Abstract
Previous studies have shown that nicorandil has a protective effect on cardiomyocytes. However, there is no study to investigate whether perioperative intravenous nicorandil can further reduce the myocardial infarct size in patients with ST-segment elevation myocardial infarction (STEMI) compared to the current standard of percutaneous coronary intervention (PCI) regimen. The CHANGE (China-Admini stration of Nicorandil Group) study is a multicenter, prospective, randomized, double-blind and parallel-controlled clinical study of STEMI patients undergoing primary PCI in China, aiming to evaluate the efficacy and safety of intravenous nicorandil in ameliorating the myocar dial infarct size in STEMI patients undergoing primary PCI and provide evidence-based support for myocardial protection strategies of STEMI patients.
Keywords: Cardiovascular disease, Myocardial infarct size, Nicorandil, Primary percutaneous coronary intervention, ST-segment elevation myocardial infarction
1. Background
As people's standard of living improves and the pace of life accelerates, the incidence of ischemic heart disease has increased significantly.[1] According to the China cardiovascular diseases report 2018, [2] the cardiovascular disease (CVD) mortality rate in China remains at the top, which accounted for more than 40% of all causes of death; the total annual hospitalization cost of acute myocardial infarction (AMI) was 19.085 billion RMB, and the coronary heart disease mortality rate among residents continued to rise in 2016. The CVDs are the number one cause of death worldwide: more people die from CVDs each year than from any other cause annually, such as an estimated 17.9 million people died as a result of CVDs in 2016, accounting for 31% of global deaths. Of the 17 million premature deaths (under 70 years of age) due to noncommunicable diseases in 2015, 37% of deaths were caused by CVDs.[3] According to the World Bank's estimation, the number of people suffering from AMI in China will soar from the current 8.1 million to 22.63 million by 2030, and the average cost of hospitalization is also the highest of the medical inpatient disease spectrum in China. Therefore, how to effectively curb the hazards of AMI is of paramount public health importance.[4]
As is well known, primary percutaneous coronary intervention (PCI) is currently the most effective treatment strategy for ST-segment elevation myocardial infarction (STEMI).[5] Primary PCI can rapidly open the infarct-related artery and effectively restore forward blood flow. However, even if the infarct-related artery is restored in some patients, the damaged myocardium is not effectively perfused with blood. This phenomenon is called no-reflow. Clinical studies suggest that the annual incidence of no-reflow after PCI is approximately 5%–50%.[6] Once no-reflow occurs, often accompanied by extensive and severe myocardial damage, patients may develop left ventricular dilatation, decreased cardiac function and malignant arrhythmias, ultimately increasing the risk of cardiovascular adverse events such as sudden death and congestive heart failure. At the same time, myocardial ischaemia-reperfusion injury that occurs when myocardial tissue recovers from hemoperfusion after a long period of ischaemia will further damage the myocardium, resulting in more pronounced and severe injury and dysfunction than before reperfusion, including reduced contractile function, decreased coronary flow and altered vascular reactivity. These are important factors that influence mortality and heart failure after PCI.[7]
At present, there is still a lack of effective measures available for the prevention and treatment of coronary microcirculation dysfunction and myocardial reperfusion injury during the perioperative period of PCI. Conventional vasodilator drugs such as nitrate agents and calcium ion antagonists cannot significantly improve micro-vessels with blood vessel diameters less than 300 μm. Nicorandil is a compound consisting of N2 (2-hydroxyethyl) nicotinamide and a partial structural linkage of organic nitrate, and it is the first ATP-sensitive potassium (KATP) channel opening agent for clinical use. Nicorandil increases the outflow of vascular smooth muscle K+ from the cell, leading to a greater negative resting membrane potential (hyperpolarization), shortening the action potential time, inhibiting the influx of calcium, and causing a decrease in intracellular calcium, thus leading to vasodilation; this pharmacological effect manifests in the expansion of coronary arteries at all levels, especially the coronary arteries.[8] With the study of ischaemia preconditioning mechanisms, mitochondrial KATP channel opening is considered to be the end effector of multiple signal transduction systems.[9] A study showed that nicorandil could open mitochondrial KATP channels, thus exerting a potent direct cardioprotective effect on cardiomyocytes.[10] Studies on atrial muscles in animals and humans have shown that the cardioprotective effect of nicorandil is eliminated by the closure of KATP channels with glibenclamide and tolbutamide.[11]
In recent years, the development of cardiac magnetic resonance imaging (MRI) has provided a reliable means to assess the myocardial infarct size and microcirculatory obstruction after reperfusion.[12] Cardiac MRI is now recognized as an important method for the assessment of myocardial tissue perfusion status and microcirculation, and it can more accurately show the myocardial infarct size and the level of microcirculatory perfusion.[13, 14] We used cardiac MRI as the main assessment method to evaluate the effect of nicorandil in perioperative primary PCI on the myocardial infarct size, surviving myocardium and microcirculatory perfusion in STEMI patients to further reduce the myocardial infarct size and improve cardiac function and the long-term prognosis after primary PCI in STEMI patients.
