Abstract
Treatment of Boerhaave’s syndrome is controversial. Formal thoracotomy and laparotomy were considered the gold standard treatment in the past. However, these approaches are associated with significant surgical trauma, stress, and postoperative pain. Recently published studies reported the application of minimally invasive surgery in the setting of such esophageal emergency. However, the application of minimally invasive surgery in the setting of Boerhaave’s syndrome is debated and evidence is puzzled. The aim of this study was to summarize the current knowledge on minimally invasive treatment of Boerhaave’s syndrome. PubMed, EMBASE, and Web of Science databases were consulted. All articles that described the management of Boerhaave’s syndrome in the setting of minimally invasive surgery (thoracoscopy or laparoscopy) were included. Sixteen studies and forty-eight patients were included. The age of the patient population ranged from 37 to 81 years old and 74% were males. The time shift period from symptoms onset to surgical treatment ranged from 5 to 240 hours with 10 patients (20.8%) having surgery more than 24 hours from symptoms onset. Vomiting (100%), chest/epigastric pain (88%), and dyspnea (62%) were the most commonly reported symptoms. The perforation size ranged from 6 to 30 mm with 96% of patients suffering from distal esophageal tear. Video-assisted thoracoscopy (VATS) was the most commonly reported surgical approach (75%), followed by laparoscopy (16.7%), and combined thoraco-laparoscopy (6.2%). In case of VATS, a left approach was adopted in 91% of patients with selective lung ventilation. Primary suture was the most commonly performed surgical procedure (60%) with interrupted single or dual-layer repair. Surgical debridement (25%), primary repair reinforced with gastric or omental patch (8%), esophageal repair over T-tube (6%), and endoscopic stenting combined with laparoscopic debridement (2%) were also reported. The postoperative morbidity was 64.5% with pneumonia (42%), pleural empyema (26%), and leak (19%) being the most commonly reported complications. The overall mortality was 8.3%. Boerhaave’s syndrome is a rare entity. Minimally invasive surgical treatment seems promising, feasible, and safe in selected patients with early presentation and stable vital signs managed in referral centers. In the management algorithm of Boerhaave’s syndrome, a definitive indication to adopt minimally invasive surgery is lacking and its potential role mandates further analysis.
Keywords: Boerhaave’s syndrome, esophageal perforation, minimally invasive surgery, thoracoscopy, laparoscopy
Introduction
Boerhaave’s syndrome is a full-thickness spontaneous esophageal rupture induced by forceful retching (1). This esophageal emergency condition results in a significant morbidity and mortality because mediastinal and pleural contamination with consequent sepsis and multi organ failure if not diagnosed and promptly treated (2). It is commonly reported that survival rate is significantly decreased when the diagnostic delay is longer than 24 hours (3).
Treatment is controversial, depends upon the time of diagnosis, patient medical condition at presentation, and may range from conservative to major resections. Formal thoracotomy and laparotomy were considered the gold standard treatment in the past (4). However, these approaches are associated with significant surgical trauma, stress, and postoperative pain. By contrast, minimally invasive surgery has been shown to be associated with reduced trauma, postoperative pain, systemic inflammatory response, postoperative endotoxemia with a preserved immune function (5,6). Previous studies demonstrated that in the setting of elective esophageal cancer surgery, minimally invasive approaches seem associated with improvements in postoperative pulmonary complication and overall morbidity compared to open surgery (7). However, the application of minimally invasive surgery in the setting of esophageal emergency, such as Boerhaave’s syndrome, is debated and evidence is puzzled. The aim of this narrative review was to summarize the current knowledge on minimally invasive treatment of Boerhaave’s syndrome. We present the following article in accordance with the Narrative review reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1020).
Methods
An extensive literature search was conducted by two authors (AA, GG) to identify all English-written published series on Boerhaave syndrome treated with minimally invasive surgery (thoracoscopy and laparoscopy). PubMed, EMBASE, and Web of Science databases were consulted matching the terms “Boerhaave syndrome” and “laparoscopy” and “thoracoscopy” with “AND” until 30th November 2019. The search was completed by consulting the listed references of each article (8). Iatrogenic, foreign body-related, external trauma, cancer-related, and motility disorder related-perforations were excluded.
