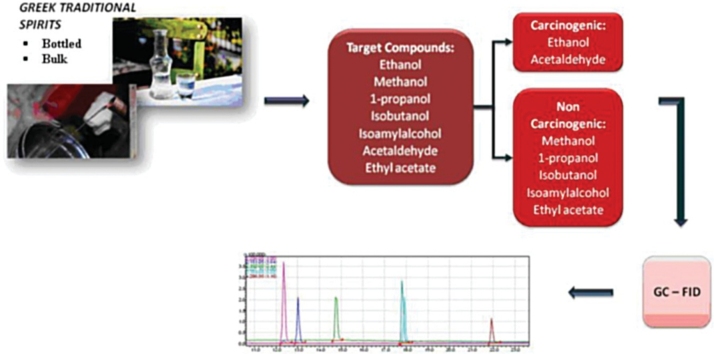
Graphical abstract
Abbreviation: BMDL, benchmark dose (lower confidence limit); GC-FID, gas chromatography coupled with flame ionization detector; NOAEL, no-observed-adverse-effect-level; IRIS, Integrated Risk Information System; MOE, margin of exposure; OAC, odor-active compounds; IARC, International Agency for Research on Cancer; EFSA, European Food Safety Authority; RfD, oral reference dose; SD, standard deviation; US EPA, United States Environmental Protection Agency; MDEQ, Michigan Department of Environmental Quality; DI, daily intake; SEAOP, Greek Federation of Spirits Producers; EDI, estimated daily intake; HRI, health risk index; MOET, combined margin of exposure
Keywords: Alcoholic beverages, Ethanol, Acetaldehyde, Higher alcohols, Esters, Methanol, Risk assessment
Highlights
-
•
Alcoholic beverages (both bottled and bulk) may introduce risk to human health, for carcinogenic compounds (MOET).
-
•
Bottled alcoholic beverages are safer than those produced in buck quantities, for noncarcinogenic compounds (HRI).
-
•
Commercial fermentation and distillation processes provide a controlled environment compared to home made distillates.
Abstract
Greek fermentation and distillation industries produce traditional spirit beverages, such as tsipouro and tsikoudia, consumed both in bottles and bulk quantities by the general population or tourists. The same spirits are also produced by individuals at home since previous centuries, as a part of the local culture but mainly due to the Greek agricultural sector unique characteristics (small cultivation areas with great number of farmers). In this study, the concentrations of carcinogenic compounds: ethanol and acetaldehyde; and noncarcinogenic: higher alcohols (1-propanol, isobutanol, and isoamyl alcohol), esters (ethyl acetate), and methanol were measured to estimate the potential cancer risk and daily intake of these compounds. The margin of exposure (MOE) of carcinogenic compounds was found to be less than 500 (mean value), well below the toxic threshold of 10,000, above which there is not public concern, as suggested by the European Food Safety Authority. Additionally, through risk assessment of noncarcinogenic compounds, we identified two specific compounds in-bulk spirits (produced by individuals), namely ethyl acetate and isobutanol, with health risk index (HRI) greater than 1 (indicating a possibility to induce side effects by consumption of high amounts). Our results indicate that bottled spirits, which are produced in a controlled environment (alcohol industries), showed higher human safety level in terms of both carcinogenic and noncarcinogenic risk assessment studies, comparing to bulk beverages produced by individuals (with out strict regulations).
1. Introduction
Traditional alcoholic beverages are produced worldwide. Greece, among other Mediterranean countries, produces wines of many varieties using fermentation followed by distillation to yield products with high alcohol content. The popular Greek tsipouro and tsikoudia are produced both in bottles and in bulk (unbottled); in fact, many locals, especially in agricultural areas, are distilling their own fermented grape pomaces for private consumption [1,2].
Fresh grape must be fermented to produce wine, which consists mainly of water (80–90 %), ethanol, trace components, acids, and a small fraction of volatiles that contribute to the wine aroma and are often described as odor-active compounds (OACs) [3]. Sugars existing in grapes are fermented by yeasts (Saccharomyces cerevisiae) in a two-stage metabolism: primary and secondary metabolisms. Primary metabolism is essential for yeast growth and cell division, producing compounds such as ethanol, glycerol, acetaldehyde, and acetic acid. Secondary metabolism yields small molecules that are nonessential for yeast growth.
The types of OACs produced depend on the grape variety and the fermentation process itself. These compounds can be divided into four main categories: esters, aldehydes, alcohols, and terpenes. Esters that form during fermentation can be further divided into two groups: ethyl-esters of organic acids and acetate-esters of higher alcohols. The most characteristic examples are ethyl acetate, isoamyl acetate, and isobutyl acetate. The aldehyde group mainly consists of acetaldehyde and diacetyl. The acetaldehyde produced from pyruvate (the end product of glycolysis) during the anaerobic pathway is further converted to ethanol by dehydrogenase enzymes.
Alcohols with more than two atoms of carbon are referred to as higher alcohols or fusel alcohols, and they have higher boiling point. Higher alcohols are produced from amino acids (through the Ehrlich pathway) or sugars (via anabolic pathway), with the formation of α-ketoacid as the vital step. The main higher alcohols produced in wine are 3-methylbutanol (isoamyl alcohol, 2-methylbutanol (active amyl alcohol), 2-methylpropanol (isobutyl alcohol), and 1-propanol (n-propyl alcohol). Higher alcohols potentially have an aromatic effect in wine (either positive or negative) and limited sensory effect. However, the aromatic effect can be of great importance in distillates owing to the increased concentration of fusel alcohols [[3], [4], [5], [6]].
The fermentation process is followed by a distillation process under heat. The produced alcohol is separated and removed, and flavorings can be added to yield specific traditional alcoholic beverages. The final product is an ethanol-water liquid matrix containing a great variety of volatile compounds at low concentrations [[7], [8], [9]].
The definition, description, presentation, labeling, and protection of geographical indications of all these traditional alcoholic beverages are described in the 110/2008 EU regulation, which has replaced the EEC 1576/89 regulation. According to this regulation, traditional alcoholic beverages produced from fermented grape pomaces are defined as grape marc spirits or grape marcs [10].
