Abstract
Many species of morning glories (Convolvulaceae) form symbioses with seed-transmitted Periglandula fungal endosymbionts, which produce ergot alkaloids and may contribute to defensive mutualism. Allocation of seed-borne ergot alkaloids to various tissues of several Ipomoea species has been demonstrated, including roots of I. tricolor. The goal of this study was to determine if infection of I. tricolor by the Periglandula sp. endosymbiont affects Southern root-knot nematode (Meloidogyne incognita) gall formation and host plant biomass. We hypothesized that I. tricolor plants infected by Periglandula (E+) would develop fewer nematode-induced galls compared to non-symbiotic plants (E-). E+ or E- status of plant lines was confirmed by testing methanol extracts from individual seeds for endosymbiont-produced ergot alkaloids. To test the effects of Periglandula on nematode colonization, E+ and E- I. tricolor seedlings were grown in soil infested with high densities of M. incognita nematodes (N+) or no nematodes (N-) for four weeks in the greenhouse before harvesting. After harvest, nematode colonization of roots was visualized microscopically, and total gall number and plant biomass were quantified. Four ergot alkaloids were detected in roots of E+ plants, but no alkaloids were found in E- plants. Gall formation was reduced by 50% in E+ plants compared to E- plants, independent of root biomass. Both N+ plants and E+ plants had significantly reduced biomass compared to N- and E- plants, respectively. These results demonstrate Periglandula’s defensive role against biotic enemies, albeit with a potential trade-off with host plant growth.
Keywords: Plant-fungal interactions, Symbiosis, Phytobiome, Defensive mutualism, Morning glory, Endosymbiont, Ipomoea tricolor, Meloidogyne incognita
Introduction
Every plant species sampled to date has been identified to harbor at least one fungal endophyte (Christian et al. 2017). Most of these endophytes colonize tissues by spores from the surrounding environment and can be easily isolated from plant tissues and grown on artificial media (Arnold et al. 2003; Mejía et al. 2014). By contrast, many vertically-transmitted fungal endophytes (endosymbionts) that are inherited from mother to offspring (Afkhami and Rudgers 2008) are difficult to isolate or unculturable (Beaulieu et al. 2015; Grum et al. 2013), requiring in vivo study of these organisms and their interactions with hosts. Strict endosymbionts that are vertically transmitted through the maternal host lineage are predicted to have a mutualistic association with their hosts given that vertically-transmitted endosymbionts with detrimental effects should be eliminated from host populations by natural selection (Clay 2014; Ewald 1987). One common mechanism of mutualism is defense of the host (Clay 1988), often characterized by endosymbiont-produced toxins active against host pests, pathogens and predators (Clay 2014; Leistner and Steiner 2018; Schardl et al. 2013A). For example, tall fescue grass (Schedonorus arundinaceus (syn. Festuca arundinacea and Lolium arundinaceum)) infected by alkaloid-producing Epichloë endosymbionts is more resistant to a range of vertebrate and invertebrate herbivores, and Epichloë increases in frequency within mixed symbiotic/non-symbiotic (E+/E-) populations when subject to high levels of herbivory (Clay et al. 2005). Endosymbiont-produced alkaloids and other chemical compounds play a protective role in many cool-season grasses and in multiple animal systems, where they increase resistance to herbivores and other natural enemies (Clay and Schardl 2002; Currie et al. 1999; Haine 2008; Kellner 2001; Lopanik et al. 2004).
The speciose morning glory family, Convolvulaceae, is globally distributed with a center of diversity in Mexico. Many taxa are herbaceous vines, including Ipomoea tricolor, which grows wild in Central America and is widely cultivated as an ornamental species. The family also includes woody lianas, trees and shrubs and contains various species representing horticultural and agricultural crops, weeds, and medicinal plants (Eserman et al. 2014). Ipomoea is the largest genus within the family, and many species are commonly infected with fungal endosymbionts classified in the genus Periglandula (Beaulieu et al. 2015; Kucht et al. 2004; Panaccione et al. 2014; Steiner et al. 2011).
Vertically-transmitted Periglandula fungi (family Clavicipitaceae) produce ergot alkaloids found in seeds and other tissues of I. tricolor and other morning glory species (Beaulieu et al. 2013; Kucht et al. 2004; Steiner et al. 2011). Periglandula spp. have only been detected in planta and have yet to be cultured in vitro. In I. tricolor, the Periglandula endosymbiont maintains an obligate association inside the plant host and does not produce epiphytic mycelia associated with oil glands commonly found in other morning glory hosts (Ahimsa-Muller et al. 2007; Beaulieu et al. 2015; Leistner and Steiner 2018), and has not been formally described or named. Periglandula spp. contain ergot alkaloid synthesis genes (Schardl et al. 2013b), and only symbiotic morning glory plants produce ergot alkaloids (Florea et al. 2017; Panaccione et al. 2014). Ergot alkaloids share a basic tetracyclic N-containing ring structure, but diverse variants fall into one of three general classes: clavines, simple amides of lysergic acid and ergopeptines, similar to the ergot alkaloids found in Claviceps and Epichloë-infected grasses (Beaulieu et al. 2013; Eich 2008). The lysergic acid amide, ergine, is the most abundant alkaloid detected in previous studies of I. tricolor (Beaulieu et al. 2013; Nowak et al. 2016). Lysergic acid amide derivatives are structurally similar to the semisynthetic psychedelic compound, lysergic acid diethylamide (LSD, Florea et al. 2017). Beaulieu et al. (2013) documented four ergot alkaloids in roots of I. tricolor seedlings (see Fig. 1), raising the possibility that the alkaloids might have activity against below-ground pests and pathogens.
Fig. 1.
