Abstract
Introduction
Immune function strongly influences the outcome of patients with non-small cell lung cancer (NSCLC). It's vital to understand the immune state of patients through detecting the percentage and number of lymphocyte subsets accurately, and helpful to evaluate conditions of prognosis and adjust treatment for patients.
Methods
We conducted a retrospective cohort study in First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China. The absolute counts and percentages of CD3+, CD3 + CD4+, CD3 + CD8+, B and NK cells were determined by single platform technologies. 172 patients received treatment including surgery or chemotherapy after surgery. The factors affecting disease progression were analyzed by Binary Logistic regression. Progression free survival (PFS) calculating survivals were with the method of Kaplan-Meier. The log-rank test and cox's proportional hazard regression (enter method) were used for univariable and multivariable analyses respectively.
Results
Relative to normal controls, patients with NSCLC at different stages showed decreased absolute lymphocyte count obviously, rather than lymphocyte percentages.
Different treatments had unlike influence on the homeostasis of lymphocytes and the effects last for a long time. Logistic regression showed CD3 + CD4+ and CD3 + CD8+ could contribute to favorable prognosis. Multivariate analysis of prognostic factors of PFS showed CD3 + CD4+ cell was independent factor for predicting PFS.
Conclusions
The absolute count of CD3+, CD3 + CD4+, CD3 + CD8+, B and NK cells were better indication of the patient's immune state than percentages of each lymphocyte subsets. Immune function was impaired in patients with non-small cell lung. The high level of baseline absolute CD3 + CD4+ cells count contributed to longer progression free survival.
Chinese Clinic Trial Registry number: ChiCTR-IOR-17014139; Registry date: 2017/12/25.
Abbreviations: NSCLC, Non-small cell lung cancer; PFS, Progression free survival; NCs, Normal controls; AL, Absolute numbers of lymphocyte subsets; DCs, Dendritic cells; PL, Persentages of lymphocyte subsets; CTLs, Cytotoxic T lymphocytes
Background
Lung cancer is the most commonly diagnosed cancer and the major cause of cancer-related mortality [1]. Non-small cell lung cancer (NSCLC) accounts for 75% of lung cancer with most of them diagnosed in the advanced stage, whose 5-year survival is only 15%. In 2015, it was estimated that a total of 733,300 new lung cancer cases and 610,200 lung cancer deaths were in China [2]. As the development of the theory of tumor immune surveillance proposed by Burnet, the study on tumor immunity is deepening, more and more researchers have realized that the occurrence and progression of tumor is closely associated with the body's immune function [3]. As we know, each stage of cancer development is exquisitely susceptible to regulation by immune cells. Whereas full activation of adaptive immune cells in response to the tumor might lead to eradication of malignant cells, actually tumor growth is inhibited by anti-tumor cytotoxic-T-cell activity and cytokine-mediated lysis of tumor cells [4]. The potential effect of a patient's immune system on clinical outcome is not only of academic interest but also has important implications for the identification of prognostic markers that predict responses to chemotherapy and radiotherapy [5]. So, the accurate assessment of the percent and absolute count of lymphocyte subsets in the peripheral circulation of cancer patients is vital, which might provide reference to redistribution of those lymphocyte subsets that mediate anti-tumor defense [6].
Absolute numbers of lymphocyte subsets (AL) in the peripheral circulation have been detected by dual-platform technologies in traditional way which couple percentages of positive cell subsets determined by flow cytometry with the absolute lymphocyte count obtained by automated hematology analyzers [6]. It is indicated that this conventional, universal technology is responsible for substantial differences in absolute lymphocyte counts reported by different laboratories [[7], [8]]. The more advanced method of single platform technologies, which are performed completely on the flow cytometer, has effectively increased the assay precision and allowed for greater uniformity of results between laboratories [[7], [8], [9]]. Here we designed to investigate both the percentages and absolute numbers of CD3+, CD3 + CD4+, CD3 + CD8+, B and NK cells in peripheral blood of patients with NSCLC using a single-platform flow cytometry-based method, and to analyze the relationship between lymphocyte subgroups and immune function of patients with NSCLC. Some researches have been proved that lymphocyte count could affect the disease-free survival in NSCLC, rectal cancer, ovarian cancer, extranodal natural killer/T-cell lymphoma, and so on [[10], [11], [12], [13]]. All of those only indicated that lymphocyte amounts was the impact factor and didn't point out which subsets. In our research, we find CD3 + CD4+ is the important factor which contributed to the disease progression. Some researches also stated that tumor-infiltrating cytotoxic T lymphocyte CD3 + CD8+ cells were essential to predict outcome in NSCLC [14], but it's difficult and inconvenient to get tumor-infiltrating lymphocytes, and only a few people could use this method in clinic. Here we try to test peripheral blood lymphocyte subgroups to evaluate the immunologic status of patients. In fact, not all patients respond to existing treatments, significant clinical responses, including durable long-term responses after treatment [15]. Clinical risk factors serve as the foundation of treatment choices and prognostic outcome so far. In our study the factors of immune function including the type of therapy and length of the post therapy period were considered, and the results suggested that regardless of these factors, lymphopenia was a persistent feature of the disease, which affected the progression of NSCLC.
Materials and methods
Clinical data
All the subjects were given the informed consent in accordance with the Declaration of Helsinki and the clinical trial was approved by the hospital ethics committee (TYLL2017[K]002) and registered at Chinese Clinic Trial Registry (ChiCTR-IOR-17014139). A total of 172 NSCLC patients and 55 age-matched normal healthy donors [normal controls (NCs)] were enrolled in First teaching hospital of Tianjin University of Traditional Chinese Medicine. Subjects who served as NCs were interrogated for the general state of health, use of medications, smoking, and alcohol consumption. The cohort of 172 patients included 130 men and 42 women with a median age of 64 years (range, 31–84 years), and the group of 55 volunteers comprised 40 males and 15 females with a median age of 63 years (range, 30-83 years). No significant differences in gender and age of the two groups (P > 0.05).