The International Agency for Research on Cancer (IARC) has classified ethanol in alcoholic beverages as carcinogenic for humans (Group 1, IARC monograph 2012, volume 96, 100E) and acetaldehyde as possibly carcinogenic (Group 2B, IARC monograph 1999, volume 36, sup7,71), [11]. The toxic effects of acetaldehyde are characterized by facial flashing, nausea, vomiting, tachycardia, and hypotension (acetaldehyde syndrome), and the severe or even fatal outcomes of these effects have been reported [[14], [15], [16]]. The chronic toxicity as well as hepatotoxic and neurotoxic effects of higher alcohols have been reported [[11], [12], [13]]. In addition, Geroyiannaki et al. underlined the toxic effects of methanol to the human body (blindness or even death), reporting that the oxidation of methanol in the body is much lower than that that of ethanol [2].
In the present study, the concentrations of cancinogenic or possibly carcinogenic compounds (ethanol and acetaldehyde), higher alcohols (1-propanol, isobutanol, and isoamyl alcohol), esters (ethyl acetate), and methanol in the most popular Greek traditional alcoholic beverages (tsipouro and tsikoudia) were measured to estimate the potential cancer risk and daily intake of the compounds. The European Food Safety Authority (EFSA) margin of exposure (MOE) was used for cancer risk characterization, and the no-observed-adverse-effect-level (NOAEL), oral reference dose (RfD), and data from the Integrated Risk Information System (IRIS) were used to assess the health risk of the compounds, in accordance with the health risk index (HRI), [[17], [18], [19], [20], [21], [22], [23], [24], [25]].
2. Materials and method
2.1. Sample collection and storage
A total of 55 drinks from the Greek market (super markets, mini markets and individual producers) were collected during 2016, stored at -20 °C, and analyzed using either a clinical chemistry analyzer or gas chromatography coupled with flame ionization detector (GC-FID).
2.2. Reagent and materials
Isobutanol (99 %), isoamyl alcohol (99 %), acetaldehyde (99 %), and 1-propanol (99.5 %) were obtained from Merck (Darmstadt, Germany). Methanol (99.9 %) and 2-propanol (99.9 %) (used as an internal standard) were purchased from Riedel- de Haën (Harvey st. Muskegon, USA). Ethyl acetate (99.7 %) was obtained from Sigma Aldrich (Steinheim, Germany). Ultrapure water was obtained by using a Direct-Q 3UV water purification system (Merck).
Stock solutions of each solvent were prepared in ultrapure water at a concentration of 50 g/L. Some analytes, such as ethyl acetate (0.083 g/mL), isobutanol (0.087 g/mL), and isoamyl alcohol (0.095 g/mL), have low water-solubility, which inhibits their aqueous dilution. In particular, a specific volume of each solvent, ranging between 0.11 mL (for ethyl acetate) and 1.28 mL (for acetaldehyde), was diluted in 20 mL water to obtain a final solution at a concentration of 50 g/L. The solutions were further diluted to prepare working mix solutions of all analytes at concentrations of 0, 25, 50, 100, 250, 500, 1000, 2500, and 5000 mg/L for methanol and of 0, 10, 25, 50, 75, 100, 250, 500, 750, and 1000 mg/L for acetaldehyde, ethyl acetate, 1-propanol, isobutanol, and isoamyl alcohol (all the above working solutions contained 2-propanol, as depicted in section 2.4). Data from the analysis of these working mix solutions were used to prepare calibration curves.
2.3. Sample pretreatment – ethanol analysis
Ethanol level was measured using an automatic clinical chemistry analyzer system (ARCHITECT c4000; ABBOTT). An 10-μl aliquot of each collected drink sample was diluted in 790 or 990 μL water (dilution factor 80 or 100) and then analyzed with no further processing.
2.4. Sample pretreatment – headspace FID
For acetaldehyde, methanol, ethyl acetate, 1-propanol, isobutanol, and isoamyl alcohol analyses, 0.5 mL of each sample was added to 1.5 mL of 2-propanol solution (0.1 %v/v) in a 10-ml head space vial, which was then sealed with a cap and placed in a GC-FID tray. Head space analysis was performed by heating the vials at 60 °C for 20 min, and then injecting 1 mL of the headspace phase into a GC-FID gas chromatography system (GC-17A; Shimadzu, Kyoto, Japan). The analytes were separated on a 60/80 CarboPack B glass column (6 ft × 50 mm × 2.6 mm) with nitrogen as a carrier gas (total flow: 25 mL/min). The temperatures of the injector port and detector were 150 °C and 190 °C, respectively. The column temperature was initially held at 60 °C for 4 min, then raised at 20 °C/min to 160 °C, and held for 3 min (total chromatographic analysis: 12 min). Under the aforementioned conditions, the retention time of each analyte was 1.17, 2.16, 3.84, 5.61, 6.23, 7.05, 8.64, and 10.64 min for acetaldehyde, methanol, ethanol, 2-propanol (IS), ethyl acetate, 1-propanol, isobutanol, and isoamyl butanol, respectively.
2.5. Statistical analysis
All alcoholic beverage samples were analyzed, and the levels of ethanol, acetaldehyde, methanol, ethyl acetate, isobutanol, 1-propanol and isoamyl alcohol were determined and evaluated using both SPSS version 19 and Excel. The results were expressed as the minimum, maximum, mean, median, and standard deviation (SD).
3. Results – discussion
3.1. Linearity and sensitivity
The linearity of the applied protocol was investigated by injecting standard mix solutions of the target solvents at the previously reported concentrations. The response of the analytical systems was found to be linear with a determination coefficient of 0.994, 0.996, 0.996, 0.997, 0.993, and 0.995 for acetaldehyde, 1-propanol, isobutanol, ethyl acetate, isoamyl alcohol, and methanol, respectively. Good sensitivity was obtained in all cases, as the limits of determination were lower than 25 mg/L for methanol and lower than 10 mg/L for the other solvents.
3.2. Data monitoring
Table 1 presents the minimum, maximum, mean, median, and SD values for bottled and in-bulk spirits. Table 1 also presents additional information such as the 25th, 50th, and 75th percentiles of the values, which were used to construct boxplot diagrams using the Excel software.
Table 1.