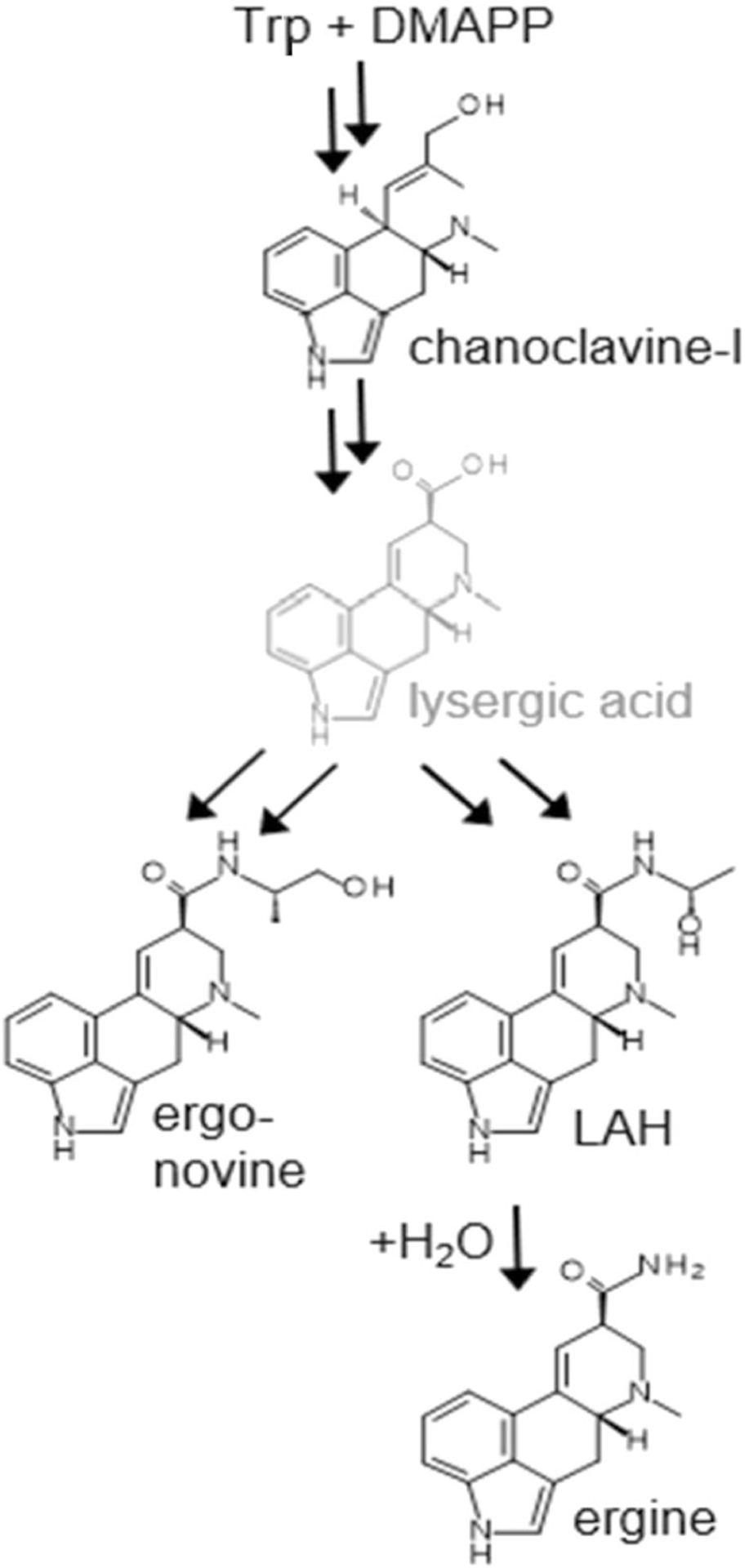
PATHWAY TO ERGOT ALKALOIDS ACCUMULATING IN Ipomoea tricolor. Abbreviations: Trp, tryptophan; DMAPP, dimethylallylpyrophosphate; LAH, lysergic acid α-hydroxyethylamide. Lysergic acid is indicated in gray to indicate that it is rapidly turned over to other alkaloids and does not accumulate. Double arrows indicate one or more omitted intermediates. Ergine results from spontaneous hydrolysis of LAH
The root-knot nematode, Meloidogyne incognita, is an agriculturally significant plant-parasitic nematode with a worldwide distribution and a wide host range, including Ipomoea spp. (Cervantes-Flores and Yencho 2002; Karuri et al. 2017; Ralmi et al. 2016). As an obligate parasite of plant roots, the root-knot nematode induces the plant to develop giant cells inside the roots which form visible galls, or knots, where the nematode lives and feeds (Escobar et al. 2015). There are multiple M. incognita races, and more than 2000 Meloidogyne species that can specialize on a particular set of host species and contribute to plant disease by damaging root systems (Tranier et al. 2014), decreasing fruit yield (Nyczepir and Meyer 2010) and making hosts more susceptible to other parasites (Bridge et al. 1990).
The goal of this study was to test if fungal endosymbiont infection affects root-knot nematode gall formation and growth of I. tricolor and to test the hypothesis that E+ I. tricolor plants would exhibit decreased gall formation on the root system compared to E- plants, consistent with the biological activity of ergot alkaloids and the defensive mutualism hypothesis (Clay 1988). We used endosymbiont-positive (E+) and negative (E-) seedlings and inoculated them with M. incognita (N+) or left them uninoculated (N-). We later quantified gall formation, plant biomass and ergot alkaloid content of plant roots.
Methods and Materials
Study System
Plants were germinated from seed stocks developed from maternal lineages of E+ or E- I. tricolor ‘Pearly Gates’. Experimental lines were created from plants derived from commercial seeds from Plantation Products Inc. (Norton, MA, USA). Half of the original plants were cured of the Periglandula sp. symbiont by treating with 1.3% pyraclostrobin fungicide (BASF) following the methods of Beaulieu et al. (2013). Since Periglandula species are not transmitted horizontally or via pollen (Leistner and Steiner 2018), plants cured of the fungus, along with their seed off-spring, remain E-. Symbiotic status was confirmed by two separate ergot alkaloid analyses of individual seeds (described below) from individual maternal plants before they were designated as E+ or E-.
