Abstract
Background
Video-assisted thoracoscopic surgery (VATS) is a commonly performed minimally invasive procedure that has led to lower levels of pain, as well as procedure-related mortality and morbidity. However, VATS requires analgesia that blocks both visceral and somatic nerve fibers for more effective pain control. This randomized controlled trial evaluated the effect of erector spinae plane block (ESPB) in the postoperative analgesia management of patients undergoing VATS.
Methods
We performed a prospective, randomized, single-center study between December 2018 and December 2019. Fifty-four patients were recruited to two equal groups (ESPB and control group). Following exclusion, 46 patients were included in the final analysis. Patients were randomly assigned to receive preoperative ultrasound-guided ESPB with either ropivacaine or saline. The primary outcome was the numeric rating scale (NRS) score, assessed 12 hours postoperatively. Secondary outcomes were the Riker Sedation-Agitation Scale (SAS) score for emergence agitation, postoperative cumulative opioid consumption, length of post-anesthesia care unit (PACU) stay, incidence of postoperative nausea and vomiting (PONV) and dizziness, and ESPB-related adverse events.
Results
The NRS in the ESPB group during the postoperative period immediately after PACU admission was significantly lower than that in the control group (5.96±1.68 and 7.59±1.18, respectively; P<0.001) and remained lower until 6 hours postoperatively (P=0.001 at 1 hour and P=0.005 at 6 hours). At 12 hours postoperatively, NRS scores were not significantly different between groups (P=0.12). The median [interquartile range (IQR)] of the postoperative rescue pethidine consumption in PACU was significantly lower [25 mg (25 mg)] in the ESPB group than that in the control group [50 mg (56.2 mg); P=0.006]. The median (IQR) of PACU residual time was significantly lower [25 min (10 min)] in the ESPB group than that in the control group [30 min (15 min); P=0.034]. The median (IQR) Riker SAS was also lower in the ESPB group [4 (1.0)] than that in the control group [5 (1.25); P<0.001] in PACU.
Conclusions
A single preoperative injection of ESPB with ropivacaine may improve acute postoperative analgesia and emergence agitation in patients undergoing VATS.
Keywords: Video-assisted thoracoscopic surgery (VATS), erector spinae plane block, thoracic epidural blockade, paravertebral block, ultrasound
Introduction
Post-thoracotomy pain syndrome (PTPS) is a serious and common problem after thoracic surgery, which has a significant effect on the quality of life in 25–60% of patients. The International Association for the Study of Pain defined chronic pain after thoracotomy as “pain that recurs or persists along a thoracotomy scar at least 2 months following surgical procedure.” (1). Video-assisted thoracoscopic surgery (VATS) is increasingly being used to manage primary lung cancer and helps reduce postoperative pain (2,3). However, it is a fact that pain following VATS can be severe and long-lasting. According to Takahiro Homma et al., 18.8% of patients who undergo VATS present with persistent pain 2 months after surgery (4). Similar to several other chronic postsurgical pain syndromes, acute postoperative pain is one of the powerful predictors of PTPS; however, its mechanism remains uncertain (5,6). Therefore, it is important to apply multimodal methods of postoperative pain control.
Numerous modalities to alleviate post-thoracic surgery pain have been described in studies, ranging from various medications for patient-controlled analgesia to diverse regional analgesic methods. Thoracic epidural analgesia (TEA) is a classic effective regional blockade to reduce pain following thoracic surgery (7). Thoracic paravertebral block (PVB) with a local anesthetic (LA), which is comparable to an epidural block for pain relief, is widely applied in thoracic surgery (8,9). The erector spinae plane block (ESPB), first described by Forero et al. in 2016 (10), is a new rising technique representative of indirect PVB methods. Several studies have shown that ESPB has strengths regarding safety and ease of use. ESPB targets a plane remote from the pleura and neuraxial structures to inject an LA into the fascial plane deep to the erector spinae muscle (Figure 1). ESPB results in blocking not only the dorsal and ventral rami of the spinal nerve in the paravertebral space via penetration of the intertransverse connection tissues but also the lateral cutaneous branches of the intercostal nerves (10,11).
Figure 1.
