10,913 (100.0) | 634 (100.0) | 16,280 (100.0) | 142,086 (100.0) |
Source 1, children studying in regular schools within the sampled districts; Source 2, children studying in regular schools outside the sampled districts; Source 3, children registered at special education schools, rehabilitation centers, or a DPF anywhere in the local city or children staying at home; Source 4, children who could not be located or ascribed to one of the above sources.
In the regular school investigation (Source 1), MC-ASRS questionnaires were collected from a total of 110,416 (96.8%) parents and 108,689 (95.3%) teachers. A total of 37,500 (32.9%) students tested positive through either the parent or teacher rating; the proportion was significantly higher among boys than among girls (37.9% vs 27.2%; P < 0.001). A total of 634 children were identified from Source 3. All of these children were asked to participate in the full ASD diagnostic assessment in the clinics of local hospitals. A total of 10,913 (8.7%) children were eligible for the study but attended schools outside the sampled districts (Source 2). The MC-ASRS screening questionnaires were mailed to these students; 44.7% (n = 4,874) of parents and teachers completed the MC-ASRS. A total of 1,870 (38.4%) students were positive for ASD according to either parent or teacher rating, and 11.8% (n = 220) participated in group face-to-face interviews in schools. Due to the poor response rate, we excluded this group from further participation in the study. The sex ratio and age distributions of children who attended the schools within or outside the sampled districts were comparable (P = 0.09).
In total, 363 participants were diagnosed with ASD according to the DSM-5 criteria; 222 (61.2%) were from regular schools and 157 (43.3%) were newly-diagnosed. The observed ASD prevalence rate was 0.29% (95% CI: 0.26%–0.32%) for the overall population, 0.44% (95% CI: 0.38%–0.49%) for boys, and 0.12% (95% CI: 0.09%–0.15%) for girls. The 363 children with ASD were aged 9.0 ± 2.0 years and the male-to-female ratio was 4.3:1.
The denominators and estimated numbers of ASD cases used for the prevalence estimation are shown by site and investigation source in Supplementary Method A7 and Table S4. The overall estimated prevalence of ASD was 0.70% (95% CI: 0.64%–0.74%) and was significantly higher in boys than in girls (0.95%; 95% CI: 0.87%–1.02% vs 0.30%; 95% CI: 0.26%–0.34%; P < 0.001); however, it did not significantly differ among ages (P = 0.19) (Table 4). Among the 867 estimated ASD cases, the contributions of Sources 1, 2, and 3 were 38.5% (334), 3.4% (29), and 58.1% (504), respectively.
Table 4.
Prevalence of ASD in Chinese children aged 6 to 12 years (per 100).
| Category | Sample size | ASD cases | Observed prevalence (95% CI) | Estimated prevalence (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 66,687 | 292 | 0.44 (0.38, 0.49) | 0.95 (0.87, 1.02) |
| Female | 59,119 | 71 | 0.12 (0.09, 0.15) | 0.30 (0.26, 0.34) |
| Age (years) | ||||
| 6 | 15,070 | 43 | 0.29 (0.20, 0.37) | / |
| 7 | 21,574 | 69 | 0.32 (0.24, 0.40) | / |
| 8 | 17,796 | 61 | 0.34 (0.26, 0.43) | / |
| 9 | 17,759 | 44 | 0.25 (0.17, 0.32) | / |
| 10 | 19,475 | 65 | 0.33 (0.25, 0.41) | / |
| 11 | 16,891 | 36 | 0.21 (0.14, 0.28) | / |
| 12 | 17,241 | 45 | 0.26 (0.18, 0.34) | |
| Total | 125,806 | 363 | 0.29 (0.26, 0.32) | 0.70 (0.64, 0.74) |
| ASD case status | ||||
| Newly diagnosed | / | 157 (43.3) | / | |
| Previously diagnosed | / | 206 (56.7) | / |
ASD, autism spectrum disorder; CI, confidence interval. The estimated prevalence of ASD was higher in boys than in girls (P < 0.001) and did not differ among ages (χ2 = 8.76, df = 6, P = 0.19).
