Echocardiography is a widespread, noninvasive, portable and powerful imaging modality which provides unparalleled anatomical and functional information on both the heart and great vessels. When used in intensive care medicine, echocardiography may be focused rather than comprehensive, is both performed and interpreted around-the-clock by the front-line physician in light of the clinical context and ongoing therapy, and frequently results in a broad and direct therapeutic impact (1). These specificities have led to define critical care echocardiography (CCE) as examinations performed and interpreted by the intensivist to make diagnoses and guide therapeutic management of the critically ill patient with cardiovascular or respiratory compromise (2). Dedicated training programs have been validated over the past decade. Despite the pivotal role gained by CCE in the intensive care unit (ICU) settings, studies assessing its impact on patient-centered outcome are scarce.
In this context, contributions such as that reported by Lan et al. (3) in the journal should be encouraged. Authors have used a large open-access database containing anonymous health-related data of 46,520 critically ill patients admitted to the Beth Israel Deaconess Medical Center in Boston (MA, USA) from 2001 to 2012 (4). Using a propensity score analysis to match patients who were assessed using CCE during the first 24 h of the septic shock onset (n=1,289) and those who were not (n=1,289), authors showed that 28-day mortality was significantly lower in the ultrasound group (33.2% vs. 37.7%: P=0.019). Improved 28-day mortality in patients assessed with CCE (OR: 0.83; 95% CI: 0.73–0.95: P=0.005) was confirmed by sensitivity analyses in the subgroup of patients who underwent a single echocardiography examination (n=2,464; OR: 0.82; 95% CI: 0.72–0.94; P=0.004) and when excluding patients monitored using either a right-heart catheter or transpulmonary thermodilution (n=2,485; OR: 0.87; 95% CI: 0.76–0.99: P=0.034) (3). These results confirm those reported by the previous contribution of Feng et al. (5) based on the same Medical Information Mart for Intensive Care (MIMIC)-III database in 6,361 patients with sepsis. Using sophisticated multivariate statistical analysis, these authors showed a significant beneficial effect of CCE assessment on 28-day mortality when performed less than 24 h before the first ICU admission (51.3%) or during ICU stay. Propensity score-matched mortality rates for CCE and no CCE groups were 25% and 30%, respectively, and adjusted odds ratio was 0.78 (95% CI: 0.68–0.90; P<0.001) (5).
What are the factors driving the performance of CCE and do they influence its association with outcome?
Scientific Societies of Intensive Care Medicine have defined the use of CCE and training content to achieve different levels of competence (2). Noticeably, CCE is suggested as the preferred modality to initially evaluate the type of shock as opposed to more invasive technologies (week recommendation; quality of experience moderate; level of evidence: B) (6). Intuitively, the therapeutic impact of CCE—hence potential benefit on patient course—heavily depends on the contextual indication of the hemodynamic assessment which will determine both its diagnostic yield and potential additional value (Figure 1). Not surprisingly, the presence of congestive heart failure was the most discriminant relative influence factor to predict the likelihood of CCE performance in the propensity score model developed by Feng et al. (5). In this study, CCE was associated with a significantly lower 28-day mortality in septic patients from the MIMIC-III database, irrespective of the five models tested (5). In the present contribution, Lan et al. (3) reported that after matching, septic patients from the same database more frequently received right-heart catheterization but not transpulmonary thermodilution when assessed using CCE. This suggests that those specific patients, who were sicker than their counterparts before matching, required a comprehensive hemodynamic assessment and monitoring according to their clinical presentation.
Figure 1.
