Abstract
The response to a zeitgeber, particularly the light/dark cycle, may vary phenotypically. Phenotypic plasticity can be defined as the ability of one genome to express different phenotypes in response to environmental variation. In this opinion paper, we present some evidence that one of the most prominent effects of the introduction of electric light to the everyday life of humans is a significant increase in phenotypic plasticity and differences in interindividual phases of entrainment. We propose that the healthy limits of phenotypic plasticity have been surpassed in contemporary society.
Keywords: Phenotypic plasticity, Light/dark cycle, Interindividual differences, Natural light
Highlights
-
•
Electric light increased phenotypic plasticity in humans and differences in interindividual phases of entrainment.
-
•
Healthy limits of phenotypic plasticity have been surpassed in contemporary society.
-
•
The correlation between biological time (DLMO) and behavioral time (MSFsc) is reduced in the population without access to electrical light.
1. Current state of the field
Widespread use of electric light, enabled by the development of electric power grids in the 1930s, has dramatically changed people's lives. The availability of light at any time of day with the flip of a switch has led to an increase in work and social activities at night, i.e., the current habits and customs of Western societies. Sociological discussions have highlighted the impact of this invention on the gap between industrial societies that have embraced electric light and preindustrial societies that have maintained connection with nature being exposed to only natural lighting.
Electric light has been shown to be a zeitgeber, an external time cue, able to entrain the human circadian timing system (Honma et al., 1987; Czeisler and Wright, 1999; Wright et al., 2005; Gronfier et al., 2007). The entrainment of biological rhythms to zeitgebers is a property of biological clocks/oscillators that contributes to the temporal adaptation of the organism to the environment. The response to a zeitgeber, however, may vary phenotypically.
Phenotypic plasticity can be defined as the ability of one genome to express different phenotypes in response to environmental variation (Oostra et al., 2018; Xue and Leibler, 2018). This ability to be modified by the environment is genetically determined (Nicoglou, 2015). Phenotypic plasticity allows evolutionary adaptation to novel environments facilitating the persistence of populations under environmental changes (Oostra et al., 2018).
The circadian system is characterized by an extraordinary phenotypic plasticity for adapting to temporal changes on multiple scales (Maggi et al., 2017). Phenotypic plasticity is a ubiquitous adaptation of organisms and has multiple dimensions (Westneat et al., 2019). The expression of phenotypic plasticity is reduced in controlled laboratory studies, whereas the expression of phenotypic plasticity in the constantly changing real-world is probably the rule (Helm et al., 2017). The phenotypic plasticity of the circadian system allows organisms to synchronize behavior to the environment through adaptation of clock mechanisms (Scheer et al., 2007; Bosler et al., 2015) resulting in changes at cellular and network levels (Girardet et al., 2010), as well as structural rearrangements (Muraro et al., 2013).
Phase of entrainment is classically defined as the difference between the phase of the circadian oscillator and the phase of the zeitgeber (Pittendrigh and Daan, 1976; e.g., the timing of melatonin onset relative to the timing of sleep/darkness onset, Wright et al., 2005). The ability of mammals to adjust their phase of entrainment to changes in the light/dark cycle is one of the best examples of phenotypic plasticity. Another example of phenotypic plasticity is the aftereffects of entrainment on circadian period (Scheer et al., 2007). Circadian period is a biological factor that interacts with light exposure to influence phase of entrainment (Wright et al., 2005; Gronfier et al., 2007). The precise environmental factors and their relative contribution on phenotypic plasticity, however, are largely unknown. Examples of environmental factors that contribute to phenotypic plasticity include food availability and perceived predation risk. For example, low food availability and decreased perceived daytime predation risk promote diurnal activity patterns (van der Vinne et al., 2014). Mammals exhibit considerable phenotypic plasticity when facing energetic challenges, with nocturnal mice becoming diurnal when exposed to cold and hunger (van der Vinne et al., 2014).
