Abstract
Sepsis and septic shock are catastrophic disease entities that portend high mortality in patients with cirrhosis. In cirrhosis, hemodynamic perturbations, immune dysregulation, and persistent systemic inflammation with altered gut microbiota in the background of portal hypertension enhance the risk of infections and resistance to antimicrobials. Patients with cirrhosis develop recurrent life-threatening infections that progress to multiple organ failure. The definition, pathophysiology, and treatment options for sepsis have been ever evolving. In this exhaustive review, we discuss novel advances in the understanding of sepsis, describe current and future biomarkers and scoring systems for sepsis, and delineate newer modalities and adjuvant therapies for the treatment of sepsis from existing literature to extrapolate the same concerning the management of sepsis in cirrhosis. We also provide insights into the role of gut microbiota in initiation and progression of sepsis and finally, propose a treatment algorithm for management of sepsis in patients with cirrhosis.
Keywords: Portal hypertension, Sequential organ failure assessment, Acute on chronic liver failure, Predisposition insult response organ-dysfunction model, Intensive care unit, Shock
Core tip: Advances in understanding sepsis have led to an uncomplicated and robust definition with prognostic importance. What has emerged is a redefinition of the clinical protocols for early and aggressive management of sepsis at hour 1 of patient presentation and identification of a novel combination of biomarkers. In addition, antimicrobial resistance has been addressed and adjuvant therapies have been identified through deep data mining, metagenomics, and machine learning-based tools for improving clinical outcomes. These advances have the potential to be extrapolated and studied in patients with cirrhosis and sepsis to improve notable catastrophic clinical outcomes seen in this unique and challenging patient population.
INTRODUCTION
Critical illness in the presence or absence of overwhelming infection leading to multiple organ failure in patients with cirrhosis is a rapid, complex, and catastrophic process that, in the majority, does not respond to conventional treatment practices laid down for the general population. In advanced cirrhosis, hyperdynamic circulation with high cardiac output, subclinical cardiomyopathy, central hypovolemia, third space fluid accumulation, and low systemic vascular resistance prevail. In decompensated patients with cirrhosis, a small proportion develops the syndrome acute on chronic liver failure (ACLF) with sepsis, which is characterized by extrahepatic organ failures requiring intensive care management with rapid progression to multiple organ failure. Such events increase in-hospital mortality and result in treatment futility even with the best supportive care.
Sepsis is defined as a “life-threatening organ dysfunction caused by a dysregulated host response to infection,” that can cause critical illness in patients with cirrhosis with different clinical consequences. In a compensated cirrhosis, the development of sepsis can cause acute decompensation that can progress to ACLF. Sepsis can develop as an intercurrent event in decompensated cirrhosis, leading to worsening of existing or new-onset decompensation, both of which can lead to ACLF. Sepsis can also develop during acute decompensation or ACLF, all of which can lead to organ failures. Sepsis is established in the presence of suspected or documented infection and an acute increase of ≥ two sequential organ failure assessment (SOFA) (a proxy for organ dysfunction) points[1-3].
In cirrhosis, an associated immune dysfunction exists with worsening severity, depending on the stages and severity of decompensation. This cirrhosis-associated immune dysfunction (CAID) is dynamic and affects both the innate and acquired immune functions, because of changes and deficiencies in both the local milieu of the liver microenvironment and systemic immunity. CAID depends on the increased and persistent systemic inflammation, liver disease severity, and portal hypertension and is central to both acute and chronic decompensation. Besides, increased gut permeability, reduction in gut motility, and altered gut microbiota promote increased bacterial translocation and subsequent endotoxemia, leading to worsening systemic inflammation in cirrhosis. Sepsis by itself is a state of profound immune dysregulation in which, during the early phase, a pro-inflammatory state, counterbalanced by an anti - inflammatory response, affects immune functions (compensatory anti - inflammatory response syndrome). In patients with dysregulated immune functions, such as those with cirrhosis, this initial phase goes unchecked, progressing to sepsis - induced immunosuppression and a stage of immune-paralysis with subsequent organ failure development. In the following sections of this review, we aim to discuss current and future aspects in the diagnosis and treatment of sepsis and extrapolate recent advances in the management of sepsis concerning critically ill patients with cirrhosis with sepsis[4,5].
DEFINING SEPSIS, SEPTIC SHOCK, AND RELATED COMPONENTS IN CIRRHOSIS
Early on, the pathogenesis of sepsis and its systemic consequences were considered a hyper-inflammatory response to microbial invasion (infection) accompanied by an evolving cytokine storm. Because of this, sepsis was defined as ”a systemic inflammatory response syndrome (SIRS) to infection” (Sepsis-1 definition). A decade later, expert consensus concluded that such a general definition did not allow for staging of sepsis-related events, and hence prediction of the host response to infection remained vague for clinical and research purposes. The SIRS criteria, even though useful for easy identification of sepsis, remained non-specific and too sensitive. This paved the way for the Sepsis-2 definition that included the PIRO [P: Predisposition; I: The type and extent of insult (infection in sepsis); R: The type and extent of host response; and O: The type and extent of organ dysfunction] model[6,7]. With PIRO, morbidity (primary organ dysfunction) because of the infection itself and morbidity developing during host response (secondary organ dysfunction) were identifiable. For example, in a patient with cirrhosis with the development of bacterial pneumonia, type 1 respiratory failure occurring early during illness can be considered primary organ dysfunction (because of the infection). In the same patient, the development of ascites and acute kidney injury during the later course of the disease can be secondary organ dysfunction (hepatorenal syndrome) because of predisposition (cirrhosis), insult (pneumonia), and host response (decompensation of cirrhosis). In such a situation, stratifying patients at risk of death depending on early and late events along with components of the PIRO model can help in improving prognostication and define specific timeframes for therapeutic interventions.
Acceptance and attempt at including the PIRO model in patients with cirrhosis are lacking in the literature. Jalan and colleagues studied the prognostic value of the PIRO model on outcomes in patients with ACLF. The authors found that in patients with organ failures, previous hospitalization (predisposition), persistence and severity of inflammation (response), and severity of organ failure (organ dysfunction) were associated with higher mortality[8,9]. Maiwall et al[10] in a prospective study of ACLF patients showed that serum creatinine, bilirubin, potassium, and blood urea at baseline (predisposition); nephrotoxic medications (insult); SIRS (response), and circulatory failure (organ dysfunction) identified those at risk of developing acute kidney injury during the disease course and death. The PIRO model could help identify patients with cirrhosis at risk of sepsis, those at risk of developing specific organ failure, and those at risk of recurrent sepsis. This could help define specific therapeutic ”windows” to improve further deterioration and reduce organ failures in patients with cirrhosis.
An expert consensus meeting re-defined sepsis (Sepsis – 3), with the omission of the terms SIRS and severe sepsis. According to the new consensus, in the absence of organ dysfunction, the event is termed an ”infection”. Septic shock was defined as hypotension unresponsive to fluid boluses and with lactate > 2 mmol/L. For defining and grading organ failures, the SOFA score was identified as the best tool. Acute organ dysfunction is identified when the SOFA score increased by two points from baseline (considered 0 before admission), and parallel identification of an infective focus defined sepsis. A new screening tool for early recognition of sepsis called the ”quick”-SOFA (qSOFA) was provided, intended for primary use in the non-intensive unit care. According to qSOFA, patients meeting two of three criteria (altered mental status, respiratory rate > 22 per min, systolic blood pressure < 100 mmHg) were suspected of having new-onset or worsening sepsis. The SOFA score was developed from the general intensive care unit population rather than from patients with cirrhosis, and hence, some of the core components (such as Glasgow coma scale and platelet count) could be influenced by the severity of the underlying liver disease[11,12].
To improve on the prediction of SOFA score in patients with cirrhosis developing acute decompensation, the European Association for Study of Liver-Chronic Liver Failure Consortium (EASL - CLIF Consortium) changed the SOFA score into CLIF - SOFA score and defined ACLF according to the new score. In the CLIF - SOFA score, six organ systems with specific changes applied regarding patients with end - stage liver disease were designated. Platelet count was replaced by the international normalized ratio of prothrombin time and the Glasgow coma scale with hepatic encephalopathy as the central nervous system criterion. It also modified the use of terlipressin as part of the cardiovascular component and renal replacement therapy within the renal parameter[13-15]. The CLIF - SOFA score also added peripheral capillary oxygen saturation/fraction of inspired oxygen in the air as an alternative to respiration parameter for patients without arterial line placed (Table 1).
Table 1.
Modification of sequential organ failure assessment score for patients with cirrhosis, the chronic liver failure-sequential organ failure assessment scoring system[13,15]
| Score | 1 | 2 | 3 | 4 |
| Respiration PaO2/FiO2 or SpO2/FiO2, mmHg | > 300 to ≤ 400 or > 357 to ≤ 512 | > 200 to ≤ 300 or > 214 to ≤ 357 | > 100 to ≤ 200 or 89 to ≤ 214 | < 100 or ≤ 89 |
| Liver bilirubin, mg/dL | 1.2-1.9 | 2.0-5.9 | 6.0-11.9 | > 12 |
| Cardiovascular hypotension | Mean arterial pressure < 70 mmHg | Dopamine ≤ 5 or any dobutamine or terlipressin | Dopamine > 5 or noradrenaline ≤ 0.1 | Dopamine > 15 or noradrenaline > 0.1 |
| Cerebral HE grades | I | II | III | IV |
| Renal creatinine (mg/dL) or urine output | 1.2-1.9 | 2.0-3.4 | 3.5-4.9 or use of renal replacement therapy | ≥ 5.0 |
| Coagulation - INR | ≥ 1.1 to < 1.25 | ≥ 1.25 to < 1.5 | ≥ 1.5 to < 2.5 | ≥ 2.5 or platelet count ≤ 20000/µL |
FiO2: Fraction of inspired oxygen; HE: Hepatic encephalopathy; INR: International normalized ratio; PaO2: Arterial oxygen pressure.
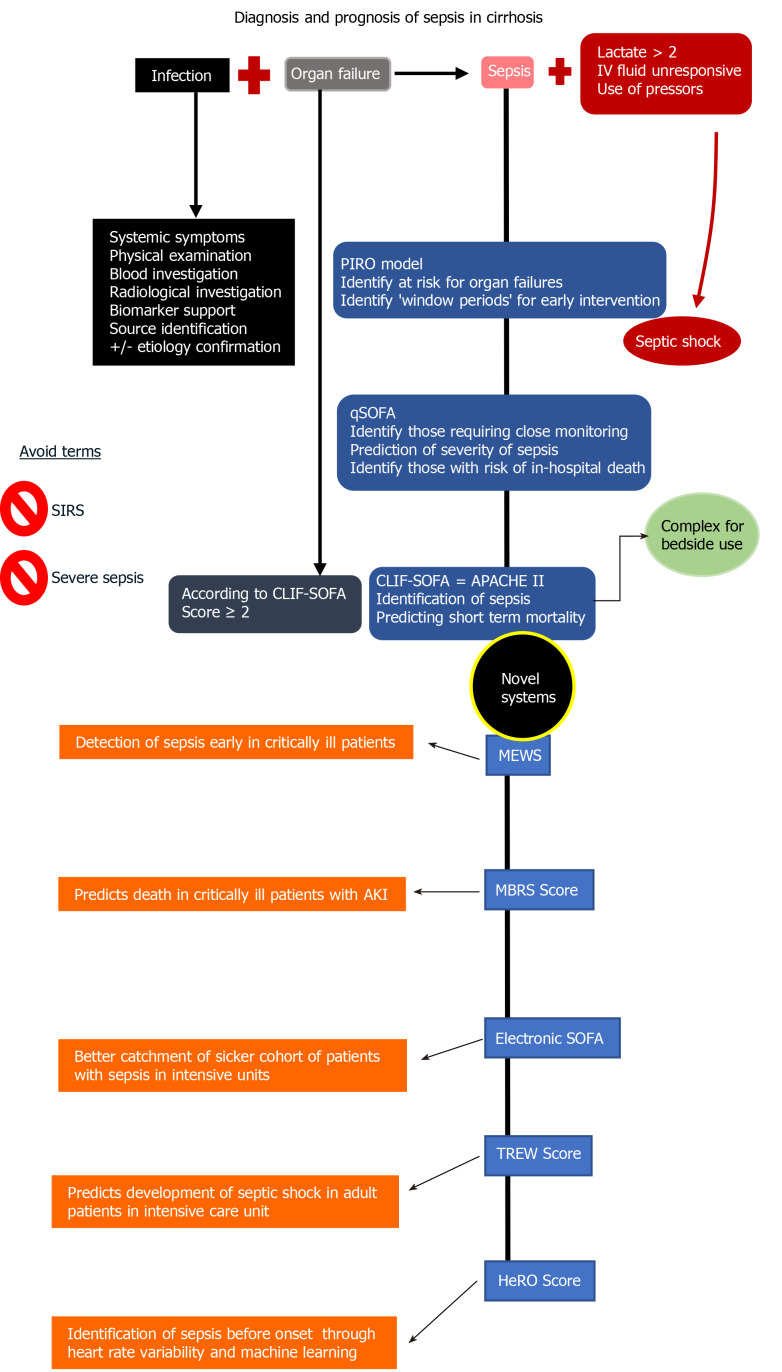
Small studies have shown that the Acute Physiology And Chronic Health Evaluation II (APACHE II) and subsequent modifications were superior to other scoring and definition systems of sepsis in cirrhosis. However, no study has shown its superiority to CLIF-SOFA. Hence, further studies pending, application of either of these scoring systems, as per operator ease is acceptable in critically ill patients with cirrhosis. To summarize, in a patient with cirrhosis, sepsis is identified when there is the fulfilment of at least two SOFA score points at presentation, in the presence of an infection, the latter proved either radiologically or microbiologically with or without blood biomarker correlation (Figure 1).