Nematode Propagation and Inoculum Production
To generate nematode-infested (and nematode-free) soil for subsequent morning glory experiments, pot cultures were derived as follows. We used a population of M. incognita race 3 that was generously provided by the Ploeg Lab (Department of Nematology, University of California Riverside, CA, USA). To establish experimental nematode populations, chopped tomato roots with nematode galls were added to a standard, sterilized greenhouse soil mixture containing 4 parts composted soil, 2 parts peat/Metro Mix (Sun Gro Horticulture), 1 part vermiculite, 1 part perlite and 1 part Osmocote slow-release fertilizer. Then an equal volume of sand was added to this soil mixture. Two-week old tomato seedlings (Solanum lycopersicum ‘Rutgers VFASt’), previously grown in sterile greenhouse soil mixture, were transplanted individually into 110 mm diameter plastic pots and grown for 60–90 days in the greenhouse with supplemental lighting on a 12 h light/12 h dark cycle. Temperature fluctuated on a daily basis depending on outside weather conditions, but room temperature was prevented from falling below 15 °C. Roots from a subsample of plants were checked for visible galls as a measure of nematode colonization. When galls were apparent, aboveground shoots were then cut off and the root mass was removed from pot, cut into smaller pieces and then mixed back into the original pot. A new set of two-week old tomato seedlings were then transplanted into these pots. This process was repeated every 60–90 days to maintain living nematode stocks. Negative controls followed the same protocol, but only nematode-free tomato roots were added to the pots by carefully confirming lack of gall formation on root systems before new seedlings were replanted. Soil from multiple pots in each treatment was homogenized to standardize nematode densities before planting experimental I. tricolor seedlings.
Quantification of Nematode Densities in Soil
30 g of nematode-infested soil was subsampled from four randomized pots in N+ treatments before planting I. tricolor by taking three random 10 g soil samples per pot. For each sample, 10 g of soil was placed into a 50 mL centrifuge tube along with 20 mL of 1.0% NaOCl solution and stirred for 30 s as modified from methods described in Byrd DW et al. (1972) and Starr and Jeger (1985). Each tube was then centrifuged at 3000 rpm for 3 min to separate the solution from the soil. The solution was then poured through a No. 80 sieve, and nematodes were collected using a No. 500 sieve. To quantify the density of the infective second-stage juvenile (J2) nematodes, 20 mL of a 38.5% sugar solution (454 g sugar per 1000 mL of water) was added and stirred for 30 s. Then the sugar solution was centrifuged for 3 min at 3000 rpm, and the solution was poured through the No. 500 sieve to collect J2 nematodes. The solution containing J2 nematodes was then visualized and quantified using microscopy at 40X magnification. Soil from four N- pots was also checked to confirm that no nematodes were detected.
Nematode Colonization of I. tricolor
The production of nematode galls on roots of I. tricolor plants was evaluated using a 2 × 2 fully factorial experimental design with nematode colonization and fungal symbiosis as main factors. Seeds from E+ or E- maternal lines were germinated on blotter paper in glass petri plates and left in natural light at ambient room temperature (24 °C) for 7 to 10 days. Seedlings were then planted in the sterile soil /sand mix, grown for one week and transplanted into 85 mm diameter plastic pots containing homogenized soil with either high nematode densities (N+) or no nematodes (N-) for 28 days before they were harvested. There were four total treatments (N + E+, N + E-, N-E+, and N-E-) with 25 replicate plants per treatment. Three plants per treatment were used for microscopy leaving 88 total experimental pots. Plants in all treatments were grown in the Indiana University greenhouse as described above. An initial harvest of three plants (one at 15 days and two at 21 days) in each treatment was made to confirm nematode colonization of roots at an earlier stage of gall formation (see Acid Fusion Staining below). Four pots in each treatment were also harvested to estimate densities of J2 nematodes in roots and potential gall formation but those data were not analyzed.
Acid Fuchsin Staining
Root samples were harvested for examination of nematode life stages within roots. A subsample of three plants was collected from each treatment, and roots were washed clean of soil and debris. Roots were then agitated in 10% bleach solution (0.525% NaOCl) for 4 min and then rinsed in running water and allowed to soak for 15 min. Roots were then heated to boiling in 30 mL water and 1 mL acid fuchsin stain (3.5 g acid fuchsin, 250 mL acetic acid and 750 mL sterile distilled water) as described by Kirkpatrick et al. (1991). Stained root tissues were then microwaved in a glycerin mixture (30 mL glycerin and 0.05 mL 5 N hydro-chloric acid) until roots became transparent, and nematodes appeared pink. Transparent roots were saved in 1-mL tubes of glycerin for subsequent microscopic examination and photography.
Gall Counts and Plant Growth Metrics
After 28 days of growth in the greenhouse, 18 plants per treatment were harvested, washed and visible gall formation quantified using a hand counter (Kirkpatrick et al. 1991; McBride et al. 1999). 15 plants per treatment were then separated into above- and below-ground tissues and dried at 60 °C for 48 h to obtain total biomass and the fraction of total biomass consisting of roots (root biomass/total biomass) for each plant. In addition, gall numbers were also quantified for three plants per treatment which were not dried and weighed but instead were used for microscopy following acid fuschin staining to confirm gall formation corresponded to nematode colonization of roots. Dried root samples were not suitable for this visualization.
Alkaloid Analyses of Roots
To quantify ergot alkaloids in roots, three random plants per treatment were assayed by the methods of Beaulieu et al. (2013). In brief, 9 to 27 mg of dried root tissue were ground by hand and mixed with 1 mL methanol (99.93% A.C.S. HPLC Grade, Sigma-Aldrich) for 3 days at room temperature, vortexing daily to extract alkaloids. 20 μL of clarified extract was then analyzed by reverse-phase HPLC on a C18 column (Prodigy 5-μm ODS3 [150 mm by 4.6 mm]; Phenomenex, Torrance, CA, USA) with dual fluorescence detectors set at excitation and emission wavelengths of 272 nm/ 372 nm and 310 nm/410 nm as described by Panaccione et al. (2012). Identity of individual peaks was confirmed by LC-MS with electrospray ionization in positive mode as described by Ryan et al. (2013). We also used commercial millet seed as a negative control, given previous studies (Beaulieu et al. 2013) showing that it is alkaloid-free.
Statistical Analyses
Total gall number of M. incognita on root systems of E+ vs. E- plants grown in nematode-infested soil (N + E+ and N + E- treatments) was analyzed using a t-test and by the nonparametric Mann-Whitney U test with N = 18 per treatment to further confirm the results of t-tests. Gall number was also analyzed by one-way ANOVA with root mass as a covariate (ANCOVA). Total plant biomass and root:shoot ratio in all four treatments were analyzed by two way ANOVA where nematode treatment and symbiotic status were the two independent variables. Levene’s tests were consistently satisfied in all ANOVA and ANCOVA’s, and t-tests, and therefore data were not transformed. All analyses were conducted in IBM SPSS Statistics version 24.