All the articles, case reports, and case series were included in this narrative review while abstracts were excluded. Two authors (AA, GG) independently extracted data from eligible studies. Data extracted included study characteristics (first author name, year, and journal of publication), number of patients included in the series, interval between perforation and diagnosis/treatment, clinical and demographic characteristics of patients’ population, type of surgical procedure, and postoperative outcomes.
The study was approved by the local Institutional Review Board (#0186-2019). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. Informed consent was not necessary for the literature review.
Results
A 49-year-old patient was admitted to our department and diagnosed with spontaneous esophageal perforation. Because the distal location a laparoscopic attempt was planned. The hiatus was opened and the mediastinum dissected. The esophagus was encircled and retracted downward. The perforation was sutured with four absorbable interrupted stitches (2.0 Vicryl®) (Figure 1). The hospital course was uneventful and the patient discharged home on postoperative day 13th.
Figure 1.
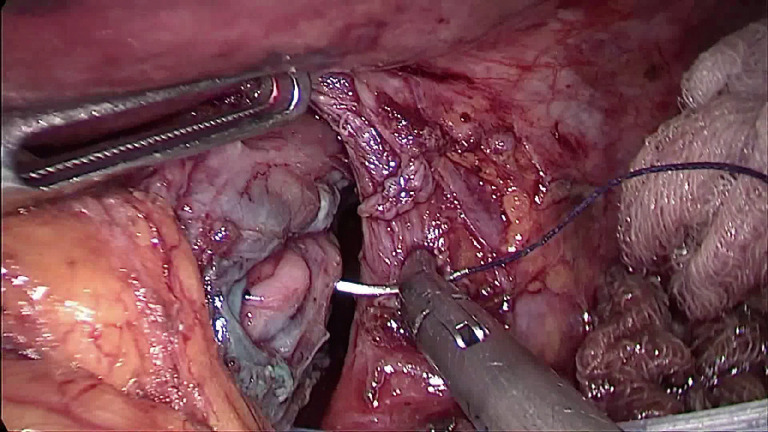
The 15 mm distal esophageal perforation was laparoscopically sutured with absorbable interrupted stitches.
Narrative review
Until 31st December 2019, 16 papers were included in this narrative review for a total of 48 patients (Table 1). The age of the patient population ranged from 37 to 81 years old and 74% were males. Patients comorbidities were reported in eight articles while none of the papers reported patient body mass index (BMI) and ASA score. The time shift period from symptoms onset to diagnosis was reported in nine studies and ranged from 2 to 48 hours. Similarly, time shift period from symptoms onset to surgical treatment was reported in nine studies and ranged from 5 to 240 hours. Overall, 10 patients (20.8%) underwent surgery more than 24 hours from symptoms onset. Vomiting (100%), chest/epigastric pain (88%), and dyspnea (62%) were the most commonly reported symptoms (Table 2). Hypotension at diagnosis was present in 7 patients (14.6%).
Table 1. Demographic, clinical, and operative data.