2. Objective
The aim of this study was to evaluate the efficacy and safety of nicorandil in improving myocardial infarct size in patients with acute myocardial infarction through a multicenter, prospective, randomized, double-blind and parallel-controlled clinical study, providing evidence-based support for the optimization of PCI treatment strategies. The primary objective of the study was to compare whether the effect of nicorandil in combination with PCI was significantly better at reducing the myocardial infarct size than that of PCI alone in AMI patients, and the secondary objective was to compare whether nicorandil combined with PCI was significantly better than PCI alone for the following events: (1) incidence of slow flow/no-reflow; (2) corrected thrombolysis in myocardial infarction (TIMI) frame count (cTFC); (3) ST-segment fall rate of electrocardiogram (ECG); (4) myocardial enzyme and troponin levels; (5) myocardial edema area; (6) microvascular obstruction; (7) left ventricular ejection fraction; and (8) composite cardiovascular events during the study: all-cause death, cardiovascular death, unplanned hospitalization for heart failure, and revascularization. Furthermore, the differences in safety between nicorandil combined with PCI and simple PCI therapy were also investigated by comparing blood pressure, heart rate and adverse events during the study.
3. Methods
3.1. Study design and population cohort
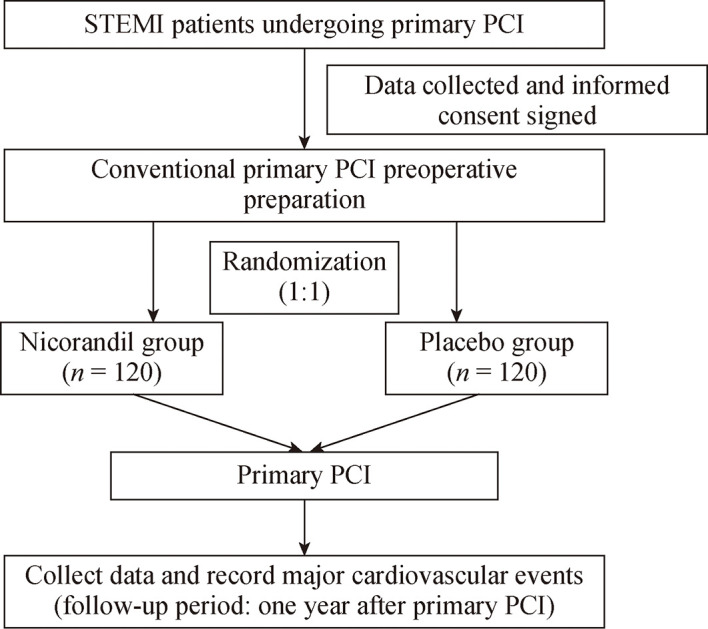
This is a multicenter, prospective, randomized, double- blind and parallel-controlled clinical study. Patients aged 18 to 80 years with STEMI undergoing primary PCI within 12 hours of onset who provided written informed consent were included from nine hospitals in China. Patients were excluded for the following reasons: (1) a systolic pressure < 80 mmHg; (2) the left main coronary artery stenosis; (3) consolidation of the aortic dissection; (4) myocardial infarction within six months; (5) revascularization (PCI or coronary artery bypass grafting) within six months; (6) patients undergoing treatment with nicorandil; (7) patients with any known allergic reaction, hypersensitivity reaction or contraindication to nicorandil or niacin; (8) contraindications to cardiac magnetic resonance imaging and MRI; (9) participation in other research projects currently or within three months; (10) pregnant and lactating women; and (11) patients with other obvious abnormal signs, laboratory examinations, and clinical disorders who were not suitable for the study according to the judgments of the clinician (Figure 1).
