Abstract
Neuropathic pain is a chronic disease state resulting from injury to the nervous system. This type of pain often responds poorly to standard treatments and occasionally may get worse instead of better over time. Patients who experience neuropathic pain report sensitivity to cold and mechanical stimuli. Since the nociceptive system of African naked mole-rats contains unique adaptations that result in insensitivity to some pain types, we investigated whether naked mole-rats may be resilient to sensitivity following nerve injury. Using the spared nerve injury model of neuropathic pain, we showed that sensitivity to mechanical stimuli developed similarly in mice and naked mole-rats. However, naked mole-rats lacked sensitivity to mild cold stimulation after nerve injury, while mice developed robust cold sensitivity. We pursued this response deficit by testing behavior to activators of transient receptor potential (TRP) receptors involved in detecting cold in naïve animals. Following mustard oil, a TRPA1 activator, naked mole-rats responded similarly to mice. Conversely, icilin, a TRPM8 agonist, did not evoke pain behavior in naked mole-rats when compared with mice. Finally, we used RNAscope to probe for TRPA1 and TRPM8 messenger RNA expression in dorsal root ganglia of both species. We found increased TRPA1 messenger RNA, but decreased TRPM8 punctae in naked mole-rats when compared with mice. Our findings likely reflect species differences due to evolutionary environmental responses that are not easily explained by differences in receptor expression between the species.
Keywords: Naked mole-rat, nociception, cold allodynia, spared nerve injury, acetone, icilin, transient receptor potential receptors
Introduction
Broadly, pain can be divided in terms of resolution—acute versus chronic—and origin—damage to tissue (nociceptive) or nerves (neuropathic).1 In contrast to acute pain, which informs of injury and warns against continued damage, chronic pain lasts beyond the tissue healing process and impairs our capacity to survive disease, trauma, and surgical procedures intended to improve quality of life. A particular problem is the treatment of chronic neuropathic pain as this type of pain is often refractory and resistant to treatment, and those therapies that do exist are associated with unwanted side effects.1 Analgesics used to treat nociceptive pain (i.e., over the counter NSAIDs and other COX-2 inhibitors) simply do not work in the case of neuropathic pain,2 while opioids—the most potent analgesics in our clinical arsenal—have reduced efficacy to control neuropathic pain and are associated with a high risk of substance abuse.3
The lack of safe and effective therapeutic options for many neuropathic pain patients highlights the urgent need for developing new and improved medications. This has led to the investigation of patients with congenital pain insensitivity that has identified several genetic mutations that render these patients unable to feel pain.4 The relevance of these genes to pain processing has been verified through the development of transgenic animal models with genetic mutations in nerve growth factor and its receptor5,6 and sodium channels7 intimately involved. However, patients with congenital pain insensitivity are rare and the generation of novel animal models can be time consuming and expensive. A novel alternative is the study of natural responses in animal species that possess unique pain adaptations. By understanding how pain processing has evolved naturally, new models and targets may be identified.