| Author, year | No. Patients | Age (years) | Male Sex |
Time onset-surgery (hrs) | Location (distal) | Size (mm) | Surgical approach | Operation | Suture techniques | Mortality (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Scott et al., 1995 (9) | 1 | 77 | 1 | 12 | 1 | nr | LVATS | Primary repair | Interrupted single layer (polyglactin) | 0 |
| Landen et al., 2002 (10) | 3 | 48–74 | 3 | range 2–5 | 3 | 30 | Laparoscopy | Primary repair [1], Primary repair + posterior fundoplication [1], posterior fundoplication [1] | Interrupted and running single-layer (polyglactin 3-0/Monocryl 2-0) | 1 |
| Toelen et al., 2007 (11) | 1 | 40 | 1 | nr | 1 | nr | LVATS/laparoscopy | Primary repair | Interrupted single layer (polyglactin 0) | 0 |
| Ashrafi et al., 2007 (12) | 1 | 42 | 1 | 5 | 1 | 30 | LVATS/laparoscopy | Primary repair + omental patch | Interrupted two-layer | 0 |
| Vaidya et al., 2010 (13) | 1 | 45 | 1 | 200 | 1 | nr | LVATS/laparoscopy/esophagostomy | Mediastinal debridement + feeding jejunostomy + cervical esophagostomy | / | 0 |
| Havemann et al., 2010 (2) | 10 | 62 [45–70] | 11 | nr | 10 | nr | VATS | Debridement and chest irrigation | / | 2 |
| Cho et al., 2011 (14) | 7 | 52±8.8 | 7 | 43 [18–78] | 7 | nr | VATS | Primary repair | Interrupted single layer (polyglactin) | 0 |
| Kimberley et al., 2011 (15) | 1 | 35 | 0 | 24 | 1 | nr | Laparoscopy | Primary repair | Interrupted single layer (polyglactin) | 0 |
| Yeo et al., 2015 (16) | 2 | 35–70 | 0 | 12 | 2 | nr | Laparoscopy | Primary repair | Interrupted single layer (polyglactin 3.0) | 0 |
| Do et al., 2016 (17) | 1 | 55 | 0 | 240 | 1 | 25 | LVATS | Primary repair over T-Tube | Interrupted single layer (polyglactin) | 0 |
| Cayci et al., 2017 (18) | 1 | 59 | 0 | 20 | 1 | 30 | Laparoscopy/endoscopy | Debridment + Hanaro stent | / | 0 |
| Nakano et al., 2018 (19) | 6 | 57.5±4 | 6 | 8.5 (5–45) | 6 | 31±6 | LVATS | Primary repair | Interrupted single and two-layer (polyglactin) | 0 |
| Aref et al., 2019 (20) | 1 | 32 | 1 | 48 | 1 | 20 | LVATS/laparoscopy | Primary repair + omental patch | Interrupted single layer (polyglactin 2.0) | 0 |
| Hayakawa et al., 2019 (21) | 1 | 70 | 1 | 11 | 1 | 20 | Laparoscopy | Primary repair | Interrupted single layer (polyglactin 3.0) | 0 |
| Elliot et al., 2019 (22) | 10 | 62 (37–81) | 8 | 27±12 | 8 | nr | LVATS [8]; RVATS [2] | Primary repair [8]; Primary repair over T-tube [2] | EndoStitch - Interrupted single layer (2-0 polyglactin) | 1 |
| Present case | 1 | 49 | 1 | 14 | 1 | 15 | Laparoscopy | Primary repair | Interrupted single layer (polyglactin 3.0) | 0 |
Data are reported as numbers, mean ± standard deviation, and median (range). LVATS, left video-assisted thoracoscopy; RVATS, right video-assisted thoracoscopy; nr, not reported.
Table 2. Patients’ symptoms.
| Symptoms | n (%) |
|---|---|
| Vomiting | 50 (100%) |
| Chest/epigastric pain | 44 (88%) |
| Dyspnea | 31 (62%) |
| Fever >38.0 °C | 7 (14%) |
| Subcutaneous emphysema | 5 (10%) |
| Haematemesis | 4 (8%) |
Data are reported as numbers and percentages (%).
The perforation size was reported in eight studies and ranged from 6 to 30 mm. All studies reported the perforation location with 95.8% of patients suffering from distal esophageal tear. VATS was the most commonly reported surgical approach (75%), followed by laparoscopy (16.7%), combined thoraco-laparoscopy (6.2%), and endo-laparoscopy (2.1%). In case of thoracoscopy, a left approach was adopted in 91% of patients and a double lumen intubation with selective contralateral lung ventilation was adopted in all patients.
Primary suture was the most commonly performed surgical procedure (59%), followed by surgical debridement (25%), primary repair reinforced with gastric or omental patch (8%), esophageal repair over T-tube (6%), and endoscopic stenting combined with laparoscopic debridement (2%). Interrupted single-layer or dual-layer repair with polyglactin were used preferentially (Table 1). Preventive diverting esophagostomy via left cervicotomy was performed in one patient. Overall, 27 patients (56%) received postoperative enteral feeding via jejunostomy and 7 patients (14%) received total parenteral nutrition.
