Abstract
Summary
This case report describes a 65-year-old man with a Rendu-Osler-Weber syndrome with secondary chronic anaemia, who received multiple intravenous (IV) iron infusions and sustained diffuse bone pain secondary to multiple insufficiency fractures. Laboratory study confirmed fibroblast growth factor 23 (FGF-23)-mediated hypophosphatemia as the main cause of a severe osteomalacia induced by ferric carboxymaltose (FCM).
After 3 months or oral phosphate replacement and switching to iron sucrose, serum phosphate levels were normalized and patient improved clinically.
Introduction
Some drugs can induce asymptomatic hypophosphatemia, which if sustained, can lead to a severe osteomalacia with multiple skeletal fractures. This complication has also been described with IV iron therapy.
Methods
This case report describes a patient with Rendu-Osler-Weber syndrome with chronic iron deficiency anaemia, recurrently treated with FCM, who developed a severe osteomalacia with multiple skeletal fractures.
Results
Laboratory study showed hypophosphatemia, with high ALP and high FGF-23. Images studies confirmed bone mass loss and multiple insufficiency fractures. A Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (18F-FDG PET/CT) did not show hidden tumor, so a diagnosis of FCM-induced hypophosphatemic osteomalacia was performed. Phosphate replacement improved clinical symptoms of the patient.
Conclusion
Intravenous iron therapy, mainly FCM form, can cause hypophosphatemia, and in some cases induce a severe osteomalacia with multiple fractures, so it seems advisable to monitor serum phosphate levels in high risk patients, as those who receive repeated dose.
Keywords: Osteomalacia, Fibroblast growth factor 23, Hypophosphatemia, Intravenous iron therapy
Highlights
-
•
Intravenous iron therapy can cause hypophosphatemia and in some cases osteomalacia.
-
•
Ferric carboxymaltose can raise fibroblast growth factor 23, a phosphaturic hormone.
-
•
Osteomalacia should be considered if a patient treated with iron therapy develops pain.
-
•
It's advisable to monitor serum phosphate in patients who receive iron therapy.
-
•
In case of persistent hypophosphatemia, switch to another iron therapy is suggested.
1. Introduction
Osteomalacia is a metabolic bone disease defined by an impairment of bone mineralization. Skeletal fractures and bone or polyarticular pain are the most frequent symptoms, being present in almost 90% of cases, with predominant location in lower extremities, pelvic ring and spine (Gifre et al., 2011). Although the main cause of osteomalacia is vitamin D deficiency, there are other less frequent causes as impaired vitamin D metabolism, vitamin D resistance or hypophosphatemia (Bhan et al., 2018).
Among the causes of hypophosphatemia, are decrease in phosphate intestinal absorption, increase in phosphate urinary excretion or phosphate redistribution (Gaasbeek and Meinders, 2005).
Tumor-induced osteomalacia (TIO), also known oncogenic osteomalacia, is a rare paraneoplastic syndrome associated with low phosphate and caused by small endocrine tumors that secrete the phosphaturic hormone, fibroblast growth factor 23 (FGF23) (Agarwal et al., 2019; Ryan and Reiss, 1984).
Some drugs can induce a decrease in serum phosphate levels through different mechanisms. Chronic treatment with nonabsorbable antacids can decrease intestinal phosphate absorption (Lotz et al., 1968). Insulin treatment of diabetic ketoacidosis, is effective to recover metabolic acidosis, but the intracellular phosphate shift decreases phosphate serum levels (Kebler et al., 1985). Some diuretics, tyrosine kinase inhibitors (e.g. imatinib), or antiviral drugs (e.g. tenofovir), can induce a renal loss of phosphate (Manghat et al., 2014).
Ferric carboxymaltose (FCM) is an iron formulation increasingly prescribed due to its effectiveness and fast infusion time. FCM can cause hypophosphatemia secondary to fibroblast growth factor 23 (FGF23) dysregulation (Glaspy et al., 2020).