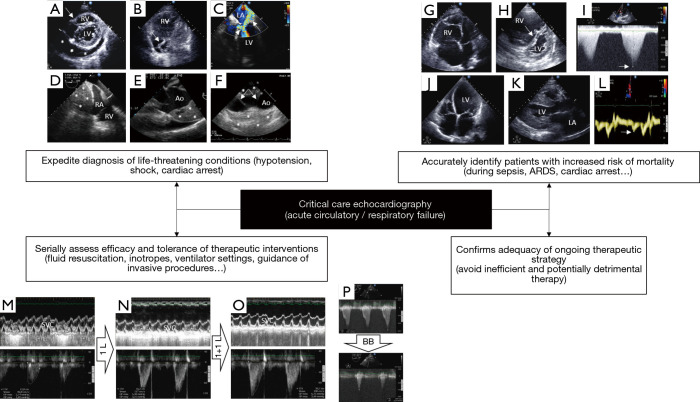
Illustrative examples of the diagnostic capabilities of critical care echocardiography in patients with cardiopulmonary compromise, when performed by the transthoracic or transesophageal approach according to clinical settings. (A) Parasternal short-axis view depicting a large pericardial effusion (asterisks) with tamponade physiology (inverted right ventricular free wall, arrow); (B) apical four-chamber view depicting enlarged right cardiac cavities and a thrombus-in-transit highly mobile within the right atrium in real time (arrow); (C) transesophageal echocardiography four-chamber view disclosing a massive eccentric mitral regurgitation due to a systolic anterior motion of the mitral valve (arrow); note that a dynamic left ventricular outflow tract obstruction is present, as reflected by a highly turbulent flow with color Doppler mapping (arrowhead); (D) transesophageal echocardiography view depicting a large mediastinal hematoma (asterisks) impeding right atrial filling and resulting in extracardiac tamponade physiology after a complicated open-heart surgery; (E) transesophageal echocardiography view showing a large intramural hematoma of the initial ascending aorta (asterisks) associated with hemopericardium in a patient ventilated for acute circulatory failure; (F) longitudinal transesophageal echocardiography view depicting a complex medial tear (arrows) associated with a subadventitial disruption of the aortic isthmus in a patient ventilated for severe blunt chest trauma. Note the large posterior hemomediastinum (asterisks) which reflects the risk of imminent lethal blood extravasation; (G) apical four-chamber view disclosing a marked enlargement of the right ventricle which size exceeds that of the left ventricle in a patient under protective ventilation for an acute respiratory distress syndrome; (H) in the same patient, the parasternal short axis view confirms the marked dilatation of the right ventricular cavity and depicts a bulging of the interventricular septum towards the left ventricle at end-systole (arrow). Taken together, these findings are consistent with a severe acute cor pulmonale; (I) maximal Doppler tricuspid regurgitation velocity (arrow) allows estimating quantitatively the systolic pressure gradient between the right atrium and right ventricle. In this patient hospitalized for a decompensated chronic respiratory insufficiency, pulmonary artery systolic pressure is estimated at approximately 110 mmHg; (J) apical four-chamber view depicting a dilated cardiomyopathy in a patient sustaining cardiogenic shock; (K) parasternal long-axis view showing a concentric hypertrophic cardiomyopathy in a patient who presented with cardiogenic pulmonary edema. Note the marked left atrial dilatation reflecting chronically elevated filling pressures; (L) in the same patient, tissue Doppler imaging at the lateral aspect of the mitral ring depicts markedly low E’ velocity (arrow), consistent with severe left ventricular diastolic dysfunction (impaired relaxation); (M) transesophageal echocardiography monitoring the effects of serial fluid challenges in a hypotensive ventilated patient with early septic shock. At baseline, marked respiratory variation of the superior vena cava with inspiratory collapse indicates preload dependence (upper panel) at the origin of reduced left ventricular stroke volume, as reflected by a decreased velocity-time integral of left ventricular outflow tract (lower panel; 12.8 cm); (N) after a 1-L fluid challenge, the velocity-time integral of left ventricular outflow tract significantly increased (lower panel; 16.2 cm) while variation of the size of the superior vena cava within the respiratory cycle remains relevant (upper panel); (O) after a second 1-L fluid challenge, the velocity-time integral of left ventricular outflow tract significantly increased (lower panel; 20.7 cm), while respiratory variation of superior vena cava decreased (upper panel); (P) left ventricular outflow tract obstruction in an elderly patient with septal hypertrophy who was assessed for weaning-induced pulmonary oedema (upper panel; maximal pressure gradient: 131 mmHg). Beta-blocker therapy markedly decreased the dynamic outflow tract obstruction, allowing successful ventilator weaning (lower panel; maximal pressure gradient: 16 mmHg). RV, right ventricle; LV, left ventricle; LA, left atrium; RA, right atrium; Ao, aorta; SVC, superior vena cava; BB, beta-blocker.
A large-scale study was recently performed on the Nationwide Inpatient Sample (NIS) which represents the largest publicly available database in the United States, with approximately 8 million hospitalizations in a sample of 20% community hospitals (7). The use of echocardiography was associated with a significant lower adjusted odds of hospital mortality in approximately half of all inpatients who were hospitalized for five of the leading six admission diagnoses (7). All of those, namely sepsis, congestive heart failure, acute myocardial infarction, acute cerebrovascular disease and cardiac dysrhythmia, are conditions which are frequently encountered in ICU patients. Overall, the potential impact of echocardiography on patient outcome is presumably influenced by the clinical presentation and suspected underlying condition, especially when severe and involving the cardiovascular system.