Results
Reduced Nematode Gall Formation in Symbiotic Plants
As predicted, the presence of the Periglandula fungal endosymbiont significantly decreased nematode total gall number by ~50% compared to gall numbers of E- plants (Fig. 2, t(34) = 7.72, P < 0.001). Mean gall number per root system was 199.50 ± 9.79 for E- plants and 95.50 ± 9.27 for E+ plants. The nonparametric Mann-Whitney U test further confirmed a highly significant difference between E+ and E- plants (P < 0.001). Gall number was also analyzed with one-way ANOVA with root mass as a covariate, which demonstrated that the symbiotic status remained a significant determinant of gall number (F(1,29) = 6.41, P < 0.018), but also that root dry weight was positively correlated with gall number (F(1,29) = 8.22, P < 0.008) and was independent of symbiotic status.
Fig. 2.

Meloidogyne incognita GALL NUMBERS ON ROOTS OF ENDOSYMBIOTIC (E+) AND NON- ENDOSYMBIOTIC (E-) Ipomoea tricolor PLANTS. Mean gall number for roots from endosymbiotic (E+) plants was 50% lower than non-endosymbiotic (E-) plants (t (34) = 7.72, P < 0.001)
Reduced Plant Biomass in Symbiotic and Nematode Treatments
Both the nematode and endosymbiont treatments significantly affected total plant biomass (F (1,56) = 25.58, P < 0.0001 and F (1,56) =4.82, P = 0.032, respectively (Fig. 3)), but there was no significant N x E interaction between treatments. Mean total biomass was 0.427 ± 0.016 g and 0.309 + 0.16 g for N- and N+ plants respectively, and 0.394 ± 0.016 g and 0.342 ± 0.16 g for E- and E+ plants, respectively. Thus, nematodes and the endosymbiont individually reduced total plant biomass by 28% and 13%, respectively. When tested with the same model, there was a significant effect of nematode treatment on root biomass fraction (F (1,56) = 79.81, P < 0.001), but no significant effect of the endosymbiont treatment or the N x E interaction on root biomass fraction. The fraction of total biomass consisting of roots was 0.167 ± 0.005 for N- plants and 0.228 ± 0.005 for N+ plants.
Fig. 3.

BIOMASS OF Ipomoea tricolor IN NEMATODE AND ENDOSYMBIONT TREATMENTS. Total plant biomass varied significantly among both nematode (N- and N+) and endosymbiont (E-and E+) treatments (F (1,56) = 25.58, P < 0.0001 and F (1,56) = 4.82, P = 0.032 respectively) with no interaction
Alkaloid Content of Roots
Ergot alkaloids were quantified in roots of E+ and E- I. tricolor plants. Detection of alkaloids in root samples agreed with predicted symbiont status, where only E+ plants had detectable ergot alkaloids. Four individual ergot alkaloids representing two classes were detected in E+ root systems (clavine: chanoclavine; lysergic acid amides: ergonovine, lysergic acid α-hydroxyethylamide (LAH), and ergine) (Table 1). The chemical structures are presented in Fig. 1. These were the same alkaloids reported by Beaulieu et al. (2013) for I. tricolor ‘Flying Saucers’ compared to ‘Pearly Gates’ tested here. The simple lysergic acid amide, ergine, was the most abundant alkaloid with the highest detected concentrations (Table 1). Among a subsample of E+ root samples (#5–7 and 11–13) total concentrations ranged from 36 to 135 μg/g dry weight of root, whereas E- root samples (Plant IDs #2–4 and 8–10; data not shown) had no detectable ergot alkaloids, nor did the millet negative control (Table 1). Although sample sizes were low, mean alkaloid concentration of N-E+ roots (110.6 μg/g) was significantly higher than mean concentration in N + E+ roots (61.03 μg/g) (t-test, P = 0.0335), suggesting there was no induction of ergot alkaloid accumulation but potential alkaloid suppression by nematode infection. Most root samples had higher total concentrations of ergot alkaloids than did a seed sample taken from an N-E+ plant.
Table 1.
ERGOT ALKALOIDS DETECTED IN Ipomoea tricolor ROOT SAMPLES
| Plant ID |
Treatment | Root Mass (mg) | ergine μg/g |
LAH μg/g |
ergonovine μg/g |
chanoclavine-I μg/g |
Total μg/g |
|---|---|---|---|---|---|---|---|
| 1 | Millet | - | 0 | 0 | 0 | 0 | 0 |
| 5 | N + E+ | 10 | 57.6 | 0.9 | 1.6 | 1.5 | 61.6 |
| 6 | N + E+ | 9 | 35.6 | 2.7 | 1.0 | 0.6 | 39.9 |
| 7 | N + E+ | 12 | 89.9 | 0.7 | 3.3 | 2.8 | 96.8 |
| 11 | N−E+ | 12 | 134.7 | 1.3 | 6.5 | 2.3 | 144.8 |
| 12 | N−E+ | 27 | 99.3 | 3.9 | 5.2 | 1.9 | 110.3 |
| 13 | N−E+ | 11 | 97.8 | 7.6 | 2.1 | 4.1 | 111.5 |
| 14 | MG seed | - | 39.7 | 1.2 | 7.1 | 8.1 | 56.1 |
Cells with zero indicate that no ergot alkaloids were detected in the specified sample (limit of detection 10 ng/g). Sample 1 is from millet seeds (15 mg, negative control) and sample 14 is morning glory (MG) seed (24 mg, positive control) from E+ I. tricolor seed, not roots
Nematode Densities in Soil and Roots
J2 nematodes were quantified in 30 g of soil sampled from four pots per treatment (with each pot containing ~ 240 g of soil) prior to planting I. tricolor seedlings. Based on nematode counts from the soil samples, we estimate that there were 1728 J2 nematodes per pot in the N+ treatments and no nematodes in the N- treatments. Nematodes were also visualized microscopically by acid fuchsin-staining both within roots and within galls (Fig. 4), suggesting growth of M. incognita was inhibited within E+ plants relative to E- roots.
Fig. 4.

