Abstract
Stem cells play an irreplaceable role in the development, homeostasis, and regeneration of the craniofacial bone. Multiple populations of tissue-resident craniofacial skeletal stem cells have been identified in different stem cell niches, including the cranial periosteum, jawbone marrow, temporomandibular joint, cranial sutures, and periodontium. These cells exhibit self-renewal and multidirectional differentiation abilities. Here, we summarized the properties of craniofacial skeletal stem cells, based on their spatial distribution. Specifically, we focused on the in vivo genetic fate mapping of stem cells, by exploring specific stem cell markers and observing their lineage commitment in both the homeostatic and regenerative states. Finally, we discussed their application in regenerative medicine.
1. Introduction
The reconstruction of craniofacial bone defects is more challenging than that of the limb bone, as it requires both functional and esthetic recovery. Traditional therapies to regenerate craniofacial bone, including autologous bone grafts, allografts, and xenografts [1–3], exhibit different limitations and often fail to meet the demands of recovery [4–6]. Stem cell-guided regenerative medicine is an alternative that is currently the most promising approach to solve this problem.
Mesenchymal stem cells (MSCs) are groups of cells residing in different tissues and niches, such as the bone marrow, adipose tissue, teeth, and umbilical cord tissue. MSCs have been extensively used in tissue repair, organ reconstruction, immunomodulation, and even in the treatment of disease [7–11]. In addition, self-cell-constituted implantation results in reduced immunogenicity, and the molecules excreted from MSCs are beneficial for tissue recovery [12, 13]. The combination of MSCs with bioscaffolds further promoted MSC-based therapy by guiding MSC proliferation and migration [14].
To identify and isolate MSCs easily in vitro, the International Society for Cellular Therapy has proposed three criteria to define MSCs [15]. First, the isolated cells can adhere to plastic plates when cultured in vitro. Second, the cells express the CD73, CD90, and CD105 surface markers but not CD34, CD45, CD14 or CD11b, CD79a or CD19, and HLA-DR. Third, the cells can differentiate into osteocytes, chondrocytes, and adipocytes. In addition to in vitro characterization, the recent application and improvement of the fluorescent reporter mouse system and lineage tracing technique make the in vivo study of stem cells feasible [16]. Importantly, the in vivo study of stem cells can aid in accurately recapitulating the niche-dependent functions and interactions of stem cells.
MSCs from bones, including the bone marrow, periosteum, growth plate, and calvarium, have been the most thoroughly studied. It is now recognized that bone MSCs are highly heterogeneous populations that display variable self-renewal and differentiation potential. MSCs that commit to skeletal lineages and express selective surface markers (e.g., leptin receptor, PDGFRα, nestin, Cxcl12, Hox11, PTHrP, Sca1, Ctsk, Axin2, and Gli1) are now defined as skeletal stem cells (SSCs). Craniofacial SSCs are subgroups of cells residing in the calvarium, maxillary and mandibular bones, and tooth-supporting tissue. These cells display the basic characteristics of SSCs and are capable of self-renewal and multilineage differentiation. They can regenerate oral tissues and repair critical defects of craniofacial bones [17–20]. However, craniofacial SSCs are distinct from long bone SSCs, which might result from the different developmental origins and stem cell microenvironments/niches. The craniofacial bone originates from the mesoderm and neural crest, and the bony structure is formed by intramembranous ossification. Long bones, on the other hand, mainly originate from the mesoderm and are formed by endochondral ossification [20, 21]. In addition, the craniofacial bone is a flat bone with limited bone marrow, but the long bone is enriched with the bone marrow. Hematopoiesis-depleted or hematopoiesis-enriched-enriched environments result in totally different stem cell niches. Therefore, craniofacial SSCs are different from SSCs in long bones or other tissues. Interestingly, studies have shown that SSCs/MSCs from craniofacial bones exhibit superior osteogenic properties compared with long bone SSCs/MSCs in craniofacial tissue reconstruction [22–24]. A pioneering study also found that postnatal lineage-restricted craniofacial SSCs reverted to their embryonic plastic state and regained a neural crest cell phenotype in response to mandibular distraction for jaw regeneration [25]. Identifying subpopulations and illustrating the properties of craniofacial SSCs are thus crucial to stem cell-guided regenerative medicine.
In this review, we summarize the cranial and maxillofacial tissues in which stem cells reside as well as the characteristics of these stem cells and advancements in their applications.
2. Periosteum
The surface of the bone is covered by the periosteum, which is a 50-150 μm two-layer membrane with an abundance of nerves and blood vessels (Figure 1). The outer fibrous layer is adjacent to the surrounding soft fibrous and muscular tissue, while the inner layer is highly vascularized and provides a niche for progenitor cells [26, 27]. A large number of studies have already shown that precursor cells residing in the craniofacial bone periosteum play an important role in bone regeneration [28]. For a long time, no specific cell marker was available to identify and isolate craniofacial periosteum-derived stem cells (PSCs) [28, 29]. Recently, two specific surface makers (Ctsk+ and Mx1+αSMA+) have been identified.
Figure 1.
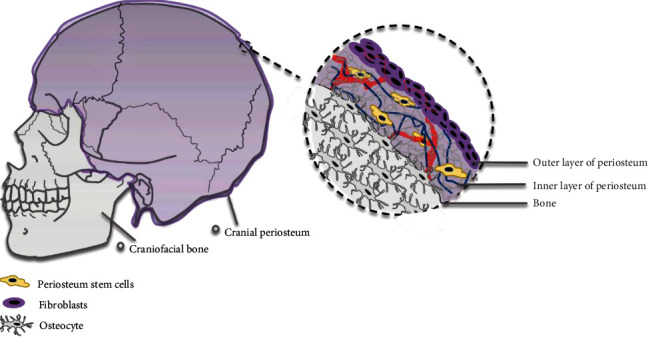
Stem cell distribution in the cranial bone. The stem cells are located in the inner layer of calvarium periosteum.