The circadian system's response to light is well-studied and the mechanisms of photic entrainment in humans have been described in detail (Duffy and Czeisler, 2009; Brown, 2020). The circadian response to light depends on the internal clock time of light exposure, as well as the intensity, duration, and wavelength of light exposure, and prior light exposure history. Specifically, the same light exposure at different biological times of day results in dissimilar responses (e.g., producing phase advance or phase delay shifts; Honma and Honma, 1988; Czeisler et al., 1989; Rüger et al., 2013). As a general rule, the brighter the light exposure (Boivin et al., 1996; Zeitzer et al., 2000; Wright et al., 2001; Wright and Czeisler, 2002; Duffy et al., 2007) and the longer the duration of light exposure (Baehr et al., 1999; Dewan et al., 2011; Rahman et al., 2017), the larger the circadian response; although responses to brief light exposures are larger on a minute per minute basis than are responses to continuous light exposure since the response to light is most efficient in the first minutes of light exposure (Gronfier et al., 2004). The circadian clock is most sensitive to blue wavelength light (Lockley et al., 2003; Revell et al., 2005; Gooley et al., 2010; Brown, 2020). However, at lower levels of intensity, the circadian timing system appears to be more sensitive to green (555 nm) than blue (460 nm) light (Gooley et al., 2010). Further, a history of exposure to dim light results in a greater response to subsequent light exposure (Chang et al., 2011).
In the past 90–100 years circadian phenotypic plasticity has been challenged by an expansion of temporal niches allowed by the availability of electricity (e.g., social and work activities later at night and 24/7 shift work operations). The latter expansion has been associated with later and irregular sleep timing, insufficient sleep, and health problems. In contemporary times, people spend more time indoors during the daytime and the resulting reduced exposure to daytime light combined with electric light after sunset contributes to increased phenotypic plasticity (Wright et al., 2013). Findings from studies of human sleep and circadian time in preindustrial societies have provided important information about the effects of electric light on circadian entrainment and, particularly, on the phenotypic plasticity of the circadian system. In most of the studies performed to date, findings show that populations not exposed to electric light have an earlier clock time of entrainment and/or earlier sleep timing when compared to those exposed to electric light (Louzada and Menna-Barreto, 2004; Wright et al., 2013; Moreno et al., 2015). In this opinion paper, we present some evidence that one of the most prominent effects of the introduction of electric light to the everyday life of humans is a significant increase in phenotypic plasticity and differences in interindividual phases of entrainment.
Louzada and Menna-Barreto (2004) studied self-reported sleep/wake patterns in adolescents living in a Brazilian rural area, some of whom did not have electricity at home. Those who did not have access to electricity showed significantly earlier bedtimes on school days and weekends compared to those with access to electricity. Additionally, adolescents with electricity at home had greater variability in sleep timing during weekends. Peixoto et al. (2009) studying adolescents living in Brazilian rural areas also showed significantly earlier bedtimes on school days compared to those with access to electricity. Although there were no differences in circadian phase between the groups when dim light melatonin onset (DLMO) was compared, DLMO variability was greater in the group that had electricity at home. Paradoxically sleep/wake patterns, however, were found to have greater variability in the group that did not have electricity at home.
Wright et al. (2013) compared the timing of the dim light melatonin onset (DLMO) and dim light melatonin offset (DLMOff) of a group of adults after exposure to only natural light living outdoors (camping) in tents for one week, to the timing of the DLMO and DLMOff of the same adults after exposure to both natural and electric lighting living in their typical social environment; i.e., going to school, work, and social activities. The authors observed a significant advance of the melatonin rhythm (DLMO and DLMOff) and reduced inter-individual variability in the timing of the DLMO and DLMOff after exposure to only natural light. Individual differences in circadian melatonin timing were thus larger after exposure to the weaker zeitgeber of dimmer daytime light exposure and electric light exposure after sunset in the modern lighting environment. Iglesia et al. (2015) studied rest/activity cycles of hunter-gatherer indigenous people in the Province of Chaco (Argentina), with and without access to electricity, and showed that access to electricity was associated with reduced sleep duration and reduced inter-individual variability in rest/activity patterns. Moreno et al. (2015) studied sleep and circadian rhythms of rubber tappers in the Amazon forest (State of Acre, Brazil), with and without electricity at home, and showed that access to electricity at home was associated with reduced sleep duration and delayed timing of the DLMO.
The timing of the DLMO, the most commonly used and reliable marker of circadian phase, has been shown to positively correlate with the timing of midsleep on free days (MSFsc) (Wright et al., 2013; Kantermann and Burgess, 2017; Facer-Childs et al., 2019). This MSFsc measure is calculated from the Munich ChronoType Questionnaire (MCTQ) (Roenneberg et al., 2003). These correlations between DLMO and MSFsc chronotype have been reported under a mixture of natural and electric light-dark conditions. The strength of the correlation between biological time (melatonin onset) and behavioral time (midsleep phase), however, was reduced after only natural light-dark conditions (no electric light) (Wright et al., 2013); likely due to the fact that exposure to the natural light-dark cycle is a stronger zeitgeber than is exposure to dimmer daytime indoor light exposure and electric light at night.
