This work provides new insight into the regulation of trichome formation by gibberellin which has not previously been described in tomato.
Keywords: Gibberellin, gibberellin biosynthesis, tomato, transcription factor, transcriptional regulation, trichome
Abstract
Trichomes are epidermal protuberances on aerial parts of plants known to play an important role in biotic and abiotic stresses. To date, our knowledge of the regulation of trichome formation in crop species is very limited. Through phenotyping of the Solanum pennellii×S. lycopersicum (cv. M82) introgression population, we identified the SlbHLH95 transcription factor as a negative regulator of trichome formation in tomato. In line with this negative role, SlbHLH95 displayed a very low expression in stems where trichomes are present at high density. Overexpression of SlbHLH95 resulted in a dramatically reduced trichome density in stems and a significant down-regulation of a set of trichome-related genes. In addition to the lower trichome density, overexpressing lines also showed pleiotropic alterations affecting both vegetative and reproductive development. While most of these phenotypes were reminiscent of gibberellin (GA)-deficient phenotypes, expression studies showed that two GA biosynthesis genes, SlGA20ox2 and SlKS5, are significantly down-regulated in SlbHLH95-OE plants. Moreover, in line with a decrease in active GA content, the glabrous and dwarf phenotypes were rescued by exogenous GA treatment. In addition, yeast one-hybrid and transactivation assays revealed that SlbHLH95 represses the expression of SlGA20ox2 and SlKS5 via direct binding to their promoters. Taken together, our study established a link between SlbHLH95, GA, and trichome formation, and uncovered the role of this gene in modulating GA biosynthesis in tomato.
Introduction
Plant trichomes are specialized epidermal cell structures present on aerial parts of plants including leaves, stems, and peduncles. Trichomes play important roles in plant physiology and development by providing physical or chemical barriers that protect plants against insect predation, UV radiation, and excessive transpiration (Ishida et al., 2008). Depending on the species, trichomes are classified as unicellular or multicellular, glandular or non-glandular, and branched or unbranched (Werker, 2000; Pattanaik et al., 2014).
Arabidopsis trichomes are typically unicellular, branched, and non-glandular. The initiation and regulation mechanisms underlying trichome formation have been thoroughly studied in Arabidopsis, and genetic and molecular analyses demonstrated that a complex network of transcription factors (TFs) is involved in this process. The R2R3 MYB, basic helix–loop–helix (bHLH), and WD40 repeat (WDR) proteins, form a trimeric MYB–bHLH–WDR complex that promotes the formation and development of trichomes by activating the expression of downstream target genes (Rerie et al., 1994; Johnson et al., 2002; Ishida et al., 2007). Other TFs including TRANSPARENT TESTA 8 (TT8) and AtMYC1, NAC, and NTM1-LIKE 8 (NTL8) proteins also play pivotal roles in trichome development (Maes et al., 2008; Symonds et al., 2011; Zhao et al., 2012; Tian et al., 2017). In addition, phytohormones, especially gibberellins (GAs), jasmonates (JAs), and cytokinins (CKs), regulate trichome formation by modulating the expression of key regulatory genes in Arabidopsis (Schellmann and Hulskamp, 2005; Yang and Ye, 2013; Qi et al., 2014). Although the regulatory network of unicellular trichome formation in Arabidopsis has been extensively studied, our knowledge of the initiation and development of trichomes in crop species remains scarce.
Considering the wide diversity of trichome types in tomato (Solanum lycopersicum), this species can potentially serve as a model system for studying multicellular and glandular trichomes, due to the extensive genetic resources available, the high-quality genome sequence, and other genomics resources along with a handful of reported trichome mutants among which are chi1, coi1, hairless, odorless-2, and inquieta (Li et al., 2004; Kang et al., 2010a, b, 2014; Jeong et al., 2017). Tomato produces eight types of trichomes which can be divided into glandular (types I, IV, VI, and VII) and non-glandular (types II, III, V, and VIII) whose characterization is giving rise to an increasing number of studies (Luckwill, 1943; Simmons and Gurr, 2005; Glas et al., 2012; Xu et al., 2018). The HD-Zip protein Woolly was reported to physically interact with a B-type cyclin protein SlCycB2 to regulate type I trichome formation (Yang et al., 2011). Moreover, the C2H2 zinc finger protein Hair (H), interacting with Woolly, was recently identified as a key regulator of type I trichome formation in tomato (Chang et al., 2018). SlMX1, a MIXTA-like MYB TF, was shown to be involved in the formation of both glandular and non-glandular trichomes in tomato (Ewas et al., 2016, 2017). In addition, down-regulation of SlIAA15 or SlARF3, two auxin-related TF genes, led to reduced trichome density in tomato (Deng et al., 2012; Zhang et al., 2015). More recently, it was shown that down-regulation of SlMYC1, a bHLH TF gene, resulted in the production of smaller type VI glandular trichomes at lower densities, and the complete knockout of this gene led to total absence of type VI trichomes (Xu et al., 2018), indicating that SlMYC1 is instrumental in type VI glandular trichome development in tomato. In addition, it was reported that JA is required for the formation and development of type VI glandular trichomes in tomato (Li, 2004; Yan et al., 2013; Bosch et al., 2014). However, the role of other hormones such as GAs in tomato trichome formation remains unclear. In spite of the progress made in uncovering some of the factors controlling trichome development in tomato, our understanding of the regulatory mechanisms underlying the development of different types of tomato trichomes remains largely incomplete.
Here, we report on a new bHLH TF, SlbHLH95, acting as a putative regulator of trichome formation identified by screening the S. pennellii×S. lycopersicum introgression population for altered trichome phenotypes. Overexpression of SlbHLH95 in tomato resulted in reduced trichome density in stem, leaf, and young fruit. Moreover, SlbHLH95-overexpressing (OE) lines exhibited GA-deficient phenotypes that were rescued by exogenous GA application. The data provide new insight into the role of bHLH TFs in controlling trichome formation in tomato through the regulation of GA biosynthesis.
Materials and methods
Plant materials and growth conditions
Tomato (S. lycopersicum, Micro-Tom) plants were grown under standard greenhouse conditions set as follows: 14 h day/10 h night cycle, 25 °C/20 °C day/night temperature, 80% relative humidity, and 250 μmol m−2 s−1 light intensity.
Plant transformation
Agrobacterium tumefaciens-mediated tomato plant transformation was performed as described in Wang et al. (2005). The transformed lines were then selected on a kanamycin-containing medium. Homozygous lines from the F3 or later generations were used in all experiments.
Subcellular localization of SlbHLH95 protein
To investigate the subcellular localization of the SlbHLH95 protein, the coding sequence of SlbHLH95 without the stop codon was amplified by PCR and then cloned into pART2a to generate the 35S::SlbHLH95-GFP (green fluorescent protein) fusion gene. The vector bearing the fusion construct SlbHLH95–GFP and the control GFP vector were individually transformed into leaves of Nicotiana benthamiana using a 2 ml needleless syringe. The transformed samples were incubated in the dark at 22 °C for 48 h, then the subcellular localization of each expressed protein was visualized using a confocal microscope (Leica, TCS SP5 II). Images of transformed tobacco cells were captured using a ×40 objective lens, using bright field, GFP (excitation/emission: 488/498–548 nm), and DAPI (excitation/emission: 405/421–523 nm) filters.
RNA extraction and RT–qPCR
Total RNAs from different tissues were isolated using a Plant RNA Purification Reagent (Invitrogen, cat. no. 12322-012) according to the manufacturer’s instructions. cDNA was synthesized from 1 μg of total RNA using a PrimeScript™ RT reagent Kit with gDNA Eraser (Takara Bio, Kusatsu, Japan, AK4201). cDNA products were diluted to 2.5 ng μl–1 and used as templates for the qPCR. Quantitative reverse tanscription–PCR (RT–qPCR) was performed using the Bio-Rad CFX384 device. Each reaction (10 μl) consisted of 5 μl of iTaq™ Universal SYBR Green Supermix (Bio-Rad, Hercules, USA, #172-5124), 1 μl each of forward and reverse primers, and 3 μl of cDNA. Thirty-nine amplification cycles were performed (pre-incubation at 95 °C for 2 min followed by each cycle consisting of 5 s at 95 °C, 10 s at 60 °C, and added melting curve analysis during 65–95 °C). The results were calculated using Bio-Rad CFX Manager software. The relative expression of each gene was calculated by the ∆∆Ct method (Schefe et al., 2006) using SlActin as internal control. The primer pairs for RT–qPCR were designed using Primer3Plus (http://www.primer3plus.com) and blasted at the NCBI database to check their specificity (the primers used for qRT–PCR are listed in Supplementary Table S3 at JXB online).