Could CCE predict mortality in ICU patients?
In a small sample size study, Heidenreich et al. (8) reported that transesophageal echocardiography (TEE) diagnosis was an independent predictor of mortality in ICU patients who were hemodynamically assessed for hypotension. Authors distinguished three groups based on the mechanism of hypotension identified by TEE: non-ventricular cardiac limitation in cardiac output (e.g., pericardial or valvular disease) (group 1); depressed ventricular systolic function (group 2); vasoplegia (hyperkinetic left ventricle) and/or hypovolemia (group 3). Patients from group 1 had a significantly higher survival rate (81%) than those of group 2 (41%) and group 3 (44%; P=0.03). Not surprisingly, direct therapeutic impact resulting from TEE assessment was more frequent in group 1 than in group 2 and 3 (75% vs. 41% vs. 33%: P=0.03), including surgery (56% vs. 7% vs. 6%: P=0.0001) (8). CCE expedites diagnosis of imminent life-threatening conditions which require immediate intervention, such as tamponade, acute and severe valvular regurgitation, thrombus-in-transit associated with massive pulmonary embolism, or acute aortic disease with extravasation signs (Figure 1). Nevertheless, these findings are occasional and tend to overestimate the overall diagnostic ability and potential prognostic impact of CCE in ICU patients.
Echocardiography is recommended during the cardiopulmonary resuscitation of cardiac arrest to help identifying potentially treatable causes and to assess myocardial contractility (Figure 1). CCE appears to predict mortality during cardiopulmonary resuscitation since the absence of cardiac activity on CCE had a pooled negative likelihood ratio of 0.18 for predicting the return of spontaneous circulation (9). In 793 out-of-hospital cardiac arrests, Gaspari et al. (10) reported that only 0.6% of patients with absence of initial cardiac activity on CCE survived to hospital discharge.
In patients receiving protective mechanical ventilation for the acute respiratory distress syndrome (ARDS), the identification of a severe acute cor pulmonale by TEE, as defined by the conjunction of a marked dilatation of the right ventricle which end-diastolic area exceeds that of the left ventricle in the long axis view of the heart and a paradoxical septal motion in the short-axis view, is independently associated with increased hospital mortality (11). In addition to its valuable ability to semi-quantitatively assess left ventricular filling pressure in patients presenting with acute respiratory failure (i.e., rule in/rule out cardiogenic pulmonary edema), CCE depicts hemodynamic consequences of both associated pulmonary microvascular involvement and positive-pressure ventilation on right ventricular function which appear prognostic when marked (Figure 1).
Although association does not prove causality, taken together these findings suggest that CCE may help the front-line clinician to identify mortality risk and take preemptive action to improve patient outcome in certain clinical settings, other than sepsis. Nevertheless, the generalization of this sound clinical approach may not hold true in all ICU patients (12).
Which CCE-induced changes in therapy could influence mortality?
In the two recent studies reporting an improved 28-day mortality in septic patients from the MIMIC-III data base who were initially assessed using CCE, a significant therapeutic impact presumably related to initial noninvasive hemodynamic assessment has been described (3,5). When compared to patients who were not evaluated by CCE, those patients who underwent an echocardiography assessment during the initial management of sepsis received significantly more fluids throughout the first three days of hospitalization (5), more inotropes (3,5), and shorter duration of vasopressors (5). In contrast, the duration of mechanical ventilation was not statistically different between groups (3,5).
In a recent randomized controlled trial (RCT) performed in pediatric septic shock, the proportion of children with successful shock reversal was significantly higher (89% vs. 67%: P=0.01) and the duration of shock shorter (3.3 vs. 4.5 days: P=0.01) in those who were serially assessed with transthoracic echocardiography (TTE) to determine both the volume status and myocardial function (13). In keeping with these results, lactate normalized faster in the intervention arm. Interestingly, earlier and higher fluid volume resuscitation was administered in children monitored with CCE, but cumulative fluid volume was significantly lower at 24 h when compared to controls (13). Moreover, inotropes were more frequently used in the experimental group (89% vs. 67%: P=0.01) with earlier starting time (12 vs. 24 h: P=0.01) than in the control group. Interestingly, mortality attributed to unresolved shock was significantly lower in children monitored with CCE (38.5% vs. 88.2%: P=0.006) (13). Similar findings were reported by Kanji et al. (14) in adult patients with vasopressor-dependent shock in a before/after study where patients were hemodynamically assessed using focused echocardiography (intervention) in addition to standard of care (control period).
