Abstract
Background
Neuropathic pain (NP) is a devastating complication following nerve injury, and it can be alleviated by regulating neuroimmune direction. We aimed to explore the neuroimmune mechanism and identify some new diagnostic or therapeutic targets for NP treatment via bioinformatic analysis.
Methods
The microarray GSE18803 was downloaded and analyzed using R. The Venn diagram was drawn to find neuroimmune-related differentially expressed genes (DEGs) in neuropathic pain. Gene Ontology (GO), pathway enrichment, and protein-protein interaction (PPI) network were used to analyze DEGs, respectively. Besides, the identified hub genes were submitted to the DGIdb database to find relevant therapeutic drugs.
Results
A total of 91 neuroimmune-related DEGs were identified. The results of GO and pathway enrichment analyses were closely related to immune and inflammatory responses. PPI analysis showed two important modules and 8 hub genes: PTPRC, CD68, CTSS, RAC2, LAPTM5, FCGR3A, CD53, and HCK. The drug-hub gene interaction network was constructed by Cytoscape, and it included 24 candidate drugs and 3 hub genes.
Conclusion
The present study helps us better understand the neuroimmune mechanism of neuropathic pain and provides some novel insights on NP treatment, such as modulation of microglia polarization and targeting bone resorption. Besides, CD68, CTSS, LAPTM5, FCGR3A, and CD53 may be used as early diagnostic biomarkers and the gene HCK can be a therapeutic target.
1. Introduction
Neuropathic pain (NP) is an abnormal painful condition caused by a lesion or disease affecting the somatosensory nervous system [1]. The pain is usually described to be persistent and unbearable and seriously compromises the patient's quality of life [2]. Interestingly, nerve injury that happened to children below 5-6 years old seldom triggers neuropathic pain [3, 4]. Working on this observation, some researchers found that neuroimmune regulation was involved in the pathological process of neuropathic pain [5, 6]. Some studies further found the activation of glial cells and the production of a large number of cytokines that happened in neuroimmune microenvironment after neuropathic pain. However, the underlying mechanism about neuroimmune in regulating pain is still unclear.
Considering the importance of neuroimmune activation for neuropathic pain, several therapies targeting those mechanisms have been developed and proved to be effective [7]. For example, the inhibition of TNF-α, a pro-inflammatory cytokine, could reduce the hyperalgesia induced by diabetic neuropathy [8]. Besides, some researchers found that the Toll-like receptor is responsible for gliosis after nerve injury and knockout of TLR2 could attenuate nociceptive hypersensitivity in mice [9]. Some other treatments such as intrathecal injection of IL-10 and gene transfer of IL-4 using a viral-mediated vector have similar results [10]. Therefore, it is valuable to find some new therapeutic targets especially the upstream regulating genes.
In recent decades, the advancement of microarray technology and bioinformatics enable researchers to study the biological mechanism more comprehensively and make it more effective to identify key therapeutic and prognostic molecules. In this study, we downloaded GSE18803 from the Gene Expression Omnibus (GEO) database datasets. Subsequently, we identified a series of differentially expressed genes using R software. We further performed a series of comprehensive bioinformatic analysis on those differentially expressed genes. Our study is aimed at providing new therapeutic targets for neuropathic pain and helping to understand the underlying neuroimmune mechanism of neuropathic pain.
2. Material and Methods
2.1. Microarray Data
The microarray dataset GSE18803 was retrieved from GEO (https://www.ncbi.nlm.nih.gov/geo/). It was deposited by Costigan et al. and contained 24 samples (adult rats, 6 in the surgery group vs. 6 in the sham group; neonate rats, 6 in the surgery group vs. 6 in the sham group) [11]. In the surgery group, the adult (8-12 weeks) and neonate (10 days) rats were performed spared nerve injury (SNI) where the tibial and common peroneal nerve was ligated with a 5.0 silk and transected distally, removing 2-4 mm of distal nerve stump while leaving the sural nerve intact. In the sham operation group, the sciatic nerve was exposed but not ligated. 7 days post-SNI, the L4-L5 lumbar dorsal horns ipsilateral to the injury were collected and processed for total RNA extraction. The platform for gene expression detection was GPL341 (Affymetrix Rat Expression 230A Array).
2.2. Data Preprocessing and DEG Identification
Firstly, the raw CEL file was downloaded and divided into two separate documents—the adult and neonate groups—uploaded to R software (v 3.6.2). RMA (Robust multiarray average) method in the Affy package was used to perform normalization and background correction. KNN (K-nearest neighbors) method in the impute package was used to supplement the missing value. Finally, a total of 15923 probes were obtained both in the adult and neonate gene expression matrices. After that, the probe ID was converted into an official gene symbol based on the GPL341 annotation file downloaded from the Affymetrix official website.
Bayes test in the limma package was used to identify DEGs between the SNI and sham injury groups in each age group. The DEGs with ∣LogFC | >0.5 and P value < 0.05 were considered to be significant. After that, Venn analysis was performed between the two lists of DEGs.