Fruit shape analysis
Full size maturing (7 d post-breaker stage) freshly harvested fruits were cut with a new razor blade longitudinally, scanned at 300 dpi, and analyzed using Tomato Analyzer v3.0 (Rodríguez et al., 2010). Then fruit shape index and fruit shape triangle, two parameters characterizing the shape of the fruit, were obtained after analysis. Fruit shape index is the ratio of the maximum height length to the maximum width of the fruit, and fruit shape triangle is the ratio of the proximal end width to the distal end width (Brewer et al., 2007).
SEM analysis
Fresh tomato leaves or stems (three biological replicates) collected from wild-type (WT) and transgenic plants were fixed for 24 h in a solution of 2.5% paraformaldehyde, 2.5% glutaraldehyde buffered with 0.1 M sodium cacodylate, pH 7.4 (Electron Microscopy Sciences, Hatfield, PA, USA). Samples were dehydrated in the following ethanol concentrations (30, 50, 70, 80, 90, 100, and 100%), each treatment lasting 10 min. Samples were then critical point dried with liquid CO2 (Quarum K850 CPD), mounted, and sputter coated with a 5 nm thin layer of platinum (LEICA EM MED 020). Images were acquired with a scanning electron microscope (SEM Quanta 250 FEG FEI) at 5 kV, spot size 3 or 2 with a working distance of 1 cm.
RNA-Seq analysis and data processing
Global expression of tomato genes was determined by replicated strand-specific Illumina RNA sequencing (RNA-Seq). Paired-end RNA-Seq was carried out using Hiseq 2500 (150 bp paired ends) by Novogene (China). WT and SlbHLH95-OE83 RNAs were extracted from 4-week-old leaves with three biological replicates. Prior to sequencing, the quality of purified RNA was checked with the Agilent2100 Bioanalyzer (RIN ≥6.3).
Raw data (raw reads) of fastq format were firstly processed through in-house perl scripts. In this step, clean data (clean reads) were obtained by removing reads containing adaptor, reads containing poly-N (>10% unknown base per read), and low-quality reads (>50% of Qphred ≤20 bases per read) from raw data. At the same time, Q20 (probability of incorrect base call, 1 in 100), Q30 (probability of incorrect base call, 1 in 1000), and GC content of the clean data were calculated. All the downstream analyses were based on the clean data with high quality. Trimmed reads were then mapped to the S. lycopersicum reference genome and gene annotation ITAG2.4 (Tomato Genome Consortium, 2012) using TopHat-2.0.12 (Trapnell et al., 2009) calling Bowtie 2.2.3 (Langmead and Salzberg, 2012).
Differential expression analysis was performed using the DESeq R package (1.18.0) (Anders and Huber, 2010). DESeq provides statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting P-values were adjusted using the Benjamini–Hochberg’s approach (Benjamini and Hochberg, 1995) for controlling the false discovery rate. Genes with an adjusted P-value < 0.05 found by DESeq were assigned as differentially expressed genes (DEGs).
Gene Ontology (GO) enrichment analysis of DEGs was implemented by the GOseq R package, in which gene length bias was corrected. GO terms with a corrected P-value <0.05 were considered as significantly enriched within DEGs (Young et al., 2010). Then, we carried out the statistical enrichment of the differential expression of DEGs in Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways using KOBAS software (Mao et al., 2005; Kanehisa et al., 2008).
All raw-sequence reads data were uploaded in the NCBI Sequence Read Archive (SRA; http://www.ncbi.nlm.nih.gov/Traces/sra) with accession number SRP144705.
GA content analysis
GA measurements were conducted by Wuhan Metware Biotechnology Co., Ltd. (Wuhan, China). Leaf material was collected from WT and SlbHLH95-OE plants at the 4-week-old stage. Three replicates were performed for each genotype. Frozen plant materials were ground using a mixer mill (MM 400, Retsch, Germany) for 1 min at 30 Hz. Then, 200 mg of the powder sample was extracted overnight at 4 °C with 1500 μl of 70% (v/v) acetonitrile, and ultrasound-assisted extraction was carried out for 30 min at room temperature with vortexing (15 s) and centrifugation (14 000 rpm for 10 min). From each sample, 1000 μl of supernatant was collected, then evaporated to dryness under a nitrogen gas stream at room temperature, reconstituted in 100 μl of 80% (v/v) methanol, and diluted to 800 μl with water. The extracts were passed through the SPE cartridge (200 mg, 3 ml; CNW) and evaporated to dryness under a nitrogen gas stream at room temperature (Ma et al., 2008; Hao et al., 2015). Samples were reconstituted in 200 μl of 80% (v/v) methanol and filtrated (PTFE, 0.22 μm; Anpel), then analyzed using an LC-ESI-MS/MS system (HPLC, Shim-pack UFLC SHIMADZU CBM30A system, http://www.shimadzu.com.cn/; MS, Applied Biosystems 6500 Triple Quadrupole, http://www.appliedbiosystems.com.cn/). A Waters Acquity UPLC BEH C18 column (2.1 mm×100 mm×1.8 µm) was used. The instrument was operated, and multiple reaction monitoring (MRM) transitions were assessed (Li et al., 2017). For all samples, peak areas for endogenous and labeled GAs were compared and combined with 200 mg of powdered sample to calculate ng g–1.
GA treatment
For application of GA to young plants growing on soil, gibberellic acid (GA4, Sangon Biotech) were first dissolved in absolute ethanol and then diluted with the nutrient solution (Miracle-Gro® Pour & Feed Plant Food, Item #1006002). A solution of 10–5 M GA4 was sprayed on the plants twice a week starting 14 d post-germination. After 2 weeks of treatment, the treated plants were compared with the control plants (treated with the same solution without GA4).
Dual-luciferase transient expression assay
The SlKS5 and SlGA20ox2 promoters were cloned into the pGreenII 0800-LUC double-reporter vector, while SlbHLH95 was cloned into the pGreenII 62-SK vector effector, as described by Hellens et al. (2005). The constructed reporter and effector plasmids were transiently expressed in tobacco (N. benthamiana) leaves as described by Hellens et al. (2005). A dual-luciferase assay kit (Promega) was used to analyze the transient expression in tobacco leaves on the third day after infiltration. Absolute firefly luciferase (LUC) reporter and renilla luciferase (REN) values were measured in a Luminoskan Ascent Microplate Luminometer (Thermo Scientific) according to the manufacturer’s instructions, with a 5 s delay and 15 s integrated measurements. The transcriptional activation activity of SlbHLH95 on the promoters of GA biosynthesis-related genes was indicated by the ratio of LUC to REN. At least six biological repeats were conducted.
Yeast one-hybrid assays
The full-length cDNA sequence of SlbHLH95 was amplified and inserted into the pDEST22 vector containing the GAL4 activation domain (AD) to form the construct pDEST22-SlbHLH95. Three tandem copies of the E-box from SlKS5 and SlGA20ox2 promoters and the promoter fragment were each cloned into the pHis-Leu2-GW vector. Various combinations of pDEST22-SlbHLH95 and different promoters were co-transformed into yeast strain Y187. Empty pDEST22 with the two promoters was used as a negative control. The transformants were cultivated on SD/-Trp/-Leu medium for 3 d. DNA–protein interactions were determined by the growth of clones on SD/-Trp/-Leu/-His with 10 mM 3-AT (3-amino-1,2,4-triazole).