Figure 1.
Definitions, diagnosis, and summary of prognostic scoring systems of sepsis[2,4,6,58,63-65]. Sepsis is defined as presence of suspected or confirmed infection in the presence of an organ failure as defined by the sequential organ failure assessment tool. After diagnosis of sepsis, a prognostic tool is utilized to identify patients at risk of worsening or death. APACHE: Acute physiology and chronic health evaluation; CLIF: Chronic liver failure; HeRO: Heart rate index; MBRS: Mean arterial pressure, bilirubin, respiratory failure, sepsis; MEWS: Modified early warning score; PIRO: Predisposition, insult, response, organ failure; qSOFA: Quick sequential organ failure assessment; SIRS: Systemic inflammatory response syndrome; SOFA: Sequential organ failure assessment; TREW: Targeted real-time early warning score.
THE LACTATE QUANDARY IN CIRRHOSIS
Serum lactate levels play a major role in defining patients with septic shock. It was shown that lactate levels were elevated in patients with cirrhosis, compared to healthy controls, related to portal pressures, and increased with severity of the liver disease. This meant that lactate kinetics in the cirrhosis population differed greatly from other patient populations[16]. A study tested lactate levels in patients with acute circulatory failure with hepatic dysfunction. No differences in pertinent variables, such as lowest systolic blood pressure, serum creatinine, and simplified APACHE scores, were noted. However, the lactate levels were higher in the group with liver disease (8.24 mmol/L vs 4.29 mmol/L, P < 0.001), with a positive correlation between lactate and aspartate aminotransferase levels. The authors concluded that there was no apparent correlation between liver dysfunction and the severity of shock as a confounder[17]. Kruse et al[18] tested the significance of blood lactate in critically ill patients with liver disease. They found that arterial lactate > 2.2 mmol/L was associated with clinical evidence of shock and significant in-hospital mortality.
In a systematic review of blood lactate as a predictor for in-hospital mortality in acutely ill patients, venous or arterial lactate > 2.5 mmol/L at admission was associated with the progression of clinical deterioration[18-20]. Sun et al[21] showed that the serum lactate levels were predictive of extrahepatic organ failure (acute kidney injury) in critically ill patients with cirrhosis. The mortality rate increased with a rise in serum lactate. In a multinational study, Drolz et al[22] showed that lactate levels reflected the severity of disease and organ failure and was independently associated with a high risk of death in the brief term in critically ill cirrhosis patients.
The addition of lactate into the CLIF-C score for ACLF patients improved its prognostic power. A serum lactate ≥ 5 mmol/L had high predictive power for short term mortality, and lactate clearance predicted 28-d mortality. Admission and 12-h lactate clearance in those with admission lactate ≥ 5 mmol/L predicted 1-y mortality. In summary, including lactate above 2 mmol/L can be extrapolated to define patients of cirrhosis with septic shock. The admission and serial lactate measurements and lactate clearance are useful in identifying those with poor prognosis even though the complex lactate dynamics remain undefined in patients with advanced cirrhosis. Thus, septic shock in cirrhosis can be identified in the presence of sepsis, the onset of hypotension requiring vasopressor support [mean arterial pressure (MAP) < 65 mmHg], and lactate > 2 mmol/L despite adequate fluid resuscitation.
TOLERANCE TO SEPSIS – A BROKEN DEAL IN CIRRHOSIS
There are three essential strategies for dealing with disease because of pathogens - avoidance, resistance, and tolerance. Of these, the first two are notable among animals (who are mobile), while the third strategy is clear in plants (since they are stationary). Tolerance results in the ability to maintain health in the presence of a pathogen(s). An example of tolerance to infection or pathogen is the case of Ms. Mary Mallon (”typhoid Mary”), causing severe Salmonellosis in persons consuming dishes she prepared and tolerance to malaria among persons with sickle-cell anaemia. In sepsis, at the core, there occurs complete dysregulation of local and systemic inflammatory and associated metabolic processes that lead to organ failure. Yet another major event in sepsis is the destruction of red blood cells through direct or indirect pathogen-based hemolysin effect. Hence, in sepsis, heme production is overwhelming, and the removal of free heme results in the formation of divalent iron (Fe2+). Excess production of Fe2+ leads to the overproduction of reactive free radicals through the Fenton reaction, resulting in the release of trivalent iron (Fe3+), which is a hydroxyl radical that promotes various secondary metabolic reactions. To prevent toxic secondary reactions, oxidized iron is removed by ferritin. Ferritin thus confers tolerance towards infections[23].
In the seminal work by Weis et al[24] on sepsis tolerance, mice with pre-deleted ferritin subunit (FTH) and those expressing FTH, underwent cecal ligation and puncture (an animal model of sepsis). The authors found that the survival of the mice depended on FTH expression on hepatocytes and macrophages. Those with FTH deficiency had inferior survival with the development of sepsis. In both FTH deficient and sufficient groups, the microbial burden and cytokine production were similar but without overt sepsis in the latter, showing tolerance to sepsis development in the presence of ferritin expression. In FTH deficient mice, the bodyweight loss was extensive, with lower body temperatures, and correlated with hypoglycaemia. Thus, the link between FTH expression and maintenance of blood glucose levels was notable in this study. When heme was infused into FTH deficient mice with sepsis, death was inevitable; with the infusion of glucose, health status, and survival improved. At the gene expression level, the activity of glucose-6-phosphatase catalytic subunit-1 (G6PC-1) was reduced, leading to curtailment of gluconeogenesis. The authors found that in the absence of ferritin expression, free heme downregulated G6PC-1 expression and reduced hepatic gluconeogenesis and glycogenolysis, leading to an increase in mortality. Use of iron-chelators, antioxidants, and iron-free ferritin restored G6PC-1 activity and induced gluconeogenesis, leading to an improvement in survival[23,24]. In an animal model of listeriosis, Medzhitov et al[25] showed that correction of hypoglycaemia using glucose infusions worsened survival because of the promotion of neurotoxicity by exacerbation of reactive oxidative species. In virus-infected mice, glucose infusions improved sepsis and survival and reduced neuronal endoplasmic reticulum stress responses. These two studies showcase an important aspect of sepsis – adaptive tolerance to sepsis in the host that was dependent on the pathogen type[25,26].
In patients with decompensated cirrhosis, Changani et al[27] showed impairment in gluconeogenesis in advanced liver disease but not in stable patients with cirrhosis. In the early stages of cirrhosis, hepatic gluconeogenesis and fatty acid oxidation are increased in the presence of reduced hepatic glycogen content, resulting in lactate and alanine production through muscle breakdown and protein degradation. With the progression of cirrhosis, liver failure sets in, leading to a reduction in gluconeogenesis, depletion of glycogen stores, amelioration in glycogenesis, and loss of muscle mass (sarcopenia) leading to a diminution in pro-glucogenic substrates[28]. Heme-oxygenase has anti-inflammatory and anti-apoptotic properties, and induction of heme-oxygenase-1 in animal models of acute or chronic liver injury showed a reduction in hepatic inflammation and fibrosis progression and partial resolution of existing fibrosis. In animal models of cirrhosis and humans with decompensated cirrhosis, the expression of heme-oxygenase increased with increasing severity of liver disease and portal hypertension[29,30]. Patients with cirrhosis have excessive erythrocyte destruction because of splenomegaly, reduced red blood cell survival, reduced red cell mass, suppression of bone marrow function, blood loss because of acute and chronic gastrointestinal bleeding events associated with portal hypertension and blunted response to erythropoietin[31,32].
Ferritin, a marker of stored iron, can be elevated in decompensated cirrhosis patients with inflammation and in those patients whose cirrhosis aetiology is secondary to alcohol use or chronic hepatitis C infection. It has been shown that even in the presence of high ferritin levels in patients with decompensated cirrhosis, the transferrin levels were low, and transferrin saturation elevated. Lower transferrin levels that represent malnutrition, the severity of cirrhosis, and inflammation are associated with poor transplant-free survival in patients with decompensated cirrhosis[33,34]. To summarize, in cirrhosis, there occurs increased red cell destruction, reduction in hepatic gluconeogenesis, lower levels of transferrin, and high dysfunctional levels of ferritin, which leads to a state of perturbed tolerance to infections and a higher risk of sepsis, loss of muscle mass, lower body temperature, and dysfunctional control over inflammation. Measures to improve tolerance to sepsis in patients with advanced cirrhosis could become an important component in the armamentarium of therapeutic options against cirrhosis with sepsis and improving survival outcomes (Figure 2).
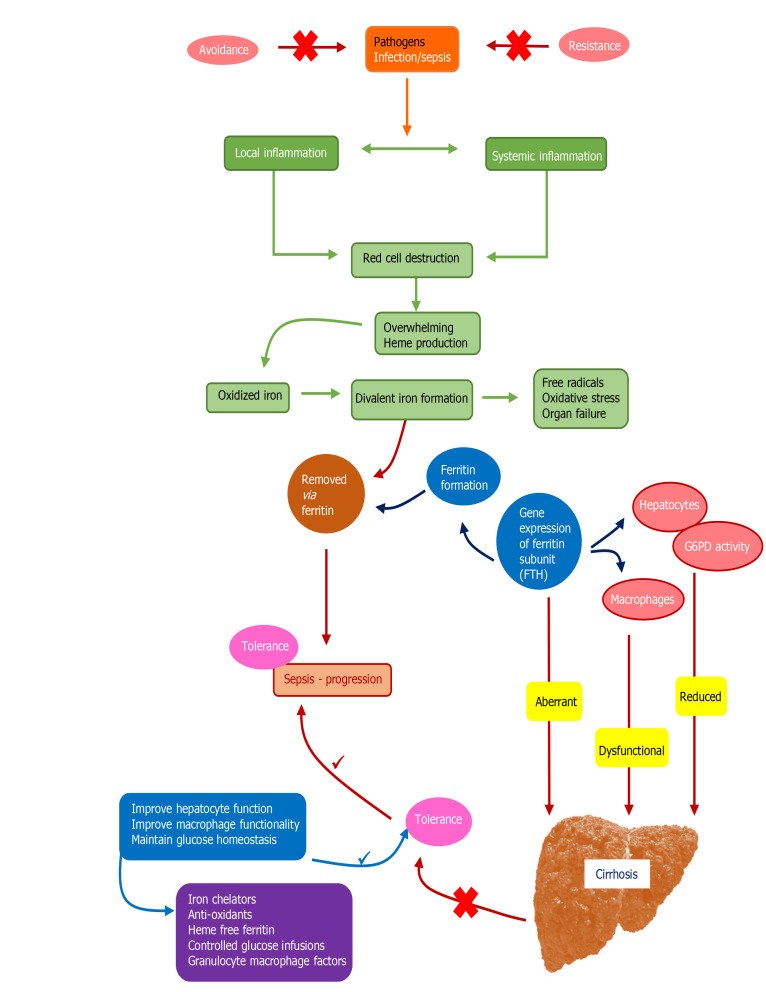
Figure 2.
