Abstract
Programmed cell death ligand-1 (PD-L1) is a type I transmembrane glycoprotein expressed on antigen-presenting cells and several tumor cells, including melanoma and lung cancer cells. A strong correlation has been reported between PD-L1 expression in tumor cells and negative prognosis in cancer patients. Previously, we established an anti-PD-L1 monoclonal antibody (mAb), L1Mab-13 (IgG1, kappa), by immunizing mice with PD-L1-overexpressing CHO-K1 cells. L1Mab-13 specifically reacts with endogenous PD-L1 in lung cancer cell lines in flow cytometry and Western blot applications, and stains a plasma membrane-like pattern in lung cancer tissues via immunohistochemical analysis. In this study, we investigated whether L1Mab-13 reacts with oral cancer cell lines and exerts antitumor activities. Because L1Mab-13 lacks antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), we first converted the subclass of L1Mab-13 from IgG1 into IgG2a (13-mG2a), and further produced a defucosylated version (13-mG2a-f) using FUT8-deficient ExpiCHO-S (BINDS-09) cells. Defucosylation of 13-mG2a-f was confirmed using fucose-binding lectins, such as Aleuria aurantia and Pholiota squarrosa lectins. The dissociation constants (KD) for 13-mG2a-f in SAS and HSC-2 oral cancer cells were determined via flow cytometry to be 2.8 × 10−9 M and 4.8 × 10−9 M, respectively, indicating that 13-mG2a-f possesses extremely high binding affinity. In vitro analysis demonstrated that 13-mG2a-f showed moderate ADCC and CDC activities against SAS and HSC-2 oral cancer cells. In vivo analysis revealed that 13-mG2a-f significantly reduced tumor development in SAS and HSC-2 xenografts in comparison to control mouse IgG, even after injection seven days post-tumor inoculation. Taken together, these data demonstrate that treatment with 13-mG2a-f may represent a useful therapy for patients with PD-L1-expressing oral cancers.
Keywords: PD-L1, Monoclonal antibody, ADCC, CDC, Antitumor activity, Oral cancer
Highlights
-
•
・PD-L1 is expressed in many tumor cells including oral cancers.
-
•
・We previously established an anti-PD-L1 mAb (L1Mab-13, mouse IgG1).
-
•
・We converted the subclass of L1Mab-13 from IgG1 into IgG2a (13-mG2a), and defucosylated it (13-mG2a-f).
-
•
・13-mG2a-f exerted ADCC, CDC, and antitumor activities against oral cancer cell lines.
Abbreviations:
- AAL
Aleuria aurantia lectin
- ADCC
antibody-dependent cellular cytotoxicity
- ATCC
American Type Culture Collection
- BSA
bovine serum albumin
- CasMab
cancer-specific mAb
- CDC
complement-dependent cytotoxicity
- CHO
Chinese hamster ovary
- Con A
concanavalin A
- DMEM
Dulbecco's Modified Eagle's Medium
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- HNSCC
head and neck squamous cell carcinoma
- JCRB
Japanese Collection of Research Bioresources Cell Bank
- mAb
monoclonal antibody
- OSCC
oral squamous cell carcinoma
- PBS
phosphate-buffered saline
- PDPN
podoplanin
- PhoSL
Pholiota squarrosa lectin
- PODXL
podocalyxin
- SCC
squamous cell carcinoma
1. Introduction
Programmed cell death ligand-1 (PD-L1) is a type I transmembrane glycoprotein expressed in many tumor types, including melanoma, brain tumor, lung cancer, breast cancer, gastric cancer, ovarian cancer, pancreatic cancer, and renal cancer [[1], [2], [3], [4], [5], [6], [7]]. PD-L1 is an immune regulatory molecule, which limits T cell effector function [8]. Programmed cell death (PD)-1/PD-L1 association inhibits activated T cell proliferation, allowing cancer cells to circumvent host immune surveillance [9,10]. A substantial correlation between PD-L1/L2 expression in cancer cells and poor prognosis has been reported in several cancers [[11], [12], [13]]. Although inhibition of PD-1 in patients with tumors is demonstrated to have therapeutic effects, detecting PD-L1 expression via immunohistochemistry would be extremely beneficial in making the clinical determination to use targeted drugs such as nivolumab or pembrolizumab for cancer treatment [14,15].
Oral cancers occupy about 2% of all cancer cases worldwide [16]. More than 350,000 individuals are diagnosed as oral cancer every year, and oral cancers are fatal for about 170,000 of these people. Increasingly, young patients are being diagnosed with oral cancers [17,18]. Oral cancers comprise several histological tumor types, such as squamous cell carcinoma (SCC), adenocarcinoma, mucoepidermoid carcinoma, and adenoid cystic carcinoma. The most effective treatment of oral SCC (OSCC), which comprises over 90% of all oral cancers, depends upon its clinical stage [19]. Early stages (stage-I and –II) are treated by surgery or radiotherapy alone. In contrast, advanced stages (stage-III and -IV) require a combination of surgery, radiotherapy, and chemotherapy [20]. For chemotherapy of OSCCs, cisplatin is the primary drug of choice, and it is usually combined with 5-fluorouracil and docetaxel [21,22]. Other anticancer agents, such as paclitaxel, methotrexate, and carboplatin can be also used for OSCCs [23], but effective molecular targeting drugs, including antibody therapies, are lacking.
PD-L1 has been utilized not only as a molecular marker of anti-PD-1 therapy, but also as a molecular target for antibody therapy. Anti-PD-L1 mAbs, such as atezolizumab, durvalumab, and avelumab has been used for patients with advanced head and neck squamous cell carcinoma (HNSCC) [24]. In 45%–87% of OSCC cases, cancer cells were PD-L1 positive, depending on the cut-off value for positivity and whether cytoplasmic staining was included as positive [24]. Anti-PD-L1 mAbs have been mainly used for PD1/PD-L1 blockade, but antitumor activities by antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) against oral cancers have not been investigated.
In our previous study, we developed a novel anti-human PD-L1 antibody, L1Mab-13 (mouse IgG1, kappa), which is useful for flow cytometry, Western blot, and immunohistochemical analysis [25]. In this study, we converted IgG1 subclass L1Mab-13 into IgG2a subclass 13-mG2a, and further produced a defucosylated version, 13-mG2a-f, using FUT8-deficient ExpiCHO-S cells (BINDS-09) [26]. We then investigated whether 13-mG2a-f exhibited ADCC, CDC, and antitumor activities against oral cancers.