ACID FUCHSIN STAINED NEMATODES INSIDE Ipomoea tricolor ROOTS 15 DAYS POST INOCULATION. Microscopic images of acid fuchsin stained nematodes inside I. tricolor roots at 15 days post inoculation. Nematodes appear magenta. A) Two Meloidogyne incognita second-stage juveniles (J2) inside N + E+ roots and B) one fourth-stage juvenile (J4) inside N + E- root gall after inducing giant cells. Both images are sized to the same scale. Scale bar, 200 μm
Discussion
Our results indicate that presence of the Periglandula fungal endosymbiont significantly decreased M. incognita gall numbers on I. tricolor root systems by 50% compared to E- plants, and that this result was not dependent on the size of the root system. E+ plants also accumulated Periglandula-produced ergot alkaloids in their roots, whereas E- plants were alkaloid free. Both symbiosis and nematode colonization had a significant effect on plant biomass where both N+ and E+ treatments decreased host plant biomass after 4 weeks of growth in the greenhouse relative to N- and E- controls. Thus, our results support the hypothesis that alkaloid production by the endosymbiont reduces pest damage, but this protection may entail a trade-off with host biomass, at least at early stages of plant growth.
To our knowledge, this is the first report of endosymbiont-produced ergot alkaloids in roots of mature I. tricolor plants (beyond the first-leaf stage reported by Beaulieu et al. 2013), or any other morning glory species. While Panaccione et al. (2006) found low concentrations of some ergot alkaloids in roots of perennial ryegrass, there is not strong evidence for accumulation of significant concentrations of ergot alkaloids in roots of any grasses infected by Epichloë endophytes. By contrast, Malinowski et al. (1999) reported high concentrations of Epichloë-produced pyrrolizidine alkaloids in tall fescue roots (1083 μg/g). Beaulieu et al. (2013) treated I. tricolor seedlings with fungicide and concluded that alkaloids in roots of young seedlings (at the first true leaf stage) originated from the seed and that de novo alkaloid production did not occur until after the first true leaf was produced. Our results indicate that ergot alkaloids are being synthesized by Periglandula in older plants and are accumulating in roots, potentially providing protection against plant-parasitic nematodes. All four of the ergot alkaloids previously reported in I. tricolor ‘Flying Saucers’ (Beaulieu et al. 2013) were also detected here in roots of ‘Pearly Gates’ in both endosymbiont treatments (N + E+, N-E+), but not in endosymbiont-free treatments (N-E-, N + E-), further confirming that the alkaloids are synthesized by Periglandula. We do not have direct evidence of where Periglandula is localized within I. tricolor. In other morning glories and in Epichloë-infected grasses, the fungus colonizes above-ground tissues, but Periglandula might potentially also colonize the roots of I. tricolor.
Our results are consistent with defensive mutualism (Clay 1988; Clay 2014; Janzen 1985) by demonstrating endosymbiont-mediated resistance of I. tricolor to a plant-parasitic nematode. In a previous study, Amor-Prats and Harborne (1993) showed that ergot alkaloids from I. parasitica, when incorporated into artificial media, reduced the growth and survival of tobacco budworm larvae (Heliothis virescens) (see also Bacetty et al. (1999A, B) and Clay and Cheplick (1989)). In addition, livestock poisonings following consumption of Ipomoea species known to be symbiotic with Periglandula and to contain ergot alkaloids have been reported, but no comparisons were made with nonsymbiotic plants nor were any toxins identified (Barbosa et al. 2006; Gardiner et al. 1965). In a recent study, Kaur et al. (2018) screened 14 species of Convolvulaceae and detected Periglandula sequences in 11 but ergot alkaloids in only five of those 11 species. Feeding trials of morning glory species with the potato psyllid (Bactericera cockerelli) demonstrated rapid mortality on plant species containing Periglandula and ergot alkaloids, but there was no effect in species with Periglandula but lacking ergot alkaloids, suggesting that ergot alkaloids may contribute to psyllid mortality. Interestingly, they report only two of the four ergot alkaloids that we detected in I. tricolor. Here, nematode gall formation was significantly decreased by 50% in E+ I. tricolor plants compared to E-plants. Ergot alkaloids in roots may deter nematode colonization or inhibit nematode development within roots, resulting in reduced gall formation in endosymbiotic I. tricolor. However, Periglandula symbiosis may also induce physiological or biochemical changes in the host plant, or produce other types of nematicidal compounds, that could potentially generate similar results (Lee et al. 2017; Malinowski et al. 1999; Panaccione et al. 2006), independently or in conjunction with antibiosis effects of ergot alkaloids.
While previous studies with grasses infected by related, ergot alkaloid-producing fungal endosymbionts have primarily reported protection against arthropod and vertebrate herbivores (Bush et al. 1997), nematode antagonism has also been reported (Schouten 2016; Zhou et al. 2018). For example, West et al. (1988) found that populations of Pratylenchus scribneri and Tylenchorhynchus acutus were significantly lower in soil in field plots of E+ tall fescue compared to E- plots. Similarly, in a greenhouse experiment, Elmi et al. (2000) found that the root-knot nematode, M. marylandi, had rapid population growth in pots with E- tall fescue plants but almost no survival in E+ pots, while Bacetty et al. (2009A) found that E+ tall fescue plants supported P. scribneri populations <1% the size of populations on E- plants. Likewise, Timper et al. (2005) reported that tall fescue grasses infected with non-ergot alkaloid producing endophyte strains supported higher numbers of nematodes than the wild-type, ergot alkaloid producing endophyte in greenhouse pots, and Jia et al. (2013) found E+ grasses of several cultivated and wild grass-endophyte associations had a negative effect on nematode chemotaxis and root penetration, and extracts from culture filtrates caused increased nematode mortality. It is important to note that in each of these greenhouse or field studies, the concentrations of ergot alkaloids were not reported. Other studies have examined nematicidal effects of pure ergot alkaloids in vitro. For example, Bacetty et al. (2009B) found that ergovaline acted as a repellent and caused complete P. scribneri mortality. Similarly, Panaccione (2005) found that the ergotamine (an ergopeptine closely related to ergovaline), but not three simpler ergot alkaloids, was nematicidal to P. scribneri. By contrast, Panaccione et al. (2006) used gene knockout Epichloë strains that lack ergot alkaloid production or had an altered alkaloid profile to examine population growth of P. scribneri but found no evidence that ergot alkaloids contribute to suppression of nematode populations and found only very low or undetectable alkaloid concentrations in roots. Thus, many, but not all, studies with grasses suggest that Epichloë infection, pure ergot alkaloids or culture filtrates inhibit nematodes, but prior to our study it has not been clearly demonstrated that ergot alkaloids occur in roots in effective concentrations and are associated with reduced nematode gall formation.