The operative time was reported in five studies and ranged from 150 to 270 minutes. The ICU length of stay was reported in eight studies and ranged from 1 to 78 days. The HLOS was reported in fourteen studies and ranged from 8 to 121 days. The time from operation to oral intake was reported in 9 studies and ranged from 4 to 94 days. The postoperative morbidity was reported in all studies and was 64.5%. Pneumonia was the most commonly reported complications (42%), followed by pleural empyema (25.8%), and leak (19.3%) (Table 3). The overall mortality was 8.3%.
Table 3. Postoperative complications.
| Complications | n=31 |
|---|---|
| Pneumonia | 13 (42%) |
| Pleural empyema | 8 (25.8%) |
| Leak | 6 (19.5%) |
| Atrial fibrillation | 5 (10%) |
| ARDS | 2 (6.4%) |
| MOF | 2 (6.4%) |
| Pelvic abscess | 2 (6.4%) |
| Other medical complications | 2 (6.4%) |
Data are reported as numbers and percentages (%). ARDS, acute respiratory distress syndrome. MOF, multi organ failure.
Discussion
The management of Boerhaave’s syndrome remains a challenge while early diagnosis and prompt treatment are determinant for a successful outcome. It is commonly reported that the survival rate is significantly decreased when the diagnostic delay is longer than 24 hours (3). The choice of the most suitable approach should be guided upon the time of diagnosis, perforation severity and patient medical condition at presentation (22). It has been shown that the Pittsburg perforation Severity Score (PSS) may be a useful tool to stratify patients into low-, intermediate-, and high-risk groups (23). The mainstay of treatment is pleural and mediastinal debridement combined with esophageal repair and spillage prevention (24). Esophageal wall closure may be achieved via primary suture with patch reinforcement or over a T-tube drainage to create a controlled esophagocutaneous fistula (12-17). Major resection with or without primary reconstruction has been described but is matter of debate especially in defeated and septic patients (4). Endoscopic treatments have been proposed in selected patients with early diagnosis, no sepsis, and good tolerance to pleural contamination (24-26). Self-expandable stents, closing with OTSC or other clipping devices, vacuum therapy, Apollo Overstitch, through-the-scope fistula lavage, local antibiotics instillation, and naso-collection or double pig-tail internal drains have been reported with different success rates and related-complications (27-30). Therefore, endoscopic treatment should be left to referral centers and a robust algorithm with precise indication for endoscopic management is lacking (24).
The development of minimally invasive techniques in the setting of elective esophageal surgery has produced significant reductions in blood loss, postoperative complications, early recovery, better health-related quality of life, reduced pain, and improved 1-year functional scores, as compared to open surgery (7). However, the adoption of minimally invasive techniques in the emergency esophageal setting is highly discussed with few published studies and puzzled evidence on safety and effectiveness (31). In order to avoid a formal thoracotomy or laparotomy, a minimally invasive approach may be beneficial in minimizing operative surgical trauma, reduce postoperative pain, improve ventilation, and facilitate early mobilization (13,14,22). In addition, the magnified camera view ideally allows a better visualization of the visceral tears compared to conventional open surgery (10).
The most commonly reported symptoms were vomiting, chest/epigastric pain and dyspnea while seven patients (14%) had hypotension at diagnosis. Tolerance to systemic inflammatory response and surgeon experience in the field of minimally invasive esophageal surgery were probably determinant for deciding on minimally invasive repair (32). Cho et al. stated that a stable blood pressure, heart rate and SaO2 at diagnosis may be indications for thoracoscopic surgery in patients with Boerhaave’s syndrome. Therefore, a clear and definitive indication to adopt minimally invasive rather than open surgery is still lacking.
