Abstract
Photoperiodic flowering responses are classified into three major types: long day (LD), short day (SD), and day neutral (DN). The inverse responses to daylength of LD and SD plants have been partly characterized in Arabidopsis and rice; however, the molecular mechanism underlying the DN response is largely unknown. Modern roses are economically important ornamental plants with continuous flowering (CF) features, and are generally regarded as DN plants. Here, RcCO and RcCOL4 were identified as floral activators up-regulated under LD and SD conditions, respectively, in the CF cultivar Rosa chinensis ‘Old-Blush’. Diminishing the expression of RcCO or/and RcCOL4 by virus-induced gene silencing (VIGS) delayed flowering time under both SDs and LDs. Interestingly, in contrast to RcCO-silenced plants, the flowering time of RcCOL4-silenced plants was more delayed under SD than under LD conditions, indicating perturbed plant responses to day neutrality. Further analyses revealed that physical interaction between RcCOL4 and RcCO facilitated binding of RcCO to the CORE motif in the promoter of RcFT and induction of RcFT. Taken together, the complementary expression of RcCO in LDs and of RcCOL4 in SDs guaranteed flowering under favorable growth conditions regardless of the photoperiod. This finding established the molecular foundation of CF in roses and further shed light on the underlying mechanisms of DN responses.
Keywords: Continuous flowering, day-neutral plants, long-day plants, photoperiod responses, Rosa chinensis, short-day plants
The complementary expression of RcCO in long days and RcCOL4 in short days guarantees flowering under favorable growth conditions regardless of the photoperiod, thus facilitating the day-neutral response of roses.
Introduction
Flowering is a biological process indicating the shift from vegetative growth to reproductive development; as such, its accurate timing is key to reproduction and survival. The timely transition from vegetative to floral meristems in higher plants is programmed by external environmental cues and endogenous signals (Langridge, 1957). So far, six genetic pathways have been identified to control plant flowering, namely photoperiod, vernalization, ambient temperature, gibberellin, age, and autonomous pathways. All these pathways finally converge on the common downstream flowering integrators FT (FLOWERING LOCUS T) and SOC1 (SUPPRESSOR OF OVEREXPRESSION OF CO1), whose expression leads to the induction of floral identity genes such as LFY (LEAFY) and AP1 (APETALA1), followed by flower bud formation and burst (Fornara et al., 2010; Srikanth and Schmid, 2011).
The photoperiod pathway refers to the regulation of flowering in response to daylength. Based on their daylength requirements, plants are classified as long day (LD), short day (SD), or day-neutral (DN) (Jeong and Clark, 2005; Srikanth and Schmid, 2011). Arabidopsis is one of the well-known LD plants and flowers much earlier under LD than SD conditions. In contrast, rice is considered a SD plant that flowers faster in SDs than in LDs. The DN plants flower interdependently of the photoperiodic conditions. The inverse responses to daylength observed between Arabidopsis (LD plant) and rice (SD plant) are partly explained by the difference between the function of CO in Arabidopsis and the rice homolog HEADING DATE 1 (Hd1) (Izawa et al., 2002; Ryosuke et al., 2003). A common role of GIGANTEA (GI)–CONSTANS (CO)–FLOWERING LOCUS T (FT) in Arabidopsis and rice has been demonstrated (Izawa et al., 2002; Ryosuke et al., 2003). Hd1 promotes the flowering under SD conditions and inhibits it under LD conditions in rice, whereas CO only accelerates flowering under LDs in Arabidopsis. Further study proves Hd1 can up-regulate Hd3a (the homolog of Arabidopsis FT in rice) expression preferably under SD conditions, and the Hd1–Hd3a pathway in rice mimics the CO–FT model in Arabidopsis (Yano et al., 2000; Reina et al., 2008; Xue et al., 2009). However, the DN response is the most poorly characterized among the three types of photoperiodic flowering responses. In the DN plant tomato (Solanum lycopersicum), the universal florigenic signal triggered by SFT (SINGLE FLOWER TRUSS), a FT homolog, is a known flowering inducer under different daylengths (Lifschitz and Eshed, 2006). Consistently, the florigen gene ZEA CENTRORADIALIS8 (ZCN8) in maize (Zea mays) is associated with the floral transition both in DN temperate maize and in SD-requiring tropical maize, and has been shown to be regulated by different chromatin modifications at the floral transition (Lazakis et al., 2011).
Roses are economically important ornamental plants with high symbolic value and great cultural importance all over the world. They are extensively used as garden plants, cut flowers, as well as potted flowers, and also for the production of essential oils in the cosmetic industry (Bendahmane et al., 2013). Overall, there are three different flowering modes in rose plants, once-flowering (OF) genotypes (such as Rosa multiflora), continuous flowering (CF) genotypes (such as Rosa chinensis cv ‘Old Blush’) which flower during the growing seasons, and occasionally re-blooming (OB) genotypes, such as the vegetative mutants of the CF genotype ‘Pompon de Paris Climbing’ (Bendahmane et al., 2013; Kurokura et al., 2013). In OF genotypes, floral transition occurs during short photoperiods in early spring. In CF genotypes, floral transition occurs during short photoperiods and long photoperiods, such as in late spring and summer, and as such they are generally considered as DN plants or photoperiod-insensitive plants. The comparison of OF and CF varieties presents a unique opportunity to investigate the DN photoperiod pathway in roses.
Rosa chinensis cv ‘Old Blush’ is one of the important progenitors of modern rose cultivars and is regarded as the main contributor of recurrent flowering (Martin et al., 2001; Bendahmane et al., 2013). In a recent study of rose plants, KSN, a TFL1 homolog of Arabidopsis, is shown to act as a floral repressor. In OF rose cultivars, KSN is repressed in winter and early spring under short photoperiods, so they bloom only once in spring. After blooming, KSN expression is activated by an as yet unknown mechanism which in turn represses further flower formation in long-photoperiod summer (Iwata et al., 2012; Randoux et al., 2012; Bendahmane et al., 2013). It is further established that the 10 kb insertion of a copia retrotransposon in the second intron of the KSN gene in the CF rose ‘Old Blush’ blocks the expression of KSN and enables its flowering regardless of daylength (Iwata et al., 2012). Interestingly, studies in a different genetic background using distinct mapping populations have identified two quantitative trait loci (QTLs) for continuous flowering, suggesting that the CF trait may be under the control of multiple regulators (Dugo et al., 2005; Shubin et al., 2015). In the present study, RcCO and RcCOL4 were identified as floral activators up-regulated under LD or SD conditions, respectively, in ‘Old Blush’. RcCOL4 physically interacted with RcCO and thereby facilitated RcCO binding to the promoter of RcFT to activate its transcription. The complementary expression of these two positive floral regulators in LDs and SDs guaranteed rose flowering under favorable growth conditions irrespective of the photoperiod. This finding provided a molecular model of the CF trait in rose and deciphered the underlying mechanism of the DN response.
Materials and methods
Plant materials and growth conditions
OF roses Rosa laevigata, Rosa berberifolia, and Rosa multiflora, and CF roses Rosa chinensis cv ‘Old Blush’, R. chinensis cv ‘Sichun’, R. chinensis cv ‘Viridiflora’, and Rosa hybrida cv ‘Molde’ were grown in the rose resource nursery of Nanjing Agricultural University. Cuttings or explants were collected from multistemmed stock plants, and the generated cutting plants or seedling in vitro were used for the present experiment. Plants propagated from cuttings were used for flower phenotyping, and were grown in plant incubators with controlled conditions (25 °C, 40% relative humidity, and 200 μmol m–2 s–1) under SDs (8:16 h, light:dark) or LDs (16:8 h, light:dark). Seedlings in vitro of R. chinensis cv ‘Old blush’ were used as starting materials for in vitro propagation, and were repeatedly subcultured every 3–4 weeks on proliferation medium [Murashige and Skoog (MS)+1.5 mg l–1 6-benzyladenine (6-BA)+0.1 mg l–1 1-naphthaleneacetic acid (NAA)+30 g l–1 sucrose+6.5 g l–1 agar, pH 5.75]. The resultant young seedlings were then used as transient transformation materials.