2.3. Function and Pathway Enrichment Analyses
GO provides a specific language to describe the attributes of the selected genes in three main domains which are cellular component (CC), molecular function (MF), and biological process (BP) [12]. GO function enrichment analysis was performed by an online web tool provided in the DAVID database (https://david.ncifcrf.gov/). The Kyoto Encyclopedia of Genes and Genomes (KEGG) database stores relevant pathway information which can help us understand cells, organisms, and ecosystems at the molecular level [13]. KEGG pathway enrichment analysis was performed by another online web tool provided in the KOBAS database (http://kobas.cbi.pku.edu.cn/). TheDEGs only found in the adult groups were uploaded in the database and performed GO and KEGG pathway enrichment analyses. P value < 0.05 was set as a cutoff.
2.4. PPI Network Construction and Analysis
The gene list was uploaded to the Multiple Protein web tool in the STRING database (https://string-db.org/) and a combined score > 0.4 was set as the cutoff. The TSV file was downloaded and submitted to Cytoscape software, an open-source software platform for visualizing complex networks, to visualize the PPI network. 12 centrality mathematical calculation methods in cytoHubba, a java plugin in Cytoscape, were all applied to screen hub genes by analyzing the topology of the constructed protein-protein interaction networks. The genes with counts over 6 were chosen as the hub gene.
Molecular Complex Detection (MCODE), an app for cluster analysis in Cytoscape, was used to find important functional modules in the constructed PPI network. The genes contained in the two selected modules were performed pathway enrichment analysis in the METASCAPE database (https://metascape.org), respectively.
2.5. Related Drug Prediction
The 8 hub genes were served as promising targets for therapeutic drug retrieval in the DGIdb database (http://dgidb.genome.wustl.edu/), a web source that helps discover drug-gene interactions. The preset filters were set to be “FDA Approved” and “Immunotherapies,” while the advanced filters were set to include 20 databases, 41 gene categories, and 51 gene interaction types. The results were visualized in Cytoscape.
3. Result
3.1. Identification of DEGs
To ensure the reliability of the analysis results, we firstly performed data background correction and normalization (Figures 1(a) and 1(b)). Then, we identified 117 DEGs in the adult rat group (116 upregulated and 1 downregulated) and 26 DEGs (all upregulated) in the neonate rat group through DEG analysis using R. The results were visualized through a heat map (Figures 2(a) and 2(b)). Venn diagram analysis demonstrated that the 24 DEGs in the neonate groups were all included in the adult group DEG list (Figure 2(c)). We aimed to explore the underlying neuroimmune mechanism of neuropathic pain and find several valuable pain biomarkers, while the neonate rats did not develop nerve pain after nerve injury [14, 15]. Therefore, we mainly focused on the DEGs dysregulated only in the adult groups.
Figure 1.
Box plot of expression value of two age groups. (a) The expression value of the adult rat group. (b) The expression value of the neonate rat group. The horizontal axis represents the sample name, while the vertical axis represents the expression value after normalization. The blue represents the sham group, while the red represents the spared nerve injury (SNI) group. The black line in the box represents the median of value, which can stand for the degree of normalization. They were all in the same line, indicating the normalization was effective.
Figure 2.

The heat map of differentially expressed genes (DEGs) in the adult rat group (a). The heat map of DEGs in the neonate rat group (b). The horizontal axis represents the name of each sample, while the left vertical axis represents the degree of gene clustering. Red stands for the upregulated genes, while blue stands for the downregulated genes (a, b). Venn diagram of DEGs. The 26 DEGs in the neonate group were all included in the adult group. 91 DEGs were only expressed in the adult group (c).
3.2. GO and KEGG Analyses
To describe the attributes of selected DEGs, we submitted the gene list to the DAVID database and performed GO function enrichment analysis. We have found 148 GO terms with the P value < 0.05 (100 BP items, 28 CC items, and 20 MF items). The top 20 GO terms, ranked by P value, were shown in Table 1. Most of the enriched terms were about BP. We further found that many BP terms were closely related to the inflammatory and immune processes, such as inflammatory response, cellular response to lipopolysaccharide, B cell receptor signaling pathway, regulation of B cell, and positive regulation of T cell activation.
Table 1.
The top 20 most significant GO terms.