In the present study, the majority of patients were diagnosed with distal esophageal perforation and mostly managed via left video-assisted thoracoscopy (LVATS). Thoracoscopy may be useful to avoid a formal thoracotomy in case of pleural contamination and food debris spillage. Pleural debridement can be achieved by pleural scraping with aspirator or gauze pads. Pus, debris, and fibrinous debris can be aspirated, the pleural cavity irrigated, and the chest tube positioned under direct vision (14). Right thoracoscopic debridement in prone position has been also described (33). A laparoscopic transhiatal approach may be considered in case of limited pleural contamination when the mediastinal pleural has not been transgressed (10). This because the contaminated posterior mediastinum under the tracheal bifurcation is amenable to debridement and drainage via transhiatal laparoscopy. In addition, the distal esophagus is readily accessible and circumferentially exposed in case of posterior laceration (34).
Primary repair was the most commonly performed surgical procedure with both single and dual-layer repair. Some authors proposed primary suture in case of both early (<24 hours) and late perforations. Edematous, stiff, and friable wound edges make primary repair technically difficult and prone to leakage (2). Suture reinforced with flap or over a T-tube may be considered in attempt to reduce leak.
In accordance with previously published studies, the overall postoperative morbidity was 64% with pneumonia, pleural empyema and leak being the most commonly reported complications (22). The overall mortality rate was 8.3%. This may reflect the downward mortality trend of recent publications. Markar and colleagues in 2015 reported a 30% and 39% 30- and 90-day mortality in 2,564 patients including a significant amount of patients with Boerhaave syndrome (82%) (35). However, the recently published PERF study reported a 15% mortality rate among all-cause esophageal perforations, including 30% Boerhaave’s syndrome (36). Additionally, Haveman et al. and Elliott et al. reported a mortality rate of 8% and 10%, respectively (2,22). These improvements may be attributed to different factors such as increased centralization of care and advances in perioperative management (37). Finally, this effect may be ideally attributed to the effect of minimally invasive approach. However, a bias related to patient heterogeneity, preoperative selection, hospital volume-outcome, and single surgeon experience should be kept in mind as possible confounders.
Principal limitations of this narrative review are the relatively limited number of included patients, the possible background selection bias related to the heterogeneity of the included studies, and study methodological quality. Patients were treated in different centers with diverse expertise and surgical skills. In addition, the included population may represent a sub-selected cohort of patients. On the other hand, because the rarity of the disease, it is challenging to perform a large prospective study and a narrative review may represent an attempt to give a comprehensive and updated analysis on a specific topic (38). It is likely that with the increasing expertise in minimally invasive esophageal surgery, the application of such minimally invasive techniques in the setting of Boerhaave’s syndrome will likely increase.
Conclusions
Boerhaave’s syndrome is a rare entity. Minimally invasive treatment seems feasible and safe in selected patients with early presentation and stable vital signs managed in referral centers. In the management algorithm of spontaneous esophageal perforation, despite preliminary promising results, a clear and definitive indication to adopt minimally invasive surgery is lacking while its role is controversial and requires additional investigations.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1020
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1020). The authors have no conflicts of interest to declare.
References