Insights into pathophysiology of tolerance toward sepsis and loss of tolerance leading to higher risk of sepsis in cirrhosis patients[4,23-25]. Apart from tolerance, loss of resistance and exposure to pathogens (shown as red crosses in the upper part of the figure) can initiate infections that can lead to development of sepsis. In patients with infections who develop sepsis, local and systemic inflammation lead to dysregulated red cell homeostasis and development of toxic oxidants especially iron ligands that are removed by ferritin. Ferritin formation and oxidant sweep are regulated systematically through hepatocyte and macrophage functions in the healthy liver through expression of glucose-6-phosphatase (G6PD) and ferritin H gene subunit (FTH). In cirrhosis, liver dysfunction results in aberrant FTH activity, defective macrophage and hepatocyte functions and reduction in G6PD activity, resulting in increased oxidant stress and loss of tolerance to infection, leading to progression of sepsis through reduction in functional ferritin (shown as red crosses at the bottom). The blue and purple boxes demonstrate steps and measures for correction of dysregulated responses in a patient with cirrhosis, respectively, so as to improve tolerance to infection and prevention of sepsis. FTH: Ferritin H gene subunit; G6PD: Glucose-6-phosphatase.
FASTING METABOLISM AND DEFENSE AGAINST INFECTIONS – IS THERE A ROLE IN EARLY CIRRHOSIS?
A proverb goes, ”feed a cold, starve a fever”. In the presence of infection, animals develop specific behavioural changes that include anorexia, sleep pattern variations, and withdrawal from social activities – asymptomatic complex referred to as ”sickness behaviours”. These patterns were considered flawed consequences of the host response to infection. Newer evidence suggests that sickness behaviours are strategic evolution in the host to ward off the harmful effects of infection and improve survival. Of these, the most important is anorexia. Anorexia modulates host metabolic requirements of stress responses pertinent for tolerance to bacterial inflammatory states.
In the seminal work by Wang et al[26], it was demonstrated in a small animal model that fasting metabolism was protective in bacterial but not virus-induced inflammation. The ketosis that develops during fasting limited the reactive oxygen species induced neuronal damage during bacterial infection-related inflammation while non-fasting or glucose infusion prevented neuronal damage in viral inflammation, showcasing the importance of host responses to aetiology of infection during fasting[26,35,36]. Force-feeding mice with lipopolysaccharide induced endotoxemia increased mortality. Intermittent fasting was shown to increase acute immune and behavioural sickness responses leading to worse outcomes in mouse models of viral infections and inflammation.
Metabolic processes in the liver microenvironment are firmly regulated by neuronal and hormonal systems such as the sympathetic and parasympathetic systems and insulin-glucagon related systems[37,38]. Patients with cirrhosis are a unique population regarding nutritional and metabolic disorders. In advanced cirrhosis, the liver cannot synthesize and store required amounts of glycogen, which creates a ”glucose deficient” state in times of stress. In this scenario, the utilization of non-carbohydrate sources for gluconeogenesis, such as glycerols from fatty tissue and amino acids from muscles, becomes remarkable. Dietary improvements rather than restriction are well known to improve outcomes in this stage, even though dietary or nutritional interventions in special situations such as in an obese patient with cirrhosis remain controversial. An overnight fast in a patient with cirrhosis patient is akin to 3-d fasting in an average person[39]. Owen et al[40] showed that after an overnight fast, hepatic glucose production in patients with cirrhosis was diminished because of low-rate glycogenolysis, but hepatic gluconeogenesis and ketogenesis were increased. After 3 d of starvation, patients with cirrhosis were found to have hepatic gluconeogenic and ketogenic profiles comparable to those of healthy patients undergoing deprivation of a similar duration. García-Compeán et al[41] showed that subclinical abnormal glucose tolerance was a predictor of death in patients with liver cirrhosis. In the study by Wang et al[26], the authors found that fasting metabolism protected against sepsis and that protection was suppressed by infused glucose[35], and Weis et al[24] discovered that mice have to maintain minimal glucose levels through gluconeogenesis for tolerating bacterial sepsis. Thus, there occurs an upper and lower limit of glucose homeostasis and blood glucose level that must be maintained through the reduced intake (anorexia) and by endogenous hepatic glucose production (gluconeogenesis) to improve outcomes in sepsis. This is a matter of further research in patients with cirrhosis.
In animal models of cirrhosis and sepsis, we need to identify outcomes related to fasting and non-fasting states. Studies on intermittent or prolonged fasting on immune functions in patients with cirrhosis and associated clinical outcomes remain an unmet need. In summary, in patients with compensated cirrhosis, the role of different modes/methods of fasting for prevention or treatment of bacterial infection could be an exciting area of research – one that needs bench work in small cirrhotic animal models for further consideration in humans (Figure 3).
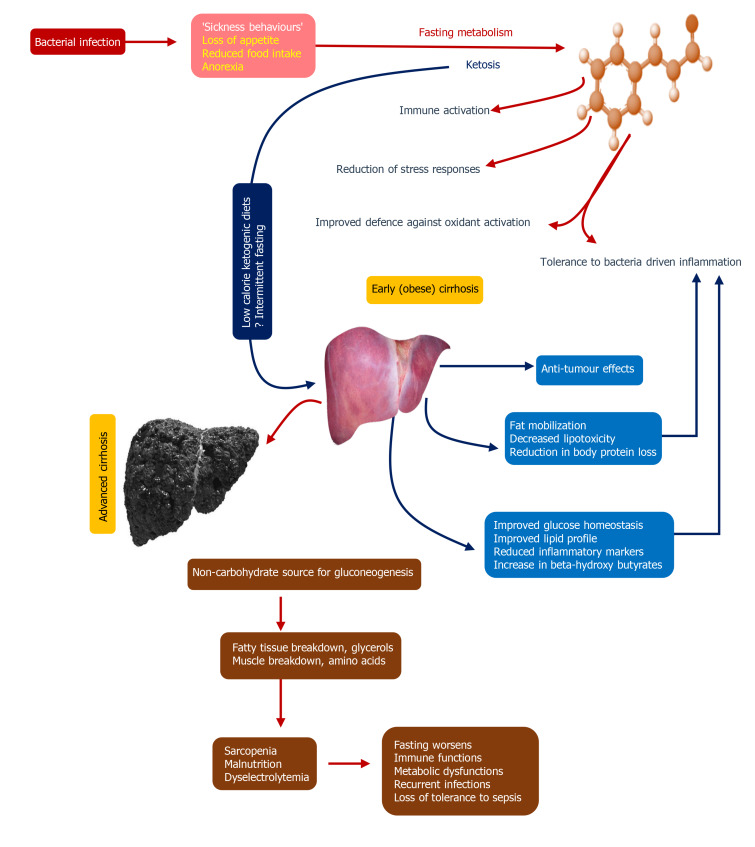
Figure 3.
Fasting metabolism and its impact on immune homeostasis and enhanced tolerance to infections[26-28]. The figure demonstrates the potential mechanisms associated with fasting metabolism on immune functions that ultimately prove beneficial for prevention of and combating infections. This could be hypothesized to have benefits in patients with early cirrhosis, especially in those who are obese, pending bench to bedside translational studies. Nonetheless, in advanced cirrhosis, on the contrary, nutritional management to improve immune functions, prevention of infections, and boosting tolerance to sepsis is of importance.
ADVANCES IN GENERAL PATHOPHYSIOLOGY OF SEPSIS AND RELEVANCE IN CIRRHOSIS
With the intrusion of a microbial entity, the initial host response is the activation of innate immunity that is comprised of macrophages, monocytes, neutrophils, and natural killer cells. This cellular activation is a result of the binding of pathogen-associated molecular patterns, which include endotoxins and fungal elements such as beta-glucans and other microbial degradation components. Apart from these direct pathogen-related activator molecules, damage-associated molecular patterns, which include intracellular material, components of dead or damaged host cells, and microbial DNA, potentiate the host response to infection. All of these activator molecules bind to specific receptors on cells (such as monocytes and macrophages) associated with mounting a counteractive immune response through toll-like receptors, C-type leptin receptors retinoic-acid inducible gene-1-like receptors and nucleotide-binding oligomerization domain-like receptors[42]. In sepsis, close interactions between the inflammatory and haemostatic pathways also affect host responses at the cellular, tissue, and organ levels. With perpetuation of host inflammatory response to an overwhelming or under controlled infection, toxicity at local and systemic levels due to inflammatory components as well as microthrombi formation in organ systems in the initial phase leads to hypoperfusion and decreased delivery of and utilization of oxygen by cellular components, leading to organ dysfunction seen in sepsis[43].
In cirrhosis, the systemic inflammation is mediated through the activation of all innate and adaptive immune cells, with the tipping of the balance towards pro-inflammatory cytokines. In compensated cirrhosis, the progression of fibrosis and hepatocyte loss release damage-associated molecular patterns that activate the immune system causing sterile systemic inflammation. In decompensated cirrhosis, worsening portal hypertension leads to bacterial translocation and release of pathogen-associated molecular patterns into the systemic circulation from the intestinal lumen into the circulation. The continuous influx of immune and inflammation activating molecules leads to a state of persistent inflammation in the host. As cirrhosis progresses and patients start developing complications of portal hypertension, and ultimately liver failure, exhaustion of the immune system occurs, along with loss of tolerance to infections, leading to the inability to mount functional innate and adaptive immune responses. This defines CAID state in which increased levels of anti-inflammatory cytokines and leukocyte inhibitory antigens predominate with loss of immune cell function. In its most extreme form, ACLF, a state of immune paralysis that is also notable in advanced stages of sepsis, is appreciable. In advanced cirrhosis with CAID, an infectious insult can rapidly lead to a state of immune exhaustion that is much more burdensome compared to a non-cirrhotic patient population. A study showed that lymphocytopenia on the 4th day after a diagnosis of sepsis was predictive of both 28-d and 1-y mortality in sepsis[42,44].
Systemic inflammation plays a central role in defining landmark events in patients with cirrhosis. Even in the absence of infection or sepsis, patients with cirrhosis are at baseline, in a state of persistent systemic inflammation. In the presence of non-infectious causes for worsening or acute severe systemic inflammatory states (for example, alcoholic hepatitis, drug-induced liver injury, or reactivation of chronic hepatitis B virus infection), acute decompensation can develop in patients with compensated cirrhosis. In cirrhosis patients with bacterial infections who developed acute decompensation and ACLF, the inflammatory markers interleukin (IL)-6, tumour necrosis factor-alpha, and IL-1 receptor antagonist were found to increase much higher than those with other stress/insults[45]. In patients with compensated and decompensated cirrhosis, in the absence of infections, persistent systemic inflammation leads to a prothrombotic or hypercoagulable state. This baseline hypercoagulability worsens organ dysfunction in patients with cirrhosis who develop infections, and in advanced stages of cirrhosis, once organ failures take full form, disseminated intravascular coagulation develops that leads to a haemorrhagic phenotype in critically ill patients with cirrhosis with septic shock[46,47]. In summary, targeting sepsis in cirrhosis is not merely targeting the pathogen but, in early stages, improving tolerance to infection and correcting of hypercoagulability; in middle stages, keeping in control the unhealthy proinflammatory storm; and late stages, improving immune regulation and abolishing immune paralysis. Timing of treatments and targeted therapy for these important events in sepsis and cirrhosis remain an unmet need and require multicentre collaboration and specific focused groups working on each aspect to define each therapeutic component that would ultimately become a primary protocol that can be generalized world over.
