Abstract
Background
This study aimed to investigate the pathological characteristics of BK polyomavirus (BKPyV)-associated nephropathy (BKPyVAN) with glomerular involvement in kidney transplant recipients.
Methods
Forty-four patients with glomerular BKPyV infection were retrospectively included for analysis. Immunohistochemical (IHC) staining was performed on paraffin sections using monoclonal mouse anti-SV40 large T antigen antibody.
Results
In BKPyV-infected glomeruli, the glomerular parietal epithelial cells (GPECs) were swollen, hyperchromatic, and enlarged, with an increased nuclear to cytoplasm (N/C) ratio and smudgy basophilic intra-nuclear viral inclusions. IHC staining revealed the distribution of BKPyV involvement in GPECs, podocytes, and shedding cells within Bowman’s space. Notably, BKPyV affected GPEC proliferation and caused crescent formation (7 biopsies, 15.9%). Three biopsies exhibited fibrous crescents and the absence of viral inclusions. The other 4 biopsies exhibited cellular and fibro-cellular crescents, with viral cytopathic changes and positive IHC staining in the proliferative GPECs. Electron microscopy showed viral particles in both GPECs and podocytes. BKPyV-infected GPECs were degenerative, with mitochondrial swelling, endoplasmic reticulum expansion, and multi-layered membranous structure formation. Twelve (27.3%) patients received repeat biopsies within 1.6 to 39.5 months (median: 13.5 months), but none revealed persistent glomerular BKPyV infection.
Conclusions
Distinct glomerular changes in BKPyVAN biopsies should raise the possibility of glomerular involvement.
Keywords: BK polyomavirus (BKPyV), kidney transplantation, glomerular infection, pathological characteristics
Introduction
BK polyomavirus (BKPyV)-associated nephropathy (BKPyVAN) is a known cause of renal allograft dysfunction and failure (1). Renal biopsy is considered the gold-standard diagnostic test, showing distinct intra-nuclear viral inclusions and SV40 large T antigen staining (2). In most biopsies, BKPyV is detected within the tubular epithelium, and occasionally in Bowman’s capsular parietal epithelium. BKPyV infection often begins in the collecting tubules and distal convoluted tubules in the medulla, and gradually spreads to the tubules in the cortical area. In mild or moderate cases, BKPyV infection is confined to the tubules (3,4). In advanced stages, BKPyV can infect the glomeruli; this is infrequent but can cause irreversible injury to the kidney. The presence of viral cytopathic changes in the glomeruli has also been associated with a poor prognosis (5). Similarly, we have found BKPyV infection in glomerular parietal epithelial cells (GPECs) in some kidney transplant (KT) recipients, and these patients had rapid deterioration of renal function and even graft loss (6). BKPyVAN with glomerular involvement exhibits specific pathological features. The glomerular changes caused by BKPyV infection are particularly distinctive but have not been described in detail.
Thus, the purpose of this study was to describe in detail the pathological characteristics of BKPyVAN with glomerular involvement. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1879).
Methods
Clinical data
With the approval from the local Institutional Review Board, we retrospectively reviewed the renal transplant biopsies performed at our center from 1/1/2008 to 9/30/2018. During this period, 189 renal transplant recipients, who received 288 biopsies, were diagnosed with BKPyVAN and GPEC involvement was observed in 44 patients. The pathological findings of patients with glomerular involvement were retrospectively analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee and the Research Board of our institution [approval No. (2009) 28]. Because of the retrospective nature, the requirement of informed consent was waived.
Pathological methods
Hematoxylin-eosin, periodic acid Schiff, Masson’s trichrome, and methenamine silver staining were performed for light microscopy evaluation. Immunofluorescence (IF) studies were performed with antibodies against IgA, IgM, IgG, C3, C1q, fibrinogen, and C4d. Nuclear expression of SV40 large T antigen was confirmed by immunohistochemical (IHC) staining with mouse anti-SV40 large T antigen monoclonal antibody (Oncogene Research Products, Cambridge, MA, USA, catalogue number DP02, clone PAb 416) as previously described (7).
Electron microscopy
A CM10 electron microscope (JEM-1400, Japanese) was used to detect viral particles, especially in tubular epithelial cells and glomerular innate cells. Infected cells were defined as those with viral particles with a diameter between 30 and 45 nm detected in the cytoplasm or nucleus.