- 1.Pate JW, Walker WA, Cole FH, Jr, et al. Spontaneous rupture of the esophagus: a 30-year experience. Ann Thorac Surg 1989;47:689-92. 10.1016/0003-4975(89)90119-7 [DOI] [PubMed] [Google Scholar]
- 2.Haveman JW, Nieuwenhuijs VB, Kobold JP, et al. Adequate debridement and drainage of the mediastinum using open thoracotomy or video-assisted thoracoscopic surgery for Boerhaave's syndrome. Surg Endosc 2011;25:2492-7. 10.1007/s00464-011-1571-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron JL, Kieffer RF, Hendrix TR, et al. Selective nonoperative management of contained intrathoracic esophageal disruptions. Ann Thorac Surg 1979;27:404-8. 10.1016/S0003-4975(10)63335-8 [DOI] [PubMed] [Google Scholar]
- 4.Lawrence DR, Ohri SK, Moxon RE, et al. Primary esophageal repair for Boerhaave's syndrome. Ann Thorac Surg 1999;67:818-20. 10.1016/S0003-4975(99)00043-0 [DOI] [PubMed] [Google Scholar]
- 5.Schietroma M, Carlei F, Cappelli S, et al. Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann Surg 2006;243:359-63. 10.1097/01.sla.0000201455.89037.f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schietroma M, Piccione F, Carlei F, et al. Peritonitis from perforated appendicitis: stress response after laparoscopic or open treatment. Am Surg 2012;78:582-90. 10.1177/000313481207800541 [DOI] [PubMed] [Google Scholar]
- 7.Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. 10.1097/SLA.0000000000002171 [DOI] [PubMed] [Google Scholar]
- 8.Goossen K, Tenckhoff S, Probst P, et al. Optimal literature search for systematic reviews in surgery. Langenbecks Arch Surg 2018;403:119-29. 10.1007/s00423-017-1646-x [DOI] [PubMed] [Google Scholar]
- 9.Scott HJ, Rosin RD. Thoracoscopic repair of a transmural rupture of the oesophagus (Boerhaave's syndrome). J R Soc Med 1995;88:414P-5P. [PMC free article] [PubMed] [Google Scholar]
- 10.Landen S, El Nakadi I. Minimally invasive approach to Boerhaave's syndrome: a pilot study of three cases. Surg Endosc 2002;16:1354-7. 10.1007/s00464-001-9185-4 [DOI] [PubMed] [Google Scholar]
- 11.Toelen C, Hendrickx L, Van Hee R. Laparoscopic treatment of Boerhaave's syndrome: a case report and review of the literature. Acta Chir Belg 2007;107:402-4. 10.1080/00015458.2007.11680082 [DOI] [PubMed] [Google Scholar]
- 12.Ashrafi AS, Awais O, Alvelo-Rivera M. Minimally invasive management of Boerhaave's syndrome. Ann Thorac Surg 2007;83:317-9. 10.1016/j.athoracsur.2006.05.111 [DOI] [PubMed] [Google Scholar]
- 13.Vaidya S, Prabhudessai S, Jhawar N, et al. Boerhaave's syndrome: Thoracolaparoscopic approach. J Minim Access Surg 2010;6:76-9. 10.4103/0972-9941.68585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho JS, Kim YD, Kim JW, et al. Thoracoscopic primary esophageal repair in patients with Boerhaave's syndrome. Ann Thorac Surg 2011;91:1552-5. 10.1016/j.athoracsur.2011.01.082 [DOI] [PubMed] [Google Scholar]
- 15.Kimberley KL, Ganesh R, Anton CK. Laparoscopic repair of esophageal perforation due to Boerhaave syndrome. Surg Laparosc Endosc Percutan Tech 2011;21:e203-5. 10.1097/SLE.0b013e3182245771 [DOI] [PubMed] [Google Scholar]
- 16.Yeo D, Hai TC, Hua TS, et al. Laparoscopic transhiatal repair of esophageal rupture in Boerhaave Syndrome is a safe and effective treatment. Available online: http://www.sciedupress.com/journal/index.php/css/article/view/8594
- 17.Do YW, Lee CY, Lee S, et al. Successful Management of Delayed Esophageal Rupture with T-Tube Drainage Using Video-Assisted Thoracoscopic Surgery. Korean J Thorac Cardiovasc Surg 2016;49:478-80. 10.5090/kjtcs.2016.49.6.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cayci HM, Erdoğdu UE, Dilektasli E, et al. An unusual approach for the treatment of oesophageal perforation: Laparoscopic-endoscopic cooperative surgery. J Minim Access Surg 2017;13:69-72. 10.4103/0972-9941.181760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano T, Onodera K, Ichikawa H, et al. Thoracoscopic primary repair with mediastinal drainage is a viable option for patients with Boerhaave's syndrome. J Thorac Dis 2018;10:784-9. 10.21037/jtd.2018.01.