2. Materials and methods
2.1. Cell lines
CHO-K1 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). CHO/PD-L1 was previously established [25]. Oral squamous carcinoma cell lines, including SAS (tongue) and HSC-2 (oral cavity), were obtained from the Japanese Collection of Research Bioresources Cell Bank (JCRB, Osaka, Japan). CHO-K1 and CHO/PD-L1 were cultured in RPMI 1640 medium (Nacalai Tesque, Inc., Kyoto, Japan). SAS and HSC-2 were cultured in Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque, Inc.). The medium is supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc., Waltham, MA, USA), 100 units/mL of penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Nacalai Tesque, Inc.) at 37 °C in a humidified atmosphere containing 5% CO2.
2.2. Antibodies
Anti-PD-L1 mAb L1Mab-13 (mouse IgG1, kappa) was developed as previously described [25]. To generate 13-mG2a, appropriate VH cDNA of mouse L1Mab-13 and CH of mouse IgG2a were subcloned into pCAG-Ble vector (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), and VL and CL cDNAs of L1Mab-13 were subcloned into pCAG-Neo vector (FUJIFILM Wako Pure Chemical Corporation). The vectors were transfected into BINDS-09 (FUT8-deficient ExpiCHO-S cells) using the ExpiCHO Expression System [26]. 13-mG2a-f was purified using Protein G-Sepharose (GE Healthcare Bio-Sciences, Pittsburgh, PA). Mouse IgG was purchased from Sigma-Aldrich Corp. (St. Louis, MO).
2.3. Flow cytometry
Cells were harvested by brief exposure to 0.25% trypsin/1 mM ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque, Inc.). After washing with 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), cells were treated with primary mAbs for 30 min at 4 °C and subsequently with Alexa Fluor 488-conjugated anti-mouse IgG (1:1000; Cell Signaling Technology, Inc., Danvers, MA, USA). Fluorescence data were collected using an EC800 Cell Analyzer (Sony Corp., Tokyo, Japan).
2.4. Determination of the binding affinity
Cells were suspended in 100 μL of serially diluted mAbs (0.3 ng/mL–5 μg/mL), followed by the addition of Alexa Fluor 488-conjugated anti-mouse IgG (1:200; Cell Signaling Technology, Inc.). Fluorescence data were collected using an EC800 Cell Analyzer (Sony Corp.). The dissociation constant (KD) was calculated by fitting binding isotherms to built-in one-site binding models in GraphPad PRISM 6 (GraphPad Software, Inc., La Jolla, CA, USA).
2.5. Enzyme-linked immunosorbent assay (ELISA)
L1Mab-13 and 13-mG2a-f were immobilized on Nunc Maxisorp 96-well immunoplates (Thermo Fisher Scientific Inc.) at 1 μg/mL for 30 min. After blocking using SuperBlock buffer (Thermo Fisher Scientific Inc.) containing 0.5 mM CaCl2, the plates were incubated with biotin-labeled lectins, such as Aleuria aurantia lectin (AAL, fucose binder; Vector Laboratories, Burlingame, CA, USA) [27], Pholiota squarrosa lectin (PhoSL, core fucose binder; J-OIL MILLS, Inc., Tokyo, Japan) [28], and Concanavalin A (ConA, mannose binder; Vector Laboratories) [29], followed by 1:3000 diluted peroxidase-conjugated streptavidin (Agilent Technologies, Santa Clara, CA, USA). The enzymatic reaction was produced using a 1-Step Ultra TMB-ELISA (Thermo Fisher Scientific Inc.). Optical density was measured at 655 nm using an iMark microplate reader (Bio-Rad Laboratories, Inc., Berkeley, CA, USA).
2.6. Animals
All animal experiments were performed in accordance with relevant guidelines and regulations to minimize animal suffering and distress in the laboratory. Animal studies for ADCC and antitumor activity were approved by the institutional committee for experiments of the Institute of Microbial Chemistry (Permit number: 2020–007). Mice were monitored for health and weight every 2 or 4 days. Experiment duration was 3 weeks. A bodyweight loss exceeding 25% and a maximum tumor size exceeding 3000 mm3 were identified as humane endpoints. Mice were euthanized by cervical dislocation, and the death was verified by respiratory arrest and cardiac arrest.
2.7. ADCC
Six 6-week-old female BALB/c nude mice were purchased from Charles River (Kanagawa, Japan). After euthanization by cervical dislocation, spleens were removed aseptically and single-cell suspensions obtained by forcing spleen tissues through a sterile cell strainer (352360, BD Falcon, Corning, New York, NY, USA) using a syringe. Erythrocytes were lysed with a 10-sec exposure to ice-cold distilled water. Splenocytes were washed with DMEM and resuspended in DMEM with 10% FBS and used as effector cells. Target cells were labeled with 10-μg/mL Calcein AM (Thermo Fisher Scientific, Inc.) and resuspended in the same medium. The target cells (2 × 104 cells/well) were plated in 96-well plates and mixed with effector cells, anti-PD-L1 antibodies, or control IgG (mouse IgG2a) (Sigma-Aldrich). After a 6.5-h incubation, the Calcein AM release of supernatant from each well was measured. Fluorescence intensity was determined using a microplate reader (Power Scan HT) (BioTek Instruments, Winooski, VT, USA) with an excitation wavelength of 485 nm and an emission wavelength of 538 nm. Cytolytic activity (as % of lysis) was calculated using the equation: % lysis = (E−S)/(M−S) × 100, where E is fluorescence of combined target and effector cells, S is spontaneous fluorescence of target cells only, and M is maximum fluorescence measured after lysing all cells with a buffer containing 0.5% Triton X-100, 10 mM Tris-HCl (pH 7.4), and 10 mM of EDTA.
2.8. CDC
To assess cell viability, cells were labeled with 10-μg/mL Calcein AM (Thermo Fisher Scientific, Inc.) and resuspended in the same medium. The cells (2 × 104 cells/well) were plated in 96-well plates and mixed with rabbit complement (final dilution 1:10; Low-Tox-M Rabbit Complement; Cedarlane Laboratories, Hornby, Ontario, Canada), anti-PD-L1 antibodies, or control IgG (mouse IgG2a) (Sigma-Aldrich Corp.). After a 6.5-h incubation, the Calcein AM release of supernatant from each well was measured. Fluorescence intensity was determined as described in the ADCC part above.