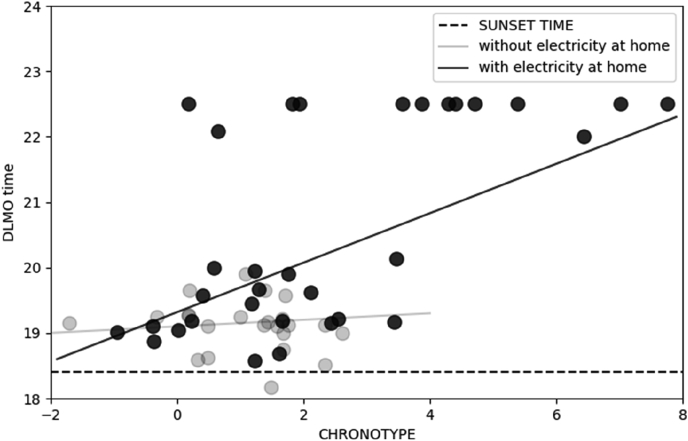
Access to electric lighting was also found to increase interindividual differences in the circadian timing (DLMO) of rubber tappers (Moreno et al., 2015) The correlation between biological time (DLMO) and behavioral time (MSFsc) was reduced in the population without access to electrical light (Fig. 1, data replotted from Moreno et al., 2015). These findings are consistent with data from Wright et al. (2013).
Fig. 1.
Correlation between Dim light Melatonin Onset (DLMO) and midsleep time (MSFsc) among rubber tappers from Amazon, Brazil. Rubber tappers with electric lighting at home (n = 31; solid symbols; r = 0.63; p < 0.05); rubber tappers without electric lighting at home (n = 23; open symbols; r = 0.22; ns).
2. Future research directions to advance sleep and circadian science
Studies carried out in communities that have compared participants with and without access to electric light provide crucial information about the effects of access to electric light on interindividual variability in circadian timing and sleep. Such phenotypic plasticity differences appear much more pronounced under conditions with access to electric light. Phenotypic plasticity is an essential property of living systems, and access to electric light increases the degree of phenotypic plasticity with potential health consequences when phenotypic plasticity results in later chronotypes in a social environment that requires early work/school start times. Further, findings from studies of night shift workers (Kecklund and Axelsson, 2016; Moreno et al., 2019) and of patients with circadian rhythm sleep/wake disorders (Sack et al., 2007) show the negative impacts of light exposure at night and associated circadian disruption on health. Such examples suggest that healthy limits of phenotypic plasticity may have been surpassed in contemporary society. Future studies should aim to understand how humans can optimize light exposure during the 24 h day (e.g. brighter days and dimmer nights) to promote health, and to reduce the negative effects of going beyond healthy phenotypic plasticity circadian entrainment limits.
CRediT authorship contribution statement
C.R.C. Moreno: Conceptualization, Writing - original draft, Writing - review & editing. K. Wright: Writing - review & editing. D.J. Skene: Writing - review & editing. F.M. Louzada: Conceptualization, Writing - original draft, Visualization, Writing - review & editing.
Declaration of competing interest
K.P.W. reports being a consultant to/and or receiving personal fees from Circadian Therapeutics, Inc., Circadian Biotherapies, Inc., Philips, Inc; and receiving research support from the NIH, the Office of Naval Research, the PAC-12 conference, outside the submitted work. C.R.C.M., D.J.S., and F.M.L. have nothing to disclose.
Acknowledgments
We thank Suleima Vasconcelos, Federal University of Acre, Brazil, for the data collection of Fig. 1, and FAPESP for funding this data collection (Grant number 2014/01514–0).