1.
The flowchart of the CHANGE study.
PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
3.2. Method of administration
A drug code was given in chronological order of entry into the cohort in each study unit, and the drugs labelled with the corresponding code were applied according to the drug code. Dissolved 12 mg of the drug in 20 mL of normal saline, injected 10 mL of intravenous bolus within 20 s, one minute before the first balloon expansion or stent implantation, and discarded the other 10 mL. Dissolved 144 mg of the drug in 480 mL of normal saline, then immediately after the bolus injection, the solution was administered by intravenous drip at a constant rate of 6 mg/h for 24 h.
3.3. Data collection
All data were obtained after the patient signed an informed consent form. Clinical examinations follow standardized protocols and use validated procedures and instruments. The follow-up data collection was conducted by physicians in the participating hospitals, who received training and standardization programs organized by the researchers. Well-trained doctors used a validated questionnaire specially designed for this study to collect basic demographic information of all participants.
3.4. Assessment of demographic variables and cardiovascular risk factors
Detailed information was collected on demographic variables (such as age, gender, previous history of diseases, and family history of diseases). Based on self-reported information, smoking is defined as cumulative smoking more than 18 packs in the last year and drinking is defined as drinking more than twice a week for more than one year. Body weight (kg), height (cm), blood pressure and heart rate were measured on admission. Previous history of diseases including stroke or transient ischemic attack, myocardial infarction, PCI, atrial fibrillation or valvular heart disease, coronary artery bypass grafting, hypertension, diabetes mellitus, and hyperlipemia is defined as a self-reported history. Family history of cardiovascular disease is restricted to biological parents and siblings. Lack of physical exercise is defined as exercise (more than 30 minutes) less than three times a week without moderate or severe physical labour.
3.5. Laboratory analysis
At each examination, blood samples were collected from the antecubital vein. Cardiac enzyme (CK, CK-MB) and troponin (cardiac troponin T, cardiac troponin I) levels were measured before and after PCI at 6, 12, 18, and 24 h. The elevation of CK-MB is defined as a postoperative maximum value more than 16 mg/dL and an increase by more than 5% compared to the preoperative value; the elevation of cardiac troponin T is defined as a postoperative maximum value more than 0.2 ng/mL and an increase by more than 0.1 ng/mL compared to the preoperative value and the cardiac troponin I elevation is defined as a postoperative maximum more than 0.1 ng/mL and an increase of more than 0.05 ng/mL compared to preoperative values. For all participants, blood routine, hepatic and renal function, B-type natriuretic peptide (BNP) or pro-BNP will be assessed.
3.6. Analysis of cardiac MRI and cTFC
The myocardial infarct size was derived by measuring the area of delay-enhanced image-enhanced MRI, which was judged and analyzed by two experienced MRI cardiac diagnosticians through Segment version 1.9 post-processing software. The Segment version 1.9 program was used to draw the intima and adventitia on the short-axis image to derive the size of the left ventricle (the papillary muscle was considered part of the left ventricular cavity). The myocardial infarct size was derived from the program's automatic analysis of delayed enhancement images. Myocardial infarct size (g) = Σ (enhanced area) × layer thickness × myocardial density (1.05 g/mL). The myocardial infarct size is expressed as grams (g) or percentage (%) (myocardial infarct size/full left ventricle size).
The area of myocardial edema was derived by two experienced MRI cardiologists through measuring the "edema" range of T2-weighted flip sequence images on cardiac MRI on the 7th day after PCI. Multifaceted short-axis scans (layers 11–13) covered the entire left ventricle as much as possible. The intimal and adventitia boundaries of the heart were delineated on each short-axis image by the Segment version 1.9 program to obtain the size of the left ventricle (excluding the papillary muscles and the gap between the papillary muscles).