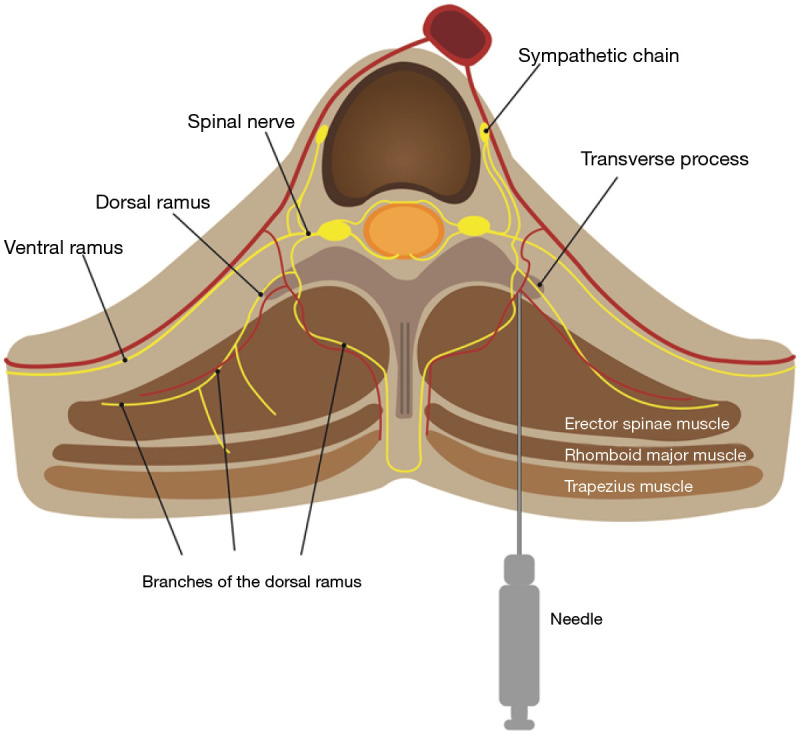
Schematic diagram of erector spinae plane block. A needle was inserted into the interfascial plane between the erector spinae muscle and transverse process of the vertebra.
Numerous clinical studies have reported that ESPB can provide effective analgesia in the thoracoabdominal region, including for breast surgery, cardiothoracic surgery, and laparoscopic cholecystectomy (12,13). There is a lack of research on randomized post-VATS ESPB studies. There are mainly case reports, with only one randomized study currently published (14). We suggest that ESPB may provide analgesia for VATS lobectomy. This study was designed to observe a multiple-angle efficacy in postoperative care. We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-689).
Methods
This prospective randomized trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by our institutional ethics committee (Kangbuk Samsung Hospital Institutional Review Board, Seoul, Republic of Korea; approval number: KBSMC 2018-09-017-004) and was registered at ClinicalTrials.gov (ID: NCT03777592) before the first patient’s participation. The authors obtained written informed consent from the participants. Between December 2018 and December 2019, we enrolled patients with American Society of Anesthesiologists physical status 1–3 and aged 19–85 years old who were scheduled for unilateral lobectomy under complete VATS with three trochar ports for lung cancer. The trochar ports were made at the fifth and sixth intercostal levels. The authors excluded patients with abnormal clotting hemostatic test results, patients on anticoagulant treatment, patients with a history of allergy to LA agents, patients with skin problems at the needle puncture site, pregnant patients, and highly obese patients (body mass index >30 kg/m2).
The participants were randomly allocated to undergo ESPB with either 30 mL of 0.5% ropivacaine (ESPB group) or 0.9% physiological saline (control group) before general anesthesia. Randomization was conducted at a 1:1 ratio using a web-based response system (http://www.randomization.com). Assignments were sealed in serially numbered envelopes. Randomization and blinding procedures were performed by an independent researcher who was not involved in the trial. The patients and physician assessing the outcomes were kept blinded to the grouping process (double-blind study).
In both groups, general anesthesia was achieved using propofol (2–2.5 mg/kg) and remifentanil (1 µg/kg). Tracheal intubation with a double lumen tube was facilitated with rocuronium (0.6–1 mg/kg). Anesthesia was maintained with desflurane (5–6%) or sevoflurane (1–2%) and a remifentanil infusion (0.01–0.1 mg/kg/min) was begun. Intraoperative monitoring, including electrocardiography, heart rate, respiratory rate, peripheral oxygen saturation, invasive arterial pressure, exhaled CO2 (end-tidal capnography), noninvasive blood pressure, and body temperature, was performed.