Inclusion criteria
NSCLC
(1) Pathologically and immunohistochemically ascertained diagnosis of NSCLC, according to the WHO classification [16]; (2) no evidence of attendant malignancies; (3) the patients in the study should have received all phases of adjuvant treatment suggested, including chemotherapy and surgical therapy; (4) all of them should have complete clinical and laboratory data during the follow-up. (5) No serious heart, liver, kidney, blood vessel, hemopoietic system and immune system disease and so on.
NCs
Physical, blood routine examination, liver functions (AST and ALT), renal functions (SCr), blood glucose levels were determined normal.
Exclusion criteria
NSCLC
(1) The diagnosis of malignant tumor was unclear; (2) patients who received adjuvant treatment had clinical evidence of acute infection, presence of haematological disorders, chronic inflammatory or autoimmune diseases, severe renal disease or any other malignancies. (3) Do not accord with medical advice.
NCs
Except those whose testing positive to HIV, systemic infection, connective tissue disease, abnormal tumor marker or cancer.
The main reagents and instruments
The detection of lymphocyte subsets were performed by using a lyse/no-wash procedure based on a single-platform technique by ten-color flow cytometry (BD FACS Canto II: U6573380-00541). The main reagents were BD Multitest IMK kit (340503) containing (BD Multitest CD3 FITC/CD8PE/CD45PerCP/CD4APC and BD Multitest CD3 FITC/CD16 + CD56PE/CD45PerCP/CD19APC), BD Multitest IMK kit lysing solution (340503); The EDTA blood collecting tubes and trucount tubes (340334) were also from BD Biosciences.
Sample collection
Two millilitres of fresh peripheral blood were obtained from NCs and the patients with NSCLC who had just been admitted to hospital, and were stored in the EDTA blood collecting tubes.
Cellular staining and analyzing
Total 227 cases collected between July 2016 and September 2019 and performed by flow cytometry. The manipulation was referred to BD operating instruction.
-
(1)
For each sample, two trucount tubes labeled with the simple identification number such as letters A and B to distinguish from each other.
-
(2)
20 μL of BD Multitest CD3/CD4/CD8/CD45 reagent was pipetted into the bottom of each tube marked A.
-
(3)
20 μL of BD Multitest CD3/CD16 + CD56/CD45/CD19 reagent were pipetted into the bottom of each tube marked B.
-
(4)
50 μL of well-mixed and anticoagulated whole blood was pipetted into the bottom of every tube using the method of reverse pipetting.
-
(5)
Then vortexed gently to mix, incubate for 15 min in dark at room temperature.
-
(6)
Finally, 450 μL of 1× BD Multitest IMK kit lysing solution was pipetted into every tube (Dilute the 10× concentrate 1:10 with room temperature deionized water). The solution was vortexed gently to mix and incubated for 15 min in dark at room temperature.
-
(7)
Samples were analyzed on the flow cytometer.
Therapeutic schedule
Patients at stage I: anatomic resection lobectomy and mediastinal lymph node dissection, stereotactic body radiation [[17], [18], [19]]; Patients at stage II: anatomic pulmonary resection and hilar and mediastinal lymph node dissection, postoperative chemotherapy (platinum+paclitaxel, platinum+pemetrexed, platinum+gemcitabine), stereotactic body radiation [[17], [18], [19]];
Patients at stage III: a lobectomy or pneumonectomy and postoperative platinum-based chemotherapy (platinum+paclitaxel, platinum+pemetrexed, platinum+ gemcitabine) and radiation [[20], [21], [22]];
Patients at stage IV: platinum-based chemotherapy or some others treatment [[20], [21], [22]].
Statistical analysis
Using the t-test to analyze the differences in percentages and absolute counts of lymphocyte subsets between patients and NCs. One Way Anova (Bonferroni test or Dunnett's test) was applied for analysis among three-group differences. For repeated measure data, analysis of covariance was used by Bonferroni or Tamhane's test. PFS was defined as freedom from any events as follows in the follow-up: newly diagnosed local or regional recurrence; distant organ metastasis; second primary malignancy. Factors affecting disease progression were analyzed by Binary Logistic regression. Calculating survivals were with the method of Kaplan-Meier. The Log-Rank test and proportional hazard regression model (enter method) were used for univariable and multivariable analyses, respectively. Variables with a P value of <0.1 by a univariable analysis were entered for a multivariable analysis. Two-sided P values <0.05 were considered statistically significant. Datas were analyzed by SPSS 24.0 and graphing were used by Graph Pad Prism version 8.00 software.
Result
Analysis of lymphocyte subsets between patients and NCs
The principle of AL detected by the single-platform was that the known total number of fluorescent microbeads were used as the standard internal parameters and fluorescent labeled antibodies added into the trucount tubes, then applied acquisition and analysis software in the flow cytometry to calculate data.