The myocardial danger zone was defined as the area of high-signal edema in the T2-weighted image (two standard deviations above the average signal intensity of the normal myocardium). Low-signal areas within the myocardial danger zone were considered to be part of the myocardial danger zone. Myocardial danger zone range (g) = Σ (area of high signal area) × layer thickness × myocardial density (1.05 g/mL). Myocardial salvage index = (size of myocardial danger zone-size of infarct range)/size of myocardial danger zone.
Local filling deficits in contrast first-over-perfusion or low-signal areas in high-signal necrotic myocardium during contrast-delayed imaging are usually considered to represent irreversible microcirculatory damage, also known as microcirculatory obstruction. Intramyocardial hemorrhage, which manifests as low-signal areas in the myocardial area of high-signal edema during T2 signal scans, was also considered to demonstrate microvascular obstruction.
The TIMI flow grade of infarct-related coronary arteries before PCI and cTFC after PCI were measured respectively. The left anterior descending branch (LAD) was analysed in the right anterior 30° + cephalic 30° position, the left circumflex branch (LCX) was analysed in the right anterior 30° + foot 30° position, and the right coronary artery (RCA) was analysed in the left anterior 45° position.
The cTFC calculation: the starting point is the number of frames in which the contrast agent completely or nearly completely filled the beginning of the coronary artery and touched both sides of the coronary vessel wall, and the end point is the number of frames in which the contrast agent entered the distal branch vessel and developed a specific anatomical marker. The LAD was marked by the distal apical "eight-stringed" bifurcation, the LCX was marked by the distal blunt branch bifurcation including the lesion site, and the RCA was marked by the first branch of the posterior left ventricular branch. The cTFC for LAD = calculated frame count/1.7.
The cTFC was measured by two cardiologists, and the average value was taken. When scanning at a rate of 15 frames per second, cTFC > 20 frames per second was used as the criterion for the diagnosis of slow blood flow after primary PCI.
3.7. Follow-up and outcome assessment
Participants will be followed up by telephone or outpatient visits one year after PCI, as shown in Table 1. It is important to note that participants will receive cardiac MRI examinations one week and six months after PCI. Telephone follow-up results were completed into a paper version of the follow-up form. All clinical data were collected and entered into the Electronic Data Capture (EDC) system by the clinical research coordinator, and the information in the EDC system were verified by the clinical research associate to ensure that the information was true and valid. The primary endpoint of the study is the myocardial infarct size, and secondary endpoints include slow flow/no-reflow incidence, corrected TIMI frame count (cTFC), ST-segment fall rate of ECG, cardiac enzymes and troponin, area of myocardial edema, microvascular obstruction, left ventricular ejection fraction, and composite cardiovascular events during the study period: all-cause death, cardiovascular death, unplanned hospitalization for heart failure, and revascularization.
1.
Follow-up programme.
| Time from baseline | Screening period | Treatment period | Follow-up period (V1) 7 ± 3 days after PCI | Follow-up period (V2) 30 ± 7 days after PCI | Follow-up period (V3) 90 ± 14 days after PCI | Follow-up period (V4) 180 ± 29 days after PCI | Follow-up period (V5) 360 ± 29 days after PCI |
| BNP: brain natriuretic peptide; MRI: magnetic resonance imaging; PCI: percutaneous coronary intervention. | |||||||
| General data | |||||||
| Informed consent | √ | ||||||
| Demographic information | √ | ||||||
| Medical history | √ | ||||||
| Height and weight | √ | ||||||
| Blood pressure | √ | √ | |||||
| Heart rate | √ | √ | |||||
| General laboratory tests | |||||||
| Blood routine | √ | ||||||
| Hepatic and renal function | √ | ||||||
| Blood biochemistry | √ | ||||||
| Myocardial enzyme | √ | √ | |||||
| Troponin | √ | √ | |||||
| BNP & pro-BNP | √ | √ | |||||
| Electrocardiogram | √ | √ | |||||
| Catheter room information | √ | ||||||
| Cardiac MRI | √ | √ | |||||
| Consolidation of medication records | √ | √ | √ | √ | √ | √ | |
| Bad incident records | √ | √ | √ | √ | √ | √ | |
| Serious adverse event reports | √ | √ | √ | √ | √ | √ | |
| Endpoint incident reports | √ | √ | √ | √ | √ | √ | |
| Final study report | √ | √ | √ | √ | √ | √ | |
3.8. Statistical analysis
The full analysis set closest to the intention-to-treat analysis and the dataset conforming to the protocol-compliant set were selected for analysis. The quantitative indicators will be described as the mean ± SD, median (interquartile range), minimum and maximum values. The differences in the quantitative indicators between groups will be compared and analyzed using the Student's t-test or analysis of variance (ANOVA). The categorical indicators will be described as the frequency and percentage, etc. The differences in the categorical indicators between groups will be analyzed by the Pearson's chi-square test and the Cochran-Mantel-Haenszel (CMH) method (center stratification). All statistical analyses were performed using SPSS software version 26.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-sided, and P-value < 0.05 was considered to be statistically significant.