Arabidopsis plants were also grown in a plant incubator with controlled conditions (22 °C, 40% relative humidity, and 180 μmol m–2 s–1) under LDs (16:8 h, light:dark).
For the phylogenetic analysis
First, the hidden Markov model of the BBX domain (PF00643) t from the Pfam database (http://pfam.xfam.org/) was used to retrieve all of the candidate members of the BBX gene family from R. chinensis, R. multiflora, and Arabidopsis by using the HMMER v3.0 program with default parameters. Then, all candidate protein sequences were further validated on InterPro (http://www.ebi.ac.uk/interpro/) and SMART (http://smart.embl-heidelberg.de/) for the integrity of their conserved domains. Multiple sequence alignments were executed by using MAFFT v7.409 (Katoh and Standley 2013) with the L-INS-I alignment strategy (most accurate). Systematic phylogenetic analysis and maximum-likelihood phylogenetic trees were constructed using FastTree software with the JTT+CAT model (http://www.microbesonline.org/fasttree/) (Price et al., 2009). The phylogenetic trees were visualized and edited using MEGA7 software (https://www.megasoftware.net/home) (Kumar et al., 2016). To explore the domain compositions of the full-length sequences of BBX domain-containing proteins, SMART (https://smart.embl-heidelberg.de/) and Pfam (https://pfam.xfam.org/) online programs were used to identify all the conserved domains with default parameters.
Plasmid constructions
The overexpression constructs were prepared by amplifying RcCO (RcChr2g0164091) and RcCOL4 (RcChr6g0299051) from the cDNA of ‘Old Blush’ using the primers listed in Supplementary Table S1 at JXB online. Subsequently, PCR products were cloned into the pENTR-D-TOPO entry vector (Invitrogen) and then cloned into the binary vector pFAST-R05 (http://www.psb.ugent.be/).
For virus-induced gene silencing (VIGS), gene-specific fragments of RcCO and RcCOL4 were amplified using the primers listed in Supplementary Table S1 and then cloned into pTRV2 to generate the VIGS constructs.
For protein–protein interaction analysis by rose transient assay, coding sequences of RcCO and RcCOL4 were inserted into pMK7-nL-WG2 or pMK7-cL-WG2 (http://www.psb.ugent.be/), respectively, by LR reaction. For promoter binding analysis, a fragment containing 1976 bp upstream of the translational start site of RcFT (RcChr4g0439111) was amplified from the genome sequence. Next, the PCR product was cloned into the pENTR-D-TOPO vector and subsequently recombined into pGBWL7 (http://www.psb.ugent.be/).
For yeast two-hybrid experiments, full coding sequences of RcCO and RcCOL4 were inserted into the pGBKT7 vector (bait, BD) or the pGADT7 vector (prey, AD). For yeast one-hybrid assay used to identify the promoter binding, 1976 bp upstream of the translational start site of RcFT was cloned into the pHIS2 vector.
To substitute Cys by Ser in Box1 and Box2 in the RcCOL4 sequence, we used the Hieff Mut™ Site-Directed Mutagenesis Kit (YEASEN, Shanghai, China) with the base substitution primers listed in Supplementary Table S1.
To induce protein in Escherichia coli, the coding sequences of RcCO and RcCOL4 were cloned into pGEX4T-1 using the Hieff Clone® Plus One Step Cloning Kit (YEASEN).
Gene expression analysis
For RNA isolation, the uppermost young leaves from 40-day-old rose plants propagated from cuttings were harvested and frozen in liquid nitrogen. Total RNA was then extracted using the FastPure Plant Total RNA Isolation Kit (VAZYME, Nanjing, China), and 1 µg of total RNA was reverse transcribed using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TRANSGEN BIOTECH, Beijing, China) according to the manufacturer’s instructions. Quantitative real-time PCR (RT-qPCR) was performed to identify gene expression levels by using TB Green™ Premix Ex Taq™ II (TaKaRa, Dalian, China) and RcGAPDH was used as reference gene as described previously (Liu et al., 2018). Every experiment was conducted with three replicates each with three technical repeats. Semi-quantitative RT-PCR was performed to identify gene expression in Arabidopsis by using the primers listed in Supplementary Table S1, and Actin2 was used as reference gene (Chang et al., 2016).
Virus-induced gene silencing
For the generation of gene-silenced plants, VIGS was performed as previously reported (Tian et al., 2014). Briefly, the Agrobacterium tumefaciens strain GV3101 carrying TRV-RcCO or TRV-RcCOL4 was grown at 28 °C in Yeast Extract Broth medium supplemented with 20 mM acetosyringone, 50 mg l–1 gentamicin, 50 mg l–1 kanamycin, and 30 mg l–1 rifampicin, and shaken on a rocking platform at 250 rpm for ~18–24 h. Subsequently, Agrobacterium cells were harvested and suspended in infiltration buffer [10 mmol l–1 MgCl2, 200 mmol l–1 acetosyringone, 10 mmol l–1 MES, 0.01% (v/v) Silwet-L77, pH 5.6]. A mixture of A. tumefaciens cultures containing pTRV1 and constructed pTRV2-RcCO/RcCOL4 in a ratio of 1:1 (v/v) was adjusted to OD600=0.6, and the mixture of pTRV1 and pTRV2 with the same concentration was also prepared as a negative control. Then, R. chinensis cv ‘Old Blush’ cuttings were submerged in infiltration buffer and exposed to a vacuum of −25 kPa twice, each for 60 s. The infiltrated cuttings were briefly washed with distilled water and planted in substrates [roseite:perlite:peat soil 1:1:1 (v/v/v)] for further analysis.
Transient transformation analysis in rose
To perform the protein–protein interaction or promoter binding analysis, transient transformations using young shoots of R. chinensis cv ‘Old Blush’ were conducted as previously described (Lu et al., 2017). Briefly, for protein–protein interaction, the A. tumefaciens strain GV3101 carrying pMK7-nL-WG2-RcCO or pMK7-cL-WG2-RcCOL4 was co-infiltrated into rose shoots to test the possibility of luciferase reconstitution. For promoter binding analysis, the A. tumefaciens strain GV3101 carrying pFAST-R05-RcCO/RcCOL4 and pGBWL7-pFT or other different combinations were co-infiltrated into rose shoots to determine changes in activity of luciferase.
Luciferase imaging
Luciferase imaging was performed using a CCD camera (Andor Technology). At 48 h after the agro-infiltration of young shoots of R. chinensis cv ‘Old Blush’ the images were acquired every 10 min for 60 min, and luciferase activity was quantified as mean counts per pixel per exposure time using Andor Solis image-analysis software (Andor Technology).
Yeast hybrid experiments
For yeast one-hybrid assay, yeast Y187 cells carrying pFT-pHis were grown on SD medium lacking tryptophan and histidine (SD/-Trp/-His) with different contents of 3-amino-1,2,4-triazole (3-AT) to optimize the concentration for inhibiting self-activation. After that, pGADT7-RcCO/pGADT7-RcCOL4 and pFT-pHis were co-transformed into yeast Y187 cells, and the binding activity was examined on SD medium lacking tryptophan, leucine, and histidine (SD/−Trp/−Leu/−His) with the proper concentration of 3-AT.
For yeast two-hybrid (Y2H) assay, the Y2H yeast strain was used according to the manufacturer’s instructions (Clontech, Mountain View, CA, USA). Yeast transformation was carried out using the lithium acetate method. The Y2H yeast cells containing prey (RcCO-AD) and bait (RcCOL4-BD/RcCOL4M1-BD/RcCOL4M2-BD) were co-cultured on SD medium lacking leucine and tryptophan (SD/−Leu/−Trp). Putative transformants were then transferred to SD medium lacking adenine, histidine, leucine, and tryptophan (SD/−Ade/−His/−Leu/−Trp; Clontech) with or without X-α-gal. At least three independent transformations were performed and three clones per transformations were used to evaluate the protein interaction.