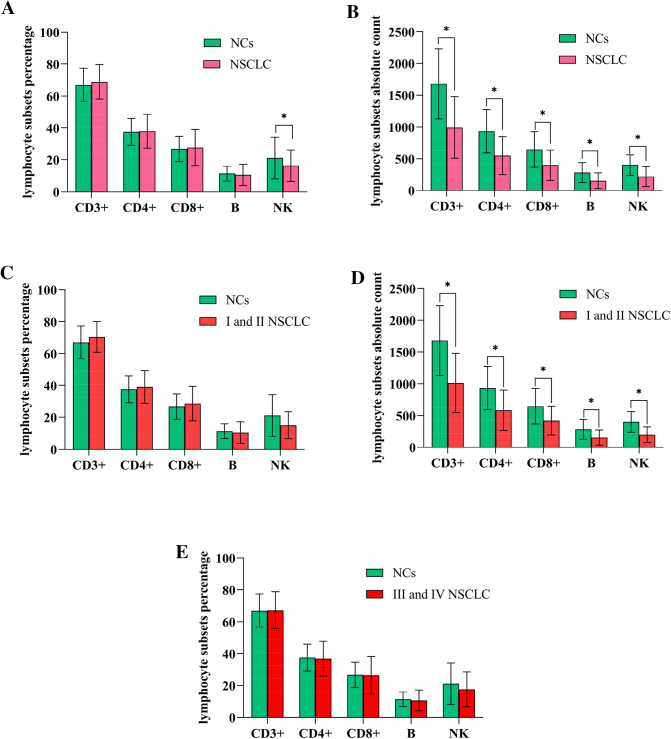
Using the method, we initially compared both the percentages (PL) and AL of CD3+, CD3 + CD4+, CD3 + CD8+, B and NK cells in all patients and NCs (normal controls). Contrasted with normal controls, no difference in percentages of each subsets (P>0.05, Fig. 1A) except NK cells but the absolute count of CD3+, CD3 + CD4+,CD3 + CD8+, B and NK cells were significantly decreased in patients (P<0.05, Fig. 1B). It suggested that it's AL not PL decreased in NSCLC patients. For example, relative to NCs, patient's PL of CD3+, CD3 + CD4+, CD3 + CD8+, B and NK cells recorded as 72%, 45%, 26%, 10% and 11% respectively, they were all normal; but AL were 437cells/μl, 266 cells/μl, 156 cells/μl, 62 cells/μl and 68 cells/μl respectively, they were all lower. As we know, PL represents the proportion or composition of each subgroups, indicating the development and differentiation of lymphocyte, while AL shows the precise amount of lymphocyte subsets in peripheral blood, suggesting proliferation of progenitor lymphocyte. The results revealed that propagation capacity of lymphocyte was impaired obviously.
Fig. 1.
Analysis of percentages and absolute count of lymphocyte subsets between NSCLC patients and NCs. A. showed that the percentages of each subsets had no significant differences between two group (P > 0.05) except NK (P < 0.05); B. revealed that NSCLC had significantly decreased absolute counts of CD3+, CD3 + CD4+, CD3 + CD8+, B and NK cells (P < 0.05). C and D. showed NSCLC patients at different stages of I and II stage had lower absolute counts of lymphocyte subsets (P < 0.01) relative to NCs, and E and F showed significantly more lower in patients with stage III~IV compared with control group (P < 0.01), no significant differences were observed in the percentages of them.(*represents significant differences).
Moreover, relative to NCs, patients with NSCLC at I~II stage had lower absolute count of lymphocyte subsets (P < 0.01, Fig. 1D), and this difference was significantly lower in patients at stage III~IV(P < 0.01, Fig. 1F), no significant differences were observed in the percentages. In brief, the patients having significantly lower absolute lymphocyte counts but not percents than NCs (Fig. 1C, E) was correlated to clinical stage.
The lymphocyte subsets in patients with different clinic stages
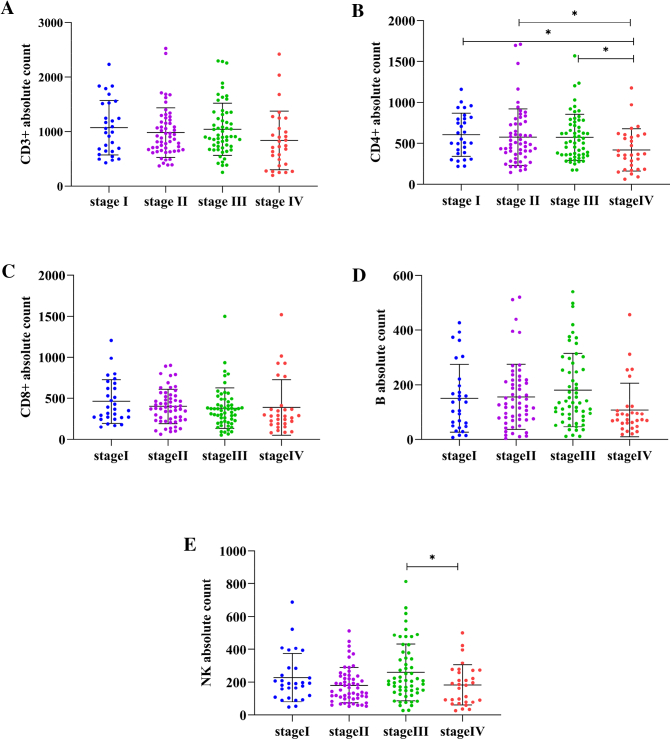
Compared with CD3+, CD3 + CD4+, CD3 + CD8+, B and NK cells absolute counts in patients at different clinical stage, we discovered that only CD3 + CD4+ and NK cells were associated with clinical stages (Fig. 2). Patients who were in phase I, II, III had consistent CD4+ absolute count and occurred sharp drop in IV (P < 0.05).
Fig. 2.
The comparison of absolute lymphocyte subsets counts in patients with different clinical stages. A, B, C, D, E showed the absolute count of CD3+,CD3 + CD4+, CD3 + CD8+,B,NK cells in patients at different stages respectively. Patients who were in phase I, II, III had consistent CD4+ absolute count and occurred sharp drop in IV (P < 0.05) (Fig. 2B). Absolute count of NK cells of patients presented pattern of wave drop with decrease in stage II and IV (P < 0.05) (Fig. 2E). CD3+, CD3 + CD8+, B cells counts didn't show the pattern changing with clinic stages (Fig. 2A, C, D). (*represents significant differences).