Results
Identification of SlbHLH95 as a putative regulator of tomato trichome formation
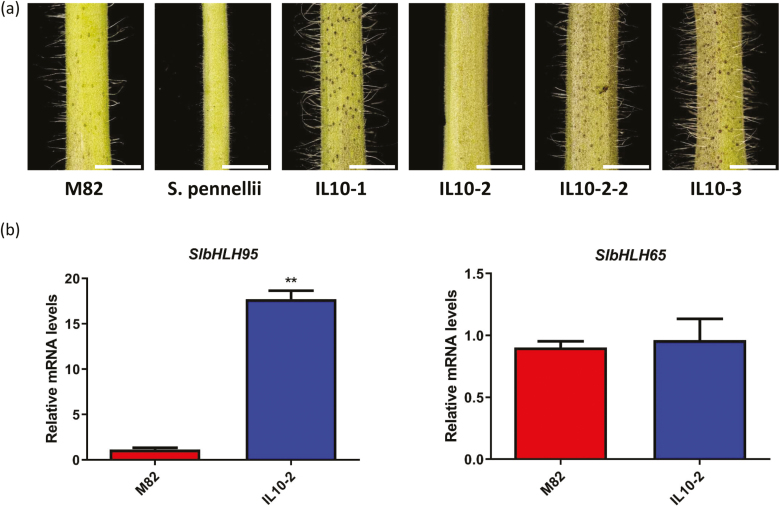
Phenotyping of the S. pennellii×S. lycopersicum (cv. M82) introgression population identified one specific introgression line (IL) on chromosome 10 (IL10-2) showing altered trichome density in the stem compared with the M82 control parental line (Fig. 1a). This suggested the presence of a putative trichome regulatory gene(s) in the replacement region specific to the IL10-2 line. The search for putative genes present in the S. pennellii genome region introgressed in IL10-2 identified 56 potential gene units resident in this region, and among these two were predicted to putatively encode bHLH TFs (Solyc10g079050, SlbHLH95 and Solyc10g079070, SlbHLH65; Supplementary Table S1). Considering that bHLH family genes have been shown to regulate trichome formation in Arabidopsis and given that SlbHLH95 displayed 15 times higher expression at the transcript level in stems of IL10-2 than in M82 (Fig. 1b), whereas SlbHLH65 exhibited similar expression in the two tomato accessions (Fig. 1b), we postulated that SlbHLH95 represents a realistic candidate gene underpinning the observed trichome phenotype. We therefore sought to address the functional significance of SlbHLH95 in the regulation of trichome formation in tomato.
Fig. 1.
Different trichome phenotypes displayed by domesticated tomato (S. lycopersicum), a wild relative (S. pennellii), and introgression lines. (a) Introgression line IL10-2 showed altered trichome density in stem compared with the parental line M82. The white bars represent 1 cm. IL10-1, IL10-2, IL10-2-2, and IL10-3 are different introgression lines. (b) RT–qPCR analysis of mRNA levels of SlbHLH95 and SlbHLH65 in M82 and the introgression line IL10-2. Values are means ±SD. Statistical significance was determined by Student’s t-test: **0.001<P<0.01.
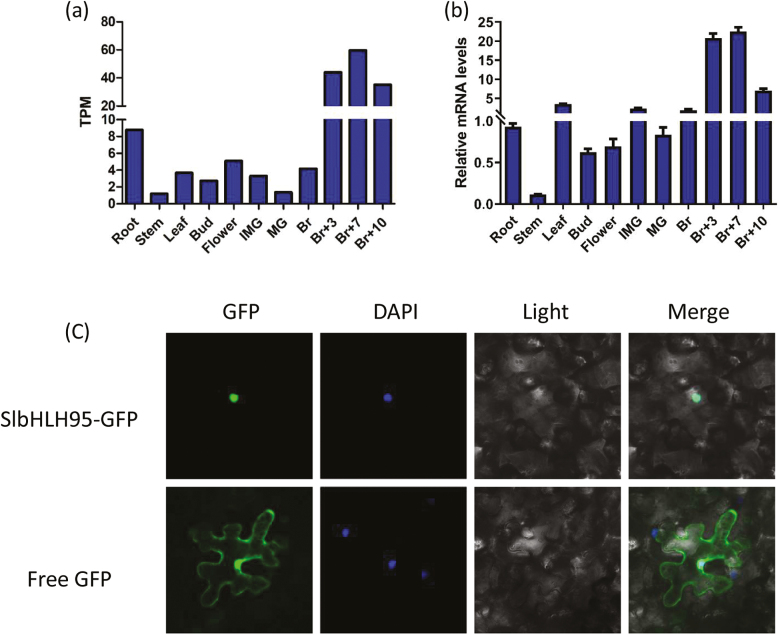
RNA-Seq and RT–qPCR studies revealed that SlbHLH95 was constitutively expressed in all tissues tested, with the highest transcript levels observed in ripe fruit and the lowest in stems where trichomes are present at high density (Fig. 2a, b), indicating that SlbHLH95 expression may negatively correlate with the presence of trichomes in the tissue. Confocal laser scanning microscopy analysis showed that SlbHLH95 strictly localized in the nucleus (Fig. 2c), consistent with its putative TF activity.
Fig. 2.
Expression pattern of SlbHLH95 and subcellular localization of SlbHLH95 protein. (a) Expression levels of the SlbHLH95 gene in different tissues. TPM, transcripts per kilobase of exon model per million mapped reads. (b) Relative mRNA levels of SlbHLH95 in different tissues. Accumulation of SlbHLH95 transcripts was assessed by RT–qPCR in root, stem, leaf, flower, mature green fruit (MG), breaker fruit (Br), 3 d post-breaker fruit (Br+3), and 7 d post-breaker fruit (Br+7). The relative mRNA levels of SlbHLH95 in the root were standardized to 1.0, referring to the SlActin gene as the internal control. Values are means ±SD of three biological replicates. (c) GFP-tagged fusion proteins were transiently expressed under the control of the 35S promoter in tobacco cells after transfection.
Overexpression of SlbHLH95 results in reduced trichome formation
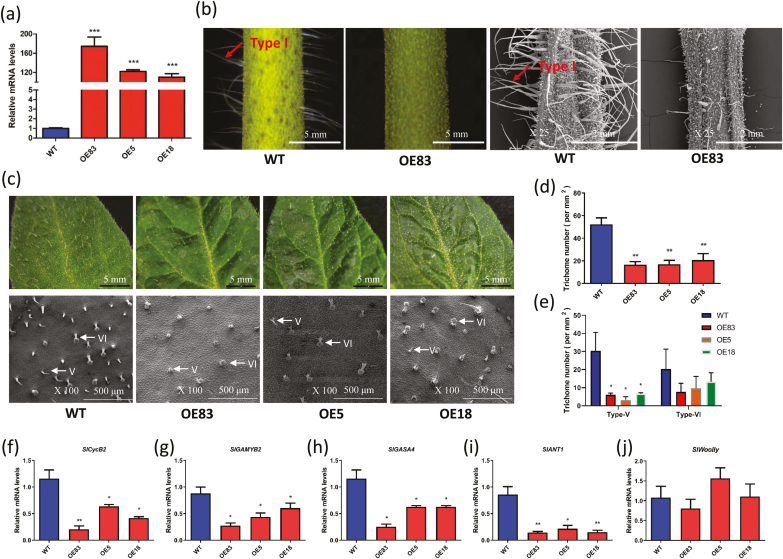
Since the expression level of SlbHLH95 is very low in stems showing a high density of trichomes, we generated tomato overexpressing lines to uncover the role of this TF in trichome formation. Twelve 35S::SlbHLH95 independent homozygous lines were obtained that showed similar phenotypes. Three lines exhibiting 100- to 120-fold increases in the SlbHLH95 transcript level were selected for further studies (Fig. 3a). SlbHLH95-OE plants exhibited a dramatic reduction in trichome number in stems compared with WT plants. Both light microscopy and SEM analyses showed that the decrease in trichome number mainly affected type I trichomes (Fig. 3b). Moreover, these analyses also revealed that the total trichome number was significantly decreased in leaves due to the decrease of type V trichomes (Fig. 3c–e) and in 7-day-old fruit of SlbHLH95-OE lines (Supplementary Fig. S1a, b). To gain insight into the molecular features underlying the reduced trichome phenotype, we assessed by RT–qPCR the transcript accumulation of genes known to be involved in trichome formation. SlCycB2, a B-type cyclin gene, shown to regulate tomato trichome formation, was significantly down-regulated in the SlbHLH95-OE lines (Fig. 3f). Other trichome-related genes, such as SlGAMYB2, SlGASA4, and SlANT1, also displayed a significant down-regulation in SlbHLH95-OE plants (Fig. 3g–i). In contrast, the transcript levels of SlWoolly, another gene reported to control trichome formation, showed no significant alteration (Fig. 3j).
Fig. 3.
