Abstract
Vertebrate hemoglobin (Hb) and myoglobin (Mb) were among the first proteins whose structures and sequences were determined over 50 years ago. In the subsequent pregenomic period, numerous related proteins came to light in plants, invertebrates and bacteria, that shared the myoglobin fold, a signature sequence motif characteristic of a 3-on-3 α-helical sandwich. Concomitantly, eukaryote and bacterial globins with a truncated 2-on-2 α-helical fold were discovered. Genomic information over the last 20 years has dramatically expanded the list of known globins, demonstrating their existence in a limited number of archaeal genomes, a majority of bacterial genomes and an overwhelming majority of eukaryote genomes. In vertebrates, 6 additional globin types were identified, namely neuroglobin (Ngb), cytoglobin (Cygb), globin E (GbE), globin X (GbX), globin Y (GbY) and androglobin (Adgb). Furthermore, functions beyond the familiar oxygen transport and storage have been discovered within the vertebrate globin family, including NO metabolism, peroxidase activity, scavenging of free radicals, and signaling functions. The extension of the knowledge on globin functions suggests that the original roles of bacterial globins must have been enzymatic, involved in defense against NO toxicity, and perhaps also as sensors of O2, regulating taxis away or towards high O2 concentrations. In this review, we aimed to discuss the evolution and remarkable functional diversity of vertebrate globins with particular focus on the variety of non-canonical expression sites of mammalian globins and their according impressive variability of atypical functions.
Keywords: Cancer, Hemoglobin, Hypoxia, Myoglobin, Nitric oxide, Oxidative stress
Abbreviations
- Adgb
androglobin
- CO
carbon monoxide
- Cygb
cytoglobin
- Cyt-c
cytochrome c
- Epo
erythropoietin
- Fgb
single domain FHb-like globin
- FHb
flavohemoglobin
- GbE
globin E
- GbX
globin X
- GbY
globin Y
- GCS
globin-coupled sensor protein
- Hb
hemoglobin
- HIF
hypoxia inducible factor
- LHb
leghemoglobin
- LPS
lipopolysaccharide
- Mb
myoglobin
- Ngb
neuroglobin
- NO
nitric oxide
- Pgb
protoglobin
- PHD
prolyl hydroxylase
- RNS
reactive nitrogen species
- RONS
reactive oxygen and nitrogen species
- ROS
reactive oxygen species
- SSDgb
sensor single domain globin
- THb
truncated hemoglobin
- VEGF
vascular endothelial growth factor
1. A historical perspective – what is a globin?
Globins are a family of proteins, characterized by the myoglobin (Mb)-fold, a 3/3 sandwich of α-helices (A, B, C and E over helices F, G and H) and the presence of a heme prosthetic group (Fe2+-complexed protoporphyrin IX) ensconced within the hydrophobic cavity formed by the fold. The hallmark of sequence conservation between taxa lies within the systematic presence of a His at position 8 of helix F, which represents the principal axial binding site of the proximal heme iron [1,2]. Furthermore, the state of the sixth iron binding site subgroups globins into two categories: in the deoxygenated state, penta-coordinated globins are characterized by an empty binding site of the heme iron atom, favoring the binding of small ligands such as O2, CO and NO, whereas in hexa-coordinated globins, the iron atom is bound to the distal amino acid of the protein chain (usually a His or Gln in position 7 of helix E) [3]. Historically, the familiar vertebrate O2-binding hemoglobin (Hb), a tetramer of α- and β-globins, and Mb were among the first proteins whose sequences and structures were determined over 50 years ago [4,5]. At that time, the Hbs in non-vertebrate metazoans were investigated mostly in cases where their presence was visible. These included the larval Hb of the insect Chironomus [6], the intracellular Hb of the polychaete annelid Glycera [7], and the nodule leghemoglobin (LHb) from the nodule of the legume Lupinus [8]. Comparison of vertebrate and invertebrate Hb structures led to the identification of a highly conserved α-helical secondary structure, the Mb-fold, underpinned by a sequence motif based on the conservation of over 30, mostly solvent-inaccessible hydrophobic residues [9,10], a conservation that can be observed even in cases of <20% identity to vertebrate globins.
It should be pointed out that the 3/3 α-helical Mb fold is a physically stable protein motif, that is also found in proteins unrelated to globins. The Mb fold is for example found in the cyanobacterial light-harvesting phycocyanins and the bacterial toxin colicin A [11,12]. Furthermore, the DNA-binding domain of the bacteriophage repressor family is also structurally similar to the globin eight-helix fold [13]. Pediococcus pentosaeus (Bacilli) glutamate racemase displays substantial sequence homology (21–52%) with mammalian Mbs and can form an inactive stoichiometric complex with hemin [14]. The classical globin fold is also suitable for binding a variety of antibiotics, as indicated by the structure of a Streptomyces TipA class of multidrug resistance transcriptional regulators [15]. Moreover, a number of non-heme globin-like proteins have also been discovered. These include the Bacillus subtilis stress response regulator RsbR, where the G and H helices bend inward, eliminating the formation of a heme-binding cavity [16]. A dimeric globin fold that does not bind heme is adopted by the sensor domain of one of several histidine kinases that regulate Bacillus anthracis sporulation [17]. It appears to be a member of a large collection of sensor histidine kinases not only in Bacilli but also in additional bacterial taxa (Chlorobi and δ-Proteobacteria).
2. Diversity of globins in living organisms
In the pregenomic era from the 1960's to the 1990's, the number of vertebrate globin sequences and structures increased rapidly, leading to the discovery of many metazoan Hbs and Mbs [18,19]. New globins were also identified in prokaryotes, such as a Hb in the β-proteobacterium Vitreoscilla [20], and in plants, including symbiotic plant Hbs other than LHbs as well as nonsymbiotic plant Hbs [[21], [22], [23]]. This period also witnessed the discovery of flavohemoglobins (FHbs), chimeras of globin and reductase domains, in bacteria, e.g. E. coli [24] and Ralstonia eutropha [25] and in yeasts [26,27]. Concomitantly, globins shorter than normal (<130aa), were found in ciliates [28,29] and the cyanobacterium Nostoc [30]. By 1995, the ~700 vertebrate globin sequences could be aligned with about 100 nonvertebrate globins [1] based on the Mb-fold. At that juncture, it was evident that globin genes occur widely in living organisms [31].
The rapid accretion of genomic information in the next two decades revealed globins in organisms with no visible sign of their presence, such as nematodes [[32], [33], [34]] and insects [35]. Moreover, the genomes of some metazoans were shown to possess a large number of genes with globin signature. The more than 30 globin genes of free-living nematodes [36], the over 40 globin genes of the insect Chironomus [37], the multiple globins of the acorn worm [38] and echinoderm [39] genomes evince development-specific and tissue-specific expressions.
3. Genomic information reveals three globin families
Over the past 20 years, the availability of numerous sequenced genomes, new bioinformatics tools and the determination of globin crystal structures enabled to classify bacterial, archaeal and eukaryote globins into three families/lineages that belong to two structural classes: globins with the canonical 3/3 α-helical fold, and globins with a 2/2 fold [40]. Furthermore, within each family, globins can be either chimeric or single domain (i.e. non-chimeric) (Fig. 1).
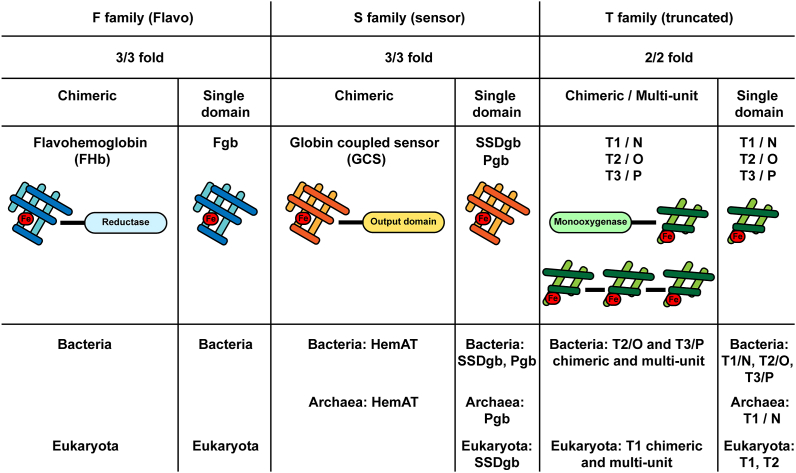
Fig. 1.
The three globin families. The F (Flavo) family, the S (Sensor) family and the T (Truncated) family can all be found in a chimeric or in a single domain configuration. T globins may further display multi-unit assemblies. Chimeric (FHb) and single-domain (Fgb) F globins are found in bacteria and eukaryotes, but absent in archaea, and are numerically preponderant. S family globins include chimeric globin-coupled sensor proteins (GCS) which carry a C-terminal output domain (including HemAT for aerotactic heme sensor), and sensor single domain globins (SSDgb) and their shorter version the protoglobins (Pgb). HemAT is found in bacteria and archaea, SSDgbs are found in bacteria and eukaryotes, and Pgb in bacteria and archaea. T family globins exist in three structural subfamilies, T1, T2 and T3, which, in bacteria, are also termed N, O and P, respectively. Chimeric and multi-unit T globins can be found in bacteria (T2/O and T3/P) and eukaryotes (T1), whereas single domain T globins appear in bacteria (T1/N, T2/O and T3/P), in archaea (T1/N) and eukaryotes (T1 and T2).
The first family, displaying the typical 3/3 fold, is the F (for flavo) family, comprising flavohemoglobins (FHbs) and related single domain FHb-like globins (Fgbs). F globins are numerically preponderant, and are present in a variety of bacterial groups, in all eukaryotes and plants, in protists and lower metazoans, but are absent in Archaea [[41], [42], [43], [44], [45], [46]] (Fig. 1).
The second family, which also displays a 3/3 fold, is the S (for sensor) family. This family includes globin-coupled sensor proteins (GCS), chimeric proteins displaying an N-terminal globin domain and a C-terminal output domain. Known and predictive GCS output domains include methyl accepting chemotaxis protein domains (MCP, including aerotactic HemAT for aerotactic heme sensor), kinase domains, diguanylate cyclase domains, adenylate cyclase domains, gene regulatory domains, and domains of yet unknown functions [[47], [48], [49], [50], [51]]. The family further includes the GCS-related sensor single domain globins (SSDgbs), and Protoglobins (Pgbs), which are themselves related to SSDgbs but shorter (~195aa) [[52], [53], [54]]. Although SSDgbs are found in primitive metazoans, such as bdelloid rotifers, the GCSs globins are generally limited to microbial organisms and some fungi [45,55] (Fig. 1).
The third family is the T (for truncated) family (THbs), which is characterized by a 2/2 α-helical fold, with an absent or abbreviated A helix, and a loop instead of the F helix [56,57]. The T family exists in three structurally distinct subfamilies, T1, T2 and T3 [58], which in bacteria, are also referred to as N, O, and P, respectively [59]. As for the two other families, T globins exist as chimeric and as single domain globins [60]. Furthermore, multi-unit T globins have been identified in silico, consisting of multiple 2/2 globin repeats [61]. T1 chimeric and multi-unit globin sequences have been detected in ciliated protozoan parasites, while T1 and T2 single domain globins occur in microbial eukaryotes (ciliates, stramenopiles, oomycetes, opisthokonts, etc.) and in green algae [62]. In plants, only T2 single domain globins are present [62,63]. So far, there has been no report of T globins in metazoans.
It has been proposed that eukaryote globins evolved from the respective bacterial lineage via horizontal gene transfer resulting from one or both of the accepted endosymbiotic events responsible for the origin of mitochondria and chloroplastids, involving an α-proteobacterium and a cyanobacterium, respectively [64,65]. Within this framework, all metazoan Hbs, as well as plant Hbs are likely to have emerged from a bacterial Fgb. This hypothesis has received experimental support from the crystal structure of a globin from the thermophilic bacterium Methylokorus infernorum, which was found to be closest to mammalian neuroglobin, despite a <20% identity in sequence [66]. Furthermore, it is evident that the F family globins that had one or more enzymatic functions in the early bacteria, evolved in multicellular eukaryotes to have new properties, including reversible binding of important diatomic ligands, such as O2, NO and sulfide, that enabled the development of transport and storage functions. Oxygen must be regarded as a friend and a foe at the same time. Thereby, it is likely that the Last Eukaryote Common Ancestor globin(s) served a purpose that was not related to oxygen transport and storage [67,68]. Indeed, the first organisms emerged in an oxygen-poor environment, whereby oxygen itself was probably representing a cellular threat that needed detoxification. In a second phase, following the appearance of mitochondria, oxygen emerged as an excellent driver of (aerobic) metabolism enabling a greatly enhanced energy production and thereby the development of larger organisms, which in turn needed a more efficient oxygen transport mechanism than solely by simple diffusion. Concomitantly, following the emergence of chloroplast and photosynthesis, the oxygen levels rose. Thus, living organisms needed both an oxygen-protective and subsequently an oxygen-transport mechanism to sustain the respiratory electron transfer chain. Globins fulfill both needs, and it is likely that their various functions represent a testimony of eukaryote and vertebrate evolution alongside Earth's environmental evolution.
4. Vertebrate globins
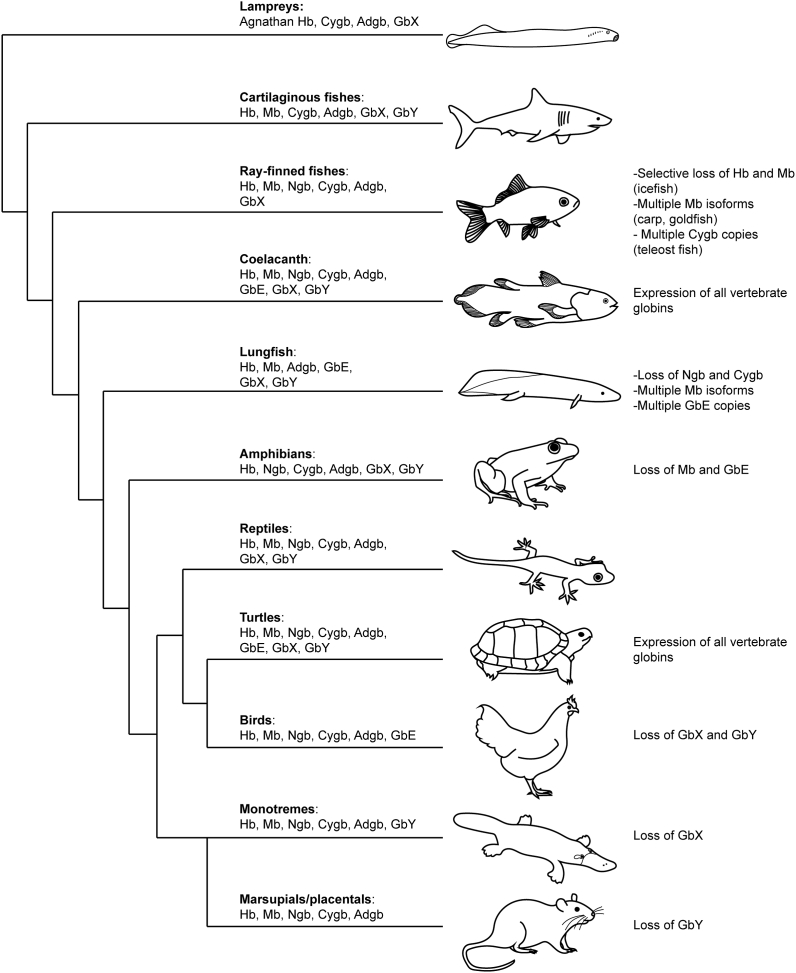
Genomic analyses have also considerably altered and greatly extended the structural and functional diversity of vertebrate globins through the discovery of 6 additional globin types, that all arose through gene duplication and sequence diversification [69]. 20 years ago, neuroglobin (Ngb) was discovered in nerve cells, followed rapidly by cytoglobin (Cygb) in fibroblasts [[70], [71], [72], [73]]. Restricted to certain taxa, globin Y (GbY) in amphibians and monotreme mammals [74], globin E (GbE) in the avian eye [75] and globin X (GbX) found in many metazoans but absent in birds and mammals [76] were further identified (Fig. 2). More recently, androglobin (Adgb), a large chimeric protein family completed the vertebrate globin repertoire [77]. Remarkably, from all vertebrates studied so far, only three species display expression of all 8 globins: the “living fossile” Latimeria chalumnae, a member of the coelacanth order of lobe-finned fishes, and closely related to lung fishes, the Chinese soft-shell turtle (Pelodiscus sinensis) and the western painted turtle (Chrysemys picta bellii) [78,79] (Fig. 2). These species, together with diverse other animals, represent invaluable models of vertebrate globin evolution, enabling to trace back through phylogenetic and syntenic analyses the gradual occurrence and separation of globin clades, thereby giving insights into their respective primary functions. It thus appears that Ngb, GbX and Adgb represent major clades, distinct from the other globin types because of their early emergence and their expression in both vertebrates and invertebrates [70,77,80,81]. This indicates that these globins emerged prior to the separation of Protostomia and Deuterostomia. Also, given this very early evolutionary origin, Ngb, GbX and Adgb likely had non-overlapping functions. Subsequently, Cygb was identified in the sea lamprey Petromyzon marinus [82], a member of the most ancient group of vertebrates, indicating that Cygb diverged from the other globin clades prior to the separation of Agnathan and Gnathostomata. Strikingly, these four globins all display hexa-coordination, suggesting that the hexa-coordinated state was possibly the original heme-binding conformation and simultaneously indicating that penta-coordination, the optimized O2-carrying configuration, evolved later. It is therefore speculated that Hb, Mb, GbE, and GbY belong to a separate clade of globins, whereby the coordination state of GbY is still unknown [69,80]. The retention, or inversely the loss, of certain globins across vertebrate taxa and species is still not fully understood, however it is likely that various parameters have influenced their presence or absence, including the adaptation to varying oxygen concentrations depending on the natural habitat of given species, functional redundancy which led to the disappearance of given globins in favor of others, or morphological changes rendering some globins indispensable or obsolete [83,84]. The study of species living in extreme conditions has given some insights into their functional plasticity. These include hypoxia-tolerant ectothermic vertebrates such as freshwater turtles and different species of the carp family of fish [85], species living at high altitude [[86], [87], [88]], in cold habitats [89], diving species [90,91], and animals living in underground tunnels [92,93]. An appropriate illustrative example is the expression of Mb in certain fish taxa. Indeed, the common carp (Cyprinus carpio) and the goldfish (Carassius auratus), both hypoxia-tolerant fish, express two isoforms of Mb [94,95]. While Mb1 resembles closely the classical muscle form of Mb with extended expression sites within the brain, kidney, liver and gills, the Mb2 isoform is exclusively expressed in the brain [94]. Functional analyses of these two isoforms have revealed that Mb1 displays a comparable O2 affinity and nitrite reductase activity as the regular vertebrate muscle Mb, whereas Mb2 bears lower O2 affinity and slower nitrite reductase activity, but shows a much faster H2O2 elimination rate, suggesting a specific function in ROS protection [96]. More extensive diversification of Mb genes exists in West African lungfish (Protopterus annectens). Lungfish harbour up to 7 distinct Mb genes that are differentially expressed in various tissues and it has been shown that the Mbs differ in their abilities to confer tolerance towards hypoxia [97]. Most recently, additional lungfish species (Protopterus dolloi, Protopterus aethiopicus and Lepidosiren paradoxa) were described to display a similar diversity of Mb genes and have orthologous Mb genes [98]. Functional studies have provided evidence for different O2 binding and enzymatic properties of lungfish Mbs, reflecting multiple subfunctionalization and neofunctionalization events that occurred early in the evolution of lungfish. It was also suggested that some Mbs may have also taken over the functions of neuroglobin and cytoglobin, which are widely expressed in vertebrates but appear to be missing in lungfish (Fig. 2). Another example of globin family members substituting for another and presumably assuming the latter's function is illustrated by the elephant shark with expression of α-globin in its nervous tissue whereas its genome appears to be devoid of Ngb [99]. In sharp contrast to the multiple Mb isoform harbouring genomes, certain Antarctic icefish species (Channichthyidae family) have completely lost the expression of Mb (and for some also Hb), whose absence is greatly compensated by an adapted cardiovascular system to ensure proper oxygen delivery to the tissues [[100], [101], [102]]. Notably, also frogs, stickleback fish and the marsupial opossum lack a Mb gene [83,103]. The presence and the functional variety of vertebrate globins is thus not limited to oxygen transport and storage, but also NO metabolism, ROS/RNS protection and detoxification, and signaling functions. It also becomes increasingly clear that globins display broader expression sites than previously anticipated (Fig. 3) and fulfil organ-specific functions. The role of each of the vertebrate globins, with special emphasis on mammalian globins and their non-canonical expression sites and according uncommon functions, will be detailed in the next chapters.
Fig. 2.
Vertebrate globins and their distribution. Schematic cladogram of the vertebrate globin repertoire. Notable differences in expression patterns are summarized on the right of each phylogenetic branch, including the selective loss of Hb and Mb in ray-finned fish subspecies or the selective duplication of Mb in ray-finned fish subspecies, and the duplications of Mb and GbE in lungfishes. Hb: hemoglobin, Mb: myoglobin, Ngb: neuroglobin, Cygb: cytoglobin, Adgb: androglobin, GbE: globin E, GbX: globin X, GbY: globin Y.