3.9. Ethics statement
The study complies with the World Medical Association Declaration of Helsinki, the current Chinese Good Clinical Practice and the relevant ethical principles of legal regulations. Ethical approval for the trial was obtained from the Ethical Committee of China PLA General Hospital and the other hospitals involved. Informed consent will be obtained from all participants prior to recruitment.
Study drugs: nicorandil for injection was approved for marketing by the National Medical Products Administration, with manufacturing batch number 2012S00559, held by Beijing Sihuan Kebao Pharmaceutical Company Limited, and its new drug certificate number is Chinese medicine certificate H20120041, held by Beijing Sihuan Kebao Pharmaceutical Company Limited.
4. Discussion
Studies have been carried out abroad on the immediate and long-term benefits of nicorandil after PCI. In a study of 81 patients with anterior wall myocardial infarction, 40 patients received nicorandil, and the results showed that nicorandil attenuated slow flow/no-reflow, improved left ventricular wall motion scores, and reduced the incidence of ventricular arrhythmias and congestive heart failure.[15] However, most of the clinical studies on nicorandil in AMI have used myocardial enzymes and myocardial radionuclide imaging as indicators of nicorandil to reduce myocardial damage, and they are single-center studies with small sample sizes. A larger randomized double-blind study found that nicorandil significantly reduced cardiovascular mortality and the incidence of congestive heart failure in 368 AMI patients.[16] However, the J-WIND study with the largest sample size of patients with nicorandil injection to date failed to observe the protective effect of nicorandil on the myocardial infarct size and long-term prognosis, the results of which were published in Lancet in 2007.[17] Therefore, there is no definitive conclusion as to whether perioperative PCI in combination with nicorandil application will further reduce the myocardial infarct size and improve clinical prognosis in patients with AMI compared to current standard PCI regimens.
Basic studies have found a significant dose-dependence of ischemic pre-protective effects of nicorandil's dilatation of coronary arteries at all levels. Furthermore, the timing of protective therapy during reperfusion is also important.[18] Post-treatment studies conducted in animal models have clearly demonstrated that protective interventions must be applied prior to sustained reperfusion and may not be protective if the intervention is delayed until blood flow is restored.[19] In view of this, this multicenter, large-sample clinical study proposes to use a highly effective and clinically accessible nicorandil regimen in AMI patients to compare the difference between PCI standard regimens and combined nicorandil regimens in improving the myocardial infarct size in patients with AMI by cardiac MRI. It can provide a basis for optimizing PCI regimens and a more direct chain of evidence for effective myocardial cell protection and long-term prognosis in the acute phase of AMI.
5. Study status
The study was registered on the ClinicalTrials.gov website on February 26, 2018 (Clinical Trial Number: NCT034 45728) and began in March 2018, and patients' follow-up visits are ongoing now. Data entry into the study was performed simultaneously during clinical data collection.
Acknowledgments
This study was supported by the National Key Research and Development program of China (2018ZX09201013) and Xinxin Merck Cardiovascular Research Fund (2017- CCA-xinxin merck fund-003). All authors had no conflicts of interest to disclose.
We appreciate all the participants and their relatives in the study. In addition, we would like to thank the members of the survey teams from every hospital involved. The authors thank the staff of the Beijing Sihuan Kebao Pharmaceutical Co., Ltd. for their important efforts.
Contributor Information
Geng QIAN, Email: 9396@263.net.
Yun-Dai CHEN, Email: cyundai@vip.163.com.