Electrophoretic mobility shift assay
The EMSA was performed using biotinylated probes (Sangon Biotech, Shanghai, China) and the Light Shift Chemiluminescence EMSA kit (Thermo Scientific, https://www.thermofisher.com/). Briefly, E. coli strain BL21 carrying pGEX4T-1-RcCO or pGEX4T-1-RcCOL4 was grown at 37 °C in Luria–Bertani medium supplemented with 100 mg l–1 ampicillin and shaken on a rocking platform to OD600=0.5. Subsequently, the E. coli cultures were supplemented with 500 μmol l–1 isopropyl-β-d-thiogalactopyranoside (IPTG) and shaken on a rocking platform at 25 °C to induce the protein expression. The obtained E. coli cells were harvested by centrifugation and re-suspended in phosphate-buffered saline (PBS), and then were broken by ultrasonication and the supernatant was isolated and purified by the GST-Tagged Protein Purification Kit (CWBIO, Beijing, China). Next, the purified RcCO/RcCOL4, RcNF-YB, and RcNF-YC proteins were mixed with probes (biotin-labeled and unlabeled) and incubated at 24 °C for 20 min, followed by separation on 6% native polyacrylamide gels in 0.5× TBE buffer. The gels were electroblotted to Hybond N+ (Millipore) nylon membranes in 0.5× TBE buffer for 210 min (120 mA) and then detected by Gel Documentation Systems (BIO-RAD Technology) (Xu et al., 2014). The probes used in this study are listed in Supplementary Table S1.
Pull-down assay
The pull-down assays were conducted using a HIS Pull-down kit (ThermoFisher Scientific) according to the manufacturer’s instruction. Briefly, the purified RcCO-His fusion protein was incubated with immobilized glutathione S-transferase (GST) and RcCOL4–GST fusion proteins in pull-down buffer (50 mmol l–1 Tris–HCl, pH 7.2, 150 mmol l–1 NaCl, 10% glycerol, 0.1% Triton X-100, 1× protease inhibitor cocktail) for 2 h at 4 °C. Then, proteins were eluted in the elution buffer, and the interaction was determined by western blot using anti-His antibody (CWBIO, Beijing, China).
Statistical analyses
To determine statistical significance, these analyses were performed by Kruskal–Wallis test. The difference was considered significant at P<0.05.
Results
Converse regulation of RcCO and RcCOL4 expression levels by photoperiod
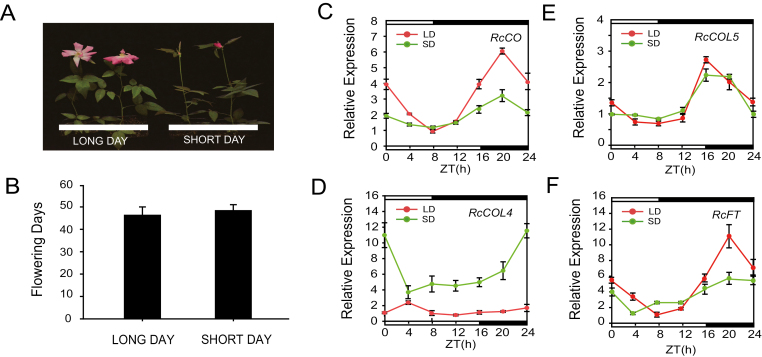
CF roses are usually considered as DN plants because they flower under any favorable environments irrespective of the photoperiodic conditions. To confirm this, we compared the flowering time of CF cuttings of R. chinensis cv ‘Old Blush’ transplanted in LD and SD conditions. As shown in Fig. 1A and B, seedling cuttings bloomed at 43 d under LDs in contrast to 44 d under SDs. The difference in flowering time between SDs and LDs was not statistically significant, supporting the DN response of rose plants.
Fig. 1.
Flowering phenotype and expression levels of RcCO, RcCOL4, RcCOL5, and RcFT under LD and SD conditions. (A) Phenotypic characterization of Rosa chinensis ‘Old Blush’ under LD (16:8 h, light:dark) and SD (8:16 h, light:dark) conditions. (B) Flowering time of R. chinensis ‘Old Blush’ under LD and SD conditiona. Error bars indicate ± the standard deviation (n=10). (C–F) Relative expression of RcCO (C), RcCOL4 (D), RcCOL5 (E), and RcFT (F) of ‘Old Blush’ under LD and SD conditions. Error bars indicate ± the standard deviation (n=3).
A conserved role of the CO/FT pathway in flowers of Arabidopsis and rice has been demonstrated, and the circadian regulation of CO as a basis for monitoring daylength and a guarantee for the induction of FT has been established (Suárez-López et al., 2001; Valverde et al., 2004). To clone CO in rose, we screened the newly published genome database of R. chinensis (https://lipm-browsers.toulouse.inra.fr/pub/RchiOBHm-V2/) (Raymond et al., 2018), and identified 18 non-redundant BBX genes that contained one or two BBX domains (Supplementary Fig. S1). Three genes (RcChr2g0164091, RcChr6g0299051, and RcChr4g0403841) were classified into the structure group I subfamily, which contained a highly conserved double B-box domain in the N-terminus and a CCT domain in the C-terminus (Supplementary Fig. S2). Accordingly, the genes were designated as RcCO (RcBBX1), RcCOL4 (RcBBX5), and RcCOL5 (RcBBX6) following the nomenclature system suggested by Khanna et al. (2009).
Subsequently, the expression levels of RcFT, RcCO, and its closest family members RcCOL4 and RcCOL5 were examined every 4 h in a 24 h cycle starting at the onset of light. The results clearly showed circadian regulation of the genes, categorizing them according to the expression differences between SD and LD conditions: higher in LDs and lower in SDs (RcCO) (Fig. 1C), lower in LDs and higher in SDs (RcCOL4) (Fig. 1D), and lower or higher alternately (RcFT and RcCOL5) in SDs and LDs (Fig. 1E, F). It was noteworthy that the higher expression of RcFT from ZT8 to ZT16 may compensate the lower expression in the rest time under SDs, which may result in the equivalent flowering time in SDs and LDs (Fig. 1F). Interestingly, RcCO and RcCOL4 displayed distinct photoperiod-dependent expression levels; that is, the expression level of RcCOL4 was higher in SDs than in LDs, whereas the level of RcCO was higher in LDs than SDs over most times of the day and light cycle. To further test the universality of this phenomenon, three OF (R. laevigata, R. berberifolia, and R. multiflora) and CF (R. chinensis cv ‘Sichun’, R. chinensis cv ‘Viridiflora’, and R. hybrida cv ‘Molde’) rose varieties were selected to characterize the time-course of RcCO, RcCOL4, and RcCOL5 expression in a 24 h cycle under both LDs and SDs. Surprisingly, RcCOL4 was expressed preferentially more highly under SDs in all the CF varieties (Supplementary Fig. S3B), in contrast to the higher expression levels of RcCO under LDs, in both OF and CF rose varieties (Supplementary Fig. S3A), while there was no obvious regularity of RcCOL4 expression under LDs in OF roses (Supplementary Fig. S3B) and of RcCOL5 in OF and CF roses (Supplementary Fig. S3C). Collectively, these results portrayed inverse responses of RcCO and RcCOL4 expression to the photoperiod and thus suggested their key roles in photoperiod-dependent flowering time.