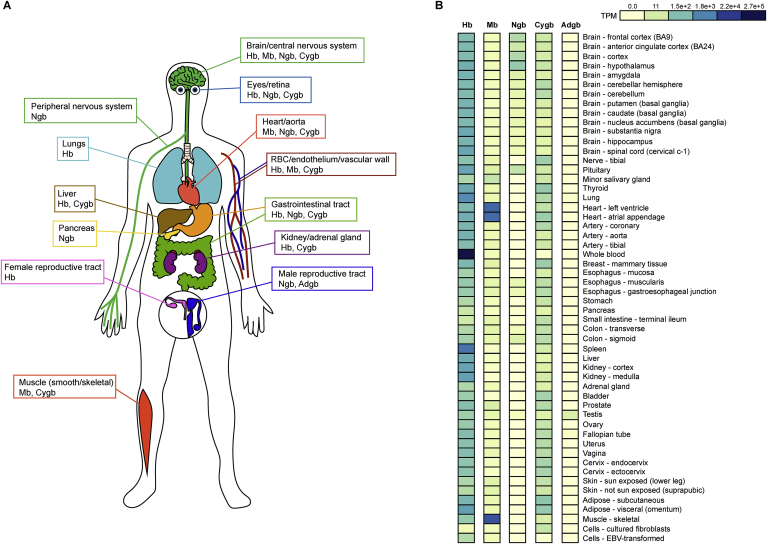
Fig. 3.
Human globin expression atlas. (A) Schematic representation of globin expression sites within the human body. (B) RNA-sequencing-based mRNA expression in TPM (transcripts per million) of Hb, Mb, Ngb, Cygb and Adgb within tissues from healthy donors. The dataset was generated and adapted from the Genotype-Tissue Expression (GTEx) project (https://www.gtexportal.org.com). Hb: hemoglobin, Mb: myoglobin, Ngb: neuroglobin, Cygb: cytoglobin, Adgb: androglobin.
5. Hemoglobin
5.1. General aspects
Beyond its well-established respiratory function, hemoglobin (Hb) with a familiar tetrameric α2β2 structure also plays a crucial function in NO metabolism. In particular, toxic NO can be converted by oxy-Hb to nitrate, whereas deoxy-Hb can reverse this conversion in conditions of hypoxia to induce blood vessel dilation [104,105]. In all jawed vertebrates (gnathostomes) except in the icefish family Channichthydiae [100] (Fig. 2), erythrocytes contain different types of hemoglobin chains throughout different developmental stages, which are differentially expressed during ontogenesis (the globin gene switching) [106]. All produce a first embryonic form in erythroid precursor cells derived from the yolk sac, some subsequently produce a fetal form in the liver, and all produce a “final” adult form in the bone marrow [106]. The different forms of developmental hemoglobins display varying affinities for oxygen, thereby ensuring a proper oxygenation flux from the mother to the embryo or the foetus [107]. The predominant adult forms of Hb display a heterotetrameric α2β2 structure, composed of two α-subunits (Hbα, HBA gene) and two β-subunits (Hbβ, HBB gene), whereby each subunit is bound to a heme prosthetic group. It has long been considered that Hb expression is exclusive to erythrocytes; however, several studies reported that α-globin and β-globin are also expressed outside of red blood cells [108] (Fig. 3). Importantly, while the HBB gene cluster, located on human chromosome 11, is in an inactive heterochromatin state in nonerythroid cells, the HBA gene cluster, located on human chromosome 16, has an open heterochromatin conformation in all known cell types [67].
5.2. Neuroprotective function in neurons of the central nervous system
The first evidence of nonerythroid Hb expression was reported in neurons, and the authors suggested that motor neuron degeneration could be linked to iron-catalyzed oxidation of neuronal Hb [109,110]. Thereafter, various groups started exploring the expression sites of Hb, and its potential relationship with different cerebral pathologies. Wu and colleagues detected Hb within pyramidal neurons of the hippocampus, parietal grey and white matter, and in cerebellum [111]. They further showed that Hb co-immunoprecipitates with Aβ oligomers in brain homogenates from Alzheimer disease (AD) patients, and that the expression of Hb is higher in AD brains than in control individuals [111]. Ferrer and coworkers reported an opposite expression profile of Hb in AD brains, as well as a downregulation in neurons [112]. Various other studies reported the same discrepancy, without providing a clear explanation despite a wide variety of plausible hypotheses [113]. In rodents, the full heme-bound Hb tetramer is found specifically in neurons of the cortex, hippocampus and cerebellum, but is totally absent in astrocytes and oligodendrocytes [114]. Additionally, Hb is upregulated by erythropoietin (Epo) in these cells and leads to a better oxygenation, supporting an Epo-dependent Hb function as neuroprotector against ischemic/hypoxic conditions [114]. In parallel, Richter and colleagues reported expression of Hbα and Hbβ in rodent nigral, cortical, and striatal neurons, and in cortex, basal ganglia, hypothalamus, hippocampus, cortical and thalamic dendrites and substantia nigra pars reticulata in rat and human brains [115]. Interestingly, systemic treatment of rats with rotenone, an inhibitor of mitochondrial complex I chain, strongly downregulated Hb mRNA expression in nigral dopaminergic neurons, pyramidal neurons and in GABAergic projection neurons of the striatum [115]. Various hypotheses were speculated on, including a possible function of Hb in mitochondrial respiration and regulation of the redox reactions, inactivation of Hif-1α due to mitochondrial complex I inhibition, or through the reduction of free heme, which is a strong activator of globin-chain transcription and whose synthesis takes place in the mitochondria [115]. Shortly thereafter, Biagioli and coworkers observed changes in gene expression related to O2 homeostasis and oxidative phosphorylation within A9 dopaminergic neurons (MN9D cell line) overexpressing Hba and Hbb, further linking Hb to mitochondrial function in neurons [116]. They confirmed expression of Hb in mouse, rat and human mesencephalic A9 dopaminergic neurons, however in contrast to another report [114], they also detected expression within cortical and hippocampal astrocytes and mature oligodendrocytes [116]. The authors concluded on a possible function of Hb as an O2 reservoir during anoxic or hypoxic conditions, thereby influencing mitochondrial function. They moreover suggested a possible link between mitochondrial Hb and the degeneration of A9 neurons observed in Parkinson's disease [116]. It was subsequently shown that in A9 neurons, Hb conserves its canonical α2β2 tetrameric structure in vitro and in vivo suggesting that neuronal Hb preserves some of its biochemical activities and biological functions associated to its role in the erythroid lineage [117]. In the case of Parkinson's disease, it was also shown that Hb forms insoluble aggregates in nucleoli and mitochondria of A9 neurons when overexpressed in vitro and in vivo and upon neurochemical mitochondrial stress [118]. This phenomenon was accompanied by decreased pre-rRNA transcription, inducing ultimately nucleolar stress and cell damage, as well as a decrease in the H3 methylation status [118]. Strikingly, mice overexpressing neuronal Hb displayed aggregates and motor behavior defects after only one month of vector injection. These observations led the authors to speculate on a function of Hb as energy status sensor of neurons, as well as on additional functions linking Hb to ribosome biogenesis, autophagy and epigenetic regulation [118]. Similar findings relating Hb to neuronal energetics and epigenetic changes to histones were proposed for multiple sclerosis, further supporting a mitochondrial-associated neuroprotective function of Hb, possibly in an erythropoietin-dependent manner [119,120].
5.3. Potential enzymatic functions in macrophages
Liu and coworkers reported expression of Hb in macrophages following stimulation with LPS and IFN-γ, both in vitro in the RAW264.7 cell line, and ex vivo after isolation of peritoneal macrophages from BALB/c mice [121]. Given that no increase in NO consumption was observed, the authors speculated on possible alternative enzymatic functions of Hb, or that Hb might serve as O2/NO sensor [121]. The expression of Hb in macrophages was confirmed a decade later by a second group, during granulation tissue formation in rats [122]. In this study, two peaks of Hbα and Hbβ mRNA were detected, a first peak identified as macrophages infiltrating the tissue, and a second peak that the authors hypothesized to be primitive precursor cells from the hematopoietic lineage [122].
5.4. ROS scavenging and cellular oxygenation of the eyes
Further evidence for nonerythroid Hb expression was found in the eye at the level of the lens [123] and of the retinal pigment epithelium [124]. In the lens, various hypotheses were proposed, including a participation in iron homeostasis via a chelating action of Hb, or as oxygen transporter/oxygen “sink” to avoid increased oxidation of nuclear proteins and ROS production in the very hypoxic environment of the lens. Both defective iron homeostasis and oxygen removal are connected to formation of cataracts, and surprisingly, increased cataract formation has been associated with sickle cell anemia and thalassemia patients [125,126], further suggesting a so far uncharacterized function of Hb in the lens. The expression of Hb was further confirmed specifically within lens epithelial cells, cortical lens fiber cells, but also in the corneal epithelium and retinal ganglion cells, however no heme group could be detected in these cells [127]. Gene expression analysis during the whole lens fiber differentiation process, concomitant with denucleation, suggested a possible function of Hb in apoptosis-like signaling processes required during lens differentiation [127]. In stark contrast with the lens, retinal pigment epithelial (RPE) cells require high amounts of oxygen [128], and primarily supply photoreceptors with oxygen and nutrients. Thereby, hypoxia-induced metabolic stress of RPE cells is sufficient to lead to photoreceptor degeneration, which is in turn associated with age-related macular degeneration [129]. Tezel and coworkers [124] were the first to observe expression of Hb in RPE cells, by analyzing the proteome of human donor eyes, and could furthermore reveal a basolateral secretion of Hb in vivo, supporting the hypothesis that Hb is required for O2 and CO2 transport between the RPE and photoreceptor cells, thereby supporting the high metabolic demands of the photoreceptors [124]. Finally, Hb expression was also reported in the retina and optic nerve head macroglia, as well as in retinal ganglion cells, with significant upregulation in hypertensive rat eyes and glaucomatous human eyes. The authors concluded that upregulation of Hb might serve as an intrinsic protective mechanism facilitating cellular oxygenation and free radical scavenging [130].
5.5. O2 sensing, NO scavenging and hypoxic protection of the lungs
Robust expression of α-globin and β-globin was further reported in lung alveolar epithelial cells (AECs) [131,132]. While comparing rat type I and type II AECs by DNA microarray analysis, Bhaskaran and colleagues [131] could detect significant expression of α-globin and β-globin specifically in type II AECs, which was lost upon differentiation of type II to type I AECs. Given the close proximity of type II AECs with environmental oxygen, the circulatory system and altered gaseous conditions, the authors proposed various functions of Hb within the epithelium, including facilitation of oxygen transport across the air-blood barrier, as O2 sensor, and as NO scavenger against oxidative and nitrosative stress [131]. This finding was confirmed shortly thereafter, with reported mRNA and protein expression in human Clara-like adenocarcinoma cells (H441), human ATII adenocarcinoma cells (A549), mouse alveolar epithelial cells (MLE-15) and rat primary ATII cells [132]. In addition to its respiratory functions, the authors suggested an intriguing hypothesis linking Hb's NO metabolism to respiratory pathologies [132]. This hypothesis was partially supported by the findings of Ishikawa and coworkers [133], who compared the proteomes of healthy lungs with lungs from patients suffering either from idiopathic pulmonary fibrosis (IPF) or chronic obstructive lung disease (COPD). Hb expression was dramatically decreased in IPF samples, but remained unaffected in COPD. Hb expression could also be detected in bronchoalveolar lavage fluid and sputum, suggesting that Hb is secreted from the lung tissue. A modified cysteine residue was further identified in a thiol group of the α-globin monomer in IPF samples, preventing the formation of Hb complexes [133]. Interestingly, type II AEC Hb expression is regulated by hypoxia as evidenced by Grek and colleagues [134]. Following 20 h of hypoxia or prolyl-4-hydroxylase (PHD) inhibition to mimic hypoxia, MLE-15 cells and primary ATII cells displayed robust induction of hypoxia inducible factor 2 (HIF-2α) together with upregulation of Hb mRNA and protein levels. The authors could further demonstrate that the hypoxia-induced Hb upregulation is GATA-1-dependent. Furthermore, hypoxia also reduced the expression levels of pro-surfactant proteins. These results suggest that HIFs and Hb might jointly be involved in the homeostatic protection of the lung epithelium against hypoxic injury, as observed with edema and subsequent hypoxia [134].
5.6. Localized expression in gut villi
While studying the spatio-temporal expression of Hb during lens formation and its link to early embryonic development, Mansergh and coworkers used gut sections to visualize normal Hb expression in erythrocytes within a strongly vascularized organ [127]. Unexpectedly, they noticed a small number of cells within villi epithelia that stained positive for Hb, and that were neither associated with stem cells nor with erythrocytes, suggesting a further moderate site of Hb expression within the gut [127].
5.7. Hb might function as heme/iron buffer in the endometrium
In the endometrium, which is particularly sensitive to oxygen and iron, Hb expression was detected in all phases of the menstrual cycle in human biopsies. The expression of Hb was not influenced by steroid hormones, neither by hypoxia during the M-phase of the cycle during which the oxygen levels are the lowest [135]. Moreover, heme oxygenase 1 (HO-1), one of the main heme-neutralizing enzymes, was equally detected in epithelial cells of the endometrium. The authors discussed the potential existence of an endometrial Hb-HO-1-system as buffer to regulate the supply of heme and iron to endometrial cells due to their critical iron requirements for proliferation [135]. They also suggested a possible oxygen-regulatory function of Hb and HO-1 during the first trimester of embryo development taking place under very low oxygen concentrations [135].
5.8. Antioxidative function in kidney
In the kidney, the major oxygen-sensing organ in the human body, Hb expression was reported in glomerular mesangial cells by in situ hybridization and immunofluorescence. Additionally, expression profiles of genes and proteins differentially regulated between normoxic and hypoxic rat kidneys revealed transient upregulation of Hbα and Hbβ following hypoxia. Furthermore, overexpression of α- and β-globin in rat primary mesangial cells significantly inhibited ROS generation following stimulation with H2O2 [136]. Similarly, induction of Hbα and Hbβ was also reported in HEK293 cells following treatment with H2O2 [137], further supporting an antioxidative function of Hb in renal cells [136,137].
5.9. Oxidative stress protection in liver
In a study focusing on the pathogenesis of non-alcoholic steatohepatitis (NASH), strong induction of Hbα and Hbβ was detected by microarray analysis in liver biopsies from NASH patients [137]. The expression of Hbα and Hbβ was confirmed in HepG2 cells, that displayed enhanced levels following H2O2 treatment, and similarly to mesangial cells, overexpression of Hbα and Hbβ protected HepG2 cells from oxidative stress. The induction was not correlated with hematopoietic transcription factors, suggesting expression in non-erythroid cells. Immunofluorescence revealed positive staining of Hbα and Hbβ in hepatocytes of NASH liver biopsies. Interestingly, stimulation of cells with H2O2 reduced the expression of GATA-1, suggesting a different mechanism of Hb regulation during oxidative stress as observed following hypoxia [137].
5.10. NO-mediated vascular tone regulation within the endothelium
Robust expression of Hb was furthermore described in systemic arterial endothelial cells, and particularly enriched at the myoendothelial junction [138]. In these cells, a specific role for Hbα was found on the regulation of NO-mediated vascular reactivity, which is dependent on the heme iron redox state. In the Fe3+ state, NO signaling is active and allows diffusion of NO to smooth muscle and vasodilation, whereas following reduction to Fe2+ by the endothelial cytochrome B5 reductase 3, NO signaling is abrogated via Hb-mediated scavenging of NO [138]. Of interest, within endothelial cells, Hbα colocalizes with eNOS in a macromolecular complex, with which it can form direct protein-protein interactions [138]. The importance of Hbα/eNOS coupling was demonstrated ex vivo by using a mimetic Hbα peptide, in which the interaction sequence was mutated, resulting in altered vasoconstriction [139]. Injection of the mimetic Hbα peptide into hypertensive mice caused a significant decrease in blood pressure, and no effect was observed in eNOS knockout mice, hence confirming the importance of the complex formation for NO scavenging [139]. The complex was further expanded with the identification of α hemoglobin stabilizing protein (AHSP) as a required chaperone for the stabilization of Hbα monomers prior to its reduction by eNOS [140]. Recently, Hbα was also detected in pulmonary endothelial cells, where an association with pulmonary hypertension and enhanced Hbα was described [141].
5.11. Vaginal and cervical antimicrobial and anti-inflammatory function
Several studies confirmed expression of Hb also in vaginal epithelial cells [108,[142], [143], [144]]. The first indirect evidence of Hb expression in vaginal epithelial cells was reported in a study focusing on the identification of antimicrobial peptides in rabbit vaginal lavage [142]. One of the peptides (RVFHbαP) was subsequently designed from Hbα, and could be robustly detected in vaginal sections within glandular epithelial cells. The result was later confirmed using full-length Hbα and Hbβ antibodies [108]. The peptide also showed activity against various microbial strains, and was able to bind LPS, suggesting an anti-inflammatory effect [142]. Li and coworkers reported expression both in normal and cancerous human cervical tissue, as well as in the SiHa and CaSki cervical cancer cell lines [143]. They further reported increased Hbα and Hbβ expression in cancerous tissue and following treatment of cells with H2O2. The overexpression of Hbα and Hbβ in these cell lines reduced the production of ROS and increased cell viability upon H2O2 treatment, which led the authors to speculate on an antioxidant function of Hb in cervical cancer cells [143]. Saha and colleagues investigated the expression and function of Hb in a human immortalized vaginal epithelial cell line (VK2/E6E7) and in human primary vaginal epithelial cells (hPVECs), and could confirm Hb expression at the mRNA and protein levels in both cell lines [144]. The expression of Hb was significantly increased by LPS, alongside with enhanced NF-κB. Consistently, following inhibition of NF-κB, the expression of Hb was back to normal levels, and direct binding of NF-κB to the Hbα promoter was identified by chromatin immunoprecipitation. These results indicate that Hb may have antimicrobial effects, and act as an NF-κB-dependent defense protein against vaginal infections [144].
5.12. Potential cytoprotective effect on cancer cells
Evidences for Hb expression in various cancer types have been reported. Gorr and colleagues observed Hb expression in breast tumors, but not in normal breast tissue [145]. Further Hb expression in cancer cells was reported in cervical carcinoma cells [143], glioblastoma cells [146] and metastasis-competent circulating tumor cells [147]. All studies concluded on a potential cytoprotective effect of Hb on the cancer cells, conferring them resistance to oxidative stress and possibly against some forms of chemotherapies.
6. Myoglobin
6.1. General aspects
Mb is a monomer, mainly found in the cytoplasm, whose established function is the transport and facilitated diffusion of oxygen from the sarcolemma to the mitochondria of skeletal and heart muscle in almost all vertebrates [148,149]. Mb also serves an oxygen-storage function: in terrestrial mammals, Mb functions as short-term oxygen storage molecule in the heart during normal systole when the blood flow is temporarily interrupted; in diving mammals and birds, high concentrations of Mb guarantee sufficient oxygen storage capacity for long-term diving performances [[150], [151], [152], [153]]. Similar to Hb in erythrocytes, Mb was long believed to be exclusively expressed in the striated muscle, i.e. restricted to the heart and skeletal muscle, but various studies have reported expression of Mb also in other cell types, including vascular smooth muscle in mammals [154] (Fig. 3). As mentioned before, all vertebrates, except for amphibians and some species of icefish [74,83,100], express at least one isoform of the Mb gene (Fig. 2). Multiple non-mammalian vertebrate model organisms have been used so far to explore the functions of Mb, which can vary greatly depending on the animal's specializations. In these model organisms, expression of Mb was also reported in kidney, liver, gills, brain and endothelial cells [149]. It was long thought that Mb serves only as oxygen storage protein, however, various other functions have been described in vertebrates, which will be further discussed in the next paragraphs.
6.2. NO scavenging and production in heart and vascular smooth muscle
Apart from its oxygen storage and supply functions, another key function of Mb is its regulation of NO and ROS levels within the cell in response to O2 homeostasis. In the heart, excessive build-up of NO can impair cardiac functions leading to heart failure [155]. As part of its cardioprotective role, Mb acts as an NO scavenger, where oxygenated Mb (oxy-Mb) converts NO into NO3- while itself being transformed to met-Mb, having the heme iron in ferric state [156,157]. Additionally, the versatility of Mb in NO homeostasis is illustrated by its ability to switch from NO scavenger to NO producer in normoxic and hypoxic cellular environments, respectively [96,[158], [159], [160], [161], [162]]. Deoxygenated Mb (deoxy-Mb) acts as a hypoxia-dependent nitrite reductase, that along with other cellular nitrite reductases, regulates cell energetics in response to low oxygen levels. One prominent importance of NO is the O2-homeostasis regulated vasodilation resulting from the generation of NO by Mb in the vascular wall. Although Mb is not the sole NO producer in vascular smooth muscles, Mb-deficient mice display impaired blood vessel dilation, with enhanced vasodilation upon NO treatment, thereby evidencing the importance of Mb as NO producer [157,[163], [164], [165]]. In addition to smooth muscles, the Mb-generated NO in cardiomyocytes is important for cardio protection following cardiac ischemia-reperfusion (I/R) injury as it minimizes mitochondrial cellular respiration and hence limits ROS generation and reduces oxidative damage [166].