Diagnosis of BKPyVAN
The diagnosis of polyomavirus-associated nephropathy (PyVAN) was established by the presence of interstitial inflammation, tubular atrophy, interstitial fibrosis, and the existence of viral cytopathic changes in the tubular epithelial cells, confirmed by IHC nuclear positivity with anti-SV40 large T antigen monoclonal antibody, as previously described (8). The histological features of PyVAN were classified using the American Society of Transplantation (AST) schema, and PyVAN was classified as stage A, B, and C based on the guidelines published by Hirsch et al. (7). Histological viral load was assessed semi-quantitatively as the percentage of tubules positive for polyomavirus using a 4-tier system (<10%, 10–25%, 25–50%, and >50%) (9). The diagnosis of BKPyVAN was confirmed on the basis of positive BK viruria and/or BK viremia, in addition to positive IHC staining for SV40 large T antigen in renal tissue. Particular attention was paid to the presence of anti-SV40 large T antigen staining in Bowman’s capsular epithelium. GPEC+ PyVAN was defined as at least 1 glomerular intrinsic cell positive for anti-SV40 large T antigen. Glomerulitis is characterized by complete or partial occlusion of ≥1 glomerular capillary with leukocyte infiltration and endothelial cell enlargement (10). All slides were reviewed by two independent pathologists, and histological scores were evaluated using the Banff criteria (10,11).
Statistical analysis
Continuous data were expressed as mean ± standard deviation (SD) and compared by Student’s t-test. If normality was not assumed, continuous data were expressed as median (range), and the Mann-Whitney U test was used for comparisons between groups. Categorical data were indicated by number and percentage (%) and compared by Pearson’s chi-square test or Fisher’s exact test between groups. All statistical tests were two-tailed, and a value of P<0.05 was considered to indicate statistical significance. All analyses were performed by using IBM SPSS version 20 software (SPSS Statistics V20, IBM Corporation, Somers, New York, USA).
Results
Baseline information
The collected biopsies came from 27 women and 17 men, ranging in age from 21 to 62 years (median: 39.2 years). The native renal diseases included IgA nephropathy (n=6), hypertension (n=1), systemic lupus erythematosus (SLE) nephritis (n=1), and chronic glomerulonephritis with an unknown cause (n=36). The immunosuppressive maintenance regimen included tacrolimus, mycophenolic acid, and prednisone. The reasons for biopsies included serum creatinine elevating ≥30% than baseline value combined with BKPyV infection (40/44, 90.9%), plasma BKPyV load ≥1,000 copies/ml (3/44, 6.8%), and serum creatinine elevating ≥30% than baseline value combined with proteinuria (1/44, 2.3%). At diagnosis of BKPyVAN, the urine BK viral load varied from 2.7×106 to 1.0×1011 copies/mL (median: 1.8×109 copies/mL), and plasma BK viral load varied from 0 to 2.1×107 copies/mL (median: 1.8×104 copies/mL). The mean serum creatinine level was 266.6±138.1 µmol/L. Urine test results were presented in Table S1. During a follow-up period of median 14.4 months (interquartile range: 17.6–42.3 months), 20 (45.5%) patients lost their graft function.
Table S1. Urine test results in 44 patients with glomerular BKPyV involvement.
| Urine-related indicators | Median | Upper quartile | Lower quartile |
|---|---|---|---|
| Urine volume (mL/24 h) | 2,700 | 2,000 | 3,000 |
| Urine specific gravity | 1.008 | 1.006 | 1.009 |
| Urinary red blood cell (/μL) | 3.0 | 1.0 | 7.9 |
| Urine protein quantitation (g/24 h) | 0.316 | 0.227 | 0.500 |
| Urine microalbumin (mg/L) | 24.10 | 9.45 | 68.78 |
| Urine albumin-to-creatinine ratio | 53.0 | 25.5 | 134.5 |
BKPyV, BK polyomavirus.
Light microscopy findings
The basic pathological features of the 44 patients with glomerular BKPyV involvement are shown in Table 1. Typical cytopathic evidence of BKPyV infection, such as intra-nuclear viral inclusions and ground-glass like nuclei were observed in the parietal epithelium of Bowman’s capsule, proximal tubular epithelium, and distal tubular epithelium. The glomerular sclerosis ratio ranged from zero to 12.8. The average number of non-sclerotic glomeruli was 11.0±10.5. Mild glomerulitis (Banff g1) was observed in 43.2% of the biopsies (Figure S1). Mononuclear inflammatory cells were mainly found in the glomerular capillary wall, and occasionally among the proliferating GPECs. Mild mesangial hyperplasia of glomeruli was also observed. Neither endothelial proliferation nor necrosis of glomerular capillary loops was identified. In some cases, glomerular ischemia and shrinkage, capsular thickening, and peri-glomerular fibrosis were observed. No viral inclusions were found in glomerular capillary endothelial cells. All biopsies exhibited mild to severe tubulitis (Banff t1 to t3) in non-atrophic intact tubules, with focal or diffuse interstitial inflammation (Banff i1 to i3; mainly lymphocytes, eosinophils, and plasma cells) (Table 2). Mild to severe interstitial fibrosis and tubular atrophy was also noted in all biopsies (Table 2).