In patients with chronic anaemia requiring long-term iron administration, a long-lasting hypophosphatemia can lead to osteomalacia with associated bone pain and skeletal fractures.
Hypophosphatemia is a widely known complication of FCM treatment. However, to our knowledge only few cases of FCM induced osteomalacia were reported (Anand and Schmid, 2017; Klein et al., 2018; Schouten et al., 2009; Tozzi and Tozzi, 2019), being this the first case in a patient with hereditary hemorrhagic telangiectasia (HHT) or Rendu-Osler-Weber syndrome.
2. Case report
A 65-year-old man was evaluated in the Internal Medicine outpatient clinic because of widespread pain.
For the last 6 months prior to admission, the patient had been suffering from increasing bone pain in both shoulders, pelvic girdle and four limbs, which worsened with movements. Pain did not produce sleep disturbance but was intensively progressing, requiring crutch and wheelchair to move. Patient didn't refer to weakness, asthenia, fever or constitutional symptoms such as weight loss.
Personal history of interest included sensorineural deafness, type 2 diabetes treated with insulin and sitagliptin; and HHT with iron deficiency chronic anaemia due to intestinal blood loss and epistaxis. Patient had been treated with oral ferrous sulphate for fifteen years, but he required monthly intravenous (IV) iron treatment with FCM in the last two years.
A previous fibrogastroscopy showed gastric and duodenal angiodysplasia, which was treated by ligation with elastic bands.
Three years before, the patient was diagnosed of osteoporosis, with low bone mineral density (BMD) (T-score −2.0 at lumbar spine and −2.2 at femoral neck) at dual-energy X-ray absorptiometry (DXA) and a mild vertebral fracture. Serum calcium and phosphate levels were within the normal range. He had been treated with risedronate, calcium (500 mg/day) and vitamin D (400 IU of cholecalciferol) since then with good adherence.
Currently retired, he worked as a jeweller. He never smoked or drank alcohol and had a normal body mass index.
On physical examination the patient was afebrile, pale, but showed good general condition. He had several facial and oral angiodysplasias. He had neither swelling nor limited joints, but showed tenderness on both shoulders, knees and femurs.
Laboratory analysis revealed calcium 9.2 mg/dL [8.8–10.2], phosphate 1.2 mg/L [2.7–4.5], alkaline phosphatase (ALP) 356 U/L [40–129], 25-hydroxyvitamin D 21.8 ng/mL [optimal levels >30], parathyroid hormone (PTH) 82 pg/mL [10–65], creatinine 0.93 mg/dL [0.7–1.2], 24-hour urine calcium 46.8 mg, phosphate 0.44 g, and fractional excretion of phosphate (FEPO4) was 5.9%; serum C-terminal FGF-23 > 419 RU/mL [reference values up to 145 RU/mL]); other laboratory test results are shown in Table 1.
Table 1.
Baseline laboratory data.
| Variable | Patient | Reference range |
|---|---|---|
| Hemoglobin, g/L | 8.4 | 130–175 |
| Calcium, mg/dL | 9.2 | 8.8–10.2 |
| Phosphate, mg/L | 1.2 | 2.7–4.5 |
| Gamma-glutamyl transferase, U/L | 10 | 8–61 |
| Alkaline phosphatase, U/L | 356 | 40–129 |
| 25-Hydroxyvitamin D, ng/mL | 21.8 | >30 |
| Parathyroid hormone, pg/mL | 82 | 10–65 |
| Creatinine, mg/dL | 0.93 | 0.7–1.2 |
| Glomerular filtration rate CKD-EPI mL/min/1.73 m2 | 87.5 | >60 |
| Thyrotropin, μU/mL | 2.15 | 0.4–4.0 |
| Cortisol, μg/dL | 17.9 | 6.02–18.4 |
| Total testosterone, ng/mL | 7.18 | 1.93–7.40 |
| Tryptase, μg/L | 5.3 | 0–11 |
| 24-Hour urine calcium, mg/d | 46.8 | 100–300 |
| 24-Hour urine phosphate, g/d | 0.44 | 0.4–1.3 |
| Fractional excretion of phosphate, % | 5.9 | <5 |
| Serum C-terminal FGF-23, RU/mL | >419 | <145 |
| Prostate specific antigen, ng/mL | 0.9 | 0–4 |
| IgG lambda, g/L | 1.21 | |
| IgM lambda, g/L | 1.48 |
FGF-23: fibroblast growth factor 23; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration.