Recent studies have described pain insensitivity in the African naked mole-rat (Heterocephalus glaber) (NMR) in response to noxious stimulation from acid8,9 and capsaicin/heat.10 The receptor for capsaicin, TRPV1, is functional in the NMR,8,10 with their lack of pain behavior thought to be due to a paucity of peptidergic C fibers and decreased connectivity with neurons in the deep layers of the dorsal horn of the spinal cord.8 NMRs also lack calcitonin gene-related peptide (CGRP) and substance P in nociceptive C fibers,11 have a conspicuous dearth of unmyelinated C fibers in cutaneous nerves,12 and possess a hypofunctional tropomyosin receptor kinase A (TRKA) receptor in the dorsal horn of the spinal cord resulting in a lack of a heat inflammatory pain response.10 The NMR also has a species-specific variant of the nociceptor-specific sodium channel NaV1.7 resulting in an insensitivity toward acid stimuli.9
Thus, we sought to explore whether unique somatosensory features of the NMR may extend to neuropathic pain sensitivity phenotypes and determine whether these adaptations permit resistance to the development of hypersensitivity following nerve injury. Using the spared nerve injury (SNI) model, we compared responses to innocuous mechanical, noxious mechanical, and cold stimuli in mice and NMRs over multiple weeks. Hypersensitivity to both types of mechanical stimuli following nerve injury was comparable in mice and NMRs; however, NMRs did not respond to the cold stimulus. Based on our initial experiments, we then compared the responses of mice and NMRs to activators of two known cold transducers belonging to the transient receptor potential family, TRPA1 and TRPM8. Nocifensive behavior was greater in NMRs compared with mice following administration of mustard oil, a TRPA1 agonist, while NMRs did not respond to icilin, a TRPM8 agonist. Behavioral experiments in the present study were followed up by in situ characterization of TRPA1 and TRPM8 messenger RNA (mRNA) in dorsal root ganglia (DRG) tissue of NMRs and mice, which revealed higher TRPA1 expression in NMRs with no difference between the species for TRPM8.
Materials and methods
Animals
Adult subordinate NMRs were selected from four different colonies and randomly assigned to cohorts of eight consisting of both sexes. Each cohort was tested together and included two distinct subclasses of NMRs. Subclass was selected by observing in-colony behavior and out-pairing an individual in a fresh cage with a small juvenile from another colony and observing aggressive behavior (e.g., biting, intrusive sniffing with shoving) toward the novel conspecific. NMRs displaying overt aggressive behavior were classified as soldiers, while NMRs that did not display aggressive behavior were classified as workers. NMRs were bred in-house (M.M. Holmes laboratory) and group‐housed in large (45.75 cm L × 24 cm W × 15.25 cm H) and small (30 cm L × 18 cm W × 13 cm H) polycarbonate cages connected by plastic tubes (25 cm L × 5 cm D) in the animal facility under a 12-:12-h light:dark photoperiod (07:00 lights on) at 29 ± 1°C and 50%–60% relative humidity. NMRs received ad libitum access to peeled, cut sweet potato, and wet 19% protein mash (Teklad; Harlan, Indianapolis, IN, USA). Adult mice were bred in-house (L.J. Martin laboratory) from outbred CD-1 (ICR:Crl) breeders obtained from Charles River Laboratories. Mice were group-housed single-sex in small (30 cm L × 18 cm W × 13 cm H) polycarbonate cages in the animal facility under a 12-:12-h light:dark photoperiod (07:00 lights on) at 22 ± 1°C. Mice received ad libitum access to 19% protein chow (Teklad; Harlan) and water. NMRs and mice were housed and tested in separate rooms for all experiments. All procedures were conducted in accordance with the animal care standards set forth by the Canadian Council on Animal Care (CCAC) and were approved by the University of Toronto Animal Care Committee.
Spared nerve injury
SNI, a surgical nerve injury designed to produce neuropathic pain, was adapted from the Decosterd and Woolf rat model.13 Briefly, animals were deeply anesthetized with isoflurane, and blunt dissection was performed through the hind thigh muscle to expose the sciatic nerve. Two tight knots of silk (PERMA-HAND® Silk suture 7–0, Ethicon) were tied around the common peroneal and tibial nerves immediately distal to the branching. The nerves were transected distal to the suture, and the dissection was closed with sutures (Vicryl suture 6–0, Ethicon). The result of the surgery left the sural and saphenous nerves unaltered such that the respective lateral and medial edges of the hind paw remained connected with the spinal cord, while the center dorsal and ventral surfaces of the paw were denervated. Following surgery, animals were allowed to recover from anesthesia and were returned to the home cage. The sham surgery consisted of the blunt dissection through the thigh muscle with care taken not to disturb the nerve.