(Fig. 2B). NK cells absolute count of patients presented pattern of wave drop with decrease in stage II and IV (P < 0.05) (Fig. 2E). CD3+, CD3 + CD8+, B cells count didn't show the pattern changes with clinical stages (Fig. 2A, C, D). Additionally, numbers of CD4+ showed significant correlations with clinical stages (correlation coefficient, Rs = −0.174, P = 0.023), which indicated that it declined upon the deteriorating of NSCLC.
Effect of surgery and chemotherapy on AL
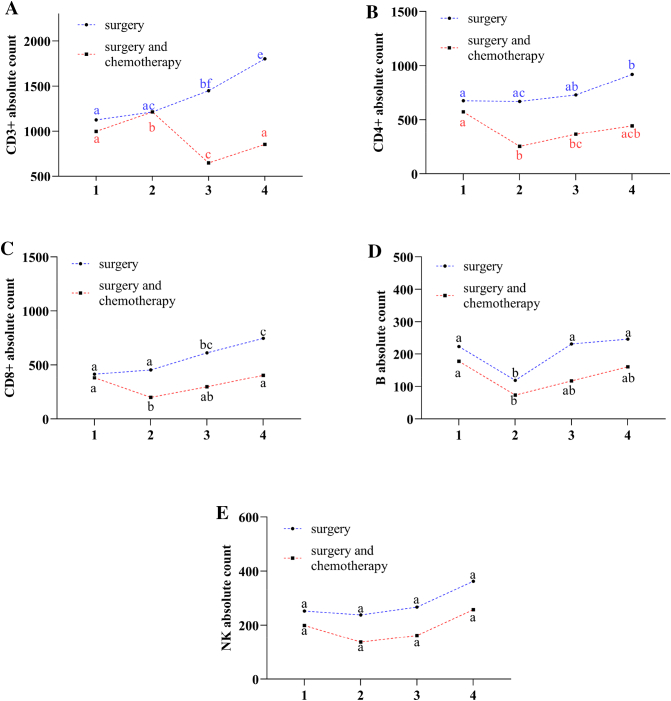
In order to study the effect of treatment on AL, we analyzed the AL levels across the whole period of diagnosis, treatments, and follow-up among 40 NSCLC patients at stage II. Those patients were divided in two groups, one received surgery (anatomic pulmonary resection and hilar and mediastinal lymph node dissection), and the other was added 2 cycles of chemotherapy (squamous: platinum+gemcitabine; nonsquamous Platinum+Pemetrexed) after surgery [[17], [18], [19]]. We could see that AL of CD3+, CD3 + CD4, CD3 + CD8+ cells in NSCLC patients exposed to surgery showed a growing trend over time, while chemotherapy after surgery revealed decline at first follow-up period, then slowly increased in the next following time, but couldn't back to the level of baseline finally (P < 0.05) (Fig. 3A, B, C). B cells showed reduced at first period in both two groups,then increased in the following time, but no significant differences relative to the first period (Fig. 3D). NK+ cells rised slowly in both group (Fig. 3E). The results suggested that it was chemotherapy but not surgery could impair the AL of lymphocyte, and both of them could harm AL of B cells.
Fig. 3.
Effect of surgery and chemotherapy on AL. Digit 1,2,3,4 on x-axis represent observe point-in-time (1 is just admitted to hospital, 2 is 1 year follow-up, 3 is 1.5 year follow-up and 4 is 2 years follow-up). We used the repeated-measures ANOVA to analyze the result. We used the alphabetic notation (a, b, c, d, e, f) to mark the significant differences in figure legend. In the same treatment group, if the any two dots with the same marking letter means there is no significant difference between two time points, any two dots without the same marking letter means there is significant difference. A. AL of CD3+ cells in patients who received surgery showed a gradual growing trend in whole time (P < 0.05), but a general trend of decrease when it was exposed to chemotherapy (P < 0.05) and lower than surgery. B. Patients of surgery, CD3 + CD4+ cells showed gradual increase in the whole follow-up period, and reach the peak at forth observe point (P < 0.05), patients of chemotherapy, CD3 + CD4+ cells declined to the minimum at second point (P < 0.05), then slowly increased (P > 0.05), but couldn't recover to the level of baseline finally. C and E. CD3 + CD8+ and NK cells showed similar trends to B for surgery and chemotherapy respectively. D. B cells showed reduced trend to the minimum at second point in both two group (P < 0.05), then increased gradually, and no significant differences at the forth point compared to the first point (P < 0.05), patients of chemotherapy kept consistent lower than that of surgery.
Effect of AL on the progression of disease
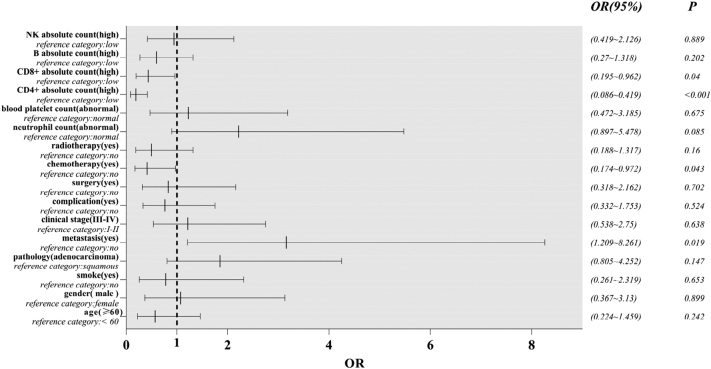
In order to ascertain whether the AL could affect the progression of disease (freedom from any events as follows: newly diagnosed local or regional recurrence; distant organ metastasis; second primary malignancy), we performed further study. Logistic regression analysis was used to analyze the prognostic factors of NSCLC. From the forest plots of subgroup analysis for progression-free, we could see CD3 + CD4+ cells (95% confidence interval 0.086–0.419, P<0.001), CD3 + CD8+ cells (95% confidence interval 0.195–0.962, P = 0.04), metastasis (95% confidence interval 1.209–8.261, P = 0.019) could affect the progression of disease (Fig. 4). High AL of CD3 + CD4+ and CD3 + CD8+ cells helpfully contributed to favorable prognosis, which showed in the picture was on the left side of the line of OR = 1. Opposite to CD3 + CD4+ and CD3 + CD8+ cells, metastasis led to unfavorable prognosis.