2.9. Antitumor activity of 13-mG2a-f in the xenografts of oral cancers
Thirty-two 6-week-old female BALB/c nude mice were purchased from Charles River (Kanagawa, Japan) and used at 7 weeks of age. SAS and HSC-2 cells (0.3 mL of 1.33 × 108 cells/mL in DMEM) were mixed with 0.5 mL BD Matrigel Matrix Growth Factor Reduced (BD Biosciences, San Jose, CA, USA). 100 μL of this suspension (5 × 106 cells) was injected subcutaneously into the left flank. After day 8, 100 μg of 13-mG2a-f and control mouse IgG (Sigma-Aldrich Corp.) in 100 μL PBS was injected intraperitoneally (i.p.) into treated and control mice, respectively. Additional antibodies were then injected on days 14 and 21. Twenty-three days after cell implantation, all mice were euthanized by cervical dislocation, and tumor diameters and volumes were determined as previously described [30].
2.10. Statistical analyses
All data were expressed as mean ± SEM. Statistical analysis used ANOVA and subsequently Sidak's multiple comparisons test for tumor volume and mouse weight, or Welch's t-test for ADCC/CDC and tumor weight using GraphPad Prism 7 (GraphPad Software, Inc.). P < 0.05 was adopted as a level of statistical significance.
3. Results
3.1. Development and characterization of 13-mG2a-f, a core-fucose-deficient mouse IgG2a-type anti-PD-L1 antibody
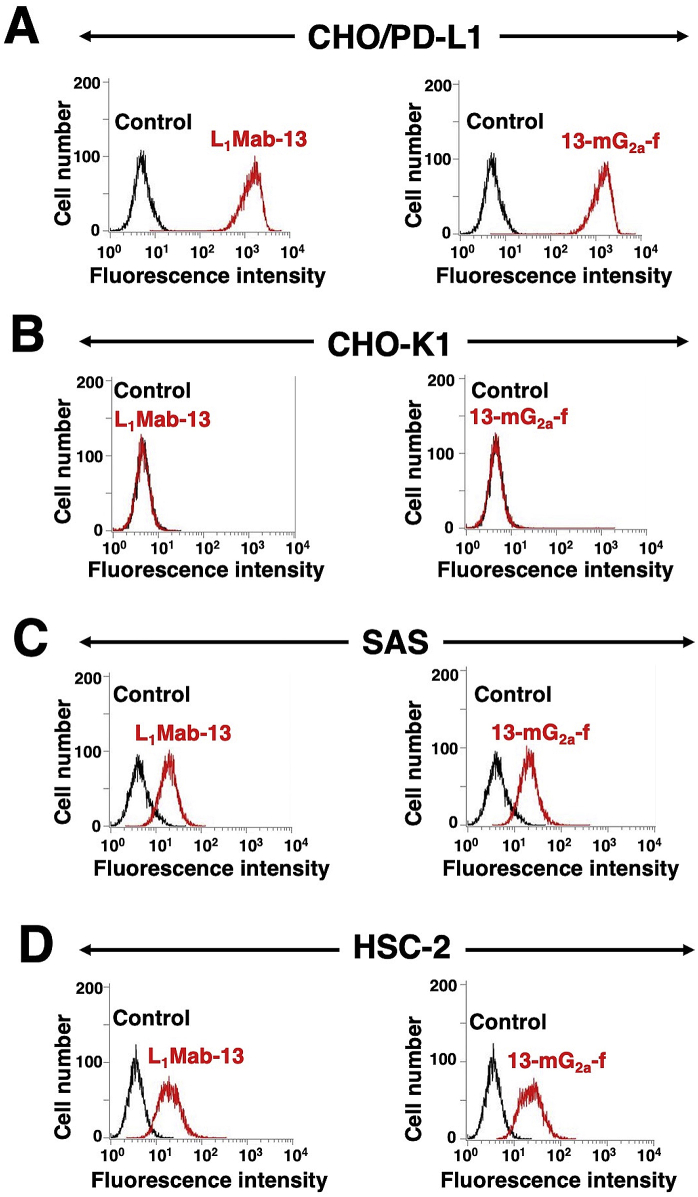
As mouse IgG2a possesses high ADCC and CDC activities [31], we first developed a mouse IgG2a version of L1Mab-13 (mouse IgG1) by subcloning appropriate VH cDNA of L1Mab-13 and CH of mouse IgG2a into pCAG-Ble vector, and the light chain of L1Mab-13 into pCAG-Neo vector. This IgG2a-type of L1Mab-13 is henceforth referred to as 13-mG2a. We additionally produced a core-fucose-deficient type of 13-mG2a, henceforth referred to as 13-mG2a-f, using the BINDS-09 cell line (FUT8-knockout ExpiCHO–S cell line) [26]. We analyzed the sensitivity of 13-mG2a-f in CHO-K1 cells expressing PD-L1 (CHO/PD-L1) and in OSCC cell lines (SAS and HSC-2) using flow cytometry. Both L1Mab-13 and 13-mG2a-f reacted with CHO/PD-L1 cells (Fig. 1A), but not with CHO-K1 cells (Fig. 1B). Both mAbs also reacted with SAS cells (Fig. 1C) and HSC-2 cells (Fig. 1D), indicating that 13-mG2a-f demonstrated high sensitivity and specificity for PD-L1.
Fig. 1.
Recognition of PD-L1 using anti-PD-L1 mAbs. (A) CHO/PD-L1 cells were treated with L1Mab-13 and 13-mG2a-f (1 μg/mL), followed by secondary antibodies. (B) CHO-K1 cells were treated with L1Mab-13 and 13-mG2a-f (1 μg/mL), followed by secondary antibodies. (C) SAS cells were treated with L1Mab-13 and 13-mG2a-f (1 μg/mL), followed by secondary antibodies. (D) HSC-2 cells were treated with L1Mab-13 and 13-mG2a-f (1 μg/mL), followed by secondary antibodies. The black line represents the negative control.