Behavioral assays
For the SNI model behavioral experiments (von Frey, acetone droplet, and pin prick: NMRs, n = 31 (males = 15: 7 SNI, 8 sham; females = 16: 8 SNI, 8 sham); CD1 mice n = 8 (males = 8 surgery)), care was taken to test animals in a random blinded fashion; however, the nature of the surgery lends to easy identification of the SNI animals due to increased guarding behavior seven days following surgery. Baseline was measured the day before surgery and testing started the day after surgery continuing for four weeks. All animals were tested using innocuous mechanical stimuli twice per week (e.g., Monday, Wednesday) and on separate days were tested for cold allodynia followed by mechanical hyperalgesia, placing the strongest stimulus last in the testing session, on alternate days of the week (e.g., Tuesday, Thursday). NMRs were tested in their respective housing room to match temperature and humidity.
Mild mechanical—von Frey test
Sham or SNI animals were habituated to clean individual cubicles on an elevated wire mesh grid for 1 h before testing. Calibrated von Frey filaments (0.008–1.4g for mice, 1.6–10 g for mole-rats, North Coast Medical Inc., Gilroy, California, USA) were applied to the lateral edge of the hind paw ipsilateral to surgery using the Chaplan Up and Down method14 until the filament bowed (50% bend) for at least 4 s. Withdrawal, meaning a strong leg muscle reflex, lifting, guarding, chewing, or licking, was scored as present or absent. Each hind paw was tested up to 10 times with at least 2 min between successive tests. Animals were tested twice per week for four weeks after surgery.
Mild cold—Acetone droplet test
Sham or SNI animals were placed in clean individual cubicles on an elevated wire mesh grid and habituated to the testing arena for 1 h before testing. We employed an acetone droplet assay to both sham and SNI animals, as previously described by Yoon et al.15 A 30 µl droplet of acetone (Caledon Laboratory Chemicals, 1201–7–10) was placed onto the plantar surface ipsilateral to surgery using a blunted 18-gauge needle and a 100 ml syringe. Care was made to touch the droplet of acetone, but not the needle, to the plantar hind paw surface. The duration of withdrawal response (i.e., licking, shaking, guarding) was measured using a minimum of 0 s and a maximum of 20 s. Animals were tested twice per week for four weeks after surgery.
Strong mechanical—Pinprick test
Using sham or SNI animals, a pinprick test was implemented as described by Tal and Bennett16 after a 20-min wait period following the last test of the cold allodynia assay. The pin was applied swiftly to the lateral edge of the paw ipsilateral to surgery to the point of skin indentation but not skin penetration. We recorded the duration of paw withdrawal using an arbitrary minimum cut-off of 0.5 s and a maximum cut-off of 10 s. The duration of hind paw response (i.e., licking, chewing, shaking, guarding) was recorded using a stopwatch. Animals were tested twice per week for four weeks after surgery.
Mustard oil and icilin tests
Without using the nerve injury model, surgically naïve animals (mustard oil: NMR n = 17 (male = 7, female = 10), CD1 mice n = 18 (male = 9, female = 9); icilin: NMR n = 16 (male = 8, female = 8), CD1 mice n = 16 (male = 8, female = 8)) were habituated to red acrylic cylinders on an elevated acrylic observation platform for 30 min. Animals were then briefly removed from the cylinder, and 20 µl intraplantar injection of either mustard oil (allyl isothiocyanate 5%, Sigma-Aldrich W203408, suspended in 80% DMSO and 15% PBS) or icilin (Cayman Chemical, 10137, 3 mg/ml (60 µg/dose) suspended in 80% DMSO and 20% normal saline) of the left hind paw was given before the animal was placed back into the cylinder. Behavior was digitally video recorded for 10 min, and video files were later scored for the total duration (s) of licking/biting of the paw.