SlbHLH95 overexpression lines show reduced trichome number on stems and leaves. (a) RT–qPCR analysis of SlbHLH95 transcripts in SlbHLH95-OE lines. (b) Light microscopy and SEM observation of trichomes on stems. Arrows show type I trichomes. (c) Light microscopy and SEM observation of leaf trichomes. Observations were made on the adaxial surface of leaves at the same stage. Arrows show type V and type VI trichomes. (d) Density of trichomes on adaxial leaves from WT and SlbHLH95-OE plants. (e) The densities of the non-glandular type V trichome and the glandular type VI trichome are shown for the adaxial leaves. (f–j) RT–qPCR relative expression of trichome-associated genes in WT and SlbHLH95-OE lines in leaves at 4 weeks old. Values are means ±SD. Statistical significancewas determined by Student’s t-test: *0.01<P<0.05; **0.001<P<0.01; ***P<0.001. OE83, OE5, and OE18 are three SlbHLH95-OE independent lines.
SlbHLH95 overexpression lines display multiple vegetative and reproductive phenotypes
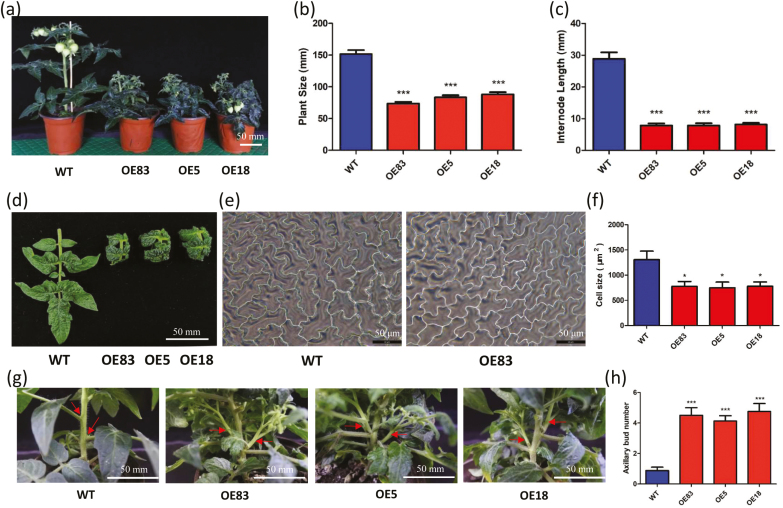
In addition to the reduced trichome formation, SlbHLH95-OE lines exhibited a stunted phenotype from early developmental stages with a severe reduction in plant size (Fig. 4a). The average height of SlbHLH95-OE adult plants was less than half of that of WT plants due to shorter internodes (Fig. 4b, c). Leaf morphology was dramatically altered in the SlbHLH95-OE lines, with twisted leaf margins and wrinkled lamina, and leaf size was significantly reduced due to smaller epidermal cells (Fig. 4d–f). In addition, SlbHLH95-OE transgenic plants exhibited more axillary buds than WT plants (Fig. 4g). Unlike the WT that produced 1–2 axillary buds, SlbHLH95-OE lines had 3–5 axillary buds per plant at the 8-week-old stage (Fig. 4h).
Fig. 4.
Vegetative phenotypes of SlbHLH95 overexpression lines. (a) Dwarf phenotype of SlbHLH95-OE plants. Photographs were taken at 80 d after germination. (b) Reduced plant size of 80-day-old SlbHLH95-OE plants. Values are means ±SD (n≥15) of three replicates. (c) Shorter internodes of 80-day-old SlbHLH95-OE plants. Values are means ±SD (n≥15) of three replicates. (d) Leaf morphology of SlbHLH95-OE plants. (e) Light microscopy observation of a leaf epidermal cell in WT and SlbHLH95-OE plants. (f) Epidermal cell size in WT and SlbHLH95-OE leaves. *0.01<P<0.05 (Student’s t-test). OE83, OE5, and OE18 are three independent SlbHLH95-OE lines. (g) Axillary bud phenotype of SlbHLH95-OE plants. Photographs were taken at the 8-week-old stage. (h) SlbHLH95-OE plants have more axillary buds than the WT. Values are the mean ±SD (n≥15) of three replicates. ***P < 0.001, (Student’s t-test).
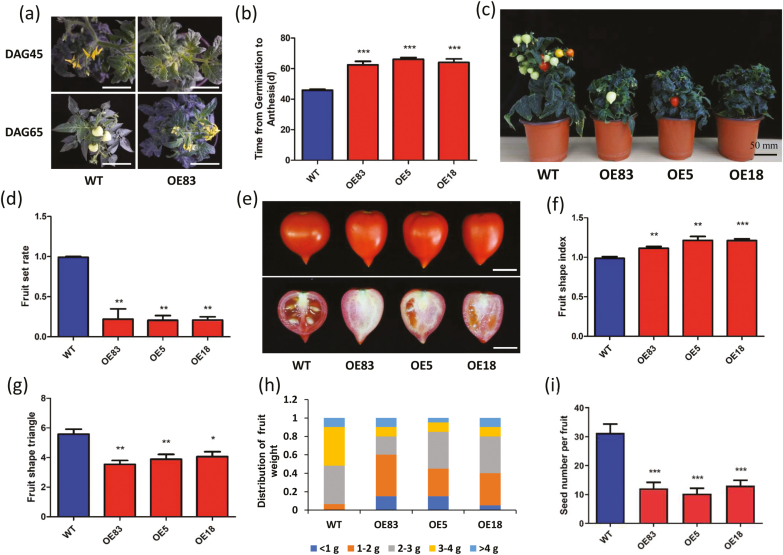
SlbHLH95-OE plants also showed large alterations in reproductive development. The time from germination to anthesis was delayed by ~20 d compared with the reference WT plants (Fig. 5a, b). Remarkably, although SlbHLH95-OE lines produced normal flower buds, the majority of these never reach the anthesis stage (Supplementary Fig. S2a), which is reminiscent of phenotypes due to a defect in GA (Nester and Zeevaart, 1988). Furthermore, the SlbHLH95-OE lines exhibited a dramatic reduction in fruit set, leading to markedly lower fruit number per plant (Fig. 5c). The fruit set rate was 15–20% in the SlbHLH95-OE lines, while 95% of the flowers successfully set fruit in WT plants grown under the same cultivation conditions (Fig. 5d). Cross-fertilization assays showed that pollen viability and female organ fertility were not affected in overexpressing lines (Supplementary Fig. S2b).
Fig. 5.
Reproductive development was dramatically altered in SlbHLH95-OE lines. (a, b) Delayed flowering of SlbHLH95-OE plants compared with the WT. DAG, days after germination. The white bars represent 5 cm. (c, d) Reduced fruit set in SlbHLH95-OE plants. (e) Altered fruit shape in SlbHLH95-OE plants. The white bars represent 1 cm. (f) Fruit shape index as the ratio of length to width in three representative independent lines. (g) Fruit shape triangle as the ratio of proximal end width to distal end width in three representative independent lines. (h) Distribution of fruit weight in WT and SlbHLH95-OE lines. n=60 fruits in WT and transgenic lines. (i) Seed number per fruit in WT and SlbHLH95-OE lines. n=60 fruits in WT and transgenic lines. Values are means ±SD. Statistical significance determined by Student’s t-test: *0.01<P<0.05; **0.001<P<0.01; ***P<0.001.
Although fruits of SlbHLH95-OE lines showed no significant difference compared with WT lines with respect to fruit development and ripening (Supplementary Fig. S2c, d), the fruit were more elongated with increased fruit shape index and decreased fruit shape triangle compared with the WT (Fig. 5e–g). The average fruit weight was also reduced, with a higher proportion of small fruits in SlbHLH95-OE lines (Fig. 5h). Moreover, the number of seeds per fruit was significantly reduced in SlbHLH95-OE lines (Fig. 5i).
Genome-wide transcriptomic profiling of SlbHLH95-OE lines
To further unveil the molecular basis of the phenotypes observed in SlbHLH95-OE lines, we performed a global gene expression profiling of 4-week-old leaves with three biological replicates. The number of raw reads ranged from 43 to 55 million with an error rate of ~0.02%, producing 6.11–7.84 G clean bases (Supplementary Data S1). On average, 96% of these reads were mapped to the ITAG-2.4 tomato reference genome, yielding 38–49 million unique mapping reads based on the sample examined (Supplementary Data S2). The expression values, indicated by fragments per kilobase of transcript per million mapped fragments (FPKM) (Supplementary Data S3), showed high correlations (Pearson correlation coefficient >0.97) among biological replicates (Supplementary Data S4) (Trapnell et al., 2010). Distribution plots showed that the distribution of the normalized expression levels was comparable between WT and OE lines (Supplementary Fig. S3).