NOVEL BIOMARKERS FOR SEPSIS DIAGNOSIS AND PROGNOSIS: USE IN CIRRHOSIS
A multitude of biomarkers has been identified that help in the diagnosis and prognosis of sepsis. Of these, the C-reactive protein (CRP), procalcitonin (PCT), and IL-6 have been most extensively studied and of clinical use currently. However, these markers have high levels of heterogeneity concerning the population studied and lack homogeneity in displaying diagnostic value under special circumstances. The Surviving Sepsis Campaign guidelines advocate that measuring PCT can help reduce the duration and promote early discontinuation, escalation, or de-escalation of antimicrobial therapy in patients with diagnosed sepsis. Even though single PCT measurements do not have strong prognostic value, serial measurements can help identify patients at risk of death due to the progression of sepsis and the emergence of septic shock[48,49].
Mid-regional pro adrenomedullin (MR-proADM), a fragment of adrenomedullin precursor (amino acids 45 to 92) with vasodilator and natriuretic properties, was found to be superior to current biomarkers and scoring systems in predicting 28-d mortality in patients with sepsis, septic shock, critically ill with new-onset fever, and respiratory tract infection. In patients with cirrhosis, MR-proADM was found to relate to portal pressures and systemic hemodynamics. It was recently shown that MR-proADM was reliable in identifying cirrhosis patients with complicated bacterial infections as well as those with a very high risk of short-term death independent of bacterial infections or SIRS criteria[50].
Remmler et al[51], in a retrospective observational study in end-stage liver disease patients, found that the model for end-stage liver disease (MELD) scores, IL-6 level, and CRP level were associated with mortality risk. The 1-y mortality was zero among patients with IL-6 levels < 5.3 pg/mL but 68% among those with IL-6 > 37.0 pg/mL. The predictive performance for 90-d mortality was excellent (area under the curve, 0.94) for IL-6 and similar to those of MELD and MELD-sodium scores and superior to those of CRP and white blood cell levels. The authors also found that the IL-6 level was an independent predictor of mortality after adjustment for the other markers[51].
Recently, the changes associated with the expression of the neutrophilic CD64 surface marker were found to predict severe inflammation and sepsis efficiently. Neutrophilic dysfunction, a hallmark of sepsis, is that in which neutrophils lose their ability to respond to chemokines leading to an alteration in the microbicidal activity. Such neutrophils have a specific motility signature that can be captured using microfluidic-based assays. From these motility signatures, the Sepsis Score was determined by [N × (O + P + R + AD)/103], where N is the neutrophil count; O the number of oscillations exhibited within the migration channels; P the time spent pausing during spontaneous motility; R the reverse migration of cells out of the device, and AD the average distance migrated by the cells. Among patients without cirrhosis, the scoring system generated an area under the curve of 0.98 for non-sepsis and sepsis patients with 96.8% sensitivity and 97.6% specificity[52].
Apart from the clinical definitions that guide diagnosing sepsis at the outset, several novel investigational tools have improved diagnosis and prognostication of sepsis. A novel biomarker, the intensive care infection score (ICIS) composed of five blood-cell-derived parameters [mean fluorescence intensity of mature (segmented) neutrophils, the difference in haemoglobin concentration between newly formed and mature red blood cells, the total segmented neutrophil count, the antibody-secreting lymphocytes, and the accurate immature granulocytes count] characterizing the early innate immune response can be routinely obtained from blood samples sent to the laboratory for cell counts. This score has been retrospectively evaluated in two pilot studies, which suggested its potential predictive value for infection. A mean ICIS value of < 3 (lower cut-off level) indicates the absence of infection. In contrast to CRP and PCT measurements, the ICIS can be determined routinely without new blood sampling and lower costs, yielding results within 15 min[53].
Recently, the monocyte distribution width, with a value > 20 U, was found to be effective for sepsis detection based on the Sepsis – 3 criteria at admission. In the presence of a raised white cell count, the value of monocyte distribution width improved diagnosing and defining early management protocols for sepsis[54]. Crawford et al[55] developed an automated deformability cytometric analysis using microfluidic cartridge and customized instrumentation. In this system, imaging of single cells at the rate of thousands/s with the high-speed camera can be studied as they undergo stretching in a controlled microfluidic flow. Deformability was defined as the length by width of a cell during its motion through the microfluidic chamber. The authors found that granulocytes in patients with sepsis fluidize and elongate much more when compared to the normal population. This can help identify patients with sepsis-associated early innate immune activation. The assay time takes less than 10 min from blood collection to final output and can be used in an emergency setting to identify those who require immediate antibiotic care. This is important because an increase in mortality has been shown with every passing hour in patients with sepsis and even more so in those with septic shock, which holds in patients with cirrhosis and sepsis at a more catastrophic level (Figure 4)[55,56].
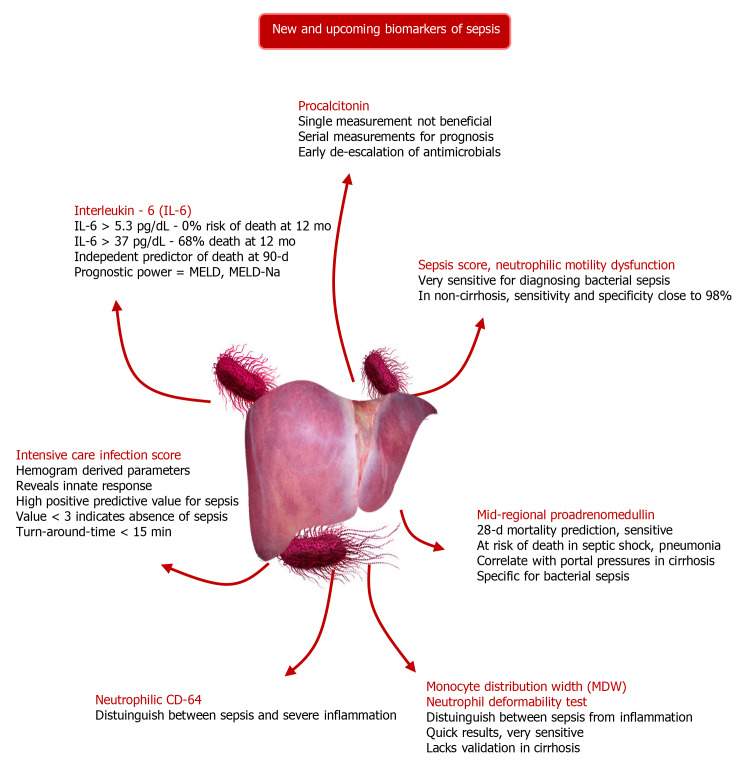
Figure 4.
A summary of new and upcoming biomarkers for sepsis[48,50-57]. CD: Cluster of differentiation; IL: Interleukin; MDW: Monocyte distribution width; MELD: Model for end-stage liver disease; Na: Sodium.
To improve on the diagnostic and prognostic accuracy of single-protein biomarkers that are currently in use, transcriptomics (the study of the whole set of RNA transcripts that are produced by the genome, under specific circumstances or in a specific cell—using high-throughput methods, to identify specific gene expressions) based biomarker panels for a broader assessment of host response to infection have become novel powerful tools. Two such transcriptomic sepsis scores, the SeptiScore™ and the Sepsis MetaScore, using set algorithms, have been validated in independent cohorts. SeptiScore™ utilizes SeptiCyte™LAB technology (ImmuneExpress, Seattle, WA, United States), which consists of four messenger-RNAs (mRNA; CEACAM4, LAMP1, PLA2G7, PLAC8) that represent sepsis-related host response gene expression based mathematical algorithm that predicts sepsis earlier than traditional methods. SeptiScore™ is United States Food and Drug Administration cleared and aids in the differentiation of infection-negative sterile systemic inflammation compared to infection-positive sepsis. The Sepsis MetaScore, which utilizes the expression of 11 host mRNAs discovered from public microarray datasets, was found to have the highest prediction power for sepsis amongst all currently studied transcriptomics-based assays. mRNA based gene expression assays have also been studied to differentiate bacterial from viral sepsis (for example, the 7-mRNA bacterial or viral Metascore). All of these novel tools (Table 2) for diagnosing and identifying the severity of sepsis appear promising but lacks validation in the patients with cirrhosis[57].
Table 2.
Transcriptomics based micro assays for diagnosis of sepsis[57]
| Assay name (manufacturer) | Technique/sample volume | Turn-around time | Highest noted sensitivity and specificity | Detection |
| SeptiFast (Roche) | Real-time PCR/1.5 mL | 4 h to 6 h | 83%/95% | > 16 bacteria, Candida and Aspergillus fumigatus |
| SeptiTest (Molzyme) | Universal PCR/1 mL | 8 h to 10 h | 87%/96% | > 345 bacteria and 13 fungi |
| SeptiCyte (ImmuneExpress) | RT-qPCR with machine learning/2.5 mL | 1 h to 6 h | -/ 95% (discriminates SIRS from sepsis) | All pathogens |
| Iridica Plex ID (Abbott) | Multiplex broad range PCR/5 mL | 6 h | 83%/94% | 780 bacteria and Candida |
| MinION (Oxford Nanopore) | Nanopore sequencing/10 ng DNA | 4 h to 6 h | -/100% | Few viruses and bacteria currently |
| U-dHRM (UCSD, United States) | Digital PCR/1 mL | 3 h | -/99.9% | 37 bacteria |
| LAMP Tech | Loop mediated isothermal amplification/30 µL | 1 h | -/100% | 1 pathogen per sample |
| Integrated droplet digital detection tech (Velox Biosystems) | DNA-zyme base sensor droplet microencapsulation 3D particle analysis | 1 h to 4 h | - | 1 pathogen per sample |
3D: Three-dimensional; PCR: Polymerase chain reaction; RT: Reverse transcriptase; SIRS: Systemic inflammatory response syndrome.
UPDATE ON PROGNOSTIC SCORING SYSTEMS FOR SEPSIS IN CIRRHOSIS
The newly described qSOFA, as per the Sepsis-3 guidelines, has become an important tool that can be utilized at the bedside for the identification of sepsis and predict mortality. More recently, Rhee et al[58] evaluated the performance of a novel electronic SOFA (eSOFA) compared to the classical SOFA score. The eSOFA was developed by the United States Centers for Disease Control and Prevention to facilitate retrospective surveillance of sepsis events and was found to identify better, a smaller but sicker cohort of patients, than classical SOFA score system. The SOFA score defines organ dysfunction across six organ systems and assigns 0-4 points for each organ system depending on the degree of dysfunction, whereas eSOFA replaces these with binary criteria for most of the same organ systems. Currently, the diagnosis of sepsis with the SOFA score to evaluate organ dysfunction in the setting of infection and the use of qSOFA to predict the severity and outcome of sepsis have been recommended by the Sepsis-3 consensus document. Müller et al[59] showed that qSOFA did not predict in-hospital mortality, intensive unit admission, or length of hospitalization in patients with decompensated cirrhosis. The application of sodium level to qSOFA (called qSOFA-Na+) improved the diagnostic ability for identifying sepsis and mortality. However, in a larger series of cirrhosis patients, Piano et al[60] found that the Sepsis-3 criteria were more accurate than SIRS criteria in predicting the severity of infections in patients with cirrhosis and that the qSOFA was a useful bedside tool in assessing risk for poor outcomes in hospital. Patients fulfilling Sepsis-3 criteria had a higher incidence of ACLF, septic shock, and transfer to an intensive unit that those without. In a more recent study, Augustinho et al[61] showed that in patients with cirrhosis hospitalized for bacterial infections, admission qSOFA was an independent predictor of survival, and for those classified as high risk for death by qSOFA, only the CLIF-SOFA predicted prognosis independently, and Sepsis-3 criteria did not play a major role in predicting risk or stratifying patients. Lan et al[62] in a large retrospective cohort found that CLIF-SOFA and CLIF-organ failure scores were better tools that qSOFA, MELD, or qCLIF-SOFA in the evaluation of prognosis of critically ill patients with cirrhosis with suspected infections.