| Category | Term | Count | P value |
|---|---|---|---|
| BP | GO:0006954~inflammatory response | 14 | 2.42E − 09 |
| BP | GO:0071222~cellular response to lipopolysaccharide | 10 | 1.41E − 07 |
| BP | GO:0050853~B cell receptor signaling pathway | 6 | 3.13E − 06 |
| BP | GO:0045577~regulation of B cell differentiation | 4 | 1.38E − 05 |
| BP | GO:0051279~regulation of release of sequestered calcium ion into cytosol | 4 | 5.14E − 05 |
| BP | GO:0050870~positive regulation of T cell activation | 4 | 9.12E − 05 |
| BP | GO:0071346~cellular response to interferon-gamma | 5 | 2.38E − 04 |
| BP | GO:0071407~cellular response to organic cyclic compound | 6 | 3.52E − 04 |
| BP | GO:0051607~defense response to virus | 6 | 4.39E − 04 |
| BP | GO:0045730~respiratory burst | 3 | 6.68E − 04 |
| BP | GO:0009611~response to wounding | 5 | 6.71E − 04 |
| BP | GO:0050665~hydrogen peroxide biosynthetic process | 3 | 8.56E − 04 |
| CC | GO:0070062~extracellular exosome | 37 | 8.92E − 10 |
| CC | GO:0005764~lysosome | 10 | 2.39E − 06 |
| CC | GO:0005615~extracellular space | 21 | 2.70E − 06 |
| CC | GO:0009986~cell surface | 12 | 1.49E − 04 |
| CC | GO:0005623~cell | 6 | 2.58E − 04 |
| CC | GO:0043020~NADPH oxidase complex | 3 | 7.86E − 04 |
| MF | GO:0008201~heparin binding | 6 | 5.76E − 04 |
| MF | GO:0016175~superoxide-generating NADPH oxidase activity | 3 | 5.81E − 04 |
GO: Gene Ontology; BP: biological process; CC: cellular component; MF: molecular component; Count: enriched gene numbers in each term; NADPH: nicotinamide adenine dinucleotide phosphate.
To further investigate the functions of the DEGs, we did KEGG pathway analysis in the KOBAS database. The top 10 pathways were shown in Table 2. The result was similar to the GO analysis. Several most significantly enriched pathways were involved in inflammatory and immune responses, such as Fc gamma R-mediated phagocytosis, natural killer cell-mediated cytotoxicity, chemokine signaling pathway, and Yersinia infection.
Table 2.
The top 10 most significant enriched KEGG pathways.
| ID | Description | No. of genes | P value | Input |
|---|---|---|---|---|
| rno04666 | Fc gamma R-mediated phagocytosis | 7 | 6.20E − 09 | Ncf1, Rac2, Arpc1b, Fcgr3a, Ptprc, Hck, Lyn |
| rno04142 | Lysosome | 7 | 4.29E − 08 | Ctsh, Hexb, Laptm5, Man2b1, Cd68, Npc2, Ctss |
| rno05140 | Leishmaniasis | 6 | 4.72E − 08 | Ncf1, Fcgr3a, Cybb, Cyba, Ifngr1, Ptpn6 |
| rno04650 | Natural killer cell-mediated cytotoxicity | 6 | 2.24E − 07 | Rac2, Cd48, Fcgr3a, Ifngr1, Lcp2, Ptpn6 |
| rno04621 | NOD-like receptor signaling pathway | 7 | 3.77E − 07 | Il18, Ccl2, Gbp2, Cybb, Cyba, Pycard, Tmem173 |
| rno04060 | Cytokine-cytokine receptor interaction | 8 | 3.84E − 07 | Il18, Ccl2, Tgfbr1, Cx3cr1, Cd4, Ifngr1, Cxcl13, Ngfr |
| rno04062 | Chemokine signaling pathway | 7 | 3.91E − 07 | Ccl2, Ncf1, Rac2, Cx3cr1, Hck, Cxcl13, Lyn |
| rno05135 | Yersinia infection | 6 | 9.44E − 07 | Il18, Ccl2, Rac2, Skap2, Pycard, Lcp2 |
| rno04380 | Osteoclast differentiation | 6 | 9.86E − 07 | Ncf1, Fcgr3a, Tgfbr1, Cyba, Ifngr1, Lcp2 |
| rno04145 | Phagosome | 6 | 1.07E − 05 | Ncf1, Cyba, Fcgr3a, Cybb, Coro1a, Ctss |
KEGG: Kyoto Encyclopedia of Genes and Genomes; NOD: nucleotide-binding oligomerization domain.
3.3. PPI Network Analysis
Based on the information from the STRING database, the current established PPI network contained 68 nodes and 299 protein pairs (Figure 3(a)). To identify the hub gene in the PPI network, we applied every calculation method in the cytoHubba plugin. The genes with number > 6 were selected as the hub gene, including protein tyrosine phosphatase receptor type C (Ptprc), Cd68 molecule (Cd68), cathepsin S (Ctss), Rac family small GTPase 2 (Rac2), lysosomal protein transmembrane 5 (Laptm5), Fc fragment of IgG receptor IIIa (Fcgr3a), CD53 molecule (Cd53), protooncogene, and hematopoietic cell kinase (Hck) (Figure 3(b)).
Figure 3.