Cathepsin K (CTSK) has long been regarded as a specific marker for osteoclasts. Using Ctsk-mGFP transgenic mice to trace cell lineages combined with single-cell RNA sequencing, Debnath et al. identified Ctsk+ periosteum stem cells as both long bone and calvarial periosteal skeletal stem cells (PSCs). Ctsk+ PSCs are capable of self-renewal, colony formation, and multilineage differentiation. Interestingly, Ctsk+ PSCs are highly plastic, as they can mediate not only intramembranous ossification but also endochondral ossification in response to bone injury [30]. In 2019, Park et al. observed that a group of postnatal long-term Mx1+αSMA+ periosteal stem cells contributed significantly to the injury repair of bone defects. In addition to being capable of self-renewal and clonal multipotency, Mx1+αSMA+ PSCs can migrate toward the injury site in response to a CCR5 ligand- (CCL5-) dependent mechanism, as visualized by in vivo real-time imaging of the calvarium [31].
3. Craniofacial Bone Marrow
Given that jawbones and teeth in the craniofacial system originate from the cranial neural crest, marrow stem cells in jawbones are considered to have characteristics different from those of long bone MSCs. Studies have been performed to compare the similarities and differences between stem cells in the craniofacial, axial, and appendicular regions. Human MSCs in the jawbone and iliac crest have been the most commonly studied, as these sites are ideal for marrow aspiration. Akintoye et al. cultured jawbone MSCs and iliac crest MSCs from the same individual and found that jawbone MSCs displayed a higher proliferation rate, delayed senescence, and greater differentiation potential. In vivo transplantation results showed that jawbone MSCs formed more bone, whereas iliac crest MSCs formed more compacted bone along with hematopoietic tissue [32]. Using tube formation assays and 3D fibrin vasculogenic tests, Du et al. found that jawbone MSCs showed stronger angiogenic propensities than iliac crest MSCs when they were cocultured with human umbilical vein endothelial cells (HUVECs). Coculture with jawbone MSCs allowed HUVECs to form more tube-like structures in vitro and larger vessels in vivo [33]. The increase in the expression of the basic fibroblast growth factor (bFGF) by jawbone MSCs is the key factor contributing to angiogenesis. However, the chondrogenic and adipogenic potential of jawbone MSCs is weaker than that of iliac crest MSCs [34, 35].
Several populations of SSCs in the long bone marrow were identified, including leptin-receptor-expressing (LepR+) SSCs, nestin-expressing (Nestin+) SSCs, Gremlin 1-expressing (Grem1+) SSCs, glioma-associated oncogene 1-expressing (Gli1+) SSCs, and CD45−Ter−119−Tie2−AlphaV+Thy−6C3−CD105−CD200+ SSCs [36–39]. However, their identity and function in the craniofacial bone remain unclear. We recently identified a quiescent population of tissue-resident LepR+ SSCs in jawbone marrow that became activated in response to tooth extraction and contributed to intramembranous bone formation [40]. Using LepR-Cre; tdTomato; Col2.3-GFP reporter mice, we found that these LepR+ cells remained quiescent in the physiological state and gradually increased in activity with age. External stimuli such as tooth extraction activated LepR+ SSCs, which rapidly proliferated and differentiated into Col2.3-expressing osteoblasts, contributing significantly to extraction socket repair. Ablation of LepR+ SSCs with diphtheria toxin dramatically impaired the bone healing process. A mechanistic study showed that alveolar LepR+ SSCs are responsive to parathyroid hormone/parathyroid hormone I receptor (PTH/PTH1R) signaling. Knockout of Pth1r in the LepR+ cell lineage disrupted the bone formation process.
4. Temporomandibular Joint
The temporomandibular joint (TMJ) is located between the temporal bone and the mandible. It is one of the most frequently used joints in humans and is the only diarthrosis in the stomatognathic system. The occurrence of TMJ osteoarthritis is highly prevalent in humans, yet the regenerative capacity of condylar cartilage is limited. Therefore, identifying and isolating stem cells in the TMJ is crucial for osteoarthritis amelioration and regeneration.
Two types of stem cells reside in the TMJ (Figure 2). One type is TMJ synovium-derived stem cells, and the other type is fibrocartilage stem cells (FCSCs). In 2011, Liu et al. isolated and cultured stem cells from human TMJ synovial fluid and found that these cells exhibit fibroblastic and spindle shapes. Flow cytometry analysis showed that these cells express MSC markers and could be induced to differentiate toward osteogenic, chondrogenic, adipogenic, and neurogenic lineages. Thus, these synovium-derived cells are stem cells [41]. Koyama et al. found STRO-1- and CD146-expressing stem cells in the TMJ synovial fluid of patients with temporomandibular joint disorder. These cells showed great potential to differentiate into chondrocytes, osteoblasts, adipocytes, and neurons [42]. Stem cells were also isolated from the radiolucent zone of TMJ ankylosis patients, but they had a slower proliferation rate and lower osteogenic differentiation capacity than BMSCs [43]. In 2014, Sun et al. isolated synovial fragment cells from the synovial fluid of temporomandibular disease patients and revealed the multilineage differentiation capacity of this group of cells [44]. Fibrocartilage stem cells (FCSCs) reside in the superficial zone of condylar cartilage. A single FCSC could generate a cartilage anlage, which then undergoes autogenous bone formation and supports a hematopoietic microenvironment. Wnt signaling impairs the FCSC niche and results in cartilage degeneration. Intra-articular injection of the Wnt inhibitor sclerostin reconstructed the stem cell niche and repaired TMJ injury, indicating a potential therapeutic strategy for patients with fibrocartilage defects and disease [45].