RcCO and RcCOL4 are essential for DN response of roses
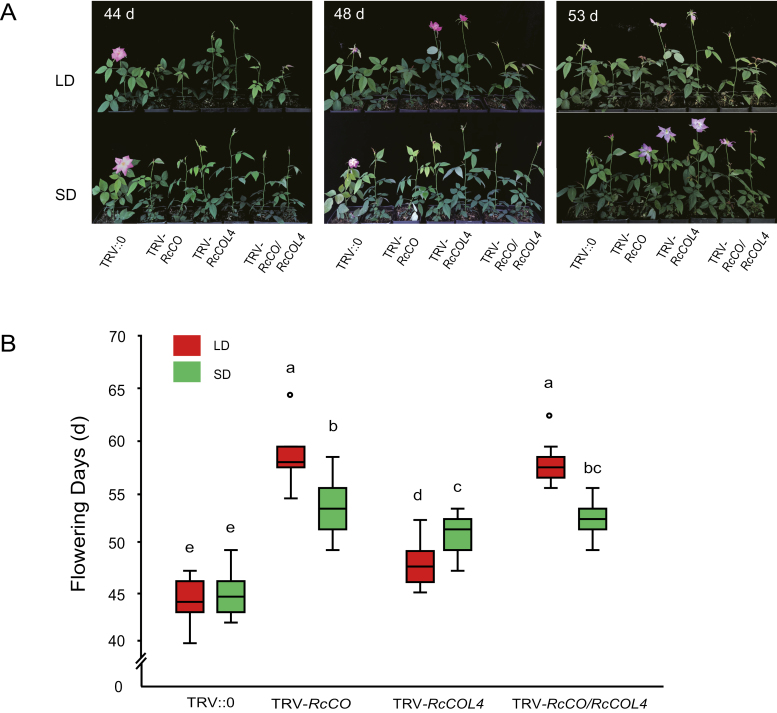
To gain the genetic evidence of the biological functions of RcCO, RcCOL4, and RcCOL5, the VIGS technique was employed to silence the genes in R. chinensis ‘Old Blush’, followed by recoding their respective flower phenotypes (Fig. 2; Supplementary Fig. S4), and measurements of gene expression under SD and LD conditions (Fig. 3, Supplementary Fig. S4). RcFT was also quantified as a marker gene of flowering time (Fig. 3C, F). As shown in Fig. 2A, B and Supplementary Fig. S4, the flowering times of RcCO-, RcCOL4-, and RcCOL5-silenced plants differed under both LD and SD conditions with the decrease of gene expression. Specifically, flowering of RcCO-silenced plants was delayed 14 d in LDs and 9 d in SDs, flowering of RcCOL4-silenced plants was delayed 4 d in LDs and 7 d in SDs, whereas flowering of RcCOL5-silenced plants was delayed 6 d in LDs and SDs. Consequently, RcCO-silenced plants flowered later in LDs (57 d) than in SDs (53 d), RcCOL4-silenced plants flowered earlier in LDs (47 d) than SDs (51 d), while RcCOL5-silenced plants flowered at the same time (50 d) under LDs and SDs. These results clearly indicated that RcCO, RcCOL4, and RcCOL5 were all flowering activators in rose plants and essential for normal flowering under SD and LD conditions. It was noteworthy that only silencing of either RcCO or RcCOL4 induced the difference in flowering time between SDs and LDs, thus disturbing the DN response of rose plants, ruling out the function of RcCOL5 in the DN response. Furthermore, the identical flowering time between RcCO/RcCOL4 double-silenced plants and RcCO-silenced single mutants implied the epistatic function of RcCOL4 and RcCO (Fig. 2A, B).
Fig. 2.
Flowering phenotype of TRV-RcCO and TRV-RcCOL4 plants under LD and SD conditions. (A) Time-course of the flowering phenotype of TRV-RcCO and TRV-RcCOL4 Rosa chinensis ‘Old Blush’ under LD and SD conditions. (B) Flowering time of TRV-silenced R. chinensis ‘Old Blush’ under LD and SD conditions. Error bars indicate ± the standard deviation (n=10). Different letters above the columns denote significant differences as determined by Kruskal–Wallis test (P<0.05).
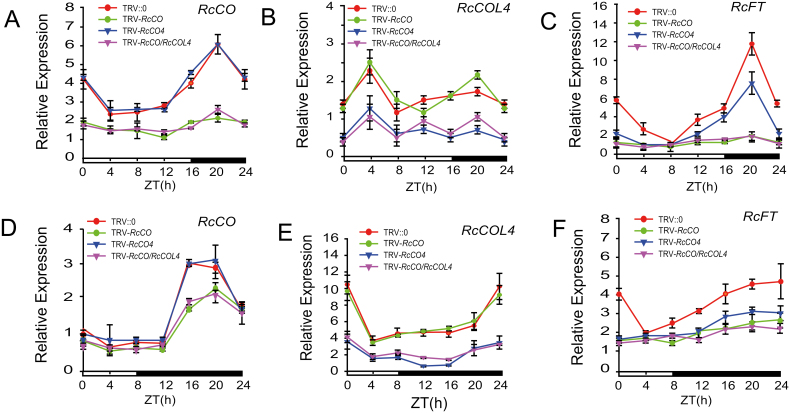
Fig. 3.
Time-course expression levels of RcCO, RcCOL4, and RcFT in TRV-RcCO and TRV-RcCOL4 plants under LD and SD conditions. (A–C) Relative expression of RcCO (A), RcCOL4 (B), and RcFT (C) in TRV-silenced Rosa chinensis ‘Old Blush’ under LDs. Error bars indicate ± the standard deviation (n=3). (D–F) Relative expression of RcCO (D), RcCOL4 (E), and RcFT (F) in TRV-silenced R. chinensis ‘Old Blush’ under SDs. Error bars indicate ± the standard deviation (n=3). The uppermost young leaves from 40-day-old plants propagated from cuttings were harvested and the total RNAs were extracted. RT-qPCR was then performed with RcGAPDH as reference gene. Every experiment was conducted with three replicates each with three technical repeats. The bar below the graphs indicates the light conditions, with day and night denoted in white and black, respectively.
As the key flowering integrator, the expression levels of RcFT were reduced significantly under both LDs and SDs in RcCO- and RcCOL4-silenced plants (Fig. 3C, F), consistent with the aforementioned flowering phenotypes (Fig. 2A, B). Specifically, the expression of RcFT in RcCO-silenced plants was higher in SDs than in LDs; in contrast, that in RcCOL4-silenced plants was lower in SDs than in LDs. This implied that an imbalance of RcFT expression could by extension compromise the DN response. These results suggested that RcCO and RcCOL4 regulate flowering time through affecting the transcription of RcFT, and that the complementary expression of RcCO in LDs and of RcCOL4 in SDs guarantees rose flowering under favorable conditions irrespective of the photoperiod, and facilitation of the DN responses of CF roses.
RcCO rather than RcCOL4 recovers the late flowering phenotype of the co mutant in Arabidopsis
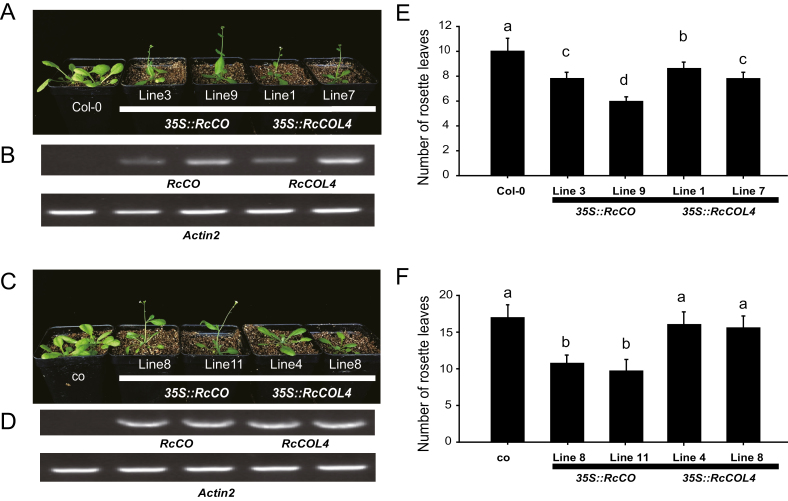
To further test the functions of RcCO and RcCOL4 in Arabidopsis, we overexpressed them in Col wild type (WT) as well as in the co mutant background. We performed semi-quantitative RT-PCR in Arabidopsis to verify the expression of RcCO and RcCOL4 (Fig. 4B, D). Based on the rosette leaf number under LD conditions, the flowering time of overexpression lines was accelerated significantly in WT backgrounds. For example, the number of rosette leaves of RcCO or RcCOL4 overexpression lines at flowering initiation was seven or eight, respectively, in contrast to 10 leaves in the WT (Fig. 4A, E). In terms of the co mutant, the flowering time was delayed significantly to 18 rosette leaves in comparison with 10 in the WT, overexpression of RcCO decreased the number of rosette leaves to nine, while no obvious phenotype was observed upon overexpression of RcCOL4 in the co mutant background (Fig. 4C, F). In summary, the complementary effect on flowering time of co mutants by RcCO demonstrated the conserved function of RcCO and AtCO in flowering regulation. Furthermore, AtCO most probably acted downstream of RcCOL4 and was essential for its function in Arabidopsis, consistent with the previous result in roses.