In guiding the therapeutic management of patients with cardiopulmonary compromise, CCE has a significant therapeutic impact which may secondarily improve patient-centered outcome. Specific therapy such as additional fluid loading or inotrope initiation may be delivered earlier and adapted serially when guided by CCE monitoring (Figure 1). This may avoid unnecessary fluids or drugs and guide therapeutic changes to better take into account the complex course of ICU patients, especially when sustaining septic shock.
Why therapeutic impact does not translate into prognostic improvement?
In Cardiology settings, appropriate use criteria have been established to optimize the rational use of TTE and potentially help the decision making of attending physicians. TTE examinations fulfilling these criteria appear to result in a more frequent therapeutic impact than when performed for inappropriate indications (86.7% vs. 14.1%: P<0.0001) (15). Despite mostly appropriate examinations (91.8%), Matulevicius et al. (16) showed that nearly half of TTEs resulted in continuation of ongoing care and less than one-third (31.8%) induced therapeutic changes. When confirming that initiated empiric therapy is adequate in ICU patients, CCE is as informative as when it provides additional information, since it avoids unnecessary and potentially detrimental treatment (e.g., excessive fluid resuscitation in non-responders) (Figure 1). In 137 ventilated patients assessed using TEE for septic shock, hemodynamic assessment confirmed the adequacy of initiated therapy in 27% of the cases (17). According to clinical settings, the respective proportion of therapeutic changes and validation of ongoing management following CCE examination is expected to vary greatly, so as resulting potential impact on outcome.
Early recognition of hemodynamic disorders and guidance to determine appropriate initial therapy aimed at reducing the duration of hypotension and tissue hypoperfusion have been shown to decrease morbidity and mortality in patients sustaining septic shock (18). Accordingly, the timing of hemodynamic assessment, including when using CCE, is crucial to consider. In patients presenting with septic shock, CCE is typically performed within the first hours following ICU admission, after initial fluid resuscitation and stabilized blood pressure under vasopressor support (17), as in the present contribution (3). CCE allows early identification of distinct cardiovascular phenotypes in patients admitted to the ICU for septic shock (19). Whether this strategy will select the subset of patients whose outcome could be improved by a specific treatment (e.g., inotropes) remains to be determined. To address this hypothesis, CCE will be used to select eligible patients with associated septic cardiomyopathy for their potential enrollment in a RCT comparing the effects of dobutamine vs. placebo on sepsis-induced organ dysfunctions (NCT04166331). In contrast, uniformly administered inodilator increases the frequency of adverse effects and fails to improve mortality when compared to placebo (20). Interestingly, early CCE assessment promises to provide valuable information to guide tailored resuscitation as soon as the diagnosis of sepsis is raised in the Emergency Department (21). Whether this precocious information may alter the early Surviving Sepsis Campaign bundle and patient-centered outcome remains to be investigated. As opposed to common practice in Cardiology, CCE is currently more considered and used to monitor both the efficacy and tolerance of therapeutic interventions rather than as a punctual diagnostic imaging modality (Figure 1) (22). Accordingly, future studies should take into account this relevant and rapidly spreading clinical practice (3).
When compared with the sole clinical assessment, hemodynamic monitoring provides relevant additional information with a potential impact on therapeutic decisions. Nevertheless, for this new information to translate into an improvement of outcome, hemodynamic assessment should be accurate, adequately interpreted and associated with a standardized therapeutic algorithm (17). In contrast, if the additional information is interpreted or applied inappropriately, resulting therapeutic interventions may prove ineffective or harmful, and outcome will not be improved or even may be worsened (23). Accordingly, the measurement of routinely used hemodynamic parameters should be reproducible and adequate training of intensivists is key, irrespective of the targeted level of competence in CCE (24). Finally, according to the clinical scenario, the use of TEE rather than TTE may alter resulting therapeutic impact due to its higher diagnostic capacity for the identification of certain conditions (e.g., extrapericardial tamponade after cardiac surgery), hence potential influence of CCE on outcome.
Are RCTs to establish the prognostic role of CCE necessary in ICU patients?