Figure 2.
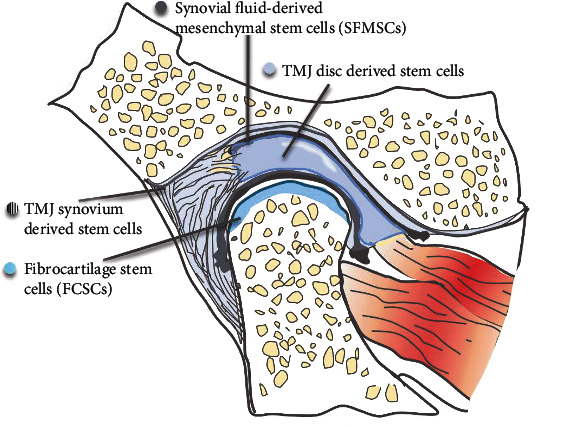
Stem cell distribution in TMJ. Stem cells are located in the synovial fluid, synovium, disc of TMJ, and surface zone of condylar cartilage.
5. Sutures
In summary, the flexible connection between paired calvarial bones permits the deformation of the skull during birth, directs the growth of the skull, and acts as a shock absorber that can cushion the load of mastication (Figure 3). Humans and mice both have four sutures. Metopic sutures (called interfrontal sutures in mice) and sagittal sutures are vertically distributed, and the osteogenic fronts about each other. Coronal sutures and lambdoid sutures are horizontally distributed, and their osteogenic fronts overlap with each other. Unossified sutures are recognized as the bone growth center of the postnatal skull vault [46], where the new bone precipitates at the edges of the bone front. Premature closure of the suture could lead to craniosynostosis. Studies have demonstrated a unique stem cell niche in cranial sutures, where multiple populations ofSeveral subpopulations of suture mesenchymal stem cells (SuSCs) were identified, including Gli1-positive (Gli1+) cells, Axin2-expressing (Axin2+) cells, and postnatal Prx1-expressing (Prx1+) cells [46–49]. All these cells possess the ability for self-renewal and continually produce skeletal cell descendants. Clonal expansion analysis demonstrated that SuSCs were capable of forming bones during calvarial development. SuSCs expand dramatically in the damaged site and contribute directly to skeletal repair; the nearer the cells are to the sutures, the better the recovery is [46, 50]. However, the spatiotemporal properties of SuSCs differ. During the early stage of postnatal development, Gli1+ cells were distributed throughout the periosteum, dura, and sutures, whereas Axin2+ cells and Prx1+ cells appeared only in the sutures. At 4 weeks of age, all of the SuSCs were restricted to the sutures. Gli1+ SuSCs were capable of trilineage differentiation into chondrocytes, osteoblasts, and adipocytes; Axin2+ SuSCs mainly gave rise to the chondro- and osteolineages, whereas Prx1+ SuSCs could only differentiate into osteoblasts. Depletion of Gli1+ or Axin2+ cells but not Prx1+ SuSCs caused craniosynostosis. Additional comparisons of the three types of cells are listed in Table 1.
Figure 3.
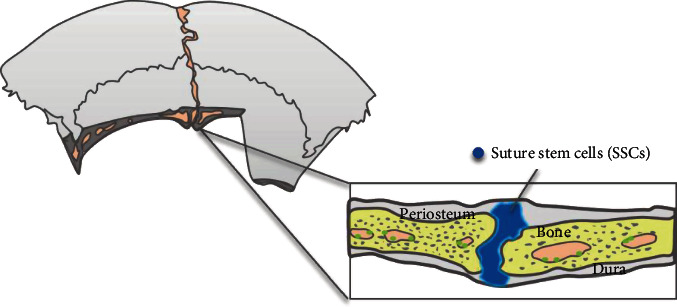
Stem cell distribution in sutures.
Table 1.
The subpopulations of suture mesenchymal stem cells and their characteristics.
| Cell types | Gli1+ cells [50, 51] | Axin2+ cells [46] | Postnatal Prx1+ cells [52] | |
|---|---|---|---|---|
| Distribution | Early stage | All over the periosteum, dura, and the craniofacial sutures | In the calvarial sutures | In the calvarial sutures |
| One month after birth | Self-renewal | Only in the sutures | / | |
| Stemness | Self-renewal | Slow-cycling cells | ||
| Contribution to other tissues | Suture mesenchyme, periosteum, dura mater, and parts of the calvarial bones | Suture mesenchyme and bone matrix near the osteogenic fronts | All calvarial tissues, except bone marrow osteoblasts | |
| Ability to repair the defect | Unequivocal and potentially exclusive contribution of the sutural mesenchyme to calvarial injury repair | |||
| Ablation | Craniosynostosis | Craniosynostosis | Did not result in craniosynostosis or any other major craniofacial phenotype | |
| MSC markers | CD90, CD73, CD44, Sca1, and CD146 | LepR | Pdgfrα and Mcam/CD146 (upregulation), Ccne2, Mcm4, and Pcna (downregulation), Itga2, Itga3, and Itga6 | |
| Differentiation | Osteoblasts | + | + (upon external stimulation) |
+ (stimulated with recombinant WNT3A) |
| Chondrocytes | + | + (upon external stimulation) |
/ | |
| Adipocytes | + | / | / | |
| Foundation of each study | Gli1 is the master transcriptional factor of hedgehog signaling and is indispensable for bone development and homeostasis. Gli1+ stem cells have been identified in canine and long bones. | Axin2 plays an irreplaceable role in the Wnt, BMP, and FGF signaling pathways; Axin2 knockout mice showed craniosynostosis. | Prx1 was previously shown to be highly expressed during limb bud formation and craniofacial development. | |
6. Periodontium
The tooth is a hard tissue containing a vascularized and nerve-rich pulp chamber and is surrounded by the tooth-supporting periodontium, including the periodontal ligament, cementum, and alveolar bone. Multiple stem cell niches exist in dental tissues, as teeth are uniquely shaped and are subject to complicated microenvironments with occlusal force and microorganisms [53]. To date, more than seven kinds of dental stem cells have been found, including dental pulp stem cells (DPSCs) [54], human exfoliated deciduous teeth (SHED) [55], periodontal ligament stem cells (PDLSCs) [56], dental follicle progenitor cells (DFPCs) [57], stem cells from dental apical papilla (SCAP) [58], tooth germ stem cells (TGSCs) [59], gingival mesenchymal stem cells (GMSCs) [60], and human natal dental pulp stem cells (NDP-SCs) (Figure 4) [61]. All of them are capable of self-renewal, proliferation, and multidirectional differentiation [62]. Among them, PDLSCs are the only kind of stem cells that can differentiate into osteoblasts in vivo and contribute to the construction of alveolar bone and tooth extraction sockets.