6.3. Cardiac and neuronal regulation of ROS
Besides NO, Mb also directly regulates ROS homeostasis, acting as both antioxidant and pro-oxidant. Mb exhibits catalase- and peroxidase-like activities thereby neutralizing the ROS-generating H2O2 while itself being converted from ferric-heme to ferryl-heme Mb [[167], [168], [169], [170]]. The role of Mb in cardiac antioxidant defense is observed in Mb knockout mice that show poor adaptation to oxidative challenge, including higher ROS in the heart upon cardiac I/R injury and slow recovery from ischemic insult [171]. Additionally, the importance of Mb as antioxidant was also reported in non-muscle cells including the neuron, which upregulates Mb upon beta amyloid peptide ROS insult [172]. Meanwhile, the brain-specific Mb2 isoform in carp is shown to be active in eliminating H2O2 [95,96]. Similar to Hb, Mb can also elicit oxidative stress via the release of ‘reactive iron’ from the heme group, that catalyzes Haber-Weiss reaction to produce damaging ROS, mostly OH radicals [173,174]. Reasonably, the oxidative assault by Mb is mostly associated with pathological conditions such as cancer and muscle damage. Muscle damage resulting from burn of injury releases Mb into the bloodstream, which can be glycated and accumulate in the kidney. The heavy release of iron (more with glycation) from Mb induces free radical damage which often results in acute renal failure [[175], [176], [177], [178]].
6.4. Fatty acid metabolism in skeletal and cardiac muscle
Another unconventional function of Mb is its role in fatty acid metabolism in both skeletal and cardiac muscles [166,179]. This function stems from its ability to bind and transport long-chain fatty acids (FAs) including oleic and linoleic acids, in its oxy-Mb form but not the deoxy-Mb form. Whereas the O2 binding on Mb is required for FA binding, the binding of FA has no effect on the O2 binding [[180], [181], [182], [183]]. The high energy demand from the perpetual beatings of the heart is primarily satisfied by lipid metabolism, and the highly expressed Mb in cardiomyocytes is involved in FA oxidation for energy generation [184,185]. The prominent role of Mb in cardiac FA metabolism is evident from the biochemical shifting from FA to glucose oxidation with concomitant upregulation of GLUT4 and glucose uptake in Mb-deficient mice [186]. Additionally, high-lipid diet in rats induces an increase in total Mb protein, similar to the upregulation of Mb in lipid-supplemented C2C12 cells and Weddell seal cells in vitro [179,187]. In this context, Mb facilitates the transport of FA to mitochondria for β-oxidation and energy production. Inversely, Mb-deficient mice display abnormal accumulation of lipid deposits in the heart with compromised heart performance [185]. Therefore, Mb seems to be also crucial for energy supply in the heart and to regulate and prevent cardiac lipotoxicity.
6.5. Antiproliferative function on cancer cell progression
Mb is also found to be ectopically expressed in several cell types and malignancies, specifically in many epithelial-derived cancer cells including breast cancer [145,[188], [189], [190], [191]], prostate cancer [191,192], non-small cell lung adenocarcinoma [193], renal cell carcinoma [194], head and neck squamous cell carcinomas [195], ovarian cancer [188], and colon cancer [188]. Mb expression status positively correlates with the overall survival rate of breast cancer patients [189], and patients with gleason score 8–10 prostatic carcinoma [192]. The expression of Mb in breast cancer cell lines and biopsies positively correlates with established hypoxia markers CAIX, FASN, VEGF and hypoxia-inducible factor HIF-2α, but not HIF-1α [189,190,192]. HIF-1α is instead found to be regulating Mb mRNA expression levels by binding to a remote enhancer element located ~2.7 kb upstream of the Mb gene, upon experimental hypoxia [190,191]. Hypoxia-induced Mb expression regulation was reported in breast, prostate and colon cancer cells [196]. These data suggest that the hypoxic tumor microenvironment contributes to the upregulation of Mb in cancer cells. In contrast, in hormone-dependent breast and prostate cancers, Mb expression was shown to be downregulated via the recruitment of repressor complex to the Mb promoter as mediated by estrogen and androgen, respectively, that bind to varying distal Mb regulatory elements [189,191]. Surprisingly, nearly twenty Mb splice variants have been identified in human, and 12 are found to be expressed in human cancer cell lines [197,198], with variants 9, 10, and 11 expressed from a hypoxia-inducible tumor-associated promoter [191,198]. Expression of Mb in cancer cells is related to its tumor inhibiting functions. In breast cancer cell lines, it was elucidated that the Mb-catalyzed production of cellular ROS exerts high oxidative stress on Parkin, a mitochondrion E3-ubiquitin ligase, which is then oxidized and degraded. The degradation of Parkin, safeguards mitofusin 1 and 2 (MFN1 and MFN2) from ubiquitination-mediated degradation. MFN1 and MFN2 are proteins localized at the outer mitochondrial membrane that promote mitochondrial fusion. Their upregulation thus leads to mitochondrial hyperfusion, which subsequently halts cell cycle progression at G1 to S phase transition [199]. Hence, Mb expression in cancer cells might attenuate cancer cell proliferation. Such oxidative-based counteraction of Mb in cancer is also hypothesized by a transcriptomic-based study which proposed that Mb-mediated ROS- and NO- production regulates specific factors in cancer cells. This includes phosphorylation of p53, which regulates its downstream target genes, resulting in cell cycle arrest and apoptosis. Furthermore, oxidative stress also inactivates PHD2 which subsequently safeguards intact HIF-α, leading to downstream shifting in metabolic activities in a cell-specific manner [196]. In these processes, Mb-dependent oxygen binding and oxidative stress generation are crucial, while any free radical scavenging activity can potentially impede the process [200]. Collectively, Mb might assume positive roles in slowing cancer progression. Most recently, targeted delivery of O2-bound myoglobin was suggested to be an effective approach to enhance the efficacy of radiotherapy via increasing intracellular oxygen levels [201].
7. Neuroglobin
7.1. General aspects
Neuroglobin (Ngb) emerged in early Metazoans around 800 million years ago from a common ancestor to Adgb and Globin X [69,77] and is found in vertebrates including ray-finned fishes, coelacanths, amphibians, lizards, turtles, birds, monotremes, marsupials and mammals, with the exception of lampreys, cartilaginous fishes and lungfishes [69,202] (Fig. 2). A number of studies have reported globin-like proteins localized to neurons of lower organisms, but it remains to be determined if these represent genuine orthologs of vertebrate Ngb [[203], [204], [205]]. Ngb is a monomeric protein that reversibly binds oxygen with a reported P50 ranging from 0.9 to 10 torr and displays a hexa-coordinated scheme [70,206,207]. Initially identified in rodent and human brain tissues [70], Ngb is expressed in the central (olfactory bulb, solitary tract nucleus, cerebellum, amygdala, hippocampus, thalamus and grey substance of spinal cord) and peripheral (sensory and autonomic ganglia) nervous system, with particularly high expression in the hypothalamus, in astrocytes, but also in the gastrointestinal tract, in endocrine tissues such as the pituitary gland, adrenal glands and islets of Langerhans, in the photoreceptor inner segments of the retina, retinal pigment epithelium, inner and outer plexiform layers, the retinal ganglion cell layer, inner and outer nuclear layers, and the optic nerve, as well as in the heart and testes [[208], [209], [210], [211], [212]] (Fig. 3). Surprisingly, in stickleback fish, Ngb is expressed in erythrocytes at higher levels than Hb [213]. Finally, several studies reported that Ngb localizes mainly in the cytosol, the inner wall of mitochondria and in the nucleus [[214], [215], [216], [217]].
Despite two decades of extensive research efforts on Ngb and a broad array of tools developed [218], its function still remains unclear. In the following paragraphs we will briefly discuss the transcriptional and posttranscriptional mechanisms regulating Ngb, and focus on the physiological processes in which neuroglobin has been suggested to be involved.
7.2. Hormone-dependent regulation of Ngb in the central nervous system
Different studies reported that several hormones and growth factors can modulate the expression of Ngb. In mouse, vascular endothelial growth factor (VEGF) increases Ngb protein expression in cortical neurons, which in turn induces a negative feed-back loop inhibiting VEGF expression [219]. Another study showed that thyroid hormone T3 supraphysiological administration in rats induces Ngb mRNA and protein expression in cortex, cerebellum and hippocampus of thyroidectomized rats [220]. Administration of 17β-estradiol (E2) was also reported to upregulate Ngb levels in mouse hippocampal neurons and primary cortical astrocytes [221,222]. Interestingly, whereas no estrogen response element is found within the Ngb promoter, Guglielmotto and colleagues demonstrated that E2 induces the recruitment of estrogen receptor alpha (ERα) to the first intron of Ngb that acts as an enhancer element, which in turn induces chromatin remodeling and leads to enhanced Ngb expression [223]. In a model of brain-induced ischemia in gerbils, administration of recombinant human neuronal erythropoietin resulted in Ngb upregulation and reduced neuronal death [224]. However, whether this upregulation is mediating the observed protective effects remains unclear.
7.3. Oxidative stress protection in brain and heart
Using purified recombinant neuroglobin and human neuroblastoma SH-SY5Y cells, Jayaraman and colleagues investigated Ngb posttranscriptional modifications occurring under stress conditions, by mimicking an ischemic insult by serum and glucose deprivation under normoxic or hypoxic conditions [225]. Interestingly, they demonstrated that under low oxygen content, Ngb phosphorylation is increased in an ERK- and PKA-dependent manner, which in turn induces 14-3-3 protein binding and prevents its dephosphorylation. Moreover, functional characterization showed that phosphorylation of Ngb substantially enhances its nitrite reductase activity, emphasizing an adaptive mechanism that may be triggered in ischemic conditions to protect the brain [225]. Of note, Ngb was shown to directly promote 14-3-3γ expression in a yet unknown way [226,227]. In line with these studies, overexpression of Ngb in several pathogenic cardiac models, including ischemia and isoproterenol-induced cardiac hypertrophy, resulted in reduced apoptosis, inflammation, hypertrophy, infarct size and promoted cardiomyocyte survival by acting on reactive oxygen and nitrogen species levels and thereby reducing oxidative stress [228,229]. Intriguingly, Tae and coworkers recently reported that rats exposed to side stream cigarette smoke displayed increased levels of Ngb, and to a lesser extent Cygb, in cerebral cortex and hippocampus, and suggested a role in the oxidative stress response [230].
7.4. Oxidative stress sensing and recruitment of Ngb to membrane lipid rafts
During oxidative stress, generation of ROS, and especially NO induces Ngb transition from ferrous (Fe2+) to ferric state (Fe3+) and conformational changes, and results in NO3- production [231,232]. Unlike other globins, Ngb was shown to lack catalase and peroxidase activity [233]. Intriguingly, Watanabe and coworkers showed that in PC12 and SH-SY5Y cells overexpressing Ngb H2O2-induced oxidative stress resulted in Ngb recruitment to membrane lipid rafts, which significantly increased cell survival as compared to Ngb-devoid cells [232]. Moreover, addition of methyl-β-cyclodextrin (MβCD), a lipid raft-disrupting agent, strikingly reduced cell viability that was restored after cholesterol addition, emphasizing the importance of Ngb recruitment to these membrane domains to exert its protective effects. Furthermore, under its ferric state, but not ferrous, Ngb binds flotillin-1, a protein shown to recruit signaling complexes to lipid rafts and to mediate transduction of several cellular processes [234,235]. In addition, Watanabe and colleagues demonstrated that in these lipid rafts Ngb, in its oxidized state, binds the GDP-bound Gαi/o and acts as guanine nucleotide dissociation inhibitor (GDI), preventing ROS-induced Gαi/o activation and favouring neuroprotective cAMP signaling [232], therefore strongly suggesting a role as oxidative stress sensor for Ngb.
7.5. Inhibition of apoptosis in neurons and retinal ganglion cells
Several studies reported that Ngb may exert its protective effects by directly acting on the intrinsic apoptotic pathway. Under oxidative stress, Ngb can bind to huntingtin (HTT) and shuttle from the cytosol to the mitochondria [236], where it associates with mitochondrial voltage-dependent anion channel 1 (VDAC), a constituent of mitochondrial permeability transition pore (mPTP), preventing mitochondrial cytochrome c release [237,238], in primary mouse cortical neurons under oxygen-glucose deprivation/reoxygenation conditions. Moreover, Ngb can directly interact, scavenge and reduce the heme iron of cytochrome c [238,239], thereby preventing cellular death [240]. Interestingly, Ngb silencing experiments performed in rat retinal ganglion cells (RGCs), as well as in vivo shRNA knockdown in rat eyes, resulted in reduced RGCs number and nerve fibre density, impaired visual ability and strongly affected mitochondrial respiratory chain complex I and III activity [241]. These effects might be explained by the association of Ngb with electron-transferring flavoprotein alpha (ETfα) that is part of the mitochondrial respiratory chain [237], which may, in a yet unknown manner, favor mitochondrial function and prevent unwanted ROS production. In another study, Khan and coworkers used isolated neurons from transgenic mice overexpressing Ngb under the control of the chicken β-actin promoter, previously shown to protect mice from brain and heart ischemia by induction of endothelial NO synthase (eNOS) in vascular endothelial cells [242]. Under hypoxic conditions, they demonstrated that the RhoGTPase Rac1, maintained in an inactive state by Rho-GDI association, is released, activated and relocated inducing actin polymerization, cytoskeletal and mitochondrial aggregation, which is prevented in cells overexpressing Ngb [243]. Moreover, Ngb associates with p21 activated kinase 1 (Pak1), a kinase promoting GDI-Rac1 dissociation, and prevents its activity [243]. Finally, several studies reported an association of Ngb with Akt and its upstream phosphatidylinositol modulator, 3,4,5-trisphosphate 3-phosphatase (PTEN), which resulted in neuroprotection and less caspase activity [[244], [245], [246]].
7.6. Regulation of ATP production in brain and heart
Beyond its role in inhibiting apoptosis, a recent study in an immortalized murine hippocampal cell line (HT-22), demonstrated that overexpression of Ngb promotes ATP production and that lipid and glycogen content was increased [247]. These effects were confirmed in heart tissues of Ngb-overexpressing transgenic mice. Moreover, they showed that these effects rely on the inhibition of AMP-activated protein kinase (AMPK) signaling, a kinase promoting catabolism, and activation of acetyl-CoA carboxylase (ACC) in brain and heart tissues of mice overexpressing Ngb [247]. These evidences support a role of Ngb in the regulation of ATP production by promoting cellular anabolism through its inhibitory effect on AMPK, though how Ngb achieves this inhibition still remains unclear.
7.7. The controversial function of Ngb during cancer progression
While neuroglobin's antioxidative functions have been extensively studied, its role and expression in cancer are still largely debated [145,248]. Cancer cells are able to survive in conditions of high intracellular levels of ROS, which could influence the cell fate by activating apoptosis or mechanisms of aberrant adaptation causing augmented cell proliferation, immortalization, metastasis and chemo-resistance. Currently, many groups are focusing on the identification of the survival factors and pathways involved in the cancer resistance and adaptation to high ROS levels. Increased Ngb levels have been found not only in brain cancer [249,250] but also in primary tumors and cancer cell lines of different origin than the central nervous tissue, as highlighted by Emara and colleagues who analyzed by microarray various human solid tumors [249]. These included, among others, ovarian, hepatocellular and breast carcinoma, for which they further showed Ngb-stained tumor sections that displayed distinct staining patterns with uniform expression in stromal tissue, regions of distinct cell types, and intensive focal areas [249]. The complexity of Ngb expression in cancer was also addressed in non-small cell lung cancer with focus on the two histological subtypes adenocarcinoma and squamous cell carcinoma [193]. Interestingly, despite being overexpressed in both cancer types, Ngb was more frequently overexpressed in squamous cell carcinoma. They further demonstrated that in 30.8% of lung cancers, the promoter of Ngb was hypermethylated, which paradoxically suppresses its mRNA expression. The methylation status did not appear determinant on Ngb expression in adenocarcinoma, while it had profound consequences on Ngb expression in squamous cell carcinoma. Various molecular mechanisms have been proposed for the role of Ngb in cancer progression, however whether Ngb is pro- or anti-oncogenic is still controversial. Indeed, it has been reported that Ngb displays antiapoptotic, antioxidant, but also antiproliferative functions. One suggested molecular mechanism behind the Ngb-mediated cancer cell protection hypothesizes the direct activation and involvement of the PI3K/AKT pathway as investigated in breast cancer and glioma [216,[251], [252], [253]]. In these cells, ERα-mediated PI3K/AKT over-activation ultimately leads to Ngb overexpression and Cyt-c scavenging, preventing the Cyt-c-mediated formation of the apoptosome. As a consequence, the cancer cells are simultaneously protected from caspase-mediated cell death, and from ROS via the overexpression of Ngb [216,[251], [252], [253]]. The combined antioxidant and antiapoptotic action of Ngb was further demonstrated in glioma cell lines and tissue, where the authors reported that under conditions of oxidative stress, Ngb overexpression protects U87 glioma cells from apoptosis [253]. The same group proposed Ngb as prognostic marker for patients affected by glioma, for its positive correlation was associated with the histological grade of the cancer and survival rate [254]. The tumor grade-dependent expression of Ngb was confirmed in an independent study, that additionally reported a negative correlation with PPARγ expression, leading to loss of Ngb upon PPARγ overexpression and vice-versa. It was suggested that enhanced Ngb might protect the glioma cells from cell death and at the same time partially reverse the pro-apoptotic and anti-proliferative actions of PPARγ [251]. An alternative molecular mechanism has been postulated in hepatocellular carcinoma (HCC), where Ngb has been found downregulated in human tumor tissue [255]. Ngb knockdown in the HCC cell line HepG2 enhanced cell growth and proliferation, whereas its overexpression both in HepG2 cells and in vivo suppressed cell growth and tumorigenicity. Ngb overexpression was found to coincide with abolished Raf/MEK/Erk signaling, an effect that could be reversed upon mutating the oxygen-binding site (H64L) of Ngb, thereby linking oxygen/ROS signaling to the Raf/MEK/Erk pathway [255].
These studies collectively illustrate that the cancer-related regulation of Ngb represents a yet-to-be-determined complex pathway, involving histological, hypoxic and epigenetic aspects, revealing Ngb to be an intriguing potential target for the development of anticancer therapies. Most recently, an additional unexpected therapeutical role of Ngb was described based on its potential function as an antidote for CO poisoning [256,257].
8. Cytoglobin
8.1. General aspects
Cygb, together with Hb and Mb, likely originated from a cellular globin ancestor present in vertebrates around 450 million years ago. Indeed, it is speculated that Cygb and Mb share a common phylogenetic clade, and that Cygb and Mb represent paralogous regions of genomic DNA (respectively regions 17q25 and 22q12 in humans), as a result of a duplication event [72]. In most vertebrates, a single Cygb gene has been found, including in mammals, birds and frogs. However, teleost fish, a sub-class of the ray-finned fish, express two Cygb genes, Cygb-1 and Cygb-2, with distinct amino acid sequences and tissue expression patterns, with a preferential neural expression for Cygb-2, whereas the expression of Cygb-1 seems to be much more ubiquitous [83,258,259] (Fig. 2). The function of this duplication is still unclear, as the physiological role of the paralogs remains unknown. However, recent studies have shown that the functions of Cygb-1 and Cygb-2 might even be species-specific, as well as tissue-specific [260]. In this line, Corti and collaborators have suggested that Cygb-2, which is most orthologous to mammalian Cygb might play a role in the protection against ROS, NO deoxygenation, nitrite reduction and also protein-protein interactions, whereby an oxygen carrier function seems less likely, except in specific tissues such as the retina. On the other hand, Cygb-1 appears to be functionally and structurally different from its paralog. Indeed, Cygb-1 displays a penta-coordinated heme group, and is robustly expressed in zebrafish red blood cells where it might function as an oxygen carrier when oxygen concentrations are low [261,262].
In mammals, the tissue expression profile of Cygb has been extensively studied [263] (Fig. 3). Cygb is predominantly expressed in fibroblasts and related cell types, but also in distinct nerve cell populations [264]. Its precise physiological function, however, remains unclear.
8.2. NO-dependent regulation of vascular tone
The role of Cygb in NO metabolism has been addressed in a large number of studies [265]. Strikingly, Cygb displays the highest rate of NO dioxygenation of all mammalian globins, and shows a faster reduction rate than other globin types in presence of reducing agents such as ascorbate, P450 reductase or b5/cytochrome b5 reductase [[266], [267], [268], [269], [270]]. The first in vitro evidence for a function of Cygb in NO dioxygenation was provided by Halligan and coworkers, who demonstrated that following stable shRNA-mediated knockdown of Cygb in NIH-3T3 cells resulted in significantly decreased cellular NO consumption and intracellular nitrate formation. This effect could be reversed by re-expression of human full-length cytogobin [271]. Interestingly, the NO dioxygenase activity of Cygb is O2-dependent, and rapidly drops under physiological hypoxia [267]. However, under anaerobic/anoxic conditions, Cygb's function switches from NO dioxygenation to nitrite reduction, thereby rather promoting perfusion and tissue oxygenation, which supports a role for Cygb as an important sensor and regulator of NO in the maintenance of vascular homeostasis in an oxygen-dependent manner [272]. In line with these studies, expression of Cygb was robustly detected in rat aortic adventitial fibroblasts, rat aortic medial smooth muscle, and in rat, mouse and human aortic vascular smooth muscle cells, which drove the authors to conclude on an important function of Cygb in the NO-dependent regulation of the vascular tone [271]. Liu et al. recently provided in vivo evidence for a role of Cygb as regulator of NO degradation and cardiovascular tone in the vascular wall using a Cygb-deficient mouse model [269]. Lilly and coworkers further suggested an involvement of Notch signaling in the regulation of Cygb within endothelial cells, thereby facilitating the ability of smooth muscle cells to metabolize NO [273].