Table 1. Proportion of various histopathologic lesion in 44 BKPyVAN patients with glomerular involvement.
| Pathological features | N (%) |
|---|---|
| Glomerulitis | |
| g0 | 25 (56.8) |
| g1 | 19 (43.2) |
| Chronic transplant glomerulopathy | |
| cg 0 | 42 (95.5) |
| cg 1 | 1 (2.3) |
| cg 2 | 1 (2.3) |
| Crescents | 7 (15.9) |
| Peri-glomerular fibrosis | 29 (65.9) |
| Extent of SV40 large T antigen | |
| 1+ | 6 (13.6) |
| 2+ | 12 (27.3) |
| 3+ | 21 (47.7) |
| 4+ | 5 (11.4) |
| Tubulitis | |
| t1 | 25 (56.8) |
| t2 | 13 (29.5) |
| t3 | 6 (13.6) |
| Interstitial inflammation | |
| i0 | 9 (20.5) |
| i1 | 18 (40.9) |
| i2 | 10 (22.7) |
| i3 | 7 (15.9) |
| Tubular atrophy | |
| ct1 | 11 (25.0) |
| ct2 | 27 (61.4) |
| ct3 | 6 (13.6) |
| Interstitial fibrosis | |
| ci1 | 11 (25.0) |
| ci2 | 23 (52.3) |
| ci3 | 10 (22.7) |
| Peri-tubular capillaritis | |
| pct0 | 25 (56.8) |
| ptc1 | 17 (38.6) |
| ptc2 | 2 (4.5) |
| Endarteritis | 0 |
| BKPyVAN stage | |
| Stage A | 0 |
| Stage B1 | 8 (18.2) |
| Stage B2 | 11 (25.0) |
| Stage B3 | 20 (45.5) |
| Stage C | 5 (11.4) |
| CNI nephrotoxicity | 2 (4.5) |
| FSGS | 2 (4.5) |
| PDG | 1/44 (2.3) |
BKPyVAN, BK polyomavirus-associated nephropathy; Ptc, peri-tubular capillaritis; CNI, calcineurin inhibitor; FSGS, focal segmental glomerular sclerosis; PDG, pediatric donor glomerulopathy.
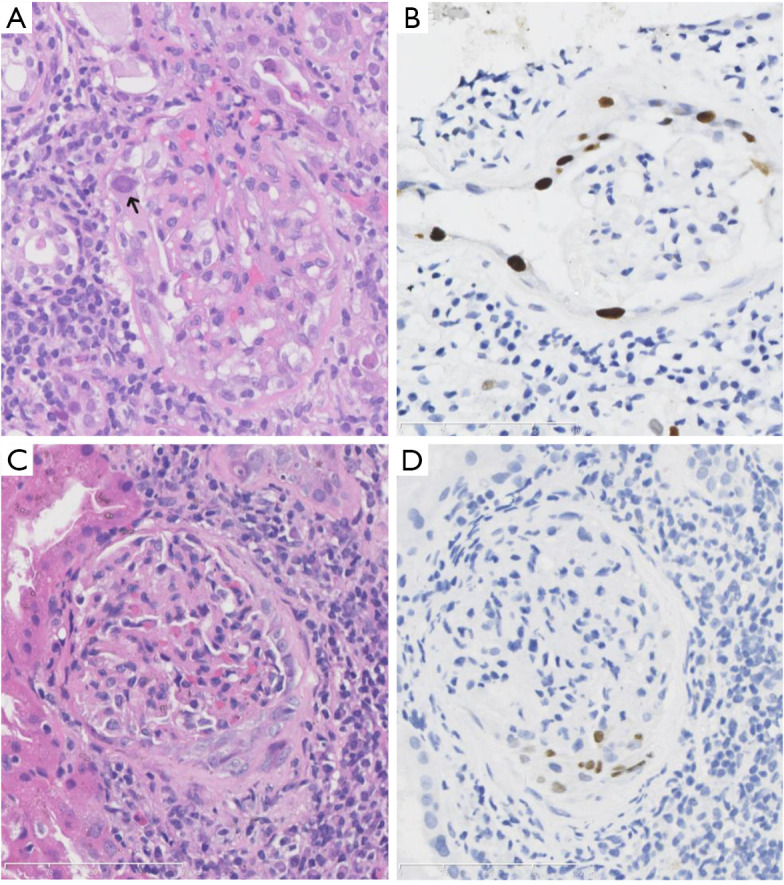
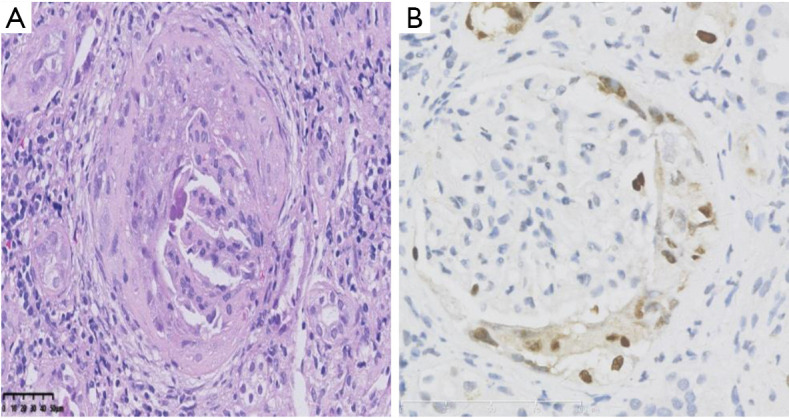
Figure S1.