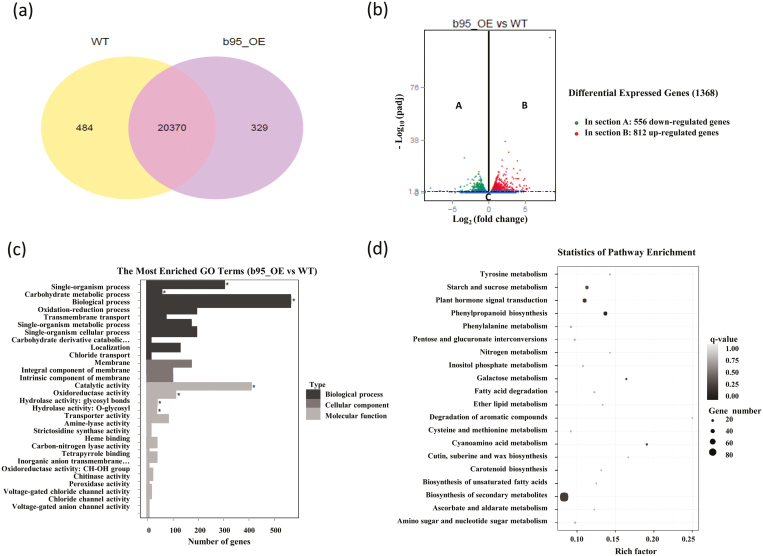
A total of 20 854 and 20 699 genes were expressed (averaged FPKM ≥1) in WT and OE lines, respectively. Among all these genes, 484 and 329 genes were uniquely expressed in WT and OE lines, respectively (Fig. 6a). A total of 1368 DEGs were identified between the WT and OE lines, of which 812 were up-regulated and 556 down-regulated in SlbHLH95-OE lines (Fig. 6b). The putative functions of the DEGs were predicted using GO analysis. The DEGs were categorized into three main categories: biological process; molecular function; and cellular component (Fig. 6c; Supplementary Data S5). Using the KEGG annotation pathway for the DEGs of SlbHLH95-OE versus the WT, those related to ‘Biosynthesis of secondary metabolites’, ‘Plant hormone signal transduction’, and ‘Phenylpropanoid biosynthesis’ were over-represented (Fig. 6d; Supplementary Data S6). Consistent with the reduced trichome number, most of the genes previously reported to regulate trichome formation in tomato were down-regulated in the SlbHLH95-OE lines (Supplementary Fig. S4).
Fig. 6.
RNA-Seq profiling of SlbHLH95-OE lines. (a) Venn diagram of the numbers of expressed genes in WT and SlbHLH95-OE lines. (b) Volcano plot showing the DEGs between two different libraries. q<0.05 was used as the threshold to determine the significance of DEGs. Dots in section A show down-regulated genes, dots in section B represent up-regulated genes, and dots below the dash-dotted line (section C) indicate transcripts that did not change significantly in the SlbHLH95-OE library compared with the WT. (c) GO enrichment analysis of DEGs between SlbHLH95-OE and the WT. The most enriched GO terms are shown. Asterisks indicate significantly enriched GO terms (q<0.05). (d) KEGG pathway enrichment analysis of DEGs between SlbHLH95-OE and the WT. The left y-axis shows the KEGG pathway. The x-axis shows the Rich factor. A high q-value is represented by light gray, and a low q-value is represented by black (q<0.05).
Considering that the phytohormone GA was reported to regulate trichome formation in Arabidopsis and that the SlbHLH95-OE lines displayed GA-deficient phenotypes such as reduced plant size, reduced trichome number, increased axillary buds, delayed flowering time, and reduced fruit set, we analyzed the expression of GA-related genes. Interestingly, among the top 20 DEGs that show down-regulation, we found two GA biosynthesis genes. Indeed, (–)-ent-kaurene synthase (SlKS5, Solyc03g006550) and gibberellin 20-oxidase-2 (SlGA20ox2, Solyc06g035530) showed significantly lower expression at the transcript level in SlbHLH95-OE lines (Table 1), suggesting that GA content might be decreased in SlbHLH95-OE plants.
Table 1.
Top 20 down-regulated genes in SlbHLH95-OE lines
| Solyc number | Log2fold | Description |
|---|---|---|
| Solyc12g056690 | –7.9933 | Serine/threonine-protein phosphatase 7 long form homolog |
| Solyc03g006550 | –4.6366 | (–)-ent-kaurene synthase (SlKS5) |
| Solyc10g085240 | –3.9082 | UDP-glucosyltransferase |
| Solyc06g009240 | –3.8717 | Cation/H(+) antiporter 15 |
| Solyc07g006560 | –3.6676 | Hypersensitive response assisting protein |
| Solyc08g080710 | –3.3537 | Carboxyl-terminal peptidase |
| Solyc06g083470 | –3.0144 | Tropinone reductase-like protein 16 |
| Solyc01g094870 | –2.9991 | MRNA clone RAFL21-92-I07 |
| Solyc04g005600 | –2.8636 | MYB transcription factor |
| Solyc11g021060 | –2.7435 | Proteinase inhibitor |
| Solyc03g121680 | –2.5768 | β-Fructofuranosidase |
| Solyc06g075460 | –2.5287 | TGF-β receptor type I/II extracellular region |
| Solyc02g077300 | –2.2942 | Peroxidase 73 |
| Solyc01g108610 | –2.2776 | Cyclin-dependent kinase inhibitor 12 |
| Solyc06g035530 | –2.1946 | Gibberellin 20-oxidase-2 (SlGA20ox2) |
| Solyc09g075790 | –2.1653 | Long-chain fatty acid CoA ligase |
| Solyc03g114830 | –2.1535 | MADS box transcription factor |
| Solyc09g072700 | –2.1518 | Peroxidase 57 |
| Solyc04g014410 | –2.1116 | Serine/threonine protein kinase-like |
| Solyc04g056450 | –2.0013 | Cyclopropane-fatty-acyl-phospholipid synthase |
All the genes were allocated a q-value <0.05.
SlbHLH95-OE lines exhibit decreased gibberellin contents
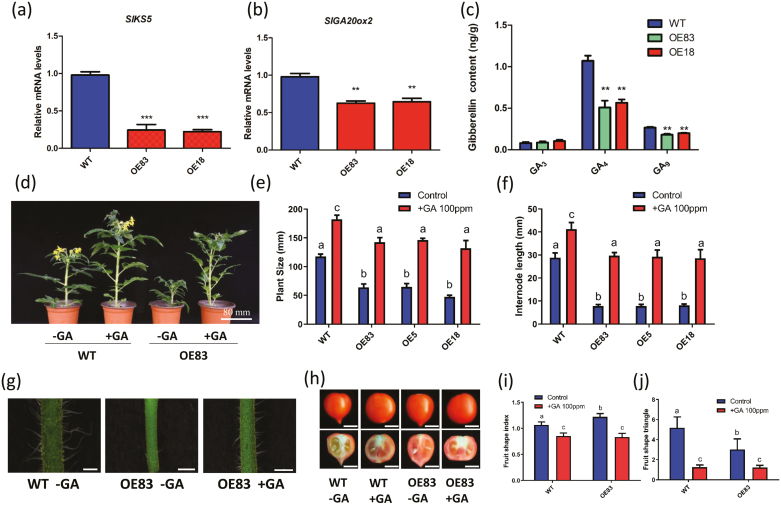
Transcript accumulation corresponding to SlKS5 and SlGA20ox2 genes was further assessed by RT–qPCR, showing a significant decrease in SlbHLH95-OE lines compared with the WT (Fig. 7a, b). Assessing the GA content indicated that although GA3, the active GA form, displayed no change, GA4, the final product of the GA bioactive form, and its precursor GA9 were significantly decreased in SlbHLH95-OE plants (Fig. 7c). These results suggested that the decreased GA levels may account for the inhibition of trichome formation and for some of the vegetative and reproductive developmental alterations in SlbHLH95-OE lines.
Fig. 7.