References
- Baehr E.K., Fogg L.F., Eastman C.I. Intermittent bright light and exercise to entrain human circadian rhythms to night work. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;277:R1598–R1604. doi: 10.1152/ajpregu.1999.277.6.R1598. [DOI] [PubMed] [Google Scholar]
- Boivin D., Duffy J., Kronauer R., Czeisler C.A. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- Bosler O., Girardet C., Franc J.L., Becquet D., François-Bellan A.M. Structural plasticity of the circadian timing system. An overview from flies to mammals. Front. Neuroendocrinol. 2015;38:50–64. doi: 10.1016/j.yfrne.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Brown T.M. Melanopic illuminance defines the magnitude of human circadian light responses under a wide range of conditions. J. Pineal Res. 2020;e12655 doi: 10.1111/jpi.12655. [DOI] [PubMed] [Google Scholar]
- Chang A.M., Scheer F.A., Czeisler C.A. The human circadian system adapts to prior photic history. J. Physiol. 2011;589:1095–1102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler C.A., Kronauer R.E., Allan J.S., Duffy J.F., Jewett M.E., Brown E.N., Ronda J.M. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Czeisler C.A., Wright K.P., Jr. Influence of light on circadian rhythmicity in humans. In: Turek F.W., Zee P.C., editors. Regulation of Sleep and Circadian Rhythms. Marcel Dekker, Inc.; New York, NY: 1999. pp. 147–180. [Google Scholar]
- Dewan K., Benloucif S., Reid K., Wolfe L.F., Zee P.C. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34(5):593–599. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.F., Zeitzer J.M., Czeisler C.A. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol. Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.F., Czeisler C.A. Effect of light on human circadian physiology. Sleep Med. Clin. 2009;4:165–177. doi: 10.1016/j.jsmc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facer-Childs E.R., Middleton B., Skene D.J., Bagshaw A.P. Resetting the late timing of 'night owls' has a positive impact on mental health and performance. Sleep Med. 2019;60:236–247. doi: 10.1016/j.sleep.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Girardet C., Becquet D., Blanchard M.P., François‐Bellan A.M., Bosler O. Neuroglial and synaptic rearrangements associated with photic entrainment of the circadian clock in the suprachiasmatic nucleus. Eur. J. Neurosci. 2010;32:2133–2142. doi: 10.1111/j.1460-9568.2010.07520.x. [DOI] [PubMed] [Google Scholar]
- Gooley J.J., Rajaratnam S.M., Brainard G.C., Kronauer R.E., Czeisler C.A., Lockley S.W. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C., Wright K.P., Jr., Kronauer R.E., Jewett M.E., Czeisler C.A. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am. J. Physiol. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C., Wright K.P., Jr., Kronauer R.E., Czeisler C.A. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm B., Visser M.E., Schwartz W., Kronfeld-Schor N., Gerkema M., Piersma T., Bloch G. Two sides of a coin: ecological and chronobiological perspectives of timing in the wild. Phil. Trans. R. Soc. B37220160246. 2017 doi: 10.1098/rstb.2016.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K., Honma S. Circabidian rhythm: its appearance and disappearance in association with a bright light pulse. Experientia. 1988;44:981–983. doi: 10.1007/BF01939893. [DOI] [PubMed] [Google Scholar]
- Honma K., Honma S., Wada T. Entrainment of human circadian rhythms by artificial bright light cycles. Experientia. 1987;43:572–574. doi: 10.1007/BF02143589. [DOI] [PubMed] [Google Scholar]
- Iglesia H.O., Fernández-Duque E., Golombek D.A., Lanza N., Duffy J.F., Czeisler C.A., Valeggia C.R. Access to electric light is associated with shorter sleep duration in a traditionally hunter-gatherer community. J. Biol. Rhythm. 2015;30:342–350. doi: 10.1177/0748730415590702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantermann T., Burgess H.J. Average mid-sleep time as a proxy for circadian phase. PsyCh J. 2017;6:290–291. doi: 10.1002/pchj.182. [DOI] [PubMed] [Google Scholar]
- Kecklund G., Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. doi: 10.1136/bmj.i5210. [DOI] [PubMed] [Google Scholar]
- Lockley S.W., Brainard G.C., Czeisler C.A. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 2003;88(9):4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Louzada F., Menna-Barreto L. Sleep–wake cycle in rural populations. Biol. Rhythm. Res. 2004;35(1–2):153–157. doi: 10.1080/09291010412331313304. [DOI] [Google Scholar]
