Abstract
Background
Human adenovirus (HAdV) can cause severe community-acquired pneumonia, but there are few studies on the associated cytokine patterns. The purpose of this study was to analyze the relationship between inflammatory cytokine and severity of adenovirus pneumonia.
Methods
This was a prospective observational study. We evaluated pneumonia patients admitted to the Armed Forces Capital Hospital in Korea. On admission, blood samples were acquired from patients who showed signs of pneumonia. HAdV infection was diagnosed using Real-Q RV detection Kit, and types of HAdV were confirmed by nucleotide blast analysis. We used enzyme-linked immunosorbent assays (ELISAs) to quantify the serum levels of cytokines [interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, γ-interferon (IFN-γ), and IL-10]. We evaluated clinical characteristics and cytokine patterns.
Results
Of 74 pneumonia patients, respiratory specimens from 43 tested positive for HAdV-55, and the other 31 tested negatives. The length of hospital stay was significantly longer in the HAdV group. The serum concentrations of IL-6, IL-8, IL-10, and IFN-γ were all significantly higher in the HAdV group. Of the 43 HAdV pneumonia patients, 6 evidenced PaO2/FiO2 (PF) ratio <300, and 37 did not. Compared to the non-hypoxemic group, the hypoxemic group showed significantly lower lymphocyte and monocyte counts, and increased IL-6 and IFN-γ concentrations. Logistic regression analysis showed that the IL-6, IL-10, and IFN-γ were significantly associated with hypoxemia in the HAdV group. The IL-6, and IFN-γ levels correlated significantly with the PF ratio.
Conclusions
We found that the levels IL-6, IL-10, and IFN-γ were significantly associated with hypoxemia in patients with HAdV-55 pneumonia.
Keywords: Adenoviridae, cytokines, hypoxia, interleukins
Introduction
Human adenoviruses (HAdV) are double-stranded, non-enveloped DNA viruses that are significant pathogens of the upper and lower respiratory tract (1). In non-immunocompromised hosts, respiratory tract infections caused by HAdV are usually accompanied by mild symptoms that resolve spontaneously. In immunocompromised patients such as organ transplant recipients, HAdV infection can be fatal (2,3). However, several studies have reported severe HAdV-related community-acquired outbreaks of pneumonia among immunocompetent patients (4,5). Such outbreaks have been reported in the military training camps of many countries (6-9). Previous studies on adenoviral pneumonia in Korean military personnel showed that the majority of patients were infected with HAdV type 55 (8-10). Of patients with pneumonia, a significant number exhibited rapid progression to acute respiratory distress syndrome (ARDS), and sometimes death (9,11,12).
To reduce fatalities among mostly young recruits, clinical characteristics and laboratory findings have been evaluated to predict unfavorable outcomes. Yoon et al. showed that initial monocytopenia was an independent predictor of rapid respiratory failure (9). The authors further considered that severe inflammation (including upregulated cytokine production) may have contributed to aberrant chemotaxis of monocytes and imbalanced production of inflammatory cells (9). However, little is known about the roles played by inflammatory cytokines in HAdV pneumonia. Wu et al. showed that HAdV infection initially induced the production of cytokines such as interleukin-6 (IL-6) and IL-8, and concurrent neutrophil recruitment. The initial inflammatory process was followed by the upregulation of chemokines that induced the synthesis of macrophage inflammatory proteins and γ-interferon-inducible protein-10, triggering monocyte infiltration (13). Several studies have indicated that severe HAdV infection was associated with marked imbalances in inflammatory cells and the cytokines IL-1ra, IL-6, IL-8, tumor necrosis factor-α (TNF-α) and γ-interferon (IFN-γ) (14-16). One study evaluating HAdV-55 patients in Chinese military camps found that IFN-γ, IL-4, IL-10, and IFN-α2 levels were higher in pneumonia patients than those with upper respiratory tract infections (17).
HAdV 55 pneumonia is frequently fatal; the condition requires intensive evaluation. Cytokines released by inflammatory cells play important early roles in the host response to community-acquired pneumonia (18,19). TNF-α, IL-6, and IL-10 increase the susceptibility to pneumonia caused by various pathogens (20-22). A comparison of the clinical characteristics and cytokine patterns between patients with adenoviral and non-adenoviral pneumonia may be of clinical utility. Furthermore, to understand why some cases of adenoviral pneumonia progress to rapid respiratory failure, comparisons between hypoxemic and non-hypoxemic patients with adenoviral pneumonia are also required. The cross-sectional study by Chen et al. did not show how differences in immunological patterns correlated with the severity of pneumonia, and the number of pneumonia patients studied was relatively small (n=34) (17); a prospective study on only pneumonia patients is essential. Here, we evaluated the clinical characteristics and cytokine patterns of pneumonia patients admitted to the military hospital of the Korean Armed Forces, and further compared hypoxemic and non-hypoxemic patients with adenoviral pneumonia.