Fig. 4.
The forest plots of factors affected the progression of disease. OR> 1 indicates variable is considered a risk factor; OR< 1 represents variables is a protective factor.
AL affecting progression-free survival
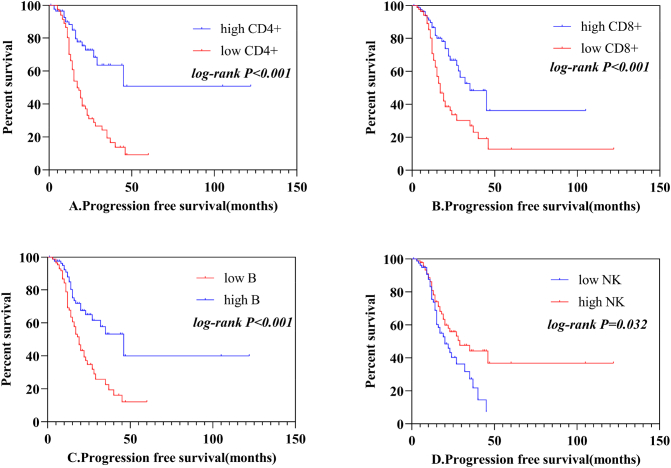
Furthermore, we used Kaplan-Meier survival and multivariate Cox's proportional hazards model to study whether AL of lymphocyte subsets could affect the progression free survival (PFS). The median AL of CD3 + CD4+ cells were 502 cells/μL,CD3 + CD8 + cells were 355 cells/μL, B cells were 117cells/μL, NK cells were187cells/μL. Patients were divided into the high AL of CD3 + CD4+ cohort (>502/μL) and low AL cohort (CD4 + ≤502cells/μL). Table 1 showed the patients' clinicopathological characteristics presented by CD3 + CD4+ cells categories. 172 patients include 130 males and 42 females who met the inclusion criteria were enrolled finally in our study. The median age of these patients was 65 years old, ranging from 31 to 84 years. Among the 172 patients, 102 (59.3%) were adenocarcinoma, 70 (40.7%) were squamous cell carcinmas. Patients with stage I ~ II and III~IV were 85 (49.4%), 87 (50.6%) respectively. 27.9% of patients had lymph node metastasis, 38.3% had complications such as diabetes, hypertension. The clinical characteristics were well balanced between the low CD3 + CD4+ cells cohort and high CD3 + CD4+ cells cohort. Table 2 showed the results of univariate analysis and multivariate analysis of prognostic factors of PFS. Univariate analysis suggested that 7 clinicopathological factors contributed to be important predictors of PFS, including clinical stage (I~II vs III~IV, P = 0.025), metastasis (yes vs no, P < 0.001), surgery (yes vs no, P = 0.004), radiotherapy (yes vs no, P = 0.004), CD3 + CD4+ cells (high vs low, P < 0.001) (Fig. 5A), CD3 + CD8 + (high vs low, P < 0.001) (Fig. 5B), B cells (high vs low, P < 0.001) (Fig. 5C), NK cells (high vs low, P = 0.032) (Fig. 5D). Multivariate analysis revealed that metastasis (Hazard ratio = 2.176, 95% confidence interval 1.313–3.608, P = 0.003) was the unfavorable prognostic factor, CD3 + CD4+ cells (Hazard ratio = 0.545, 95% confidence interval 0.300–0.991, P = 0.047) was the favorable prognostic factor. Kaplan–Meier survival curve of PFS and the cutoff value were shown in Fig. 5. The PFS of patients with CD3 + CD4+ absolute count >502 cells/μL were longer than CD4+ absolute count ≤502cells/μL. With the increment of time, metastasis and low CD3 + CD4+ absolute count increase the risk of PFS.
Table 1.
Patients' characteristics of the two cohorts.
| Characteristics | Overall, n (%) | CD4+ absolute count |
P-value | |
|---|---|---|---|---|
| Low (≤502 cells/μL) | High (>502cells/μL) | |||
| Age | 0.372 | |||
| >60 | 130 (75.5%) | 66 | 64 | |
| ≤60 | 42 (24.5%) | 18 | 24 | |
| Gender | 0.524 | |||
| Male | 121 (70.4%) | 61 | 60 | |
| Female | 51(29.6%) | 23 | 28 | |
| Family history | ||||
| Yes | 44 (25.6%) | 22 | 22 | 0.858 |
| No | 128 (74.4%) | 62 | 66 | |
| Smoke | ||||
| Yes | 106 (61.6%) | 53 | 53 | 0.699 |
| No | 66 (38.4%) | 31 | 35 | |
| Pathology | 0.713 | |||
| Adenocarcinomas | 102 (59.3%) | 51 | 51 | |
| Squamous carcinoma | 70 (40.7%) | 33 | 37 | |
| Clinical stage | 0.645 | |||
| I~II | 85 (49.4%) | 40 | 45 | |
| III~IV | 87 (50.6%) | 44 | 43 | |
| Metastasis | 0.384 | |||
| Yes | 48 (27.9%) | 26 | 22 | |
| No | 124 (72.1%) | 58 | 66 | |
| Complication | 0.051 | |||
| Yes | 66(38.3%) | 26 | 40 | |
| No | 166 (61.6%) | 58 | 48 | |
| Surgery | 0.226 | |||
| Yes | 48(27.9%) | 21 | 27 | |
| No | 124 (72.1%) | 67 | 57 | |
| Chemotherapy | 0.381 | |||
| Yes | 109 (63.4%) | 56 | 53 | |
| No | 63 (36.6%) | 28 | 35 | |
| Radiotherapy | 0.172 | |||
| Yes | 51 (29.7) | 29 | 22 | |
| No | 121 (70.3) | 55 | 66 | |
Table 2.