Glomerulus involved by BK polyomavirus presenting with glomerulitis. In this glomerulus, a cellular crescent was formed, composed of the proliferated parietal epithelial cells with nuclear enlargement and intra-nucleic inclusion. Meanwhile, segmental glomerulitis can be identified with mononuclear cell infiltrates and endothelium swelling (arrow) (hematoxylin-eosin, ×400). Immunohistochemistry staining revealed SV40 large T antigen expression in the proliferated parietal epithelium (immunohistochemistry, ×400).
Table 2. The scores of tubular interstitial lesion in 44 patients with glomerular BKPyV involvement.
| Pathological indicators | Banff grading scores |
|---|---|
| Extent of SV40 large T antigen | 2.6±0.9 |
| Tubulitis | 1.5±0.8 |
| Interstitial inflammation | 1.3±1.0 |
| Tubular atrophy | 1.9±0.7 |
| Interstitial fibrosis | 2.0±0.7 |
BKPyV, BK polyomavirus.
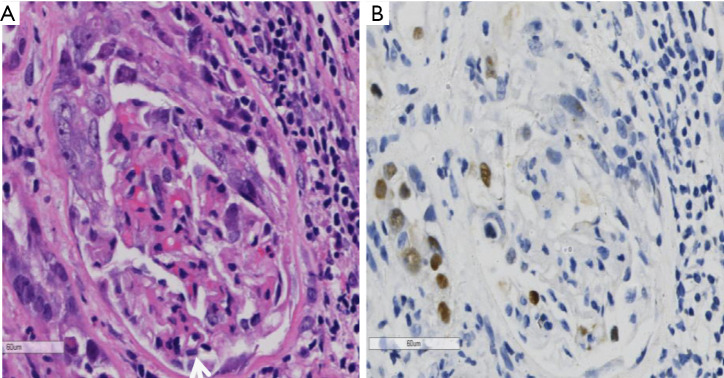
In BKPyV-infected glomeruli, the GPECs were swollen, hyperchromatic, and enlarged, with an increased nuclear to cytoplasm (N/C) ratio, and showed typical BKPyV infection changes such as ground-glass nuclei and smudgy basophilic intra-nuclear viral inclusions (Figure 1A). The BKPyV-infected GPECs were continuous with the proximal tubular epithelium. Scattered cell debris was observed in the glomerular space. IHC staining for SV40 large T antigen showed mild to moderate positivity in tubular epithelial cell nuclei (Table 1), the parietal epithelium of Bowman’s capsule, and the contiguous proximal tubular epithelium (Figure 1B). In two biopsy specimens, viral inclusions were aggregated within the GPECs and the podocytes (Figure 1C), and SV40 large T antigen staining was positive in shedding cells in Bowman’s space and podocytes (Figure 1D). There was no positive IHC staining for cytomegalovirus.
Figure 1.
BK polyomavirus (BKPyV) infection in glomeruli. (A) The BKPyV-infected glomerular parietal epithelial cells presented with typical cytological changes in the peripheral tubular epithelium, with enlarged and ground-glass like nuclei and intra-nuclear inclusions (arrow) [hematoxylin-eosin (HE), ×400]. (B) Immunohistochemistry (IHC) staining showed SV40 large T antigen positivity in the BKPyV-infected glomerular parietal epithelial cells, and the contiguous proximal tubular epithelium (B, IHC, ×400). (C,D) BKPyV infection in both the visceral and parietal epithelium of Bowman’s capsule, with enlarged and hyperchromatic nuclei (C, HE, ×400) and SV40 large T antigen expression (D, IHC, ×400).
Notably, BKPyV could affect GPEC proliferation and cause crescent formation (7 biopsy specimens, 15.9%). Three biopsies specimens exhibited only fibrous crescents and the absence of viral inclusions. Four specimens had cellular and fibro-cellular crescents, with evident viral cytopathic changes (Figure 2A), and positive SV40 large T staining in the proliferative GPECs (Figure 2B). In particular, one patient had crescent formation in 11 of 26 glomeruli, including 4 cellular, 2 fibro-cellular, and 5 fibrous crescents. In BKPyV infections, the cellular components exhibited smudgy basophilic inclusions. The involved glomeruli showed no disruption of the capillary basement membranes.
Figure 2.
Crescent formation in BK polyomavirus-infected glomeruli. The proliferated glomerular parietal epithelial cells exhibited enlarged and ground-glass like nuclei (A, hematoxylin-eosin, ×400) and SV40 large T antigen expression (B, immunohistochemistry, ×400).