Methods
Study design
This was a prospective observational study. Consecutive patients with pneumonia admitted to the Armed Forces Capital Hospital (AFCH) between September 2017 and August 2018 were enrolled. The inclusion criteria were: (I) confirmed pneumonia and aged older than 18 years; (II) provision of informed consent; and, (III) fever >37.6 °C at initial presentation (23). The exclusion criteria were: (I) significant possibility of pulmonary tuberculosis at initial presentation; and (II) lung inflammation that was not attributable to a bacterial or viral infection (such as acute eosinophilic pneumonia). After pneumonia was confirmed radiologically by both pulmonologists and radiologists, blood and sputum samples were acquired with patient consent. Blood samples were obtained on either day 1 or 2 of admission. Serum samples were stored at −70 °C before subsequent analyses. Sputum samples were acquired at admission and were analyzed for the pathogen causative of pneumonia. Initial clinical symptoms including cough, dyspnea, chest pain, and significant fever [body temperature (BT) ≥38.3 °C] (8) were compared between those with HAdV and non-HAdV pneumonia, and hypoxemic and non-hypoxemic pneumonia. Hospital days, total days of fever (BT ≥38.3 °C at least once during the day) (8), total days of oxygen supply, oxygen saturation levels, and laboratory data were also compared.
Study population
All patients were military personnel on active duty in the Korean army; most were conscripts aged 19–26 years; all were diagnosed with pneumonia based on the presenting symptoms and radiological findings. Our study center (AFCH) is an 874-bed, tertiary military referral hospital; we admit not only patients with mild-to-moderate pneumonia but also those with severe pneumonia transferred from other military hospitals in Korea.
Definition of pneumonia and categorization of adenoviral pneumonia and non-adenoviral pneumonia
Patients with acute fever (>37.6 °C) (23), cough, sputum, and lung parenchymal consolidations evident on chest imaging were diagnosed with pneumonia; the chest X-ray and computed tomographic (CT) findings were confirmed by both pulmonologists and radiologists. All patients underwent chest CT and chest X-ray. Of all patients, those positive for HAdV on the initial sputum polymerase chain reaction (PCR) test were diagnosed with adenoviral pneumonia and all others with non-adenoviral pneumonia.
Indications for hospitalization and intensive care unit (ICU) admission for pneumonia
To prevent an outbreak amongst the conscripts and unscreened ARDS cases, our center (AFCH) have extended indications for hospitalization and ICU admission for pneumonia patients. Pneumonia patients with (I) high fever (BT ≥38.3 °C) lasting more than 3 days, (II) fever unresponsive to antipyretics lasting more than 3 days, or (III) rapid aggravation of radiologic findings were to be hospitalized. Pneumonia patients requiring high oxygen supply or impending ARDS cases were admitted to ICU. The ICU cases requiring prolonged mechanical ventilation or extracorporeal membrane oxygenation were transferred to other university hospitals with larger ICU facilities.
Multiplex real-time PCR
Multiplex PCR detecting human respiratory viruses was performed using an AdvanSure™ RV real-time PCR Kit (LG Life Sciences, Daejeon, Korea). The assay targets 12 types of pathogenic RNA viruses: rhinoviruses A/B/C, influenza viruses A/B, parainfluenza viruses 1/2/3, coronaviruses 229E/NL63/OC43, respiratory syncytial viruses (RSVs) A/B, and metapneumovirus; and two types of DNA viruses: bocavirus and adenovirus (12). We also used multiplex PCR to detect respiratory bacterial pathogens (Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila); we used the Seeplex1PneumoBacter ACE Detection assay (Seegene, Seoul, Korea). Streptococcus pneumoniae and Haemophilus influenzae PCR were not performed from respiratory specimens because we could not differentiate true infection from colonization of these pathogens (12).
Determination of HAdV types
For the diagnosis of HAdV infection, DNA were extracted from sputum using NX-48 viral NA Kit (Genolution, Seoul, Korea), and multiplex real-time PCR for 13 respiratory viruses including HAdV was conducted using Real-Q RV Detection Kit (Biosewoom, Seoul, Korea). Then, PCR targeting the hexon gene was performed again with type-specific primers. The primer’s product size was 544 bp. The Forward sequence is 5'-CCC ATG GCN CAC ACC AC-3', and the Reverse sequence is 5'-CTC ATG GGC TGG AAG TT-3. The primer sequence position is Forward 1843-1859, and Reverse 2371-2387. The PCR products were purified Wizard MagneSil Sequencing Reaction Clean-Up System (Promega, Madison, WI, USA), and then sequenced using the ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA). Types of HAdV were confirmed by nucleotide blast analysis using the BLASTn program (National Center for Biotechnology Information, Bethesda, MD, USA).
Cytokine measurement
Quantification of five cytokines (IL-6, IL-8, TNF-α, IFN-γ and IL-10) in sera was performed with the ELISA (Quantikine HS Human IL-6 Immunoassay, HS Human TNF-α, Human CXCL8/IL-8 Immunoassay, Quantikine Human IFN-γ and Human IL-10 Quant HS. ELISA kit, R&D, USA). Assays were performed following the manufacturer’s instructions.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the institutional review board of the Armed Forces Capital Hospital (AFMC IRB-2017-03-02). All patients gave their written, informed consent to participate in the study. All patients consented to blood and sputum sampling.