References
- 1.Moran AE, Forouzanfar MH, Roth GA, et al. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma LY, Chen WW, Gao RL, et al. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol. 2020;17:1–8. doi: 10.11909/j.issn.1671-5411.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. About cardiovascular diseases (CVDs). World Health Organization of cardiovascular dis eases Web site. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed August 8, 2020).
- 4.The World Bank. Toward a healthy and harmonious life in China: stemming the rising tide of non-communicable dis eases, 2011. The World Bank Web site. https://www.worldbank.org/content/dam/Worldbank/document/NCD_report_en.pdf#:~:text=Toward%20a%20Healthy%20and%20Harmonious%20Life%20in%20China%3A,the%20response%20over%20the%20medium%20and%20longer%20terms (accessed Aug 8, 2020).
- 5.Kaul P, Ezekowitz JA, Armstrong PW, et al. Incidence of heart failure and mortality after acute coronary syndromes. Am Heart J. 2013;165:379–385. doi: 10.1016/j.ahj.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Niccoli G, Cosentino N, Spaziani C, et al. No-reflow: in ci dence and detection in the cath-lab. Curr Pharm Des. 2013;19:4564–4575. doi: 10.2174/1381612811319250005. [DOI] [PubMed] [Google Scholar]
- 7.Salinas P, Jimenez-Valero S, Moreno R, et al. Update in phar macological management of coronary no-reflow phenomenon. Cardiovasc Hematol Agents Med Chem. 2012;10:256–264. doi: 10.2174/187152512802651024. [DOI] [PubMed] [Google Scholar]
- 8.Jahangir A, Terzic A. K(ATP) channel therapeutics at the bedside. J Mol Cell Cardiol. 2005;39:99–112. doi: 10.1016/j.yjmcc.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granfeldt A, Lefer DJ, Vinten-Johansen J. Protective ischae mia in patients: preconditioning and postconditioning. Cardio vasc Res. 2009;83:234–246. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsubara T, Minatoguchi S, Matsuo H, et al. Three minute, but not one minute, ischemia and nicorandil have a precon ditioning effect in patients with coronary artery disease. J Am Coll Cardiol. 2000;35:345–351. doi: 10.1016/S0735-1097(99)00539-2. [DOI] [PubMed] [Google Scholar]
- 11.Lader JM, Vasquez C, Bao L, et al. Remodeling of atrial ATP-sensitive K+ channels in a model of salt-induced elevated blood pressure. Am J Physiol Heart Circ Physiol. 2011;301:H964–H974. doi: 10.1152/ajpheart.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nazir SA, McCann GP, Greenwood JP, et al. Strategies to attenuate micro-vascular obstruction during P-PCI: the rando mized reperfusion facilitated by local adjunctive therapy in ST-elevation myocardial infarction trial. Eur Heart J. 2016;37:1910–1919. doi: 10.1093/eurheartj/ehw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jogiya R, Kozerke S, Morton G, et al. Validation of dynamic 3-dimensional whole heart magnetic resonance myocardial perfusion imaging against fractional flow reserve for the detection of significant coronary artery disease. J Am Coll Cardiol. 2012;60:756–765. doi: 10.1016/j.jacc.2012.02.075. [DOI] [PubMed] [Google Scholar]
- 14.Hamirani YS, Wong A, Kramer CM, et al. Effect of micro vascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial in farc tion: a systematic review and meta-analysis. JACC Cardio vasc Imaging. 2014;7:940–952. doi: 10.1016/j.jcmg.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito H, Taniyama Y, Iwakura K, et al. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction. J Am Coll Cardiol. 1999;33:654–660. doi: 10.1016/S0735-1097(98)00604-4. [DOI] [PubMed] [Google Scholar]
- 16.Ishii H, Ichimiya S, Kanashiro M, et al. Impact of a single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction. Circulation. 2005;112:1284–1288. doi: 10.1161/CIRCULATIONAHA.104.530329. [DOI] [PubMed] [Google Scholar]
- 17.Kitakaze M, Asakura M, Kim J, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 18.Akai K, Wang Y, Sato K, et al. Vasodilatory effect of nico randil on coronary arterial microvessels: its dependency on vessel size and the involvement of the ATP-sensitive potas sium channels. J Cardiovasc Pharmacol. 1995;26:541–547. doi: 10.1097/00005344-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]