Figure 4.
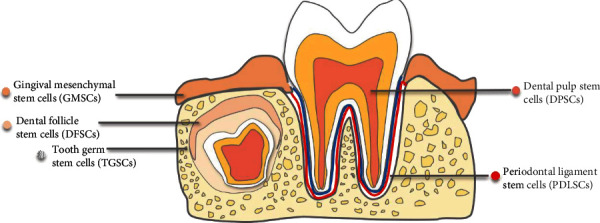
Stem cell distribution in dental tissues. Stem cells are located in gingiva, dental follicle, tooth germ, dental pulp, and periodontal ligament.
As reported in 2004, Seo et al. isolated and identified PDLSCs from human impacted wisdom teeth for the first time [56]. In addition to wisdom teeth, PDLSCs can be extracted from permanent tooth root surfaces [63], deciduous tooth [64–66], or even inflammatory periodontal tissues [67]. However, PDLSCs derived from different environments display different properties related to proliferation and osteogenic potential. For example, studies have found that PDLSCs from deciduous teeth promote osteoclastogenesis and lead to root absorption [68]. PDLSCs from inflammatory tissue are predisposed to a pathological local microenvironment [69]. The regulatory mechanism of PDLSC biological behavior remains to be revealed.
To exploit the osteogenic potential of PDLSCs, the osteogenic mechanism of PDLSCs needs to be clarified. It has been reported that antidifferentiation noncoding RNA (ANCR) [70], long noncoding RNAs (lncRNAs) [71], and microRNA-182 and microRNA-214 [72, 73] regulate the proliferation and osteogenic differentiation of PDLSCs. He et al. [74] and Yan et al. [75] found that hypoxia and cannabinoid receptor I (CB1) could alter the activity of PDLSCs through the p38/MAPK pathway. Meanwhile, the PI3K-AKT-mTOR pathway [76] and NF-κB axis [77] are also involved in the modulation of PDLSCs. In clinical practice, additional topics, such as how metformin contributes to the osteogenic potential of PDLSCs [78] and how nicotine [79, 80] and Porphyromonas gingivalis [81] weaken the osteogenic potential of PDLSCs, remain to be investigated.
PDLSCs exhibit high potential for tissue regeneration and are capable of giving rise to osteoblast-/cementoblast-like cells, adipocytes, chondrogenic cells, neurogenic lineage cells, endothelial cells, cardiac myocytes, and Schwann cells in vitro [62]. For stem cell identity and fate commitment, Roguljic and colleagues identified αSMA as a marker of PDLSCs in vivo [82]. αSMA+ PDLSCs expanded over time and mainly gave rise to cells in the apical region. Following periodontal ligament injury, PDLSCs proliferated and generated mature cementoblasts, osteoblasts, and fibroblasts within the periodontium. However, αSMA+ PDLSCs only made minor contributions to periodontium homeostasis and repair. Yuan and colleagues used Axin2 to track progenitor cells in the periodontal ligament and reported that PDLSCs are responsive to Wnt signaling [83]. Axin2+ PDLSCs remain quiescent under physiological conditions and differentiate into osteoblastic cells for alveolar bone repair when tooth extraction injury occurs. Most recently, using genetic fate mapping, Men et al. identified Gli1+ PDLSCs in adult mouse molars that gave rise to periodontal ligament, alveolar bone, and cementum in both the homeostatic state and during injury repair [84]. Gli1+ PDLSCs are enriched in the apical tooth region and surround the neurovascular bundle, and they are activated by canonical Wnt signaling. Sclerostin secreted by alveolar bone osteocytes inhibits Wnt signaling. Occlusal force can inhibit sclerostin secretion. Therefore, a feedback loop that regulates stem cell activities is present in the stem cell niche, where occlusal force-mediated inhibition of sclerostin secretion by alveolar bone osteocytes promotes Gli1+ cell maintenance and activation. The authors also compared other stem cell markers with Gli1. They concluded that labeling of Gli1 more efficiently identified PDLSCs compared to labeling of αSMA, LepR, NG2, and Pdgfrα. Using an inducible Cre system and immunostaining, they also reported that LepR+, NG2+, and Pdgfrα+ cells are descendants of Gli1+ cells in the periodontal ligament.