Statistical analysis
All analyses were performed using Statistical Package for Social Sciences software program version 20.0 (SPSS Inc., Chicago, IL, USA). Distribution of continuous variables was tested using the Shapiro-Wilk normality test. Continuous parameters were compared using the Mann-Whitney U test, and categorical variables using the chi-squared test. The initial serum cytokine concentrations were compared using the Mann-Whitney U test. Possible associations between hypoxemia and other parameters assessed by logistic regression analysis. Continuous variables including BT, lymphocyte count, monocyte count, and platelet count were converted to categorical variables based on the previous publication (9). While there were no established cutoffs for the cytokine concentration levels, cytokines were entered in the logistic regression as continuous variables, and odds ratio was calculated for a one-unit increase (24,25). Factors that were significant on univariate analysis were subjected to multivariate analysis. Correlations between the variables were analyzed with the Spearman correlation coefficient. A P value <0.05 was considered to reflect statistical significance.
Results
Comparisons between patients with adenoviral and non-adenoviral pneumonia
During the study period, 208 patients with pneumonia were admitted, and 86 met our inclusion criteria. Of these, 12 were excluded because their samples were of poor quality (e.g., blood samples exhibited hemolysis). After radiological confirmation of pneumonia, we finally evaluated 74 patients. The patient selection process was shown in Figure 1. Table 1 lists their baseline clinical characteristics and laboratory data. Of the 74 patients, 43 tested positive for HAdV on initial virus PCR of their respiratory specimens, and 31 patients tested negative. Table 2 shows the results of respiratory virus PCR testing and the atypical pathogens found in both groups. The adenovirus genotype of 43 pneumonia patients was HAdV-55. In the non-adenovirus group, there were 5 cases of rhinovirus, 1 case of coronavirus 220E, 2 cases of RSV-B, 4 cases of influenza A and 2 cases of influenza B. Furthermore, 3 patients were positive for streptococcus pneumoniae antigen.
Figure 1.
Flow diagram for the patient selection process.
Table 1. Baseline clinical characteristics of patients.
| Clinical parameters | Adenoviral pneumonia | Non-adenoviral pneumonia | P value |
|---|---|---|---|
| Number of patients | 43 | 31 | |
| Median age (range) | 20 [20–21] | 21 [20–22] | 0.015 |
| Sex (male) | 43 [100] | 31 [100] | N/A |
| Hospital days | 7.0 [6.0–10.0] | 6.0 [6.0–7.0] | 0.012 |
| ICU care required | 9 (20.9) | 0 (0.0) | 0.007 |
| Presenting symptom | |||
| Cough | 40 (93.0) | 30 (96.8) | 0.481 |
| Dyspnea | 13 (30.2) | 5 (16.1) | 0.163 |
| Chest pain | 12 (27.9) | 6 (19.4) | 0.398 |
| Fever (≥38.3 °C) | 38 (88.4) | 17 (54.8) | 0.001 |
| Sputum | 35 (81.4) | 24 (77.4) | 0.639 |
| Initial room air saturation (%) | 98.0 [97.0–98.0] | 98.0 [97.0–98.0] | 0.690 |
| Total fever days | 3.0 [2.0–4.0] | 1.0 [0.0–2.0] | <0.001 |
| Total days requiring oxygen supply | 3.0 [0.5–5.0] | 0.0 [0.0–3.5] | 0.098 |
| Initial laboratory findings | |||
| White blood cell count (/μL) | 5,190 [3,770–6,890] | 9,620 [5,710–13,690] | <0.001 |
| Neutrophil count (/μL) | 3,412 [2,254–4,926] | 7,162 [3,866–10,746] | <0.001 |
| Lymphocyte count (/μL) | 1,170 [809–1,389] | 1,628 [922–2,108] | 0.009 |
| Monocyte count (/μL) | 571 [291–799] | 685 [462–1,016] | 0.036 |
| Hemoglobin (g/dL) | 13.9 [13.6–14.6] | 14.3 [13.4–15.0] | 0.292 |
| Platelet count (103/μL) | 164k [131k–184k] | 216k [185k–247k] | <0.001 |
| C-reactive protein (mg/dL) | 8.5 [4.3–10.5] | 7.7 [2.9–14.8] | 0.705 |
| Biomarkers | |||
| IL-6 (pg/mL) | 15.6 [9.4–16.8] | 10.2 [2.6–15.9] | 0.005 |
| IL-8 (pg/mL) | 24.3 [16.8–33.1] | 16.0 [11.5–27.2] | 0.024 |
| TNF-α (pg/mL) | 1.73 [1.47–1.99] | 1.48 [1.16–1.88] | 0.138 |
| IFN-γ (pg/mL) | 21.8 [6.9–47.4] | 0.1 [0.1–11.49] | <0.001 |
| IL-10 (pg/mL) | 1.61 [0.44–4.83] | 0.27 [0.01–1.29] | 0.002 |
| Initial chest radiograph finding | 0.057 | ||
| Unilateral pneumonia | 36 (83.7) | 20 (64.5) | |
| Bilateral pneumonia | 7 (16.3) | 11 (35.5) | |
| Effusion | 8 (18.6) | 1 (3.3) | 0.051 |
dl, deciliter; g, gram; ICU, intensive care unit; IFN, interferon; IL, interleukin; k, thousand; PF, PaO2/FiO2; TNF, tumor necrosis factor.