IF staining
IF staining results are shown in Table 3. Sparse granular deposition of IgA, IgG, IgM, C3, C1q, and fibrinogen was occasionally observed in the mesangium but did not exhibit a particular pattern in glomeruli with or without BKPyV infection. In 18 (40.9%) of the biopsy specimens, sparse C4d staining was found along glomerular or tubule basement membranes. No C4d deposition was found along peri-tubular capillaries. The frequency of C4d in tubular basement membranes and glomerular basement membranes (GBMs) was similar between viremia (9/14) and non-viremia cases (15/30) (P=0.375). No immunoglobulins or complements were found in Bowman’s capsule.
Table 3. Results of immunofluorescence staining from 44 patients with glomerular BKPyV involvement.
| IF staining findings | N (%) |
|---|---|
| Glomerular mesangial area and capillary loops | |
| IgA | 3 (6.8) |
| IgM | 11 (25.0) |
| IgG | 2 (4.5) |
| C3 | 2 (4.5) |
| C1q | 6 (13.6) |
| Fibrinogen | 3 (6.8) |
| C4d deposition | |
| Along GBM | 6 (13.6) |
| Along TBM | 18 (40.9) |
| Along ptc | 0 |
BKPyV, BK polyomavirus; IF, immunofluorescence; GBM, glomerular basement membranes; TBM, tubular basement membranes; ptc, peri-tubular capillaries.
Ultrastructural changes
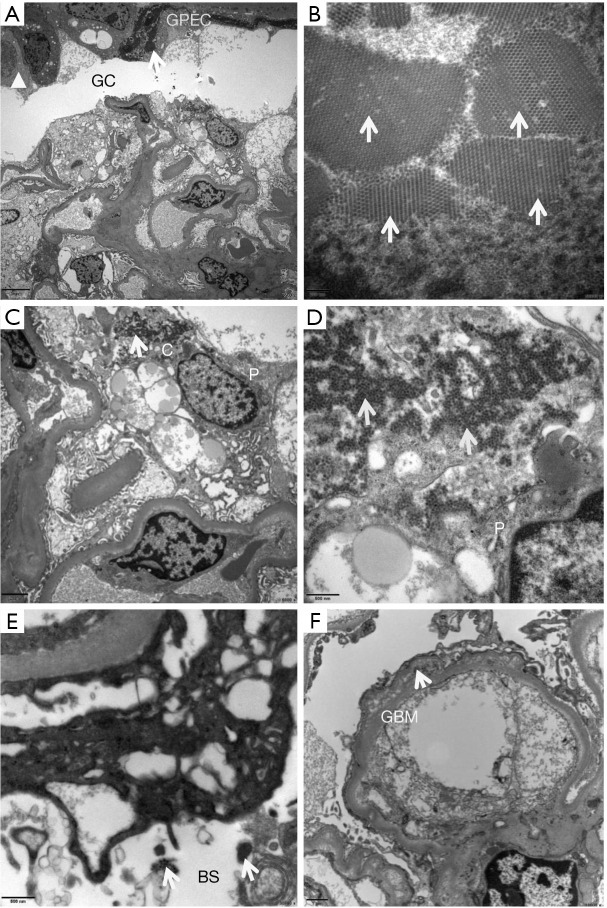
Each ultrathin section contained at least 1 non-sclerotic, non-ischemic glomerulus. Five (11.4%) biopsy specimens had viral particles within the glomeruli. The capsular walls of the BKPyV-infected glomeruli showed changes similar to those of the basement membranes of BKPyV-infected tubules. The wall of the glomerular capsule was thickened and layered, with high electron-dense interposition, composed of uniform dense structures (Figure 3A) or membranous cell debris. Most BKPyV-infected parietal epithelial cells were degenerative, with mitochondrial swelling, endoplasmic reticulum expansion, and multi-layered membranous structure formation. The nuclei were enlarged and fusiform with intact nuclear membranes. Large numbers of virus particles were found in the nucleus (Figure 3B) and cytomembrane of the partial epithelial cells, podocytes, and tubular epithelial cells. The viral particles were identified as tightly packed hexagonal arrays of naked, round, electron-dense structures that measure between 30 and 45 nm in diameter. The BKPyV-infected visceral epithelial cells (podocytes) showed scattered virus particles in the cytoplasm (Figure 3C), and on the surface of the foot processes (Figure 3D). Typical viral particles were occasionally identified in Bowman’s space (Figure 3E) and along the GBM (Figure 3F). No viral particles were found in the mesangial area, glomerular capillary lumen, or endothelial cells. There was no GBM breakage, foot process fusion, or electron-dense deposition in the mesangial or subendothelial areas.
Figure 3.