ADOS and ADI-R assessments were offered to all 363 children who were diagnosed with ASD based on the DSM-5 criteria. Among them, 318 (87.6%) were assessed with the ADOS (164 children, 45.2%) and the ADI-R (154 parents, 42.4%). The agreement between the DSM-5-based diagnosis and a positive score on the secondary assessment was 91.5% for the ADOS, 90.3% for the ADI-R, and 96.4% for both the ADOS and the ADI-R. Among the 363 confirmed ASD cases, 185 (51.0%) children also received the WISC-C to evaluate their cognitive function.
Among the 185 children who received the WISC-C test, 35.7% had a normal cognitive performance with an intelligence quotient (IQ) ≥ 85, 18.9% had a borderline IQ (70–85), 11.4% had mild intellectual disability (ID) (IQ, 50–69), and 34.0% had moderate or severe ID (IQ < 50) (Table S5). However, according to the WISC-C, boys with ASD had significantly higher IQs than girls with ASD (73.9 ± 28.7 vs 55.7 ± 20.9, P < 0.001) (Table S6).
Among the 157 children newly diagnosed with ASD, 90.4% were attending regular schools (Sources 1 and 2), which was markedly higher than the proportion from Source 3 (9.6%; P < 0.001). The children newly diagnosed with ASD had significantly higher IQs (~ 1 SD higher; 79.1 ± 25.2 vs 58.4 ± 27.9, P < 0.001) than the previously-diagnosed ASD cases. The mean scores on the ADOS and ADI-R were significantly lower (P < 0.001) for newly-diagnosed cases than for previously-diagnosed cases, which indicates less severe ASD in the newly-diagnosed cases (Table S7). In fact, for 11 of the 12 comparisons of mean scores on clinical measurements, newly-diagnosed children showed less severe impairments than previously-diagnosed children.
Neuropsychiatric comorbidities were assessed using the MINI-kids in a subset of 102 (28.1%) ASD cases, 68.8% of which had at least one comorbid neuropsychiatric disorder (Table S8). The common neuropsychiatric comorbidities included attention deficit hyperactivity disorder (ADHD, 43.1%), followed by specific phobia (10.6%), agoraphobia (7.5%), obsessive–compulsive disorder (6.4%), social phobia (6.3%), mania (5.3%), and tic disorder (5.3%). For the ASD cases (28.9%) with medical records available to review for the occurrence of other common comorbidities, gastrointestinal problems (GI, 41.4%), sleep disorders (19.2%), allergic diseases (15.8%), febrile seizure (6.7%), and epilepsy (5.7%) were also reported. There was no difference in the pattern of psychiatric comorbidity between newly- and previously-diagnosed cases as well as between boys and girls. We found that in children with ASD who had ID (IQ < 70), the frequency of comorbidity was not significantly different from that of children with ASD without ID (IQ ≥ 70).
Discussion
We conducted the first nationwide and the largest cross-sectional epidemiological study of ASD using the total population of children aged 6 to 12 years residing in eight representative cities in China from 2014 to 2016. This study provided a national estimated ASD prevalence of 0.70% (95% CI: 0.64%–0.74%), corresponding to ~ 1 in 143 children. For the first time, we present a reliable estimate of the disease burden of ASD in China. The prevalence was estimated as 0.95% (1 in 105; 95% CI: 0.87%–1.02%) in boys and 0.30% (1 in 333; 95% CI: 0.26%–0.34%) in girls. Importantly, 43.3% of the children with ASD were newly diagnosed, had a milder presentation, attended regular schools at the time of the study, and 2/3 (68.8%) of them had at least one neuropsychiatric comorbidity. Our findings provide valuable guidance to the medical community and policy-makers to develop a strategic plan for the care of children with ASD and to support future research on ASD in China.