Table 2. Virus PCR results from initial respiratory specimens of the patients.
| Virus PCR | Adenovirus (n=43) | Non-adenovirus (n=31) |
|---|---|---|
| Adenovirus | 43 | 0 |
| HAdV-55 | 43 | – |
| Rhinovirus | 1 | 5 |
| Coronavirus 229E | 2 | 1 |
| RSV-B | 1 | 2 |
| Influenza virus A | 0 | 4 |
| Influenza virus B | 0 | 2 |
| Metapneumovirus | 0 | 1 |
| Parainfluenza virus 1 | 0 | 1 |
| None | 0 | 17 |
| Streptococcus pneumoniae antigen | 0 | 3 |
| Legionella pneumophila | 0 | 0 |
| Atypical bacterial pathogen | ||
| Mycoplasma pneumoniae | 0 | 0 |
| Chlamydophila pneumoniae | 0 | 0 |
HAdV, human adenovirus virus; PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
All patients were military personnel and male. The median age of the adenoviral pneumonia group was 20 years, which was younger than that of the non-adenoviral pneumonia group (P=0.015). The hospital stay was significantly longer for the adenoviral than the non-adenoviral pneumonia group (P=0.012). While a total of 9 (20.9%) patients were admitted to the ICU, none received ICU care in the non-adenoviral pneumonia group (P=0.007). The proportion of patients who complained of cough, dyspnea, chest pain, and sputum production did not differ significantly between the two groups. However, on admission, a significantly higher proportion of patients showed significant fever in adenoviral pneumonia when compared to the non-adenoviral pneumonia group (P=0.001). Initial room air saturation showed no statistical significance between the two groups. Total fever day during admission was longer for the adenoviral pneumonia group with statistical significance (P<0.001), while the total days requiring oxygen supply showed no significant difference between the two groups.
Initial laboratory findings were also compared between the groups. Total white blood cells, absolute neutrophil, lymphocyte, and monocyte count were significantly lower for the adenoviral pneumonia group (P<0.001, P<0.001, P=0.009, and P=0.036, respectively). Platelet count was significantly lower for the adenoviral pneumonia group (P<0.001). The cytokine levels measured from blood samples at admission were compared. IL-6, IL-8, IL-10, and IFN-γ were all significantly higher for the adenoviral pneumonia group (P=0.005, P=0.024, P=0.002 and P<0.001, respectively). Radiologic findings from initial chest CT did not show a significant difference between the two groups.
Comparisons between the hypoxemic and non-hypoxemic groups
A total of 43 HAdV pneumonia patients were further categorized according to the value of the PF ratio (Table 3). Six patients showed a PF ratio of less than 300, while 37 patients showed otherwise. The hypoxemia group were hospitalized longer than the non-hypoxemia group (P<0.001). When the presenting symptoms were evaluated, five of six patients from the hypoxemia group complaint chest pain (83.3%), while only 18.9% of the non-hypoxemic group had chest pain at presentation (P=0.001). Total fever days were significantly longer for the hypoxemia group than the non-hypoxemia group (5.0 vs. 2.0, P<0.001).
Table 3. Comparison of clinical characteristics and inflammatory cytokines between hypoxemia non- hypoxemia group in 43 type-55 adenovirus pneumonia.
| Clinical parameters | PF ratio <300 | PF ratio >300 | P value |
|---|---|---|---|
| Number of patients | 6 | 37 | |
| Median age, range | 20.0 [20.0–22.0] | 20.0 [19.0–21.0] | 0.482 |
| Sex (male) | 6 [100] | 37 [100] | – |
| Hospital days | 12.5 [10.0–14.8] | 7.0 [6.0–9.0] | <0.001 |
| Presenting symptom | |||
| Cough | 6 (100.0) | 34 (91.9) | 0.470 |
| Dyspnea | 3 (50.0) | 10 (27.0) | 0.256 |
| Chest pain | 5 (83.3) | 7 (18.9) | 0.001 |
| Fever (≥38.3 °C) | 6 (100.0) | 32 (86.5) | 0.338 |
| Sputum | 5 (83.3) | 30 (81.1) | 0.895 |
| Initial room air saturation (%), mean | 96.0 [90.0–98.0] | 98 [97.0–98.0] | 0.162 |
| Total fever days | 5.0 [4.5–6.5] | 2.0 [2.0–3.0] | <0.001 |
| Initial laboratory findings | |||
| White blood cell count (/μL) | 3,980 [2,585–6,195] | 5,440 [3,840–7,040] | 0.015 |
| Neutrophil count (/μL) | 3,144 [1,934–4,737] | 3,342 [2,312–5,131] | 0.694 |
| Lymphocyte count (/μL) | 664 [494–870] | 1270 [906–1430] | 0.004 |
| Monocyte count (/μL) | 264 [100–442] | 539 [338–868] | 0.015 |
| Hemoglobin (g/dL) | 14.0 [13.6–14.3] | 13.9 [13.6–14.7] | 0.932 |
| Platelet count (103/μL) | 125,330±63,503 | 164,970±48,294 | 0.218 |
| C-reactive protein (mg/dL) | 10.0 [7.2–10.5] | 8.5 [3.5–10.5] | 0.206 |
| Serum cytokine concentration | |||
| IL-6 (pg/mL) | 17.3 [16.7–18.0] | 13.7 [8.4–16.5] | <0.001 |
| IL-8 (pg/mL) | 28.1 [20.0–58.6] | 23.8 [16.7–32.0] | 0.420 |
| TNF-α (pg/mL) | 1.87 [1.61–2.54] | 1.71 [1.47–1.98] | 0.293 |
| IFN- γ (pg/mL) | 103.1 [30.8–157.2] | 19.9 [6.0–41.6] | 0.035 |
| IL-10 (pg/mL) | 7.33 [1.53–13.05] | 1.13 [0.37–3.87] | 0.070 |
| Initial chest radiograph finding | 0.016 | ||
| Unilateral pneumonia | 3 (50.0) | 33 (89.2) | |
| Bilateral pneumonia | 3 (50.0) | 4 (10.8) | |
| Effusion | 4 (66.7) | 4 (10.8) | 0.001 |
dl, deciliter; g, gram; IFN, interferon; IL, interleukin; PF, PaO2/FiO2; TNF, tumor necrosis factor.