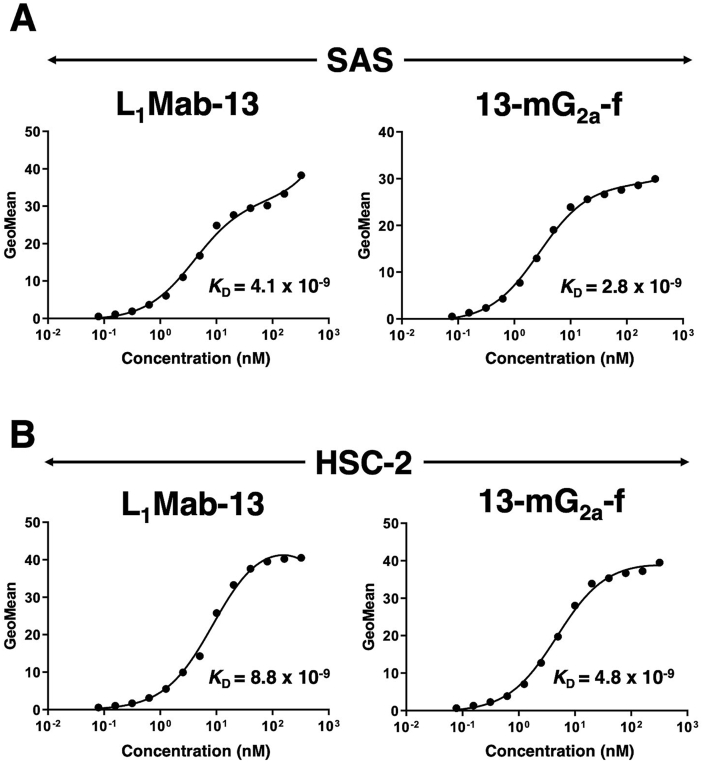
We conducted a kinetic analysis of the interactions of L1Mab-13 and 13-mG2a-f with SAS and HSC-2 oral cancer cell lines using flow cytometry. The dissociation constant (KD) for L1Mab-13 in SAS cells was determined to be 4.1 × 10−9 M (Fig. 2A). In contrast, the KD for 13-mG2a-f in SAS cells was 2.8 × 10−9 M. The binding affinity of 13-mG2a-f in SAS cells was 1.5-fold higher than that of L1Mab-13. Likewise, the KD for L1Mab-13 against HSC-2 cells was 8.8 × 10−9 M (Fig. 2B). By contrast, the KD for 13-mG2a-f in HSC-2 cells was 4.8 × 10−9 M. The binding affinity of 13-mG2a-f in HSC-2 cells was 1.8-fold higher than that of L1Mab-13. The binding affinity of 13-mG2a-f in SAS cells was 1.7-fold higher than that in HSC-2 cells.
Fig. 2.
Determination of the binding affinity of anti-PD-L1 mAbs for oral cancer cells using flow cytometry. (A) SAS cells were suspended in 100 μl of serially diluted mAbs (0.3 ng/mL - 5 μg/mL), followed by the addition of Alexa Fluor 488-conjugated anti-mouse IgG, and fluorescence data were collected. (B) HSC-2 cells were suspended in 100 μL of serially diluted mAbs (0.3 ng/mL - 5 μg/mL), followed by the addition of Alexa Fluor 488-conjugated anti-mouse IgG, and fluorescence data were collected.
Defucosylation of 13-mG2a-f was confirmed using lectins such as AAL (which binds to fucose) and PhoSL (which binds to core fucose). ConA (which binds to mannose) was used as a positive control. Both L1Mab-13 and 13-mG2a-f were detected using ConA (Fig. 3A). L1Mab-13, but not 13-mG2a-f, was detected using AAL (Fig. 3B) and PhoSL (Fig. 3C), demonstrating that 13-mG2a-f was defucosylated.
Fig. 3.
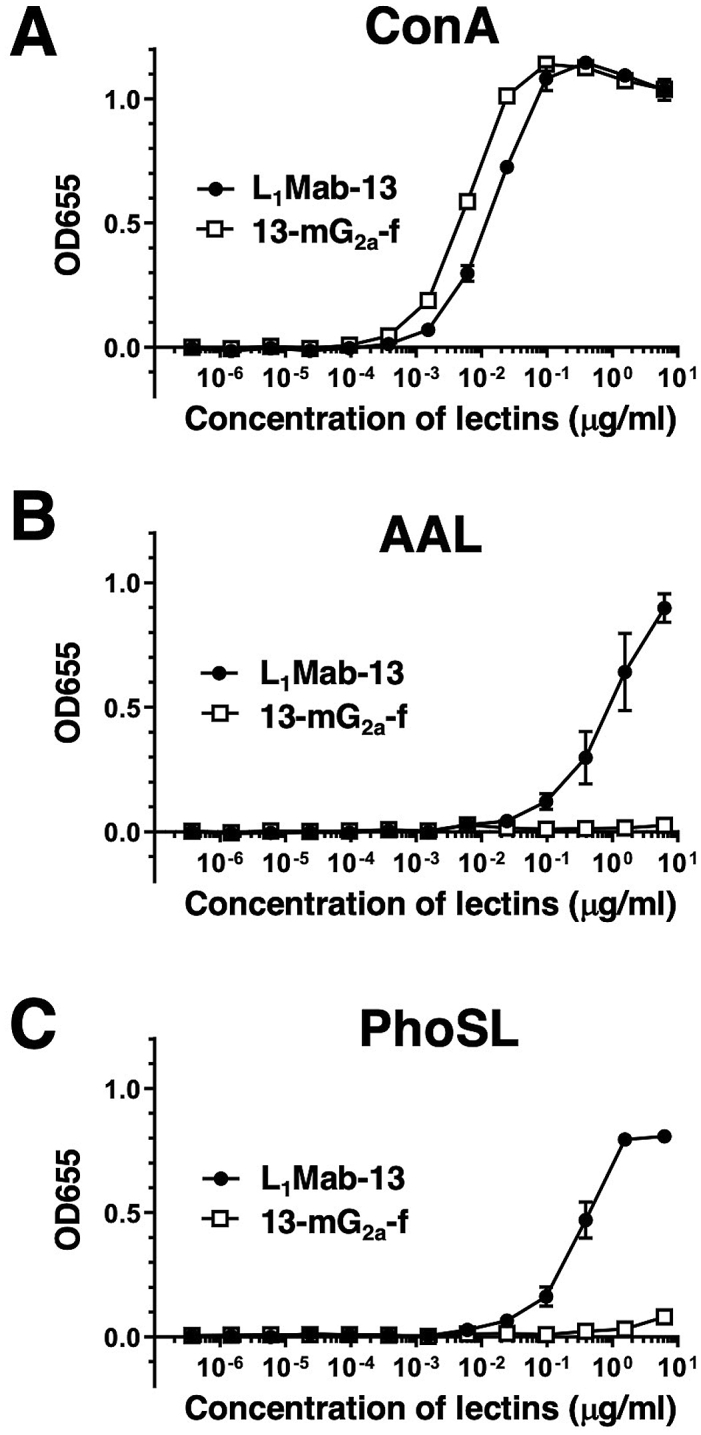
Confirmation of defucosylation of 13-mG2a-f by enzyme-linked immunosorbent assay (ELISA) using lectins. (A) L1Mab-13 and 13-mG2a-f were immobilized and incubated with biotin-labeled concanavalin A (Con A), followed by peroxidase-conjugated streptavidin. OD655 was measured as a function of Con A concentration. (B) L1Mab-13 and 13-mG2a-f were immobilized and incubated with biotin-labeled Aleuria aurantia lectin (AAL), followed by peroxidase-conjugated streptavidin. OD655 was measured as a function of AAL concentration. (C) L1Mab-13 and 13-mG2a-f were immobilized and incubated with biotin-labeled Pholiota squarrosa lectin (PhoSL), followed by peroxidase-conjugated streptavidin. OD655 was measured as a function of PhoSL concentration.