Our study has several major strengths. First, this study provides a national estimate of ASD prevalence in a population-based, multi-center, epidemiological study. To the best of our knowledge, ours was the largest sample (125,806) ever surveyed in China or in any published ASD prevalence study using similar methodology [20, 31]. Second, instead of restricting the sampling framework to a school population, as in most previous studies [22], our strategy was based on households and used the most inclusive registration system. This sampling scheme ensured maximal coverage of the target population. Third, we achieved a high response rate for the screening step (~ 90%); this rate was higher than that of any other published population-based study in the literature [4, 14, 16]. Fourth, we used a modified Chinese version of the ASRS for screening, the DSM-5 as the diagnostic tool, and the ADOS and ADI-R for in-depth diagnostic evaluations. In fact, this was the first large ASD prevalence study to use the DSM-5 since it was released in 2013 [32]. In addition, this was the first study to use a combination of screening and diagnostic tools, the MC-ASRS and the ADOS/ADI-R, in Chinese children. The multi-informant approach that combined parent and teacher ratings for the initial ASRS screening followed by a professional group interview is a unique feature of our study design that improved the quality of the screening process. Altogether, we believe that these significant strengths in design and execution have ensured the best estimate of ASD prevalence in China to date.
Our estimated prevalence rate of 0.70% is consistent with the results of several meta-analyses and individual reviews but is lower than the prevalence reported in several recent epidemiological studies that had prevalence rates ranging from 1% to 2% [33, 34]. For instance, the estimated prevalence rate of 1.46% for children aged 8 years was from the CDC’s monitoring network in 2014 [10] and 1.68% in a more recent report [3], and the estimate of 2.47% among children in the USA aged 3 to 17 years from 2014 to 2016 was from the National Health Interview [4]. A prevalence of 1.57% was found in a school-based survey in the UK [13], the prevalence was 2.5% in an Australian birth cohort aged 6–7 years [35], and a prevalence of 2.64% was obtained for South Korean children aged 7–12 years in a population-based survey [14].
Several explanations may account for the lower estimated prevalence in our study. First, the level of cultural and public awareness of ASD should be considered. Although studies have generally found that the clinical phenotypes of ASD show little variation with country or culture [36], a lower ASD prevalence has been consistently reported in Hispanic and African American populations and in populations associated with low socioeconomic status in the USA in surveys by the CDC surveillance program [17, 37], presumably due to less access to specialized medical care and educational services. It is possible that the specific cultural heritage and the level of awareness of ASD in China may have influenced the prevalence estimation in the present study. The first autism case report in China was published in 1987 [38], 40 years after Kanner’s seminal report [39]. However, public awareness in China did not truly emerge until 2010 [40]. Further research may be warranted to assess the impact of these cultural factors. Second, the use of the DSM-5 may have contributed to a lower estimate of ASD prevalence, as suggested in two other population-based studies [41, 42]. These studies have shown that, other things being equal, the prevalence estimate is reduced by 15%–20% when the DSM-5 criteria are used instead of the DMS-IV-TR criteria for case determination. Finally, while the response rate for screening was very high and satisfactory (~ 90%), the participation rate of ~ 80% among children who were screened as positive by the MS-ARS during the in-person diagnostic assessment was slightly lower. The exact reasons why individuals failed to attend in-person assessment need to be fully investigated as it is unclear whether non-participation was more or less common among families with children with ASD. From a cultural standpoint, it is possible that some parents might be afraid of their child being diagnosed with ASD due to social stigma and the fear of being ridiculed by others [40].
In contrast, the 0.70% prevalence reported in this study is significantly higher than the ~ 0.12% (95% CI: 0.08%–0.15%) arising from 25 pooled studies of various age groups from 1987 to 2011 in mainland China [22]. The much lower prevalence in previous studies is likely due to methodological issues, such as the sampling procedures, sample size, and diagnostic criteria used. However, it remains conceptually possible that the higher prevalence in this study may actually reflect a true increase in the incidence of ASD, as suggested in other countries [4, 43].
Consistent with the results reported in the literature [14, 34], the male-to-female ratio of children with ASD in our study was 4.3:1. Similar to the findings of some studies [34], females with ASD had significantly lower cognitive function and more severe impairment in social interactions and other autistic behaviors than males with ASD. Interestingly, children newly diagnosed with ASD had higher IQs than previously-diagnosed children. This is consistent with the fact that most of these newly-diagnosed children were attending regular schools and that their behavioral problems had not drawn sufficient attention from parents or teachers to lead to testing for ASD [14].