When the initial laboratory findings were compared, the hypoxemia group showed significantly lower total white blood cell, lymphocyte, and monocyte counts (P=0.015, P=0.004 and P=0.015, respectively) when compared to the non-hypoxemia group. Of the serum cytokines evaluated, IL-6 and IFN-γ were significantly increased in the hypoxemia group (P<0.001 and P=0.035, respectively).
A larger proportion of patients showed bilateral pneumonic infiltration from the radiologic findings in the hypoxemia group (50% vs. 10.8%, P=0.016). Furthermore, 66.7% of the hypoxemia group showed pleural effusions, while only 10.8% showed effusions in the non-hypoxemia group (P=0.001).
Association with hypoxemia in patients with HAdV pneumonia
In 43 patients with HAdV pneumonia, baseline parameters were evaluated in terms of possible significant associations with hypoxemia (PF <300). The BT at admission, initial lymphocyte, monocyte, and platelet counts which were converted to categorical variables, and initial concentrations of IL-8, IL-10, IL-6, and IFN-γ as continuous variables were entered into univariate analysis (9,24,25). The concentrations of IL-10, IL-6, and IFN-γ were significant from univariate analysis, as well as the initial lymphocyte count. In multivariate analysis, the lymphocyte count and one of the three cytokines significant in the univariate analysis was entered into different multivariate models (Table 4). In the model including IL-10, IL-10 showed a significant association with hypoxemia (P=0.035, OR =1.299; 95% CI: 1.018–1.656). IFN-γ showed a significant association with hypoxemia (P=0.038, OR =1.014; 95% CI: 1.001–1.027), when IFN-γ was included in analysis. In the model including IL-6, it showed a significant association with hypoxemia (P=0.042, OR =17.953; 95% CI: 1.110–298.46).
Table 4. Association with hypoxemia (PF ratio <300) in 43 patients with HAdV-55 pneumonia.
| Characteristics | Univariate | Multivariate (Model 1) | Multivariate (Model 2) | Multivariate (Model 3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | ||||
| BT at admission (≥38.3/<38.3 °C) | 1.524 | 0.247–9.383 | 0.650 | ||||||||||||
| IL-8* | 1.033 | 0.978–1.091 | 0.246 | ||||||||||||
| IL-10* | 1.319 | 1.062–1.637 | 0.012 | 1.299 | 1.018–1.656 | 0.035 | |||||||||
| IL-6* | 13.489 | 1.369–132.876 | 0.026 | 17.953 | 1.110–298.46 | 0.042 | |||||||||
| IFN-γ* | 1.013 | 1.000–1.026 | 0.046 | 1.014 | 1.001–1.027 | 0.038 | |||||||||
| Lymphocytopenia (<1,000/≥1,000 μL) | 10.417 | 1.093–99.293 | 0.042 | 8.926 | 0.806–98.89 | 0.074 | 7.557 | 0.444–128.52 | 0.123 | 13.66 | 0.975–191.5 | 0.052 | |||
| Monocytopenia (<150/≥150 μL) | 8.750 | 0.954–80.259 | 0.055 | ||||||||||||
| Thrombocytopenia (<150,000/≥150,000/μL) | 1.846 | 0.325–10.485 | 0.489 | ||||||||||||
*, indicate risk associated with 1 pg/mL increase. BT, body temperature; CI, confidence interval; HAdV, human adenovirus; IFN, interferon; IL, interleukin; OR, odds ratio; PF, PaO2/FiO2.
Correlation between clinical parameters and laboratory findings of HAdV pneumonia patients
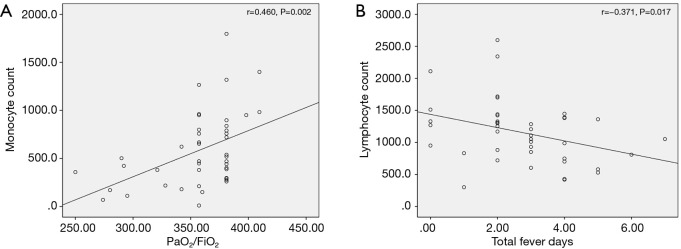
Correlations between initial laboratory values and outcome parameters were evaluated. Absolute monocyte count showed significant ascending linear correlation with PF ratio (r=0.460, P=0.002) (Figure 2A), however, absolute lymphocyte count did not show a significant correlation (r=0.294, P=0.056). Also, absolute lymphocyte count showed a correlation with the total fever days (r=−0.371, P=0.017) (Figure 2B).
Figure 2.
Correlation between clinical parameters and laboratory findings of HAdV pneumonia. (A) Absolute monocyte count showed significant linearly ascending correlation with PF ratio (r=0.460, P=0.002). (B) Absolute lymphocyte count showed a significant correlation with the total fever days (r=−0.371, P=0.017). HAdV, human adenovirus.