Application of exogenous GA rescued the dwarf phenotype in SlbHLH95-OE plants. (a, b) Relative expression of GA biosynthesis-related genes in the WT and SlbHLH95-OE lines in leaves at 4 weeks old. Statistical significance was determined by Student’s t-test: **0.001<P<0.01; ***P<0.001. (c) GA content was decreased in SlbHLH95-OE plants. **0.001< P<0.01 (Student’s t-test). (d) GA application rescued SlbHLH95-OE plant size. (e) Plant size of SlbHLH95-OE compared with WT plants after GA application. Values are means ±SD (n≥15) of three replicates. The error bars and letters in the graph indicate the SEs among samples and significant differences in plant size evaluated by Tukey’s test (α<0.05). (f) Internode length in SlbHLH95-OE plants treated with exogenous GA compared with that of WT plants. Values are the mean ±SD (n≥15) of three replicates. (g) Trichome density was partially rescued by exogenous GA application in SlbHLH95-OE lines. The white bars represent 2 mm. (h) Fruit shape rescued by exogenous GA application in SlbHLH95-OE lines. The white bars represent 1 cm. (i) The fruit shape index of GA4-treated and untreated plants. Values are means ±SD (n≥10) of three replicates. The error bars and letters in the graph indicate the SEs among samples and significant differences in plant size evaluated by Tukey’s test (α<0.05). (j) The fruit shape triangle of GA4-treated and untreated plants. Values are means ±SD (n≥10) of three replicates. The error bars and letters in the graph indicate the SEs among samples and significant differences in plant size evaluated by Dunnett’s T3 test (α<0.05).
To further investigate the contribution of the reduced GA levels to the phenotypes of low trichome density and altered vegetative and reproductive development in SlbHLH95-OE plants, we applied exogenous GA4 to 2-week-old plants since GA4 levels were found to be significantly decreased in the transgenic lines. The plant size of SlbHLH95-OE plants was fully rescued by GA4 treatment (Fig. 7d, e), with the internode length becoming comparable with those of untreated WT plants (Fig. 7f). Moreover, trichome density was enhanced in SlbHLH95-OE lines treated with exogenous GA4 (Fig. 7g). Interestingly, the fruit shape of GA-treated plants became more round compared with untreated SlbHLH95-OE plants (Fig. 7h) and, accordingly, both fruit shape index and fruit shape triangle were significantly decreased (Fig. 7i, j). These results indicated that the reduced trichome density, dwarf plant phenotype, and elongated fruit shape displayed by SlbHLH95-OE lines are likely to be due to the decreased GA levels.
SlbHLH95 directly regulates SlGA20ox2 and SlKS5 via binding to their promoters
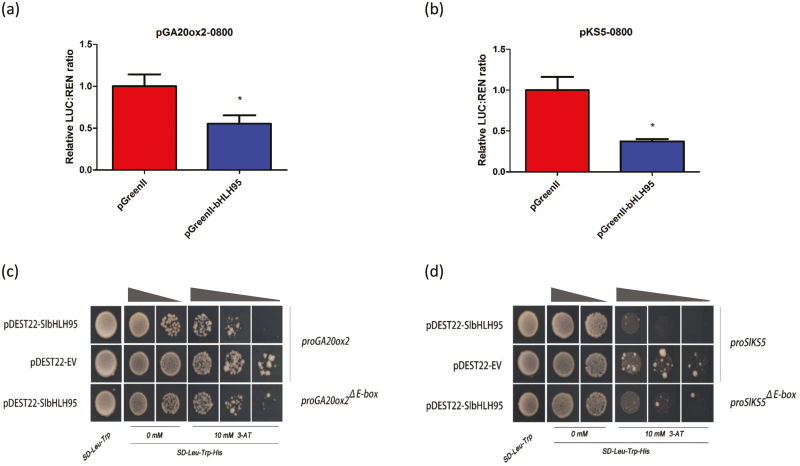
The decreased GA content together with the down-regulated expression of SlKS5 and SlGA20ox2 in SlbHLH95-OE lines motivated the investigation of a possible regulation of SlKS5 and SlGA20ox2 transcription by SlbHLH95. An in silico search for typical regulatory motifs in the SlKS5 and SlGA20ox2 promoter sequences revealed the presence of conserved E-box (CANNTG) and E-box like cis-elements known as putative targets of bHLH type TFs (Supplementary Table S2). To examine whether SlbHLH95 could repress the transcription of a reporter gene driven by the SlKS5 and SlGA20ox2 promoters, transactivation assays using the dual-luciferase reporter system were performed. The dual luciferase reporter plasmids harboring the SlKS5 and SlGA20ox2 promoters that contain the E-box-like motif were fused to the LUC reporter, and the REN reporter driven by the 35S promoter was used as an internal control. The expression of SlbHLH95 resulted in reduced activity of both SlKS5 and SlGA20ox2 promoters, indicating that SlbHLH95 exerts a negative regulation on the transcription of the two GA biosynthesis genes (Fig. 8a, b).
Fig. 8.
SlbHLH95 represses the expression of SlGA20ox2 and SlKS5 through direct binding to their cis-regulatory elements. (a, b) Dual-luciferase reporter assay. Interactions of SlbHLH95 with the promoters of SlGA20ox2 and SlKS5 were assessed in vivo by transient assays in tobacco leaves. The trans-repression ability of SlbHLH95 is indicated by the LUC:REN ratio. Each value represents the mean of six biological replicates, and vertical bars represent the SE. Significant difference (Student’s t-test): *0.01<P<0.05; LUC, firefly luciferase; REN, renilla luciferase. (c, d) SlbHLH95 represses the activity of the SlGA20ox2 and SlKS5 promoters as indicated by yeast one-hybrid assays. Yeast cells were co-transformed with a bait vector, containing a promoter fragment (proSlGA20ox2 and mutated proSlGA20ox2; proSlKS5 and mutated proSlKS5) fused to a HIS2 reporter gene, and a prey vector, containing SlbHLH95 fused to the GAL4 activation domain. Cells were grown in liquid medium to OD600 of 0.1 (10–1) and diluted in a 10× dilution series (10–2 to 10–3). Of each dilution, 5 µl was spotted on media selecting for both plasmids (SD-Leu-Trp) and selecting for interaction (SD-Leu-Trp-His), supplemented with 10 mM 3-AT to suppress background growth and test the strength of the interaction.
To assess whether SlbHLH95 directly binds to the SlKS5 and SlGA20ox2 promoters, we then expressed SlbHLH95 fused to the GAL4 AD in a yeast one-hybrid system to test its ability to bind to the two promoters fused to the HIS2 reporter. The results showed that SlbHLH95 significantly reduced yeast growth compared with the control (Fig. 8c, d), whereas when challenged with mutated E-box sequences SlbHLH95 failed to significantly affect yeast growth (Fig. 8c, d), indicating that SlbHLH95 represses the activities of SlKS5 and SlGA20ox2 promoters via binding to the E-box cis-elements. Collectively, these data indicate that SlbHLH95 acts as a negative regulator of GA biosynthesis through direct binding to the E-box motif within the SlKS5 and SlGA20ox2 promoters. This is probably the causal factor leading to reduced trichome formation and other vegetative and reproductive developmental phenotypes.
Discussion
Development of unicellular non-glandular trichomes is known to be regulated by a network of TFs in Arabidopsis (Schellmann and Hulskamp, 2005; Yang and Ye, 2013; Qi et al., 2014), and phytohormones such as GA, JA, and CK were reported to play an essential role in this process acting in a TF complex-dependent manner (Pattanaik et al., 2014). In contrast, less is known about regulatory networks that control the development of trichomes in crop species. Our present study shows that the TF SlbHLH95 is involved in the regulation of trichome development in tomato through the negative regulation of GA biosynthesis. Overexpression of SlbHLH95 resulted in reduced trichome density, reduced plant size, increased axillary buds, delayed flowering time, poor fruit set, and elongated fruit shape that resemble GA-deficient phenotypes. Supporting this notion, GA content was significantly reduced in SlbHLH95-OE lines due to lower expression of two key GA biosynthesis genes, SlGA20ox2 and SlKS5. The role of GA is further sustained by the rescue of the phenotypes by exogenous treatment of SlbHLH95-OE lines with the hormone. Yeast one-hybrid and transient expression assays showed that SlbHLH95 regulates the expression of SlGA20ox2 and KS5 via binding to their promoters. Altogether, these data validate the link between SlbHLH95, GA, and trichome formation in tomato.