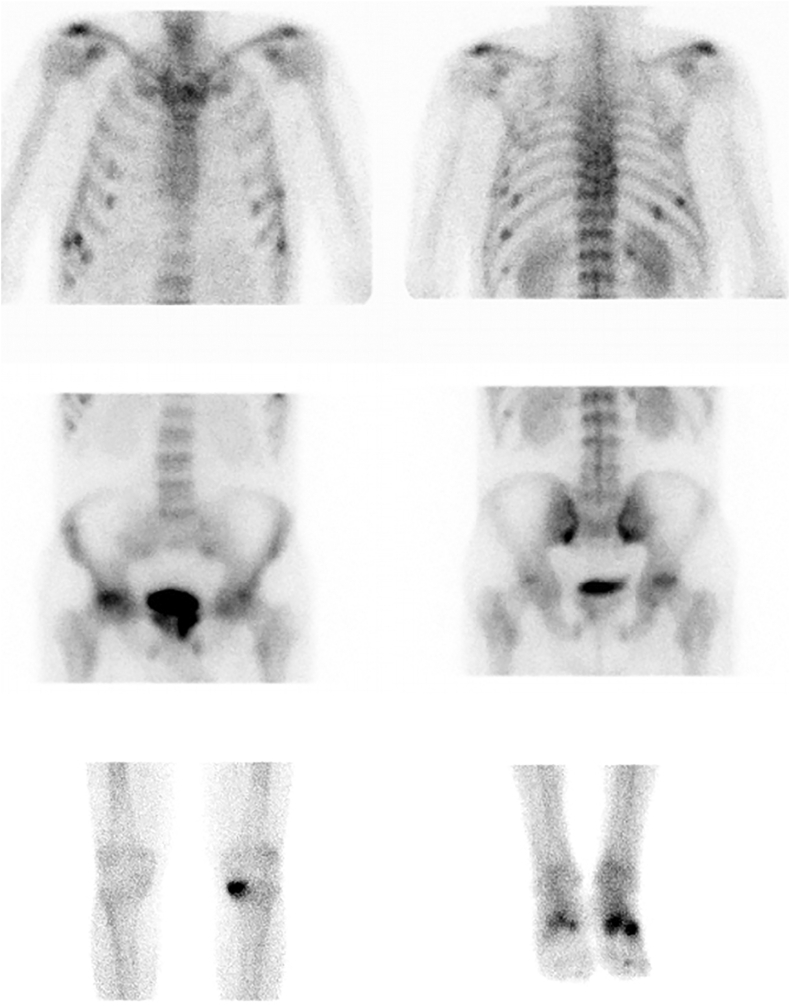
99m Tc-MDP bone scan showed multiple hot spots located in several ribs, left scapula, bilateral sacral ala, ischiopubic and iliopubic branches, right femoral head, left tibia internal plateau and both tarsi. All the findings were suspicious of insufficiency fractures or Looser's zones (Fig. 1). Whole-body computed tomography and whole-body magnetic resonance imaging excluded metastasis and confirmed multiple insufficiency fractures.
Fig. 1.
Bone scintigraphy with revealed a characteristic metabolic pattern with a generalized increased uptake in axial skeleton. Multiple hypercaptive foci consistent with insufficiency fractures or Looser's zones were observed in multiple bilateral rib, left scapula, bilateral sacral ala, ischiopubic and iliopubic branches, right femoral head, left tibia internal plateau and both tarsi.
A DXA scan showed a significant loss of BMD (T-score −3.14 at lumbar spine and −3.86 at femoral neck).