At the end of surgery, anesthesia was discontinued, and extubation of the patient was completed after reversal of muscle relaxant by pyridostigmine (0.25 mg/kg) and glycopyrrolate (0.012 mg/kg). The patients were then transferred to the post-anesthesia care unit (PACU).
Application of ESPB
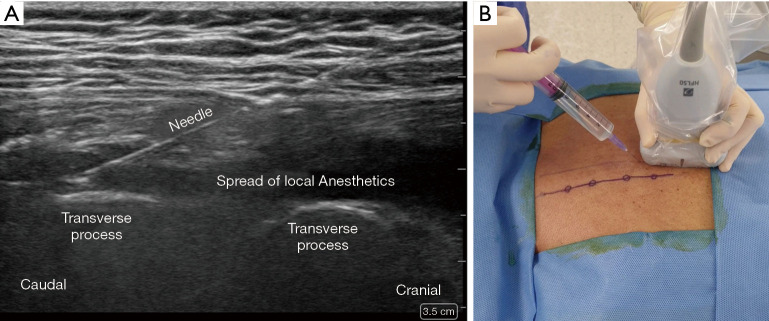
ESPB was performed in the prone position before general anesthesia induction under standardized monitoring. The patient’s back was sterilized and draped in a sterile fashion. After an initial anatomic scan to confirm the thoracic levels, appearance, and depth of structures, the procedural site was identified. A 5–12-MHz linear array ultrasound transducer (Sonosite® X-porte-) was placed in a sterile sheath. US-guided ESPB was administered at the T5 vertebral level. An in-plane paramedian longitudinal block was performed with the probe (approximately 2–3 cm lateral to the midline). After visualizing the trapezius, rhomboid major, and erector spinae muscles, a 60-mm 23-gauge b-bevel needle was inserted into the interfascial plane between the erector spinae muscle and transverse process of the vertebra using an in-plane technique. After the correct location was confirmed by hydrodissection of the interfascial plane with 2 mL of physiological saline solution, 25 mL of 0.5% ropivacaine or saline was injected (Figure 2).
Figure 2.
Sonoanatomy and technique of the erector spinae plane block. (A) The ultrasound transducer is placed longitudinally, 2 to 3 cm lateral to the T5 transverse process, under aseptic conditions. The needle is inserted in a cranial-to-caudal direction in-plane; (B) correct needle tip placement is identified by hypoechoic local anesthetic under the erector spinae muscle, setting it apart from the transverse processes.
Postoperative analgesia management
Postoperative pain management was performed in an identical manner in the two groups according to our institutional protocol. An intravenous patient-controlled analgesia (IVPCA) device (Ambix Anaplus® AP 1020, E-Wha Fresenius Kabi Inc., Gunpo, Republic of Korea) was connected at the PACU and was maintained postoperatively using the following protocol: 2 mL/h (fentanyl 5 µg/mL) basal infusion with 0.5-mL bolus and 15-minute lockout time. Meperidine 25 mg was administered intravenously as a rescue analgesic on demand (when the numeric rating scale (NRS) score was ≥4). The side effects of postoperative opioid consumption, such as a nausea, vomiting, breathing depression, sedation, urinary retention, and itching, were also recorded.
Outcome measurement
The data collected included the NRS score for pain (primary outcome) to assess the quality of effective analgesia upon immediate arrival at the PACU, and 1, 6, and 12 h postoperatively. Secondary outcomes included the Riker SAS score [1= minimal or no response to noxious stimuli; 2= arousal to physical stimuli but non-communicative; 3= difficult to arouse but awakens to verbal stimuli or gentle shaking; 4= calm and follows commands; 5= anxious or physically agitated but calms on verbal instructions; 6= requires restraint and frequent verbal reminders of limits; and 7= attempting to remove tracheal tube or catheters or striking at staff (15)] to assess emergence agitation, postoperative cumulative opioid consumption, length of PACU stay, incidence of PONV and dizziness, and ESPB-related adverse events. A single trained researcher blinded to group assignments assessed all outcomes.