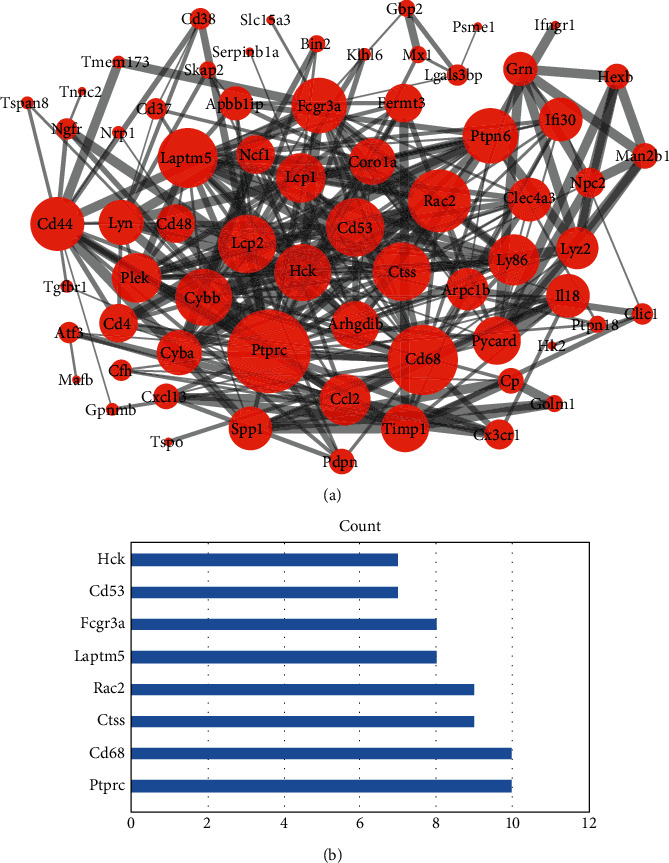
Protein-protein interaction network of 91 neuroimmune-related genes. The size of each protein is determined by its connection degree to other proteins. The width of each edge is determined by the combined score between the related two proteins (a). The 8 hub genes screened by 12 centrality mathematical calculation methods (b).
To further analyze the PPI network, we found two important clusters using the MCODE plugin in Cytoscape and performed pathway enrichment in the METASCAPE database (Figures 4(a)–4(d)). The first cluster was mainly enriched in the innate immune system, bone resorption, Fc gamma R-mediated phagocytosis, and antigen processing and presentation. The second cluster was enriched in neutrophil degranulation and lysosome.
Figure 4.
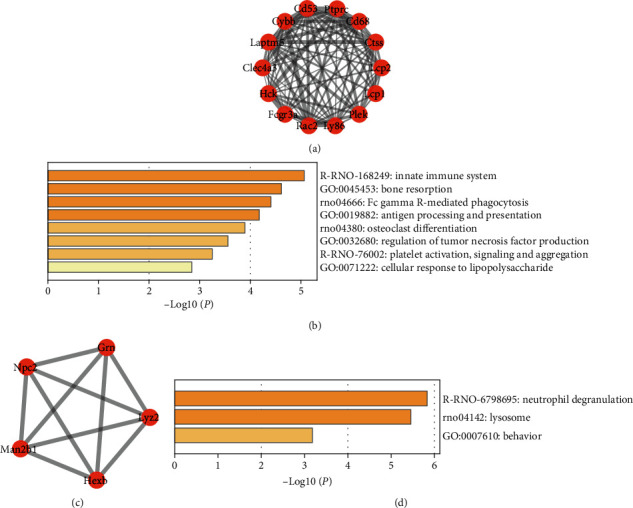
The first module (a) and the pathway enrichment analysis of this module (b). The second module (c) and the pathway enrichment analysis of this module (d).
3.4. Related Drug Prediction
Using the DGIdb database, the potential drugs targeting the selected genes were obtained. The results showed that a total of 24 candidate drugs included PTPRC (prednisone, fluorouracil, adalimumab, infliximab, and etanercept), FCGR3A (rituximab, muromonab-Cd3, alefacept, natalizumab, daclizumab, Ibritumomab tiuxetan, etanercept, tositumomab, adalimumab, basiliximab, ciclosporin, alemtuzumab, thalidomide, cytarabine, cyclophosphamide, vincristine, and infliximab), and HCK (dasatinib and ibrutinib), as shown in Figure 5. Further use of literature retrieval found that prednisone, adalimumab, infliximab, etanercept, alemtuzumab, and thalidomide have been clinically used for NP treatment. Four drugs (rituximab, natalizumab, daclizumab, and ibrutinib) have a role in neuropathy.
Figure 5.
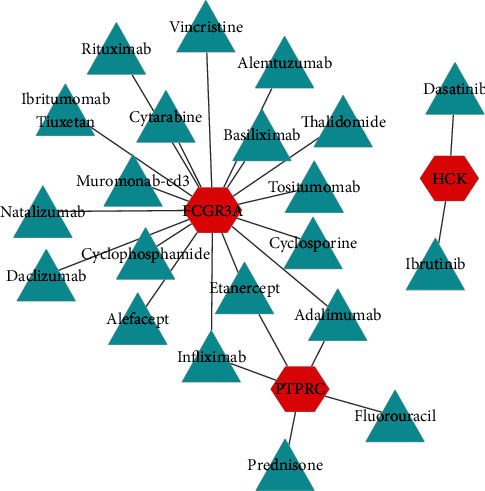
The drug-hub gene interaction network. The red nodes represent the hub genes, while the blue nodes represent the relevant drugs. A total of 3 hub genes and 24 drugs were included in the network.