A Korean study revealed that qSOFA had limited utility in predicting adverse outcomes in cirrhosis patients with sepsis at medical emergency team activation in the general wards or rooms. Another scoring system, called the modified early warning score (Table 3), detected sepsis early in these patients[63]. Pan et al[64] showed that the “MAP, bilirubin, respiratory failure, sepsis” score, a simple prognostic model consisting of MAP, serum bilirubin level, assessment of acute respiratory failure, and sepsis [calculated using the following predictors: MAP, < 80 mmHg; serum bilirubin level, > 80 µmol/L (4.7 mg/dL); type 1 respiratory failure, and fulfilment of definition of sepsis; defined as the sum of the values of the individual predictors, each value ranging from 0 to 4], analysed on the 1st day of admission to the intensive care unit in critically ill patients with cirrhosis with acute kidney injury, was useful in predicting short term mortality in-hospital better than current complex scoring systems including the commonly used CTP and MELD scores. A novel approach to predicting sepsis and severity is by coupling electronic medical records data with machine learning algorithms. As an example of this modality, researchers have identified a novel targeted, real-time early warning score called the TREWScore that predicts the development of septic shock in adult intensive care patients 28 h before clinical onset. The HeRO score algorithm (Medical Predictive Science Corp, Charlottesville, VA, United States) utilized subtle changes and irregularities in heart rate variability to predict poor outcomes before the actual onset. However, this technology has not been fully validated and lacks power in the identification of sepsis and bloodstream infections. Such novel approaches have the potential to be of great value in diagnosing sepsis and improving outcomes in this difficult to manage cohort of patients[65].
Table 3.
The modified early warning scoring system for identification of sepsis[63]
| Score | 3 | 2 | 1 | 0 | 1 | 2 | 3 |
| Respiratory rate per min | ≤ 8 | 9-14 | 15-20 | 21-29 | > 29 | ||
| Heart rate per min | ≤ 40 | 41-50 | 51-100 | 101-110 | 111-129 | > 129 | |
| Systolic blood pressure, mmHg | ≤ 70 | 71-80 | 81-100 | 101-199 | ≥ 200 | ||
| Urine output, mL/(kg·h) | Nil | < 0.5 | |||||
| Temperature, °C | ≤ 35 | 35.1-36 | 36.1-38 | 38.1-38.5 | ≥ 38.6 | ||
| Neurological, subjective | Alert | Reacting to voice | Reacting to pain | Unresponsive |
TREATING SEPSIS IN CIRRHOSIS – CURRENT RECOMMENDATIONS AND NEWER APPROACHES
Current updated guidelines recommend that the treatment of sepsis, along with needful resuscitation, should commence immediately at the identification of sepsis and related clinical outcomes. This includes appropriate antibiotics (based on region-specific community and hospital-related pathogen patterns) and other source control measures. The Surviving Sepsis Campaign currently recommends the ”Hour-1 Bundle”, which includes broad-spectrum antimicrobials, intravenous fluid management, measurement of serum lactate level and inotropes, and vasopressor support in those not responding to fluid resuscitation. There is no role of early goal-directed treatment in sepsis, as was considered previously as three large multicentre trials in three major countries reported absence of benefit with such an intervention. The use of crystalloid or colloid as the initial resuscitation fluid also remains an enigma. Even though guidelines suggest that crystalloid, possibly normal saline or a buffered salt solution such as Plasmalyte need to be utilized at 30 mL/kg over 3 h, this practice is currently undergoing further scrutiny to improve on protocolized management. In those patients in whom crystalloids do not improve MAP, the addition of human albumin may be considered. However, no such recommendations exist, and the choice of fluid and its further modification rightfully rests on the common sense directed therapeutic decisions of the treating physician, based on close follow up of clinical parameters in the intensive care unit. Generalizability of Surviving Sepsis Campaign recommendations in the patients with cirrhosis needs validation. This is because goals of treatment may be different in patients with cirrhosis since they are frailer, have lower MAP at baseline due to the use of beta-blockers for portal hypertension, have higher central venous oxygen saturation due to a hyperdynamic circulation with lower haematocrit, and abnormal lactate metabolism[66,67].
Philips et al[68] conducted an open-label trial in 308 patients with cirrhosis (published in abstract form) with sepsis-induced hypotension and randomized them to receive either 5% human albumin or normal saline. The primary endpoint was the reversal of hypotension (MAP > 65 mmHg) at 3 h, and the secondary endpoints included effects on heart rate, arterial lactate, urine output, and survival at 1 wk. The authors found that the reversal of hypotension was higher in patients receiving 5% albumin than saline at the end of 1 h [25.3% and 11.7% respectively, P = 0.03, odds ratio (95%CI): - 1.9 (1.08-3.42)] and 3 h [11.7% and 3.2% respectively, P = 0.008, 3.9 (1.42-10.9)]. Sustained reduction in heart rate and lactate levels were greater in patients receiving albumin, without statistically significant changes in the urine output or adverse events between the groups. At the end of 1 wk, the proportion of patients surviving in the albumin group was higher than that in those who received saline (43.5% vs 38.3%, P = 0.03).
Regarding antibiotic therapy, some authors suggest that in community-acquired infections, the initial antibiotic of choice be a third-generation cephalosporin or amoxicillin-clavulanic acid and carbapenem or piperacillin and tazobactam combination in nosocomial infections in regions with a high and low prevalence of multiresistant bacteria, respectively, with or without a glycopeptide. Novel antimicrobial strategies are an area of active research. This includes targeting resistance mechanisms in pathogens. For example, the novel small molecule Inh2-B1, which targets serine-threonine protein kinase of methicillin-resistant Staphylococcus aureus, makes the pathogen susceptible to ceftriaxone and cefotaxime. Another example is antibiotic pairing with novel beta-lactamase or carbapenemase, as is seen with ceftazidime-avibactam and meropenem-vaborbactum, both of which were approved by the United States Food and Drug Administration for use in Enterobacteriaceae infections. Pathogen targeted antibody therapy is also a novel strategy to improve antimicrobial susceptibility. An example of this is the development of a monoclonal antibody against extremely drug-resistant Acinetobacter baumannii[43].
A haemoglobin threshold of 7 g/dL to 8 g/dL could be considered ideal in patients with cirrhosis with sepsis, as is endorsed by Baveno VI guidelines in those with acute variceal bleeding with a restrictive strategy of blood transfusion. In general, the septic shock population, the use of noradrenaline with or without vasopressin and adrenaline in a staged manner, has been recommended to maintain MAP. In a randomized controlled trial in patients with cirrhosis, Choudhury et al[69] demonstrated that terlipressin was as effective as noradrenaline as a vasopressor in patients with cirrhosis with septic shock and additionally provided early survival benefit with reduction in risk of variceal bleeding. In cirrhosis patients, the use of dopamine does not come highly recommended due to the high risk of inducing arrhythmias, and the administration of dobutamine is not supported because patients with cirrhosis have high cardiac output at baseline, which worsens with sepsis. Dobutamine is recommended in patients with clinically significant myocardial dysfunction. With regards to vasopressor hypo responsiveness and adrenal insufficiency in a general population of septic shock patients, the ADRENAL trial found no difference in 90-d all-cause mortality even though patients in the hydrocortisone group had faster resolution of shock, had a shorter duration of the initial episode of mechanical ventilation, and were less likely to receive blood transfusions. However, the APPROCHS (Activated Protein C and Corticosteroids for Human Septic Shock) trial showed that the 90-d all-cause mortality was lower among those who received hydrocortisone plus fludrocortisone than among those who received a placebo. A systematic review and meta-analysis in septic shock patients showed that corticosteroids possibly caused a small reduction in mortality and reduced duration of shock and intensive unit treatment but an increase in neuromuscular complications[70,71].
In patients with cirrhosis, a randomized study did not show any benefit on mortality and shock reversal with the use of intravenous hydrocortisone[72]. Since protective ventilation (low tidal volumes of 6 mL/kg of ideal body weight and plateau pressures < 30 cm H2O) improves survival in general patients with adult respiratory distress syndrome, patients with cirrhosis who require mechanical ventilation should also be treated on the same lines. However, the sedation in such circumstances must ideally be with drugs with short half-lives such as propofol and remifentanil with avoidance of benzodiazepines[73-75]. In patients with cirrhosis, profound distributive shock leads to the development of refractoriness (a state of ”vasoplegia”) to inotrope, and pressor support is higher than that seen in non-liver patients with septic shock due to increased sympathetic drive, use of beta-blockers, and more severe relative adrenal insufficiency.
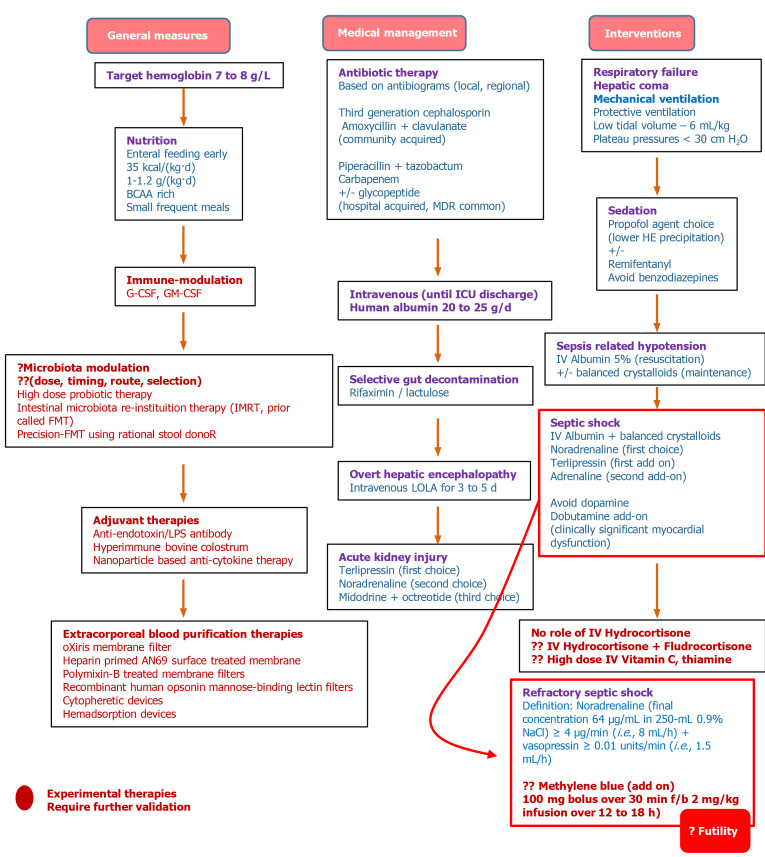
Case reports and small series in general patients have shown that methylene blue (MB) (a selective inhibitor of guanylate cyclase improves vascular tone and tissue perfusion) infusion could improve refractory septic shock. Ahamed et al[76] recently studied the role of MB in patients with cirrhosis with refractory septic shock (published in abstract form) compared to those on a standard of care. The authors defined refractory septic shock as requirement of noradrenaline (final concentration 64 μg/mL in 250 mL 0.9% normal saline) ≥ 4 μg/min (i.e. 8 mL/h) + vasopressin ≥ 0.01 units/min (i.e. 1.5 mL/h). On retrospective analysis, they found that improvement in systolic blood pressure was significantly better in the MB group from baseline at the end of 24-, 72-, and 120 h compared to those on a standard of care. Improvement in diastolic blood pressure was notable in the MB group from baseline at the end of 24- and 72 h; between 24- and 72 h and 24- and 120 h while the increase in MAP was significantly higher in patients receiving MB from baseline at 24- and 72 h. Significant reduction in dose of noradrenaline and vasopressin dosing was also noted from baseline at the end of 24- and 72 h in the MB group. The need for additional inotropes was significantly higher at the end of 24- and 72 h in patients continued on the standard of care. The total hospital stay duration was significantly lower in the MB group (8 d vs 10 d, P < 0.05), however, without significant differences in short-term-survival (1 wk, 14 d, and 28 d) between groups[76]. A proposed algorithm for the treatment of sepsis in cirrhosis is shown in Figure 5[43,66,73,74].
Figure 5.
The proposed treatment algorithm for sepsis in cirrhosis[66,68,70-72,75]. BCAA: Branched-chain amino acids; FMT: Faecal microbiota transplantation; G-CSF: Granulocyte-colony stimulating factor; GM: Granulocyte-macrophage; ICU: Intensive care unit; IMRT: Intestinal microbiota re-instituition therapy; IV: Intravenous; LOLA: L-ornitine L-aspartate; LPS: Lipopolysaccharide; MDR: Multidrug resistant.