3.2. ADCC and CDC activities of 13-mG2a-f in oral cancer cell lines
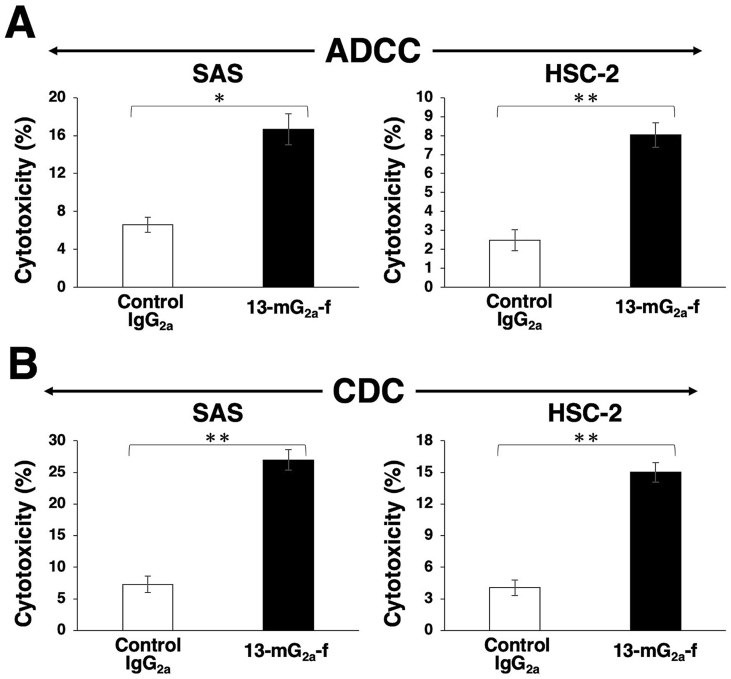
Because the mouse IgG1 subclass L1Mab-13 does not possess ADCC or CDC activities, we created a mouse IgG2a subclass mAb, and further defucosylated it to enhance those activities. In this study, we examined whether the developed 13-mG2a-f induced ADCC and CDC in PD-L1-expressing oral cancer cell lines, such as SAS and HSC-2 cells. 13-mG2a-f exhibited higher ADCC (17% cytotoxicity) in SAS cells compared with that of control mouse IgG2a treatment (6.6% cytotoxicity; P < 0.05) (Fig. 4A). Similarly, 13-mG2a-f exhibited higher ADCC (8.0% cytotoxicity) in HSC-2 cells compared with that of control mouse IgG2a treatment (2.5% cytotoxicity; P < 0.01) (Fig. 4A).
Fig. 4.
Evaluation of ADCC and CDC activities of 13-mG2a-f in SAS and HSC-2 cells. (A) ADCC activities of 13-mG2a-f and control mouse IgG2a in SAS and HSC-2 cells. (B) CDC activities by 13-mG2a-f and control mouse IgG2a in SAS and HSC-2 cells. Values are mean ± SEM. Asterisk indicates statistical significance (**P < 0.01, *P < 0.05, n.s: not significant, Welch's t-test).
Furthermore, 13-mG2a-f exhibited higher CDC activity (27% cytotoxicity) in SAS cells compared with control mouse IgG2a treatment (7.3% cytotoxicity; P < 0.01; Fig. 4B). Similarly, 13-mG2a-f exhibited higher CDC activity (15% cytotoxicity) in HSC-2 cells compared with control mouse IgG2a treatment (4.1% cytotoxicity; P < 0.01; Fig. 4B). Although ADCC/CDC activities of 13-mG2a-f in oral cancer cells are not outstanding, it remained to be seen whether 13-mG2a-f may exert antitumor activity against oral cancer cells in vivo.
3.3. Antitumor activities of 13-mG2a-f in the mouse xenografts of SAS oral cancer cells
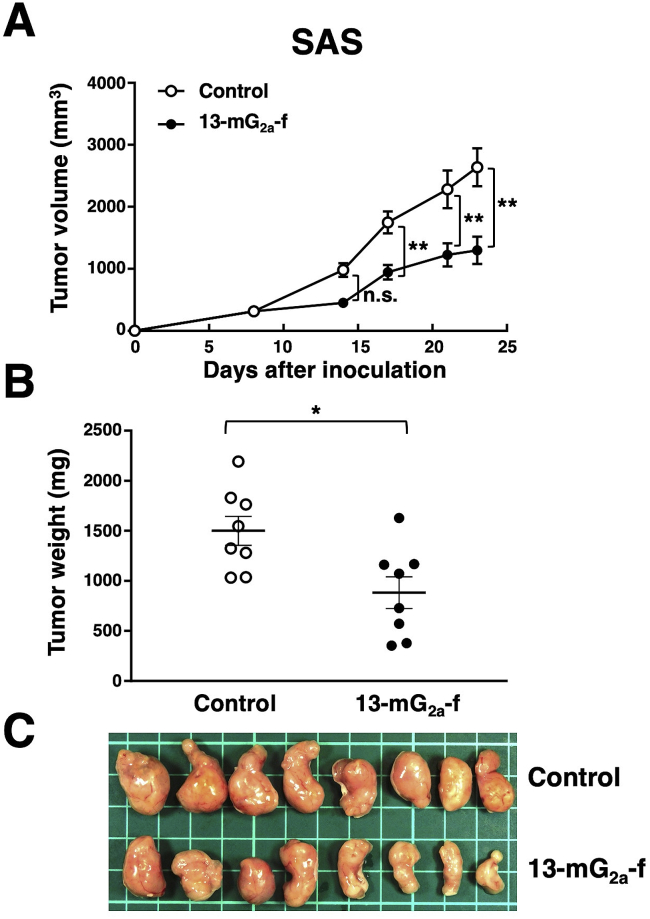
In the SAS xenograft models, tumor formation in 16 SAS-injected mice was observed after eight days. These 16 SAS tumor-bearing mice were then divided into a 13-mG2a-f-treated group and a control group. On days 8, 14, and 21 after SAS cell injections into the mice, 13-mG2a-f (100 μg) and control mouse IgG (100 μg) were injected i.p. into the mice. Tumor formation was observed in mice in both treated and control groups. Tumor volume was measured on days 8, 14, 17, 21, and 23 after SAS cell injection. 13-mG2a-f-treated mice showed significantly reduced tumor development on day 17 (P < 0.01), day 21 (P < 0.01), and day 23 (P < 0.01) in comparison to IgG-treated control mice (Fig. 5A). Tumor volume reduction by 13-mG2a-f treatment was 51% on day 23. Tumors from 13-mG2a-f-treated mice weighed significantly less than tumors from IgG-treated control mice (41% reduction, P < 0.05; Fig. 5B). Resected tumors on day 23 are depicted (Fig. 5C). Total body weights did not significantly differ between the two groups (data not shown). These results indicate that 13-mG2a-f reduced the growth of SAS xenografts effectively, even when 13-mG2a-f was injected eight days post-SAS cell injections in mice.