4. Discussion
The nerve injury occurred to neonate rats seldom triggers neuropathic pain, while in adults it does [16, 17]. Research on DEGs between the neonate and adult rats after SNI might help us better understand the underlying mechanism of neuropathic pain. In the current study, we downloaded the GSE18803 dataset from GEO and identified 91 DEGs particularly expressed in adult rats after SNI. The 91 DEGs were then used to perform GO, pathway, and PPI network analyses. We also determined several hub genes and constructed the hub gene-drug network. Our results might provide some novel insights in neuropathic pain treatment.
Previous studies demonstrated that the activation of neuroimmune mechanisms after nerve injury was one of the main reasons to cause neuropathic pain [18, 19]. The results of GO and pathway enrichment analyses have shown similar trends, since many enriched terms or pathways were immune related. What is more, according to the results of enrichment analysis, we hypothesize that the infiltration of immune cells, including B cells, T cells, and natural killer cells, was closely related to the development of neuropathic pain.
We identified 8 hub genes in the PPI network: PTPRC, CD68, CTSS, RAC2, LAPTM5, FCGR3A, CD53, and HCK. Interestingly, five among them (CD68, CTSS, LAPTM5, FCGR3A, and CD53) were involved in microglia activation. CD68 is a widely used biomarker for activated macrophages or microglia. Its overexpression represents the functional phenotype of macrophages/microglia changing to proinflammatory M1 cells which directly induce neuropathic pain [20, 21]. Both CTSS and LAPTM5 were lysosome-associated proteins in the microglia. The critical role of CTSS played in maintaining neuropathic pain has been proved, while the function of LAPTM5 in neuropathic pain needs further elucidation [22–24]. FGCR3A was also identified as a crucial gene in neuropathic pain before [25]. Besides, the expression of FGCR3A was positively correlated with microglia phagocytic capacity [26]. CD53, the cell surface adhesion molecule, was overexpressed in the microglia after nerve injury and might mediate microglial migration [27]. Considering the early response of the microglia in the generation of neuropathic pain, the five identified genes have the potential to be the early diagnostic biomarkers of neuropathic pain [28].
Multiple studies have suggested that activated microglia were a critical contributor to the initiation and development of neuropathic pain [29, 30]. However, there are two different states of microglia activation, the proinflammatory M1 and anti-inflammatory M2. As opposed to M1 microglia which releases proinflammatory or neurotoxic factors, inducing NP, M2 microglia release anti-inflammatory factors, dampening inflammation and subsequently alleviating NP. A recent study showed that although both M1 and M2 microglia are activated after nerve injury, the microglia skewed towards M1 phenotype as neuralgia develops [31]. This might explain why inhibiting microglia activation could alleviate NP, regardless of its phenotype [32, 33]. What is more, some researchers found microglia polarization to the M2 phenotype could reduce neuropathic pain [34, 35]. Therefore, modulation of microglia polarization, inhibiting proinflammatory M1 microglia reaction while simultaneously promoting M2 microglia anti-inflammatory effect could be another treatment for neuropathic pain.
HCK belongs to the Src protein family (SFK). SFKs extensively participate in multiple signal transduction pathways such as cell growth, migration, and apoptosis. Recently, some researchers have found that the spinal cord injury could result in SFK activation in the microglia, and administration of the SFK inhibitor could attenuate mechanical hypersensitivity following nerve injury [36]. Specifically, as a member of SFKs, HCK was also found upregulated during microglia activation [37]. Previous studies demonstrated that HCK could mediate TLR4-induced proinflammatory mediator production via AP-1 [38]. TLR4, a specific pattern recognition receptor expressed on the microglia surface, was reported to play an important role during the pathological process of neuropathic pain [39, 40]. Therefore, it is reasonable to speculate that HCK is associated with the generation of neuropathic pain. The overexpressed HCK might activate the microglia and increase the production of proinflammatory cytokines via the TLR/AP-1 pathway, subsequently inducing neuropathic pain. What is more, the gene therapy targeting HCK might attenuate the severe neuropathic pain.
The pathway enrichment analysis of the top 2 modules showed those genes were mainly enriched in the innate immune system, bone resorption, Fc gamma R-mediated phagocytosis, antigen processing and presentation, neutrophil degranulation, and lysosome. It is reasonable that most of the enriched terms were immune related. Besides, bone resorption attracted our attention. Bisphosphonates, the compounds commonly used for osteoporosis treatment, have recently been found to also alleviate neuropathic pain [41]. Bisphosphonate compounds can inhibit bone resorption by inhibiting osteoclast activation and inducing osteoclast apoptosis. The osteoclasts and microglia both belong to the mononuclear macrophage family, and they are both differentiated from hematopoietic stem cells [42]. The bisphosphonate compounds targeting receptors on osteoclasts such as CD45 were also expressed on the microglia [43, 44]. Yao et al. reported alendronate, one of the bisphosphonate compounds, could inhibit CD45 downstream signaling pathways, attenuate microglial activation, and subsequently alleviate neuropathic pain [44]. Therefore, the studies on bone resorption can not only help treat osteoporosis, another serious complication following nerve injury, but also provide a novel therapeutic target for neuropathic pain.