7. Application of Craniofacial Stem Cells
Stem cells for tissue regeneration have been widely exploited and are mainly applied in three ways: direct transplantation of a specific type of stem cells, combined application of different types of stem cells, and the use of stem cells in combination with biological scaffolds. However, the regenerative techniques used in the long bone cannot be easily extended to craniofacial applications because the microenvironment of craniofacial tissue is quite different from that of the long bone. Oral pathological factors (e.g., microorganisms, nicotine, and bisphosphonate) greatly affect the biological behavior of craniofacial stem cells. For instance, Kim et al. found that excessive nicotine intake will induce the vacuolation of jawbone MSCs and impair their proliferation and differentiation capacity [85]. Akintoye et al. found that jawbone MSC self-renewal and proliferation ability were impaired when the MSCs were treated with bisphosphonate, which might be associated with the pathogenesis of bisphosphonate-associated osteonecrosis. Therefore, to minimize potential damage, the use of appropriate ways to amplify and induce stem cells to differentiate toward the osteoblast lineage is particularly important. Wang et al. reported that vitamin C and vitamin D were ideal stimulants of craniofacial PDLSCs for osteoblast differentiation in vitro [26]. Naung et al. proposed a protocol to cultivate palate periosteum-derived MSCs in serum-free and xeno-free medium, which could become a useful source of MSCs for clinical applications [86]. Moreover, a recent study found that induced pluripotent stem cells (iPSCs) generated from human jaw periosteum cells expressed MSC markers and possessed strong mineralization ability [87].
The application of MSCs for regenerative medicine should be performed with caution because the stem cells obtained and expanded with plastic culture are highly heterogeneous and might not be intrinsically multipotent [88]. It should be noted that the minimal criteria, including adherence to the plastic plate, induction of multidirectional differentiation, and detection of appropriate surface marker profiles, are not sufficient to identify bona fide stem cells [89]. Some quiescent stem cells might not readily adhere to the plastic, and the heterogeneous cell mixture contains lineage-committed stem cells that give rise to cells of native tissue origin. For instance, bone marrow stem cells show an intrinsic propensity for differentiation toward osteolineages [90]. The surface markers of bone marrow stem cells are specific for fibroblast-like cells rather than stem cells [88]. Therefore, in vivo clonal assays and fate mapping are essential to identify bona fide stem cells [38]. Future studies are warranted to identify the stem cell properties of craniofacial stem cells, which will benefit clinical applications.
8. Conclusions
This review describes the stem cells found in the craniofacial periosteum, craniofacial bone marrow, TMJ, cranial suture, and periodontal ligament. Craniofacial stem cells express specific cell surface determinants, possess a low self-renewal rate, and show multidifferentiation ability. They rapidly proliferated and differentiated into osteoblasts in response to injury. In addition, craniofacial tissues are easily obtained from human jawbones with minimal invasiveness when clinicians perform implant surgeries, tooth extractions, and periodontal surgeries. All these results indicated that craniofacial stem cells are an ideal resource for tissue engineering.
However, the current research on craniofacial stem cells is still inadequate and lacks depth. Specific cell markers for the isolation of stem cells are still lacking. In vivo clonal assays and lineage fate mapping are warranted in future studies, which could facilitate the therapeutic application of craniofacial stem cells in vivo and in the clinic.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Number: NSFC 81901042) and China Postdoctoral Science Foundation (Grant Number: 2019M653443) to Shiwen Zhang.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Geru Zhang and Qiwen Li contributed equally to this work.
References
- 1.Boehm K. S., al-Taha M., Morzycki A., Samargandi O. A., al-Youha S., LeBlanc M. R. Donor site morbidities of iliac crest bone graft in craniofacial surgery: a systematic review. Annals of Plastic Surgery. 2019;83(3):352–358. doi: 10.1097/SAP.0000000000001682. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W., Yelick P. C. Craniofacial tissue engineering. Cold Spring Harbor Perspectives in Medicine. 2018;8(1) doi: 10.1101/cshperspect.a025775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearon J. A., Griner D., Ditthakasem K., Herbert M. Autogenous bone reconstruction of large secondary skull defects. Plastic and Reconstructive Surgery. 2017;139(2):427–438. doi: 10.1097/PRS.0000000000002941. [DOI] [PubMed] [Google Scholar]
- 4.Melville J. C., Mañón V. A., Blackburn C., Young S. Current methods of maxillofacial tissue engineering. Oral and Maxillofacial Surgery Clinics of North America. 2019;31(4):579–591. doi: 10.1016/j.coms.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Fillingham Y., Jacobs J. Bone grafts and their substitutes. The Bone & Joint Journal. 2016;98-B(1_Supple_A):6–9. doi: 10.1302/0301-620X.98B.36350. [DOI] [PubMed] [Google Scholar]
- 6.Dimitriou R., Jones E., McGonagle D., Giannoudis P. V. Bone regeneration: current concepts and future directions. BMC Medicine. 2011;9(1) doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrzejewska A., Lukomska B., Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells. 2019;37(7):855–864. doi: 10.1002/stem.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu X., Liu G., Halim A., Ju Y., Luo Q., Song G. Mesenchymal stem cell migration and tissue repair. Cell. 2019;8(8):p. 784. doi: 10.3390/cells8080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang R., Yu T., Zhou Y. Interplay between craniofacial stem cells and immune stimulus. Stem Cell Research & Therapy. 2017;8(1):p. 147. doi: 10.1186/s13287-017-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spees J. L., Lee R. H., Gregory C. A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Research & Therapy. 2016;7(1):p. 125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrell C., Fellabaum C., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cell. 2019;8(5):p. 467. doi: 10.3390/cells8050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Luca M., Aiuti A., Cossu G., Parmar M., Pellegrini G., Robey P. G. Advances in stem cell research and therapeutic development. Nature Cell Biology. 2019;21(7):801–811. doi: 10.1038/s41556-019-0344-z. [DOI] [PubMed] [Google Scholar]