The mean, minimum, maximum, and median concentrations (mg/L) of ethanol, methanol, acetaldehyde, ethyl acetate, 1-propanol, isobutanol, and isoamyl alcohol in bottled and in-bulk spirits.
| Bottled spirits (26 samples) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol (g/100 mL) | Ethanol (vol%) | Ethanol (103 mg/l) | Acetaldehyde (mg/l) | Methanol (mg/l) | Ethyl acetate (mg/l) | 1-propanol (mg/l) | Isobutanol (mg/l) | Isoamyl alcohol (mg/l) | |||||
| Min | 26.50 | 33.59 | 265.04 | 41.18 | 314.57 | 46.75 | 104.56 | 113.62 | 174.84 | ||||
| Max | 47.35 | 60.02 | 473.52 | 853.51 | 2184.98 | 1306.77 | 223.08 | 408.53 | 1450.28 | ||||
| Mean | 35.44 | 44.92 | 354.42 | 297.58 | 698.02 | 420.16 | 168.15 | 197.64 | 732.01 | ||||
| Median | 33.29 | 42.19 | 332.91 | 296.87 | 545.73 | 396.47 | 176.58 | 169.51 | 634.01 | ||||
| SD | 7.55 | 9.56 | 75.46 | 176.79 | 426.69 | 290.45 | 33.24 | 80.13 | 309.91 | ||||
| mean ± SD | 42.99 | 54.48 | 429.87 | 474.37 | 1124.71 | 710.62 | 209.82 | 277.78 | 1041.92 | ||||
| Q1 (25th percentile) | 28.44 | 284.38 | 146.62 | 433.37 | 200.27 | 139.89 | 139.45 | 539.89 | |||||
| Q2 (50th percentile) | 33.29 | 332.91 | 296.87 | 545.73 | 396.47 | 176.58 | 169.51 | 634.01 | |||||
| Q3 (75th percentile) | 43.18 | 431.83 | 367.56 | 776.47 | 585.10 | 191.19 | 247.07 | 804.37 | |||||
| Q1-min | 1.93 | 19.34 | 105.45 | 118.80 | 153.52 | 35.34 | 25.83 | 365.06 | |||||
| Median-Q1 | 4.85 | 284.38 | 150.25 | 112.37 | 196.20 | 36.69 | 30.06 | 94.11 | |||||
| Q3-median | 9.89 | 98.92 | 70.68 | 230.74 | 188.63 | 14.61 | 77.56 | 170.36 | |||||
| Max-Q3 | 4.17 | 41.70 | 485.95 | 1408.51 | 721.67 | 31.89 | 161.46 | 645.91 | |||||
| In-bulk spirits (29 samples) | |||||||||||||
| Min | 30.36 | 38.48 | 303.60 | 57.53 | 342.48 | 117.11 | 91.53 | 114.17 | 250.11 | ||||
| Max | 47.82 | 60.61 | 478.24 | 556.45 | 1685.17 | 4978.06 | 301.08 | 566.90 | 2022.50 | ||||
| Mean | 36.60 | 46.39 | 366.02 | 199.75 | 781.20 | 1067.66 | 173.64 | 307.14 | 930.19 | ||||
| Median | 35.92 | 45.53 | 359.20 | 164.46 | 741.62 | 740.90 | 170.23 | 304.64 | 906.66 | ||||
| SD | 4.38 | 5.56 | 43.85 | 121.71 | 271.67 | 1211.82 | 48.69 | 86.22 | 345.57 | ||||
| Mean ± SD | 40.99 | 51.95 | 409.87 | 321.46 | 1052.87 | 2279.48 | 140.23 | 393.36 | 1275.76 | ||||
| Q1 (25th percentile) | 33.32 | 333.20 | 124.94 | 673.82 | 348.88 | 151.66 | 266.61 | 696.96 | |||||
| Q2 (50th percentile) | 35.92 | 359.20 | 164.46 | 741.62 | 740.90 | 170.23 | 304.64 | 906.66 | |||||
| Q3 (75th percentile) | 38.60 | 386.00 | 226.87 | 903.26 | 1172.90 | 188.73 | 334.33 | 1107.50 | |||||
| Q1-min | 2.96 | 29.60 | 67.41 | 331.34 | 231.77 | 60.12 | 152.44 | 446.85 | |||||
| Median-Q1 | 2.60 | 26.00 | 39.52 | 67.80 | 392.01 | 18.58 | 38.03 | 209.70 | |||||
| Q3-median | 2.68 | 26.80 | 62.41 | 161.64 | 432.00 | 18.49 | 29.70 | 200.84 | |||||
| Max-Q3 | 9.22 | 92.24 | 329.58 | 781.91 | 3805.17 | 112.35 | 232.57 | 915.00 | |||||
Excel was used to construct boxplots: Q1, median-Q1, Q3-median with Q1-min and max-Q3 as minimum whiskers and maximum whiskers, respectively.
Q2 (the 50th percentile = median).
SD: standard deviation.
The detected ethanol concentrations (g/100 mL) were converted to alcoholic strength in volume (vol%) by dividing them with ethanol density (789 g/l) (Table 1). The European Legislation [10] clearly states the alcoholic strength specifications for grape marc spirits: the minimum is at least 37.5 % and the maximum is less than 86 % by volume. All spirits produced in bulk by individuals met these requirement, presenting slightly higher alcohol concentration than spirits produced by commercial establishments (bottled beverages). Four out of 26 bottled spirits had an alcohol level of less than 37.5 %; however, the median value of all spirit samples was 40 %, which is exactly the same as the value printed on the label of each commercial product. It was obvious that commercial products, which were fermented and distilled under well-specified industrial procedures, were able to fulfill the law requirements with lower production costs. In contrast, individuals producing spirits in bulk cannot control all the necessary parameters, yielding spirits with higher alcoholic content (median value of in-bulk beverages: 46 %) with increased costs and higher probability of producing low-quality spirits.
The concentration of ethanol exceeded that of the other five compounds up to four orders of magnitude. This was an expected result because the other five compounds were present as a small fraction of volatiles (OAC). Acetaldehyde concentrations in both bottled
and in-bulk spirits were lower than those reported by other researchers [2,15,18,26] with a maximum value of less than 1000 mg/l. Similarly, the maximum concentrations of the higher alcohols isobutanol and isoamyl alcohol never exceeded 1000 mg/l and 2022.5 mg/l, respectively. The highest concentration of methanol was observed in bottled spirits (2185 mg/l) and that of ethyl acetate was observed in in-bulk spirits (4978 mg/l).