The DGIdb database makes it convenient to screen therapeutic agents that might relieve neuralgia. Mounting epidemiological studies demonstrated TNF-α blockade dampening inflammation and subsequently alleviating NP. Adalimumab, infliximab, and etanercept are all TNF-α inhibitors, and they have been clinically used for NP treatment [45–47]. However, during the treatment, neutralizing antibodies would develop to adalimumab and infliximab, attenuating treatment efficacy, while etanercept would not [48]. Thus, etanercept could be in consideration for some refractory NP treatment. Thalidomide is another promising approach for NP treatment. Some animal studies have demonstrated that oral or intraperitoneal injection of thalidomide can not only reduce NP responses but also mediate depressive-like behaviors associated with NP [49, 50]. The others, including natalizumab, rituximab, daclizumab, and ibrutinib, have not been reported as NP therapeutic drugs. However, these drugs are commonly used in some neurological diseases, including multiple mononeuropathy (MM), pediatric multiple sclerosis (PMS), and paraneoplastic disorders of the peripheral nervous system, of which NP is a frequent complication [51–53]. Therefore, the treatment efficacies of these drugs in NP deserve further study.
Several limitations should be acknowledged in our study. First, the sample size was relatively small. Besides, the results were all based on bioinformatic analysis. The experimental and clinical verifications are lacking. In the future, we will perform some more in-depth study around the screened hub genes and drugs.
5. Conclusion
Taken together, the present study deepens our knowledge about the neuroimmune mechanism of neuropathic pain. The study could instruct the treatment of neuropathic pain, such as modulation of microglia polarization and targeting bone resorption. We also identified several hub genes and predicted relevant therapeutic drugs. Among them, CD68, CTSS, LAPTM5, FCGR3A, and CD53 may be used as the early diagnostic biomarkers while the gene HCK can be a therapeutic target.
Acknowledgments
We thank Costigan and his coworkers very much, the authors who deposited their dataset (GSE18803) in the public GEO database. This study was supported by the International Cooperation Program of the National Natural Science Foundation of China (81620108018), National Natural Science Foundation of China (81772342, 81871766, and 81930070), Key Program of Natural Science Foundation of Tianjin (19JCZDJC36300), and Tianjin key research and development plan, key projects for science and technology support (19YFZCSY006600).
Data Availability
The data used to support the findings of the study are included in this article.
Conflicts of Interest
The authors declared no conflict of interests.
Authors' Contributions
HY and YL contributed in the study design and data analysis. HY wrote the paper. CL, QW, JHW, ZQX, and SQF contributed in the critical revision of the manuscript for intellectual content. SQF helped in raising funds. Hao Yu, Yang Liu, and Chao Li contributed equally.
References
- 1.St. John Smith E. Advances in understanding nociception and neuropathic pain. Journal of Neurology. 2018;265(2):231–238. doi: 10.1007/s00415-017-8641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colloca L., Ludman T., Bouhassira D., et al. Neuropathic pain. Nature Reviews. Disease Primers. 2017;3(1) doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard R. F., Wiener S., Walker S. M. Neuropathic pain in children. Archives of Disease in Childhood. 2014;99(1):84–89. doi: 10.1136/archdischild-2013-304208. [DOI] [PubMed] [Google Scholar]
- 4.Walco G. A., Dworkin R. H., Krane E. J., LeBel A. A., Treede R. D. Neuropathic pain in children: special considerations. Mayo Clinic Proceedings. 2010;85(3):S33–S41. doi: 10.4065/mcp.2009.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss A., Beggs S., Vega-Avelaira D., et al. Spinal microglia and neuropathic pain in young rats. Pain. 2007;128(3):215–224. doi: 10.1016/j.pain.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Costigan M., Scholz J., Woolf C. J. Neuropathic pain: a maladaptive response of the nervous system to damage. Annual Review of Neuroscience. 2009;32(1):1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mata M., Hao S., Fink D. J. Gene therapy directed at the neuroimmune component of chronic pain with particular attention to the role of TNFα. Neuroscience Letters. 2008;437(3):209–213. doi: 10.1016/j.neulet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamakawa I., Kojima H., Terashima T., et al. Inactivation of TNF-α ameliorates diabetic neuropathy in mice. Endocrinology and Metabolism. 2011;301(5):E844–E852. doi: 10.1152/ajpendo.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D., Kim M. A., Cho I. H., et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. The Journal of Biological Chemistry. 2007;282(20):14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- 10.Hao S., Mata M., Glorioso J. C., Fink D. J. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Molecular Pain. 2006;2, article 1744-8069-2-6 doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costigan M., Moss A., Latremoliere A., et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(46):14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gene Ontology Consortium. The Gene Ontology (GO) project in 2006. Nucleic acids research. 2006;34(90001):D322–D326. doi: 10.1093/nar/gkj021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanehisa M., Sato Y., Furumichi M., Morishima K., Tanabe M. New approach for understanding genome variations in KEGG. Nucleic Acids Research. 2019;47(D1):D590–D595. doi: 10.1093/nar/gky962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard R. F., Walker S. M., Mota M. P., Fitzgerald M. The ontogeny of neuropathic pain: postnatal onset of mechanical allodynia in rat spared nerve injury (SNI) and chronic constriction injury (CCI) models. Pain. 2005;115(3):382–389. doi: 10.1016/j.pain.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 15.McKelvey R., Berta T., Old E., Ji R. R., Fitzgerald M. Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2015;35(2):457–466. doi: 10.1523/JNEUROSCI.2315-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald M., McKelvey R. Nerve injury and neuropathic pain - a question of age. Experimental Neurology. 2016;275, Part 2:296–302. doi: 10.1016/j.expneurol.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeinali H., Manaheji H., Zaringhalam J., Bahari Z., Nazemi S., Sadeghi M. Age-related differences in neuropathic pain behavior and spinal microglial activity after L5 spinal nerve ligation in male rats. Basic and Clinical Neuroscience Journal. 2016;7(3):203–212. doi: 10.15412/J.BCN.03070305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coraggio V., Guida F., Boccella S., et al. Neuroimmune-driven neuropathic pain establishment: a focus on gender differences. International Journal of Molecular Sciences. 2018;19(1) doi: 10.3390/ijms19010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin P. J., Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. Journal of Neuroimmunology. 2010;229(1-2):26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Hendrickx D. A. E., van Eden C. G., Schuurman K. G., Hamann J., Huitinga I. Staining of HLA-DR, Iba 1 and CD68 in human microglia reveals partially overlapping expression depending on cellular morphology and pathology. Journal of Neuroimmunology. 2017;309:12–22. doi: 10.1016/j.jneuroim.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Xu N., Tang X. H., Pan W., et al. Spared nerve injury increases the expression of microglia M1 markers in the prefrontal cortex of rats and provokes depression-like behaviors. Frontiers in Neuroscience. 2017;11 doi: 10.3389/fnins.2017.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barclay J., Clark A. K., Ganju P., et al. Role of the cysteine protease cathepsin S in neuropathic hyperalgesia. Pain. 2007;130(3):225–234. doi: 10.1016/j.pain.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Clark A. K., Yip P. K., Grist J., et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(25):10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Origasa M., Tanaka S., Suzuki K., Tone S., Lim B., Koike T. Activation of a novel microglial gene encoding a lysosomal membrane protein in response to neuronal apoptosis. Molecular Brain Research. 2001;88(1-2):1–13. doi: 10.1016/S0169-328X(01)00005-5. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y. K., Lu X. B., Wang Y. H., Yang M. M., Jiang D. M. Identification crucial genes in peripheral neuropathic pain induced by spared nerve injury. European Review for Medical and Pharmacological Sciences. 2014;18(15):2152–2159. [PubMed] [Google Scholar]
- 26.Sivagnanam V., Zhu X., Schlichter L. C. Dominance of E. coli phagocytosis over LPS in the inflammatory response of microglia. Journal of Neuroimmunology. 2010;227(1-2):111–119. doi: 10.1016/j.jneuroim.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Inoue H., Sawada M., Ryo A., et al. Serial analysis of gene expression in a microglial cell line. GLIA. 1999;28(3):265–271. doi: 10.1002/(SICI)1098-1136(199912)28:3<265::AID-GLIA10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Grace P. M., Hutchinson M. R., Maier S. F., Watkins L. R. Pathological pain and the neuroimmune interface. Nature Reviews. Immunology. 2014;14(4):217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G., Zhang Y. Q., Qadri Y. J., Serhan C. N., Ji R. R. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100(6):1292–1311. doi: 10.1016/j.neuron.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Echeverry S., Shi X. Q., Yang M., et al. Spinal microglia are required for long-term maintenance of neuropathic pain. Pain. 2017;158(9):1792–1801. doi: 10.1097/j.pain.0000000000000982. [DOI] [PubMed] [Google Scholar]
- 31.Xu F., Huang J., He Z., et al. Microglial polarization dynamics in dorsal spinal cord in the early stages following chronic sciatic nerve damage. Neuroscience Letters. 2016;617:6–13. doi: 10.1016/j.neulet.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 32.Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacological Reports. 2008;60(3):297–307. [PubMed] [Google Scholar]