Fig. 4.
Flowering phenotype of RcCO- and RcCOL4-overexpressing Arabidopsis in the Col and co background. (A and E) Flowering phenotypes and rosette leaf numbers of RcCO- and RcCOL4-overexpressing plants in the Col background. (B and D) Expression of RcCO and RcCOL4 measured by semi-quantitative RT-PCR in Arabidopsis. Actin2 was used as reference gene. (C and F) Flowering phenotypes and rosette leaf numbers of RcCO- and RcCOL4-overexpressing plants in the co mutant background. Error bars indicate ± the standard deviation (n=3). Different letters above the columns denote significant differences at P<0.05.
RcCO regulates RcFT by directly binding to the CORE motif in the promoter
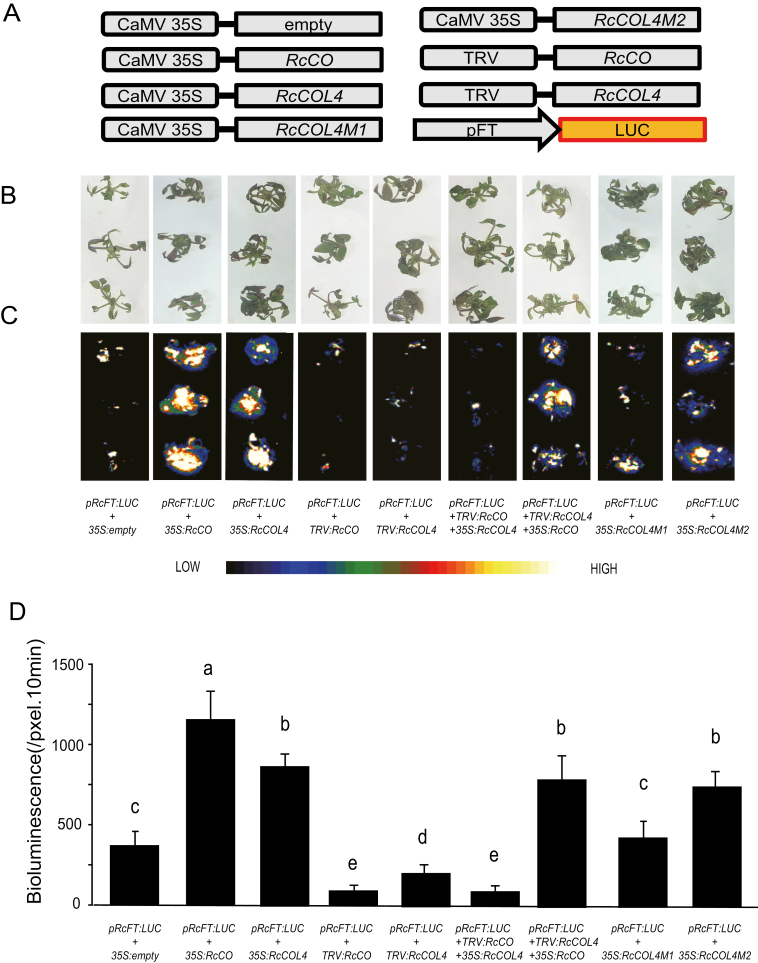
In Arabidopsis, AtCO activates AtFT transcription through direct binding to CO-responsive (CORE) elements in the FT promoter (Seung Kwan et al., 2005; Wenkel et al., 2006; Tiwari et al., 2010; Cao et al., 2014). This prompted us to test the relationship between RcCO and RcFT in roses. We performed transient A. tumefaciens infiltration assays in the young rose shoots as previously described (Lu et al., 2017). Specifically, a construct containing the FT promoter region fused to firefly luciferase (pRcFT:LUC) was infiltrated into ‘Old Blush’ young shoots together with an empty vector control, or in 35S:RcCO or 35S:RcCOL4 (Fig. 5A). The results clearly demonstrated a notable induction above the LUC bioluminescence background in rose shoots co-infiltrated with pRcFT:LUC plus 35S:RcCO or 35S:RcCOL4 (Figs. 5B–D), suggesting that RcCO and RcCOL4 might activate the expression of RcFT. Consistently, the base level of LUC bioluminescence reflecting the promoter activity of RcFT was suppressed significantly by silencing of RcCO or RcCOL4. Interestingly, the promotion effect of RcCOL4 on pRcFT:LUC was almost eliminated in RcCO-silenced seedlings, while enhanced pRcFT:LUC activity by RcCO was not affected in RcCOL4-silenced seedlings. Collectively these results suggested that RcCO and RcCOL4 activate the expression of RcFT, thus accelerating flowering time, and furthermore with a functional RcCOL4 depending on RcCO. Subsequently, the binding of RcCO to the RcFT promoter was further confirmed in yeast one-hybrid assays; however, RcCOL4 was not shown to directly bind to the promoter of RcFT in yeast cells (Supplementary Fig. S5).
Fig. 5.
Transient transformation analysis of transcriptional activation of RcFT by RcCO and RcCOL4. (A) Schematic diagram of the reporter and effectors used in the Rosa chinensis ‘Old Blush’ transient transformation assays. (B and C) Representative images of transient expression assays in R. chinensis ‘Old Blush’ displayed by bright field (B) and dark field (C) of rose shoots expressing pRcFT-LUC together with 35S:empty, 35S:RcCO, 35S:RcCOL4, TRV-CO, TRV-COL4, TRV-CO+35S:RcCOL4, TRV-COL4+ 35S:RcCO, mutated RcCOL4-M1, and RcCOL4-M2. (D) Intensities of the LUC bioluminescence presented in (C) using Andor Solis image analysis software. Data are means ± SE (n=10). Different letters above the columns denote significant differences at P<0.05.
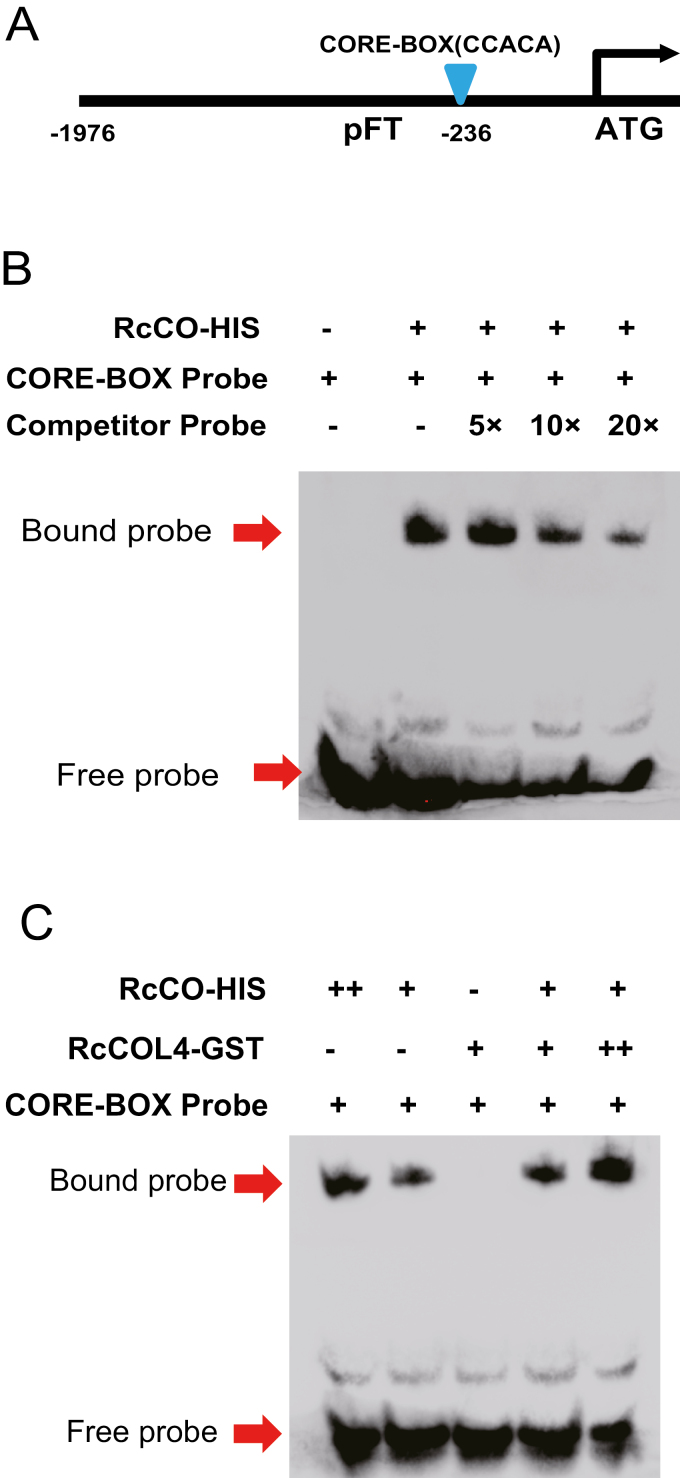
To further clarify the binding details, we screened the promoter of RcFT and found the CORE motif located at –236 bp upstream of the start codon (Fig. 6). Next we generated biotinylated probes across the motif for EMSA. The EMSA result (Fig. 6B) clearly demonstrated RcCO direct binding to the CORE motif in the RcFT promoter as determined by mobility shift, and the binding activities decreased dose-dependently by competitive probes. To further differentiate the function of RcCO and RcCOL4 using the EMSA system, RcCO and RcCOL4 proteins were incubated with the labeled probes in different combinations. The results clearly showed that only RcCO and not RcCOL4 bound to the CORE motif in the promoter of RcFT; however, the combination of RcCO with RcCOL4 certainly enhanced the binding activity (Fig. 6C).