8.3. Antioxidative function in liver, retina and kidney
Cygb was furthermore proposed as protector against oxidative stress [265]. Various in vitro studies indicated that Cygb is able to scavenge free radicals, and overexpression of Cygb in renal immortalized fibroblasts [274,275] as well as in various other cellular models preserves cell viability under conditions of oxidative stress [[276], [277], [278], [279], [280], [281]]. Moreover, Thuy le et al. [317] confirmed the antioxidant function of Cygb using a global knockout mouse model which displays age-dependent development of multiple organ abnormalities. Recently, the same group further validated the antioxidative role of Cygb by establishing a Cygb transgenic (TG) mouse line that overexpresses Cygb [282]. Using this model, they demonstrated for the first time that following induction of membrane damage and oxidative stress in the liver using thioacetamide, TG mice were better withstanding the increase in ROS and inflammation, displaying reduced neutrophil accumulation and release of inflammation-related cytokines, less hepatic fibrosis with decreased activation of hepatic stellate cells compared to wild-type mice [282]. Kwon et al. very recently showed that Cygb-deficiency affects retinal degeneration in mice with defects in the expression of Crumbs1, caused by a point mutation in the rd8 gene [283]. Indeed, Cygb-/-rd8/rd8 mice displayed a more rapid degeneration of the retina than Cygb+/+rd8/rd8 mice, with a more marked photoreceptor degeneration and defective lamination of progenitor cells, Muller glia and new photoreceptors. Kwon and colleagues suggested a ROS scavenging-related role for Cygb for the regulation of the polarity of photoreceptor and progenitor cells in the retina [283]. Most recently, additional evidence for the antioxidative function of Cygb in the kidney was demonstrated by using a Cygb-deficient murine model, displaying reduced kidney function and podocyte number [284]. Mechanistic studies using shRNA-mediated knockdown experiments in human podocyte-derived cell lines with abundant Cygb expression levels, further confirmed an antiapoptotic and antioxidative role of Cygb [284].
8.4. Cygb protects against fibrosis
Nakatani et al. detected expression of Cygb in rat kidney, mainly in fibroblasts, but also in the glomerulus [285]. Upon cyclosporine A-induced nephropathy, Cygb expression was upregulated in fibrotic lesions of the kidney, specifically in interstitial fibroblasts positive for the renal cortical fibroblast markers CD73 and αSMA [285], similarly to the first findings by Kawada and colleagues, back when Cygb was first described as stellate cell activation-associated protein (STAP) and its involvement in hepatic fibrosis [71]. Thuy le and coworkers also observed substantially increased fibrosis in Cygb knockout mice, mostly in the liver, but also in the kidney, suggesting an antifibrotic role of Cygb [317]. The same group could further show that Cygb deficiency enhanced hepatocyte damage and inflammation following bile duct ligation, and increased fibrosis possibly due to altered NO metabolism [286]. Similarly, Mimura et al. and Nishi et al. employed Cygb-overexpressing transgenic rats in disease models of remnant kidney and renal ischemia/reperfusion, respectively, to provide evidence for an antifibrotic role of Cygb in kidney fibroblasts, potentially via a ROS scavenging function [274,275].
8.5. Tumor suppressive function
Consistent with a general globin role in hypoxia adaptation, also the function of Cygb was extensively investigated under conditions of reduced oxygen supply. Several groups explored the relationship between hypoxia and Cygb on the level of gene regulation as well as on the level of activity in a tumor context [249,[287], [288], [289], [290]]. In the same line, McRonald et al. and Langan et al. have identified Cygb in a background of tylosis with esophageal cancer [291,292]. Additionally, Thuy le et al. [315] and Yassin et al. [293] both reported an implication of Cygb in tumorigenesis using in vivo models. Thuy le et al. described in a murine Cygb knockout model that the absence of expression of Cygb resulted in an increased incidence of tumor development in the liver and lungs [316]. In a follow-up paper, the group showed that Cygb-deficiency leads to an increase in oxidative stress and, in a situation of non-alcoholic steatohepatitis, leads to a nearly certain development of hepatocellular carcinoma [315]. Yassin and colleagues found that Cygb is important for the suppression of colorectal tumors [293]. In an independently generated Cygb knockout mouse model, Cygb deficiency exacerbated chemically induced colitis and augmented the establishment of colonic tumors. Cygb-deficient mice were more susceptible to severe inflammation, leading to a significant loss of colonic crypts and colonic epithelial cells [293]. By employing cell line studies and patient breast and lung cancer material, further evidence was given for a tumor suppressive role of Cygb [294]. Furthermore, Shaw and colleagues described regulation of Cygb expression by promoter hypermethylation, as well as by tumor hypoxia [295]. Accordingly, Oleksiewicz et al. described a similar bimodal role for Cygb in lung tumorigenesis, dependent on cell type and microenvironment. Using lung cancer cell lines Cygb is found to function as tumor suppressor under normoxic conditions and as oncogene in cells exposed to stress [296], revealing Cygb as a potential versatile and attractive target for tumor therapy.
8.6. Cytoprotective lipid peroxidase activity
Interestingly, a recent study explored the influence of cold atmospheric plasma treatment, a novel tool in cancer therapy relying on increased RONS production, on the structure of Cygb. Application of cold atmospheric plasma revealed the formation of inter- and intra-molecular disulfide bridges, illustrating the importance of the oxidation of two Cygb cysteine residues in regulating its ligand-binding properties [297]. In this context, a difference is observed in the ability of Cygb to bind lipids: monomers with intramolecular disulfide bridges bind lipids more easily as compared to dimers, as the result of the formation of a more open heme pocket structure [298,299]. This notion further supports that the cold atmospheric plasma-induced conformational change is part of its cytoprotective role under oxidative stress conditions via its lipid peroxidase activity [297].
8.7. Tissue repair and regeneration
Unexpectedly, Cygb seems to display a function in tissue repair and remodeling. Singh and colleagues [300] suggested a major role of Cygb in muscle repair and regeneration, as myogenic progenitor cells derived from a mouse model in which Cygb was specifically knocked out in skeletal muscle, were severely deficient in their ability to form myotubes. The regenerative function of Cygb was also proposed during tail re-growth in the house gecko [301]. It was further shown that hypertension is associated with loss of Cygb in rat aorta, whereas its overexpression promoted the proliferation and migration of vascular smooth muscle cells, suggesting a role in vascular wall remodeling [302]. A similar finding was reported by Jourd'heuil and coworkers in the rat balloon angioplasty model and in Cygb knockout mice subjected to carotid ligation, whereby the expression of Cygb appeared crucial for the regulation of apoptosis during vascular remodeling [303].
9. Androglobin
9.1. General aspects
In 2012, the fifth mammalian globin was described and termed androglobin (Adgb) due to its predominant expression in testis tissue [77] (Fig. 3). This youngest member of the globin gene family represents a unique type of chimeric protein with a very high phylogenetic conservation that can be traced back to early metazoans and choanoflagellates, thereby defining Adgb as a major clade of globins together with Ngb and GbX that likely emerged before the separation of Protostomia and Deuterostomia [69,77,80] (Fig. 2). This furthermore suggests that these 3 globin clades exhibited very distinct and non-overlapping functions. In contrast to all other known globins, Adgb displays a peculiar protein architecture including an N-terminal calpain-like cysteine protease domain, a central permuted globin domain, an IQ calmodulin-binding motif, and a long C-terminal tail without detectable motifs. The central globin domain exhibits an unprecedented α-helical rearrangement, with helices C to H placed before and split from helices A and B. However, the globin domain is functional, and displays an absorption spectrum characteristic of hexa-coordination of the heme iron [77,304].
9.2. Physiology and pathophysiology
The physiological function of Adgb remains still largely unknown, but a role in spermatogenesis is conceivable given its strong expression in vertebrate testis. Furthermore, evidence exists that Adgb expression is reduced in infertile versus fertile men [77]. A single in vitro study reported a potential oncogenic function of Adgb in glioma, whereby lentiviral knockdown of Adgb led to inhibition of proliferation and increased apoptosis in the U251 glioma cell line [305]. At present, further studies are required to assess with certainty the physiological function of this unique globin type, and to explore its relationship towards oxygen, NO scavenging, calcium-mediated signaling or potential proteolytic activity.
10. Globin E
Initially identified in birds’ eye [75,83], globin E (GbE) was thereafter also found in turtles and coelacanth [78,79] (Fig. 2). Blank and coworkers demonstrated that GbE localizes in photoreceptor cells in Gallus gallus sp. (domestic chicken) [306]. Later, GbE expression was also detected in bird erythrocytes [213]. GbE displays a heme penta-coordinated scheme and analysis of oxygen binding kinetics showed that GbE exhibits a high oxygen binding affinity with a P50 of about 5.8 and 15 torr, respectively at 25 °C and 41 °C [306]. Interestingly, these data indicate that GbE shows a higher affinity for oxygen compared to myoglobin in a variety of terrestrial and diving mammals and aquatic birds (P50 = 2.4–4.85 torr at 37 °C) [307] and suggests that GbE plays an important role in oxygen supply in avian eyes. More recently, a study showed that lungfish bear also multiple copies of GbE. Surprisingly, in contrast to avian location, the major sites of expression were ovaries and oocytes, whereas no detectable mRNA was found in the eye [202]. The physiological role of GbE in lungfish and more particularly in oocytes is still unclear, but it is conceivable that its main function might be to retrieve oxygen from water and support embryonic development.
11. Globin Y
Globin Y (GbY) was reported in reptiles, platypus, ray-finned fishes, coelacanth, spotted gar, elephant shark and amphibians [69,308] (Fig. 2). Genomic analyses showed that GbY localizes at the 3’ end of the α-globin gene cluster [309]. Moreover, the sites of expression vary among species: gill and spleen in spotted gar; intestine, gill and spleen in elephant shark; a low expression in several tissues including kidney, lung, brain or blood in western painted turtle [79,308]. Up to date, due to the lack of experimental evidence for the physiological role of GbY, its function remains obscure.
12. Globin X
12.1. General aspects
During evolution, Globin X (GbX) emerged in early Metazoans from a common ancestor around 800 million years ago and shows 25–30% sequence identity to Ngb [76,79,308]. In vertebrates its expression is restricted to certain taxa, including lampreys, fish, amphibians and some reptiles, whereas no detectable expression was reported in birds and mammals [69,82,310] (Fig. 2). However, the sites and levels of expression may be variable depending on the species and include brain, eye, intestine, liver, spleen, and heart [79,308]. In zebrafish, GbX is mainly expressed in the eye and the central nervous system, and its levels are oxygen-dependently regulated, with a significant decrease under hypoxic conditions [311,312].
12.2. Membrane anchoring-dependent ROS protection
In silico and biochemical analyses reported that GbX is myristoylated at Gly2 and palmitoylated at Cys3, which results in its anchoring to the membrane [312]. Spectroscopic and oxygen binding analyses showed that GbX has a hexacoordinated scheme with a deoxy-spectrum similar to Ngb and Cygb and a P50 between 1.3 and 12.5 torr at 20 °C depending on the pH and the presence of DTT, which strongly suggests the formation of disulfide bridges that may control heme reactivity, as well as an electron transfer function rather than oxygen binding [312]. In a more recent study, overexpression was performed of Mb, wild-type GbX and a mutated form of GbX, where the Gly2 was replaced by Ala and the Cys3 by a Ser, in the mouse neuronal HN33 cell line lacking GbX and Mb, and the viability of cells and ROS production was compared under hypoxic conditions or in the presence of H2O2. Under low oxygen content, Mb showed significantly greater preservation of cellular viability reflected by the higher mitochondrial dehydrogenase activity and ATP production as compared to GbX or mutated GbX. However, in the presence of various amounts of H2O2, overexpression of Mb had only minor impact, whereas GbX and mutant GbX were clearly more protective. Intriguingly, loss of membrane anchoring resulted in increased ROS production upon H2O2 treatment and in lower cell viability as compared to the wild-type form, emphasizing the importance of its membrane localization in order to sustain optimal function [310]. However, it remains unclear how GbX exerts these protective effects and if it may protect the membrane lipids by reducing peroxides resulting from the accumulation of ROS, as suggested by several studies [80,312].
12.3. GbX-mediated nitrite reduction and inhibition of platelet activation
Surprisingly, two other studies have recently reported that GbX is present in fish erythrocytes and localizes in the cytoplasm and nuclear pores [313,314]. Nitric oxide (NO) formation experiments demonstrated that deoxy-GbX is capable of nitrite reductase activity with a NO production that is around 100-fold higher than Hb. These data were confirmed by silencing experiments performed in cultured fish red blood cells (RBCs), which exhibited a significant reduction of NO production. Moreover, physiologically the absence of GbX in RBCs completely abolishes the inhibitory effect on platelet activation [313]. Altogether, these data indicate that in nucleated fish RBCs, GbX has a specialized function in nitrite reduction under lower oxygen content resulting in the generation of NO and inhibition of platelet activation. However, whether GbX may act on other intracellular or extracellular processes through NO signaling has still to be elucidated.
13. Conclusion
The genome era has revolutionized our knowledge on multiple aspects of modern biology and medicine, and has greatly enhanced the tools for exploring vertebrate evolution. Until 20 years ago, Hb and Mb were the only known globin types in mammals, and their function was believed to be mainly restricted to oxygen transport and supply. With the emergence of genomic data, globin research witnessed an explosion, not only of novel globin types, but also of unexpected functions. These functions include an increasing variety of oxygen-independent actions, leaving the globin field perplexed and intrigued at the same time. Still 20 years later, new functions are being discovered and novel research questions with accompanying open avenues arise. Indisputably, the major diversity in globin expression sites warrants substantial functional versatility. Future research on globin function and diversification will certainly give rise to even more insights into their astonishing complexity, but will undoubtedly also reserve some unexpected surprises to the research field.
Funding
This work was supported by the Swiss National Science Foundation (Grant 31003A_173000).
Declaration of competing interests
The authors declare that no competing interests exist.
References
- 1.Kapp O.H., Moens L., Vanfleteren J., Trotman C.N., Suzuki T., Vinogradov S.N. Alignment of 700 globin sequences: extent of amino acid substitution and its correlation with variation in volume. Protein Sci. 1995;4:2179–2190. doi: 10.1002/pro.5560041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gell D.A. Structure and function of haemoglobins. Blood Cells Mol. Dis. 2018;70:13–42. doi: 10.1016/j.bcmd.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 3.de Sanctis D., Pesce A., Nardini M., Bolognesi M., Bocedi A., Ascenzi P. Structure-function relationships in the growing hexa-coordinate hemoglobin sub-family. IUBMB Life. 2004;56:643–651. doi: 10.1080/15216540500059640. [DOI] [PubMed] [Google Scholar]
- 4.Kendrew J.C., Bodo G., Dintzis H.M., Parrish R.G., Wyckoff H., Phillips D.C. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature. 1958;181:662–666. doi: 10.1038/181662a0. [DOI] [PubMed] [Google Scholar]
- 5.Muirhead H., Perutz M.F. Structure of haemoglobin. A three-dimensional fourier synthesis of reduced human haemoglobin at 5-5 a resolution. Nature. 1963;199:633–638. doi: 10.1038/199633a0. [DOI] [PubMed] [Google Scholar]
- 6.Huber R., Epp O., Steigemann W., Formanek H. The atomic structure of erythrocruorin in the light of the chemical sequence and its comparison with myoglobin. Eur. J. Biochem. 1971;19:42–50. doi: 10.1111/j.1432-1033.1971.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 7.Padlan E., Love W. Three-dimensional structure of hemoglobin from the polychaete annelid, Glycera dibranchiata, at 2.5 A resolution. J. Biol. Chem. 1974;249:4067–4078. [PubMed] [Google Scholar]
- 8.Vainshtein B.K., Harutyunyan E.H., Kuranova I.P., Borisov V.V., Sosfenov N.I., Pavlovsky A.G., Grebenko A.I., Konareva N.V. Structure of leghaemoglobin from lupin root nodules at 5 angstrom resolution. Nature. 1975;254:163–164. doi: 10.1038/254163a0. [DOI] [PubMed] [Google Scholar]
- 9.Lesk A.M., Chothia C. How different amino acid sequences determine similar protein structures: the structure and evolutionary dynamics of the globins. J. Mol. Biol. 1980;136:225–270. doi: 10.1016/0022-2836(80)90373-3. [DOI] [PubMed] [Google Scholar]
- 10.Bashford D., Chothia C., Lesk A.M. Determinants of a protein fold. Unique features of the globin amino acid sequences. J. Mol. Biol. 1987;196:199–216. doi: 10.1016/0022-2836(87)90521-3. [DOI] [PubMed] [Google Scholar]
- 11.Holm L., Sander C. Structural alignment of globins, phycocyanins and colicin A. FEBS Lett. 1993;315:301–306. doi: 10.1016/0014-5793(93)81183-z. [DOI] [PubMed] [Google Scholar]
- 12.Holm L., Sander C. Globin fold in a bacterial toxin. Nature. 1993;361:309. doi: 10.1038/361309a0. [DOI] [PubMed] [Google Scholar]
- 13.Subbiah S., Laurents D.V., Levitt M. Structural similarity of DNA-binding domains of bacteriophage repressors and the globin core. Curr. Biol. 1993;3:141–148. doi: 10.1016/0960-9822(93)90255-m. [DOI] [PubMed] [Google Scholar]
- 14.Choi S.Y., Esaki N., Ashiuchi M., Yoshimura T., Soda K. Bacterial glutamate racemase has high sequence similarity with myoglobins and forms an equimolar inactive complex with hemin. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10144–10147. doi: 10.1073/pnas.91.21.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahmann J.D., Sass H.J., Allan M.G., Seto H., Thompson C.J., Grzesiek S. Structural basis for antibiotic recognition by the TipA class of multidrug-resistance transcriptional regulators. EMBO J. 2003;22:1824–1834. doi: 10.1093/emboj/cdg181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray J.W., Delumeau O., Lewis R.J. Structure of a nonheme globin in environmental stress signaling. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17320–17325. doi: 10.1073/pnas.0506599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stranzl G.R., Santelli E., Bankston L.A., La Clair C., Bobkov A., Schwarzenbacher R., Godzik A., Perego M., Grynberg M., Liddington R.C. Structural insights into inhibition of Bacillus anthracis sporulation by a novel class of non-heme globin sensor domains. J. Biol. Chem. 2011;286:8448–8458. doi: 10.1074/jbc.M110.207126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinogradov S.N., Walz D.A., Pohajdak B., Moens L., Kapp O.H., Suzuki T., Trotman C.N. Adventitious variability? The amino acid sequences of nonvertebrate globins. Comp. Biochem. Physiol. B. 1993;106:1–26. doi: 10.1016/0305-0491(93)90002-m. [DOI] [PubMed] [Google Scholar]