We included assessments of neuropsychiatric and medical comorbidity in our study. This is a first among all prevalence studies conducted in Chinese children in China or other regions in Asia. Over two-thirds of the children with ASD had at least one comorbid psychiatric disorder, including ADHD and social phobia. The overall pattern of comorbidity is similar to that found in other reports that used the same assessment tool, with one exception: the rate of anxiety disorder was much lower in our study than in a previous study [44]. Over 40% of the children with ASD had co-occurring medical conditions, such as GI problems. The pattern of medical comorbidity is similar to previous reports, except that the frequency of seizures is lower than the 20%–25% reported in other studies [45].
Limitations
The study has three major limitations. First, we deliberately selected the eight participating sites using several predetermined criteria, including prior research experience with ASD, the quality of infrastructure and facilities, and the level of collaboration between the school and medical communities to implement the study. Consequently, the survey population was mainly composed of urban residents, although there was a fair representation of diverse social and economic strata. Given the rapid urbanization of China over the last two decades, 60% of the population lived in urban areas between 2014 and 2016. Nevertheless, the results may not be generalizable to rural population. The inclusion of children living in rural regions should be considered in future national epidemiological studies when it is technically feasible. Second, the assumption of an equal risk of ASD in participants versus non-participants, particularly the children who failed to present in person for diagnostic assessment, may lead to bias in both directions. For instance, this assumption may underestimate the prevalence if parents of children with ASD were more likely to be non-participants. However, without further investigation, it remains uncertain what impact the 10% and 20% non-response rates in the screening and diagnostic phases, respectively, could have had on the final prevalence estimate in this study. Finally, the response rates differed among sources 1, 2, and 3, although we adjusted for unequal participation from each source in our prevalence calculations.
Based on these limitations, the rural population should be included in future national epidemiological studies, in order to truly represent the prevalence in China. The propagation, extension, and awareness campaigns and science popularization by the ASD network are expanding the public awareness of ASD, and may increase the response rate in the target population.
Conclusions
We report an estimated 0.70% prevalence of ASD among 6- to 12-year-old children in the largest population-based study to date, with > 120,000 children from eight representative cities in China. This estimate translates into a total of ~ 700,000 children aged 6–12 years with ASD in China, based on the national census data for 2016 (http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm). The finding that almost half of the confirmed ASD cases were previously-undiagnosed children attending regular schools has important implications for the medical community, the educational system, and society in China. Our findings support the rising public concern about and awareness of ASD over the last decade in China and provide, for the first time, valid and reliable data to inform public health and government agencies in their efforts to design evidence-based policies regarding the care of children with ASD in China.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Health Commission of the People’s Republic of China (201302002, Clinical Trial NCT02200679). We thank all the parents who participated in this study. For data collection, we would like to acknowledge the members of the National Health Commission listed in the supplement in the LATENT-NHC Study Team member list.
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Members of the LATENT-NHC Study Team are listed in the Supplementary Materials.
Hao Zhou, Xiu Xu, Weili Yan contributed equally.