It was reported that the bHLH TFs GL3 and EGL3 regulate trichome development in a partially redundant manner (Payne et al., 2000; Zhang, 2003), and other members of the bHLH gene family, such as AtMYC1 and TRANSPARENT TESTA 8 (TT8), have also been shown to positively regulate trichome development in Arabidopsis (Maes et al., 2008; Zhao et al., 2012). The present study indicates that bHLH-type TFs are not only essential for trichome formation in Arabidopsis, but they are also involved in this process in tomato. This finding is consistent with recent reports on the role of SlMYC1, another bHLH family gene, that positively regulates type VI glandular trichome formation in tomato (Xu et al., 2018). Interestingly, unlike other reported bHLH TFs, such as SlMYC1 which play a positive role in type VI trichome development (Payne et al., 2000; Zhang, 2003; Maes et al., 2008; Zhao et al., 2012; Shangguan et al., 2016; Xu et al., 2018), overexpression of SlbHLH95 in tomato resulted in dramatically reduced type I trichomes on stems and significantly fewer type V trichomes on leaves.
Mutation in the C2H2 ZFP gene (Solyc10g078970) has been suggested to account for the glabrous phenotype of IL10-2 and the hair absent (h) mutant LA3172 (Chang et al., 2018). Our results demonstrate that the TF gene SlbHLH95 (Solyc10g079050), mapping to the same region on chromosome 10 as C2H2 ZFP, also plays an active role in tomato trichome formation. Interestingly, in addition to the glabrous phenotype, overexpression of SlbHLH95 in Micro-Tom induced pleiotropic alterations associated with GA deficiency, including reduced plant size, more axillary buds, lower fruit set, and elongated fruit shape, which are absent in S. pennellii and IL10-2, probably due to the different expression levels of SlbHLH95 in these lines. It is also worth pointing out that the cultivar used in this study is Micro-Tom. Although the Micro-Tom cultivar is a brassinosteroid mutant and its trichome formation might be different from that other tomato cultivars, it has been shown to be a good model to study the regulation of trichome formation (Campos et al., 2009; Zhang et al., 2015).
In Arabidopsis, GAs play an essential role in unicellular trichome development as shown by the GA-deficient mutant ga1-3 that displays glabrous leaves, and treatment with exogenous GAs restores trichome formation (Perazza et al., 1998). Likewise, mutations of GA signaling-related genes can also affect trichome development in Arabidopsis (Chien and Sussex, 1996; Dill and Sun, 2001; Gan et al., 2007). GA transport and distribution were recently reported to be important in trichome formation (Matías-Hernández et al., 2016). While it is now established that GAs promote unicellular trichome initiation and development in Arabidopsis, the putative role of GAs in trichome formation in a crop species such as tomato remains largely unknown. Our data show that GA content was significantly decreased in SlbHLH95 overexpression plants along with the down-regulation of two key GA biosynthesis genes, consistent with the displayed GA-deficient phenotypes. Moreover, application of exogenous GA to these plants restored several WT phenotypes including trichome formation, plant size, and axillary bud number, thus suggesting that similar to the situation for unicellular trichomes in Arabidopsis, GA may also play an important role in the formation of different types of trichomes in tomato. Interestingly, several TF genes including SlIAA15, SlARF3, and SlMYC1 reported to positively regulate trichome formation in tomato (Deng et al., 2012; Zhang et al., 2015; Xu et al., 2018) showed a down-regulation tendency in SlbHLH95-OE lines (Supplementary Fig. S4). In addition, a number of MYB, bHLH, and WDR gene family members also showed altered transcript levels in SlbHLH95-OE plants (Supplementary Data S7). Taken together, these results suggest that the way in which GAs regulate trichome formation in tomato might be through modulating the expression of key trichome regulators as in Arabidopsis.
In Arabidopsis, several TFs have been shown to affect GA content via the regulation of GA biosynthesis genes, such as the MADS domain protein AGAMOUS that promotes the expression of AtGA3ox1 in developing flowers (Gomez-Mena, 2005), the bHLH TF INDETERMINATE, reported to directly regulate AtGA3ox1 in the valve margins and septum of the Arabidopsis silique (Arnaud et al., 2010), and the MADS protein SVP which delays flowering by repressing GA biosynthesis via reducing expression of AtGA20ox2 (Andrés et al., 2014). In contrast, very little is known about the direct regulation of GA biosynthesis genes in tomato. Our data reveal that SlbHLH95 regulates the expression of two key GA biosynthesis genes, SlGA20ox2 and SlKS5, via direct binding to their promoters, thus extending our understanding of the regulation of GA biosynthesis in tomato. While the present data reveal that GA is involved in trichome development, additional studies are needed to uncover the molecular mechanisms underlying GA regulation of trichome formation in tomato. In particular, it remains to be investigated whether trichome formation involving SlbHLH95 is also mediated by a complex similar to the MYB–bHLH–WDR complex described in Arabidopsis.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. The glabrous phenotype exhibited by SlbHLH95-OE young fruit.
Fig. S2. Reproductive development of SlbHLH95- overexpressing lines.
Fig. S3. The gene expression distribution (FPKM) in WT and SlbHLH95-OE lines.
Fig. S4. Expression levels of trichome-related genes in WT and SlbHLH95-OE lines.
Table S1. Genes contained in the S. pennellii genome fragment introgressed in M82 to give rise to the IL10-2 line.
Table S2. Putative bHLH-binding cis-elements present in the promoter regions of SlGA20ox2, SlKS5.
Table S3. List of the primers used in the study.
Dataset S1. Quality assessment of sequencing data.
Dataset S2. Reads mapping to the reference genome.
Dataset S3. The expression levels of genes in SlbHLH95-OE and wild-type (WT) lines.
Dataset S4. Pearson correlation coefficient analysis.
Dataset S5. GO enrichment analysis of DEGs between SlbHLH95-OE (b95_OE) and WT lines.
Dataset S6. KEGG enrichment analysis of DEGs between SlbHLH95-OE (b95_OE) and WT lines.
Dataset S7. The expression levels of MYB, bHLH and WD40 family genes in SlbHLH95-OE and WT lines.
Acknowledgements
This research was supported by the National Key R&D Program of China (2016YFD0400100) and the National Natural Science Foundation of China (31772372) to ML, and this project is also supported by the Fundamental Research Funds for the Central Universities (SCU2019D013). Yao Chen was sponsored by the China Scholarship Council.
Author contributions
ML planned and designed the research; Yao Chen, DS, JL, SY, and HD performed the experiments; XH, YZ, YL, Ya Chen, JP, and YL analyzed the data; and YC, ML, and MB wrote the manuscript.
Data availability
All relevant data are within the paper and its supplementary files.