- 19.Weber R.E., Vinogradov S.N. Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol. Rev. 2001;81:569–628. doi: 10.1152/physrev.2001.81.2.569. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi S., Matsubara H., Webster D.A. Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. Nature. 1986;322:481–483. doi: 10.1038/322481a0. [DOI] [PubMed] [Google Scholar]
- 21.Appleby C.A., Tjepkema J.D., Trinick M.J. Hemoglobin in a nonleguminous plant, parasponia: possible genetic origin and function in nitrogen fixation. Science. 1983;220:951–953. doi: 10.1126/science.220.4600.951. [DOI] [PubMed] [Google Scholar]
- 22.Sowa A.W., Guy P.A., Sowa S., Hill R.D. Nonsymbiotic haemoglobins in plants. Acta Biochim. Pol. 1999;46:431–445. [PubMed] [Google Scholar]
- 23.Hunt P.W., Watts R.A., Trevaskis B., Llewelyn D.J., Burnell J., Dennis E.S., Peacock W.J. Expression and evolution of functionally distinct haemoglobin genes in plants. Plant Mol. Biol. 2001;47:677–692. doi: 10.1023/a:1012440926982. [DOI] [PubMed] [Google Scholar]
- 24.Vasudevan S.G., Armarego W.L., Shaw D.C., Lilley P.E., Dixon N.E., Poole R.K. Isolation and nucleotide sequence of the hmp gene that encodes a haemoglobin-like protein in Escherichia coli K-12. Mol. Gen. Genet. 1991;226:49–58. doi: 10.1007/BF00273586. [DOI] [PubMed] [Google Scholar]
- 25.Ermler U., Siddiqui R.A., Cramm R., Schroder D., Friedrich B. Crystallization and preliminary X-ray diffraction studies of a bacterial flavohemoglobin protein. Proteins. 1995;21:351–353. doi: 10.1002/prot.340210408. [DOI] [PubMed] [Google Scholar]
- 26.Iwaasa H., Takagi T., Shikama K. Amino acid sequence of yeast hemoglobin. A two-domain structure. J. Mol. Biol. 1992;227:948–954. doi: 10.1016/0022-2836(92)90236-d. [DOI] [PubMed] [Google Scholar]
- 27.Zhu H., Riggs A.F. Yeast flavohemoglobin is an ancient protein related to globins and a reductase family. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5015–5019. doi: 10.1073/pnas.89.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwaasa H., Takagi T., Shikama K. Protozoan myoglobin from Paramecium caudatum. Its unusual amino acid sequence. J. Mol. Biol. 1989;208:355–358. doi: 10.1016/0022-2836(89)90395-1. [DOI] [PubMed] [Google Scholar]
- 29.Iwaasa H., Takagi T., Shikama K. Protozoan hemoglobin from Tetrahymena pyriformis. Isolation, characterization, and amino acid sequence. J. Biol. Chem. 1990;265:8603–8609. [PubMed] [Google Scholar]
- 30.Potts M., Angeloni S.V., Ebel R.E., Bassam D. Myoglobin in a cyanobacterium. Science. 1992;256:1690–1691. doi: 10.1126/science.256.5064.1690. [DOI] [PubMed] [Google Scholar]
- 31.Hardison R.C. A brief history of hemoglobins: plant, animal, protist, and bacteria. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5675–5679. doi: 10.1073/pnas.93.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman D.R., Guinn B., Perdok M.M., Goldberg D.E. Components of sterol biosynthesis assembled on the oxygen-avid hemoglobin of Ascaris. Science. 1992;258:1930–1932. doi: 10.1126/science.1470914. [DOI] [PubMed] [Google Scholar]
- 33.Hoogewijs D., Geuens E., Dewilde S., Moens L., Vierstraete A., Vinogradov S., Vanfleteren J. Genome-wide analysis of the globin gene family of C. elegans. IUBMB Life. 2004;56:697–702. doi: 10.1080/15216540500037562. [DOI] [PubMed] [Google Scholar]
- 34.Tilleman L., Germani F., De Henau S., Geuens E., Hoogewijs D., Braeckman B.P., Vanfleteren J.R., Moens L., Dewilde S. Globins in Caenorhabditis elegans. IUBMB Life. 2011;63:166–174. doi: 10.1002/iub.443. [DOI] [PubMed] [Google Scholar]
- 35.Burmester T., Storf J., Hasenjager A., Klawitter S., Hankeln T. The hemoglobin genes of Drosophila. FEBS J. 2006;273:468–480. doi: 10.1111/j.1742-4658.2005.05073.x. [DOI] [PubMed] [Google Scholar]
- 36.Hoogewijs D., Geuens E., Dewilde S., Vierstraete A., Moens L., Vinogradov S., Vanfleteren J.R. Wide diversity in structure and expression profiles among members of the Caenorhabditis elegans globin protein family. BMC Genomics. 2007;8:356. doi: 10.1186/1471-2164-8-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burmester T., Hankeln T. The respiratory proteins of insects. J. Insect Physiol. 2007;53:285–294. doi: 10.1016/j.jinsphys.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann F.G., Opazo J.C., Hoogewijs D., Hankeln T., Ebner B., Vinogradov S.N., Bailly X., Storz J.F. Evolution of the globin gene family in deuterostomes: lineage-specific patterns of diversification and attrition. Mol. Biol. Evol. 2012;29:1735–1745. doi: 10.1093/molbev/mss018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen A.B., Herman J.L., Elphick M.R., Kober K.M., Janies D., Linchangco G., Semmens D.C., Bailly X., Vinogradov S.N., Hoogewijs D. Phylogeny of echinoderm hemoglobins. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinogradov S.N., Hoogewijs D., Bailly X., Arredondo-Peter R., Guertin M., Gough J., Dewilde S., Moens L., Vanfleteren J.R. Three globin lineages belonging to two structural classes in genomes from the three kingdoms of life. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11385–11389. doi: 10.1073/pnas.0502103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu G., Wainwright L.M., Poole R.K. Microbial globins. Adv. Microb. Physiol. 2003;47:255–310. doi: 10.1016/s0065-2911(03)47005-7. [DOI] [PubMed] [Google Scholar]
- 42.Farres J., Rechsteiner M.P., Herold S., Frey A.D., Kallio P.T. Ligand binding properties of bacterial hemoglobins and flavohemoglobins. Biochemistry. 2005;44:4125–4134. doi: 10.1021/bi047389d. [DOI] [PubMed] [Google Scholar]
- 43.Vinogradov S.N., Hoogewijs D., Bailly X., Arredondo-Peter R., Gough J., Dewilde S., Moens L., Vanfleteren J.R. A phylogenomic profile of globins. BMC Evol. Biol. 2006;6:31. doi: 10.1186/1471-2148-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vazquez-Limon C., Hoogewijs D., Vinogradov S.N., Arredondo-Peter R. The evolution of land plant hemoglobins. Plant Sci. 2012;191–192:71–81. doi: 10.1016/j.plantsci.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Vinogradov S.N., Bailly X., Smith D.R., Tinajero-Trejo M., Poole R.K., Hoogewijs D. Microbial eukaryote globins. Adv. Microb. Physiol. 2013;63:391–446. doi: 10.1016/B978-0-12-407693-8.00009-1. [DOI] [PubMed] [Google Scholar]
- 46.Vinogradov S.N., Tinajero-Trejo M., Poole R.K., Hoogewijs D. Bacterial and archaeal globins - a revised perspective. Biochim. Biophys. Acta. 2013;1834:1789–1800. doi: 10.1016/j.bbapap.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 47.Thijs L., Vinck E., Bolli A., Trandafir F., Wan X., Hoogewijs D., Coletta M., Fago A., Weber R.E., Van Doorslaer S., Ascenzi P., Alam M., Moens L., Dewilde S. Characterization of a globin-coupled oxygen sensor with a gene-regulating function. J. Biol. Chem. 2007;282:37325–37340. doi: 10.1074/jbc.M705541200. [DOI] [PubMed] [Google Scholar]
- 48.Martinkova M., Kitanishi K., Shimizu T. Heme-based globin-coupled oxygen sensors: linking oxygen binding to functional regulation of diguanylate cyclase, histidine kinase, and methyl-accepting chemotaxis. J. Biol. Chem. 2013;288:27702–27711. doi: 10.1074/jbc.R113.473249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guimaraes W.G., Gondim A.C.S., Costa P., Gilles-Gonzalez M.A., Lopes L.G.F., Carepo M.S.P., Sousa E.H.S. Insights into signal transduction by a hybrid FixL: denaturation study of on and off states of a multi-domain oxygen sensor. J. Inorg. Biochem. 2017;172:129–137. doi: 10.1016/j.jinorgbio.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Sousa E.H.S., Gilles-Gonzalez M.A. Haem-based sensors of O2: lessons and perspectives. Adv. Microb. Physiol. 2017;71:235–257. doi: 10.1016/bs.ampbs.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Walker J.A., Rivera S., Weinert E.E. Mechanism and role of globin-coupled sensor signalling. Adv. Microb. Physiol. 2017;71:133–169. doi: 10.1016/bs.ampbs.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou S., Freitas T., Larsen R.W., Piatibratov M., Sivozhelezov V., Yamamoto A., Meleshkevitch E.A., Zimmer M., Ordal G.W., Alam M. Globin-coupled sensors: a class of heme-containing sensors in Archaea and Bacteria. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9353–9358. doi: 10.1073/pnas.161185598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freitas T.A., Hou S., Dioum E.M., Saito J.A., Newhouse J., Gonzalez G., Gilles-Gonzalez M.A., Alam M. Ancestral hemoglobins in archaea. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6675–6680. doi: 10.1073/pnas.0308657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freitas T.A., Saito J.A., Hou S., Alam M. Globin-coupled sensors, protoglobins, and the last universal common ancestor. J. Inorg. Biochem. 2005;99:23–33. doi: 10.1016/j.jinorgbio.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 55.Hoogewijs D., Dewilde S., Vierstraete A., Moens L., Vinogradov S.N. A phylogenetic analysis of the globins in fungi. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pesce A., Couture M., Dewilde S., Guertin M., Yamauchi K., Ascenzi P., Moens L., Bolognesi M. A novel two-over-two alpha-helical sandwich fold is characteristic of the truncated hemoglobin family. EMBO J. 2000;19:2424–2434. doi: 10.1093/emboj/19.11.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pesce A., Bolognesi M., Nardini M. The diversity of 2/2 (truncated) globins. Adv. Microb. Physiol. 2013;63:49–78. doi: 10.1016/B978-0-12-407693-8.00002-9. [DOI] [PubMed] [Google Scholar]
- 58.Wittenberg J.B., Bolognesi M., Wittenberg B.A., Guertin M. Truncated hemoglobins: a new family of hemoglobins widely distributed in bacteria, unicellular eukaryotes, and plants. J. Biol. Chem. 2002;277:871–874. doi: 10.1074/jbc.R100058200. [DOI] [PubMed] [Google Scholar]
- 59.Vuletich D.A., Lecomte J.T. A phylogenetic and structural analysis of truncated hemoglobins. J. Mol. Evol. 2006;62:196–210. doi: 10.1007/s00239-005-0077-4. [DOI] [PubMed] [Google Scholar]
- 60.Bonamore A., Attili A., Arenghi F., Catacchio B., Chiancone E., Morea V., Boffi A. A novel chimera: the "truncated hemoglobin-antibiotic monooxygenase" from Streptomyces avermitilis. Gene. 2007;398:52–61. doi: 10.1016/j.gene.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 61.Hade M.D., Kaur J., Chakraborti P.K., Dikshit K.L. Multidomain truncated hemoglobins: new members of the globin family exhibiting tandem repeats of globin units and domain fusion. IUBMB Life. 2017;69:479–488. doi: 10.1002/iub.1630. [DOI] [PubMed] [Google Scholar]
- 62.Becana M., Yruela I., Sarath G., Catalan P., Hargrove M.S. Plant hemoglobins: a journey from unicellular green algae to vascular plants. New Phytol. 2020;227:1618–1635. doi: 10.1111/nph.16444. [DOI] [PubMed] [Google Scholar]
- 63.Hoy J.A., Hargrove M.S. The structure and function of plant hemoglobins. Plant Physiol. Biochem. 2008;46:371–379. doi: 10.1016/j.plaphy.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 64.Vinogradov S.N., Hoogewijs D., Bailly X., Mizuguchi K., Dewilde S., Moens L., Vanfleteren J.R. A model of globin evolution. Gene. 2007;398:132–142. doi: 10.1016/j.gene.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 65.Vinogradov S.N., Fernandez I., Hoogewijs D., Arredondo-Peter R. Phylogenetic relationships of 3/3 and 2/2 hemoglobins in Archaeplastida genomes to bacterial and other eukaryote hemoglobins. Mol. Plant. 2011;4:42–58. doi: 10.1093/mp/ssq040. [DOI] [PubMed] [Google Scholar]
- 66.Teh A.H., Saito J.A., Baharuddin A., Tuckerman J.R., Newhouse J.S., Kanbe M., Newhouse E.I., Rahim R.A., Favier F., Didierjean C., Sousa E.H., Stott M.B., Dunfield P.F., Gonzalez G., Gilles-Gonzalez M.A., Najimudin N., Alam M. Hell's Gate globin I: an acid and thermostable bacterial hemoglobin resembling mammalian neuroglobin. FEBS Lett. 2011;585:3250–3258. doi: 10.1016/j.febslet.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Hardison R. Hemoglobins from bacteria to man: evolution of different patterns of gene expression. J. Exp. Biol. 1998;201:1099–1117. doi: 10.1242/jeb.201.8.1099. [DOI] [PubMed] [Google Scholar]
- 68.Storz J.F. Oxford University Press BPS; GB: 2018. Hemoglobin: Insights into Protein Structure, Function, and Evolution. [Google Scholar]
- 69.Burmester T., Hankeln T. Function and evolution of vertebrate globins. Acta Physiol. (Oxf) 2014;211:501–514. doi: 10.1111/apha.12312. [DOI] [PubMed] [Google Scholar]
- 70.Burmester T., Weich B., Reinhardt S., Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 71.Kawada N., Kristensen D.B., Asahina K., Nakatani K., Minamiyama Y., Seki S., Yoshizato K. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J. Biol. Chem. 2001;276:25318–25323. doi: 10.1074/jbc.M102630200. [DOI] [PubMed] [Google Scholar]
- 72.Burmester T., Ebner B., Weich B., Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol. Biol. Evol. 2002;19:416–421. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- 73.Trent J.T., 3rd, Hargrove M.S. A ubiquitously expressed human hexacoordinate hemoglobin. J. Biol. Chem. 2002;277:19538–19545. doi: 10.1074/jbc.M201934200. [DOI] [PubMed] [Google Scholar]
- 74.Fuchs C., Burmester T., Hankeln T. The amphibian globin gene repertoire as revealed by the Xenopus genome. Cytogenet. Genome Res. 2006;112:296–306. doi: 10.1159/000089884. [DOI] [PubMed] [Google Scholar]
- 75.Kugelstadt D., Haberkamp M., Hankeln T., Burmester T. Neuroglobin, cytoglobin, and a novel, eye-specific globin from chicken. Biochem. Biophys. Res. Commun. 2004;325:719–725. doi: 10.1016/j.bbrc.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 76.Roesner A., Fuchs C., Hankeln T., Burmester T. A globin gene of ancient evolutionary origin in lower vertebrates: evidence for two distinct globin families in animals. Mol. Biol. Evol. 2005;22:12–20. doi: 10.1093/molbev/msh258. [DOI] [PubMed] [Google Scholar]
- 77.Hoogewijs D., Ebner B., Germani F., Hoffmann F.G., Fabrizius A., Moens L., Burmester T., Dewilde S., Storz J.F., Vinogradov S.N., Hankeln T. Androglobin: a chimeric globin in metazoans that is preferentially expressed in Mammalian testes. Mol. Biol. Evol. 2012;29:1105–1114. doi: 10.1093/molbev/msr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwarze K., Burmester T. Conservation of globin genes in the "living fossil" Latimeria chalumnae and reconstruction of the evolution of the vertebrate globin family. Biochim. Biophys. Acta. 2013;1834:1801–1812. doi: 10.1016/j.bbapap.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 79.Schwarze K., Singh A., Burmester T. The full globin repertoire of turtles provides insights into vertebrate globin evolution and functions. Genome Biol. Evol. 2015;7:1896–1913. doi: 10.1093/gbe/evv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blank M., Burmester T. Widespread occurrence of N-terminal acylation in animal globins and possible origin of respiratory globins from a membrane-bound ancestor. Mol. Biol. Evol. 2012;29:3553–3561. doi: 10.1093/molbev/mss164. [DOI] [PubMed] [Google Scholar]
- 81.Droge J., Pande A., Englander E.W., Makalowski W. Comparative genomics of neuroglobin reveals its early origins. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwarze K., Campbell K.L., Hankeln T., Storz J.F., Hoffmann F.G., Burmester T. The globin gene repertoire of lampreys: convergent evolution of hemoglobin and myoglobin in jawed and jawless vertebrates. Mol. Biol. Evol. 2014;31:2708–2721. doi: 10.1093/molbev/msu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoffmann F.G., Opazo J.C., Storz J.F. Differential loss and retention of cytoglobin, myoglobin, and globin-E during the radiation of vertebrates. Genome Biol. Evol. 2011;3:588–600. doi: 10.1093/gbe/evr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffmann F.G., Opazo J.C., Storz J.F. Whole-genome duplications spurred the functional diversification of the globin gene superfamily in vertebrates. Mol. Biol. Evol. 2012;29:303–312. doi: 10.1093/molbev/msr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fago A. Functional roles of globin proteins in hypoxia-tolerant ectothermic vertebrates. J. Appl. Physiol. 2017;123:926–934. doi: 10.1152/japplphysiol.00104.2017. 1985. [DOI] [PubMed] [Google Scholar]
- 86.Weber R.E. High-altitude adaptations in vertebrate hemoglobins. Respir. Physiol. Neurobiol. 2007;158:132–142. doi: 10.1016/j.resp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Projecto-Garcia J., Natarajan C., Moriyama H., Weber R.E., Fago A., Cheviron Z.A., Dudley R., McGuire J.A., Witt C.C., Storz J.F. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20669–20674. doi: 10.1073/pnas.1315456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Storz J.F. Hemoglobin-oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend? J. Exp. Biol. 2016;219:3190–3203. doi: 10.1242/jeb.127134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campbell K.L., Roberts J.E., Watson L.N., Stetefeld J., Sloan A.M., Signore A.V., Howatt J.W., Tame J.R., Rohland N., Shen T.J., Austin J.J., Hofreiter M., Ho C., Weber R.E., Cooper A. Substitutions in woolly mammoth hemoglobin confer biochemical properties adaptive for cold tolerance. Nat. Genet. 2010;42:536–540. doi: 10.1038/ng.574. [DOI] [PubMed] [Google Scholar]
- 90.Ponganis P.J. Diving mammals. Comp. Physiol. 2011;1:447–465. doi: 10.1002/cphy.c091003. [DOI] [PubMed] [Google Scholar]
- 91.Helbo S., Fago A. Functional properties of myoglobins from five whale species with different diving capacities. J. Exp. Biol. 2012;215:3403–3410. doi: 10.1242/jeb.073726. [DOI] [PubMed] [Google Scholar]
- 92.Avivi A., Gerlach F., Joel A., Reuss S., Burmester T., Nevo E., Hankeln T. Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21570–21575. doi: 10.1073/pnas.1015379107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campbell K.L., Storz J.F., Signore A.V., Moriyama H., Catania K.C., Payson A.P., Bonaventura J., Stetefeld J., Weber R.E. Molecular basis of a novel adaptation to hypoxic-hypercapnia in a strictly fossorial mole. BMC Evol. Biol. 2010;10:214. doi: 10.1186/1471-2148-10-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fraser J., de Mello L.V., Ward D., Rees H.H., Williams D.R., Fang Y., Brass A., Gracey A.Y., Cossins A.R. Hypoxia-inducible myoglobin expression in nonmuscle tissues. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2977–2981. doi: 10.1073/pnas.0508270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roesner A., Mitz S.A., Hankeln T., Burmester T. Globins and hypoxia adaptation in the goldfish, Carassius auratus. FEBS J. 2008;275:3633–3643. doi: 10.1111/j.1742-4658.2008.06508.x. [DOI] [PubMed] [Google Scholar]
- 96.Helbo S., Dewilde S., Williams D.R., Berghmans H., Berenbrink M., Cossins A.R., Fago A. Functional differentiation of myoglobin isoforms in hypoxia-tolerant carp indicates tissue-specific protective roles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R693–R701. doi: 10.1152/ajpregu.00501.2011. [DOI] [PubMed] [Google Scholar]
- 97.Koch J., Ludemann J., Spies R., Last M., Amemiya C.T., Burmester T. Unusual diversity of myoglobin genes in the lungfish. Mol. Biol. Evol. 2016;33:3033–3041. doi: 10.1093/molbev/msw159. [DOI] [PubMed] [Google Scholar]
- 98.Ludemann J., Fago A., Falke S., Wisniewsky M., Schneider I., Fabrizius A., Burmester T. Genetic and functional diversity of the multiple lungfish myoglobins. FEBS J. 2020;287:1598–1611. doi: 10.1111/febs.15094. [DOI] [PubMed] [Google Scholar]
- 99.Opazo J.C., Lee A.P., Hoffmann F.G., Toloza-Villalobos J., Burmester T., Venkatesh B., Storz J.F. Ancient duplications and expression divergence in the globin gene superfamily of vertebrates: insights from the elephant shark genome and transcriptome. Mol. Biol. Evol. 2015;32:1684–1694. doi: 10.1093/molbev/msv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sidell B.D., O'Brien K.M. When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. J. Exp. Biol. 2006;209:1791–1802. doi: 10.1242/jeb.02091. [DOI] [PubMed] [Google Scholar]