The concentrations of all compounds increased in bottled spirits compared with those in-bulk spirits, except for acetaldehyde, which showed a slight decrease from 297.58 ± 176.79 mg/l to 199.75 ± 121.71 mg/l. To explain this difference, it is important to examine the fermentation process conducted before distillation. Fermentation is an anaerobic process where sugars are converted to pyruvate by yeast via the glycolytic pathway, decarboxylated to form acetaldehyde and carbon dioxide, and then further reduced to ethanol. Additional amounts of acetaldehyde can be produced if sulfur dioxide is added to the reaction or if the fermentation pH or temperature is increased. Furthermore, different commercial strains can produce higher or lower levels of acetaldehyde [27]. All these parameters are controlled in the fermentation-distillation industry (that produces bottled spirits), and variations in the processing conditions lead to the different organoleptic characteristics of the end products; hence, the acetaldehyde variations. Differences during fermentation and distillation by individuals indicated that their control of the processing parameters was restricted to the basics.
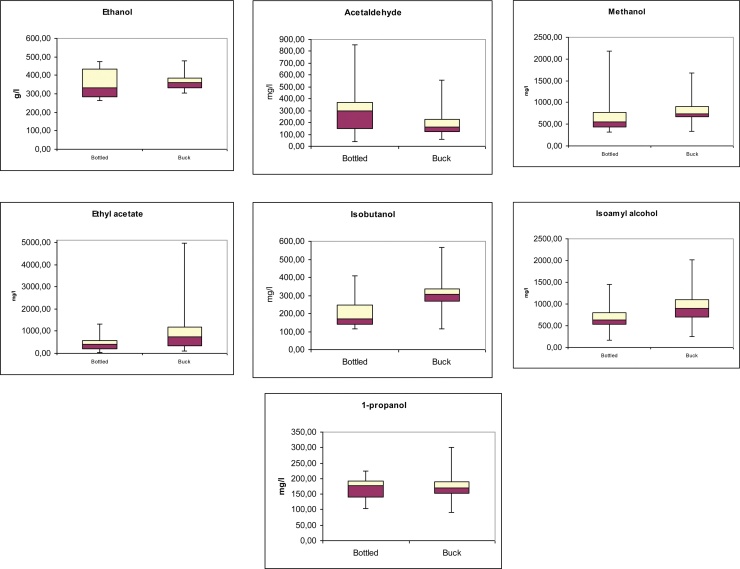
Fig. 1 shows the boxplot (or box and whiskers plot) diagrams of all compound concentrations for both bottled and in-bulk spirits. The median values, a measure of central tendency, for all compounds except for acetaldehyde were greater in-bulk spirits than in bottled spirits and close to the mean values in both types of spirits, except for those of isoamyl alcohol (bottled) and ethyl acetate (in-bulk). This difference in the mean and median values can be explained by the raw data (not shown), in which a small number of extreme concentration values of isoamyl alcohol and ethyl acetate were observed in the distillates. The same conclusion was drawn by evaluating the box size for ethanol, acetaldehyde, methanol, 1-propanol, and isobutanol, which was shorter in bulk spirits than in bottled spirits, whereas that for ethyl acetate and isoamyl alcohol box was shorter in bottled spirits, indicating lower values.
Fig. 1.
Comparison of measured compound concentrations in bottled and in-bulk spirits.
Following data analysis, we conducted descriptive statistics and Spearman’s rank correlation analysis (Supplemented Data, ST1 and ST2) to examine the normal distribution of the distillate values. Skewness and Kurtosis coefficients were all found to be different from zero, indicating abnormal data distribution. By examining the absolute value of Skewness and Kurtosis coefficients compared with twice the values of standard errors, we identified a relatively normal distribution for ethanol, 1-propanol, and isoamyl alcohol in bottled spirits as well as for ethanol and 1-propanol in in-bulk spirits. Finally, Spearman’s rank correlation analysis between compounds in bottled and in-bulk spirits and between ethanol and the other compounds showed moderate or weak correlation in most cases (Supplemented Data, ST2).
3.3. Risk assessment of noncarcinogenic compounds
Identification of methanol, ethyl acetate, 1-propanol, isobutanol, and isoamyl alcohol was conducted in accordance with the United States Environmental Protection Agency (US EPA) approach to assess the health risk of chemical substances [19,28,29]. According to this methodology, HRI is estimated by the formula HRI = EDI/RfD, where EDI is the estimated daily intake (EDI) in mg/kg body weight and RfD is the oral reference dose. Compounds with HRI values more than 1 are considered unsafe for human health, with possible side effects of nausea, dizziness, headache, and stupor (US National Library of Chemicals Pubchem), [30]. The RfD values of methanol, ethyl acetate, and isobutanol were obtained from data published in the IRIS, whereas that of 1-propanol was obtained from the Michigan Department of Environmental Quality [31]. The RfD values are 2, 0.9, 0.3, and 2 mg/kg/day, respectively. The RfD value of isoamyl alcohol was calculated to be 2.95 mg/kg/day using the equation RfD = NOAEL/(UF1 × UF2), with NOAEL = 295 and UF1 = 10 for human variability and UF2 = 10 for animal studies [32]. EDI values were estimated by the formula EDI = C × DI / BW, where C is the concentration (mg/l) of the identified compound, DI is the daily intake (ml/day) of alcoholic spirit, and BW is body weight, which was assumed to be 70 kg, as suggested by the EFSA Scientific Panels and Committee [33]. To estimate the DI of alcoholic spirits (ml/day), we used recommendations from health authorities for safe or low-risk drinking. The Center for Disease Control and Prevention [34] suggests one drink per day for women and two for men, for moderate drinking. Five or more drinks for men and four or more for women within 2 h will bring blood alcohol concentration to the United States legal driving limit of 0.08 %. Alcohol unit in the US is equal to 14 g of pure ethanol, whereas that in many European countries, including Greece, is equal to 10 g. We used the recommendation from the Greek Federation of Spirits Producers [35] namely three standard alcoholic drinks per day (30 g of ethanol), which is calculated to be equal to consumption of roughly 100 mL alcoholic spirit (of 40 %).
EDI and HRI values are shown in Table 2. All compounds from bottled distillates had lower HRI values than those from in-bulk spirits in terms of mean and median estimates, indicating that bottled alcoholic beverages were safer than those produced in bulk. The HRI mean and median values of two compounds found in in-bulk spirits, namely ethyl acetate and isobutanol, were estimated to be higher than 1, indicating possible side effects according to the US National Library of Medicine. All mean and median compound values of bottled samples and individual values of 1-propanol and isoamyl alcohol for both types of spirits
Table 2.