Fig. 5.
Evaluation of antitumor activity of 13-mG2a-f in SAS xenografts. (A) Tumor volume of SAS xenografts. SAS cells (5 × 106 cells) were injected subcutaneously into the left flank. After eight days, 100 μg of 13-mG2a-f or control mouse IgG in 100 μl PBS were injected i.p. into treated and control mice, respectively. Additional antibodies were then injected on days 14 and 21. Tumor volume was measured on days 8, 14, 17, 21, and 23. Values are mean ± SEM. Asterisk indicates statistical significance (**P < 0.01, n.s: not significant, ANOVA and Sidak's multiple comparisons test) (B) Tumor weights of SAS xenografts. Tumors of SAS xenografts were resected from 13-mG2a-f and control mouse IgG groups. Tumor weight on day 23 was measured from excised xenografts. Values are mean ± SEM. Asterisk indicates statistical significance (*P < 0.05, Welch's t-test). (C) Resected tumors of SAS xenografts from 13-mG2a-f and control mouse IgG groups on day 23.
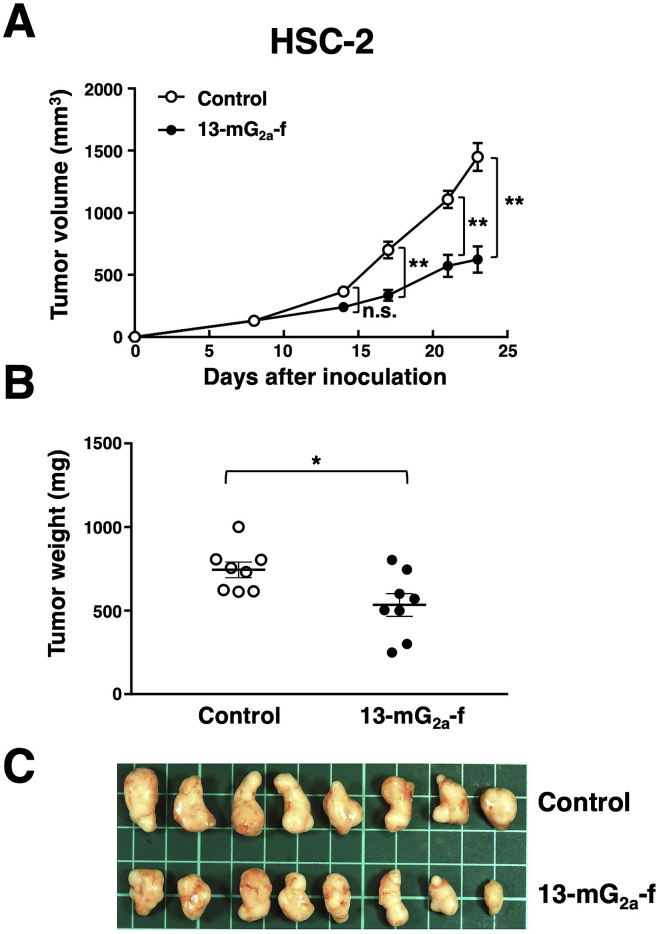
3.4. Antitumor activities of 13-mG2a-f in mouse xenografts of HSC-2 oral cancer cells
In the HSC-2 xenograft models, tumor formation of 16 HSC-2-injected mice was observed after eight days. These 16 HSC-2-bearing mice were then divided into a 13-mG2a-f-treated group and a control group. On days 8, 14, and 21 after cell injections into the mice, 13-mG2a-f (100 μg) and control mouse IgG (100 μg) were injected i.p. into the mice. Tumor volume was assessed on days 8, 14, 17, 21, and 23. 13-mG2a-f-treated mice showed significantly reduced tumor development on day 17 (P < 0.01), day 21 (P < 0.01), and day 23 (P < 0.01) in comparison to IgG-treated control mice (Fig. 6A). Tumor volume reduction in 13-mG2a-treated mice was 57% on day 23. Tumors from 13-mG2a-f-treated mice weighed significantly less than tumors from IgG-treated control mice (28% reduction, P < 0.05; Fig. 6B). Resected tumors on day 23 are shown in Fig. 6C. Total body weights did not significantly differ between the two groups (data not shown). These results indicate that 13-mG2a-f reduced the growth of HSC-2 xenografts effectively, even when 13-mG2a-f was injected eight days post–HSC–2 cell injections in mice.
Fig. 6.
Evaluation of antitumor activity of 13-mG2a-f in HSC-2 xenografts. (A) Tumor volume of HSC-2 xenografts. HSC-2 cells (5 × 106 cells) were injected subcutaneously into the left flank. After eight days, 100 μg of 13-mG2a-f or control mouse IgG in 100 μl PBS were injected i.p. into treated and control mice, respectively. Additional antibodies were then injected on days 14 and 21. Tumor volume was measured on days 8, 14, 17, 21, and 23. Values are mean ± SEM. Asterisk indicates statistical significance (**P < 0.01, n.s: not significant, ANOVA and Sidak's multiple comparisons test) (B) Tumor weights of HSC-2 xenografts. Tumors of HSC-2 xenografts were resected from 13-mG2a-f and control mouse IgG groups. Tumor weight on day 23 was measured from excised xenografts. Values are mean ± SEM. Asterisk indicates statistical significance (*P < 0.05, Welch's t-test). (C) Resected tumors of HSC-2 xenografts from 13-mG2a-f and control mouse IgG groups on day 23.