Univariate and multivariate analysis for PFS.
| Characteristics | Univariate |
Multivariate |
||
|---|---|---|---|---|
| P-value | HR | HR and 95% CI | P-value | |
| Age (>60 vs≤60) | 0.226 | 0.943 | ||
| Gender (male vs female) | 0.179 | 2.029 | ||
| Smoke (yes vs no) | 0.437 | 0.616 | ||
| Pathology (squamous carcinoma vs adenocarcinomas) | 0.749 | 0.997 | ||
| Clinical stage (I ~ II vs III ~ IV) | 0.025 | 0.807 | 0.667 (0.405–1.099) | 0.112 |
| Metastasis (yes vs no) | <0.001 | 2.425 | 2.176 (1.313–3.608) | 0.003 |
| Complication (yes vs no) | 0.273 | 1.056 | ||
| Surgery (yes vs no) | 0.004 | 1.330 | 1.470 (0.778–2.776) | 0.236 |
| Chemotherapy (yes vs no) | 0.182 | 0.550 | ||
| Radiotherapy (yes vs no) | 0.004 | 0.614 | 0.714 (0.417–1.220) | 0.217 |
| Neutrophil-count (normal vs abnormal) | 0.079 | 1.166 | 1.202(0.749–1.931) | 0.446 |
| Blood platelet count (normal vs abnormal) | 0.116 | 1.048 | ||
| CD4+ absolute count (high vs low) | <0.001 | 0.494 | 0.545 (0.300–0.991) | 0.047 |
| CD8+ absolute count (high vs low) | <0.001 | 0.624 | 0.663(0.378–1.162) | 0.151 |
| B absolute count (high vs low) | <0.001 | 0.706 | 0.642(0.372–1.107) | 0.111 |
| NK absolute count (high vs low) | 0.032 | 0.981 | 0.869(0.532–1.419) | 0.574 |
Fig. 5.
Progression free survival curves of 172 NSCLC patients. A, B, C, D represents the effects of CD3 + CD4+, CD3 + CD8+, B and NK cells absolute count on progression free survival respectively.
Discussion
It is feasible to apply the single-platform flow cytometry to analyze independently the percentages and absolute numbers of lymphocyte subsets in the patients' peripheral blood. At present, the clinical significance of detection of percentages and absolute numbers of lymphocyte subsets are different. If assay of the percentages of lymphocyte subsets was performed clinically only, some problems may be neglected. Just as the data we showed, comparing NCs to patients, absolute numbers of CD3+, CD3 + CD4+, CD3 + CD8+, B and NK cells decreased in the patients obviously, but the percentages of them were normal. So, in this study, we stressed the necessity of detecting absolute counts of lymphocyte subsets in patients, not percentages. The common report point up cell percentages are misleading because they do not consider total white blood cell count, which might constantly change in these patients, especially who are receiving anticancer therapies [6]. Therefore, we should detect the absolute numbers of lymphocyte subsets in clinic, it will help us to know the changes in the patient's immunologic function, analyze clinical condition and predict curative effect of patients for clinicians [23].
NSCLC patients have the lower absolute count of lymphocyte subsets, it states that patients' immune function are impaired. Patients' impaired immune system can't indeed prevent tumor progression [24]. During the clinical therapy, we should pay attention to patient's immune state, and improving the immune function may enhance their own anti-tumor ability. Some researchers had proved that using Astragalus polysaccharides (PG2), an ingredient from dried roots of astragalus membranaceus, could actively improve the immune function and quality of life of patients, as well as synergistically enhance the anticancer effect of conventional chemotherapeutic agent. The mechanism of it could promote the functional maturation of dendritic cells (DCs) with consequent enhancement of T cell-mediated anticancer immune responses [25].
Interestingly, we found that NSCLC patients at stage of I, II, III had higher CD3 + CD4+ cells absolute count than IV, it varied with clinical stage and declined upon the development of lung cancer. The multivariate analysis revealed that CD3 + CD4+ cells absolute count was the favorable prognostic factor of PFS rather than CD3 + CD8+ cells. Higher CD3 + CD4+ cells absolute count led to longer PFS. Therefore, we had to attach more importance to CD3 + CD4+ cells in the tumor immunity. Contribution of CD3 + CD4+ cells had been demonstrated in the laboratory, but now, we find CD3 + CD4+ cells plays an important role in the clinic [26]. CD3 + CD4+ cells promote trigger and boost the effector functions and the memory functions of CD3 + CD8+ cytotoxic T lymphocytes (CTLs) and help CTLs to overcome negative regulation, which provides an opportunity to amplify the T cell response against tumor-associated antigens without deleterious autoimmunity [27]. CD3 + CD4+ T cells can target tumor cells in various ways, either directly by eliminating tumor cells through cytolytic mechanisms or indirectly by modulating the tumor microenvironment [[26], [27], [28]]. Furthermore, in secondary lymphoid organs, CD3 + CD4+ cells increase the magnitude and quality of B cell and CTLs responses [[29], [30]]. Helping for the CTL response [[29], [30], [31], [32]] is delivered in a newly recognized second T cell-priming step [[32], [33]], in which CD3 + CD4+ cells and CD3 + CD8+ cells both recognize their respective antigens on the same DCs. Antigen-specific contacting with the CD3 + CD4+ cells enables the DCs to optimize antigen presentation and to deliver specific cytokine and costimulatory signals to the CD3 + CD8+ cells that promote its clonal expansion and its differentiation into an effector or memory T cells [34]. CD3 + CD4+ cells help to initiate a gene expression program in CD3 + CD8+ cells that enhances CTL function by multiple molecular mechanisms, enabling CTLs to overcome the obstacles that typically hamper antitumour immunity [35].