Risk assessment of non carcinogenic and carcinogenic compounds.
| Health risk assessment of non carcinogenic compounds | ||||||||
|---|---|---|---|---|---|---|---|---|
| Estimated daily intake (mg /kg BW) |
Health risk index |
|||||||
| Min | Max | Mean | Median | Min | Max | Mean | Median | |
| Bottled spirits | ||||||||
| Methanol | 0.45 | 3.12 | 1.00 | 0.78 | 0.22 | 1.56 | 0.50 | 0.39 |
| Ethyl acetate | 0.07 | 1.87 | 0.60 | 0.57 | 0.07 | 2.07 | 0.67 | 0.63 |
| 1-Propanol | 0.15 | 0.32 | 0.24 | 0.25 | 0.07 | 0.16 | 0.12 | 0.13 |
| Isobutanol | 0.16 | 0.58 | 0.28 | 0.24 | 0.54 | 1.95 | 0.94 | 0.81 |
| Isoamyl alcohol | 0.25 | 2.07 | 1.05 | 0.91 | 0.08 | 0.70 | 0.35 | 0.31 |
| In-bulk spirits | ||||||||
| Methanol | 0.49 | 2.41 | 1.12 | 1.06 | 0.24 | 1.20 | 0.56 | 0.53 |
| Ethyl acetate | 0.17 | 7.11 | 1.53 | 1.06 | 0.19 | 7.90 | 1.69 | 1.18 |
| 1-Propanol | 0.13 | 0.43 | 0.25 | 0.24 | 0.07 | 0.22 | 0.12 | 0.12 |
| Isobutanol | 0.16 | 0.81 | 0.44 | 0.44 | 0.54 | 2.70 | 1.46 | 1.45 |
| Isoamyl alcohol | 0.36 | 2.89 | 1.33 | 1.30 | 0.12 | 0.98 | 0.45 | 0.44 |
| Human exposure to ethanol and acetaldehyde and margin of exposure (MOE) estimates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Human exposure (mg /kg BW) |
MOE |
Combined MOE (MOET) |
||||||
| Min | Max | Mean | Median | Mean | Median | Mean | Median | |
| Bottled spirits | ||||||||
| Ethanol | 378.63 | 676.46 | 506.29 | 475.57 | 4.74 | 5.05 | 4.67 | 4.96 |
| Acetaldehyde | 0.06 | 1.22 | 0.43 | 0.42 | 294.04 | 294.74 | ||
| In-bulk spirits | ||||||||
| Ethanol | 433.71 | 683.20 | 522.89 | 513.14 | 4.59 | 4.68 | 4.54 | 4.64 |
| Acetaldehyde | 0.08 | 0.79 | 0.29 | 0.23 | 438.04 | 532.04 | ||
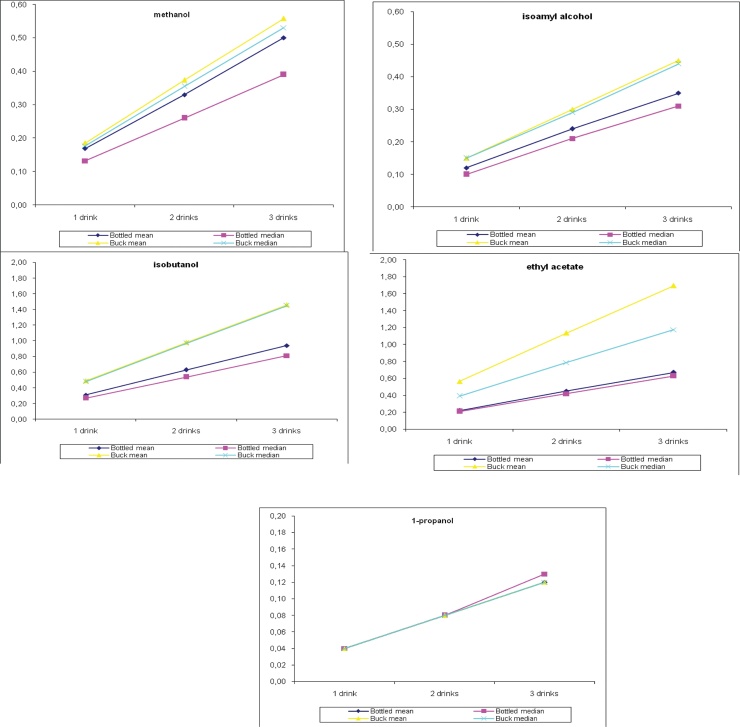
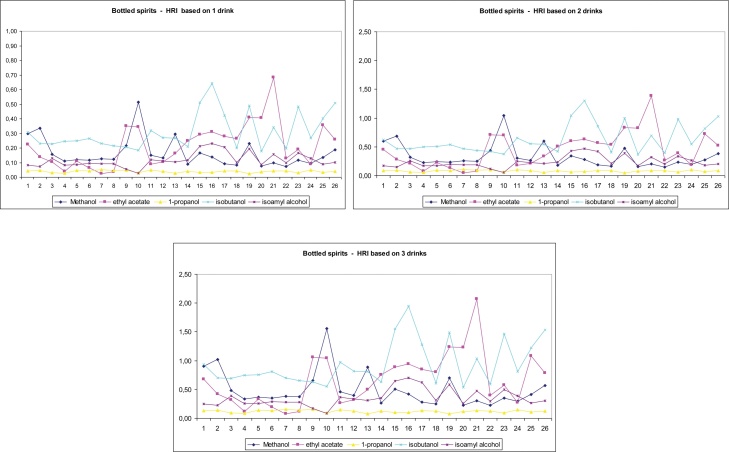
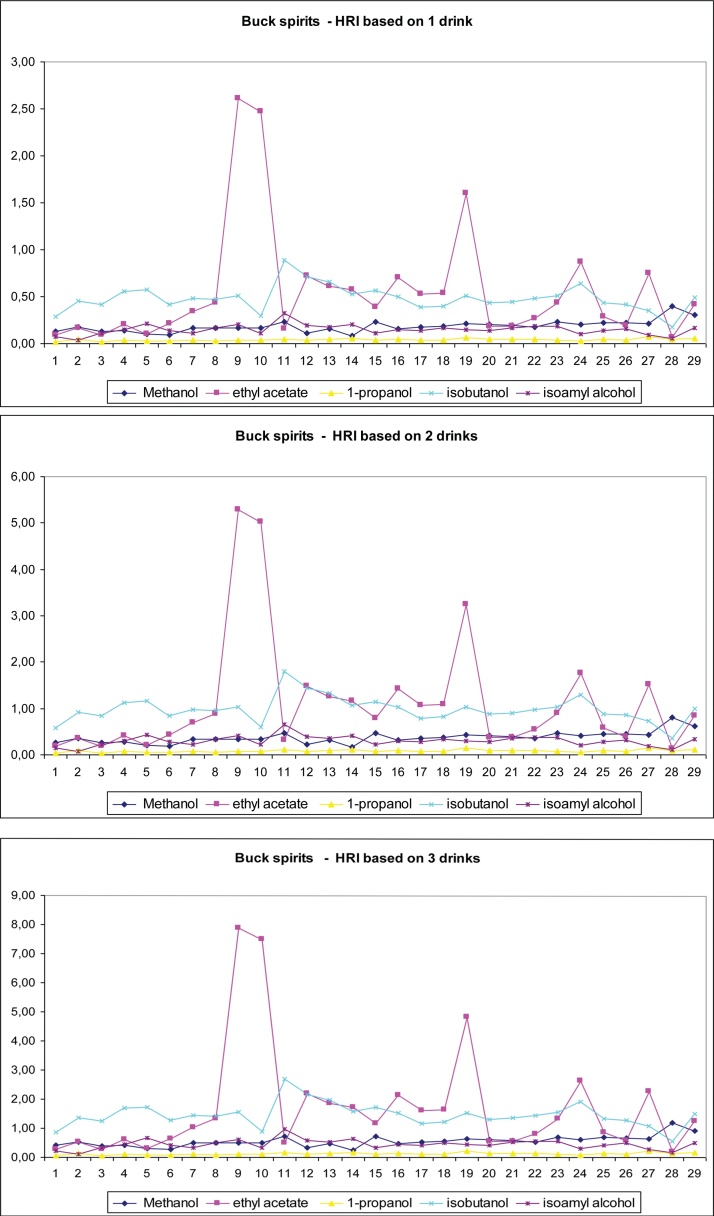
were found to be lower than 1. To further investigate the effect of these compounds on human health, we repeated the previous calculations (with 30 g of ethanol) with regard to two other scenarios; HRI was estimated with 10 and 20 g daily consumption of ethanol corresponding to one and two drinks, respectively. As shown in Fig. 2, the results showed that methanol, 1-propanol, and isoamyl alcohol were safe even with moderate daily consumption of three drinks. Ethyl acetate and isobutanol were safe for consumption of up to three drinks for bottled spirits, whereas only two drinks of in-bulk spirits can induce mild health side effects. Fig. 3, Fig. 4 show the calculated HRI values for the one-, two-, and three-drink scenarios for all individual samples analyzed for both types of spirits. As shown in Fig. 3 (bottled spirits), 1 (methanol), 2 (isobutanol), and 1 (ethyl acetate) sample suited for the two-drink scenario, as well as 2 (methanol), 8 (isobutanol), and 6 (ethyl acetate) samples suited for the three-drink scenario were found to have HRI index greater than 1. As shown in Fig. 4 (in-bulk spirits), 3 (ethyl acetate, one drink), 11 (ethyl acetate and isobutanol, two drinks), 16 (ethyl acetate, three drinks), and 25 (isobutanol, three drinks) samples had HRI values greater than 1.
Fig. 2.