The reduction of plant biomass with nematode colonization was expected given the status of M. incognita as a significant pest in agriculture, and the negative effect of plant-parasitic nematodes in other systems (van der Putten et al. 1993; Yeates 1999). It was less expected that the endosymbiont would reduce plant growth, but its metabolic costs to the host plant may be relatively higher in younger vs. older plants. For example, inhibition of growth of E+ tall fescue and perennial ryegrass (Lolium perenne) (vs. E- plants) at the seedling stage shifted to growth enhancement of E+ plants at later developmental stages (Cheplick et al. 1989). Here experimental plants were grown for 4 weeks in the greenhouse, but longer-term studies are required to determine the long-term fitness impacts of endosymbiosis on later life history stages. If Periglandula infection has long-term detrimental effects on host plant growth and survival, we would not expect it to persist or to occur at high frequencies in I. tricolor and other morning glory species.
Our results suggest that other morning glory species should be examined to determine whether alkaloid accumulation in roots and antagonism of nematodes extend to other convolvulaceous Periglandula hosts. In addition, the potential inhibitory effects on other root-feeding pests or pathogens should also be examined. For example, in Epichloë-infected turfgrasses, there are reduced populations of root-feeding grass grubs (Costelytra zealandica) and Japanese beetle grubs (Popillia japonica Newman) in soil compared to E- controls (Patchett et al. 2011; Potter et al. 1992). Moreover, other types of fungal alkaloids such as loline alkaloids might also occur in roots and have insecticidal or nematicidal effects. For example, Lee et al. (2017) reported the occurrence of indole diterpene alkaloids in two other Periglandula-infected Ipomoea species, and those alkaloids could potentially occur in roots of I. tricolor as well.
E+ I. tricolor could potentially have significant impacts on plant community dynamics and trophic interactions relative to non-symbiotic E- plants. For example, ergot alkaloids may leach from the roots and influence the rhizosphere community and co-occurring plant species. In particular, E+ I. tricolor could potentially reduce nematode damage in other, co-occurring plant species when intermixed (Vandermeer 1989) or planted as a companion plant (Franck 1983), consistent with the concept of associational resistance (Barbosa et al. 2009), or when planted as a rotation crop. For example, Anaya et al. (1990) report that peasant farmers in Mexico grow I. tricolor as a cover crop before planting sugar cane in order to reduce weed populations. They suggest that I. tricolor has allelopathic effects on weed species but it might also suppress nematode populations in the soil to the benefit of sugar cane (Saccharum officinarum). However, some morning glory species are noxious weeds themselves, and growers might be reluctant to plant them for biocontrol of crop pests. Our results show reduced colonization of I. tricolor root tissues by nematodes and suggest that E+ I. tricolor could potentially decrease overall soil densities of M. incognita and their infestation of other co-occurring species.
Acknowledgements
Authors are grateful to Yuan-Zheng Zhao, Chuwen Li, Michael Frisby, Clay lab members, Indiana University (IU) Greenhouse staff, IU Light Microscopy Imaging Center, Dr. Erik Ragsdale and lab members (IU) and Dr. Antoon Ploeg (University of California-Riverside) for all contributions and technical support for the success of this research. L.D. was supported by a National Science Foundation Graduate Research Fellowship. D.G.P. was supported by National Institutes of Health grant 2R15GM114774-2 and Hatch funds. This research was financially supported in part by grant 429440 from the Simons Foundation to the Smithsonian Tropical Research Institute (W. Wcislo, P.I.).
References
- Afkhami ME, Rudgers JA (2008) Symbiosis lost: imperfect vertical transmission of fungal endophytes in grasses. Am Nat 172:405–416 [DOI] [PubMed] [Google Scholar]
- Ahimsa-Muller MA, Markert A, Hellwig S, Knoop V, Steiner U, Drewke C, Leistner E (2007) Clavicipitaceous fungi associated with ergoline alkaloid-containing Convolvulaceae. J Nat Prod 70:1955–1960 [DOI] [PubMed] [Google Scholar]
- Amor-Prats D, Harborne JB (1993) Allelochemical effects of ergoline alkaloids from Ipomoea parasitica on Heliothis virescens. Chemoecology 4:55–61 [Google Scholar]
- Anaya AL, Calera MR, Mata R, Pereda-Miranda R (1990) Allelopathic potential of compounds isolated from Ipomoea tricolor Cav. (Convolvulaceae). J Chem Ecol 16:2145–2152 [DOI] [PubMed] [Google Scholar]
- Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA (2003) Fungal endophytes limit pathogen damage in a tropical tree. Proc Natl Acad Sci U S A 100:15649–15654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacetty AA, Snook ME, Glenn AE, Noe JP, Hill N, Culbreath A, Timper P, Nagabhyru P, Bacon CW (2009a) Toxicity of endophyte-infected tall fescue alkaloids and grass metabolites on Pratylenchus scribneri. Phytopathology 99:1336–1345 [DOI] [PubMed] [Google Scholar]
- Bacetty AA, Snook ME, Glenn AE, Noe JP, Nagabhyru P, Bacon CW (2009b) Chemotaxis disruption in Pratylenchus scribneri by tall fescue root extracts and alkaloids. J Chem Ecol 35:844–850 [DOI] [PubMed] [Google Scholar]
- Barbosa RC, Riet-Correa F, Medeiros RM, Lima EF, Barros SS, Gimeno EJ, Molyneux RJ, Gardner DR (2006) Intoxication by Ipomoea sericophylla and Ipomoea riedelii in goats in the state of Paraíba, northeastern Brazil. Toxicon 47:371–379 [DOI] [PubMed] [Google Scholar]