References
- 1.Harris J. Leo Kanner and autism: a 75-year perspective. Int Rev Psychiatry. 2018;30:3–17. doi: 10.1080/09540261.2018.1455646. [DOI] [PubMed] [Google Scholar]
- 2.Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45:601–613. doi: 10.1017/S003329171400172X. [DOI] [PubMed] [Google Scholar]
- 3.Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67:1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu G, Strathearn L, Liu B, Bao W. Prevalence of autism spectrum disorder among US children and adolescents, 2014–2016. JAMA. 2018;319:81–82. doi: 10.1001/jama.2017.17812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health. 2017;38:81–102. doi: 10.1146/annurev-publhealth-031816-044318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maenner MJ, Durkin MS. Trends in the prevalence of autism on the basis of special education data. Pediatrics. 2010;126:e1018–e1025. doi: 10.1542/peds.2010-1023. [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein E, Daniels J, Schieve LA, Christensen DL, Van Naarden BK, Rice CE, et al. Trends in special education eligibility among children with autism spectrum disorder, 2002–2010. Public Health Rep. 2018;133:85–92. doi: 10.1177/0033354917739582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandell DS, Barry CL, Marcus SC, Xie M, Shea K, Mullan K, et al. Effects of autism spectrum disorder insurance mandates on the treated prevalence of autism spectrum disorder. JAMA Pediatr. 2016;170:887–893. doi: 10.1001/jamapediatrics.2016.1049. [DOI] [PubMed] [Google Scholar]
- 9.Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ 2007, 56: 12–28. [PubMed]
- 10.Christensen DL, Baio J, Van Naarden BK, Bilder D, Charles J, Constantino JN, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years–autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu G, Strathearn L, Liu B, Bao W. Corrected prevalence of autism spectrum disorder among US children and adolescents. JAMA. 2018;319:505. doi: 10.1001/jama.2018.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schendel DE, Bresnahan M, Carter KW, Francis RW, Gissler M, Gronborg TK, et al. The International Collaboration for Autism Registry Epidemiology (iCARE): multinational registry-based investigations of autism risk factors and trends. J Autism Dev Disord. 2013;43:2650–2663. doi: 10.1007/s10803-013-1815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194:500–509. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- 14.Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- 15.Charman T. The highs and lows of counting autism. Am J Psychiatry. 2011;168:873–875. doi: 10.1176/appi.ajp.2011.11060897. [DOI] [PubMed] [Google Scholar]
- 16.Poovathinal SA, Anitha A, Thomas R, Kaniamattam M, Melempatt N, Anilkumar A, et al. Prevalence of autism spectrum disorders in a semiurban community in south India. Ann Epidemiol. 2016;26:663–665. doi: 10.1016/j.annepidem.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Durkin MS, Maenner MJ, Baio J, Christensen D, Daniels J, Fitzgerald R, et al. Autism spectrum disorder among US children (2002–2010): Socioeconomic, Racial, and ethnic disparities. Am J Public Health. 2017;107:1818–1826. doi: 10.2105/AJPH.2017.304032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice CE, Lee LC. Expanding the global reach of research in autism. Autism. 2017;21:515–517. doi: 10.1177/1362361317704603. [DOI] [PubMed] [Google Scholar]
- 19.Huang AX, Jia M, Wheeler JJ. Children with autism in the People’s Republic of China: diagnosis, legal issues, and educational services. J Autism Dev Disord. 2013;43:1991–2001. doi: 10.1007/s10803-012-1722-6. [DOI] [PubMed] [Google Scholar]
- 20.Feng L, Li C, Chiu H, Lee TS, Spencer MD, Wong JC. Autism spectrum disorder in Chinese populations: a brief review. Asia Pac Psychiatry. 2013;5:54–60. doi: 10.1111/appy.12079. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Allison C, Wei L, Matthews FE, Auyeung B, Wu YY, et al. Autism prevalence in China is comparable to Western prevalence. Mol Autism. 2019;10:7. doi: 10.1186/s13229-018-0246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X, Allison C, Matthews FE, Sharp SJ, Auyeung B, Baron-Cohen S, et al. Prevalence of autism in mainland China, Hong Kong and Taiwan: a systematic review and meta-analysis. Mol Autism. 2013;4:7. doi: 10.1186/2040-2392-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H , Zhang L, Wu L, Zou X, Luo X, Xia K, et al. Validity and reliability analysis of the Chinese parent version of the Autism Spectrum Rating Scale (6–18 years) Psychiatry Res. 2015;230:255–261. doi: 10.1016/j.psychres.