Health risk index estimates for one, two, and three drinks per day consumption of both bottled and in-bulk spirits.
Fig. 3.
Health risk index (HRI) values of all examined bottled spirits for the one-, two-, and three-drink scenarios.
Fig. 4.
Health risk index (HRI) values of all examined in-bulk spirits for the one-, two-, and three-drink scenario.
3.4. Risk assessment of carcinogenic compounds
The concentrations of carcinogenic compounds (ethanol and acetaldehyde), were measured to estimate their potential cancer risk. In addition to ethanol, which is the primary toxic compound of any alcoholic beverage, acetaldehyde was evaluated because the threshold of its mechanisms of toxicity and carcinogenicity is currently unclear [36]. The EFSA MOE approach was used for cancer risk characterization using the formula MOE = NOAEL or BMDL / human exposure. NOAEL is defined as the dose at which a small but measurable adverse effect in experimental studies is first observed, and BMDL is the benchmark dose (lower 95 % confidence limit) corresponding to a concentration that produces a predetermined change in response to an adverse effect [37]. The NOAEL values of ethanol have been reported in a dietary study in rats to be approximately 2400 mg/kg body weight/day [38] or 1730 mg/kg bw/day according to the European Chemical Agency [39]. The Scientific Committee on Consumer Safety (European Commission SCCS/1468/12) suggests that the NOAEL value of acetaldehyde is 125 mg/kg bw/day [40]. BMDL values were obtained from previous studies [18,36,41], but they are in lower magnitude to those of NOAEL. We decided to use the higher values of NOAEL for MOE estimation to avoid underestimating the potential cancer risk. Human exposure data were estimated with the same approach as EDI estimation for noncarcinogenic compounds. Moreover, to estimate the individual MOE values of ethanol and acetaldehyde, we calculated the combined margin of exposure (MOET), which assumes additional risks owing to similar mechanism, by using the formula MOET = 1 / (1/MOEeth + 1/MOEacet), [42,43]. The data are shown in Table 2. The MOE of carcinogenic compounds, such as ethanol and acetaldehyde, was found to be less than 500 (mean value), well below 10,000, which is suggested by the EFSA to be the lower border of the toxic threshold of the consumed substances. Finally, the MOET estimates were also found to be below 10,000, indicating risk for public health. However, as shown in Table 2, the MOET of both compounds was similar to the MOE of ethanol, clearly indicating the potent effect of ethanol on the cumulative assessment of the combined MOE. The risk of acetaldehyde would be minor compared with that of ethanol.
4. Conclusions
The MOE value of carcinogenic compounds, such as ethanol and acetaldehyde, was found to be less than 500 (mean value), well below to the toxic threshold of 10,000, above which there is not public concern, as suggested by the European Food Safety Authority. In addition, the MOET estimates were found to be affected mainly by ethanol, and the effect of acetaldehyde was thus judged to be negligible.