Correlation of serum cytokine levels with laboratory findings and clinical parameters in HAdV pneumonia
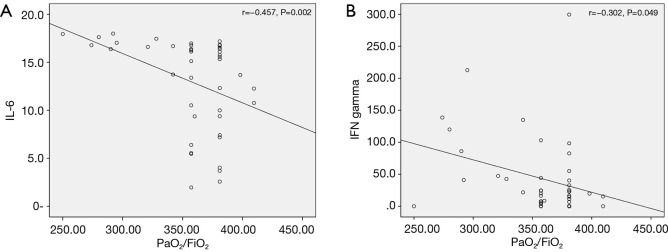
Serum cytokine levels were evaluated for their correlations with the PF ratio. IL-8, IL-10, and TNF-α did not show a significant correlation. IL-6 showed a significant correlation with PF ratio (r=−0.457, P=0.002) (Figure 3A). IFN-γ also showed a significant correlation with PF ratio (r=−0.302, P=0.049) (Figure 3B). As for total fever days, IL-6, IL-8, IL-10, and IFN-γ showed significant correlations. However, no significant correlation with TNF-α was observed.
Figure 3.
Correlations of serum cytokine levels with PF ratio in HAdV pneumonia (A) IL-6 showed a significant correlation with PF ratio (r=−0.457, P=0.002). (B) IFN-γ also showed a significant correlation with PF ratio (r=−0.302, P=0.049). HAdV, human adenovirus; IFN, interferon; IL, interleukin; PF, PaO2/FiO2.
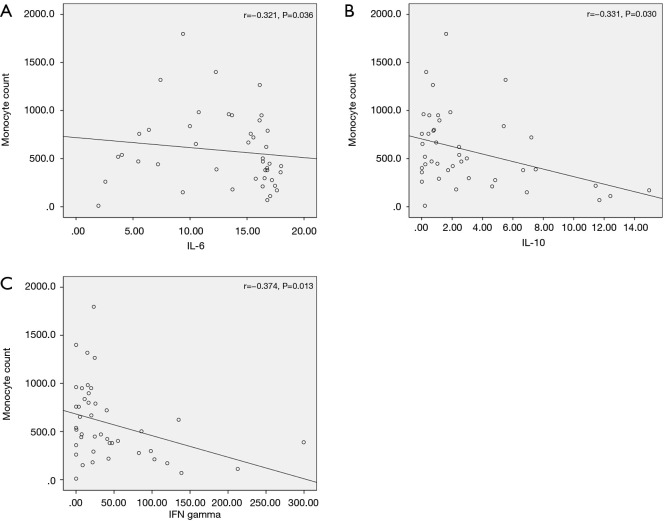
Correlation analysis was also performed for association between cytokine levels and other initial laboratory values. IL-6, IL-10 and IFN-γ showed significant correlations with monocyte count (r=−0.321, P=0.036, r=−0.331, P=0.030 and r=−0.374, P=0.013, respectively) (Figure 4A,B,C, respectively). Regarding lymphocyte count, no cytokine showed a significant correlation.
Figure 4.
Correlation between serum cytokine levels and other initial laboratory values. (A) IL-6, (B) IL-10, and (C) IFN-γ showed significant correlations with monocyte count (r=−0.321, P=0.036, r=−0.331, P=0.030, and r=−0.374, P=0.013, respectively). IFN, interferon; IL, interleukin.
Discussion
The present study compared clinical characteristics and laboratory results of HAdV pneumonia group to non-HAdV pneumonia group, and further evaluated the roles of inflammatory cytokines in HAdV-55 patients. We found that higher serum IL-6, IL-10, and IFN-γ were associated with acute hypoxemia in HAdV-55 pneumonia.
Compared to non-adenoviral pneumonia, our results showed that patients with HAdV-55 pneumonia had a prolonged hospital stay and fever, while showing more frequent abnormal laboratory findings. In the previous studies on HAdV pneumonia among Korean military personnel, respiratory failure occurred in a considerable number of patients (26,27). In the present study, the adenoviral group also showed increased concentrations of IL-6, IL-8, IL-10, and IFN-γ, suggesting more active cytokine-mediated inflammations than in non-adenoviral pneumonia. Wu et al. showed that the levels of IL-6 and IL-8 increased markedly after adenovirus-7 infection (13), and this may also be true of HAdV-55 infection.
The clinical features and laboratory findings showed contrasting differences even among the HAdV pneumonia group, after they were grouped according to the presence of hypoxemia. The hypoxemic HAdV pneumonia patients were hospitalized longer and had more prolonged fever than the less severe group. Our results showed also showed that monocyte count was significantly lower in the hypoxemic group, and this was consistent with the previous study in which monocytopenia was an independent predictor for respiratory failure (9).
In the 43 HAdV-55 group, IL-6, IL-8, and IFN-γ showed significant associations with hypoxemia. Moreover, IL-6 and IFN-γ showed significant descending linear correlations with the PF ratio, while IL-8 showed a significant correlation with total fever days. In a previous study, IL-6 was shown to play a significant role in susceptibility to pneumonia (21). Furthermore, an elevated IL-6 was reported to have associations with severe pneumonia and ARDS (16,28). In the case of IL-10, a study on elderly patients with community-acquired pneumonia demonstrated that high IL-10 levels were shown to predict 30-day mortality (29), and IL-10 levels were also related to CURB-65 scores in pneumonia (30).