Video analysis
Videos were analyzed by behavioral coders blinded to treatment conditions. Videos were scored using an open-source key-stroke ethogram software mice_notes.py, Copyright 2019 Jack Poulson, and Sandra Poulson under the open-source BSD 2-Clause License, available on GitHub https://github.com/sandrapoulson/mice_notes. The use of this software is similar to using a handheld stopwatch, except that multiple behaviors can be scored real time with the click of different keys representing different behaviors, and the data are stored in time bins such that it can be easily graphed using the freely available graphing software like Matplotlib in Python. The time animals spent licking or chewing the left hind paw was graphically represented in raster plots made using the Python Matplotlib function eventplot.17
RNAscope
Without using the nerve injury model, surgically naïve animals (NMR n = 1 male, CD1 mouse n = 1 male) were deeply anesthetized (avertin at 40 mg/100g i.p. for NMRs, pentobarbital at 80 mg/kg i.p. for mice) and transcardially perfused with sterile PBS and then 4% paraformaldehyde. Lumbar DRG were removed and post-fixed in 4% PFA for 24 h and then treated with 10%, 20%, and then 30% sucrose in PBS until the tissue settled at the base of the tube. Tissue was then embedded in Tissue-Plus O.C.T. Compound (Fisher Healthcare, 4585) and kept at −80°C for one month or less. Tissue was cut via cryostat (CryoStar™ NX50 Cryostat, Thermo Scientific) at 14 µm and mounted directly onto Superfrost Plus slides (Fisherbrand, 1255015) and kept frozen until the RNA in situ hybridization assay (RNAscope). After tissue preparation and slide-mounting, DRG tissue was prepared according to the manufacturer’s technical note (Advanced Cell Diagnostics (ACD), catalog no. 302535). Briefly, RNAscope was applied to the slides using the version 1 fluorescent multiplex reagent kit (ACD, catalog no. 320850) and RNAscope procedure (ACD, 320293). Slides were cover-slipped using ProLong Gold Antifade Mountant (Invitrogen, P36930) and imaged using a Zeiss LSM 800 confocal with Airyscan at 20× magnification. Punctae staining was counted using the three-dimensional viewer and marker function in ZEN lite (3.1 blue edition, Zeiss). RNAscope signal is revealed as a punctate staining, with little or no background, and previous studies demonstrated that each punctum corresponds to one molecule of the intended target mRNA.18 Thus, punctae were counted in each positively labeled cell and compared between NMR and mouse DRG tissue for TRPM8, TRPA1, and TRPV1 mRNA staining (see Table 1 for probe specifications). Quantification of the number of puncta per cell offered a direct measurement of expression probe targets.
Table 1.
Advanced cell diagnostics RNAscope target ZZ probes.
| Target | Catalog no. | Length | Target region | Accession no. | Species |
|---|---|---|---|---|---|
| Hg-TRPM8 | 537651 | 20ZZ | 1510–2485 | XM_021247317.1 | Naked mole-rat |
| Hg-TRPA1 | 537661-C2 | 20ZZ | 898–1948 | XM_004842180.2 | Naked mole-rat |
| Hg-TRPV1 | 537671-C3 | 20ZZ | 548–1491 | NM_001279859.1 | Naked mole-rat |
| Mm-TRPM8 | 420451-C3 | 20ZZ | 440–1350 | NM_134252.3 | Mouse |
| Mm-TRPA1 | 400211-C2 | 20ZZ | 77–1047 | NM_177781.4 | Mouse |
| Mm-TRPV1 | 313331 | 20ZZ | 1162–2155 | NM_001001445.1 | Mouse |
| Hg-Actb (positive) | 540491 | 12ZZ | 20–1821 | XM_004840381.2 | Naked mole-rat |
| 3-plex positive control probe-Mm | 320881 | NA | NA | NA | Mouse |
NA: not applicable.