Currently, no definitive RCT aimed at assessing the prognostic impact of CCE in ICU patients has been fully conducted. Reasons are numerous (Table 1). The only published RCT evaluated the influence of point-of-care ultrasonography on mortality of patients presenting to the Emergency Department with undifferentiated (i.e., without a clearly evident cause) non traumatic hypotension or shock (25). This study failed to find any benefit to perform early point-of-care ultrasonography on patient-centered outcome, when compared to standard of care. Interestingly, this RCT was interrupted prematurely at interim analysis because of slow recruitment due to concerns about randomization to the control group from physicians and perceived futility of continuing (25). In addition to its lack of power, this study suffered from substantial limitations: exclusion of clear mechanisms of hypotension or shock (potential bias), absence of specific clinical questions to address (unknown presumptive diagnoses), and lack of associated predefined therapeutic algorithm (random therapeutic impact). Not surprisingly, the volume of fluid resuscitation and proportion of patients receiving inotropes were not statistically significant between groups (25).
Table 1. Illustrative reasons potentially limiting the development of randomized controlled trials to establish the impact of CCE on patient-centered outcome.
| Parameters | Limitations | Alternatives |
|---|---|---|
| Primary criterion | Mortality is multifactorial | Consider intermediate criteria, such as organ dysfunction scores1 |
| Study population | ICU patients constitute a highly heterogeneous population | Determine distinct cardiovascular phenotypes to select a homogeneous population of interest |
| Paradigm | CCE guides therapy, hence influences outcome | CCE identifies eligible patients for a specific, potentially beneficial therapeutic intervention |
| Modality of CCE | Level of competence is variable TTE and TEE are not interchangeable CCE can be used punctually or repeatedly |
Use the same CCE level uniformly Adapt CCE modality to clinical setting2 Stratify on participating centers3 |
| Hemodynamic indices | Large numbers of quantitative parameters Inter- and intra-observer variability No consensual definition of relevant variations |
Use simple indices (external validity) Use reproducible indices (<10%) Select meaningful, validated threshold values |
| Therapeutic impact | CCE-related therapeutic changes are variable CCE-confirmed therapeutic adequacy is frequent Adequate CCE interpretation may still lead to various (potentially inadequate) therapeutic changes |
Focus on predefined organs support4 Record exhaustively (severe) adverse effects5 Combine a standardized therapeutic algorithm with CCE diagnostic work-up |
1, for example, Sepsis-related Organ Failure Assessment (SOFA) score; 2, for example, TEE should be used in the perioperative period of cardiac surgery; 3, CCE may be routinely used either punctually or serially to monitor therapeutic interventions according to center; 4, for example, fluid resuscitation, inotropes, vasopressors, mechanical ventilation, renal replacement therapy; 5, especially those potentially induced by therapy (may be decreased in case of CCE confirmation of therapeutic adequacy). CCE, critical care echocardiography; ICU, intensive care unit; TTE, transthoracic echocardiography; TEE, transesophageal echocardiography.
Wrap-up
Since echocardiography is the most ubiquitous, portable, cost-effective and well-tolerated imaging modality with an infinitesimal risk-to-benefit ratio, the high therapeutic impact resulting from its routine use in ICU patients may be interpreted as beneficial for the patient. Although CCE-guided management of patients with cardiopulmonary compromise has not yet been demonstrated to improve outcome by RCTs, its unparalleled diagnostic capacity will continue to expedite recognition of life-threatening hemodynamic derangements, identify patients at high risk of increased mortality, select potential candidates for targeted therapy, quantify the effects of therapeutic changes and reduce potentially harmful interventions. As such, CCE will undoubtedly contribute to tailor therapy and improve critically ill patient outcomes.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3208). The author has no conflicts of interest to declare.