Ultrastructural changes of BK polyomavirus (BKPyV)-infected glomeruli. The involved glomerular capsule (GC) was thickened and layered (triangle), within which uniform dense structures or membranous cell debris (arrow) was found [A, electron microscopy (EM), ×2,500]. The nuclei of glomerular parietal epithelial cells (GPEC) were enlarged and fusiform, with a large number of virus particles (arrows) (B, EM, ×40,000). Podocytes (P) were occasionally infected by BKPyV. The uniform viral particles (arrows) were found inside the cytoplasm (C, EM, ×6,000), the foot processes (FP) (D, EM, ×20,000), Bowman’s space (BS) (E, EM, ×20,000), and the glomerular basement membrane (GMB) (F, EM, ×10,000).
Repeat biopsies
Twelve (27.3%) patients received repeat biopsies within 1.6 to 39.5 months (median: 13.5 months) after the diagnosis of BKPyVAN. None of the repeat biopsies revealed persistent glomerular BKPyV involvement. SV40 large T antigen staining in the tubular epithelial cells became negative in 4 patients, and the degree of staining decreased remarkably in the other 8 patients. Two patients with cellular crescents received repeat biopsies, and the cellular crescents were found to be replaced by fibrous crescents, suggestive of late changes of previous epithelial proliferation. Compared with the initial biopsies, the repeat biopsies revealed decreased tubulitis and had lower interstitial inflammation scores, but exhibited increased tubular atrophy and interstitial fibrosis scores (Table 4). No repeat biopsy showed pathological signs of rejection.
Table 4. Comparison of pathological damage between initial biopsies and repeat biopsies.
| Pathological lesions | Initial biopsy (N=12) | Repeat biopsy (N=12) | P |
|---|---|---|---|
| Glomerular involvement | 12/12 | 0/12 | <0.001 |
| Glomerulitis | 0.5±0.3 | 0.4±0.1 | 0.572 |
| Chronic allograft injury | 0 | 0.2±0.1 | 0.328 |
| Peri-glomerular fibrosis | 6/12 | 8/12 | 0.408 |
| Cellular crescents | 2/12 | 0/12 | 0.460 |
| Fibrous crescents | 0/12 | 2/12 | 0.460 |
| Extent of SV40 large T antigen | 2.5±0.9 | 1.1±0.9 | 0.001 |
| Tubulitis | 1.3±0.5 | 1.1±0.9 | 0.408 |
| Interstitial inflammation | 1.3±1.2 | 0.6±0.9 | 0.103 |
| Tubular atrophy | 1.9±0.7 | 2.5±0.8 | 0.010 |
| Interstitial fibrosis | 2.0±0.7 | 2.5±0.8 | 0.027 |
Discussion
In a review of 124 biopsies, Celik et al. reported that 29% of biopsies had glomerular BKPyV infection (4). Similarly, in our study, glomerular BKPyV infection was identified in 24.4% (44/180) of the biopsy specimens, suggesting that glomerular BKPyV involvement is not uncommon. In general, medullary collecting ducts and distal tubules are involved at the onset of BKPyVAN (7). Occasionally, virus-associated cytopathic features can also be noted in the GPECs, especially in severely infected cases and at later stages (12). This pathological phenomenon may suggest a worse prognosis.
Crescentic glomerulonephritis is uncommon in renal transplant recipients. Most cases are caused by recurrent disease, and less frequently, by de novo disease (13). Preexisting crescents from the donor kidney also can be identified in the transplanted kidney in early post-transplantation biopsy specimens (14). Rarely, pathogen infection-related crescents have been reported in cases of streptococcus (15), cytomegalovirus (16), and Epstein-Barr virus infection (17). In several case reports (18), the presence of glomerular crescents harboring BKPyV-infected cells has been described in renal transplant recipients. It is known that crescents are formed after breakage of capillary loops and fibrin leakage into Bowman’s space, leading to parietal epithelial cell proliferation. In the present study, there was no evidence of capillary loop breakage or fibrin leakage into Bowman’s space. In addition, immune deposits were absent in glomeruli under both IF microscopy and electron microscopy. We had assumed that crescent formation might be due to BKPyV infection or recurrence of the primary kidney disease. Laboratory examination and repeat biopsies ruled out the latter possibility. Therefore, it is most likely that crescents represent a florid proliferation of GPECs induced by direct infection of BKPyV through contiguous spread from the adjacent proximal tubule. Positive SV40 large T antigen staining within glomerular crescents and adjacent proximal tubules support a hypothesis that BKPyV-associated tubule-interstitial nephritis and crescent formation are causally related (13). Therefore, we suggest that BKPyV infection should be included in the differential diagnosis of crescentic glomerulonephritis in KT recipients to avoid unnecessary modulation of immunosuppressants.