4. Discussion
In this study, we investigated whether anti-PD-L1 mAbs are advantageous for the treatment of oral cancers by ADCC/CDC activities, rather than by neutralization of PD-L1/PD1 interaction, as human PD-L1 does not react with mouse PD-1. We had previously developed a sensitive and specific anti-PD-L1 mAb, L1Mab-13 [25], but were unable to investigate antitumor activity as the IgG1 subclass does not possess ADCC/CDC activities. Therefore, we converted L1Mab-13 into an IgG2a subclass antibody, and increased ADCC activity via defucosylation. We demonstrated that 13-mG2a-f exerts ADCC/CDC activities in vitro (Fig. 4), and antitumor activities against oral cancer xenografts in vivo (Fig. 5, Fig. 6). Importantly, 13-mG2a-f efficaciously reduced the growth of SAS xenografts (Fig. 5) and HSC-2 xenografts (Fig. 6), even when 13-mG2a-f was injected eight days after cell implantations into the mice. However, SAS and HSC-2 tumor volume reduction on day 23 by 13-mG2a-f treatment only reached 51% and 57%, respectively, indicating that anti-PD-L1 therapy might not be sufficient for solo treatment of most oral cancers. One potential reason for this weak antitumor activity is low ADCC activity (Fig. 4A) and CDC activity (Fig. 4B) of 13-mG2a-f, in spite of the high binding affinity in SAS cells (KD: 2.8 × 10−9 M; Fig. 2A) and HSC-2 cells (KD: 4.8 × 10−9 M; Fig. 2B). The binding affinity of 13-mG2a-f in SAS cells was 1.5-fold higher than that of L1Mab-13. Likewise, the binding affinity of 13-mG2a-f in HSC-2 cells was 1.8-fold higher than that of L1Mab-13. These results are consistent with our previous observation that the mouse IgG2a-type mAb 47-mG2a-f also shows a higher affinity for PODXL than the original PcMab-47 (mouse IgG1), indicating that fragment crystallizable (Fc) portion of mouse IgG could be important for the binding affinity for target molecules; or, recombinant mAbs might possess high purity compared to hybridoma-derived mAbs [32].
We recently developed a sensitive and specific mAb against EGFR (clone EMab-17, mouse IgG2a), and examined its ADCC/CDC and antitumor activities against SAS and HSC-2 xenografts [30]. In another recent study, HER2 was shown to be expressed in oral cancers, and an anti-HER2 mAb (clone H2Mab-19, mouse IgG2b) showed antitumor activity against SAS and HSC-2 xenografts [33]. Further, we previously investigated whether PODXL may be a therapeutic target in OSCC using anti-PODXL mAbs [32]. We converted an anti-PODXL mAb of IgG1 subclass (PcMab-47) into a mouse IgG2a-type mAb (47-mG2a) to increase ADCC. We developed 47-mG2a-f, a core fucose-deficient variant of 47-mG2a to increase its ADCC. In vivo analysis demonstrated that 47-mG2a-f, but not 47-mG2a, exerted antitumor activity in SAS and HSC-2 xenograft models at a dose of 100 μg/mouse/week administered three times. Although both 47-mG2a and 47-mG2a-f exhibited antitumor activity in HSC-2 xenograft models at a dose of 500 μg/mouse/week administered twice, 47-mG2a-f also demonstrated higher antitumor activity than 47-mG2a, indicating that a core fucose-deficient anti-PODXL mAb could be profitable for antibody-based therapy against PODXL-expressing OSCCs.
Targeting multiple targets, such as PODXL, EGFR, HER2, and PD-L1 may be needed for effective therapy to cure oral cancers. Another important goal is to target cancer-specific antigens using a cancer-specific mAb (CasMab). We previously established CasMab against PDPN, which is expressed in many cancers, including oral cancers [34]. In xenograft models with HSC-2 cells, a mouse-human chimeric mAb, chLpMab-23, exerted antitumor activity using human natural killer cells, indicating that chLpMab-23 may be advantageous for antibody therapy against PDPN-expressing oral cancers [35]. In the future, cancer-specific anti-PD-L1 mAbs may also be developed that can reduce the adverse effects of traditional antibody therapy.
Funding
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Numbers: JP20am0401013 (Y.K.), JP20am0101078 (Y.K.), and JP20ae0101028 (Y.K.).
Acknowledgments
We thank Ms. Saori Handa and Mr. Yu Komatsu (Department of Antibody Drug Development, Tohoku University Graduate School of Medicine) for technical assistance of in vitro experiments, and Ms. Akiko Harakawa (Institute of Microbial Chemistry (BIKAKEN), Numazu, Microbial Chemistry Research Foundation) for technical assistance of animal experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100801.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Jilaveanu L.B., Shuch B., Zito C.R., Parisi F., Barr M., Kluger Y., Chen L., Kluger H.M. PD-L1 expression in clear cell renal cell carcinoma: an analysis of nephrectomy and sites of metastases. J. Canc. 2014;5:166–172. doi: 10.7150/jca.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., Carlsson R., Ambjorn M., Hasan M., Badn W., Darabi A., Siesjo P., Issazadeh-Navikas S. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J. Neurosci. 2013;33:14231–14245. doi: 10.1523/JNEUROSCI.5812-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maine C.J., Aziz N.H., Chatterjee J., Hayford C., Brewig N., Whilding L., George A.J., Ghaem-Maghami S. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol. Immunother. 2014;63:215–224. doi: 10.1007/s00262-013-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merelli B., Massi D., Cattaneo L., Mandala M. Targeting the PD1/PD-L1 axis in melanoma: biological rationale, clinical challenges and opportunities. Crit. Rev. Oncol. Hematol. 2014;89:140–165. doi: 10.1016/j.critrevonc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Mittendorf E.A., Philips A.V., Meric-Bernstam F., Qiao N., Wu Y., Harrington S., Su X., Wang Y., Gonzalez-Angulo A.M., Akcakanat A., Chawla A., Curran M., Hwu P., Sharma P., Litton J.K., Molldrem J.J., Alatrash G. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Zhang J., Xu K., Xiao Z., Sun J., Xu J., Wang J., Tang Q. PTEN/PI3K/mTOR/B7-H1 signaling pathway regulates cell progression and immuno-resistance in pancreatic cancer. Hepato-Gastroenterology. 2013;60:1766–1772. [PubMed] [Google Scholar]