References
- 1.Vignon P. What is new in critical care echocardiography? Crit Care 2018;22:40. 10.1186/s13054-018-1970-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest 2009;135:1050-60. 10.1378/chest.08-2305 [DOI] [PubMed] [Google Scholar]
- 3.Lan P, Wang TT, Li HY, et al. Utilization of echocardiography during septic shock was associated with a decreased 28-day mortality: a propensity score-matched analysis of the MIMIC-III database. Ann Transl Med 2019;7:662. 10.21037/atm.2019.10.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. 10.1038/sdata.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng M, McSparron JI, Kien DT, et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med 2018;44:884-92. 10.1007/s00134-018-5208-7 [DOI] [PubMed] [Google Scholar]
- 6.Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. 10.1007/s00134-014-3525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papolos A, Narula J, Bavishi C, et al. U.S. Hospital Use of Echocardiography: Insights From the Nationwide Inpatient Sample. J Am Coll Cardiol 2016;67:502-11. 10.1016/j.jacc.2015.10.090 [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich PA, Stainback RF, Redberg RF, et al. Transesophageal echocardiography predicts mortality in critically ill patients with unexplained hypotension. J Am Coll Cardiol 1995;26:152-8. 10.1016/0735-1097(95)00129-N [DOI] [PubMed] [Google Scholar]
- 9.Blyth L, Atkinson P, Gadd K, et al. Bedside focused echocardiography as predictor of survival in cardiac arrest patients: a systematic review. Acad Emerg Med 2012;19:1119-26. 10.1111/j.1553-2712.2012.01456.x [DOI] [PubMed] [Google Scholar]
- 10.Gaspari R, Weekes A, Adhikari S, et al. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation 2016;109:33-9. 10.1016/j.resuscitation.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 11.Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862-70. 10.1007/s00134-015-4141-2 [DOI] [PubMed] [Google Scholar]
- 12.Sawchuk CW, Wong DT, Kavanagh BP, et al. Transthoracic echocardiography does not improve prediction of outcome over APACHE II in medical-surgical intensive care. Can J Anaesth 2003;50:305-10. 10.1007/BF03017803 [DOI] [PubMed] [Google Scholar]
- 13.El-Nawawy AA, Abdelmohsen AM, Hassouna HM. Role of echocardiography in reducing shock reversal time in pediatric septic shock: a randomized controlled trial. J Pediatr (Rio J) 2018;94:31-9. 10.1016/j.jped.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 14.Kanji HD, McCallum J, Sirounis D, et al. Limited echocardiography-guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care 2014;29:700-5. 10.1016/j.jcrc.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 15.Ballo P, Bandini F, Capecchi I, et al. Application of 2011 American College of Cardiology Foundation/American Society of Echocardiography appropriateness use criteria in hospitalized patients referred for transthoracic echocardiography in a community setting. J Am Soc Echocardiogr 2012;25:589-98. 10.1016/j.echo.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 16.Matulevicius SA, Rohatgi A, Das SR, et al. Appropriate use and clinical impact of transthoracic echocardiography. JAMA Intern Med 2013;173:1600-7. 10.1001/jamainternmed.2013.8972 [DOI] [PubMed] [Google Scholar]
- 17.Vignon P, Begot E, Mari A, et al. Hemodynamic Assessment of Patients With Septic Shock Using Transpulmonary Thermodilution and Critical Care Echocardiography: A Comparative Study. Chest 2018;153:55-64. 10.1016/j.chest.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 18.Gu WJ, Wang F, Bakker J, et al. The effect of goal-directed therapy on mortality in patients with sepsis - earlier is better: a meta-analysis of randomized controlled trials. Crit Care 2014;18:570. 10.1186/s13054-014-0570-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Géri G, Vignon P, Aubry A, et al. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med 2019;45:657-67. 10.1007/s00134-019-05596-z [DOI] [PubMed] [Google Scholar]
- 20.Gordon AC, Perkins GD, Singer M, et al. Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis. N Engl J Med 2016;375:1638-48. 10.1056/NEJMoa1609409 [DOI] [PubMed] [Google Scholar]
- 21.Lafon T, Appert A, Hadj M, et al. Comparative Early Haemodynamic Profiles in Patients Presenting to the Emergency Department With Septic and Non-Septic Acute Circulatory Failure Using Focused Echocardiography. Shock 2020;53:695-700. 10.1097/SHK.0000000000001449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vignon P. Continuous cardiac output assessment or serial echocardiography during septic shock resuscitation? Ann Transl Med 2020;8:797. 10.21037/atm.2020.04.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent JL, Rhodes A, Perel A, et al. Clinical review: Update on hemodynamic monitoring--a consensus of 16. Crit Care 2011;15:229. 10.1186/cc10291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vignon P. PRO: physician-performed ultrasound: the time has come for routine use in acute care medicine. Anesth Analg 2012;115:999-1003. 10.1213/ANE.0b013e31826bfa7d [DOI] [PubMed] [Google Scholar]
- 25.Atkinson PR, Milne J, Diegelmann L, et al. Does Point-of-Care Ultrasonography Improve Clinical Outcomes in Emergency Department Patients With Undifferentiated Hypotension? An International Randomized Controlled Trial From the SHoC-ED Investigators. Ann Emerg Med 2018;72:478-89. 10.1016/j.annemergmed.2018.04.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as