- 14.Ho-Shui-Ling A., Bolander J., Rustom L. E., Johnson A. W., Luyten F. P., Picart C. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–162. doi: 10.1016/j.biomaterials.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominici M., le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 16.Kassem M., Bianco P. Skeletal stem cells in space and time. Cell. 2015;160(1-2):17–19. doi: 10.1016/j.cell.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Kawecki F., Clafshenkel W. P., Fortin M., Auger F. A., Fradette J. Biomimetic tissue-engineered bone substitutes for maxillofacial and craniofacial repair: the potential of cell sheet technologies. Advanced Healthcare Materials. 2018;7(6, article e1700919) doi: 10.1002/adhm.201700919. [DOI] [PubMed] [Google Scholar]
- 18.Sharpe P. T. Dental mesenchymal stem cells. Development. 2016;143(13):2273–2280. doi: 10.1242/dev.134189. [DOI] [PubMed] [Google Scholar]
- 19.Liu J., Yu F., Sun Y., et al. Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. 2015;33(3):627–638. doi: 10.1002/stem.1909. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H., Chai Y. Stem cells in teeth and craniofacial bones. Journal of Dental Research. 2015;94(11):1495–1501. doi: 10.1177/0022034515603972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Gilbert J. R., Zhang X., Zhao B., Ker D. F. E., Cooper G. M. Calvarial versus long bone: implications for tailoring skeletal tissue engineering. Tissue Engineering Part B: Reviews. 2020;26(1):46–63. doi: 10.1089/ten.teb.2018.0353. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Monjaraz B., Santiago-Osorio E., Monroy-García A., Ledesma-Martínez E., Mendoza-Núñez V. Mesenchymal stem cells of dental origin for inducing tissue regeneration in periodontitis: a mini-review. International Journal of Molecular Sciences. 2018;19(4):p. 944. doi: 10.3390/ijms19040944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang M., Zhang H., Gangolli R. Advances of mesenchymal stem cells derived from bone marrow and dental tissue in craniofacial tissue engineering. Current Stem Cell Research & Therapy. 2014;9(3):150–161. doi: 10.2174/1574888X09666140213142258. [DOI] [PubMed] [Google Scholar]
- 24.Alge D. L., Zhou D., Adams L. L., et al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. Journal of Tissue Engineering and Regenerative Medicine. 2010;4(1):73–81. doi: 10.1002/term.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ransom R. C., Carter A. C., Salhotra A., et al. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature. 2018;563(7732):514–521. doi: 10.1038/s41586-018-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y.-L., Hong A., Yen T.-H., Hong H.-H. Isolation of mesenchymal stem cells from human alveolar periosteum and effects of vitamin D on osteogenic activity of periosteum-derived cells. Journal of Visualized Experiments. 2018;135, article e57166 doi: 10.3791/57166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lageneste O. D., Julien A., Abou-Khalil R., et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by periostin. Nature Communications. 2018;9(1) doi: 10.1038/s41467-018-03124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Z., Fateh A., Salem D. M., Intini G. Periosteum. Journal of Dental Research. 2013;93(2):109–116. doi: 10.1177/0022034513506445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouraret S., von Kaeppler E., Bardet C., et al. The potential for vertical bone regeneration via maxillary periosteal elevation. Journal of Clinical Periodontology. 2014;41(12):1170–1177. doi: 10.1111/jcpe.12310. [DOI] [PubMed] [Google Scholar]
- 30.Debnath S., Yallowitz A. R., McCormick J., et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562(7725):133–139. doi: 10.1038/s41586-018-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortinau L. C., Wang H., Lei K., et al. Identification of functionally distinct Mx1+αSMA+ periosteal skeletal stem cells. Cell Stem Cell. 2019;25(6):784–796.e5. doi: 10.1016/j.stem.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akintoye S. O., Lam T., Shi S., Brahim J., Collins M. T., Robey P. G. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38(6):758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 33.Du Y., Jiang F., Liang Y., et al. The angiogenic variation of skeletal site-specific human BMSCs from same alveolar cleft patients: a comparative study. Journal of Molecular Histology. 2016;47(2):153–168. doi: 10.1007/s10735-016-9662-7. [DOI] [PubMed] [Google Scholar]
- 34.Kanawa M., Igarashi A., Fujimoto K., et al. Genetic markers can predict chondrogenic differentiation potential in bone marrow-derived mesenchymal stromal cells. Stem Cells International. 2018;2018:9. doi: 10.1155/2018/9530932.9530932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsubara T., Suardita K., Ishii M., et al. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. Journal of Bone and Mineral Research. 2005;20(3):399–409. doi: 10.1359/JBMR.041117. [DOI] [PubMed] [Google Scholar]