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aref H, Yunus T, Alhallaq O. Laparoscopic Management of Boerhaave's syndrome: a case report with an intraoperative video. BMC Surg 2019;19:109. 10.1186/s12893-019-0576-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayakawa S, Mitsui A, Kato Y, et al. Laparoscopic transhiatal suture closure for spontaneous esophageal rupture: a case report. Surg Case Rep 2019;5:149. 10.1186/s40792-019-0711-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott JA, Buckley L, Albagir M, et al. Minimally invasive surgical management of spontaneous esophageal perforation (Boerhaave's syndrome). Surg Endosc 2019;33:3494-502. 10.1007/s00464-019-06863-2 [DOI] [PubMed] [Google Scholar]
- 23.Schweigert M, Sousa HS, Solymosi N, et al. Spotlight on esophageal perforation: A multinational study using the Pittsburgh esophageal perforation severity scoring system. J Thorac Cardiovasc Surg 2016;151:1002-9. 10.1016/j.jtcvs.2015.11.055 [DOI] [PubMed] [Google Scholar]
- 24.Chirica M, Kelly MD, Siboni S, et al. Esophageal emergencies: WSES guidelines. World J Emerg Surg 2019;14:26. 10.1186/s13017-019-0245-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bona D, Aiolfi A, Rausa E, et al. Management of Boerhaave's syndrome with an over-the-scope clip. Eur J Cardiothorac Surg 2014;45:752-4. 10.1093/ejcts/ezt363 [DOI] [PubMed] [Google Scholar]
- 26.Loske G, Schorsch T, van Ackeren V, et al. Endoscopic vacuum therapy in Boerhaave's syndrome with open-pore polyurethane foam and a new open-pore film drainage. Endoscopy 2015;47 Suppl 1 UCTN:E410-1. [DOI] [PubMed]
- 27.Dickinson KJ, Buttar N, Wong Kee Song LM, et al. Utility of endoscopic therapy in the management of Boerhaave syndrome. Endosc Int Open 2016;4:E1146-50. 10.1055/s-0042-117215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raju G S, Shibukawa G, Ahmed I, et al. Endoluminal suturing may overcome the limitations of clip closure of a gaping wide colon. Gastrointest Endosc 2007;65:906-11. 10.1016/j.gie.2006.08.048 [DOI] [PubMed] [Google Scholar]
- 29.Schaheen L, Blackmon SH, Nason KS. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 2014;208:536-43. 10.1016/j.amjsurg.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bona D, Aiolfi A, Siboni S, et al. Giant leiomyoma of the gastroesophageal junction: technique and results of endoscopic full-thickness resection. Clin Exp Gastroenterol 2011;4:263-7. 10.2147/CEG.S26119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonavina L, Aiolfi A, Siboni S, et al. Thoracoscopic removal of dental prosthesis impacted in the upper thoracic esophagus. World J Emerg Surg 2014;9:5. 10.1186/1749-7922-9-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffin SM, Lamb PJ, Shenfine J, et al. Spontaneous rupture of the oesophagus. Br J Surg 2008;95:1115-20. 10.1002/bjs.6294 [DOI] [PubMed] [Google Scholar]
- 33.Dapri G, Dumont H, Roman A, et al. A delayed Boerhaave's syndrome diagnosis treated by thoracoscopy in prone position. Minerva Chir 2008;63:237-40. [PubMed] [Google Scholar]
- 34.Bonavina L, Asti E, Sironi A, et al. Hybrid and total minimally invasive esophagectomy: how I do it. J Thorac Dis 2017;9:S761-72. 10.21037/jtd.2017.06.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markar SR, Mackenzie H, Wiggins T, et al. Management and Outcomes of Esophageal Perforation: A National Study of 2,564 Patients in England. Am J Gastroenterol 2015;110:1559-66. 10.1038/ajg.2015.304 [DOI] [PubMed] [Google Scholar]
- 36.Ali JT, Rice RD, David EA, et al. Perforated esophageal intervention focus (PERF) study: a multi-center examination of contemporary treatment. Dis Esophagus 2017;30:1-8. 10.1093/dote/dox093 [DOI] [PubMed] [Google Scholar]
- 37.Kuppusamy MK, Hubka M, Felisky CD, et al. Evolving management strategies in esophageal perforation: surgeons using nonoperative techniques to improve outcomes. J Am Coll Surg 2011;213:164-71. 10.1016/j.jamcollsurg.2011.01.059 [DOI] [PubMed] [Google Scholar]
- 38.Ferrari R. Writing Narrative Style Literature Reviews. Medical Writing 2015;24:230-5. 10.1179/2047480615Z.000000000329 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