References
- Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biology 11, R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés F, Porri A, Torti S, Mateos J, Romera-Branchat M, García-Martínez JL, Fornara F, Gregis V, Kater MM, Coupland G. 2014. SHORT VEGETATIVE PHASE reduces gibberellin biosynthesis at the Arabidopsis shoot apex to regulate the floral transition. Proceedings of the National Academy of Sciences, USA 111, E2760–E2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud N, Girin T, Sorefan K, Fuentes S, Wood TA, Lawrenson T, Sablowski R, Østergaard L. 2010. Gibberellins control fruit patterning in Arabidopsis thaliana. Genes & Development 24, 2127–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300. [Google Scholar]
- Bosch M, Wright LP, Gershenzon J, Wasternack C, Hause B, Schaller A, Stintzi A. 2014. Jasmonic acid and its precursor 12-oxophytodienoic acid control different aspects of constitutive and induced herbivore defenses in tomato. Plant Physiology 166, 396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer MT, Moyseenko JB, Monforte AJ, van der Knaap E. 2007. Morphological variation in tomato: a comprehensive study of quantitative trait loci controlling fruit shape and development. Journal of Experimental Botany 58, 1339–1349. [DOI] [PubMed] [Google Scholar]
- Campos ML, de Almeida M, Rossi ML, Martinelli AP, Litholdo Junior CG, Figueira A, Rampelotti-Ferreira FT, Vendramim JD, Benedito VA, Peres LE. 2009. Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. Journal of Experimental Botany 60, 4347–4361. [DOI] [PubMed] [Google Scholar]
- Chang J, Yu T, Yang Q, et al. 2018. Hair, encoding a single C2H2 zinc-finger protein, regulates multicellular trichome formation in tomato. The Plant Journal 96, 90–102. [DOI] [PubMed] [Google Scholar]
- Chien JC, Sussex IM. 1996. Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiology 111, 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Yang Y, Ren Z, Audran-Delalande C, Mila I, Wang X, Song H, Hu Y, Bouzayen M, Li Z. 2012. The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytologist 194, 379–390. [DOI] [PubMed] [Google Scholar]
- Dill A, Sun T. 2001. Synergistic derepression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics 159, 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewas M, Gao Y, Ali F, Nishawy EM, Shahzad R, Subthain H, Amar M, Martin C, Luo J. 2017. RNA-seq reveals mechanisms of SlMX1 for enhanced carotenoids and terpenoids accumulation along with stress resistance in tomato. Science Bulletin 62, 476–485. [DOI] [PubMed] [Google Scholar]
- Ewas M, Gao Y, Wang S, et al. 2016. Manipulation of SlMXl for enhanced carotenoids accumulation and drought resistance in tomato. Science Bulletin 61, 1413–1418. [DOI] [PubMed] [Google Scholar]
- Gan Y, Yu H, Peng J, Broun P. 2007. Genetic and molecular regulation by DELLA proteins of trichome development in Arabidopsis. Plant Physiology 145, 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas JJ, Schimmel BC, Alba JM, Escobar-Bravo R, Schuurink RC, Kant MR. 2012. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. International Journal of Molecular Sciences 13, 17077–17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Mena C, de Folter S, Costa MM, Angenent GC, Sablowski R. 2005. Transcriptional program controlled by the floral homeotic gene AGAMOUS during early organogenesis. Development 132, 429–438. [DOI] [PubMed] [Google Scholar]
- Hao YH, Zhang Z, Wang L, Liu C, Lei AW, Yuan BF, Feng YQ. 2015. Stable isotope labeling assisted liquid chromatography-electrospray tandem mass spectrometry for quantitative analysis of endogenous gibberellins. Talanta 144, 341–348. [DOI] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA. 2005. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Hattori S, Sano R, et al. 2007. Arabidopsis TRANSPARENT TESTA GLABRA2 is directly regulated by R2R3 MYB transcription factors and is involved in regulation of GLABRA2 transcription in epidermal differentiation. The Plant Cell 19, 2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Kurata T, Okada K, Wada T. 2008. A genetic regulatory network in the development of trichomes and root hairs. Annual Review of Plant Biology 59, 365–386. [DOI] [PubMed] [Google Scholar]
- Jeong N-R, Kim H, Hwang I-T, Howe GA, Kang J-H. 2017. Genetic analysis of the tomato inquieta mutant links the ARP2/3 complex to trichome development. Journal of Plant Biology 60, 582–592. [Google Scholar]
- Johnson CS, Kolevski B, Smyth DR. 2002. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. The Plant Cell 14, 1359–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Araki M, Goto S, et al. 2008. KEGG for linking genomes to life and the environment. Nucleic Acids Research 36, D480–D484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Liu G, Shi F, Jones AD, Beaudry RM, Howe GA. 2010a The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiology 154, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, McRoberts J, Shi F, Moreno JE, Jones AD, Howe GA. 2014. The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiology 164, 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Shi F, Jones AD, Marks MD, Howe GA. 2010b Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. Journal of Experimental Botany 61, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nature Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Guo Z, Liu C, Li J, Xu W, Chen Y. 2017. Quantification of near-attomole gibberellins in floral organs dissected from a single Arabidopsis thaliana flower. The Plant Journal 91, 547–557. [DOI] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA. 2004. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. The Plant Cell 16, 126–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckwill LC. 1943. The genus Lycopersicon: a historical, biological, and taxonomic survey of the wild and cultivated tomatoes. Aberdeen: Aberdeen University Press. [Google Scholar]
- Ma Z, Ge L, Lee AS, Yong JW, Tan SN, Ong ES. 2008. Simultaneous analysis of different classes of phytohormones in coconut (Cocos nucifera L.) water using high-performance liquid chromatography and liquid chromatography-tandem mass spectrometry after solid-phase extraction. Analytica Chimica Acta 610, 274–281. [DOI] [PubMed] [Google Scholar]
- Maes L, Inzé D, Goossens A. 2008. Functional specialization of the TRANSPARENT TESTA GLABRA1 network allows differential hormonal control of laminal and marginal trichome initiation in Arabidopsis rosette leaves. Plant Physiology 148, 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Cai T, Olyarchuk JG, Wei L. 2005. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 21, 3787–3793. [DOI] [PubMed] [Google Scholar]
- Matías-Hernández L, Aguilar-Jaramillo AE, Cigliano RA, Sanseverino W, Pelaz S. 2016. Flowering and trichome development share hormonal and transcription factor regulation. Journal of Experimental Botany 67, 1209–1219. [DOI] [PubMed] [Google Scholar]
- Nester JE, Zeevaart JAD. 1988. Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. American Journal of Botany 75, 45. [Google Scholar]
- Pattanaik S, Patra B, Singh SK, Yuan L. 2014. An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Frontiers in Plant Science 5, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CT, Zhang F, Lloyd AM. 2000. GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics 156, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazza D, Vachon G, Herzog M. 1998. Gibberellins promote trichome formation by up-regulating GLABROUS1 in Arabidopsis. Plant Physiology 117, 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T, Huang H, Wu D, Yan J, Qi Y, Song S, Xie D. 2014. Arabidopsis DELLA and JAZ proteins bind the WD-repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy. The Plant Cell 26, 1118–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerie WG, Feldmann KA, Marks MD. 1994. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes & Development 8, 1388–1399. [DOI] [PubMed] [Google Scholar]
- Rodríguez GR, Moyseenko JB, Robbins MD, Huarachi Morejón N, Francis DM, van der Knaap E. 2010. Tomato analyzer: a useful software application to collect accurate and detailed morphological and colorimetric data from two-dimensional objects. Journal of Visualized Experiments (37), e1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. 2006. Quantitative real-time RT-PCR data analysis: current concepts and the novel ‘gene expression’s C T difference’ formula. Journal of Molecular Medicine 84, 901–910. [DOI] [PubMed] [Google Scholar]
- Schellmann S, Hülskamp M. 2005. Epidermal differentiation: trichomes in Arabidopsis as a model system. International Journal of Developmental Biology 49, 579–584. [DOI] [PubMed] [Google Scholar]
- Shangguan XX, Yang CQ, Zhang XF, Wang LJ. 2016. Functional characterization of a basic helix–loop–helix (bHLH) transcription factor GhDEL65 from cotton (Gossypium hirsutum). Physiologia Plantarum 158, 200–212. [DOI] [PubMed] [Google Scholar]
- Simmons AT, Gurr GM. 2005. Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agricultural and Forest Entomology 7, 265–276. [Google Scholar]
- Symonds VV, Hatlestad G, Lloyd AM. 2011. Natural allelic variation defines a role for ATMYC1: trichome cell fate determination. PLoS Genetics 7, e1002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Wang X, Guo H, Cheng Y, Hou C, Chen J-G, Wang S. 2017. NTL8 regulates trichome formation in Arabidopsis by directly activating R3 MYB genes TRY and TCL11. Plant Physiology 174, 2363–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomato Genome Consortium 2012. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology 28, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech JC, Bouzayen M. 2005. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. The Plant Cell 17, 2676–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker E. 2000. Trichome diversity and development. Advances in Botanical Research. 31, 1–35. [Google Scholar]
- Xu J, van Herwijnen ZO, Dräger DB, Sui C, Haring MA, Schuurink RC. 2018. SlMYC1 regulates type VI glandular trichome formation and terpene biosynthesis in tomato glandular cells. The Plant Cell 30, 2988–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Zhai Q, Wei J, et al. 2013. Role of tomato lipoxygenase D in wound-induced jasmonate biosynthesis and plant immunity to insect herbivores. PLoS Genetics 9, e1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Li H, Zhang J, et al. 2011. A regulatory gene induces trichome formation and embryo lethality in tomato. Proceedings of the National Academy of Sciences, USA 108, 11836–11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ye Z. 2013. Trichomes as models for studying plant cell differentiation. Cellular and Molecular Life Sciences 70, 1937–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MD, Wakefield MJ, Smyth GK, Oshlack A. 2010. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biology 11, R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A. 2003. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130, 4859–4869. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yan F, Tang Y, Yuan Y, Deng W, Li Z. 2015. Auxin response gene SlARF3 plays multiple roles in tomato development and is involved in the formation of epidermal cells and trichomes. Plant & Cell Physiology 56, 2110–2124. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wang X, Zhu D, Cui S, Li X, Cao Y, Ma L. 2012. A single amino acid substitution in IIIf subfamily of basic helix–loop–helix transcription factor AtMYC1 leads to trichome and root hair patterning defects by abolishing its interaction with partner proteins in Arabidopsis. Journal of Biological Chemistry 287, 14109–14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its supplementary files.