IFN-γ was another cytokine that showed association with hypoxemia in our study. IFN- γ plays an important role in inflammation and immune reaction in infection (31), and is produced by the immune cells, including natural killer cells, T lymphocytes, and macrophages (32,33). In the study comparing viral and pneumococcal pneumonia, IFN-γ was significantly elevated in viral community-acquired pneumonia (24). Increased level of IFN-γ was predictive of more frequent extrapulmonary complications in the study of pediatric mycoplasma pneumonia (34). We assume that the upregulation of cytokine productions would result in more active inflammations in lung parenchyme and eventually, hypoxemia.
Monocyte and lymphocyte counts were significantly decreased in the hypoxemic group when compared to the non-hypoxemic group. In the previous study on HAdV pneumonia, monocytopenia was an independent predictor for respiratory failure (9). Our correlation analysis showed that IL-6, IL-10, and IFN-γ showed significant correlations with monocyte level, suggesting the possibility of inflammatory cytokines’ contribution to an imbalance of inflammatory cells. The study on HAdV infection by Chen et al., showed that lymphocyte and monocyte counts, and inflammatory cytokines concentrations showed significant differences between a pneumonia group and a relatively milder upper respiratory infection group (17), suggesting possible interrelations between immune cells, inflammatory cytokines and disease severity. The exact mechanism underlying the interrelationship between immune cells and cytokines in HAdV infection is not exactly known and needs further studies.
Our study had several limitations. First, it used a single-center prospective design and included a relatively small number of patients. The study patients were consecutively enrolled, but we believe that the analyses performed using this group should be reconfirmed in future studies including a larger population of adenoviral pneumonia. Second, we evaluated only male military personnel; our findings may not be generalizable to other populations. However, HAdV pneumonia is prevalent in military training camps
Conclusions
We found that the levels IL-6, IL-10, and IFN-γ were significantly associated with hypoxemia in patients with HAdV-55 pneumonia. A future study involving serial measurements of inflammatory cytokine levels is required to understand an association between acute inflammation and the prognosis of adenoviral pneumonia.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the Korean Military Medical Research Project funded by the Republic of Korea Ministry of National Defense (ROK-MND-2017-KMMRP-031).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the institutional review board of the Armed Forces Capital Hospital (AFMC IRB-2017-03-02). All patients gave their written, informed consent to participate in the study.
Footnotes
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-19-4067
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-19-4067). CKR serves as an unpaid editorial board member of Journal of Thoracic Disease. The authors have no conflicts of interest to declare.
References
- 1.Lynch JP, 3rd, Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med 2011;32:494-511. 10.1055/s-0031-1283287 [DOI] [PubMed] [Google Scholar]
- 2.Simsir A, Greenebaum E, Nuovo G, et al. Late fatal adenovirus pneumonitis in a lung transplant recipient. Transplantation 1998;65:592-4. 10.1097/00007890-199802270-00027 [DOI] [PubMed] [Google Scholar]
- 3.Doan ML, Mallory GB, Kaplan SL, et al. Treatment of adenovirus pneumonia with cidofovir in pediatric lung transplant recipients. J Heart Lung Transplant 2007;26:883-9. 10.1016/j.healun.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 4.Gu L, Liu Z, Li X, et al. Severe community-acquired pneumonia caused by adenovirus type 11 in immunocompetent adults in Beijing. J Clin Virol 2012;54:295-301. 10.1016/j.jcv.2012.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hijikata N, Takayanagi N, Sato S, et al. Adenovirus pneumonia in an immunocompetent adult. J Infect Chemother 2012;18:780-5. 10.1007/s10156-012-0367-x [DOI] [PubMed] [Google Scholar]
- 6.Brosch L, Tchandja J, Marconi V, et al. Adenovirus serotype 14 pneumonia at a basic military training site in the United States, spring 2007: a case series. Mil Med 2009;174:1295-9. 10.7205/MILMED-D-03-0208 [DOI] [PubMed] [Google Scholar]
- 7.Chmielewicz B, Benzler J, Pauli G, et al. Respiratory disease caused by a species B2 adenovirus in a military camp in Turkey. J Med Virol 2005;77:232-7. 10.1002/jmv.20441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon H, Jhun BW, Kim H, et al. Characteristics of Adenovirus Pneumonia in Korean Military Personnel, 2012-2016. J Korean Med Sci 2017;32:287-95. 10.3346/jkms.2017.32.2.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon H, Jhun BW, Kim SJ, et al. Clinical characteristics and factors predicting respiratory failure in adenovirus pneumonia. Respirology 2016;21:1243-50. 10.1111/resp.12828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SJ, Kim K, Park SB, et al. Outcomes of early administration of cidofovir in non-immunocompromised patients with severe adenovirus pneumonia. PLoS One 2015;10:e0122642. 10.1371/journal.pone.0122642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafolie J, Mirand A, Salmona M, et al. Severe Pneumonia Associated with Adenovirus Type 55 Infection, France, 2014. Emerg Infect Dis 2016;22:2012-4. 