Our study also showed clinical treatment could affect CD3+, CD3 + CD4, CD3 + CD8+ cells absolute count. Surgery group showed a growing trend of lymphocyte subsets during the following time; chemotherapy after surgery group showed decrease in CD3 + CD4, CD3 + CD8+ cells in follow-up period, then increase in the rest of time, but they didn't reach the baseline level finally. The data suggested that effects of the chemotherapy on the homeostasis of lymphocytes was long-term. It has been recently demonstrated that immune monitoring of peripheral lymphocyte cells might lead to the identification of biomarkers, which could serve to predict prognosis and therapy response [36]. CD3 + CD4+ absolute number also affects the treatment effect and prognosis of disease. Predicting long-term PFS of cancer patients immediately after surgical resection of clinically localized and advanced disease is of great value for patient counseling, identifying poor risk-group who might benefit from enrollment in adjuvant therapy protocols [37]. Prognosis of cancer patients is based not only on tumor-related factors, but also on host-related factors, including systemic immune cell activation [38]. All in all, we should pay more attention to the AL changes of lymphocyte subsets in clinic, it may give us important references on treatment.
Conclusion
The clinical significance of detection of percentages and absolute numbers of lymphocyte subsets are different, we stressed the necessity of detecting absolute counts of lymphocyte subsets in patients. Compared to NCs, patients with NSCLC at different stages showed decreased absolute lymphocyte count obviously, it states that patients' immune function are impaired. Our study also showed clinical treatment could affect CD3+, CD3 + CD4+,CD3 + CD8+ cells absolute count. CD3 + CD4+ absolute number also affects the treatment effect and prognosis of disease. Pay a attention to the immune state of patients through detection of the level of lymphocyte subsets including percentage and number timely and accurately, it will help us to evaluate conditions of prognosis and adjust the treatment program for patients.
Ethics approval and consent to participate
The clinical trial was approved by the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine ethics committee: (TYLL2017[K]002) and registered at Chinese Clinic Trial Registry (ChiCTR-IOR-17014139).
Consent for publication
Not applicable.
Availability of data and materials
All data generated and analyzed during this study are available from the corresponding author in response to reasonable requests.
Funding
The work was supported by Specialized Research Fund for the Doctoral Program of Higher Education (No.20121210110010), Traditional Chinese Medicine and Western Medicine Research Project of Tianjin Health and Family Planning Commission (No. 2017003) and the Scientific Research Plan Project of Tianjin Education Commission (No. 2018KJ034).
CRediT authorship contribution statement
Jianchun Yu: Conceptualization, Methodology. Ying Xia, Wentao Li, Yongmin Li: Writing - Original Draft, Formal analysis, Writing - Review & Editing. Yunhe Liu, Songshan Ye, Aqing Liu: Data Curation, Validation. Yingjie Jia, Xu Liu, Huayu Chen, Yongtie Guo: Resources. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no potential conflicts of interest.
Acknowledgement
The work was supported by Specialized Research Fund for the Doctoral Program of Higher Education (No. 20121210110010) and Scientific Research Plan Project of Tianjin Education Commission (No.2018KJ034).
References
- 1.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018:GLOBOC-OCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W. Cancer statistics in China, 2015.CA Cancer J. Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Burnet F.M. Immunological aspects of malignant disease. Lancet. 1967;1(7501):1171–1174. doi: 10.1016/s0140-6736(67)92837-1. [DOI] [PubMed] [Google Scholar]
- 4.de Visser Karin E., Eichten Alexandra, Coussens Lisa M. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 5.Fridman W.H1., Pagès F., Sautès-Fridman C. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 6.Iris Kuss I., Hathaway B., Ferris R.L. Decreased absolute counts of T lymphocy-te subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10(11):3755–3762. doi: 10.1158/1078-0432.CCR-04-0054. [DOI] [PubMed] [Google Scholar]
- 7.Brando B., Barnett D., Janossy G. Cytofluorometric methods for assessing absolute numbers of cell subsets in blood. European Working Group on clinical cell analysis. Cytometry. 2000;42:327–346. doi: 10.1002/1097-0320(20001215)42:6<327::aid-cyto1000>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.O'Gorman M.R., Nicholson J.K. Adoption of single-platform technologies for enumeration of absolute T-lymphocyte subsets in peripheral blood. Clin Diag Lab Immunol. 2000;7:333–335. doi: 10.1128/cdli.7.3.333-335.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reimann K.A., O'Gorman M.G., Spritzler J. Multisite comparison of CD4 and CD8 T-lymphocyte counting by single-versus multiple-platform methodologies: evaluation of Beckman Coulter Flow Count fluorospheres and the tetraONE system. Clin Diag Lab Immunol. 2000;7:344–351. doi: 10.1128/cdli.7.3.344-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seung Yeop Oh., Heo Jaesung, Noh O. Kyu. Absolute lymphocyte count in preoperative chemoradiotherapy for rectal cancer: changes over time and progn-ostic significance. Technol Cancer Res Treat. 2018;17:1–18. doi: 10.1177/1533033818780065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Na, Zhang Li, Song Hao-lan, Zhang Jing. et al.Prognostic impact of absolute lymphocyte count/absolute monocyte count ratio and prognostic score in patients with nasal-type, extranodal natural killer/T-cell lymphoma. Tumour Biol. 2017;39(5):1–11. doi: 10.1177/1010428317705503. [DOI] [PubMed] [Google Scholar]