- Maggi S., Balzani E., Lassi G., Garcia-Garcia C., Plano A., Espinoza S., Mus L., Tinarelli F., Nolan P.M., Gainetdinov R.R., Balci F., Nieus T., Tucci V. The after-hours circadian mutant has reduced phenotypic plasticity in behaviors at multiple timescales and in sleep homeostasis. Sci. Rep. 2017;7:17765. doi: 10.1038/s41598-017-18130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Vasconcelos S., Marqueze E., Lowden A., Middleton B., Fischer F.M., Louzada F.M., Skene D.J. Sleep patterns in Amazon rubber tappers with and without electric light at home. Sci. Rep. 2015;5:14074. doi: 10.1038/srep14074N.I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C.R.C., Marqueze E.C., Sargent C., Wright K.P., Jr., Ferguson S.A., Tucker P. Working Time Society consensus statements: evidence-based effects of shift work on physical and mental health. Ind. Health. 2019;57:139–157. doi: 10.2486/indhealth.SW-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro N.I., Pírez N., Ceriani M.F. The circadian system: plasticity at many levels. Neuroscience. 2013;247:280–293. doi: 10.1016/j.neuroscience.2013.05.036. [DOI] [PubMed] [Google Scholar]
- Nicoglou A. The evolution of phenotypic plasticity: genealogy of a debate in genetics. Stud. Hist. Philos. Sci. C. 2015;50:67–76. doi: 10.1016/j.shpsc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Oostra V., Saastamoinen M., Zwaan B.J., Wheat C.W. Strong phenotypic plasticity limits potential for evolutionary responses to climate change. Nat. Commun. 2018;9:1005. doi: 10.1038/s41467-018-03384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto C.A.T., Silva A.G.T., Mary A., Carskadon M.A., Louzada F.M. Adolescents living in homes without electric lighting have earlier sleep times. Behav. Sleep Med. 2009;7:73–80. doi: 10.1080/15402000902762311. [DOI] [PubMed] [Google Scholar]
- Pittendrigh C.S., Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J. Comp. Physiol. 1976;106:291–331. doi: 10.1007/BF01417857. [DOI] [Google Scholar]
- Rahman S.A., St Hilaire M.A., Chang A.M. Circadian phase resetting by a single short-duration light exposure. JCI Insight. 2017;2 doi: 10.1172/jci.insight.89494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell V.L., Arendt J., Terman M., Skene D.J. Short-wavelength sensitivity of the human circadian system to phase-advancing light. J. Biol. Rhythm. 2005;20:270–272. doi: 10.1177/0748730405275655. [DOI] [PubMed] [Google Scholar]
- Roenneberg T., Wirz-Justice A., Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J. Biol. Rhythm. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Rüger M., St Hilaire M.A., Brainard G.C., Khalsa S.B.S., Kronauer R.E., Czeisler C.A., Lockley S.W. Human phase response curve to a single 6.5 h pulse of short‐wavelength light. J. Physiol. 2013;591:353–363. doi: 10.1113/jphysiol.2012.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R.L., Auckley D., Auger R., Carskadon M.A., Wright K.P., Jr., Vitiello M.V., Zhdanova I.V. Circadian rhythm sleep disorders: Part I, basic principles, shift work and jet lag disorders. Sleep. 2007;30:1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer F.A., Wright K.P., Jr., Kronauer R.E., Czeisler C.A. Plasticity of the intrinsic period of the human circadian timing system. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vinne V., Riede S.J., Gorter J.A., Willem G., Eijer W.G., Sellix M.T., Menaker M., Daan S., Pilorz V., Hut R.A. Cold and hunger induce daytime activity. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:15256–15260. doi: 10.1073/pnas.141313511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westneat D.F., Potts L.J., Sasser K.L., Shaffer J.D. Causes and consequences of phenotypic plasticity in complex environments. Trends Ecol. Evol. 2019;34:p555–568. doi: 10.1016/j.tree.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Wright K.P., Jr., Czeisler C.A. Absence of circadian phase resetting in response to bright light behind the knees. Science. 2002;297:571. doi: 10.1126/science.1071697. [DOI] [PubMed] [Google Scholar]
- Wright K.P., Jr., Gronfier C., Duffy J.F., Czeisler C.A. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. Rhythm. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.P., Jr., Hughes R.J., Kronauer R.E., Dijk D.J., Czeisler C.A. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.P., Jr., McHill A.W., Birks B.R., Griffin B.R., Rusterholz T., Chinoy E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Leibler S. Benefits of phenotypic plasticity for population growth in varying environments. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115:12745–12750. doi: 10.1073/pnas.1813447115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer J.M., Dijk D.J., Kronauer R.E., Brown E.N., Czeisler C.A. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J. Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]