10.3201/eid2211.160728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JY, Kim BJ, Lee EJ, et al. Clinical Features and Courses of Adenovirus Pneumonia in Healthy Young Adults during an Outbreak among Korean Military Personnel. PLoS One 2017;12:e0170592. 10.1371/journal.pone.0170592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu W, Booth JL, Duggan ES, et al. Human lung innate immune cytokine response to adenovirus type 7. J Gen Virol 2010;91:1155-63. 10.1099/vir.0.017905-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mistchenko AS, Koch ER, Kajon AE, et al. Lymphocyte subsets and cytokines in adenoviral infection in children. Acta Paediatr 1998;87:933-9. 10.1111/j.1651-2227.1998.tb01761.x [DOI] [PubMed] [Google Scholar]
- 15.Díaz PV, Calhoun WJ, Hinton KL, et al. Differential effects of respiratory syncytial virus and adenovirus on mononuclear cell cytokine responses. Am J Respir Crit Care Med 1999;160:1157-64. 10.1164/ajrccm.160.4.9804075 [DOI] [PubMed] [Google Scholar]
- 16.Mistchenko AS, Diez RA, Mariani AL, et al. Cytokines in adenoviral disease in children: association of interleukin-6, interleukin-8, and tumor necrosis factor alpha levels with clinical outcome. J Pediatr 1994;124:714-20. 10.1016/S0022-3476(05)81360-5 [DOI] [PubMed] [Google Scholar]
- 17.Chen WW, Nie WM, Xu W, et al. Cross-sectional study of the relationship of peripheral blood cell profiles with severity of infection by adenovirus type 55. BMC Infect Dis 2014;14:147. 10.1186/1471-2334-14-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolling UK, Hansen F, Braun J, et al. Leucocyte response and anti-inflammatory cytokines in community acquired pneumonia. Thorax 2001;56:121-5. 10.1136/thorax.56.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endeman H, Meijvis SC, Rijkers GT, et al. Systemic cytokine response in patients with community-acquired pneumonia. Eur Respir J 2011;37:1431-8. 10.1183/09031936.00074410 [DOI] [PubMed] [Google Scholar]
- 20.Kerr AR, Irvine JJ, Search JJ, et al. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect Immun 2002;70:1547-57. 10.1128/IAI.70.3.1547-1557.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan AQ, Shen Y, Wu ZQ, et al. Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae. Infect Immun 2002;70:749-61. 10.1128/IAI.70.2.749-761.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preston JA, Beagley KW, Gibson PG, et al. Genetic background affects susceptibility in nonfatal pneumococcal bronchopneumonia. Eur Respir J 2004;23:224-31. 10.1183/09031936.03.00081403 [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain JM, Terndrup TE, Alexander DT, et al. Determination of normal ear temperature with an infrared emission detection thermometer. Ann Emerg Med 1995;25:15-20. 10.1016/S0196-0644(95)70349-7 [DOI] [PubMed] [Google Scholar]
- 24.Burgmeijer EH, Duijkers R, Lutter R, et al. Plasma cytokine profile on admission related to aetiology in community-acquired pneumonia. Clin Respir J 2019;13:605-13. 10.1111/crj.13062 [DOI] [PubMed] [Google Scholar]
- 25.Kwan J, Horsfield G, Bryant T, et al. IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp Gerontol 2013;48:960-5. 10.1016/j.exger.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 26.Yoo H, Oh J, Park C. Characteristics of fever and response to antipyretic therapy in military personnel with adenovirus-positive community-acquired pneumonia. Mil Med Res 2020;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko JH, Lim JU, Choi JY, et al. Early cidofovir administration might be associated with a lower probability of respiratory failure in treating human adenovirus pneumonia: a retrospective cohort study. Clin Microbiol Infect 2020;26:646.e9-14. 10.1016/j.cmi.2019.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schütte H, Lohmeyer J, Rosseau S, et al. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Respir J 1996;9:1858-67. 10.1183/09031936.96.09091858 [DOI] [PubMed] [Google Scholar]
- 29.Pinargote-Celorio H, Miralles G, Cano M, et al. Cytokine levels predict 30-day mortality in octogenarians and nonagenarians with community-acquired pneumonia: a retrospective observational study. Eur J Clin Microbiol Infect Dis 2020;39:299-307. 10.1007/s10096-019-03725-6 [DOI] [PubMed] [Google Scholar]
- 30.Zobel K, Martus P, Pletz MW, et al. Interleukin 6, lipopolysaccharide-binding protein and interleukin 10 in the prediction of risk and etiologic patterns in patients with community-acquired pneumonia: results from the German competence network CAPNETZ. BMC Pulm Med 2012;12:6. 10.1186/1471-2466-12-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 2004;75:163-89. 10.1189/jlb.0603252 [DOI] [PubMed] [Google Scholar]
- 32.Robinson CM, O'Dee D, Hamilton T, et al. Cytokines involved in interferon-gamma production by human macrophages. J Innate Immun 2010;2:56-65. 10.1159/000247156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng CG, Kaviratne M, Rothfuchs AG, et al. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol 2006;177:7086-93. 10.4049/jimmunol.177.10.7086 [DOI] [PubMed] [Google Scholar]
- 34.Jin X, Zhu Y, Zhang Y, et al. Assessment of levels of D-dimer and interferon-gamma in pediatric patients with Mycoplasma pneumoniae pneumonia and its clinical implication. Exp Ther Med 2018;16:5025-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as