- 101.O'Brien K.M. New lessons from an old fish: what Antarctic icefishes may reveal about the functions of oxygen-binding proteins. Integr. Comp. Biol. 2016;56:531–541. doi: 10.1093/icb/icw062. [DOI] [PubMed] [Google Scholar]
- 102.Daane J.M., Giordano D., Coppola D., di Prisco G., Detrich H.W., 3rd, Verde C. Adaptations to environmental change: globin superfamily evolution in Antarctic fishes. Mar. Genomics. 2020;49:100724. doi: 10.1016/j.margen.2019.100724. [DOI] [PubMed] [Google Scholar]
- 103.Xi Y., Obara M., Ishida Y., Ikeda S., Yoshizato K. Gene expression and tissue distribution of cytoglobin and myoglobin in the Amphibia and Reptilia: possible compensation of myoglobin with cytoglobin in skeletal muscle cells of anurans that lack the myoglobin gene. Gene. 2007;398:94–102. doi: 10.1016/j.gene.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 104.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X., Huang K.T., Shields H., Kim-Shapiro D.B., Schechter A.N., Cannon R.O., 3rd, Gladwin M.T. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 105.Shiva S., Rassaf T., Patel R.P., Gladwin M.T. The detection of the nitrite reductase and NO-generating properties of haemoglobin by mitochondrial inhibition. Cardiovasc. Res. 2011;89:566–573. doi: 10.1093/cvr/cvq327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iarovaia O.V., Kovina A.P., Petrova N.V., Razin S.V., Ioudinkova E.S., Vassetzky Y.S., Ulianov S.V. Genetic and epigenetic mechanisms of beta-globin gene switching. Biochemistry (Mosc.) 2018;83:381–392. doi: 10.1134/S0006297918040090. [DOI] [PubMed] [Google Scholar]
- 107.Bank A. Regulation of human fetal hemoglobin: new players, new complexities. Blood. 2006;107:435–443. doi: 10.1182/blood-2005-05-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saha D., Patgaonkar M., Shroff A., Ayyar K., Bashir T., Reddy K.V. Hemoglobin expression in nonerythroid cells: novel or ubiquitous? Int. J. Inflamm. 2014 doi: 10.1155/2014/803237. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ohyagi Y., Goto I. Does iron-catalyzed oxidation of neuronal hemoglobin contribute to motor neuron degeneration? J. Neurol. Sci. 1994;126:237–239. doi: 10.1016/0022-510x(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 110.Ohyagi Y., Yamada T., Goto I. Hemoglobin as a novel protein developmentally regulated in neurons. Brain Res. 1994;635:323–327. doi: 10.1016/0006-8993(94)91455-9. [DOI] [PubMed] [Google Scholar]
- 111.Wu C.W., Liao P.C., Yu L., Wang S.T., Chen S.T., Wu C.M., Kuo Y.M. Hemoglobin promotes Abeta oligomer formation and localizes in neurons and amyloid deposits. Neurobiol. Dis. 2004;17:367–377. doi: 10.1016/j.nbd.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 112.Ferrer I., Gomez A., Carmona M., Huesa G., Porta S., Riera-Codina M., Biagioli M., Gustincich S., Aso E. Neuronal hemoglobin is reduced in Alzheimer's disease, argyrophilic grain disease, Parkinson's disease, and dementia with Lewy bodies. J. Alzheimers Dis. 2011;23:537–550. doi: 10.3233/JAD-2010-101485. [DOI] [PubMed] [Google Scholar]
- 113.Altinoz M.A., Guloksuz S., Schmidt-Kastner R., Kenis G., Ince B., Rutten B.P.F. Involvement of hemoglobins in the pathophysiology of Alzheimer's disease. Exp. Gerontol. 2019;126:110680. doi: 10.1016/j.exger.2019.110680. [DOI] [PubMed] [Google Scholar]
- 114.Schelshorn D.W., Schneider A., Kuschinsky W., Weber D., Kruger C., Dittgen T., Burgers H.F., Sabouri F., Gassler N., Bach A., Maurer M.H. Expression of hemoglobin in rodent neurons. J. Cerebr. Blood Flow Metabol. 2009;29:585–595. doi: 10.1038/jcbfm.2008.152. [DOI] [PubMed] [Google Scholar]
- 115.Richter F., Meurers B.H., Zhu C., Medvedeva V.P., Chesselet M.F. Neurons express hemoglobin alpha- and beta-chains in rat and human brains. J. Comp. Neurol. 2009;515:538–547. doi: 10.1002/cne.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Biagioli M., Pinto M., Cesselli D., Zaninello M., Lazarevic D., Roncaglia P., Simone R., Vlachouli C., Plessy C., Bertin N., Beltrami A., Kobayashi K., Gallo V., Santoro C., Ferrer I., Rivella S., Beltrami C.A., Carninci P., Raviola E., Gustincich S. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:15454–15459. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Russo R., Zucchelli S., Codrich M., Marcuzzi F., Verde C., Gustincich S. Hemoglobin is present as a canonical alpha2beta2 tetramer in dopaminergic neurons. Biochim. Biophys. Acta. 2013;1834:1939–1943. doi: 10.1016/j.bbapap.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 118.Codrich M., Bertuzzi M., Russo R., Francescatto M., Espinoza S., Zentilin L., Giacca M., Cesselli D., Beltrami A.P., Ascenzi P., Zucchelli S., Persichetti F., Leanza G., Gustincich S. Neuronal hemoglobin affects dopaminergic cells' response to stress. Cell Death Dis. 2017;8:e2538. doi: 10.1038/cddis.2016.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brown N., Alkhayer K., Clements R., Singhal N., Gregory R., Azzam S., Li S., Freeman E., McDonough J. Neuronal hemoglobin expression and its relevance to multiple sclerosis neuropathology. J. Mol. Neurosci. 2016;59:1–17. doi: 10.1007/s12031-015-0711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singhal N.K., Alkhayer K., Shelestak J., Clements R., Freeman E., McDonough J. Erythropoietin upregulates brain hemoglobin expression and supports neuronal mitochondrial activity. Mol. Neurobiol. 2018;55:8051–8058. doi: 10.1007/s12035-018-0971-6. [DOI] [PubMed] [Google Scholar]
- 121.Liu L., Zeng M., Stamler J.S. Hemoglobin induction in mouse macrophages. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6643–6647. doi: 10.1073/pnas.96.12.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tommila M., Stark C., Jokilammi A., Peltonen V., Penttinen R., Ekholm E. Hemoglobin expression in rat experimental granulation tissue. J. Mol. Cell Biol. 2011;3:190–196. doi: 10.1093/jmcb/mjq036. [DOI] [PubMed] [Google Scholar]
- 123.Wride M.A., Mansergh F.C., Adams S., Everitt R., Minnema S.E., Rancourt D.E., Evans M.J. Expression profiling and gene discovery in the mouse lens. Mol. Vis. 2003;9:360–396. [PubMed] [Google Scholar]
- 124.Tezel T.H., Geng L., Lato E.B., Schaal S., Liu Y., Dean D., Klein J.B., Kaplan H.J. Synthesis and secretion of hemoglobin by retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2009;50:1911–1919. doi: 10.1167/iovs.07-1372. [DOI] [PubMed] [Google Scholar]
- 125.Bloomfield S.E., Markenson A.L., Miller D.R., Peterson C.M. Lens opacities in thalassemia. J. Pediatr. Ophthalmol. Strabismus. 1978;15:154–156. doi: 10.3928/0191-3913-19780501-08. [DOI] [PubMed] [Google Scholar]
- 126.Babalola O.E., Danboyi P., Abiose A.A. Hereditary congenital cataracts associated with sickle cell anaemia in a Nigerian family. Trop. Doct. 2000;30:12–14. doi: 10.1177/004947550003000107. [DOI] [PubMed] [Google Scholar]
- 127.Mansergh F.C., Hunter S.M., Geatrell J.C., Jarrin M., Powell K., Evans M.J., Wride M.A. Developmentally regulated expression of hemoglobin subunits in avascular tissues. Int. J. Dev. Biol. 2008;52:873–886. doi: 10.1387/ijdb.082597fm. [DOI] [PubMed] [Google Scholar]
- 128.Miceli M.V., Newsome D.A., Schriver G.W. Glucose uptake, hexose monophosphate shunt activity, and oxygen consumption in cultured human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1990;31:277–283. [PubMed] [Google Scholar]
- 129.Kurihara T., Westenskow P.D., Gantner M.L., Usui Y., Schultz A., Bravo S., Aguilar E., Wittgrove C., Friedlander M., Paris L.P., Chew E., Siuzdak G., Friedlander M. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife. 2016;5 doi: 10.7554/eLife.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tezel G., Yang X., Luo C., Cai J., Kain A.D., Powell D.W., Kuehn M.H., Pierce W.M. Hemoglobin expression and regulation in glaucoma: insights into retinal ganglion cell oxygenation. Invest. Ophthalmol. Vis. Sci. 2010;51:907–919. doi: 10.1167/iovs.09-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bhaskaran M., Chen H.F., Chen Z.M., Liu L. Hemoglobin is expressed in alveolar epithelial type II cells. Biochem. Biophys. Res. Commun. 2005;333:1348–1352. doi: 10.1016/j.bbrc.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Newton D.A., Rao K.M., Dluhy R.A., Baatz J.E. Hemoglobin is expressed by alveolar epithelial cells. J. Biol. Chem. 2006;281:5668–5676. doi: 10.1074/jbc.M509314200. [DOI] [PubMed] [Google Scholar]
- 133.Ishikawa N., Ohlmeier S., Salmenkivi K., Myllarniemi M., Rahman I., Mazur W., Kinnula V.L. Hemoglobin alpha and beta are ubiquitous in the human lung, decline in idiopathic pulmonary fibrosis but not in COPD. Respir. Res. 2010;11:123. doi: 10.1186/1465-9921-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grek C.L., Newton D.A., Spyropoulos D.D., Baatz J.E. Hypoxia up-regulates expression of hemoglobin in alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2011;44:439–447. doi: 10.1165/rcmb.2009-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dassen H., Kamps R., Punyadeera C., Dijcks F., de Goeij A., Ederveen A., Dunselman G., Groothuis P. Haemoglobin expression in human endometrium. Hum. Reprod. 2008;23:635–641. doi: 10.1093/humrep/dem430. [DOI] [PubMed] [Google Scholar]
- 136.Nishi H., Inagi R., Kato H., Tanemoto M., Kojima I., Son D., Fujita T., Nangaku M. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J. Am. Soc. Nephrol. 2008;19:1500–1508. doi: 10.1681/ASN.2007101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu W., Baker S.S., Baker R.D., Nowak N.J., Zhu L. Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Straub A.C., Lohman A.W., Billaud M., Johnstone S.R., Dwyer S.T., Lee M.Y., Bortz P.S., Best A.K., Columbus L., Gaston B., Isakson B.E. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491:473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Straub A.C., Butcher J.T., Billaud M., Mutchler S.M., Artamonov M.V., Nguyen A.T., Johnson T., Best A.K., Miller M.P., Palmer L.A., Columbus L., Somlyo A.V., Le T.H., Isakson B.E. Hemoglobin alpha/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 2014;34:2594–2600. doi: 10.1161/ATVBAHA.114.303974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lechauve C., Butcher J.T., Freiwan A., Biwer L.A., Keith J.M., Good M.E., Ackerman H., Tillman H.S., Kiger L., Isakson B.E., Weiss M.J. Endothelial cell alpha-globin and its molecular chaperone alpha-hemoglobin-stabilizing protein regulate arteriolar contractility. J. Clin. Invest. 2018;128:5073–5082. doi: 10.1172/JCI99933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alvarez R.A., Miller M.P., Hahn S.A., Galley J.C., Bauer E., Bachman T., Hu J., Sembrat J., Goncharov D., Mora A.L., Rojas M., Goncharova E., Straub A.C. Targeting pulmonary endothelial hemoglobin alpha improves nitric oxide signaling and reverses pulmonary artery endothelial dysfunction. Am. J. Respir. Cell Mol. Biol. 2017;57:733–744. doi: 10.1165/rcmb.2016-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Patgaonkar M., Aranha C., Bhonde G., Reddy K.V. Identification and characterization of anti-microbial peptides from rabbit vaginal fluid. Vet. Immunol. Immunopathol. 2011;139:176–186. doi: 10.1016/j.vetimm.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 143.Li X., Wu Z., Wang Y., Mei Q., Fu X., Han W. Characterization of adult alpha- and beta-globin elevated by hydrogen peroxide in cervical cancer cells that play a cytoprotective role against oxidative insults. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saha D., Koli S., Patgaonkar M., Reddy K.V. Expression of hemoglobin-alpha and beta subunits in human vaginal epithelial cells and their functional significance. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gorr T.A., Wichmann D., Pilarsky C., Theurillat J.P., Fabrizius A., Laufs T., Bauer T., Koslowski M., Horn S., Burmester T., Hankeln T., Kristiansen G. Old proteins - new locations: myoglobin, haemoglobin, neuroglobin and cytoglobin in solid tumours and cancer cells. Acta Physiol. (Oxf) 2011;202:563–581. doi: 10.1111/j.1748-1716.2010.02205.x. [DOI] [PubMed] [Google Scholar]
- 146.Emara M., Turner A.R., Allalunis-Turner J. Adult, embryonic and fetal hemoglobin are expressed in human glioblastoma cells. Int. J. Oncol. 2014;44:514–520. doi: 10.3892/ijo.2013.2186. [DOI] [PubMed] [Google Scholar]
- 147.Zheng Y., Miyamoto D.T., Wittner B.S., Sullivan J.P., Aceto N., Jordan N.V., Yu M., Karabacak N.M., Comaills V., Morris R., Desai R., Desai N., Emmons E., Milner J.D., Lee R.J., Wu C.L., Sequist L.V., Haas W., Ting D.T., Toner M. Expression of beta-globin by cancer cells promotes cell survival during blood-borne dissemination. Nat. Commun. 2017;8 doi: 10.1038/ncomms14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wittenberg J.B., Wittenberg B.A. Myoglobin function reassessed. J. Exp. Biol. 2003;206:2011–2020. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]
- 149.Helbo S., Weber R.E., Fago A. Expression patterns and adaptive functional diversity of vertebrate myoglobins. Biochim. Biophys. Acta. 2013;1834:1832–1839. doi: 10.1016/j.bbapap.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 150.Kooyman G.L., Ponganis P.J. The physiological basis of diving to depth: birds and mammals. Annu. Rev. Physiol. 1998;60:19–32. doi: 10.1146/annurev.physiol.60.1.19. [DOI] [PubMed] [Google Scholar]
- 151.Ponganis P.J., Kreutzer U., Stockard T.K., Lin P.C., Sailasuta N., Tran T.K., Hurd R., Jue T. Blood flow and metabolic regulation in seal muscle during apnea. J. Exp. Biol. 2008;211:3323–3332. doi: 10.1242/jeb.018887. [DOI] [PubMed] [Google Scholar]
- 152.Endeward V., Gros G., Jurgens K.D. Significance of myoglobin as an oxygen store and oxygen transporter in the intermittently perfused human heart: a model study. Cardiovasc. Res. 2010;87:22–29. doi: 10.1093/cvr/cvq036. [DOI] [PubMed] [Google Scholar]
- 153.Godecke A. Myoglobin: safeguard of myocardial oxygen supply during systolic compression? Cardiovasc. Res. 2010;87:4–5. doi: 10.1093/cvr/cvq126. [DOI] [PubMed] [Google Scholar]
- 154.Qiu Y., Sutton L., Riggs A.F. Identification of myoglobin in human smooth muscle. J. Biol. Chem. 1998;273:23426–23432. doi: 10.1074/jbc.273.36.23426. [DOI] [PubMed] [Google Scholar]
- 155.Godecke A., Molojavyi A., Heger J., Flogel U., Ding Z., Jacoby C., Schrader J. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J. Biol. Chem. 2003;278:21761–21766. doi: 10.1074/jbc.M302573200. [DOI] [PubMed] [Google Scholar]
- 156.Brunori M. Nitric oxide moves myoglobin centre stage. Trends Biochem. Sci. 2001;26:209–210. doi: 10.1016/s0968-0004(01)01824-2. [DOI] [PubMed] [Google Scholar]
- 157.Flogel U., Merx M.W., Godecke A., Decking U.K., Schrader J. Myoglobin: a scavenger of bioactive NO. Proc. Natl. Acad. Sci. U. S. A. 2001;98:735–740. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakamura M., Nakamura S. Conversion of metmyoglobin to NO myoglobin in the presence of nitrite and reductants. Biochim. Biophys. Acta. 1996;1289:329–335. doi: 10.1016/0304-4165(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 159.Rassaf T., Flogel U., Drexhage C., Hendgen-Cotta U., Kelm M., Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 2007;100:1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 160.Shiva S., Huang Z., Grubina R., Sun J., Ringwood L.A., MacArthur P.H., Xu X., Murphy E., Darley-Usmar V.M., Gladwin M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 161.Cossins A., Berenbrink M. Physiology: myoglobin's new clothes. Nature. 2008;454:416–417. doi: 10.1038/454416a. [DOI] [PubMed] [Google Scholar]
- 162.Flogel U., Fago A., Rassaf T. Keeping the heart in balance: the functional interactions of myoglobin with nitrogen oxides. J. Exp. Biol. 2010;213:2726–2733. doi: 10.1242/jeb.041681. [DOI] [PubMed] [Google Scholar]
- 163.Ormerod J.O., Ashrafian H., Maher A.R., Arif S., Steeples V., Born G.V., Egginton S., Feelisch M., Watkins H., Frenneaux M.P. The role of vascular myoglobin in nitrite-mediated blood vessel relaxation. Cardiovasc. Res. 2011;89:560–565. doi: 10.1093/cvr/cvq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Totzeck M., Hendgen-Cotta U.B., Luedike P., Berenbrink M., Klare J.P., Steinhoff H.J., Semmler D., Shiva S., Williams D., Kipar A., Gladwin M.T., Schrader J., Kelm M., Cossins A.R., Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Totzeck M., Hendgen-Cotta U.B., Kelm M., Rassaf T. Crosstalk between nitrite, myoglobin and reactive oxygen species to regulate vasodilation under hypoxia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hendgen-Cotta U.B., Merx M.W., Shiva S., Schmitz J., Becher S., Klare J.P., Steinhoff H.J., Goedecke A., Schrader J., Gladwin M.T., Kelm M., Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Giulivi C., Cadenas E. Ferrylmyoglobin: formation and chemical reactivity toward electron-donating compounds. Methods Enzymol. 1994;233:189–202. doi: 10.1016/s0076-6879(94)33022-0. [DOI] [PubMed] [Google Scholar]
- 168.Egawa T., Shimada H., Ishimura Y. Formation of compound I in the reaction of native myoglobins with hydrogen peroxide. J. Biol. Chem. 2000;275:34858–34866. doi: 10.1074/jbc.M004026200. [DOI] [PubMed] [Google Scholar]
- 169.Ahmad G., Chami B., El Kazzi M., Wang X., Moreira M.T.S., Hamilton N., Maw A.M., Hambly T.W., Witting P.K. Catalase-like antioxidant activity is unaltered in hypochlorous acid oxidized horse heart myoglobin. Antioxidants. 2019;8:414. doi: 10.3390/antiox8090414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mannino M.H., Patel R.S., Eccardt A.M., Perez Magnelli R.A., Robinson C.L.C., Janowiak B.E., Warren D.E., Fisher J.S. Myoglobin as a versatile peroxidase: implications for a more important role for vertebrate striated muscle in antioxidant defense. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019;234:9–17. doi: 10.1016/j.cbpb.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Flogel U., Godecke A., Klotz L.O., Schrader J. Role of myoglobin in the antioxidant defense of the heart. Faseb. J. 2004;18:1156–1158. doi: 10.1096/fj.03-1382fje. [DOI] [PubMed] [Google Scholar]
- 172.Clementi M.E., Sampaolese B., Giardina B. S100b induces expression of myoglobin in APbeta treated neuronal cells in vitro: a possible neuroprotective mechanism. Curr. Aging Sci. 2016;9:279–283. doi: 10.2174/1874609809666160222112850. [DOI] [PubMed] [Google Scholar]
- 173.Gutteridge J.M.C. Iron promoters of the fenton reaction and lipid-peroxidation can Be released from hemoglobin by peroxides. FEBS Lett. 1986;201:291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- 174.Panter S.S. Release of iron from hemoglobin. Methods Enzymol. 1994;231:502–514. doi: 10.1016/0076-6879(94)31034-3. [DOI] [PubMed] [Google Scholar]
- 175.Roy A., Sen S., Chakraborti A.S. In vitro nonenzymatic glycation enhances the role of myoglobin as a source of oxidative stress. Free Radic. Res. 2004;38:139–146. doi: 10.1080/10715160310001638038. [DOI] [PubMed] [Google Scholar]
- 176.Parry S.N., Ellis N., Li Z., Maitz P., Witting P.K. vol. 31. Kidney Blood Press Res; 2008. pp. 16–28. (Myoglobin Induces Oxidative Stress and Decreases Endocytosis and Monolayer Permissiveness in Cultured Kidney Epithelial Cells without Affecting Viability). [DOI] [PubMed] [Google Scholar]
- 177.Plotnikov E.Y., Chupyrkina A.A., Pevzner I.B., Isaev N.K., Zorov D.B. Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney's mitochondria. Biochim. Biophys. Acta. 2009:796–803. doi: 10.1016/j.bbadis.2009.06.005. 1792. [DOI] [PubMed] [Google Scholar]
- 178.Panizo N., Rubio-Navarro A., Amaro-Villalobos J.M., Egido J., Moreno J.A. vol. 40. Kidney Blood Press Res; 2015. pp. 520–532. (Molecular Mechanisms and Novel Therapeutic Approaches to Rhabdomyolysis-Induced Acute Kidney Injury). [DOI] [PubMed] [Google Scholar]
- 179.Schlater A.E., De Miranda M.A., Jr., Frye M.A., Trumble S.J., Kanatous S.B. Changing the paradigm for myoglobin: a novel link between lipids and myoglobin. J. Appl. Physiol. 2014;117(1985):307–315. doi: 10.1152/japplphysiol.00973.2013. [DOI] [PubMed] [Google Scholar]