Fig. 6.
RcCOL4 facilitates the binding of RcCO to the CORE motif in the promoter of RcFT. (A) Location of the CORE motif in the promoter of RcFT. (B) Direct binding of RcCO to the CORE motif of the RcFT promoter in in vitro EMSA. Biotin-labeled probes were incubated with RcCO-HIS protein, and the free and bound probes were separated on an acrylamide gel. 5×, 10×, and 20× represent the dilution multiples of the competitor probe. (C) RcCOL4 enhances the binding ability of RcCO to the CORE motif in the promoter of RcFT. Biotin-labeled probes were incubated with RcCO-HIS alone or together with RcCOL4–GST protein, and the free and bound probes were separated on an acrylamide gel.
Collectively, these results from the transient binding analysis, yeast one-hybrid assay, and EMSA provided solid evidences that RcCO directly bound to the promoter of RcFT via the CORE motif to activate its expression, and that binding was enhanced by RcCOL4, suggesting that RcCO is the downstream target of RcCOL4 that is indispensable for a functional RcCOL4.
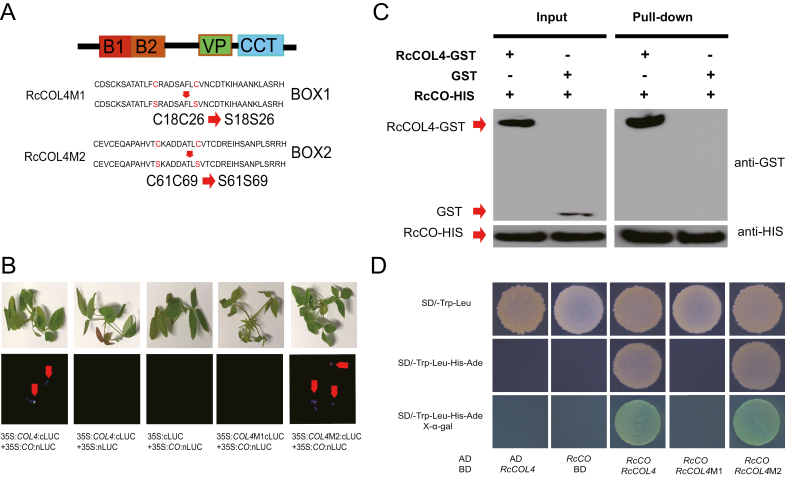
The Box1 motif of RcCOL4 is indispensable for the RcCO–RcCOL4 interaction
Given that RcCOL4 enhanced binding of RcCO to the promoter of RcFT, we questioned whether RcCOL4 could physically interact with RcCO. Thus, we conducted split luciferase complementation assays by fusing RcCOL4 and RcCO to the N- or C-terminal fragments of luciferase, respectively. Subsequently, these constructs were used in agroinfiltration-based transient assays in ‘Old Blush’ seedlings as we previously described (Lu et al., 2017). The outcome clearly revealed reconstitution of luciferase activity in the rose shoots co-infiltrated with RcCOL4–cLUC and RcCO–nLUC (Fig. 7B), thus, showing physical interaction between RcCOL4 and RcCO. Moreover, the interaction results were also verified using a pull-down assay and a Y2H assay (Fig. 7C, D).
Fig. 7.
The Box1 motif of RcCOL4 is indispensable for the RcCO–RcCOL4 protein interaction. (A) Schematic representation of the amino acid sequences and mutations of the B-box motif in RcCOL4. (B) Representative images of the spilt luciferase complementation assays in Rosa chinensis ‘Old Blush’ displayed by bright field and dark field of rose seedlings co-expressing RcCO and RcCOL4, and RcCO and mutated RcCOL4. (C) Pull-down assays prove the interaction between RcCOL4 and RcCO. The purified RcCO-HIS fusion protein was incubated with immobilized GST and RcCOL4–GST fusion proteins in pull-down buffer and the interaction was determined by western blot. (D) Yeast two-hybrid assay verifies the interaction between RcCOL4 and RcCO, and of RcCOL4-M2, but not RcCOL4-M1, and RcCO.
Next, we performed luciferase activity reconstitution assays with two independently mutated RcCOL4 constructs to better understand the role of individual B-boxes in the RcCOL4–RcCO protein interaction. One mutant construct contained Cys18 and Cys26 substituted to Ser in Box1 (named RcCOL4M1) and the other contained the analogous substitution Cys61 and Cys69 to Ser in Box2 (named RcCOL4M2) (Fig. 7A). These data clearly showed that the interaction between RcCOL4 and RcCO was eliminated in rose shoots co-infiltrated with RcCO and RcCOL4M1, whereas the protein–protein interaction remained unaffected by mutation in Box2 (Fig. 7B). These results were verified using a yeast hybrid assay (Fig. 7D).
Because of the critical role of Box1 in RcCOL4 for protein interaction, we further investigated whether the mutated RcCOL4 still possesses the ability to regulate FT transcription. Thus, we performed transient assays in rose seedlings using pFT:LUC plus empty vector control, 35S:RcCOL4, 35S:RcCOL4-M1, or 35S:RcCOL4-M2. Comparing the LUC bioluminescence in the rose shoots, the enhancement of LUC activity by 35S:RcCOL4 was almost abolished in 35S:RcCOL4-M1 but not in 35S:RcCOL4-M2 (Fig. 5B, C). This finding further defined the indispensable role of Box1 for the function of RcCOL4 in RcFT promotion and flowering time regulation in R. chinensis.
Discussion
The timing of flowering is an essential determinant for the adaptation to different environments by plants. The transition from vegetative to reproductive development is triggered by a leaf-derived, mobile floral-promoting signal named florigen (Chailakhyan, 1937; Giakountis and Coupland, 2008). The florigen-encoding FT genes are extensively identified and their functions are conserved among SD, LD, and DN plants. Conditional accumulations of FT to the threshold required for flowering are critical and common to all three types of photoperiodic response plants. Orthologs of the FT gene accelerate flowering in the LD Arabidopsis and the SD rice. The universal florigenic signal triggered by FT homologs is known to regulate growth and flowering cycles in perennial DN tomato (S. lycopersicum) (Lifschitz and Eshed, 2006). In maize (Z. mays), the florigen gene ZCN8 is associated with the floral transition in both DN temperate maize and SD-requiring tropical maize, and has been shown to be regulated by different chromatin modifications at the floral transition (Lazakis et al., 2011).