- 7.Zheng Z., Bu Z., Liu X., Zhang L., Li Z., Wu A., Wu X., Cheng X., Xing X., Du H., Wang X., Hu Y., Ji J. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin. J. Canc. Res. 2014;26:104–111. doi: 10.3978/j.issn.1000-9604.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blank C., Gajewski T.F., Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol. Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamanishi J., Mandai M., Iwasaki M., Okazaki T., Tanaka Y., Yamaguchi K., Higuchi T., Yagi H., Takakura K., Minato N., Honjo T., Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okazaki T., Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 13.Thompson R.H., Gillett M.D., Cheville J.C., Lohse C.M., Dong H., Webster W.S., Krejci K.G., Lobo J.R., Sengupta S., Chen L., Zincke H., Blute M.L., Strome S.E., Leibovich B.C., Kwon E.D. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passiglia F., Bronte G., Bazan V., Natoli C., Rizzo S., Galvano A., Listi A., Cicero G., Rolfo C., Santini D., Russo A. PD-L1 expression as predictive biomarker in patients with NSCLC: a pooled analysis. Oncotarget. 2016;7:19738–19747. doi: 10.18632/oncotarget.7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callahan M.K., Postow M.A., Wolchok J.D. Targeting T cell Co-receptors for cancer therapy. Immunity. 2016;44:1069–1078. doi: 10.1016/j.immuni.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 17.Tota J.E., Anderson W.F., Coffey C., Califano J., Cozen W., Ferris R.L., John M. St, Cohen E.E.W., Chaturvedi A.K. Rising incidence of oral tongue cancer among white men and women in the United States, 1973–2012. Oral Oncol. 2017;67:146–152. doi: 10.1016/j.oraloncology.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Hussein A.A., Helder M.N., de Visscher J.G., Leemans C.R., Braakhuis B.J., de Vet H.C.W., Forouzanfar T. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: a systematic review. Eur. J. Canc. 2017;82:115–127. doi: 10.1016/j.ejca.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Rivera C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015;8:11884–11894. [PMC free article] [PubMed] [Google Scholar]
- 20.Guneri P., Epstein J.B. Late stage diagnosis of oral cancer: components and possible solutions. Oral Oncol. 2014;50:1131–1136. doi: 10.1016/j.oraloncology.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Vokes E.E. Induction chemotherapy for head and neck cancer: recent data. Oncol. 2010;15(Suppl 3):3–7. doi: 10.1634/theoncologist.2010-S3-03. [DOI] [PubMed] [Google Scholar]
- 22.Marcazzan S., Varoni E.M., Blanco E., Lodi G., Ferrari M. Nanomedicine, an emerging therapeutic strategy for oral cancer therapy. Oral Oncol. 2018;76:1–7. doi: 10.1016/j.oraloncology.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Furness S., Glenny A.-M., Worthington H.V., Pavitt S., Oliver R., Clarkson J.E., Macluskey M., Chan K.K., Conway D.I. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.cd006386.pub3. 10.1002/14651858.cd006386.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Kok V.C. Current understanding of the mechanisms underlying immune evasion from PD-1/PD-L1 immune checkpoint blockade in head and neck cancer. Front Oncol. 2020;10:268. doi: 10.3389/fonc.2020.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada S., Itai S., Nakamura T., Yanaka M., Chang Y.W., Suzuki H., Kaneko M.K., Kato Y. Monoclonal antibody L1Mab-13 detected human PD-L1 in lung cancers. Monoclon. Antibodies Immunodiagn. Immunother. 2018;37:110–115. doi: 10.1089/mab.2018.0004. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y., Mizuno T., Yamada S., Nakamura T., Itai S., Yanaka M., Sano M., Kaneko M.K. Establishment of P38Bf, a core-fucose-deficient mouse-canine chimeric antibody against dog podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2018;37:218–223. doi: 10.1089/mab.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wimmerova M., Mitchell E., Sanchez J.F., Gautier C., Imberty A. Crystal structure of fungal lectin: six-bladed beta-propeller fold and novel fucose recognition mode for Aleuria aurantia lectin. J. Biol. Chem. 2003;278:27059–27067. doi: 10.1074/jbc.M302642200. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi Y., Tateno H., Dohra H., Moriwaki K., Miyoshi E., Hirabayashi J., Kawagishi H. A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J. Biol. Chem. 2012;287:33973–33982. doi: 10.1074/jbc.M111.327692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumner J.B., Howell S.F., Zeissig A. Concanavalin a and hemagglutination. Science. 1935;82:65–66. doi: 10.1126/science.82.2116.65. [DOI] [PubMed] [Google Scholar]
- 30.Takei J., Kaneko M.K., Ohishi T., Kawada M., Harada H., Kato Y. A novel anti-EGFR monoclonal antibody (EMab-17) exerts antitumor activity against oral squamous cell carcinomas via antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Oncol Lett. 2020;19:2809–2816. doi: 10.3892/ol.2020.11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato Y., Kunita A., Fukayama M., Abe S., Nishioka Y., Uchida H., Tahara H., Yamada S., Yanaka M., Nakamura T., Saidoh N., Yoshida K., Fujii Y., Honma R., Takagi M., Ogasawara S., Murata T., Kaneko M.K. Antiglycopeptide mouse monoclonal antibody LpMab-21 exerts antitumor activity against human podoplanin through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:20–24. doi: 10.1089/mab.2016.0045. [DOI] [PubMed] [Google Scholar]
- 32.Itai S., Ohishi T., Kaneko M.K., Yamada S., Abe S., Nakamura T., Yanaka M., Chang Y.W., Ohba S.I., Nishioka Y., Kawada M., Harada H., Kato Y. Anti-podocalyxin antibody exerts antitumor effects via antibody-dependent cellular cytotoxicity in mouse xenograft models of oral squamous cell carcinoma. Oncotarget. 2018;9:22480–22497. doi: 10.18632/oncotarget.25132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takei J., Kaneko M.K., Ohishi T., Kawada M., Harada H., Kato Y. H2Mab-19, an anti-human epidermal growth factor receptor 2 monoclonal antibody exerts antitumor activity in mouse oral cancer xenografts. Exp. Ther. Med. 2020:846–853. doi: 10.3892/etm.2020.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato Y., Kaneko M.K. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci. Rep. 2014;4:5924. doi: 10.1038/srep05924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaneko M.K., Nakamura T., Kunita A., Fukayama M., Abe S., Nishioka Y., Yamada S., Yanaka M., Saidoh N., Yoshida K., Fujii Y., Ogasawara S., Kato Y. ChLpMab-23: cancer-specific human-mouse chimeric anti-podoplanin antibody exhibits antitumor activity via antibody-dependent cellular cytotoxicity. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:104–112. doi: 10.1089/mab.2017.0014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.