- 180.Gotz F.M., Hertel M., Groschel-Stewart U. Fatty acid binding of myoglobin depends on its oxygenation. Biol. Chem. Hoppe Seyler. 1994;375:387–392. doi: 10.1515/bchm3.1994.375.6.387. [DOI] [PubMed] [Google Scholar]
- 181.Shih L., Chung Y., Sriram R., Jue T. Palmitate interaction with physiological states of myoglobin. Biochim. Biophys. Acta. 2014;1840:656–666. doi: 10.1016/j.bbagen.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chintapalli S.V., Bhardwaj G., Patel R., Shah N., Patterson R.L., van Rossum D.B., Anishkin A., Adams S.H. Molecular dynamic simulations reveal the structural determinants of Fatty Acid binding to oxy-myoglobin. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jue T., Simond G., Wright T.J., Shih L., Chung Y., Sriram R., Kreutzer U., Davis R.W. Effect of fatty acid interaction on myoglobin oxygen affinity and triglyceride metabolism. J. Physiol. Biochem. 2016;73:359–370. doi: 10.1007/s13105-017-0559-z. [DOI] [PubMed] [Google Scholar]
- 184.Baron C.P., Andersen H.J. Myoglobin-induced lipid oxidation. A review. J. Agric. Food Chem. 2002;50:3887–3897. doi: 10.1021/jf011394w. [DOI] [PubMed] [Google Scholar]
- 185.Hendgen-Cotta U.B., Esfeld S., Coman C., Ahrends R., Klein-Hitpass L., Flogel U., Rassaf T., Totzeck M. A novel physiological role for cardiac myoglobin in lipid metabolism. Sci. Rep. 2017;7:43219. doi: 10.1038/srep43219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Flogel U., Laussmann T., Godecke A., Abanador N., Schafers M., Fingas C.D., Metzger S., Levkau B., Jacoby C., Schrader J. Lack of myoglobin causes a switch in cardiac substrate selection. Circ. Res. 2005;96:e68–75. doi: 10.1161/01.RES.0000165481.36288.d2. [DOI] [PubMed] [Google Scholar]
- 187.De Miranda M.A., Jr., Schlater A.E., Green T.L., Kanatous S.B. In the face of hypoxia: myoglobin increases in response to hypoxic conditions and lipid supplementation in cultured Weddell seal skeletal muscle cells. J. Exp. Biol. 2012;215:806–813. doi: 10.1242/jeb.060681. [DOI] [PubMed] [Google Scholar]
- 188.Flonta S.E., Arena S., Pisacane A., Michieli P., Bardelli A. Expression and functional regulation of myoglobin in epithelial cancers. Am. J. Pathol. 2009;175:201–206. doi: 10.2353/ajpath.2009.081124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kristiansen G., Rose M., Geisler C., Fritzsche F.R., Gerhardt J., Luke C., Ladhoff A.M., Knuchel R., Dietel M., Moch H., Varga Z., Theurillat J.P., Gorr T.A., Dahl E. Endogenous myoglobin in human breast cancer is a hallmark of luminal cancer phenotype. Br. J. Canc. 2010;102:1736–1745. doi: 10.1038/sj.bjc.6605702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kristiansen G., Hu J., Wichmann D., Stiehl D.P., Rose M., Gerhardt J., Bohnert A., ten Haaf A., Moch H., Raleigh J., Varia M.A., Subarsky P., Scandurra F.M., Gnaiger E., Gleixner E., Bicker A., Gassmann M., Hankeln T., Dahl E., Gorr T.A. Endogenous myoglobin in breast cancer is hypoxia-inducible by alternative transcription and functions to impair mitochondrial activity: a role in tumor suppression? J. Biol. Chem. 2011;286:43417–43428. doi: 10.1074/jbc.M111.227553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bicker A., Brahmer A.M., Meller S., Kristiansen G., Gorr T.A., Hankeln T. The distinct gene regulatory network of myoglobin in prostate and breast cancer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Meller S., Bicker A., Montani M., Ikenberg K., Rostamzadeh B., Sailer V., Wild P., Dietrich D., Uhl B., Sulser T., Moch H., Gorr T.A., Stephan C., Jung K., Hankeln T., Kristiansen G. Myoglobin expression in prostate cancer is correlated to androgen receptor expression and markers of tumor hypoxia. Virchows Arch. 2014;465:419–427. doi: 10.1007/s00428-014-1646-y. [DOI] [PubMed] [Google Scholar]
- 193.Oleksiewicz U., Daskoulidou N., Liloglou T., Tasopoulou K., Bryan J., Gosney J.R., Field J.K., Xinarianos G. Neuroglobin and myoglobin in non-small cell lung cancer: expression, regulation and prognosis. Lung Canc. 2011;74:411–418. doi: 10.1016/j.lungcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 194.Behnes C.L., Bedke J., Schneider S., Kuffer S., Strauss A., Bremmer F., Strobel P., Radzun H.J. Myoglobin expression in renal cell carcinoma is regulated by hypoxia. Exp. Mol. Pathol. 2013;95:307–312. doi: 10.1016/j.yexmp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 195.Meller S., Vane A., Gevensleben H., Bicker A., Hankeln T., Gorr T.A., Sailer V., Droge F., Schrock F., Bootz F., Schrock A., Perner S., Dietrich D., Kristiansen G. Ectopic myoglobin expression is associated with a favourable outcome in head and neck squamous cell carcinoma patients. Anticancer Res. 2016;36:6235–6241. doi: 10.21873/anticanres.11217. [DOI] [PubMed] [Google Scholar]
- 196.Bicker A., Nauth T., Gerst D., Aboouf M.A., Fandrey J., Kristiansen G., Gorr T.A., Hankeln T. The role of myoglobin in epithelial cancers: insights from transcriptomics. Int. J. Mol. Med. 2020;45:385–400. doi: 10.3892/ijmm.2019.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Weller P., Jeffreys A.J., Wilson V., Blanchetot A. Organization of the human myoglobin gene. EMBO J. 1984;3:439–446. doi: 10.1002/j.1460-2075.1984.tb01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bicker A., Dietrich D., Gleixner E., Kristiansen G., Gorr T.A., Hankeln T. Extensive transcriptional complexity during hypoxia-regulated expression of the myoglobin gene in cancer. Hum. Mol. Genet. 2014;23:479–490. doi: 10.1093/hmg/ddt438. [DOI] [PubMed] [Google Scholar]
- 199.Braganza A., Quesnelle K., Bickta J., Reyes C., Wang Y., Jessup M., St Croix C., Arlotti J., Singh S.V., Shiva S. Myoglobin induces mitochondrial fusion, thereby inhibiting breast cancer cell proliferation. J. Biol. Chem. 2019;294:7269–7282. doi: 10.1074/jbc.RA118.006673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Galluzzo M., Pennacchietti S., Rosano S., Comoglio P.M., Michieli P. Prevention of hypoxia by myoglobin expression in human tumor cells promotes differentiation and inhibits metastasis. J. Clin. Invest. 2009;119:865–875. doi: 10.1172/JCI36579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang Z., Heater B.S., Cuddington C.T., Palmer A.F., Lee M.M.M., Chan M.K. Targeted myoglobin delivery as a strategy for enhancing the sensitivity of hypoxic cancer cells to radiation. iScience. 2020;23:101158. doi: 10.1016/j.isci.2020.101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ludemann J., Verissimo K.M., Dreger K., Fago A., Schneider I., Burmester T. Globin E is a myoglobin-related, respiratory protein highly expressed in lungfish oocytes. Sci. Rep. 2019;9:280. doi: 10.1038/s41598-018-36592-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hoogewijs D., De Henau S., Dewilde S., Moens L., Couvreur M., Borgonie G., Vinogradov S.N., Roy S.W., Vanfleteren J.R. The Caenorhabditis globin gene family reveals extensive nematode-specific radiation and diversification. BMC Evol. Biol. 2008;8:279. doi: 10.1186/1471-2148-8-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Persson A., Gross E., Laurent P., Busch K.E., Bretes H., de Bono M. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature. 2009;458:1030–1033. doi: 10.1038/nature07820. [DOI] [PubMed] [Google Scholar]
- 205.Lechauve C., Jager M., Laguerre L., Kiger L., Correc G., Leroux C., Vinogradov S., Czjzek M., Marden M.C., Bailly X. Neuroglobins, pivotal proteins associated with emerging neural systems and precursors of metazoan globin diversity. J. Biol. Chem. 2013;288:6957–6967. doi: 10.1074/jbc.M112.407601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dewilde S., Kiger L., Burmester T., Hankeln T., Baudin-Creuza V., Aerts T., Marden M.C., Caubergs R., Moens L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J. Biol. Chem. 2001;276:38949–38955. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- 207.Ascenzi P., di Masi A., Leboffe L., Fiocchetti M., Nuzzo M.T., Brunori M., Marino M. Neuroglobin: from structure to function in health and disease. Mol. Aspect. Med. 2016;52:1–48. doi: 10.1016/j.mam.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 208.Schmidt M., Giessl A., Laufs T., Hankeln T., Wolfrum U., Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J. Biol. Chem. 2003;278:1932–1935. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- 209.Ostojic J., Sakaguchi D.S., de Lathouder Y., Hargrove M.S., Trent J.T., 3rd, Kwon Y.H., Kardon R.H., Kuehn M.H., Betts D.M., Grozdanic S. Neuroglobin and cytoglobin: oxygen-binding proteins in retinal neurons. Invest. Ophthalmol. Vis. Sci. 2006;47:1016–1023. doi: 10.1167/iovs.05-0465. [DOI] [PubMed] [Google Scholar]
- 210.Fabrizius A., Andre D., Laufs T., Bicker A., Reuss S., Porto E., Burmester T., Hankeln T. Critical re-evaluation of neuroglobin expression reveals conserved patterns among mammals. Neuroscience. 2016;337:339–354. doi: 10.1016/j.neuroscience.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 211.Fiocchetti M., Cipolletti M., Brandi V., Polticelli F., Ascenzi P. Neuroglobin and friends. J. Mol. Recogn. 2017;30 doi: 10.1002/jmr.2654. [DOI] [PubMed] [Google Scholar]
- 212.Van Acker Z.P., Luyckx E., Dewilde S. Neuroglobin expression in the brain: a story of tissue homeostasis preservation. Mol. Neurobiol. 2019;56:2101–2122. doi: 10.1007/s12035-018-1212-8. [DOI] [PubMed] [Google Scholar]
- 213.Gotting M., Nikinmaa M. More than hemoglobin - the unexpected diversity of globins in vertebrate red blood cells. Phys. Rep. 2015;3 doi: 10.14814/phy2.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hundahl C.A., Allen G.C., Hannibal J., Kjaer K., Rehfeld J.F., Dewilde S., Nyengaard J.R., Kelsen J., Hay-Schmidt A. Anatomical characterization of cytoglobin and neuroglobin mRNA and protein expression in the mouse brain. Brain Res. 2010;1331:58–73. doi: 10.1016/j.brainres.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 215.De Marinis E., Fiocchetti M., Acconcia F., Ascenzi P., Marino M. Neuroglobin upregulation induced by 17beta-estradiol sequesters cytochrome c in the mitochondria preventing H2O2-induced apoptosis of neuroblastoma cells. Cell Death Dis. 2013;4:e508. doi: 10.1038/cddis.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fiocchetti M., Nuzzo M.T., Totta P., Acconcia F., Ascenzi P., Marino M. Neuroglobin, a pro-survival player in estrogen receptor alpha-positive cancer cells. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fiocchetti M., Camilli G., Acconcia F., Leone S., Ascenzi P., Marino M. ERbeta-dependent neuroglobin up-regulation impairs 17beta-estradiol-induced apoptosis in DLD-1 colon cancer cells upon oxidative stress injury. J. Steroid Biochem. Mol. Biol. 2015;149:128–137. doi: 10.1016/j.jsbmb.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 218.Luyckx E., Van Acker Z.P., Ponsaerts P., Dewilde S. Neuroglobin expression models as a tool to study its function. Oxid. Med. Cell Longev. 2019 doi: 10.1155/2019/5728129. 2019: 5728129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jin K., Mao X., Xie L., Greenberg D.A. Interactions between vascular endothelial growth factor and neuroglobin. Neurosci. Lett. 2012;519:47–50. doi: 10.1016/j.neulet.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Oliveira K.C., da Conceicao R.R., Piedade G.C., de Souza J.S., Sato M.A., de Barros Maciel R.M., Giannocco G. Thyroid hormone modulates neuroglobin and cytoglobin in rat brain. Metab. Brain Dis. 2015;30:1401–1408. doi: 10.1007/s11011-015-9718-5. [DOI] [PubMed] [Google Scholar]
- 221.De Marinis E., Ascenzi P., Pellegrini M., Galluzzo P., Bulzomi P., Arevalo M.A., Garcia-Segura L.M., Marino M. 17beta-estradiol--a new modulator of neuroglobin levels in neurons: role in neuroprotection against H(2)O(2)-induced toxicity. Neurosignals. 2010;18:223–235. doi: 10.1159/000323906. [DOI] [PubMed] [Google Scholar]
- 222.De Marinis E., Acaz-Fonseca E., Arevalo M.A., Ascenzi P., Fiocchetti M., Marino M., Garcia-Segura L.M. 17beta-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor beta-mediated neuroglobin up-regulation. J. Neuroendocrinol. 2013;25:260–270. doi: 10.1111/jne.12007. [DOI] [PubMed] [Google Scholar]
- 223.Guglielmotto M., Reineri S., Iannello A., Ferrero G., Vanzan L., Miano V., Ricci L., Tamagno E., De Bortoli M., Cutrupi S. E2 regulates epigenetic signature on neuroglobin enhancer-promoter in neuronal cells. Front. Cell. Neurosci. 2016;10:147. doi: 10.3389/fncel.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gao Y., Mengana Y., Cruz Y.R., Munoz A., Teste I.S., Garcia J.D., Wu Y., Rodriguez J.C., Zhang C. Different expression patterns of Ngb and EPOR in the cerebral cortex and hippocampus revealed distinctive therapeutic effects of intranasal delivery of Neuro-EPO for ischemic insults to the gerbil brain. J. Histochem. Cytochem. 2011;59:214–227. doi: 10.1369/0022155410390323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jayaraman T., Tejero J., Chen B.B., Blood A.B., Frizzell S., Shapiro C., Tiso M., Hood B.L., Wang X., Zhao X., Conrads T.P., Mallampalli R.K., Gladwin M.T. 14-3-3 binding and phosphorylation of neuroglobin during hypoxia modulate six-to-five heme pocket coordination and rate of nitrite reduction to nitric oxide. J. Biol. Chem. 2011;286:42679–42689. doi: 10.1074/jbc.M111.271973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ye S.Q., Zhou X.Y., Lai X.J., Zheng L., Chen X.Q. Silencing neuroglobin enhances neuronal vulnerability to oxidative injury by down-regulating 14-3-3gamma. Acta Pharmacol. Sin. 2009;30:913–918. doi: 10.1038/aps.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dong Y., Zhao R., Chen X.Q., Yu A.C. 14-3-3gamma and neuroglobin are new intrinsic protective factors for cerebral ischemia. Mol. Neurobiol. 2010;41:218–231. doi: 10.1007/s12035-010-8142-4. [DOI] [PubMed] [Google Scholar]
- 228.Liu Z.F., Zhang X., Qiao Y.X., Xu W.Q., Ma C.T., Gu H.L., Zhou X.M., Shi L., Cui C.X., Xia D., Chen Y.G. Neuroglobin protects cardiomyocytes against apoptosis and cardiac hypertrophy induced by isoproterenol in rats. Int. J. Clin. Exp. Med. 2015;8:5351–5360. [PMC free article] [PubMed] [Google Scholar]
- 229.Luyckx E., Everaert B.R., Van der Veken B., Van Leuven W., Timmermans J.P., Vrints C.J., De Meyer G.R.Y., Martinet W., Dewilde S. Cytoprotective effects of transgenic neuroglobin overexpression in an acute and chronic mouse model of ischemic heart disease. Heart Ves. 2018;33:80–88. doi: 10.1007/s00380-017-1065-5. [DOI] [PubMed] [Google Scholar]
- 230.Tae B., Oliveira K.C., Conceicao R.R., Valenti V.E., de Souza J.S., Laureano-Melo R., Sato M.A., Maciel R.M., Giannocco G. Evaluation of globins expression in brain, heart, and lung in rats exposed to side stream cigarette smoke. Environ. Toxicol. 2017;32:1252–1261. doi: 10.1002/tox.22321. [DOI] [PubMed] [Google Scholar]
- 231.Brunori M., Giuffre A., Nienhaus K., Nienhaus G.U., Scandurra F.M., Vallone B. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8483–8488. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Watanabe S., Takahashi N., Uchida H., Wakasugi K. Human neuroglobin functions as an oxidative stress-responsive sensor for neuroprotection. J. Biol. Chem. 2012;287:30128–30138. doi: 10.1074/jbc.M112.373381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Trandafir F., Hoogewijs D., Altieri F., Rivetti di Val Cervo P., Ramser K., Van Doorslaer S., Vanfleteren J.R., Moens L., Dewilde S. Neuroglobin and cytoglobin as potential enzyme or substrate. Gene. 2007;398:103–113. doi: 10.1016/j.gene.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 234.Staubach S., Hanisch F.G. Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert Rev. Proteomics. 2011;8:263–277. doi: 10.1586/epr.11.2. [DOI] [PubMed] [Google Scholar]
- 235.Mollinedo F., Gajate C. Lipid rafts as major platforms for signaling regulation in cancer. Adv. Biol. Regul. 2015;57:130–146. doi: 10.1016/j.jbior.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 236.Nuzzo M.T., Fiocchetti M., Totta P., Melone M.A.B., Cardinale A., Fusco F.R., Gustincich S., Persichetti F., Ascenzi P., Marino M. Huntingtin polyQ mutation impairs the 17beta-estradiol/neuroglobin pathway devoted to neuron survival. Mol. Neurobiol. 2017;54:6634–6646. doi: 10.1007/s12035-016-0337-x. [DOI] [PubMed] [Google Scholar]
- 237.Yu Z., Xu J., Liu N., Wang Y., Li X., Pallast S., van Leyen K., Wang X. Mitochondrial distribution of neuroglobin and its response to oxygen-glucose deprivation in primary-cultured mouse cortical neurons. Neuroscience. 2012;218:235–242. doi: 10.1016/j.neuroscience.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yu Z., Zhang Y., Liu N., Yuan J., Lin L., Zhuge Q., Xiao J., Wang X. Roles of neuroglobin binding to mitochondrial complex III subunit cytochrome c1 in oxygen-glucose deprivation-induced neurotoxicity in primary neurons. Mol. Neurobiol. 2016;53:3249–3257. doi: 10.1007/s12035-015-9273-4. [DOI] [PubMed] [Google Scholar]
- 239.Tejero J. Negative surface charges in neuroglobin modulate the interaction with cytochrome c. Biochem. Biophys. Res. Commun. 2020;523:567–572. doi: 10.1016/j.bbrc.2019.12.089. [DOI] [PubMed] [Google Scholar]
- 240.Fago A., Mathews A.J., Moens L., Dewilde S., Brittain T. The reaction of neuroglobin with potential redox protein partners cytochrome b5 and cytochrome c. FEBS Lett. 2006;580:4884–4888. doi: 10.1016/j.febslet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 241.Lechauve C., Augustin S., Cwerman-Thibault H., Bouaita A., Forster V., Celier C., Rustin P., Marden M.C., Sahel J.A., Corral-Debrinski M. Neuroglobin involvement in respiratory chain function and retinal ganglion cell integrity. Biochim. Biophys. Acta. 2012;1823:2261–2273. doi: 10.1016/j.bbamcr.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 242.Khan A.A., Wang Y., Sun Y., Mao X.O., Xie L., Miles E., Graboski J., Chen S., Ellerby L.M., Jin K., Greenberg D.A. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Khan A.A., Mao X.O., Banwait S., DerMardirossian C.M., Bokoch G.M., Jin K., Greenberg D.A. Regulation of hypoxic neuronal death signaling by neuroglobin. Faseb. J. 2008;22:1737–1747. doi: 10.1096/fj.07-100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Antao S.T., Duong T.T., Aran R., Witting P.K. Neuroglobin overexpression in cultured human neuronal cells protects against hydrogen peroxide insult via activating phosphoinositide-3 kinase and opening the mitochondrial K(ATP) channel. Antioxidants Redox Signal. 2010;13:769–781. doi: 10.1089/ars.2009.2977. [DOI] [PubMed] [Google Scholar]
- 245.Li L., Liu Q.R., Xiong X.X., Liu J.M., Lai X.J., Cheng C., Pan F., Chen Y., Yu S.B., Yu A.C., Chen X.Q. Neuroglobin promotes neurite outgrowth via differential binding to PTEN and Akt. Mol. Neurobiol. 2014;49:149–162. doi: 10.1007/s12035-013-8506-7. [DOI] [PubMed] [Google Scholar]
- 246.Li Y., Dai Y.B., Sun J.Y., Xiang Y., Yang J., Dai S.Y., Zhang X. Neuroglobin attenuates beta amyloid-induced apoptosis through inhibiting caspases activity by activating PI3K/Akt signaling pathway. J. Mol. Neurosci. 2016;58:28–38. doi: 10.1007/s12031-015-0645-z. [DOI] [PubMed] [Google Scholar]
- 247.Cai B., Li W., Mao X., Winters A., Ryou M.G., Liu R., Greenberg D.A., Wang N., Jin K., Yang S.H. Neuroglobin overexpression inhibits AMPK signaling and promotes cell anabolism. Mol. Neurobiol. 2016;53:1254–1265. doi: 10.1007/s12035-014-9077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fiocchetti M., Fernandez V.S., Montalesi E., Marino M. Neuroglobin: a novel player in the oxidative stress response of cancer cells. Oxid. Med. Cell Longev. 2019 doi: 10.1155/2019/6315034. 2019: 6315034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Emara M., Turner A.R., Allalunis-Turner J. Hypoxic regulation of cytoglobin and neuroglobin expression in human normal and tumor tissues. Canc. Cell Int. 2010;10:33. doi: 10.1186/1475-2867-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Qin H., Guo Y., Zhang C., Zhang L., Li M., Guan P. The expression of neuroglobin in astrocytoma. Brain Tumor Pathol. 2012;29:10–16. doi: 10.1007/s10014-011-0066-9. [DOI] [PubMed] [Google Scholar]