Statistical analysis
According to our preliminary study, the sample size was calculated on the basis of the mean difference in NRS between the ESPB-treated group and control group [ESPB group: mean ± standard deviation (SD) =4.1±1.59, n=10; control group: mean ± SD =5.5±1.35, n=10] collected retrospectively from 20 consecutive cases. We estimated that 27 subjects would be needed per group to provide a type I error of 0.05, power of 90%, and predicted dropout rate of 20% to detect a 1-point difference, which was considered clinically relevant, between the two groups.
SPSS Statistics 24.0 for Windows (IBM Corp, Armonk, NY, USA) was used to process the clinical data and perform statistical analyses. Continuous variables are expressed as mean ± SD or median (interquartile range). Frequency and percentages are used as appropriate for categorical variables. The Kolmogorov-Smirnov test was used to assess the assumption of normality. The chi-squared, Student t-test, or Mann-Whitney test were used to test significance according to the normality and types of variables.
The postoperative pain scores were analyzed using repeated measures analysis of variance (ANOVA) to evaluate the relationship between the NRS pain scores over time and groups. Post hoc testing after repeated measures ANOVA was performed to compare groups at each time point using Bonferroni correction.
Results
Fifty-four patients were equally randomized between the two groups as shown in the Consolidated Standards of Reporting Trials (CONSORT) flow-chart (Figure 3). Seven patients were excluded from the study because the operation technique was changed to emergency exploratory thoracotomy during surgery. One patient underwent wedge resection rather than lobectomy. Forty-six patients were included in the analysis. Demographic data and surgical durations were comparable between the two groups (Table 1).
Figure 3.
CONSORT flow diagram. ESPB, erector spinae plane block.
Table 1. Patients’ characteristics and duration of surgery.
| ESPB group | Control group | P value | |
|---|---|---|---|
| Sex (M/F) | 10/11 | 15/7 | 0.071 |
| Age (year) | 62.8±10.2 | 62.4±10.0 | 0.876 |
| Weight (kg) | 62.8±11.8 | 66.1±9.8 | 0.206 |
| Body mass index (kg/m2) | 23.9±2.4 | 24.2±2.1 | 0.663 |
| Comorbidities (n, %) | |||
| Hypertension | 12 (50.0) | 9 (40.9) | 0.536 |
| Diabetes mellitus | 2 (8.3) | 2 (9.1) | 1.000 |
| Coronary heart disease | 0 (0) | 3 (13.6) | 0.101 |
| Cerebrovascular disease | 2 (8.3) | 3 (13.6) | 0.659 |
| Chronic obstructive pulmonary disease | 2 (8.3) | 1 (4.5) | 1.000 |
| Hepatic disease | 2 (8.3) | 2 (9.1) | 1.000 |
| Resected lobe | 0.594 | ||
| LUL | 6 (25.0) | 6 (27.3) | |
| LLL | 2 (8.3) | 2 (9.1) | |
| RUL | 10 (41.7) | 4 (18.2) | |
| RUL and RML | 1 (4.2) | 1 (4.5) | |
| RML | 2 (8.3) | 6 (13.0) | |
| RLL | 3 (12.5) | 8 (17.4) | |
| Blood loss (mL) | 136.2±71.1 | 113.6±57.0 | 0.291 |
| Mean hospital stay, days | 10.6±4.6 | 0.053 | |
| Duration of surgery (min) | 124.8±34.2 | 125.5±29.9 | 0.945 |
ESPB, erector spinae plane block; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe.