In terms of the HRI values, noncarcinogenic compounds, such as alcohols, aldehydes, and esters, in bottled alcoholic beverages were found to have lower mean and median estimates than those in in-bulk beverages, indicating that bottled alcoholic beverages were safer than those produced in bulk. Among the scenarios of one, two, and three drinks per day, the one drink per day scenario appeared to be the safest, but one would have to exceed three drinks per day to experience mild health side effects. All noncarcinogenic compounds, except for acetaldehyde, were found in greater quantities in in-bulk spirits than in bottled spirits; nevertheless, spirits were found to be safe for human consumption.
Commercial fermentation and distillation provide a controlled environment that yields products of great variety in terms of organoleptic characteristics. Following legislation guidelines, a certified amount of alcohol content is presented to consumers, helping them to decide their own drinking pattern. Consumers should prefer bottled spirits although they are more expensive than in-bulk spirits.
Author statement
All authors have equally contributed for the preparation of this manuscript
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.08.017.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Apostolopoulou A.A., Flouros A.I., Demertzis P.G., Akrida-Demertzi K. Differences in concentration of principal volatile constituents in traditional Greek distillates. Food Control. 2005;16:157–164. doi: 10.1016/j.foodcont.2004.01.005. [DOI] [Google Scholar]
- 2.Geroyiannaki M., Komaitis M.E., Stavrakas D.E., Polysiou M., Athanasopoulos P.E., Spanos M. Evaluation of acetaldehyde and methanol in Greek traditional alcoholic beverages from varietal fermented grape pomaces (Vitis vinifera L.) Food Control. 2007;18(8):988–995. doi: 10.1016/j.foodcont.2006.06.005. [DOI] [Google Scholar]
- 3.Mina M., Tsaltas D. Intech; 2017. Contribution of Yeast in Wine Aroma and Flavour. Chapter 5. [DOI] [Google Scholar]
- 4.King E.S., Stoumen M., Buscema F., Hjelmeland A.K., Ebeler S.E., Heymann H., Boulton R.B. Regional sensory and chemical characteristics of Malbec wines from Mendoza and California. Food Chem. 2014;143:256–267. doi: 10.1016/j.foodchem.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 5.de-la-Fuente-Blanco A., Sáenz-Navajas M.P., Ferreira V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016;210:107–114. doi: 10.1016/j.foodchem.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Capone D.L., Wilkinson K.L., Jeffery D.W.A.U.- Chemical and sensory profiles of rosé wines from Australia. Food Chem. 2016;196:682–693. doi: 10.1016/j.foodchem.2015.09.111. [DOI] [PubMed] [Google Scholar]
- 7.Hazelwood L.A., Daran J.M., Van Maris A., Pronk J., Dickinson J.R. The ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74(8):2259–2266. doi: 10.1128/AEM.00934-08. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steward G. The production of secondary metabolites with flavour potential during brewing and distilling wort fermentations. Fermentation. 2017;3(4):63. doi: 10.3390/fermentation3040063. [DOI] [Google Scholar]
- 9.Puentes C., Joulia X., Vidal J.P., Esteban-Decloux M. Simulation of spirits distillation for a better understanding of volatile aroma compounds behavior: application to Armagnac production. Food Bioprod. Process. 2018;112:31–62. doi: 10.1016/j.fbp.2018.08.010. [DOI] [Google Scholar]
- 10.EC . 2008. Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Spirit Drinks and Repealing Council Regulation (EEC) No 1576/89.https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008R0110 [Google Scholar]
- 11.IARC . Vol. 1-124. International Agency for Research on Cancer; 2019. https://monographs.iarc.fr/agents-classified-by-the-iarc/ (IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Agents Classified by the IARC Monographs). [Google Scholar]
- 12.Jang G.R., Harris R.Z. Drug interactions involving ethanol and alcoholic beverages. Expert Opin. Drug Metab. Toxicol. 2007;3(5):719–731. doi: 10.1517/17425255.3.5.719. [DOI] [PubMed] [Google Scholar]
- 13.Borja Oliveira C.R. Alcohol-medication interactions: the acetaldehyde syndrome. J. Pharmacovigil. 2014;2(5) doi: 10.4172/2329-6887.1000145. [DOI] [Google Scholar]
- 14.Paine A.J., Dayan A.D. Defining a tolerable concentration of methanol in alcoholic drinks. Hum. Exp. Toxicol. 2001;20:563–568. doi: 10.1191/096032701718620864. [DOI] [PubMed] [Google Scholar]
- 15.Lachenmeier D.W., Haupt S., Schulz K. Defining maximum levels of higher alcohols in alcoholic beverages and surrogate alcohol products. Regul. Toxicol. Pharmacol. 2008;50(3):313–321. doi: 10.1016/j.yrtph.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Lachenmeier D.W., Rehm J. What is the main source of human exposure to higher alcohols and is there a link to immunotoxicity? Immunopharmacol. Immunotoxicol. 2013;35(3):451–453. doi: 10.3109/08923973.2013.794147. [DOI] [PubMed] [Google Scholar]
- 17.US EPA . United States Environmental Protection Agency, EPA/635/R-09/013; 2010. IRIS Toxicological Review of Methanol (External Review Draft; December 2009)https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=56521 [Google Scholar]
- 18.Paiano V., Bianchi G., Davoli E., Negri E., Fanelli R., Fattore E. Risk assessment for the Italian population of acetaldehyde in alcoholic and non-alcoholic beverages. Food Chem. 2014;154:26–31. doi: 10.1016/j.foodchem.2013.12.098. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadabadi A.N., Sedaghat M., Ranjbar A., Poorolajal J., Nasiripour H., Ahmadabadi M.N. Quantitative analysis and health risk assessment of methanol in medicinal herbal drinks marketed in Hamadan, Iran. J. Appl. Pharm. Sci. 2016;6(7):49–52. doi: 10.7324/JAPS.2016.60707. [DOI] [Google Scholar]