- 36.Zhou B. O., Yue R., Murphy M. M., Peyer J. G., Morrison S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worthley D. L., Churchill M., Compton J. T., et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1-2):269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan C. K. F., Seo E. Y., Chen J. Y., et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1-2):285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y., He G., Lee W. C., McKenzie J. A., Silva M. J., Long F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nature Communications. 2017;8(1):p. 2043. doi: 10.1038/s41467-017-02171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D., Zhang S., Wang J., et al. LepR-expressing stem cells are essential for alveolar bone regeneration. Journal of Dental Research. 2020;(article 002203452093283) doi: 10.1177/0022034520932834. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z., Long X., Li J., Wei L., Gong Z., Fang W. Differentiation of temporomandibular joint synovial mesenchymal stem cells into neuronal cells in vitro: an in vitro study. Cell Biology International. 2011;35(1):87–91. doi: 10.1042/CBI20100144. [DOI] [PubMed] [Google Scholar]
- 42.Koyama N., Okubo Y., Nakao K., Osawa K., Fujimura K., Bessho K. Pluripotency of mesenchymal cells derived from synovial fluid in patients with temporomandibular joint disorder. Life Sciences. 2011;89(19-20):741–747. doi: 10.1016/j.lfs.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Xiao E., Li J. M., Yan Y. B., et al. Decreased osteogenesis in stromal cells from radiolucent zone of human TMJ ankylosis. Journal of Dental Research. 2013;92(5):450–455. doi: 10.1177/0022034513483471. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y. P., Zheng Y. H., Liu W. J., Zheng Y. L., Zhang Z. G. Synovium fragment-derived cells exhibit characteristics similar to those of dissociated multipotent cells in synovial fluid of the temporomandibular joint. PLoS One. 2014;9(7, article e101896) doi: 10.1371/journal.pone.0101896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Embree M. C., Chen M., Pylawka S., et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nature Communications. 2016;7(1) doi: 10.1038/ncomms13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maruyama T., Jeong J., Sheu T. J., Hsu W. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nature Communications. 2016;7(1) doi: 10.1038/ncomms10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durham E., Howie R. N., Larson N., LaRue A., Cray J. Pharmacological exposures may precipitate craniosynostosis through targeted stem cell depletion. Stem Cell Research. 2019;40:p. 101528. doi: 10.1016/j.scr.2019.101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durham E. L., Howie R. N., Houck R., et al. Involvement of calvarial stem cells in healing: a regional analysis of large cranial defects. Wound Repair and Regeneration. 2018;26(5):359–365. doi: 10.1111/wrr.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doro D. H., Grigoriadis A. E., Liu K. J. Calvarial suture-derived stem cells and their contribution to cranial bone repair. Frontiers in Physiology. 2017;8:p. 956. doi: 10.3389/fphys.2017.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park S., Zhao H., Urata M., Chai Y. Sutures possess strong regenerative capacity for calvarial bone injury. Stem Cells and Development. 2016;25(23):1801–1807. doi: 10.1089/scd.2016.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao H., Feng J., Ho T. V., Grimes W., Urata M., Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nature Cell Biology. 2015;17(4):386–396. doi: 10.1038/ncb3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilk K., Yeh S. C. A., Mortensen L. J., et al. Postnatal calvarial skeletal stem cells expressing PRX1 reside exclusively in the calvarial sutures and are required for bone regeneration. Stem Cell Reports. 2017;8(4):933–946. doi: 10.1016/j.stemcr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akintoye S. O. The distinctive jaw and alveolar bone regeneration. Oral Diseases. 2018;24(1-2):49–51. doi: 10.1111/odi.12761. [DOI] [PubMed] [Google Scholar]
- 54.Gronthos S., Mankani M., Brahim J., Robey P. G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miura M., Gronthos S., Zhao M., et al. SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seo B.-M., Miura M., Gronthos S., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 57.Morsczeck C., Götz W., Schierholz J., et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biology. 2005;24(2):155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Sonoyama W., Liu Y., Yamaza T., et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. Journal of Endodontia. 2008;34(2):166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikeda E., Yagi K., Kojima M., et al. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76(5):495–505. doi: 10.1111/j.1432-0436.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q. Z., Su W. R., Shi S. H., et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karaöz E., Doğan B. N., Aksoy A., et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochemistry and Cell Biology. 2010;133(1):95–112. doi: 10.1007/s00418-009-0646-5. [DOI] [PubMed] [Google Scholar]
- 62.Aydin S., Sahin F. Stem cells derived from dental tissues. Advances in Experimental Medicine and Biology. 2019;1144:123–132. doi: 10.1007/5584_2018_333. [DOI] [PubMed] [Google Scholar]
- 63.Chen F. M., Gao L. N., Tian B. M., et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Research & Therapy. 2016;7(1) doi: 10.1186/s13287-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winning L., El Karim I. A., Lundy F. T. A comparative analysis of the osteogenic potential of dental mesenchymal stem cells. Stem Cells and Development. 2019;28(15):1050–1058. doi: 10.1089/scd.2019.0023. [DOI] [PubMed] [Google Scholar]
- 65.Khoshhal M., Amiri I., Gholami L. Comparison of in vitro properties of periodontal ligament stem cells derived from permanent and deciduous teeth. Journal of Dental Research, Dental Clinics, Dental Prospects. 2017;11(3):140–148. doi: 10.15171/joddd.2017.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song J. S., Kim S. O., Kim S. H., et al. In vitro and in vivo characteristics of stem cells derived from the periodontal ligament of human deciduous and permanent teeth. Tissue Engineering. Part A. 2012;18(19-20):2040–2051. doi: 10.1089/ten.tea.2011.0318. [DOI] [PubMed] [Google Scholar]
- 67.Liu N., Shi S., Deng M., et al. High levels of β-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. Journal of Bone and Mineral Research : the official journal of the American Society for Bone and Mineral Research. 2011;26(9):2082–2095. doi: 10.1002/jbmr.440. [DOI] [PubMed] [Google Scholar]