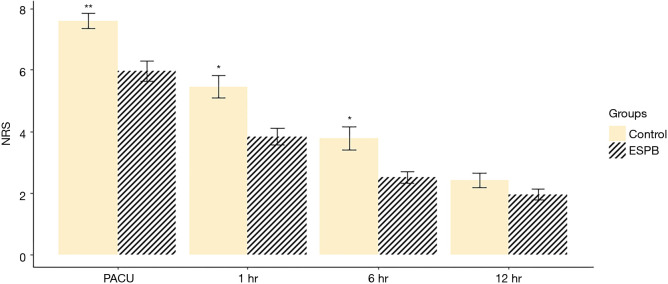
The repeated measures ANOVA of NRS pain scores showed that the NRS pain scores over time were significantly different between the two groups (P=0.030). The NRS scores in the ESPB group in the postoperative period immediately after PACU admission were significantly lower than those in the control group (5.96±1.68 and 7.59±1.18, respectively; P<0.001) and remained lower until 6 hours postoperatively (P=0.001 at 1 hour and P=0.005 at 6 hours). At 12 hours, the NRS scores were not significantly different between the two groups (P=0.12) Though seven patients who had been converted to open thoracotomy during surgery were excluded from the final analysis considering initial design of study to examine the effect of ESPB in patients undergoing VATS- lobectomy, NRS pain scores were analyzed by including them, and the results including them showed similar differences (P=0.002 at PACU, P=0.022 at 1 hour, P=0.020 at 6 hours and P=0.388 at 12 hours) (Figure 4). The median [interquartile range (IQR)] of postoperative rescue pethidine consumption in PACU was significantly lower [25 mg (25 mg)] in the ESPB group than that in the control group [50 mg (56.2 mg); P=0.006]. The median (IQR) of the length of PACU stay was significantly lower [25 min (10 min)] in the ESPB group than that in the control group [30 min (15 min); P=0.034]. The median (IQR) Riker SAS score was lower in the ESPB group [4 (1.0)] than that in the control group [5 (1.25); P<0.001] in PACU (Table 2).
Figure 4.
Comparison of postoperative NRS scores, for ESPB Group and Control Group at various time points of follow up. (A) Data in which the 7 patients with conversion of VATS to open thoracotomy were excluded; (B) all patient data including 7 patients with conversion of VATS to open thoracotomy. **P<0.001, *P<0.05. ESPB, erector spinae plane block; PACU, post-anesthesia care unit; NRS, numeric rating scale; VATS, video-assisted thoracoscopic surgery.
Table 2. Riker SAS score, length of stay, and perioperative analgesic consumption in PACU.
| ESPB group (n=24) | Control group (n=22) | P value | |
|---|---|---|---|
| Riker SAS score | 4±1 | 5±1.25 | <0.001 |
| Length of PACU stay | 25±10 | 30±15 | 0.034 |
| Postoperative pethidine consumption (mg) | 25±25 | 50±56.25 | 0.006 |
SAS, sedation-agitation scale; PACU, post-anesthesia care unit; ESPB, erector spinae plane block.
Postoperative nausea and vomiting occurred in one patient in the ESPB group, while no complications were detected in the control group. There were no complications such as pneumothorax, LA systemic toxicity, or hematoma in either group.
Discussion
Our study has shown that ultrasound-guided unilateral single shot ESPB performed before general anesthesia induction in VATS patients significantly lowered NRS at rest in the first 6 h postoperatively when compared to that in a control group. ESPB helps reduce pain, as well as opioid consumption, in PACU. Reduction of opioid administration in the PACU could also reduce the patients’ residual time in the PACU, as shown in this study. Notably, ESPB could help the incidence of emergence agitation, which is a post-anesthetic condition and indication for physical or chemical restraint in order to avoid serious consequences for the patient, such as physical injury, increased pain, hemorrhage, and removal of catheters. According to Fields et al., a chest tube results in a higher incidence of emergence agitation (16). The chest tube increases the chance of developing emergence agitation, which may cause functional problems concerning the chest tube due to the violent movement of the patient. Therefore, it is important that ESPB lowers the incidence of emergence agitation in thoracic surgery. ESPB lowers pain scores immediately after surgery, reducing opioid administration in the PACU, and thus reducing retention time in the PACU and improving patient safety. In addition, the pain relieving effect of ESPB is maintained for 6 hours; thus, a sufficient analgesic effect can be expected during the most painful time after surgery.
TEA or PVB has been used for thoracic analgesia since many years. ESPB, as an alternative to PVB, is a peri-paravertebral block and relatively safer method in which the transverse process plays the role of an anatomical barrier and avoids needle insertion into the pleura. This may mean reducing the risk of pneumothorax, the most worrying complication of PBV. In addition, it is not technically difficult to locate the target point, the interfascial plane between the erector spinae muscle and transverse process, using ultrasound. Anatomical dissection in cadaveric investigation and magnetic resonance imaging (MRI) in imaging study following ESPB showed spread of the injectant from the epidural and neural foraminal spaces over two to five levels to intercostal spaces over five to nine levels (17,18). In addition to these anatomic and MRI studies, ESPB has effectively controlled somatic and visceral pain in breast, laparoscopic cholecystectomy, and ventral hernia surgery in several studies (12,19-21). Therefore, ESPB analgesia can be effective in treating not only somatic, but also visceral pain originating from lung resection and port entry sites.