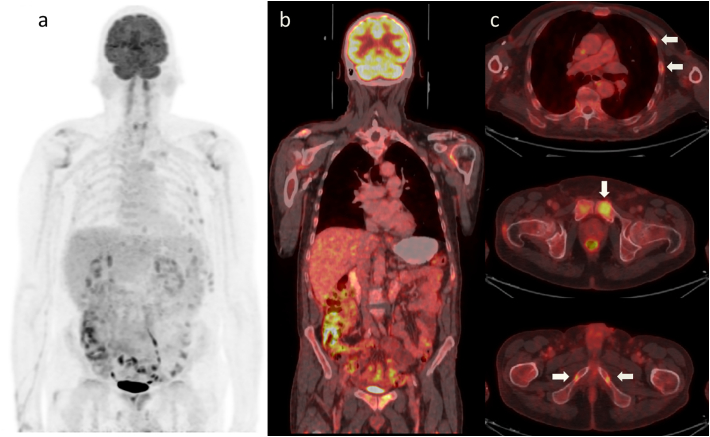
With the diagnosis of FGF-23-mediated hypophosphatemic osteomalacia probably induced by FCM, a tumoral origin (TIO) was likewise excluded with an 18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (18F-FDG PET/CT) (Fig. 2).
Fig. 2.
Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (18F-FDG PET/CT). A hidden tumor was ruled out, but multiple skeletal fractures were highlighted (arrows). a Whole body maximum intensity projection. b Fused coronal view. c Fused axial slices.
Oral phosphate replacement with 3.56 g of sodium phosphate three times a day initially, and adjusting doses in the follow-up kept serum phosphate levels within the normal range (2.8 mg/dL and 2.7 mg dL at the third and tenth weeks after starting the replacement, respectively –reference values [2.7–4.5 mg/dL] with a time interval between oral phosphate intake and blood extraction of 14 h). Patient also received calcium and vitamin D, risedronate was discontinued and FCM was switched to iron sucrose. During follow-up, patient's pain and mobility gradually improved.
3. Discussion
FCM-induced hypophosphatemia is highly underdiagnosed and may lead to a skeletal impairment with severe consequences in comorbidity and quality of life of patients.
Although FCM-induced hypophosphatemia can be asymptomatic, when it remains in time, as occurs in chronic conditions may result in severe osteomalacia, as in our patient.
Our patient was previously diagnosed of osteoporosis with a mild vertebral fracture, but with his BMD in the range of osteopenia. He had a normal serum calcium and phosphate and was treated with oral bisphosphonates, calcium and vitamin D for the last 3 years.
Although he didn't suffer any new vertebral or non-vertebral fracture, a progressive impairment of bone mineralization was the cause of a widespread bone pain as first symptom of osteomalacia. Bone scan showed multiple insufficiency fractures.
Osteomalacia has been reported with non-nitrogen containing bisphosphonates but not with third generation bisphosphonates, like risedronate.
The presence of hypophosphatemia, 24-hour urine phosphate >100 mg and FEPO4 > 5% and high FGF-23, indicated renal phosphate wasting, hence considering the history of recurrent previous infusions of FCM made us suspect this complication.
Several diseases can cause chronic blood loss requiring IV iron therapy, mainly erosive gastritis, peptic ulcer, diverticulitis, benign tumors, inflammatory bowel disease, angiodysplasia, haemorrhoids and chronic haemolysis (Camaschella, 2015).
Our patient had a HHT, an autosomal dominant inherited disease in which patients commonly had anaemia due to chronic digestive losses as hematemesis or melena in 33% (Kjeldsen and Kjeldsen, 2000). As far as we are concerned, this is the first case in the literature of FCM-induced hypophosphatemic osteomalacia in a patient with HHT.