The fact that 19 biopsy specimens with Banff g1 glomerulitis, of which 11 had concurrent peri-tubular capillaritis calls for comment. The coexistence of glomerulitis and peri-tubular capillaritis may suggest antibody-mediated rejection (ABMR) (10). However, since C4d deposition along the peri-tubular capillaries was absent, and donor-specific antibody was negative, a diagnosis of ABMR is unlikely. Glomerulitis has been reported in other viral infections, such as cytomegalovirus (16), hepatitis virus (19), and B19 virus (20). In our long-term observations, we found that some BKPyVAN cases, with or without glomerular involvement, had mild lymphocyte infiltration in glomerular and peri-tubular capillaries. Mild glomerulitis in BKPyV-infected glomeruli in this study might be associated with viral replication in the glomeruli, and the immune response against BKPyV. However, this supposition requires further investigation.
The identification of intra-nuclear inclusions by light microscopy and positive IHC staining for SV40 large T antigen establishes a diagnosis of BKPyVAN. Electron microscopy can severe as a supplement to diagnosis via direct visualization of viral particles within tubule epithelium and glomeruli. Celik et al. examined the glomerular ultrastructure in 16 biopsy specimens from renal transplant recipients with BKPyVAN, but no viral particles were detected (4). In our study, viral particles within the glomeruli were found in only 11.4% of all BKPyVAN biopsy specimens. These data indicate that finding evidence of BKPyV in glomeruli by electron microscopy is challenging. Interestingly, in one biopsy specimen, we found viral particles in GPECs and podocytes. The process of BKPyV infection in parietal epithelial cells and podocytes is similar to the process of BKPyV infection in tubular cells described in detail by Drachenberg et al. (21). In a case report, BKPyV was identified within the GBM presenting as subepithelial depositions, suggesting a mechanism for antigen clearance via deposition formation and cytoplasmic processing by podocytes (22). This phenomenon was also observed in our study. In one of our cases, electron microscopy revealed that virus particles were enveloped by protein casts within the glomerular space. However, no virus particles were found in the mesangial area, glomerular capillary lumen, or endothelial cells. Therefore, we can speculate that virus particles shed from BKPyV-infected tubular epithelial cells formed a complex with Tamm-Horsfall protein (THP) in the tubule, which was occasionally refluxed into the glomerular space (23), eventually resulting in glomerular involvement. This finding partially supports the speculation that the distribution of BKPyV within the glomerulus is mainly derived from the adjacent tubular epithelium.
It is believed that BKPyV is initially latent in the ureteral transitional epithelium (24). Under powerful immunosuppression, the virus activates and spreads in an ascending manner via from collecting ducts to distal tubules and proximal tubules (7). If the infection is not effectively controlled, the virus will eventually infect the glomeruli, since the glomerular capsule is contiguous with the proximal tubules. Bedside, BKPyV can easily infect Bowman’s capsular epithelium because it has the same origin as tubular epithelium, i.e., the nephrogenic vesicle.
In conclusion, BKPyV can infect the glomeruli and causes a series of glomerular pathological changes. However, the correlation between this unique pathological phenomenon and clinical prognosis needs further study.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors are grateful to the nursing staff of our center for collaboration and dedication to the patients.
Funding: This work was supported by grants from the National Natural Science Foundation of China (81770749); the Natural Science Foundation of Guangdong Province (2017A030313710); the China Organ Transplantation Development Foundation (2019JYJH07); and the Fundamental Research Funds for the Central Universities (17ykpy29). We would like to give special thanks to the Guangdong Provincial Key Laboratory of Organ Donation and Transplant Immunology (2013A061401007, 2017B030314018), and the Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002) for providing experimental platforms.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee and the Research Board of our institution [Approval No. (2009) 28]. Because of the retrospective nature, the requirement of informed consent was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1879
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1879
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1879). The authors have no conflicts of interest to declare.