Previous studies showed the efficacy in reducing postoperative pain following cardiothoracic surgery. For the first time, Forero et al. in 2016 demonstrated a successful application of ESPB in two cases of thoracic pain after VATS (10). Irem Kaplan et al. reported a case of continuous erector spinae plane catheter for analgesia following thoracotomy in an infant (22). Following cardiac surgery, good results for continuous erector spinae plane catheter insertion were reported in five cases (23). Since the initial publications in 2016, studies that applied ESPB to acute and thoracic pain steadily for the past three years have been conducted; however, these are mainly case studies. There is only one randomized study of ESPB for VATS published in 2019 (14), and there is one randomized study comparing PBV and ESPB for VATS, which showed no difference between the two groups (24). Our study found a conclusion consistent with that of the randomized study comparing ESPB and control groups and found that ESPB is effective in controlling pain after VATS.
The previous RCT study concluded that the time for pain reduction after ESPB was significant for 24 hours. However, our study showed a different duration of analgesia after ESPB. In our study, the significant difference in NRS between the two groups was present for 6 h. Therefore, our study concludes that single shot ESPB alone may not be sufficient to sustain the analgesic effect. Continuous catheterization might be considered for more lasting pain control according to our study.
Our study has several limitations. In this study, follow-up was performed until 12 hours after surgery. If follow-up for 2 months after surgery could be performed, the relationship of acute pain after surgery and chronic pain could be considered. In other words, it could have provided evidence to support the preemptive analgesic mechanisms of ESPB, which is assumed that performing ESPB before the application of noxious stimuli may prevent sensitization of the nervous system and reduce the incidence of PTPS. The measurement of opioid consumption was accurate up to the time in the PACU; however, the consumption in the ward was not due to use of IVPCA and opioids as routine rescue analgesic. Therefore, this may be the cause of the difference between the two groups in the amount of opioid used in PACU, but not in the amount of opioid used in the ward.
Conclusions
Ultrasound-guided ESPB leads to effective analgesia in first 6 h postoperatively in patients undergoing VATS. ESPB was helpful in reducing rescue analgesic opioid consumption and recovery time in PACU. Performing ESPB as routine for pain control after VATS has the disadvantage that the analgesic effect is maintained for a short period of time. However, ESPB can be considered a multimodal postoperative pain analgesic method because it is easy and safe to perform.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work supported by the Medical Research Funds from Kangbuk Samsung Hospital.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This prospective randomized trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Institutional Review Board (IRB) of the Kangbuk Samsung Hospital Institutional Review Board, Seoul, Republic of Korea (KBSMC 2018-09-017-004). An informed consent was obtained from all patients.
Footnotes
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-689
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-689
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-689). The authors have no conflicts of interest to declare.