- 20.Goumenou M., Tsatsakis A.M. Proposing new approaches for the risk characterisation of single chemicals and chemical mixtures: the source related Hazard Quotient (HQS) and Hazard Index (HIS) and the adversity specific Hazard Index (HIA) Toxicol. Rep. 2019;6(2019):632–636. doi: 10.1016/j.toxrep.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taghizadeh S.F., Faezeh S., Goumenou M., Rezaee R., Alegakis T., Kokaraki V., Anesti O., Sarigiannis D.A., Tsatsakis A., Karimi G. Cumulative risk assessment of pesticide residues in different Iranian pistachio cultivars: applying the source specific HQS and adversity specific HIA approaches in Real Life Risk Simulations (RLRS) Toxicol. Lett. 2019;313:91–100. doi: 10.1016/j.toxlet.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Taghizadeh S.F., Faezeh S., Rezaee R., Davarynejad G., Asili J., Nemati S.H., Goumenou M., Tsakiris I., Tsatsakis A.M., Shirani K., Karimi G. Risk assessment of exposure to aflatoxin B1 and ochratoxin A through consumption of different Pistachio (Pistacia vera L.) cultivars collected from four geographical regions of Iran. Environ. Toxicol. Pharmacol. 2018;61:61–66. doi: 10.1016/j.etap.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Taghizadeh S.F., Faezeh S., Davarynejad G., Asili J., Nemati S.H., Rezaee R., Goumenou M., Tsatsakis A.M., Karimi G. Health risk assessment of heavy metals via dietary intake of five pistachio (Pistacia vera L.) cultivars collected from different geographical sites of Iran. Food Chem. Toxicol. 2017;107:99–107. doi: 10.1016/j.fct.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Ioanna K., Tzatzarakis M., Alegakis A., Karzi V., Hatzidaki E., Stavroulaki A., Vakonaki E. Phthalate metabolites concentrations in amniotic fluid and maternal urine: cumulative exposure and risk assessment. Toxicol. Rep. 2020;7:529–538. doi: 10.1016/j.toxrep.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsatsakis A.M., Vassilopoulou L., Kovatsi L., Tsitsimpikou C., Karamanou M., Leon G., Liesivuori J., Hayes A.W., Spandidos D.A. The dose response principle from philosophy to modern toxicology: the impact of ancient philosophy and medicine in modern toxicology science. Toxicol. Rep. 2018;5:1107–1113. doi: 10.1016/j.toxrep.2018.10.001. ISSN 2214-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon C.S. Estimations of the lethal and exposure doses for representative methanol symptoms in humans. Ann. Occup. Environ. Med. 2017;29:44. doi: 10.1186/s40557-017-0197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vintessential Labs . 2019. Vintessential Laboratories. Acetaldehyde – How to Limit Its Formation During Fermentation.https://www.vintessential.com.au/acetaldehyde-how-to-limit-its-formation-during-fermentation/ Articles (accessed 8/8/2019) [Google Scholar]
- 28.US EPA . United States Environmental Protection Agency; 2000. Guidelines for the Health Risk Assessment of Chemical Mixtures. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. Risk Assessment Forum Technical Panel.https://www.epa.gov/risk/guidelines-health-risk-assessment-chemical-mixtures EPA/630/R-00/002. [Google Scholar]
- 29.Sultana M.S., Rana S., Yamazaki S., Aono T., Yoshida S. Health risk assessment for carcinogenic and noncarcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ. Sci. 2017;3 doi: 10.1080/23311843.2017.1291107. [DOI] [Google Scholar]
- 30.US PubChem . 2019. United States National Library of Medicine, National Center for Biotechnology Information.https://pubchem.ncbi.nlm.nih.gov [Google Scholar]
- 31.MDEQ, Michigan Department of Environmental Quality (MDEQ) 2015. Chemical Update Worksheet. RRD Toxicology Unit. Propyl Alcohol.https://www.michigan.gov/documents/deq/deq-rrd-chem-PropylAlcoholDatasheet_527601_7.pdf [Google Scholar]
- 32.EC . 2016. European Commission SCOEL/REC/177. Isoamyl Alcohol. Recommendation from the Scientific Committee on Occupational Exposure Limits. [DOI] [Google Scholar]
- 33.EFSA SCIENTIFIC OPINION Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. Efsa J. 2012;10(3):2579. doi: 10.2903/j.efsa.2012.2579. European Food Safety Authority. EFSA Scientific Committee, 2012. [DOI] [Google Scholar]
- 34.CDC . Centers for Disease Control and Prevention (CDC); 2019. Alcohol and Public Health. Fact Sheets – Alcohol Use and Your Health.https://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm [Google Scholar]
- 35.SEAOP, Greek Federation of Spirits Producers (SEAOP) 2019. Responsible Consumption. General Information.http://www.seaop.gr/en/responsible-consumption/enjoy-responsibly/general-info [Google Scholar]
- 36.Lachenmeier D.W., Kanteres F., Rehm J. Carcinogenicity of acetaldehyde in alcoholic beverages: risk assessment outside ethanol metabolism. Addiction. 2009;104:533–550. doi: 10.1111/j.1360-0443.2009.02516.x. [DOI] [PubMed] [Google Scholar]
- 37.EFSA Opinion of the Scientific Committee on a request from EFSA related to a harmonized approach for risk assessment of substances which are both genotoxic and carcinogenic. Efsa J. 2005;282(2005):1–131. doi: 10.2903/j.efsa.2005.282. European Food Safety Authority. [DOI] [Google Scholar]
- 38.INCHEM I.P.C.S. International programme on chemical safety (IPCS) and the canadian centre for occupational health and safety (CCOHS) Internationally Peer Reviewed Chem. Safety Inform. 2019 [Google Scholar]
- 39.ECHA . European Chemicals Agency; 2019. Ethanol – Toxicological Summary.https://echa.europa.eu/ [Google Scholar]
- 40.SCCS, European Commission . 2012. Scientific Committee on Consumer Safety (SCCS) OPINION ON Acetaldehyde. SCCS/1468/12.https://ec.europa.eu/health/scientific_committees/consumer_safety_en [Google Scholar]
- 41.Lachenmeier D.W., Gill J.S., Chick J., Rehn J. The total margin of exposure of ethanol and acetaldehyde for heavy drinkers consuming cider or vodka. Food Chem. Toxicol. 2015;83:210–214. doi: 10.1016/j.fct.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 42.US EPA . United States Environmental Protection Agency; 2001. General Principles for Performing Aggregate Exposure and Risk Assessments for Pesticides. Office of Pesticide Programs.https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/general-principles-performing-aggregate-exposure-and [Google Scholar]
- 43.Wilkinson C.F., Christoph G.R., Julien E., Kelley J.M., Kronenberg J., McCarthy J., Reiss R. Assessing the risks of exposures to multiple chemicals with a common mechanism of toxicity: how to cumulate? Regul. Toxicol. Pharmacol. 2000;31(1):30–43. doi: 10.1006/rtph.1999.1361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.