- 12.Katy Milne, Cheryl Alexander, John R Webb.Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumorinfiltrating lymphocytes.J Transl Med. 2012;10:33. doi: 10.1186/1479-5876-10-33. [DOI] [PMC free article] [PubMed]
- 13.Zhang Jian, Huang Shao-Hong, Li Hui. Preoperative lymphocyte count is a favorable prognostic factor of disease-free survival in non-small-cell lung cancer. Med Oncol. 2013;30(1):352. doi: 10.1007/s12032-012-0352-3. [DOI] [PubMed] [Google Scholar]
- 14.Törmänen-Näpänkangas U., Soini Y. High number of tumor-infiltrating lymphocytes is associated with apoptosis in non-small cell lung carcinoma. APMIS. 2001;109(7–8):525–532. doi: 10.1111/j.1600-0463.2001.907806.x. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita Tomonari, Kudo-Saito Chie, Muramatsu Reiko. Determination of poor prognostic immune features of tumour microenvironment in non-smoking patients with lung adenocarcinoma. European Journal of Cancer. 2017;86:15–27. doi: 10.1016/j.ejca.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Askin Frederic, Gabrielson Edward. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of nonsmall cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(Pt 1):103–109. doi: 10.1016/j.semcancer.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howington John A., Blum Matthew G., Chang Andrew C. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. CHEST. 2013;143(5Suppl):e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 18.Timmerman Rebecca Paulus, Galvin James, Michalski Jeffrey. et al.Stereotactic body radiation therapy for inoperable early stage lung cancer robert. JAMA. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vansteenkiste J., Crinò L., Dooms C., Douillard J.Y. 2nd ESMO consensus conference on lung cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann oncol. 2014;25(8):1462–1474. doi: 10.1093/annonc/mdu089. [DOI] [PubMed] [Google Scholar]
- 20.Eberhardt W.E.E., De Ruysscher D., Weder W. 2nd ESMO consensus conference in lung cancer: locally advanced stage iii non-small-cell lung cancer. Ann Oncol. 2015;26(8):1573–1588. doi: 10.1093/annonc/mdv187. [DOI] [PubMed] [Google Scholar]
- 21.Rusch Valerie W., Giroux Dorothy J., Kraut Michael J. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of southwest oncology group trial 9416 (intergroup trial 0160) J Clin Oncol. 2007;25(3):313–318. doi: 10.1200/JO.2006.08.2826. [DOI] [PubMed] [Google Scholar]
- 22.Arriagada Rodrigo, Bergman Bengt, Dunant Ariane. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 23.Karaman H., Karaman A., Erden A., Poyrazoglu O.K. et al.Relationship between colonic polyp type and the neutrophil/lymphocyte ratio as a biomarker. Asian Pac J Cancer Prev. 2013;14(5):3159–3161. doi: 10.7314/apjcp.2013.14.5.3159. [DOI] [PubMed] [Google Scholar]
- 24.Dunn Gavin P., Bruce Allen T., Ikeda Hiroaki. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 25.Oluwaseun Adebayo Bamodu, Kuang-Tai Kuo,et al.Astragalus polysaccharides (PG2) enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer.Nutrients. 2019;11(10).pii: E2264. doi: 10.3390/nu11102264. [DOI] [PMC free article] [PubMed]
- 26.Melssen M. Slingluff CL Jr.Vaccines targeting helper T cells for cancer immunotherapy. Curr Opin Immunol. 2017;47:85–92. doi: 10.1016/j.coi.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borst J., Ahrends T., Bąbała N. Melief CJM.CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy R., Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol. Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 29.Bevan M.J. Helping the CD8+ T cell response. Nat. Rev. Immunol. 2004:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 30.Castellino F., Germain R.N. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu. Rev. Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 31.Bedoui S., Heath W.R., Mueller S.N. CD4+T cell help amplifies innate signals for primary CD8+ T cell immunity. Immunol. Rev. 2016;272:52–64. doi: 10.1111/imr.12426. [DOI] [PubMed] [Google Scholar]
- 32.Eickhoff S. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell. 2015;162:1322–1337. doi: 10.1016/j.cell.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitano M. Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc. Natl Acad. Sci. USA. 2016;113:1044–1049. doi: 10.1073/pnas.1513607113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laidlaw B.J., Craft J.E., Kaech S.M. The multifaceted role of CD4+ T cells in CD8+ T cell memory. Nat. Rev. Immunol. 2016;16:102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahrends T. CD4+ T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity. 2017;47:848–861. doi: 10.1016/j.immuni.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Gustafson M.P., Lin Y., LaPlant B. Immune monitoring using the predictive power of immune profiles.J Immunother. Cancer. 2013;1:7. doi: 10.1186/2051-1426-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riemann D., Cwikowski M., Turzer S. et al.Blood immune cell biomarkers in lung cancer. Clin Exp Immunol. 2019;195(2):179–189. doi: 10.1111/cei.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Showe M.K., Kossenkov A.V., Showe L.C. The peripheral immune response and lung cancer prognosis. Oncoimmunology. 2012;1:1414–1416. doi: 10.4161/onci.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are available from the corresponding author in response to reasonable requests.