References
- 1.Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg 2009;36:170-80. 10.1016/j.ejcts.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 2.Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg 1994;107:1079-85; discussion 1085-6. 10.1016/S0022-5223(94)70384-1 [DOI] [PubMed] [Google Scholar]
- 3.Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. 10.1093/ejcts/ezv154 [DOI] [PubMed] [Google Scholar]
- 4.Homma T, Doki Y, Yamamoto Y, et al. Risk factors of neuropathic pain after thoracic surgery. J Thorac Dis 2018;10:2898-907. 10.21037/jtd.2018.05.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blichfeldt-Eckhardt MR, Andersen C, Ording H, et al. From acute to chronic pain after thoracic surgery: the significance of different components of the acute pain response. J Pain Res 2018;11:1541-8. 10.2147/JPR.S161303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoda Y, Kambara N, Sakai T, et al. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain 2001;5:89-96. 10.1053/eujp.2001.0225 [DOI] [PubMed] [Google Scholar]
- 7.Kaplowitz J, Papadakos PJ. Acute pain management for video-assisted thoracoscopic surgery: an update. J Cardiothorac Vasc Anesth 2012;26:312-21. 10.1053/j.jvca.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 8.Abd-Elshafy SK, Abdallal F, Kamel EZ, et al. Paravertebral Dexmedetomidine in Video-Assisted Thoracic Surgeries for Acute and Chronic Pain Prevention. Pain Physician 2019;22:271-80. [PubMed] [Google Scholar]
- 9.Yeung JH, Gates S, Naidu BV, et al. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev 2016;2:CD009121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forero M, Adhikary SD, Lopez H, et al. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med 2016;41:621-7. 10.1097/AAP.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 11.Chin KJ. Thoracic wall blocks: From paravertebral to retrolaminar to serratus to erector spinae and back again - A review of evidence. Best Pract Res Clin Anaesthesiol 2019;33:67-77. 10.1016/j.bpa.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 12.Gurkan Y, Aksu C, Kus A, et al. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: A randomized controlled study. J Clin Anesth 2018;50:65-8. 10.1016/j.jclinane.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 13.Tulgar S, Kapakli MS, Senturk O, et al. Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: A prospective, randomized, controlled clinical trial. J Clin Anesth 2018;49:101-6. 10.1016/j.jclinane.2018.06.019 [DOI] [PubMed] [Google Scholar]
- 14.Ciftci B, Ekinci M, Celik EC, et al. Efficacy of an Ultrasound-Guided Erector Spinae Plane Block for Postoperative Analgesia Management After Video-Assisted Thoracic Surgery: A Prospective Randomized Study. J Cardiothorac Vasc Anesth 2020;34:444-9. 10.1053/j.jvca.2019.04.026 [DOI] [PubMed] [Google Scholar]
- 15.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med 1999;27:1325-9. 10.1097/00003246-199907000-00022 [DOI] [PubMed] [Google Scholar]
- 16.Fields A, Huang J, Schroeder D, et al. Agitation in adults in the post-anaesthesia care unit after general anaesthesia. Br J Anaesth 2018;121:1052-8. 10.1016/j.bja.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 17.Adhikary SD, Bernard S, Lopez H, et al. Erector Spinae Plane Block Versus Retrolaminar Block: A Magnetic Resonance Imaging and Anatomical Study. Reg Anesth Pain Med 2018;43:756-62. [DOI] [PubMed] [Google Scholar]
- 18.Yang HM, Choi YJ, Kwon HJ, et al. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia 2018;73:1244-50. 10.1111/anae.14408 [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Li H, He Q, et al. Efficacy of ultrasound-guided erector spinae plane block on postoperative quality of recovery and analgesia after modified radical mastectomy: randomized controlled trial. Reg Anesth Pain Med 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Aksu C, Gurkan Y. Opioid sparing effect of Erector Spinae Plane block for pediatric bilateral inguinal hernia surgeries. J Clin Anesth 2018;50:62-3. 10.1016/j.jclinane.2018.06.048 [DOI] [PubMed] [Google Scholar]
- 21.Hong B, Bang S, Chung W, et al. Multimodal analgesia with multiple intermittent doses of erector spinae plane block through a catheter after total mastectomy: a retrospective observational study. Korean J Pain 2019;32:206-14. 10.3344/kjp.2019.32.3.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan I, Jiao Y, AuBuchon JD, et al. Continuous Erector Spinae Plane Catheter for Analgesia After Infant Thoracotomy: A Case Report. A A Pract 2018;11:250-2. 10.1213/XAA.0000000000000799 [DOI] [PubMed] [Google Scholar]
- 23.Adhikary SD, Prasad A, Soleimani B, et al. Continuous Erector Spinae Plane Block as an Effective Analgesic Option in Anticoagulated Patients After Left Ventricular Assist Device Implantation: A Case Series. J Cardiothorac Vasc Anesth 2019;33:1063-7. 10.1053/j.jvca.2018.04.026 [DOI] [PubMed] [Google Scholar]
- 24.Taketa Y, Irisawa Y, Fujitani T. Comparison of ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia after video-assisted thoracic surgery: a randomized controlled non-inferiority clinical trial. Reg Anesth Pain Med 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as