- Barbosa P, Hines J, Kaplan I, Martinson H, Szczepaniec A, Szendrei Z (2009) Associational resistance and associational susceptibility: having right or wrong neighbors. Ann Rev Ecol Evol Syst 40:1–20 [Google Scholar]
- Beaulieu WT, Panaccione DG, Hazekamp CS, Mckee MC, Ryan KL, Clay K (2013) Differential allocation of seed-borne ergot alkaloids during early ontogeny of morning glories (Convolvulaceae). J Chem Ecol 39:919–930 [DOI] [PubMed] [Google Scholar]
- Beaulieu WT, Panaccione DG, Ryan KL, Kaonongbua W, Clay K (2015) Phylogenetic and chemotypic diversity of Periglandula species in eight new morning glory hosts (Convolvulaceae). Mycologia 107: 667–678 [DOI] [PubMed] [Google Scholar]
- Bridge J, Plowright RA, Peng D (1990) Nematode parasites of rice In: Luc M (ed) Plant parasitic nematodes in subtropical and tropical agriculture. CABI Publishing, Wallingford, UK, pp 69–108 [Google Scholar]
- Bush LP, Wilkinson HH, Schardl CL (1997) Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol 114:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DW Jr, Ferris HO, Nusbaum CJ (1972) A method for estimating numbers of eggs of Meloidogyne spp. in soil. J Nematology 4:266–269 [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Flores JC, Yencho GC (2002) Host reactions of sweetpotato genotypes to root-knot nematodes and variation in virulence of Meloidogyne incognita populations. HortScience. 37:1112–1116 [Google Scholar]
- Cheplick GP, Clay K, Wray S (1989) Interactions between fungal endophyte infection and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytol 111:89–97 [Google Scholar]
- Christian N, Herre EA, Mejia LC, Clay K (2017) Exposure to the leaf litter microbiome of healthy adults protects seedlings from pathogen damage. Proc R Soc B 284:20170641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay K (1988) Fungal endophytes of grasses: a defensive mutualism between plants and fungi. Ecology 69:10–16 [Google Scholar]
- Clay K (2014) Defensive symbiosis: a microbial perspective. Funct Ecol 28:293–298 [Google Scholar]
- Clay K, Cheplick GP (1989) Effect of ergot alkaloids from fungal endophyte-infected grasses on the fall armyworm (Spodoptera frugiperda). J Chem Ecol 15:169–182 [DOI] [PubMed] [Google Scholar]
- Clay K, Schardl C (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160:S99–S127 [DOI] [PubMed] [Google Scholar]
- Clay K, Holah J, Rudgers JA (2005) Herbivores cause a rapid increase in hereditary symbiosis and alter plant community composition. Proc Natl Acad Sci U S A 102:12465–12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie CR, Scott JA, Summerbell RC, Malloch D (1999) Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398:701 [Google Scholar]
- Eich E (2008) Solanaceae and Convolvulaceae: secondary metabolites. Springer-Verlag, Berlin [Google Scholar]
- Elmi AA, West CP, Robbins RT, Kirkpatrick TL (2000) Endophyte effects on reproduction of a root-knot nematode (Meloidogyne marylandi) and osmotic adjustment in tall fescue. Grass Forage Sci 55:166–172 [Google Scholar]
- Escobar C, Barcala M, Cabrera J, Fenoll C (2015) Overview of root-knot nematodes and giant cells. Adv Bot Res 73:1–32 [Google Scholar]
- Eserman LA, Tiley GP, Jarret RL, Leebens-Mack JH, Miller RE (2014) Phylogenetics and diversification of morning glories (tribe Ipomoeeae, Convolvulaceae) based on whole plastome sequences. Am J Bot 101:92–103 [DOI] [PubMed] [Google Scholar]
- Ewald PW (1987) Transmission modes and evolution of the parasitismmutualism continuum. Annals NY Acad Sci 503:295–306 [DOI] [PubMed] [Google Scholar]
- Florea S, Panaccione DG, Schardl CL (2017) Ergot alkaloids of the family Clavicipitaceae. Phytopathology 107:504–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck G (1983) Companion planting: successful gardening the organic way. Thorsons Publishing, London [Google Scholar]
- Gardiner MR, Royce R, Oldroyd B (1965) Ipomoea muelleri intoxication of sheep in Western Australia. Brit Vet J 121:272–277 [Google Scholar]
- Grum DS, Cook D, Baucom D, Mott IW, Gardner DR, Creamer R, Allen JG (2013) Production of the alkaloid swainsonine by a fungal endophyte in the host Swainsona canescens. J Nat Prod 76:1984–1988 [DOI] [PubMed] [Google Scholar]
- Haine ER (2008) Symbiont-mediated protection. Proc Roy Soc B 275: 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen DH (1985) The natural history of mutualisms In: Boucher D (ed) The biology of mutualism: ecology and evolution. Oxford University Press, Oxford, pp 40–99 [Google Scholar]
- Jia C, Ruan WB, Zhu MJ, Ren AZ, Gao YB (2013) Potential antagonism of cultivated and wild grass–endophyte associations towards Meloidogyne incognita. Biol Control 64:225–230 [Google Scholar]
- Karuri HW, Olago D, Neilson R, Mararo E, Villinger J (2017) A survey of root knot nematodes and resistance to Meloidogyne incognita in sweet potato varieties from Kenyan fields. Crop Prot 92:114–121 [Google Scholar]
- Kaur N, Cooper WR, Duringer JM, Badillo-Vargas IE, Esparza-Diaz G, Rashed A, Horton DR (2018) Survival and development of potato psyllid (Hemiptera: Triozidae) on Convolvulaceae: effects of a plant-fungus symbiosis (Periglandula). PLoS One 13:e0201506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner RL (2001) Suppression of pederin biosynthesis through antibiotic elimination of endosymbionts in Paederus sabaeus. J Insect Physiol 47:475–483 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick TL, Oosterhuis DM, Wullschleger SD (1991) Interaction of Meloidogyne incognita and water stress in two cotton cultivars. J Nematol 23:462. [PMC free article] [PubMed] [Google Scholar]
- Kucht S, Groß J, Hussein Y, Grothe T, Keller U, Basar S, König WA, Steiner U, Leistner E (2004) Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta 219:619–625 [DOI] [PubMed] [Google Scholar]