References
- 1.Drachenberg CB, Papadimitriou JC, Chaudhry MR, et al. Histological Evolution of BK Virus-Associated Nephropathy: Importance of Integrating Clinical and Pathological Findings. Am J Transplant 2017;17:2078-91. 10.1111/ajt.14314 [DOI] [PubMed] [Google Scholar]
- 2.Nickeleit V, Singh HK, Randhawa P, et al. The Banff Working Group Classification of Definitive Polyomavirus Nephropathy: Morphologic Definitions and Clinical Correlations. J Am Soc Nephrol 2018;29:680-93. 10.1681/ASN.2017050477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lusco MA, Fogo AB, Najafian B, et al. AJKD Atlas of Renal Pathology: Polyomavirus Nephropathy. Am J Kidney Dis 2016;68:e37-8. 10.1053/j.ajkd.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 4.Celik B, Randhawa PS. Glomerular changes in BK virus nephropathy. Hum Pathol 2004;35:367-70. 10.1016/j.humpath.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 5.Hariharan S. BK virus nephritis after renal transplantation. Kidney Int 2006;69:655-62. 10.1038/sj.ki.5000040 [DOI] [PubMed] [Google Scholar]
- 6.Chen XT, Yang SC, Chen WF, et al. Glomerular Parietal Epithelial Cells Infection Is Associated With Poor Graft Outcome in Kidney Transplant Recipients With BK Polyomavirus-Associated Nephropathy. J Infect Dis 2019;219:1879-86. 10.1093/infdis/jiz022 [DOI] [PubMed] [Google Scholar]
- 7.Hirsch HH, Randhawa P, Infectious Diseases AST. Community of Practice. BK polyomavirus in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:179-88. 10.1111/ajt.12110 [DOI] [PubMed] [Google Scholar]
- 8.Huang G, Wu LW, Yang SC, et al. Factors Influencing Graft Outcomes Following Diagnosis of Polyomavirus -Associated Nephropathy after Renal Transplantation. PLoS One 2015;10:e0142460. 10.1371/journal.pone.0142460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang G, Wang CX, Zhang L, et al. Monitoring of polyomavirus BK replication and impact of preemptive immunosuppression reduction in renal-transplant recipients in China: a 5-year single-center analysis. Diagn Microbiol Infect Dis 2015;81:21-6. 10.1016/j.diagmicrobio.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 10.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 2018;18:293-307. 10.1111/ajt.14625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sis B, Mengel M, Haas M, et al. Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 2010;10:464-71. 10.1111/j.1600-6143.2009.02987.x [DOI] [PubMed] [Google Scholar]
- 12.Drachenberg CB, Hirsch HH, Ramos E, et al. Polyomavirus disease in renal transplantation: review of pathological findings and diagnostic methods. Hum Pathol 2005;36:1245-55. 10.1016/j.humpath.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 13.Nair R, Katz DA, Thomas CP. Diffuse glomerular crescents and peritubular immune deposits in a transplant kidney. Am J Kidney Dis 2006;48:174-8. 10.1053/j.ajkd.2006.04.064 [DOI] [PubMed] [Google Scholar]
- 14.Kiser RL, Thomas DB, Andreoni K, et al. Preexisting crescentic glomerulonephritis in the renal allograft. Am J Kidney Dis 2003;42:E20-6. 10.1016/j.ajkd.2003.07.013 [DOI] [PubMed] [Google Scholar]
- 15.Hikita T, Arai K, Inokami T, et al. A case of fulminant acute poststreptococcal glomerulonephritis showing mesangiolysis and crescent formation preceded by erysipelas. Nihon Jinzo Gakkai Shi 2002;44:558-63. [PubMed] [Google Scholar]
- 16.Detwiler RK, Singh HK, Bolin P, Jr, et al. Cytomegalovirus-induced necrotizing and crescentic glomerulonephritis in a renal transplant patient. Am J Kidney Dis 1998;32:820-4. 10.1016/S0272-6386(98)70139-8 [DOI] [PubMed] [Google Scholar]
- 17.Mirsaeidi M, Syed F, Jaffe ES. Antineutrophil Cytoplasmic Autoantibody Associated Systemic Vasculitis Is Associated with Epstein - Barr virus in the Setting of HIV Infection. Infect Dis Clin Pract (Baltim Md) 2013;21:50-3. 10.1097/IPC.0b013e3182601ea1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon HJ, Sun IO, Yeo MK, et al. Severe Crescentic BK Nephropathy: A Case Report. J Korean Soc Transplant 2016;30:35-7. 10.4285/jkstn.2016.30.1.35 [DOI] [Google Scholar]
- 19.Yamabe H, Nakamura N, Nakamura M, et al. Hepatitis C virus-associated glomerulonephritis without hepatitis C virus in the blood. Am J Kidney Dis 2005;46:e65-9. 10.1053/j.ajkd.2005.06.023 [DOI] [PubMed] [Google Scholar]
- 20.Nakazawa T, Tomosugi N, Sakamoto K, et al. Acute glomerulonephritis after human parvovirus B19 infection. Am J Kidney Dis 2000;35:E31. 10.1016/S0272-6386(00)70070-9 [DOI] [PubMed] [Google Scholar]
- 21.Drachenberg CB, Papadimitriou JC, Wali R, et al. BK polyoma virus allograft nephropathy: ultrastructural features from viral cell entry to lysis. Am J Transplant 2003;3:1383-92. 10.1046/j.1600-6135.2003.00237.x [DOI] [PubMed] [Google Scholar]
- 22.Brealey JK. Ultrastructural observations in a case of BK virus nephropathy with viruses in glomerular subepithelial humps. Ultrastruct Pathol 2007;31:1-7. 10.1080/01913120600854418 [DOI] [PubMed] [Google Scholar]
- 23.Kawanishi K, Honda K, Koike J, et al. A Preliminary Study Into the Significance of Intrarenal Reflux in BK Virus Nephropathy After Kidney Transplantation. Transplant Direct 2016;2:e64. 10.1097/TXD.0000000000000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamarche C, Orio J, Collette S, et al. BK Polyomavirus and the Transplanted Kidney: Immunopathology and Therapeutic Approaches. Transplantation 2016;100:2276-87. 10.1097/TP.0000000000001333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as