The mechanisms underlying plant photoperiodic responses can be explained by the external coincidence model. In this model, the coincidence of a photoperiodic signal perceived by photoreceptors and internal gene expression during a specific phase determines flowering (Searle and Coupland, 2014). CO is a transcription factor which acts as a time keeper. In Arabidopsis, the circadian regulation of CO transcript levels in conjunction with the light-induced stabilization of CO protein peaking at dusk is an established basis for monitoring daylength and a guarantee for the induction of FT under LDs (Suárez-López et al., 2001; Valverde et al., 2004). The CO–FT module also controls photoperiodic flowering in rice and poplar, but in the SD plant rice, Hd3a (the homolog of Arabidopsis FT in rice) is induced when the CO homolog Hd1 peaks during the night (Shoko et al., 2002; Henrik et al., 2006). The reverse response to daylength observed between Arabidopsis (LD plant) and rice (SD plant) is partly explained by the difference in the function of CO in Arabidopsis and the rice homolog Hd1 (Izawa et al., 2002; Ryosuke et al., 2003). However, the DN response is the most poorly characterized among the three types of photoperiodic flowering responses.
Although there are three different flowering modes (OF, CF, and OB) in rose plants, the CF trait is much more popular and plays an essential role in the tremendous success of modern roses. In contrast to OF, CF (also called recurrent, perpetual, everbearing, or remontant flowering) rose varieties start to flower in spring and continuously initiate new flowers until late autumn (Sønsteby and Heide, 2007; Foucher et al., 2008), and hence are generally considered as DN plants (Zieslin and Moe, 1985). In the present study, normal flower initiation in CF rose R. chinensis ‘Old Blush’ occurred under both SD and LD conditions, supporting roses as DN plants. In line with the flowering phenotype, the expression level of RcFT was higher under LDs and SDs alternately in the day and night cycle. Additionally, the CO–FT module in R. chinensis was also conserved in flowering time regulation, which was in agreement with the published literature in other species (Izawa et al., 2002; Ryosuke et al., 2003). RcCO was expressed more under LD conditions and directly bound to the CORE motif of the RcFT promoter to activate its expression (Figs 5, 6); accordingly, silencing of RcCO in R. chinensis by VIGS delayed flowering time significantly under both SDs and LDs (Fig. 2). Interestingly, RcCOL4, a close member of subgroup I of the BBX gene family, showed higher expression under SDs in the CF rose and physically interacted with RcCO to enhance its binding to the promoter of RcFT (Figs 1, 6, 7). As a result, flowering time of RcCOL4-silenced plants was later in SDs than in LDs. In contrast, RcCO-silenced plants flowered earlier in SDs than in LDs upon perturbation of the DN response, implying the important role of RcCOL4 under SDs and RcCO under LDs in rose flowering. Collectively, these data suggested that the alternate expression of RcCOL4 in SDs and of RcCO in LDs facilitates the CF trait and DN response of R. chinensis.
In Arabidopsis, control of flowering time is not limited to CO/BBX1, since other BBX family members also regulate flowering through distinct and overlapping as well as antagonistic functions (Cheng and Wang, 2005; Datta et al., 2006; Hassidim et al., 2009; Park et al., 2011; Li et al., 2014). In contrast, the function of BBX family members has never been characterized in rose plants. Here, we identified three BBX genes, RcCO, RcCOL4, and RcCOL5, as flowering accelerators in R. chinensis. We further showed that three BBXs were required for rose normal flowering under LD and SD conditions. Silencing either of RcCO, RcCOL4, or RcCOL5 delayed flowering under both LDs and SDs; however, RcCOL4-silenced plants flowered later in SDs than in LDs, in contrast to RcCO-silenced plants which flowered later in LDs than in SDs. The results established the distinct roles of RcCO and RcCOL4 in response to different photoperiods and they coordinately enabled the DN response of R. chinensis. A previous study showed that the CF phenotype of roses was mainly caused by a dysfunctional flowering repressor KSN, a TFL1 homolog of Arabidopsis, which was specifically inhibited by SDs and activated by LDs in Fragaria vesca (Koskela et al., 2012). The identification of RcCOL4 as a floral promoter preferably in SDs provided a new angle to better understand the mechanism of the CF trait, supporting the notion that the CF trait may be controlled by multiple regulators.
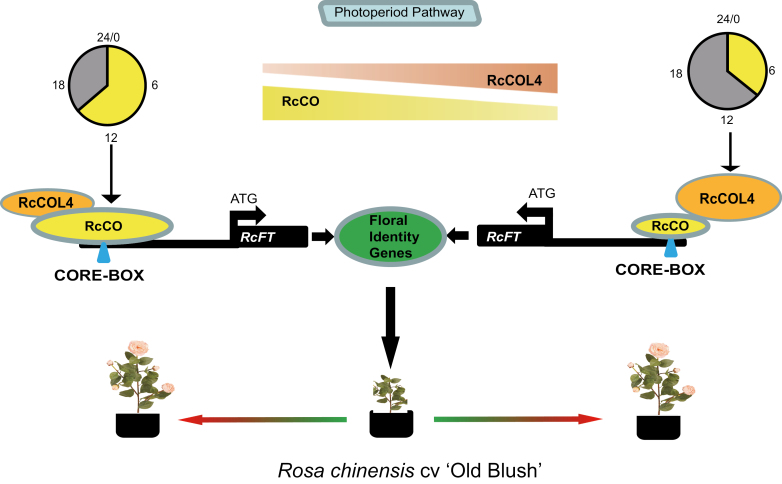
In conclusion, the present results lead to the proposal of a new model for the continuous flowering in CF cultivar R. chinensis ‘Old Blush’ (Fig. 8). Under LD conditions, RcCO was more highly expressed and played a prominent role in flowering promotion via direct binding to the promoter of RcFT to activate its expression. Under SD conditions, RcCO expression levels were reduced, but RcCOL4 enabled accelerated flowering via physically interacting with RcCO to enhance its binding to RcFT. Consequently, R. chinensis could continuously flower under both SDs and LDs irrespective of the photoperiodic conditions.
Fig. 8.
Simplified schematic model of flowering time regulation by RcCOL4–RcCO in Rose chinensis under different daylengths. Under LD conditions, RcCO is expressed more highly and plays a prominent role in flowering promotion via direct binding to the promoter of RcFT to activate its expression; under SD conditions, RcCO is down-regulated while RcCOL4 increases and accelerates flowering via physically interacting with RcCO to enhance its binding to FT. Consequently, R. chinensis ‘Old Blush’ could flower under both LDs and SDs.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Identification of RcBBX family genes in Rosa chinensis.
Fig. S2. Amino acid sequence comparison of CO, COL4, and COL5 across different species
Fig. S3. Expression level of CO, COL4, and COL5 in three OF and three CF roses under LD and SD conditions.
Fig. S4. Flowering phenotype and gene expression levels of RcCOL5-silenced plants under LDs and SDs.
Fig. S5. RcCO not RcCOL4 binds to the promoter of RcFT in yeast one-hybrid assay.
Table S1. Primers used in this study,
Acknowledgements
We thank Professor Katayoon Dehesh from UC Riverside for her critical review of the article. This work was supported the National Key Research and Development Program of China (2019YFD1000400), National Nature Science Foundation of China (31972449), and Joint Foundation of National Nature Science Foundation Committee of China and Xinjiang (U1803102) granted to CW, and the National Nature Science Foundation of China (31801890) granted to JL.
Author contributions
JL designed and performed the experiments; JS, AJ, MB, and CF executed parts of the experiments; JL performed the bioinformatic analysis; GN revised the paper; and CW wrote the manuscript.