- Lee ST, Gardner DR, Cook D (2017) Identification of indole diterpenes in Ipomoea asarifolia and Ipomoea muelleri, plants tremorgenic to livestock. J Ag Food Chem 65:5266–5277 [DOI] [PubMed] [Google Scholar]
- Leistner E, Steiner U (2018) The genus Periglandula and its symbiotum with morning glory plants (Convolvulaceae) In: Anke T, Schuffler A (eds) The Mycota XV. Springer, Berlin, pp 131–147 [Google Scholar]
- Lopanik N, Lindquist N, Targett N (2004) Potent cytotoxins produced by a microbial symbiont protect host larvae from predation. Oecologia 139:131–139 [DOI] [PubMed] [Google Scholar]
- Malinowski DP, Belesky DP, Fedders JM (1999) Endophyte infection may affect the competitive ability of tall fescue grown with red clover. J Agron Crop Sci 183:91–101 [Google Scholar]
- McBride RG, Mikkelsen RL, Barker KR (1999) A comparison of three methods for determining root-knot nematode infection of cotton roots. Nematropica 29:147–151 [Google Scholar]
- Mejía LC, Herre EA, Sparks JP, Winter K, García MN, Van Bael SA, Stitt J, Shi Z, Zhang Y, Guiltinan MJ, Maximova SN (2014) Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front Microbiol 12:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J, Woźniakiewicz M, Klepacki P, Sowa A, Kościelniak P (2016) Identification and determination of ergot alkaloids in morning glory cultivars. Anal Bioanal Chem 408:3093–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyczepir AP, Meyer SL (2010) Host status of endophyte-infected and noninfected tall fescue grass to Meloidogyne spp. J Nematol 42:151. [PMC free article] [PubMed] [Google Scholar]
- Panaccione DG (2005) Origins and significance of ergot alkaloid diversity in fungi. FEMS Microbiol Lett 251:9–17 [DOI] [PubMed] [Google Scholar]
- Panaccione DG, Kotcon JB, Schardl CL, Johnson RD, Morton JB (2006) Ergot alkaloids are not essential for endophytic fungus-associated population suppression of the lesion nematode, Pratylenchus scribneri, on perennial ryegrass. Nematology 8:583–590 [Google Scholar]
- Panaccione DG, Ryan KL, Schardl CL, Florea S (2012) Analysis and modification of ergot alkaloid profiles in fungi. Meth Enzymol 515:267–290 [DOI] [PubMed] [Google Scholar]
- Panaccione DG, Beaulieu WT, Cook D (2014) Bioactive alkaloids in vertically transmitted fungal endophytes. Funct Ecol 28:299–314 [Google Scholar]
- Patchett B, Gooneratne R, Chapman B, Fletcher L (2011) Effects of loline-producing endophyte-infected meadow fescue ecotypes on New Zealand grass grub (Costelytra zealandica). NZ J Agric Res 54:303–313 [Google Scholar]
- Potter DA, Patterson CG, Redmond CT (1992) Influence of turfgrass species and tall fescue endophyte on feeding ecology of Japanese beetle and southern masked chafer grubs (Coleoptera: Scarabaeidae). J Econ Entomol 85:900–909 [Google Scholar]
- Ralmi NH, Khandaker MM, Mat N (2016) Occurrence and control of root knot nematode in crops: a review. Aust J Crop Sci 11:1649 [Google Scholar]
- Ryan KL, Moore CT, Panaccione DG (2013) Partial reconstruction of the ergot alkaloid pathway by heterologous gene expression in Aspergillus nidulans. Toxins 5:445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl CL, Young CA, Pan J, Florea S, Takach JE, Panaccione DG, Farman ML, Webb JS, Jaromczyk J, Charlton ND, Nagabhyru P (2013a) Currencies of mutualisms: sources of alkaloid genes in vertically transmitted epichloae. Toxins 5:1064–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl CL, Young CA, Hesse U, Amyotte SG, Andreeva K, Calie PJ, Fleetwood DJ, Haws DC, Moore N, Oeser B, Panaccione DG, Schweri K, Voisey CR, Farman ML, Jaromczyk JW, Roe BA, O’Sullivan DM, Scott B, Tudzynski P, An Z, Arnaoudova EG, Bullock CT, Charlton ND, Chen L, Cox M, Dinkins RD, Florea S, Glenn AE, Gordon A, Güldener U, Harris DR, Hollin W, Jaromczyk J, Johnon RD, Khan AK, Leistner E, Li C, Liu JG, Liu J, Liu M, Mace W, Machado C, Nagabhyru P, Pan J, Schmid J, Sugawara K, Steiner U, Takach JE, Tanaka E, Webb JS, Wilson EV, Wiseman JL, Yoshida R, Zeng Z (2013b) Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet 28:e1003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten A (2016) Mechanisms involved in nematode control by endophytic fungi. Annu Rev Phytopathol 54:121–142 [DOI] [PubMed] [Google Scholar]
- Starr JL, Jeger MJ (1985) Dynamics of winter survival of eggs and juveniles of Meloidogyne incognita and M. arenaria. J Nematol 17:252. [PMC free article] [PubMed] [Google Scholar]
- Steiner U, Leibner S, Schardl CL, Leuchtmann A, Leistner E (2011) Periglandula, a new fungal genus within the Clavicipitaceae and its association with Convolvulaceae. Mycologia 103:1133–1114 [DOI] [PubMed] [Google Scholar]
- Timper P, Gates RN, Bouton JH (2005) Response of Pratylenchus spp. in tall fescue infected with different strains of the fungal endophyte Neotyphodium coenophialum. Nematology 7:105–110 [Google Scholar]
- Tranier MS, Pognant-Gros J, Quiroz RD, González CN, Mateille T, Roussos S (2014) Commercial biological control agents targeted against plant-parasitic root-knot nematodes. Braz Arch Biol Tech 57:831–841 [Google Scholar]
- van der Putten WH, Van Dijk C, Peters BAM (1993) Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature 362:53 [Google Scholar]
- Vandermeer JH (1989) The ecology of intercropping. Cambridge University Press, Cambridge [Google Scholar]
- West CP, Izekor E, Oosterhuis DM, Robbins RT (1988) The effect of Acremonium coenophialum on the growth and nematode infestation of tall fescue. Plant Soil 112:3–6 [Google Scholar]
- Yeates GW (1999) Effects of plants on nematode community structure. Annu Rev Phytopathol 37:127–149 [DOI] [PubMed] [Google Scholar]
- Zhou W, Wheeler TA, Starr JL, Valencia CU, Sword GA (2018) A fungal endophyte defensive symbiosis affects plant-nematode interactions in cotton. Plant Soil 422:251–266 [Google Scholar]