Walley et al[77] showed that the proprotein convertase subtilisin/kexin type-9 (PCSK9) was a critical regulator of the innate immune response, and septic shock outcome and reduction in PCSK9 function were associated with increased pathogen lipid clearance through the low-density lipoprotein receptors with a decrease in the inflammatory response. Repurposing drugs for newer indications and genomic approaches to improving outcomes in septic shock is an area of active research[77]. Other promising treatment modalities in sepsis include a combination of vitamin C, hydrocortisone, thiamine, short-acting beta-blockade therapy using esmolol in patients with sepsis and persistent tachycardia, and toxin removal and inflammation control using hemadsorption techniques that utilize specialized membranes such as those with polymyxin B and finally, immune-stimulation with growth factors such as granulocyte and granulocyte-macrophage colony-stimulating factors. The role of nanoparticle-based adjuvant therapies is gaining widespread attention as a novel area with beneficial strategic output in the treatment of sepsis. Nanoparticles have small size and sizeable surface area to volume ratio and can be utilized as antibacterial agents, structure platforms for adsorbents that bind and sequester endotoxins and cytokines to restore homeostasis[78,79]. A summary of novel adjuvant therapies for sepsis is shown in Table 4.
Table 4.
| Therapy | Mechanism | Systemic effect in sepsis |
| Eritoran; resatorvid | Toll-like receptor 4 antagonist; Eritoran is structurally similar to lipopolysaccharide – A of Gram-negative bacteria. Resatorvid is a direct antagonist of toll like receptor 4 | Anti-inflammatory; Immunomodulation |
| Polymixin B fibre column; CytoSorb | Hemoperfusion; CytoSorb has hemadsorption properties | Removal of circulating endotoxin and bacterial components |
| Plasma exchange; Whole blood exchange; Coupled plasma filtration adsorption; Hemofiltration | Exchange of plasma or blood with or without sorbent adsorption; either continuous or intermittent; low or high volume | Removal of endotoxins and circulating cytokines |
| Macrolides | Nuclear factor kB and AP-1 signalling suppression, inhibition of ERK-1 and 2 pathways | Anti-inflammatory and immunomodulating properties |
| Interferon-gamma | Increase in monocyte HLA-DR expression | Restores immune regulation, abolishes immunoparalysis by restoring monocyte function |
| Immunoglobulins | Increase in IgA and IgM levels | Boosts humoral immunity |
| Granulocyte macrophage colony stimulating factor | Promotes maturation and differentiation of neutrophils, monocytes, macrophages, dendritic cells, T lymphocytes and plasma cells | Improves immune regulation, reduces immunoparalysis |
| Anti-MIF | Antagonizes macrophage migration inhibition factor | Immunomodulation through boosting activity of endogenous glucocorticoids |
| Super-Antigen-Antagonist | Suppression of pro-inflammatory gene expression by inhibition of T cell activation | Th1 blockade and prevention of lethal shock |
| Heparin and its analogues | Anti-thrombotic, immunomodulation | Prevents early disseminated intravascular coagulation, prevents early organ failures due to diffuse system microvascular thrombosis |
| Naloxone | Opioid receptor antagonism | Improves hemodynamic instability |
| Pentoxifylline | Decreases erythrocyte aggregation and deformability, anti TNF-alpha effect | Improvement in arterial oxygen tension by improving fractionated oxygen exchange |
| GTS-21 | Selective alpha-7-nicotinic acetylcholine receptor agonist, blocks nuclear factor – kB and cytokines downstream | Activates cholinergic anti-inflammatory pathway |
| Interleukin 7 and 2 | Pro-inflammatory cytokines | Prevents immunoparalysis |
| Programmed cell death-1 (PD-1) and ligand (PD-L1) antagonist | Prevention of lymphocyte depletion, improvement in pro-inflammatory mediators and increased bacterial clearance | Immune modulation |
| B and T cell lymphocyte attenuator antagonism (BTLA) | Increases activity and proliferation of T cells | Increases resistance to endotoxin and prevention of endotoxin mediated shock |
| Antagonism of cytotoxic T lymphocyte antigen 4 (CTLA-4) | Increased activity and proliferation of T cells | Abolishes endotoxemia and associated toxic shock |
| Methylthiouracil | Suppresses high mobility group box – 1 (HMGB-1) | Anti-inflammatory |
| Structurally nanoengineered antimicrobial peptide polymers; Ceria – zirconia nanoparticles; Piceatannol-loaded albumin nanoparticles; Sialic-acid decorated nanoparticles; Exsosomes loaded with MFGE8, miR-223; Red blood cells and macrophage coated nanoparticles; Liposomes tagged to antimicrobials; Opsonin bound magnetic nanobeads | Nanoparticle technology (pre-clinical studies) | Antibacterial; Antioxidant; Anti-inflammatory; Endotoxin antagonist; Extracorporeal blood cleansing; Clearance of apoptotic cells |
AP: Activator protein; BTLA: B and T lymphocyte associated; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; ERK: Extracellular signal-regulated kinases; HLA-DR: Human leukocyte antigen – DR isotype; HMGB: High-mobility group box; Ig: Immunoglobulins; MFGE8: Milk fat globule epidermal growth factor 8 protein; MIF: Macrophage migration inhibitory factor; miR: Micro-RNA; PD-L: Programmed death receptor ligand; TNF: Tumour necrosis factor.
THE ROLE OF AND MODULATION OF GUT MICROBIOTA IN SEPSIS AND CIRRHOSIS
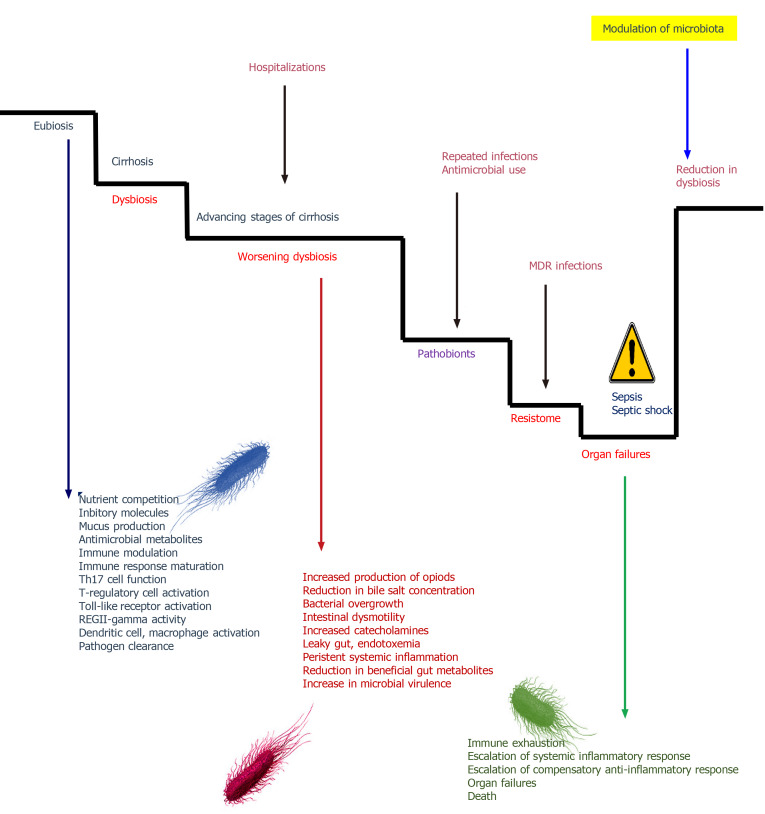
An intact intestinal barrier and commensal balance are imperative for proper maturation and development of the immune system. In health, gut microbiota antagonize pathogens by competing with nutritional components, produce antimicrobial peptides and metabolites, and render the local milieu hostile by modifying bile salts. Not only at the local sites, but at a systemic level, immune-regulation is an important task of the healthy microbiota. Various structural components of the gut microbes called the microbe-associated molecular patterns, or MAMPs, can promote a systemic inflammatory response by activating and further maturing the innate and adaptive immune system. The microbial metabolites such as short-chain fatty acids (SCFAs) help in modulating both pro and anti-inflammatory responses to maintain immune and inflammatory balance in the host. SCFAs like butyrate and propionate activate regulatory T cells that ameliorate systemic inflammation. Other gut metabolites such as desaminotyrosine boosts type 1 interferon responses leading to improved viral pathogen clearance. In patients who develop dysbiosis, due to environmental and host factors, gut microbial health deteriorates, leading to increased risk of infections. Furthermore, the use of antibiotics increases dysbiosis that enters the host into a vicious cycle of infections and organ dysfunction. It is well known that patients with cirrhosis have dysbiosis that worsens with the severity of liver disease. In its worst form, pathobionts prevail, and the intestinal microbiota after that functions as a repository for antimicrobial resistance (a state called ”resistome”). This further endangers immune function at the local and systemic level predisposing the host to not only infectious insults but also severe inflammatory states leading to organ failures. The best proof that sepsis can be improved with modulation of gut microbiota stems from studies of healthy donor faecal microbiota transplantation (currently rechristened intestinal microbiota reinstitution therapy or intestinal microbiota re-instituition therapy) in patients with recurrent and severe Clostridium difficile infections[80-82]. Modulation of microbiota in a similar fashion in patients with liver disease has been shown to improve outcomes related to hepatic encephalopathy, alcoholic hepatitis with infections, ACLF, and primary sclerosing cholangitis with recurrent cholangitis[83-86]. In patients with cirrhosis, understanding the gut microbiota and its modulation are still in foetal stages. In such patients, the first step is to identify, through omics-based research, those at risk for the development of infections before sepsis development. Modulating microbiota at this stage can help prevent infections by restoring a eubiotic microbiome. In those patients with sepsis, addressing dysbiosis through active modulation of the microbiota can help improve outcomes related to sepsis and organ failures. In those surviving sepsis, modulation of microbiota to restore homeostatic balance can help prevent dysbiosis driven infectious insults in the future (Figure 6). Recently, in a randomized controlled pilot trial, Stadlbauer et al[87] demonstrated that dysbiosis in early sepsis could be modulated by utilizing a multispecies probiotic (Winclove 607 based on Omnibiotic® 10 AAD) with improvement in clinical outcomes.
Figure 6.
The role of gut microbiota in driving and worsening sepsis and cirrhosis[78,80,81]. Gut microbiota modulation is an interesting approach to management of sepsis in the future. Reducing dysbiotic bacterial communities and favouring commensals that improve host immune functions, promote endogenous antimicrobial metabolite formation and resist pathogenic colonization could be achieved through high dose probiotics or intestinal microbiota re-institution therapy. MDR: Multidrug resistant.
CONCLUSION
Sepsis and septic shock are conditions associated with high mortality in the general population, more so in patients with cirrhosis due to specific hemodynamic and immune system-related changes affecting the latter. Over the past few decades, the definition of sepsis and its application in clinical practice has seen a major change, which has helped to identify better patients at risk. The sepsis care protocols have evolved over the past few years to incorporate the best clinical practices that would improve clinical outcomes in affected patients. Even though specific guidelines for sepsis identification and treatments do not exist in patients with cirrhosis, real-world evidence from the non-liver population has been of great help in managing sepsis in this difficult to treat cohort. Basic science work has identified novel areas such as the role of nutrition, immune regulation, genomics-based and nanomedicine-based approaches, as well as microbiota modulation in improving adjuvant treatments for sepsis, which could become an integral part in the management of severe infections. Novel antimicrobial strategies for combating resistance and the role of machine learning and deep data mining are also major tools currently in development as armamentarium against sepsis. In this exciting era of basic science work driven bench to bedside strategies, the improvements in challenges during the management of sepsis in cirrhosis in the future looks promising.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: March 22, 2020
First decision: April 12, 2020
Article in press: June 27, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pavides M, Takata H S-Editor: Yan JP L-Editor: Filipodia P-Editor: Wang LL
Contributor Information
Cyriac Abby Philips, The Liver Unit and Monarch Liver Lab, Cochin Gastroenterology Group, Ernakulam Medical Center, Kochi 682028, Kerala, India. abbyphilips@gmail.com.