Severe hypophosphatemia is an IV iron treatment side effect firstly described by Okada M et al. in 1983 (Okada et al., 1983). Despite the heterogeneous methodology of the studies and the different definition of hypophosphatemia, FCM is the form of IV iron most frequently associated with this complication. In a recent systematic literature review hypophosphatemia associated to IV iron therapies was reported in 19 randomized control trials (Glaspy et al., 2020). This complication has been observed in 0.0–92.1% patients treated with FCM (18 studies), 0.0–40.0% treated with iron sucrose (5 studies), 0.4% treated with ferumoxytol (1 study), and 0.0% treated with low-molecular-weight iron dextran (1 study).
FCM is a IV effective and safe iron formulation taken up by the reticuloendothelial cells and released gradually to the serum, with little risk of release of cytotoxic free iron into the circulating blood (Ikuta et al., 2019).
Despite the mechanism by which FCM raises FGF-23 remains unknown it has been proposed that certain carbohydrate moieties present in FCM can inhibit the degradation of FGF-23 (Wolf et al., 2013), resulting in proximal tubular reabsorption of phosphate reduction and active form of vitamin D decrease (Holick, 2007; Wolf et al., 2020). Secondary hyperparathyroidism, as we saw in our patient, due to hypocalcemia caused by decreased 1,25-dihydroxyvitamin D also can make a part in increased phosphate clearance. However, high PTH actually leads to normal calcium levels and hypophosphatemia. In addition animal models have shown that FGF-23 reduces 1,25-dihydroxyvitamin D by reducing its production and increasing its degradation (Shimada et al., 2004).
FCM-induced hypophosphatemia has been reported to be transient and asymptomatic in most cases, so serum phosphate levels of patients treated with FCM do not need to be checked or monitored in most cases (Ikuta et al., 2019). Nevertheless, in patients with existing risk factors, such as severe malnutrition, vitamin D deficiency or after prolonged exposure to high dose IV iron, as in the case of our patient, hypophosphatemia may require medical attention.
Another cause of hypophosphatemic osteomalacia is TIO, which can be identical to FCM-induced osteomalacia, in which indolent mesenchymal tumors, with slow growth are responsible of an overproduction of FGF-23 leading to an increase urinary phosphate excretion (Ryan and Reiss, 1984). Although not completely ruled it out, a whole-body 18F-FDG PET/CT did not show a hidden tumor.
Vitamin D plays an important role in bone mineralization, and low vitamin D levels can contribute to osteomalacia. Although our patient was receiving vitamin D supplementation (400 IU/day of cholecalciferol) he had vitamin D insufficiency (21 ng/mL) at presentation. Maybe the dose of cholecalciferol was insufficient to keep higher 25-hydroxyvitamin D levels.
25-Hydroxyvitamin D and phosphate serum levels were normalized after vitamin D and sodium phosphate replacement in addition to switching to another iron therapy. Serum phosphate levels often fluctuate in patients with oral phosphate supplementation and it depends on the time since the last dose. However, in our patient serum phosphate levels were within the normal range in several blood tests with a regular time interval between oral intake and blood extraction. Patient also received calcium and vitamin D, risedronate was discontinued and FCM was switched to iron sucrose. During follow-up, patient's pain and mobility gradually improved.
This case highlights that although a single or few infusions of IV iron therapy is usually safe, in cases requiring multiple doses it could be advisable to monitor closely phosphate serum levels and normalize 25-hydroxyvitamin D deficiency.
Informed consent
Informed consent was obtained from the participant included in this study.
Source of support
None.
Transparency document
Transparency document.