References
- Bendahmane M, Dubois A, Raymond O, Bris ML. 2013. Genetics and genomics of flower initiation and development in roses. Journal of Experimental Botany 64, 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Kumimoto RW, Gnesutta N, Calogero AM, Mantovani R, Holt BF. 2014. A distal CCAAT/NUCLEAR FACTOR Y complex promotes chromatin looping at the FLOWERING LOCUS T promoter and regulates the timing of flowering in Arabidopsis. The Plant Cell 26, 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chailakhyan MK. 1937. Hormonal theory of plant development. Comptes Rendus (Doklady) de l’Academie des Sciences des l’URSS 13, 79–83 [Google Scholar]
- Chang MM, Li A, Feissner R, Ahmad T. 2016. RT-qPCR demonstrates light-dependent AtRBCS1A and AtRBCS3B mRNA expressions in Arabidopsis thaliana leaves. Biochemistry & Molecular Biology Education 44, 405–411. [DOI] [PubMed] [Google Scholar]
- Cheng XF, Wang ZY. 2005. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. The Plant Journal 43, 758–768. [DOI] [PubMed] [Google Scholar]
- Datta S, Hettiarachchi GH, Deng XW, Holm M. 2006. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. The Plant Cell 18, 70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugo ML, Satovic Z, Millán T, Cubero JI, Rubiales D, Cabrera A, Torres AM. 2005. Genetic mapping of QTLs controlling horticultural traits in diploid roses. Theoretical and Applied Genetics 111, 511–520. [DOI] [PubMed] [Google Scholar]
- Fornara F, de Montaigu A, Coupland G. 2010. SnapShot: control of flowering in Arabidopsis. Cell 141, 550.e1–550.e2. [DOI] [PubMed] [Google Scholar]
- Foucher F, Chevalier M, Corre C, Soufflet-Freslon V, Legeai F, Hibrand-Saint Oyant L. 2008. New resources for studying the rose flowering process. Genome 51, 827–837. [DOI] [PubMed] [Google Scholar]
- Giakountis A, Coupland G. 2008. Phloem transport of flowering signals. Current Opinion in Plant Biology 11, 687–694. [DOI] [PubMed] [Google Scholar]
- Hassidim M, Harir Y, Yakir E, Kron I, Green RM. 2009. Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis. Planta 230, 481–491. [DOI] [PubMed] [Google Scholar]
- Henrik BH, Tao H, Laurence CC, Brunner AM, Stefan J, Strauss SH, Ove N. 2006. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312, 1040–1043. [DOI] [PubMed] [Google Scholar]
- Iwata H, Gaston A, Remay A, Thouroude T, Jeauffre J, Kawamura K, Oyant LH, Araki T, Denoyes B, Foucher F. 2012. The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. The Plant Journal 69, 116–125. [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K. 2002. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes & Development 16, 2006–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Clark SE. 2005. Photoperiod regulates flower meristem development in Arabidopsis thaliana. Genetics 169, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Kronmiller B, Maszle DR, Coupland G, Holm M, Mizuno T, Wu SH. 2009. The Arabidopsis B-box zinc finger family. The Plant Cell 21, 3416–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela EA, Mouhu K, Albani MC, Kurokura T, Rantanen M, Sargent DJ, Battey NH, Coupland G, Elomaa P, Hytönen T. 2012. Mutation in TERMINAL FLOWER1 reverses the photoperiodic requirement for flowering in the wild strawberry Fragaria vesca. Plant Physiology 159, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokura T, Mimida N, Battey NH, Hytönen T. 2013. The regulation of seasonal flowering in the Rosaceae. Journal of Experimental Botany 64, 4131–4141. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J. 1957. Effect of day-length and gibberellic acid on the flowering of Arabidopsis. Nature 180, 36–37. [Google Scholar]
- Lazakis CM, Coneva V, Colasanti J. 2011. ZCN8 encodes a potential orthologue of Arabidopsis FT florigen that integrates both endogenous and photoperiod flowering signals in maize. Journal of Experimental Botany 62, 4833–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Sun J, Wang D, Bai S, Clarke AK, Holm M. 2014. The B-box family gene STO (BBX24) in Arabidopsis thaliana regulates flowering time in different pathways. PLoS One 9, e87544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz E, Eshed Y. 2006. Universal florigenic signals triggered by FT homologues regulate growth and flowering cycles in perennial day-neutral tomato. Journal of Experimental Botany 57, 3405–3414. [DOI] [PubMed] [Google Scholar]
- Liu J, Fu X, Dong Y, Lu J, Ren M, Zhou N, Wang C. 2018. MIKC(C)-type MADS-box genes in Rosa chinensis: the remarkable expansion of ABCDE model genes and their roles in floral organogenesis. Horticulture Research 5, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bai M, Ren H, Liu J, Wang C. 2017. An efficient transient expression system for gene function analysis in rose. Plant Methods 13, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Piola F, Chessel D, Jay M, Heizmann P.. 2001. The domestication process of the modern rose: genetic structure and allelic composition of the rose complex. Theoretical & Applied Genetics 102, 398–404. [Google Scholar]
- Park HY, Lee SY, Seok HY, Kim SH, Sung ZR, Moon YH. 2011. EMF1 interacts with EIP1, EIP6 or EIP9 involved in the regulation of flowering time in Arabidopsis. Plant & Cell Physiology 52, 1376–1388. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. 2009. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Molecular Biology and Evolution 26, 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randoux M, Jeauffre J, Thouroude T, Vasseur F, Hamama L, Juchaux M, Sakr S, Foucher F. 2012. Gibberellins regulate the transcription of the continuous flowering regulator, RoKSN, a rose TFL1 homologue. Journal of Experimental Botany 63, 6543–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond O, Gouzy J, Just J, et al. 2018. The Rosa genome provides new insights into the domestication of modern roses. Nature Genetics 50, 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina K, Akiko I, Shojiro T, Shuji Y, Ko S. 2008. Hd3a and RFT1 are essential for flowering in rice. Development 135, 767–774. [DOI] [PubMed] [Google Scholar]
- Ryosuke H, Shuji Y, Shojiro T, Masahiro Y, Ko S. 2003. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422, 719. [DOI] [PubMed] [Google Scholar]
- Sønsteby A, Heide OM. 2007. Long-day control of flowering in everbearing strawberries. Journal of Horticultural Science & Biotechnology 82, 875–884. [Google Scholar]
- Searle I, Coupland G. 2014. Induction of flowering by seasonal changes in photoperiod. EMBO Journal 23, 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung Kwan Y, Kyung Sook C, Joonki K, Jeong Hwan L, Sung Myun H, Seong Jeon Y, So Yeon Y, Jong Seob L, Hoon AJ. 2005. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiology 139, 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoko K, Yuji T, Yasushi K, Lisa M, Takuji S, Takashi A, Masahiro Y. 2002. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant & Cell Physiology 43, 1096–1105. [DOI] [PubMed] [Google Scholar]
- Shubin LI, Zhou N, Zhou Q, Yan H, Jian H, Wang Q, Chen M, Qiu X, Zhang H, Wang S. 2015. Inheritance of perpetual blooming in Rosa chinensis ‘Old Blush’. Horticultural Plant Journal 1, 108–112. [Google Scholar]
- Srikanth A, Schmid M. 2011. Regulation of flowering time: all roads lead to Rome. Cellular and Molecular Life Sciences 68, 2013–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. 2001. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410, 1116–1120. [DOI] [PubMed] [Google Scholar]
- Tian J, Pei H, Zhang S, Chen J, Chen W, Yang R, Meng Y, You J, Gao J, Ma N. 2014. TRV–GFP: a modified Tobacco rattle virus vector for efficient and visualizable analysis of gene function. Journal of Experimental Botany 65, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Shen Y, Chang HC, et al. 2010. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytologist 187, 57–66. [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. 2004. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303, 1003–1006. [DOI] [PubMed] [Google Scholar]
- Wenkel S, Turck F, Singer K, Gissot L, Le Gourrierec J, Samach A, Coupland G. 2006. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. The Plant Cell 18, 2971–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Li J, Gangappa SN, Hettiarachchi C, Lin F, Andersson MX, Jiang Y, Deng XW, Holm M. 2014. Convergence of light and ABA signaling on the ABI5 promoter. PLoS Genetics 10, e1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, Yu Z, Tang W, Lei W, Zhou H, Yu S, Xu C, Li X. 2009. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nature Genetics 40, 761–767. [DOI] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, et al. 2000. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. The Plant Cell 12, 2473–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieslin N, Moe R. 1985. Rosa. In: Halevy AH, ed. Handbook of flowering. Boca Raton, FL: CRC Press, 214–225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.