Rizwan Ahamed, Gastroenterology and Advanced G.I Endoscopy, Cochin Gastroenterology Group, Ernakulam Medical Center, Kochi 682028, Kerala, India.
Sasidharan Rajesh, Division of Hepatobiliary Interventional Radiology, Cochin Gastroenterology Group, Ernakulam Medical Center, Kochi 682028, Kerala, India.
Tom George, Division of Hepatobiliary Interventional Radiology, Cochin Gastroenterology Group, Ernakulam Medical Center, Kochi 682028, Kerala, India.
Meera Mohanan, Anaesthesia and Critical Care, Cochin Gastroenterology Group, Ernakulam Medical Center, Kochi 682028, Kerala, India.
Philip Augustine, Gastroenterology and Advanced G.I Endoscopy, Cochin Gastroenterology Group, Ernakulam Medical Center, Kochi 682028, Kerala, India.
References
- 1.Dickmann P, Bauer M. Sepsis 2019 - New Trends and Their Implications for Multiple Trauma Patients. Z Orthop Unfall. 2020;158:81–89. doi: 10.1055/a-0853-2054. [DOI] [PubMed] [Google Scholar]
- 2.Barrier KM. Summary of the 2016 International Surviving Sepsis Campaign: A Clinician's Guide. Crit Care Nurs Clin North Am. 2018;30:311–321. doi: 10.1016/j.cnc.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Simpson N, Lamontagne F, Shankar-Hari M. Septic shock resuscitation in the first hour. Curr Opin Crit Care. 2017;23:561–566. doi: 10.1097/MCC.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 4.Rahmel T. [SSC International Guideline 2016 - Management of Sepsis and Septic Shock] Anasthesiol Intensivmed Notfallmed Schmerzther. 2018;53:142–148. doi: 10.1055/s-0043-114639. [DOI] [PubMed] [Google Scholar]
- 5.Plevin R, Callcut R. Update in sepsis guidelines: what is really new? Trauma Surg Acute Care Open. 2017;2:e000088. doi: 10.1136/tsaco-2017-000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 7.Scala R, Schultz M, Bos LDJ, Artigas A. New Surviving Sepsis Campaign guidelines: back to the art of medicine. Eur Respir J. 2018;52:1701818. doi: 10.1183/13993003.01818-2017. [DOI] [PubMed] [Google Scholar]
- 8.Gunsolus IL, Sweeney TE, Liesenfeld O, Ledeboer NA. Diagnosing and Managing Sepsis by Probing the Host Response to Infection: Advances, Opportunities, and Challenges. J Clin Microbiol. 2019;57:e00425–19. doi: 10.1128/JCM.00425-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalan R, Stadlbauer V, Sen S, Cheshire L, Chang YM, Mookerjee RP. Role of predisposition, injury, response and organ failure in the prognosis of patients with acute-on-chronic liver failure: a prospective cohort study. Crit Care. 2012;16:R227. doi: 10.1186/cc11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiwall R, Sarin SK, Kumar S, Jain P, Kumar G, Bhadoria AS, Moreau R, Kedarisetty CK, Abbas Z, Amarapurkar D, Bhardwaj A, Bihari C, Butt AS, Chan A, Chawla YK, Chowdhury A, Dhiman R, Dokmeci AK, Ghazinyan H, Hamid SS, Kim DJ, Komolmit P, Lau GK, Lee GH, Lesmana LA, Jamwal K, Mamun-Al-Mahtab, Mathur RP, Nayak SL, Ning Q, Pamecha V, Alcantara-Payawal D, Rastogi A, Rahman S, Rela M, Saraswat VA, Shah S, Shiha G, Sharma BC, Sharma MK, Sharma K, Tan SS, Chandel SS, Vashishtha C, Wani ZA, Yuen MF, Yokosuka O, Duseja A, Jafri W, Devarbhavi H, Eapen CE, Goel A, Sood A, Ji J, Duan Z, Chen Y of the APASL ACLF Research Consortium (AARC) working party. Development of predisposition, injury, response, organ failure model for predicting acute kidney injury in acute on chronic liver failure. Liver Int. 2017;37:1497–1507. doi: 10.1111/liv.13443. [DOI] [PubMed] [Google Scholar]
- 11.Medlej K. Calculated decisions: qSOFA (quick SOFA) score for sepsis. Emerg Med Pract. 2018;20:CD2–CD4. [PubMed] [Google Scholar]
- 12.Marik PE, Taeb AM. SIRS, qSOFA and new sepsis definition. J Thorac Dis. 2017;9:943–945. doi: 10.21037/jtd.2017.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelmann C, Thomsen KL, Zakeri N, Sheikh M, Agarwal B, Jalan R, Mookerjee RP. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care. 2018;22:254. doi: 10.1186/s13054-018-2156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, Huang C, Yu KK, Lu Q, Shi GF, Zheng JM. Validation of prognostic scores to predict short-term mortality in patients with HBV-related acute-on-chronic liver failure: The CLIF-C OF is superior to MELD, CLIF SOFA, and CLIF-C ACLF. Medicine (Baltimore) 2017;96:e6802. doi: 10.1097/MD.0000000000006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva PE, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516–1523. doi: 10.1111/liv.12597. [DOI] [PubMed] [Google Scholar]
- 16.Jeppesen JB, Mortensen C, Bendtsen F, Møller S. Lactate metabolism in chronic liver disease. Scand J Clin Lab Invest. 2013;73:293–299. doi: 10.3109/00365513.2013.773591. [DOI] [PubMed] [Google Scholar]
- 17.De Jonghe B, Cheval C, Misset B, Timsit JF, Garrouste M, Montuclard L, Carlet J. Relationship between blood lactate and early hepatic dysfunction in acute circulatory failure. J Crit Care. 1999;14:7–11. doi: 10.1016/s0883-9441(99)90002-3. [DOI] [PubMed] [Google Scholar]
- 18.Kruse JA, Zaidi SA, Carlson RW. Significance of blood lactate levels in critically ill patients with liver disease. Am J Med. 1987;83:77–82. doi: 10.1016/0002-9343(87)90500-6. [DOI] [PubMed] [Google Scholar]
- 19.Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74. doi: 10.1186/1757-7241-19-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterling SA, Puskarich MA, Jones AE. The effect of liver disease on lactate normalization in severe sepsis and septic shock: a cohort study. Clin Exp Emerg Med. 2015;2:197–202. doi: 10.15441/ceem.15.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun DQ, Zheng CF, Lu FB, Van Poucke S, Chen XM, Chen YP, Zhang L, Zheng MH. Serum lactate level accurately predicts mortality in critically ill patients with cirrhosis with acute kidney injury. Eur J Gastroenterol Hepatol. 2018;30:1361–1367. doi: 10.1097/MEG.0000000000001189. [DOI] [PubMed] [Google Scholar]
- 22.Drolz A, Horvatits T, Rutter K, Landahl F, Roedl K, Meersseman P, Wilmer A, Kluwe J, Lohse AW, Kluge S, Trauner M, Fuhrmann V. Lactate Improves Prediction of Short-Term Mortality in Critically Ill Patients With Cirrhosis: A Multinational Study. Hepatology. 2019;69:258–269. doi: 10.1002/hep.30151. [DOI] [PubMed] [Google Scholar]
- 23.Chervonsky AV. Just a Spoonful of Sugar Helps the Tolerance Go Up. Cell. 2017;169:1170–1172. doi: 10.1016/j.cell.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Weis S, Carlos AR, Moita MR, Singh S, Blankenhaus B, Cardoso S, Larsen R, Rebelo S, Schäuble S, Del Barrio L, Mithieux G, Rajas F, Lindig S, Bauer M, Soares MP. Metabolic Adaptation Establishes Disease Tolerance to Sepsis. Cell. 2017;169:1263–1275.e14. doi: 10.1016/j.cell.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, Medzhitov R. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell. 2016;166:1512–1525.e12. doi: 10.1016/j.cell.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Changani KK, Jalan R, Cox IJ, Ala-Korpela M, Bhakoo K, Taylor-Robinson SD, Bell JD. Evidence for altered hepatic gluconeogenesis in patients with cirrhosis using in vivo 31-phosphorus magnetic resonance spectroscopy. Gut. 2001;49:557–564. doi: 10.1136/gut.49.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845–859. doi: 10.1007/s00535-019-01605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumi M, Takahashi T, Fujii H, Ohashi I, Kaku R, Nakatsuka H, Shimizu H, Morita K, Hirakawa M, Inagaki M, Sadamori H, Yagi T, Tanaka N, Akagi R. Increased heme oxygenase-1 gene expression in the livers of patients with portal hypertension due to severe hepatic cirrhosis. J Int Med Res. 2002;30:282–288. doi: 10.1177/147323000203000309. [DOI] [PubMed] [Google Scholar]
- 30.Sass G, Barikbin R, Tiegs G. The multiple functions of heme oxygenase-1 in the liver. Z Gastroenterol. 2012;50:34–40. doi: 10.1055/s-0031-1282046. [DOI] [PubMed] [Google Scholar]
- 31.Lv Y, Yee Lau W, Wu H, Han X, Gong X, Liu N, Yue J, Li Q, Li Y, Deng J. Causes of peripheral cytopenia in hepatitic cirrhosis and portal hypertensive splenomegaly. Exp Biol Med (Maywood) 2017;242:744–749. doi: 10.1177/1535370217693113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Ripoll C, Maurer R, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Makuch R, Rendon G Portal Hypertension Collaborative Group. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689–695. doi: 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gkamprela E, Deutsch M, Pectasides D. Iron deficiency anemia in chronic liver disease: etiopathogenesis, diagnosis and treatment. Ann Gastroenterol. 2017;30:405–413. doi: 10.20524/aog.2017.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viveiros A, Finkenstedt A, Schaefer B, Mandorfer M, Scheiner B, Lehner K, Tobiasch M, Reiberger T, Tilg H, Edlinger M, Zoller H. Transferrin as a predictor of survival in cirrhosis. Liver Transpl. 2018;24:343–351. doi: 10.1002/lt.24981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenbaum M, Hall KD, Guo J, Ravussin E, Mayer LS, Reitman ML, Smith SR, Walsh BT, Leibel RL. Glucose and Lipid Homeostasis and Inflammation in Humans Following an Isocaloric Ketogenic Diet. Obesity (Silver Spring) 2019;27:971–981. doi: 10.1002/oby.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayres JS. Disease Tolerance Trick or Treat: Give Your Brain Something Good to Eat. Cell. 2016;166:1368–1370. doi: 10.1016/j.cell.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 37.Schieber AM, Lee YM, Chang MW, Leblanc M, Collins B, Downes M, Evans RM, Ayres JS. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science. 2015;350:558–563. doi: 10.1126/science.aac6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eghtesad S, Poustchi H, Malekzadeh R. Malnutrition in liver cirrhosis:the influence of protein and sodium. Middle East J Dig Dis. 2013;5:65–75. [PMC free article] [PubMed] [Google Scholar]
- 40.Owen OE, Reichle FA, Mozzoli MA, Kreulen T, Patel MS, Elfenbein IB, Golsorkhi M, Chang KH, Rao NS, Sue HS, Boden G. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest. 1981;68:240–252. doi: 10.1172/JCI110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, González-González JA, Muñoz-Espinosa LE, Villarreal-Pérez JZ, Maldonado-Garza HJ. Subclinical abnormal glucose tolerance is a predictor of death in liver cirrhosis. World J Gastroenterol. 2014;20:7011–7018. doi: 10.3748/wjg.v20.i22.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;7:2050312119835043. doi: 10.1177/2050312119835043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varon J, Baron RM. A current appraisal of evidence for the approach to sepsis and septic shock. Ther Adv Infect Dis. 2019;6:2049936119856517. doi: 10.1177/2049936119856517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dirchwolf M, Ruf AE. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis. World J Hepatol. 2015;7:1974–1981. doi: 10.4254/wjh.v7.i16.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249–1264. doi: 10.1002/hep.28740. [DOI] [PubMed] [Google Scholar]