- 251.Hu J., Cao X., Pang D., Luo Q., Zou Y., Feng B., Li L., Chen Z., Huang C. Tumor grade related expression of neuroglobin is negatively regulated by PPARgamma and confers antioxidant activity in glioma progression. Redox Biol. 2017;12:682–689. doi: 10.1016/j.redox.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fiocchetti M., Cipolletti M., Ascenzi P., Marino M. Dissecting the 17beta-estradiol pathways necessary for neuroglobin anti-apoptotic activity in breast cancer. J. Cell. Physiol. 2018;233:5087–5103. doi: 10.1002/jcp.26378. [DOI] [PubMed] [Google Scholar]
- 253.Zhang B., Liu Y., Li Y., Zhe X., Zhang S., Zhang L. Neuroglobin promotes the proliferation and suppresses the apoptosis of glioma cells by activating the PI3K/AKT pathway. Mol. Med. Rep. 2018;17:2757–2763. doi: 10.3892/mmr.2017.8132. [DOI] [PubMed] [Google Scholar]
- 254.Zhang B., Chang M., Wang J., Liu Y. Neuroglobin functions as a prognostic marker and promotes the tumor growth of glioma via suppressing apoptosis. Biomed. Pharmacother. 2017;88:173–180. doi: 10.1016/j.biopha.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 255.Zhang J., Lan S.J., Liu Q.R., Liu J.M., Chen X.Q. Neuroglobin, a novel intracellular hexa-coordinated globin, functions as a tumor suppressor in hepatocellular carcinoma via Raf/MAPK/Erk. Mol. Pharmacol. 2013;83:1109–1119. doi: 10.1124/mol.112.083634. [DOI] [PubMed] [Google Scholar]
- 256.Azarov I., Wang L., Rose J.J., Xu Q., Huang X.N., Belanger A., Wang Y., Guo L., Liu C., Ucer K.B., McTiernan C.F., O'Donnell C.P., Shiva S., Tejero J., Kim-Shapiro D.B., Gladwin M.T. Five-coordinate H64Q neuroglobin as a ligand-trap antidote for carbon monoxide poisoning. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aah6571. 368ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rose J.J., Bocian K.A., Xu Q., Wang L., DeMartino A.W., Chen X., Corey C.G., Guimaraes D.A., Azarov I., Huang X.N., Tong Q., Guo L., Nouraie M., McTiernan C.F., O'Donnell C.P., Tejero J., Shiva S., Gladwin M.T. A neuroglobin-based high-affinity ligand trap reverses carbon monoxide-induced mitochondrial poisoning. J. Biol. Chem. 2020;295:6357–6371. doi: 10.1074/jbc.RA119.010593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fuchs C., Luckhardt A., Gerlach F., Burmester T., Hankeln T. Duplicated cytoglobin genes in teleost fishes. Biochem. Biophys. Res. Commun. 2005;337:216–223. doi: 10.1016/j.bbrc.2005.08.271. [DOI] [PubMed] [Google Scholar]
- 259.Rochon E.R., Corti P. Globins and nitric oxide homeostasis in fish embryonic development. Mar. Genomics. 2020;49:100721. doi: 10.1016/j.margen.2019.100721. [DOI] [PubMed] [Google Scholar]
- 260.Chao Y., Xia M., Wu R., Chen Q., Zheng Z., Qi D. Molecular characterization and expression changes of cytoglobin genes in response to hypoxia in a Tibetan schizothoracine fish, Schizopygopsis pylzovi. Fish Physiol. Biochem. 2019;45:863–872. doi: 10.1007/s10695-018-0582-1. [DOI] [PubMed] [Google Scholar]
- 261.Corti P., Ieraci M., Tejero J. Characterization of zebrafish neuroglobin and cytoglobins 1 and 2: zebrafish cytoglobins provide insights into the transition from six-coordinate to five-coordinate globins. Nitric Oxide. 2016;53:22–34. doi: 10.1016/j.niox.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 262.Amdahl M.B., Petersen E.E., Bocian K., Kaliszuk S.J., DeMartino A.W., Tiwari S., Sparacino-Watkins C.E., Corti P., Rose J.J., Gladwin M.T., Fago A., Tejero J. The zebrafish cytochrome b5/cytochrome b5 reductase/NADH system efficiently reduces cytoglobins 1 and 2: conserved activity of cytochrome b5/cytochrome b5 reductases during vertebrate evolution. Biochemistry. 2019;58:3212–3223. doi: 10.1021/acs.biochem.9b00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hankeln T., Ebner B., Fuchs C., Gerlach F., Haberkamp M., Laufs T.L., Roesner A., Schmidt M., Weich B., Wystub S., Saaler-Reinhardt S., Reuss S., Bolognesi M., De Sanctis D., Marden M.C., Kiger L., Moens L., Dewilde S., Nevo E., Avivi A. Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J. Inorg. Biochem. 2005;99:110–119. doi: 10.1016/j.jinorgbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 264.Reuss S., Wystub S., Disque-Kaiser U., Hankeln T., Burmester T. Distribution of cytoglobin in the mouse brain. Front. Neuroanat. 2016;10:47. doi: 10.3389/fnana.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mathai C., Jourd'heuil F.L., Lopez-Soler R.I., Jourd'heuil D. Emerging perspectives on cytoglobin, beyond NO dioxygenase and peroxidase. Redox Biol. 2020;32:101468. doi: 10.1016/j.redox.2020.101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Liu X., Follmer D., Zweier J.R., Huang X., Hemann C., Liu K., Druhan L.J., Zweier J.L. Characterization of the function of cytoglobin as an oxygen-dependent regulator of nitric oxide concentration. Biochemistry. 2012;51:5072–5082. doi: 10.1021/bi300291h. [DOI] [PubMed] [Google Scholar]
- 267.Liu X., Tong J., Zweier J.R., Follmer D., Hemann C., Ismail R.S., Zweier J.L. Differences in oxygen-dependent nitric oxide metabolism by cytoglobin and myoglobin account for their differing functional roles. FEBS J. 2013;280:3621–3631. doi: 10.1111/febs.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Amdahl M.B., Sparacino-Watkins C.E., Corti P., Gladwin M.T., Tejero J. Efficient reduction of vertebrate cytoglobins by the cytochrome b5/cytochrome b5 reductase/NADH system. Biochemistry. 2017;56:3993–4004. doi: 10.1021/acs.biochem.7b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Liu X., El-Mahdy M.A., Boslett J., Varadharaj S., Hemann C., Abdelghany T.M., Ismail R.S., Little S.C., Zhou D., Thuy L.T., Kawada N., Zweier J.L. Cytoglobin regulates blood pressure and vascular tone through nitric oxide metabolism in the vascular wall. Nat. Commun. 2017;8:14807. doi: 10.1038/ncomms14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zweier J.L., Ilangovan G. Regulation of nitric oxide metabolism and vascular tone by cytoglobin. Antioxidants Redox Signal. 2020;32:1172–1187. doi: 10.1089/ars.2019.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Halligan K.E., Jourd'heuil F.L., Jourd'heuil D. Cytoglobin is expressed in the vasculature and regulates cell respiration and proliferation via nitric oxide dioxygenation. J. Biol. Chem. 2009;284:8539–8547. doi: 10.1074/jbc.M808231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Li H., Hemann C., Abdelghany T.M., El-Mahdy M.A., Zweier J.L. Characterization of the mechanism and magnitude of cytoglobin-mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J. Biol. Chem. 2012;287:36623–36633. doi: 10.1074/jbc.M112.342378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lilly B., Dammeyer K., Marosis S., McCallinhart P.E., Trask A.J., Lowe M., Sawant D. Endothelial cell-induced cytoglobin expression in vascular smooth muscle cells contributes to modulation of nitric oxide. Vasc. Pharmacol. 2018;110:7–15. doi: 10.1016/j.vph.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mimura I., Nangaku M., Nishi H., Inagi R., Tanaka T., Fujita T. Cytoglobin, a novel globin, plays an antifibrotic role in the kidney. Am. J. Physiol. Ren. Physiol. 2010;299:F1120–F1133. doi: 10.1152/ajprenal.00145.2010. [DOI] [PubMed] [Google Scholar]
- 275.Nishi H., Inagi R., Kawada N., Yoshizato K., Mimura I., Fujita T., Nangaku M. Cytoglobin, a novel member of the globin family, protects kidney fibroblasts against oxidative stress under ischemic conditions. Am. J. Pathol. 2011;178:128–139. doi: 10.1016/j.ajpath.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fordel E., Thijs L., Martinet W., Lenjou M., Laufs T., Van Bockstaele D., Moens L., Dewilde S. Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci. Lett. 2006;410:146–151. doi: 10.1016/j.neulet.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 277.Xu R., Harrison P.M., Chen M., Li L., Tsui T.Y., Fung P.C., Cheung P.T., Wang G., Li H., Diao Y., Krissansen G.W., Xu S., Farzaneh F. Cytoglobin overexpression protects against damage-induced fibrosis. Mol. Ther. 2006;13:1093–1100. doi: 10.1016/j.ymthe.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 278.Li D., Chen X.Q., Li W.J., Yang Y.H., Wang J.Z., Yu A.C. Cytoglobin up-regulated by hydrogen peroxide plays a protective role in oxidative stress. Neurochem. Res. 2007;32:1375–1380. doi: 10.1007/s11064-007-9317-x. [DOI] [PubMed] [Google Scholar]
- 279.Hodges N.J., Innocent N., Dhanda S., Graham M. Cellular protection from oxidative DNA damage by over-expression of the novel globin cytoglobin in vitro. Mutagenesis. 2008;23:293–298. doi: 10.1093/mutage/gen013. [DOI] [PubMed] [Google Scholar]
- 280.Stagner J.I., Seelan R.S., Parthasarathy R.N., White K. Reduction of ischemic cell death in cultured Islets of Langerhans by the induction of cytoglobin. Islets. 2009;1:50–54. doi: 10.4161/isl.1.1.8936. [DOI] [PubMed] [Google Scholar]
- 281.Zhang S., Li X., Jourd'heuil F.L., Qu S., Devejian N., Bennett E., Jourd'heuil D., Cai C. Cytoglobin promotes cardiac progenitor cell survival against oxidative stress via the upregulation of the NFkappaB/iNOS signal pathway and nitric oxide production. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-11342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Thi Thanh Hai N., Thuy L.T.T., Shiota A., Kadono C., Daikoku A., Hoang D.V., Dat N.Q., Sato-Matsubara M., Yoshizato K., Kawada N. Selective overexpression of cytoglobin in stellate cells attenuates thioacetamide-induced liver fibrosis in mice. Sci. Rep. 2018;8:17860. doi: 10.1038/s41598-018-36215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kwon Y.S., Tham A., Lopez A.J., Edwards S., Woods S., Chen J., Wong-Fortunato J., Quiroz Alonso A., Javier S., Au I., Clarke M., Humpal D., Lloyd K.C.K., Thomasy S., Murphy C., Glaser T.M., Moshiri A. Cytoglobin deficiency potentiates Crb1-mediated retinal degeneration in rd8 mice. Dev. Biol. 2020;458:141–152. doi: 10.1016/j.ydbio.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Randi E.B., Vervaet B., Tsachaki M., Porto E., Vermeylen S., Lindenmeyer M.T., Thuy L.T.T., Cohen C.D., Devuyst O., Kistler A.D., Szabo C., Kawada N., Hankeln T., Odermatt A., Dewilde S., Wenger R.H., Hoogewijs D. The antioxidative role of cytoglobin in podocytes: implications for a role in chronic kidney disease. Antioxidants Redox Signal. 2020;32:1155–1171. doi: 10.1089/ars.2019.7868. [DOI] [PubMed] [Google Scholar]
- 285.Nakatani K., Okuyama H., Shimahara Y., Saeki S., Kim D.H., Nakajima Y., Seki S., Kawada N., Yoshizato K. Cytoglobin/STAP, its unique localization in splanchnic fibroblast-like cells and function in organ fibrogenesis. Lab. Invest. 2004;84:91–101. doi: 10.1038/labinvest.3700013. [DOI] [PubMed] [Google Scholar]
- 286.Van Thuy T.T., Thuy L.T., Yoshizato K., Kawada N. Possible involvement of nitric oxide in enhanced liver injury and fibrogenesis during cholestasis in cytoglobin-deficient mice. Sci. Rep. 2017;7:41888. doi: 10.1038/srep41888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Fordel E., Geuens E., Dewilde S., Rottiers P., Carmeliet P., Grooten J., Moens L. Cytoglobin expression is upregulated in all tissues upon hypoxia: an in vitro and in vivo study by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004;319:342–348. doi: 10.1016/j.bbrc.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 288.Fordel E., Geuens E., Dewilde S., De Coen W., Moens L. Hypoxia/ischemia and the regulation of neuroglobin and cytoglobin expression. IUBMB Life. 2004;56:681–687. doi: 10.1080/15216540500037406. [DOI] [PubMed] [Google Scholar]
- 289.Fordel E., Thijs L., Martinet W., Schrijvers D., Moens L., Dewilde S. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene. 2007;398:114–122. doi: 10.1016/j.gene.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 290.Guo X., Philipsen S., Tan-Un K.C. Study of the hypoxia-dependent regulation of human CYGB gene. Biochem. Biophys. Res. Commun. 2007;364:145–150. doi: 10.1016/j.bbrc.2007.09.108. [DOI] [PubMed] [Google Scholar]
- 291.Langan J.E., Cole C.G., Huckle E.J., Byrne S., McRonald F.E., Rowbottom L., Ellis A., Shaw J.M., Leigh I.M., Kelsell D.P., Dunham I., Field J.K., Risk J.M. Novel microsatellite markers and single nucleotide polymorphisms refine the tylosis with oesophageal cancer (TOC) minimal region on 17q25 to 42.5 kb: sequencing does not identify the causative gene. Hum. Genet. 2004;114:534–540. doi: 10.1007/s00439-004-1100-3. [DOI] [PubMed] [Google Scholar]
- 292.McRonald F.E., Liloglou T., Xinarianos G., Hill L., Rowbottom L., Langan J.E., Ellis A., Shaw J.M., Field J.K., Risk J.M. Down-regulation of the cytoglobin gene, located on 17q25, in tylosis with oesophageal cancer (TOC): evidence for trans-allele repression. Hum. Mol. Genet. 2006;15:1271–1277. doi: 10.1093/hmg/ddl042. [DOI] [PubMed] [Google Scholar]
- 293.Yassin M., Kissow H., Vainer B., Joseph P.D., Hay-Schmidt A., Olsen J., Pedersen A.E. Cytoglobin affects tumorigenesis and the expression of ulcerative colitis-associated genes under chemically induced colitis in mice. Sci. Rep. 2018;8:6905. doi: 10.1038/s41598-018-24728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Shivapurkar N., Stastny V., Okumura N., Girard L., Xie Y., Prinsen C., Thunnissen F.B., Wistuba, Czerniak B., Frenkel E., Roth J.A., Liloglou T., Xinarianos G., Field J.K., Minna J.D., Gazdar A.F. Cytoglobin, the newest member of the globin family, functions as a tumor suppressor gene. Canc. Res. 2008;68:7448–7456. doi: 10.1158/0008-5472.CAN-08-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shaw R.J., Omar M.M., Rokadiya S., Kogera F.A., Lowe D., Hall G.L., Woolgar J.A., Homer J., Liloglou T., Field J.K., Risk J.M. Cytoglobin is upregulated by tumour hypoxia and silenced by promoter hypermethylation in head and neck cancer. Br. J. Canc. 2009;101:139–144. doi: 10.1038/sj.bjc.6605121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Oleksiewicz U., Liloglou T., Tasopoulou K.M., Daskoulidou N., Bryan J., Gosney J.R., Field J.K., Xinarianos G. Cytoglobin has bimodal: tumour suppressor and oncogene functions in lung cancer cell lines. Hum. Mol. Genet. 2013;22:3207–3217. doi: 10.1093/hmg/ddt174. [DOI] [PubMed] [Google Scholar]
- 297.De Backer J., Razzokov J., Hammerschmid D., Mensch C., Hafideddine Z., Kumar N., van Raemdonck G., Yusupov M., Van Doorslaer S., Johannessen C., Sobott F., Bogaerts A., Dewilde S. The effect of reactive oxygen and nitrogen species on the structure of cytoglobin: a potential tumor suppressor. Redox Biol. 2018;19:1–10. doi: 10.1016/j.redox.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Reeder B.J., Svistunenko D.A., Wilson M.T. Lipid binding to cytoglobin leads to a change in haem co-ordination: a role for cytoglobin in lipid signalling of oxidative stress. Biochem. J. 2011;434:483–492. doi: 10.1042/BJ20101136. [DOI] [PubMed] [Google Scholar]
- 299.Beckerson P., Wilson M.T., Svistunenko D.A., Reeder B.J. Cytoglobin ligand binding regulated by changing haem-co-ordination in response to intramolecular disulfide bond formation and lipid interaction. Biochem. J. 2015;465:127–137. doi: 10.1042/BJ20140827. [DOI] [PubMed] [Google Scholar]
- 300.Singh S., Canseco D.C., Manda S.M., Shelton J.M., Chirumamilla R.R., Goetsch S.C., Ye Q., Gerard R.D., Schneider J.W., Richardson J.A., Rothermel B.A., Mammen P.P. Cytoglobin modulates myogenic progenitor cell viability and muscle regeneration. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E129–E138. doi: 10.1073/pnas.1314962111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Novianti T., Juniantito V., Jusuf A.A., Arida E.A., Sadikin M., Jusman S.W.A. High expressions of the cytoglobin and PGC-1alpha genes during the tissue regeneration of house gecko (Hemidactylus platyurus) tails. BMC Dev. Biol. 2020;20:11. doi: 10.1186/s12861-020-00214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Li L., Xie Y., Li S., Tan J., Qin Y., Yan Z. Cytoglobin overexpression facilitates proliferation and migration of vascular smooth muscle cells. Arch. Biol. Sci. 2020;72:165–172. [Google Scholar]
- 303.Jourd'heuil F.L., Xu H., Reilly T., McKellar K., El Alaoui C., Steppich J., Liu Y.F., Zhao W., Ginnan R., Conti D., Lopez-Soler R., Asif A., Keller R.K., Schwarz J.J., Thanh Thuy L.T., Kawada N., Long X., Singer H.A., Jourd'heuil D. The hemoglobin homolog cytoglobin in smooth muscle inhibits apoptosis and regulates vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2017;37:1944–1955. doi: 10.1161/ATVBAHA.117.309410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bracke A., Hoogewijs D., Dewilde S. Exploring three different expression systems for recombinant expression of globins: Escherichia coli, Pichia pastoris and Spodoptera frugiperda. Anal. Biochem. 2018;543:62–70. doi: 10.1016/j.ab.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 305.Huang B., Lu Y.S., Li X., Zhu Z.C., Li K., Liu J.W., Zheng J., Hu Z.L. Androglobin knockdown inhibits growth of glioma cell lines. Int. J. Clin. Exp. Pathol. 2014;7:2179–2184. [PMC free article] [PubMed] [Google Scholar]
- 306.Blank M., Kiger L., Thielebein A., Gerlach F., Hankeln T., Marden M.C., Burmester T. Oxygen Supply from the Bird's Eye Perspective: globin E is a respiratory protein in the chicken retina. J. Biol. Chem. 2011;286:26507–26515. doi: 10.1074/jbc.M111.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wright T.J., Davis R.W. Myoglobin oxygen affinity in aquatic and terrestrial birds and mammals. J. Exp. Biol. 2015;218:2180–2189. doi: 10.1242/jeb.119321. [DOI] [PubMed] [Google Scholar]
- 308.Gallagher M.D., Macqueen D.J. Evolution and expression of tissue globins in ray-finned fishes. Genome Biol. Evol. 2017;9:32–47. doi: 10.1093/gbe/evw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Hoffmann F.G., Vandewege M.W., Storz J.F., Opazo J.C. Gene turnover and diversification of the alpha- and beta-globin gene families in sauropsid vertebrates. Genome Biol. Evol. 2018;10:344–358. doi: 10.1093/gbe/evy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Koch J., Burmester T. Membrane-bound globin X protects the cell from reactive oxygen species. Biochem. Biophys. Res. Commun. 2016;469:275–280. doi: 10.1016/j.bbrc.2015.11.105. [DOI] [PubMed] [Google Scholar]
- 311.Roesner A., Hankeln T., Burmester T. Hypoxia induces a complex response of globin expression in zebrafish (Danio rerio) J. Exp. Biol. 2006;209:2129–2137. doi: 10.1242/jeb.02243. [DOI] [PubMed] [Google Scholar]
- 312.Blank M., Wollberg J., Gerlach F., Reimann K., Roesner A., Hankeln T., Fago A., Weber R.E., Burmester T. A membrane-bound vertebrate globin. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Corti P., Xue J., Tejero J., Wajih N., Sun M., Stolz D.B., Tsang M., Kim-Shapiro D.B., Gladwin M.T. Globin X is a six-coordinate globin that reduces nitrite to nitric oxide in fish red blood cells. Proc. Natl. Acad. Sci. U. S. A. 2016;113:8538–8543. doi: 10.1073/pnas.1522670113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Gotting M., Nikinmaa M.J. Transcriptomic analysis of young and old erythrocytes of fish. Front. Physiol. 2017;8:1046. doi: 10.3389/fphys.2017.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Thuy le TT, Matsumoto Y., Thuy T.T., Hai H., Suoh M., Urahara Y., Motoyama H., Fujii H., Tamori A., Kubo S., Takemura S., Morita T., Yoshizato K., Kawada N. Cytoglobin deficiency promotes liver cancer development from hepatosteatosis through activation of the oxidative stress pathway. Am. J. Pathol. 2015;185:1045–1060. doi: 10.1016/j.ajpath.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 316.Thuy le TT, Morita T., Yoshida K., Wakasa K., Iizuka M., Ogawa T., Mori M., Sekiya Y., Momen S., Motoyama H., Ikeda K., Yoshizato K., Kawada N. Promotion of liver and lung tumorigenesis in DEN-treated cytoglobin-deficient mice. Am. J. Pathol. 2011;179:1050–1060. doi: 10.1016/j.ajpath.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Thuy le TT, Van Thuy T.T., Matsumoto Y., Hai H., Ikura Y., Yoshizato K., Kawada N. Absence of cytoglobin promotes multiple organ abnormalities in aged mice. Sci. Rep. 2016;6:24990. doi: 10.1038/srep24990. [DOI] [PMC free article] [PubMed] [Google Scholar]