- 68.Li B., Zhang Y., Wang Q., et al. Periodontal ligament stem cells modulate root resorption of human primary teeth via Runx2 regulating RANKL/OPG system. Stem Cells and Development. 2014;23(20):2524–2534. doi: 10.1089/scd.2014.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kato H., Taguchi Y., Tominaga K., Umeda M., Tanaka A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Archives of Oral Biology. 2014;59(2):167–175. doi: 10.1016/j.archoralbio.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Jia Q., Jiang W., Ni L. Down-regulated non-coding RNA (lncRNA-ANCR) promotes osteogenic differentiation of periodontal ligament stem cells. Archives of Oral Biology. 2015;60(2):234–241. doi: 10.1016/j.archoralbio.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Wang L., Wu F., Song Y., et al. Long noncoding RNA related to periodontitis interacts with miR-182 to upregulate osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients. Cell Death & Disease. 2016;7(8, article e2327) doi: 10.1038/cddis.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao F., Zhan J., Chen X., Zhang K., Lai R., Feng Z. miR-214 promotes periodontal ligament stem cell osteoblastic differentiation by modulating Wnt/β-catenin signaling. Molecular Medicine Reports. 2017;16(6):9301–9308. doi: 10.3892/mmr.2017.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu X., Li M., Jin Y., Liu D., Wei F. Identification and integrated analysis of differentially expressed lncRNAs and circRNAs reveal the potential ceRNA networks during PDLSC osteogenic differentiation. BMC Genetics. 2017;18(1):p. 100. doi: 10.1186/s12863-017-0569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He Y., Jian C. X., Zhang H. Y., et al. Hypoxia enhances periodontal ligament stem cell proliferation via the MAPK signaling pathway. Genetics and Molecular Research. 2016;15(4) doi: 10.4238/gmr15048965. [DOI] [PubMed] [Google Scholar]
- 75.Yan W., Cao Y., Yang H., et al. CB1 enhanced the osteo/dentinogenic differentiation ability of periodontal ligament stem cells via p38 MAPK and JNK in an inflammatory environment. Cell Proliferation. 2019;52(6, article e12691) doi: 10.1111/cpr.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu X. Y., He X. T., Wang J., et al. Role of the P2X7 receptor in inflammation-mediated changes in the osteogenesis of periodontal ligament stem cells. Cell Death & Disease. 2019;10(1):p. 20. doi: 10.1038/s41419-018-1253-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Xu Y., Ren C., Zhao X., Wang W., Zhang N. microRNA-132 inhibits osteogenic differentiation of periodontal ligament stem cells via GDF5 and the NF-κB signaling pathway. Pathology - Research and Practice. 2019;215(12):p. 152722. doi: 10.1016/j.prp.2019.152722. [DOI] [PubMed] [Google Scholar]
- 78.Zhang R., Liang Q., Kang W., Ge S. Metformin facilitates the proliferation, migration, and osteogenic differentiation of periodontal ligament stem cells in vitro. Cell Biology International. 2019;44(1):70–79. doi: 10.1002/cbin.11202. [DOI] [PubMed] [Google Scholar]
- 79.Chen Z., Liu H. L. Restoration of miR-1305 relieves the inhibitory effect of nicotine on periodontal ligament-derived stem cell proliferation, migration, and osteogenic differentiation. Journal of Oral Pathology & Medicine. 2017;46(4):313–320. doi: 10.1111/jop.12492. [DOI] [PubMed] [Google Scholar]
- 80.Ng T. K., Huang L., Cao D., et al. Cigarette smoking hinders human periodontal ligament-derived stem cell proliferation, migration and differentiation potentials. Scientific Reports. 2015;5(1):p. 7828. doi: 10.1038/srep07828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramenzoni L. L., Russo G., Moccia M. D., Attin T., Schmidlin P. R. Periodontal bacterial supernatants modify differentiation, migration and inflammatory cytokine expression in human periodontal ligament stem cells. PLoS One. 2019;14(7, article e0219181) doi: 10.1371/journal.pone.0219181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roguljic H., Matthews B. G., Yang W., Cvija H., Mina M., Kalajzic I. In vivo identification of periodontal progenitor cells. Journal of Dental Research. 2013;92(8):709–715. doi: 10.1177/0022034513493434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuan X., Pei X., Zhao Y., Tulu U. S., Liu B., Helms J. A. A Wnt-responsive PDL population effectuates extraction socket healing. Journal of Dental Research. 2018;97(7):803–809. doi: 10.1177/0022034518755719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Men Y., Wang Y., Yi Y., et al. Gli1+ periodontium stem cells are regulated by osteocytes and occlusal force. Developmental Cell. 2020 doi: 10.1016/j.devcel.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 85.Kim B. S., Kim S. J., Kim H. J., et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sciences. 2012;90(3-4):109–115. doi: 10.1016/j.lfs.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 86.Naung N. Y., Duncan W., Silva R. D., Coates D. Localization and characterization of human palatal periosteum stem cells in serum-free, xeno-free medium for clinical use. European Journal of Oral Sciences. 2019;127(2):99–111. doi: 10.1111/eos.12603. [DOI] [PubMed] [Google Scholar]
- 87.Umrath F., Steinle H., Weber M., et al. Generation of iPSCs from jaw periosteal cells using self-replicating RNA. International Journal of Molecular Sciences. 2019;20(7):p. 1648. doi: 10.3390/ijms20071648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ambrosi T. H., Longaker M. T., Chan C. K. F. A revised perspective of skeletal stem cell biology. Frontiers in Cell and Development Biology. 2019;7:p. 189. doi: 10.3389/fcell.2019.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bianco P., Robey P. G. Skeletal stem cells. Development. 2015;142(6):1023–1027. doi: 10.1242/dev.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rauch A., Haakonsson A. K., Madsen J. G. S., et al. Osteogenesis depends on commissioning of a network of stem cell transcription factors that act as repressors of adipogenesis. Nature Genetics. 2019;51(4):716–727. doi: 10.1038/s41588-019-0359-1. [DOI] [PubMed] [Google Scholar]