- 46.Harrison MF. The Misunderstood Coagulopathy of Liver Disease: A Review for the Acute Setting. West J Emerg Med. 2018;19:863–871. doi: 10.5811/westjem.2018.7.37893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novotny AR, Reim D, Assfalg V, Altmayr F, Friess HM, Emmanuel K, Holzmann B. Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology. 2012;217:616–621. doi: 10.1016/j.imbio.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, González Del Castillo J, Jensen JU, Kanizsai PL, Kwa ALH, Krueger S, Luyt CE, Oppert M, Plebani M, Shlyapnikov SA, Toccafondi G, Townsend J, Welte T, Saeed K. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57:1308–1318. doi: 10.1515/cclm-2018-1181. [DOI] [PubMed] [Google Scholar]
- 49.Branche A, Neeser O, Mueller B, Schuetz P. Procalcitonin to guide antibiotic decision making. Curr Opin Infect Dis. 2019;32:130–135. doi: 10.1097/QCO.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 50.Reuken PA, Kiehntopf M, Stallmach A, Bruns T. Mid-regional pro-adrenomedullin (MR-proADM): an even better prognostic biomarker than C-reactive protein to predict short-term survival in patients with decompensated cirrhosis at risk of infection? J Hepatol. 2012;57:1156–8; author reply 1158-9. doi: 10.1016/j.jhep.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 51.Remmler J, Schneider C, Treuner-Kaueroff T, Bartels M, Seehofer D, Scholz M, Berg T, Kaiser T. Increased Level of Interleukin 6 Associates With Increased 90-Day and 1-Year Mortality in Patients With End-Stage Liver Disease. Clin Gastroenterol Hepatol. 2018;16:730–737. doi: 10.1016/j.cgh.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Ellett F, Jorgensen J, Marand AL, Liu YM, Martinez MM, Sein V, Butler KL, Lee J, Irimia D. Diagnosis of sepsis from a drop of blood by measurement of spontaneous neutrophil motility in a microfluidic assay. Nat Biomed Eng. 2018;2:207–214. doi: 10.1038/s41551-018-0208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Geest PJ, Mohseni M, Linssen J, Duran S, de Jonge R, Groeneveld AB. The intensive care infection score - a novel marker for the prediction of infection and its severity. Crit Care. 2016;20:180. doi: 10.1186/s13054-016-1366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crouser ED, Parrillo JE, Seymour CW, Angus DC, Bicking K, Esguerra VG, Peck-Palmer OM, Magari RT, Julian MW, Kleven JM, Raj PJ, Procopio G, Careaga D, Tejidor L. Monocyte Distribution Width: A Novel Indicator of Sepsis-2 and Sepsis-3 in High-Risk Emergency Department Patients. Crit Care Med. 2019;47:1018–1025. doi: 10.1097/CCM.0000000000003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crawford K, DeWitt A, Brierre S, Caffery T, Jagneaux T, Thomas C, Macdonald M, Tse H, Shah A, Di Carlo D, O'Neal HR. Rapid Biophysical Analysis of Host Immune Cell Variations Associated with Sepsis. Am J Respir Crit Care Med. 2018;198:280–282. doi: 10.1164/rccm.201710-2077LE. [DOI] [PubMed] [Google Scholar]
- 56.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 57.Mangioni D, Peri AM, Rossolini GM, Viaggi B, Perno CF, Gori A, Bandera A. Toward Rapid Sepsis Diagnosis and Patient Stratification: What's New From Microbiology and Omics Science. J Infect Dis. 2020;221:1039–1047. doi: 10.1093/infdis/jiz585. [DOI] [PubMed] [Google Scholar]
- 58.Rhee C, Zhang Z, Kadri SS, Murphy DJ, Martin GS, Overton E, Seymour CW, Angus DC, Dantes R, Epstein L, Fram D, Schaaf R, Wang R, Klompas M CDC Prevention Epicenters Program. Sepsis Surveillance Using Adult Sepsis Events Simplified eSOFA Criteria Versus Sepsis-3 Sequential Organ Failure Assessment Criteria. Crit Care Med. 2019;47:307–314. doi: 10.1097/CCM.0000000000003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Müller M, Schefold JC, Leichtle AB, Srivastava D, Lindner G, Exadaktylos AK, Pfortmueller CA. qSOFA score not predictive of in-hospital mortality in emergency patients with decompensated liver cirrhosis. Med Klin Intensivmed Notfmed. 2019;114:724–732. doi: 10.1007/s00063-018-0477-z. [DOI] [PubMed] [Google Scholar]
- 60.Piano S, Bartoletti M, Tonon M, Baldassarre M, Chies G, Romano A, Viale P, Vettore E, Domenicali M, Stanco M, Pilutti C, Frigo AC, Brocca A, Bernardi M, Caraceni P, Angeli P. Assessment of Sepsis-3 criteria and quick SOFA in patients with cirrhosis and bacterial infections. Gut. 2018;67:1892–1899. doi: 10.1136/gutjnl-2017-314324. [DOI] [PubMed] [Google Scholar]
- 61.Augustinho FC, Zocche TL, Borgonovo A, Maggi DC, Rateke ECM, Matiollo C, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Applicability of Sepsis-3 criteria and quick Sequential Organ Failure Assessment in patients with cirrhosis hospitalised for bacterial infections. Liver Int. 2019;39:307–315. doi: 10.1111/liv.13980. [DOI] [PubMed] [Google Scholar]
- 62.Lan P, Wang SJ, Shi QC, Fu Y, Xu QY, Chen T, Yu YX, Pan KH, Lin L, Zhou JC, Yu YS. Comparison of the predictive value of scoring systems on the prognosis of cirrhotic patients with suspected infection. Medicine (Baltimore) 2018;97:e11421. doi: 10.1097/MD.0000000000011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Son J, Choi S, Huh JW, Lim CM, Koh Y, Kim KM, Shim JH, Lim YS, Hong SB. The quick sepsis-related organ failure score has limited value for predicting adverse outcomes in sepsis patients with liver cirrhosis. Korean J Intern Med. 2019 doi: 10.3904/kjim.2018.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan HC, Jenq CC, Tsai MH, Fan PC, Chang CH, Chang MY, Tian YC, Hung CC, Fang JT, Yang CW, Chen YC. Risk models and scoring systems for predicting the prognosis in critically ill cirrhotic patients with acute kidney injury: a prospective validation study. PLoS One. 2012;7:e51094. doi: 10.1371/journal.pone.0051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sinha M, Jupe J, Mack H, Coleman TP, Lawrence SM, Fraley SI. Emerging Technologies for Molecular Diagnosis of Sepsis. Clin Microbiol Rev. 2018;31:e00089–17. doi: 10.1128/CMR.00089-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernández J, Aracil C, Solà E, Soriano G, Cinta Cardona M, Coll S, Genescà J, Hombrados M, Morillas R, Martín-Llahí M, Pardo A, Sánchez J, Vargas V, Xiol X, Ginès P. [Evaluation and treatment of the critically ill cirrhotic patient] Gastroenterol Hepatol. 2016;39:607–626. doi: 10.1016/j.gastrohep.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 67.Thompson K, Venkatesh B, Finfer S. Sepsis and septic shock: current approaches to management. Intern Med J. 2019;49:160–170. doi: 10.1111/imj.14199. [DOI] [PubMed] [Google Scholar]
- 68.Philips CA, Choudhury AK, Sahney A, Maiwall R, Mitra LG, Sarin SK. Comparison and outcomes of 5% albumin vs 0.9% normal saline fluid resuscitation in cirrhotics presenting with sepsis induced hypotension - a randomized controlled trial - fluid resuscitation in septic shock in cirrhosis (FRISC Protocol) Hepatology. 2015;62:261A. [Google Scholar]
- 69.Choudhury A, Kedarisetty CK, Vashishtha C, Saini D, Kumar S, Maiwall R, Sharma MK, Bhadoria AS, Kumar G, Joshi YK, Sarin SK. A randomized trial comparing terlipressin and noradrenaline in patients with cirrhosis and septic shock. Liver Int. 2017;37:552–561. doi: 10.1111/liv.13252. [DOI] [PubMed] [Google Scholar]
- 70.Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D'Aragon F, Duan E, English S, Gossack-Keenan K, Alghuroba M, Szczeklik W, Menon K, Alhazzani W, Sevransky J, Vandvik PO, Annane D, Guyatt G. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med. 2018;46:1411–1420. doi: 10.1097/CCM.0000000000003262. [DOI] [PubMed] [Google Scholar]
- 71.Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R, He J, Xu P, Faramand A, Xu J, You C. Association of Corticosteroid Treatment With Outcomes in Adult Patients With Sepsis: A Systematic Review and Meta-analysis. JAMA Intern Med. 2019;179:213–223. doi: 10.1001/jamainternmed.2018.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, Knawy BA, Hajeer AH, Tamimi W, Cherfan A. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;182:1971–1977. doi: 10.1503/cmaj.090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Philips CA, Sarin SK. Sepsis in cirrhosis: emerging concepts in pathogenesis, diagnosis and management. Hepatol Int. 2016;10:871–882. doi: 10.1007/s12072-016-9753-2. [DOI] [PubMed] [Google Scholar]
- 74.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Simonetto DA, Piccolo Serafim L, Gallo de Moraes A, Gajic O, Kamath PS. Management of Sepsis in Patients With Cirrhosis: Current Evidence and Practical Approach. Hepatology. 2019;70:418–428. doi: 10.1002/hep.30412. [DOI] [PubMed] [Google Scholar]
- 76.Ahamed R, Philips CA, Rajesh S, George T, Padsalgi G, Kumbar S, Augustine P. Methylene blue in critically ill cirrhosis patients with sepsis induced severe hypotension. J Gastroenterol Hepatol. 2019;34:S3. [Google Scholar]
- 77.Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF, Christie JD, Nakada TA, Fjell CD, Thair SA, Cirstea MS, Boyd JH. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med. 2014;6:258ra143. doi: 10.1126/scitranslmed.3008782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rello J, Valenzuela-Sánchez F, Ruiz-Rodriguez M, Moyano S. Sepsis: A Review of Advances in Management. Adv Ther. 2017;34:2393–2411. doi: 10.1007/s12325-017-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuk SA, Sanchez-Rodriguez DA, Tsifansky MD, Yeo Y. Recent advances in nanomedicine for sepsis treatment. Ther Deliv. 2018;9:435–450. doi: 10.4155/tde-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Philips CA, Ahamed R, Rajesh S, Augustine P. 'You know my name, but not my story' - Deciding on an accurate nomenclature for faecal microbiota transplantation. J Hepatol. 2020;72:1212–1213. doi: 10.1016/j.jhep.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2:135–143. doi: 10.1016/S2468-1253(16)30119-4. [DOI] [PubMed] [Google Scholar]
- 82.Haak BW, Prescott HC, Wiersinga WJ. Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis. Front Immunol. 2018;9:2042. doi: 10.3389/fimmu.2018.02042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:235–246. doi: 10.1038/s41575-018-0099-1. [DOI] [PubMed] [Google Scholar]
- 84.Philips CA, Augustine P, Yerol PK, Ramesh GN, Ahamed R, Rajesh S, George T, Kumbar S. Modulating the Intestinal Microbiota: Therapeutic Opportunities in Liver Disease. J Clin Transl Hepatol. 2020;8:87–99. doi: 10.14218/JCTH.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Philips CA, Phadke N, Ganesan K, Ranade S, Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol. 2018;37:215–225. doi: 10.1007/s12664-018-0859-4. [DOI] [PubMed] [Google Scholar]
- 86.Philips CA, Augustine P, Padsalgi G, Ahamed R, Jose A, Rajesh S. Only in the darkness can you see the stars: Severe alcoholic hepatitis and higher grades of acute-on-chronic liver failure. J Hepatol. 2019;70:550–551. doi: 10.1016/j.jhep.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Stadlbauer V, Horvath A, Komarova I, Schmerboeck B, Feldbacher N, Klymiuk I, Durdevic M, Rainer F, Blesl A, Stiegler P, Leber B. Dysbiosis in early sepsis can be modulated by a multispecies probiotic: a randomised controlled pilot trial. Benef Microbes. 2019;10:265–278. doi: 10.3920/BM2018.0067. [DOI] [PubMed] [Google Scholar]