Declaration of competing interest
Eduardo L Callejas Moraga, Enrique Casado, Marta Gomez Nuñez and Ana P Caresia Aroztegui declare that they have no conflict of interest.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Agarwal N., Kale S.S., Kumari K. Tumor-induced Osteomalacia due to a phosphaturic mesenchymal tumor in the cervical spine: a case report and literature review. Neurol. India. 2019;67:1334–1340. doi: 10.4103/0028-3886.271274. [DOI] [PubMed] [Google Scholar]
- Anand G., Schmid C. Severe hypophosphataemia after intravenous iron administration. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-219160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan A., Qiu S., Rao S.D. Bone histomorphometry in the evaluation of osteomalacia. Bone Rep. 2018;8:125–134. doi: 10.1016/j.bonr.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaschella C. Iron-deficiency anemia. N. Engl. J. Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- Gaasbeek A., Meinders A.E. Hypophosphatemia: an update on its etiology and treatment. Am. J. Med. 2005;118:1094–1101. doi: 10.1016/j.amjmed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Gifre L., Peris P., Monegal A., Martinez de Osaba M.J., Alvarez L., Guanabens N. Osteomalacia revisited: a report on 28 cases. Clin. Rheumatol. 2011;30:639–645. doi: 10.1007/s10067-010-1587-z. [DOI] [PubMed] [Google Scholar]
- Glaspy J.A., Lim-Watson M.Z., Libre M.A., Karkare S.S., Hadker N., Bajic-Lucas A., Strauss W.E., Dahl N.V. Hypophosphatemia associated with intravenous iron therapies for iron deficiency anemia: a systematic literature review. Ther. Clin. Risk Manag. 2020;16:245–259. doi: 10.2147/TCRM.S243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Ito H., Takahashi K., Masaki S., Terauchi M., Suzuki Y. Safety and efficacy of intravenous ferric carboxymaltose in Japanese patients with iron-deficiency anemia caused by digestive diseases: an open-label, single-arm study. Int. J. Hematol. 2019;109:50–58. doi: 10.1007/s12185-018-2529-9. [DOI] [PubMed] [Google Scholar]
- Kebler R., McDonald F.D., Cadnapaphornchai P. Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am. J. Med. 1985;79:571–576. doi: 10.1016/0002-9343(85)90053-1. [DOI] [PubMed] [Google Scholar]
- Kjeldsen A.D., Kjeldsen J. Gastrointestinal bleeding in patients with hereditary hemorrhagic telangiectasia. Am. J. Gastroenterol. 2000;95:415–418. doi: 10.1111/j.1572-0241.2000.01792.x. [DOI] [PubMed] [Google Scholar]
- Klein K., Asaad S., Econs M., Rubin J.E. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-222851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Zisman E., Bartter F.C. Evidence for a phosphorus-depletion syndrome in man. N. Engl. J. Med. 1968;278:409–415. doi: 10.1056/NEJM196802222780802. [DOI] [PubMed] [Google Scholar]
- Manghat P., Sodi R., Swaminathan R. Phosphate homeostasis and disorders. Ann. Clin. Biochem. 2014;51:631–656. doi: 10.1177/0004563214521399. [DOI] [PubMed] [Google Scholar]
- Okada M., Imamura K., Iida M., Fuchigami T., Omae T. Hypophosphatemia induced by intravenous administration of Saccharated iron oxide. Klin. Wochenschr. 1983;61:99–102. doi: 10.1007/BF01496662. [DOI] [PubMed] [Google Scholar]
- Ryan E.A., Reiss E. Oncogenous osteomalacia. Review of the world literature of 42 cases and report of two new cases. Am. J. Med. 1984;77:501–512. doi: 10.1016/0002-9343(84)90112-8. [DOI] [PubMed] [Google Scholar]
- Schouten B.J., Doogue M.P., Soule S.G., Hunt P.J. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann. Clin. Biochem. 2009;46:167–169. doi: 10.1258/acb.2008.008151. [DOI] [PubMed] [Google Scholar]
- Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Fujita T., Nakahara K., Fukumoto S., Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- Tozzi D., Tozzi J. Osteomalacia and insufficiency fractures secondary to intravenous iron therapy: a case report. J. Orthop. Case Rep. 2019 doi: 10.13107/jocr.2019.v10.i01.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Koch T.A., Bregman D.B. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 2013;28:1793–1803. doi: 10.1002/jbmr.1923. [DOI] [PubMed] [Google Scholar]
- Wolf M., Rubin J., Achebe M., Econs M.J., Peacock M., Imel E.A., Thomsen L.L., Carpenter T.O., Weber T., Brandenburg V., Zoller H. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323:432–443. doi: 10.1001/jama.2019.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.