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhou H, Zhang L, Zou X, Luo X, Xia K, Wu L, et al. Chinese Norms for the Autism Spectrum Rating Scale. Neurosci Bull. 2017;33:161–167. doi: 10.1007/s12264-017-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Zhang L, Luo X, Wu L, Zou X, Xia K, et al. Modifying the Autism Spectrum Rating Scale (6–18 years) to a Chinese Context: An exploratory factor analysis. Neurosci Bull. 2017;33:175–182. doi: 10.1007/s12264-017-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 27.Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 28.Li D, Jin Y, Vandenberg SG, Zhu YM, Tang CH. Report on Shanghai norms for the Chinese translation of the Wechsler Intelligence Scale for Children-Revised. Psychol Rep. 1990;67:531–541. doi: 10.2466/pr0.1990.67.2.531. [DOI] [PubMed] [Google Scholar]
- 29.Shen YM, Chan B, Liu JB, Zhou YY, Cui XL, He YQ, et al. The prevalence of psychiatric disorders among students aged 6–16 years old in central Hunan, China. BMC Psychiatry. 2018;18:243. doi: 10.1186/s12888-018-1823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furukawa TA, Leucht S. How to obtain NNT from Cohen’s d: comparison of two methods. PLoS One. 2011;6:e19070. doi: 10.1371/journal.pone.0019070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong VC, Hui SL. Epidemiological study of autism spectrum disorder in China. J Child Neurol. 2008;23:67–72. doi: 10.1177/0883073807308702. [DOI] [PubMed] [Google Scholar]
- 32.McGuinness TM, Johnson K. DSM-5 changes in the diagnosis of autism spectrum disorder. J Psychosoc Nurs Ment Health Serv. 2013;51:17–19. doi: 10.3928/02793695-20130220-01. [DOI] [PubMed] [Google Scholar]
- 33.Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5:160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 35.Randall M, Sciberras E, Brignell A, Ihsen E, Efron D, Dissanayake C, et al. Autism spectrum disorder: Presentation and prevalence in a nationally representative Australian sample. Aust N Z J Psychiatry. 2016;50:243–253. doi: 10.1177/0004867415595287. [DOI] [PubMed] [Google Scholar]
- 36.Charman T, Loth E, Tillmann J, Crawley D, Wooldridge C, Goyard D, et al. The EU-AIMS Longitudinal European Autism Project (LEAP): clinical characterisation. Mol Autism. 2017;8:27. doi: 10.1186/s13229-017-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen A, Pettygrove S, Meaney FJ, Mancilla K, Gotschall K, Kessler DB, et al. Prevalence of autism spectrum disorders in Hispanic and non-Hispanic white children. Pediatrics. 2012;129:e629–e635. doi: 10.1542/peds.2011-1145. [DOI] [PubMed] [Google Scholar]
- 38.Tao KT. Infantile autism in China. J Autism Dev Disord. 1987;17:289–296. doi: 10.1007/BF01495062. [DOI] [PubMed] [Google Scholar]
- 39.Kanner L. Irrelevant and metaphorical language in early infantile autism. Am J Psychiatry. 1946;103:242–246. doi: 10.1176/ajp.103.2.242. [DOI] [PubMed] [Google Scholar]
- 40.Tang L, Bie B. The stigma of autism in China: an analysis of newspaper portrayals of autism between 2003 and 2012. Health Commun. 2016;31:445–452. doi: 10.1080/10410236.2014.965381. [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Fombonne E, Koh YJ, Kim SJ, Cheon KA, Leventhal BL. A comparison of DSM-IV pervasive developmental disorder and DSM-5 autism spectrum disorder prevalence in an epidemiologic sample. J Am Acad Child Adolesc Psychiatry. 2014;53:500–508. doi: 10.1016/j.jaac.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maenner MJ, Rice CE, Arneson CL, Cunniff C, Schieve LA, Carpenter LA, et al. Potential impact of DSM-5 criteria on autism spectrum disorder prevalence estimates. JAMA Psychiatry. 2014;71:292–300. doi: 10.1001/jamapsychiatry.2013.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fombonne E. Estimated prevalence of autism spectrum conditions in Cambridgeshire is over 1% Evid Based Ment Health. 2010;13:32. doi: 10.1136/ebmh.13.1.32. [DOI] [PubMed] [Google Scholar]
- 44.Verheij C, Louwerse A, van der Ende J, Eussen ML, Van Gool AR, Verheij F, et al. The stability of comorbid psychiatric disorders: A 7 year follow up of children with pervasive developmental disorder-not otherwise specified. J Autism Dev Disord. 2015;45:3939–3948. doi: 10.1007/s10803-015-2592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas S, Hovinga ME, Rai D, Lee BK. Brief report: Prevalence of co-occurring epilepsy and autism spectrum disorder: The U.S. National Survey of Children’s Health 2011–2012. J Autism Dev Disord 2017, 47: 224–229. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.