- 33.Lee S., Shi X. Q., Fan A., West B., Zhang J. Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain. Molecular Pain. 2018;14, article 174480691876497 doi: 10.1177/1744806918764979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin J., Guo J., Cai H., et al. M2-like microglia polarization attenuates neuropathic pain associated with Alzheimer's disease. Journal of Alzheimer's Disease. 2020;76(4):1255–1265. doi: 10.3233/jad-200099. [DOI] [PubMed] [Google Scholar]
- 35.Gong X., Chen Y., Fu B., Jiang J., Zhang M. Infant nerve injury induces delayed microglial polarization to the M1 phenotype, and exercise reduces delayed neuropathic pain by modulating microglial activity. Neuroscience. 2017;349:76–86. doi: 10.1016/j.neuroscience.2017.02.051. [DOI] [PubMed] [Google Scholar]
- 36.Katsura H., Obata K., Mizushima T., et al. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(34):8680–8690. doi: 10.1523/JNEUROSCI.1771-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krady J. K., Basu A., Levison S. W., Milner R. J. Differential expression of protein tyrosine kinase genes during microglial activation. GLIA. 2002;40(1):11–24. doi: 10.1002/glia.10101. [DOI] [PubMed] [Google Scholar]
- 38.Smolinska M. J., Page T. H., Urbaniak A. M., Mutch B. E., Horwood N. J. Hck tyrosine kinase regulates TLR4-induced TNF and IL-6 production via AP-1. Journal of Immunology. 2011;187(11):6043–6051. doi: 10.4049/jimmunol.1100967. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Zhang Y., Pan R., et al. Lentiviral-mediated inducible silencing of TLR4 attenuates neuropathic pain in a rat model of chronic constriction injury. Molecular Medicine Reports. 2018;18(6):5545–5551. doi: 10.3892/mmr.2018.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L., Liu Y., Sun Y., Li H., Mi W., Jiang Y. Analgesic effects of TLR4/NF-κB signaling pathway inhibition on chronic neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Biomedicine & pharmacotherapy. 2018;107:526–533. doi: 10.1016/j.biopha.2018.07.116. [DOI] [PubMed] [Google Scholar]
- 41.Hsu E. S. Practical management of complex regional pain syndrome. American Journal of Therapeutics. 2009;16(2):147–154. doi: 10.1097/MJT.0b013e3181715671. [DOI] [PubMed] [Google Scholar]
- 42.Ash P., Loutit J. F., Townsend K. M. Osteoclasts derived from haematopoietic stem cells. Nature. 1980;283(5748):669–670. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- 43.Fu K.-Y., Tan Y. H., Sung B., Mao J. Peripheral formalin injection induces unique spinal cord microglial phenotypic changes. Neuroscience Letters. 2009;449(3):234–239. doi: 10.1016/j.neulet.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Y., Tan Y. H., Light A. R., Mao J., Yu A. C. H., Fu K. Y. Alendronate attenuates spinal microglial activation and neuropathic pain. The Journal of Pain. 2016;17(8):889–903. doi: 10.1016/j.jpain.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Dahl E., Cohen S. P. Perineural injection of etanercept as a treatment for postamputation pain. The Clinical Journal of Pain. 2008;24(2):172–175. doi: 10.1097/AJP.0b013e31815b32c8. [DOI] [PubMed] [Google Scholar]
- 46.Tobinick E. L. Targeted etanercept for treatment-refractory pain due to bone metastasis: two case reports. Clinical Therapeutics. 2003;25(8):2279–2288. doi: 10.1016/S0149-2918(03)80219-9. [DOI] [PubMed] [Google Scholar]
- 47.Andrade P., Hoogland G., del Rosario J. S., Steinbusch H. W., Visser-Vandewalle V., Daemen M. A. Tumor necrosis factor-α inhibitors alleviation of experimentally induced neuropathic pain is associated with modulation of TNF receptor expression. Journal of Neuroscience Research. 2014;92(11):1490–1498. doi: 10.1002/jnr.23432. [DOI] [PubMed] [Google Scholar]
- 48.Marques I. B., Giovannoni G., Marta M. Mononeuritis multiplex as the first presentation of refractory sarcoidosis responsive to etanercept. BMC Neurology. 2014;14(1):p. 237. doi: 10.1186/s12883-014-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H., Dang S. J., Cui Y. Y., et al. Systemic injection of thalidomide prevent and attenuate neuropathic pain and alleviate neuroinflammatory response in the spinal dorsal horn. Journal of Pain Research. 2019;12:3221–3230. doi: 10.2147/JPR.S213112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nascimento F. P., Macedo-Júnior S. J., Borges F. R. M., et al. Thalidomide reduces mechanical hyperalgesia and depressive-like behavior induced by peripheral nerve crush in mice. Neuroscience. 2015;303:51–58. doi: 10.1016/j.neuroscience.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 51.Simone M., Chitnis T. Use of disease-modifying therapies in pediatric MS. Current Treatment Options in Neurology. 2016;18(8) doi: 10.1007/s11940-016-0420-7. [DOI] [PubMed] [Google Scholar]
- 52.Antoine J.-C., Camdessanché J.-P. Treatment options in paraneoplastic disorders of the peripheral nervous system. Current Treatment Options in Neurology. 2013;15(2):210–223. doi: 10.1007/s11940-012-0210-9. [DOI] [PubMed] [Google Scholar]
- 53.Rivière E., Cohen Aubart F., Maisonobe T., et al. Clinicopathological features of multiple mononeuropathy associated with systemic lupus erythematosus: a multicenter study. Journal of Neurology. 2017;264(6):1218–1226. doi: 10.1007/s00415-017-8519-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of the study are included in this article.