Statistical analysis
Mild mechanical (von Frey), mild cold (acetone droplet), and strong mechanical (pinprick) assays were analyzed with repeated measures analysis of variance (ANOVA), followed where appropriate with multiple independent t-tests with Bonferroni correction. A two-tailed independent t-test after a Levene’s Test for Equality of Variances was used to compare nocifensive behavior in the mustard oil and icilin assays. A two-way ANOVA was used to test whether sex or social status affected the decrease in threshold after nerve injury in NMRs. Since the RNAscope data violated assumptions of normality (Shapiro-Wilk: TRPM8, W= p < 0.0001; TRPA1, W = 0.77, p < 0.0001; TRPV1, W = 0.74, p < 0.0001) and homogeneity of variance (Levene’s test: TRPM8, F = 5.24, p < 0.0001; TRPA1, F = 56.56, p < 0.0001), data were analyzed for outliers. Cells with punctate counts 3 SD (or more) away from the mean were removed and remaining data transformed (square root, reciprocal, z-score); however, statistical assumptions were still violated. Thus, RNAscope data—with outliers removed—were analyzed using the Mann–Whitney U test, a non-parametric test that does not assume normality and is robust against violations of homogeneity of variance. All data are presented as mean ± standard error of the mean (SEM), and differences were considered statistically significant at a p-value of less than 0.05. Statistical analyses were performed using IBM SPSS Statistics (Version 24, IBM Corp), and graphical representations of data were made using Prism 8 for Max OS X (GraphPad Software, Inc).
Results
Effect of innocuous mechanical stimuli after nerve injury
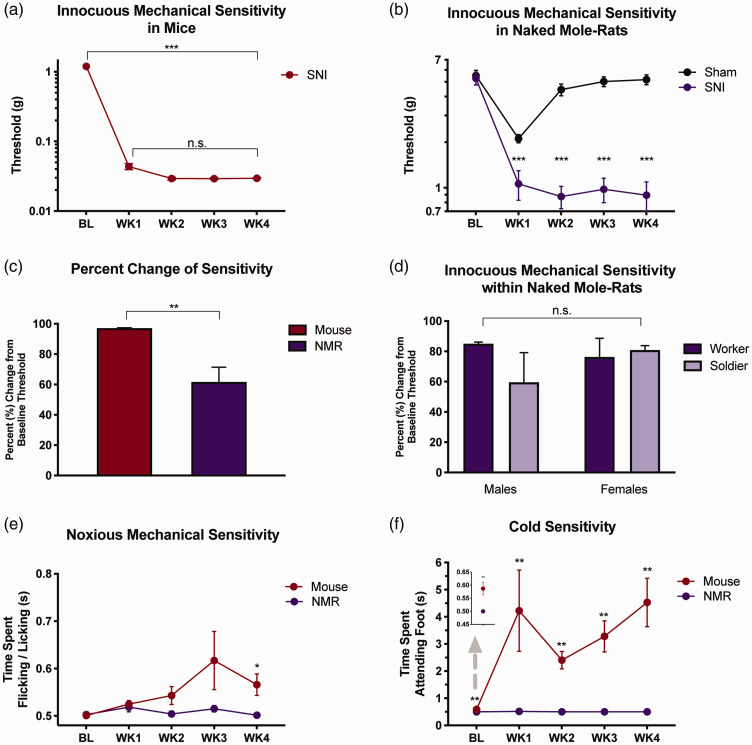
Mice that underwent SNI surgery displayed a significant decrease in von Frey mechanical thresholds across all four-week time points post-surgery (Figure 1(a), one-way ANOVA, F1,7 = 210.11, p = 0.001). In NMRs, SNI significantly decreased von Frey thresholds across all four-week time points (Figure 1(b), two-way ANOVA, surgery × time interaction, F2,55 = 22.7, p = 0.001). Sham-operated NMRs showed a decrease in von Frey thresholds during the first week of testing (t15 = 7.07, p < 0.001) followed by a return to baseline, whereas threshold measurements in NMRs were significantly decreased at every time point post-SNI surgery (week 1, t29 = 3.971; week 2, t19 = 8.476; week 3, t22 = 9.744; week 4, t22 = 9.834, all p values < 0.001). Despite the difference in threshold measurements between mice and NMRs, mice exhibited a greater decrease from baseline thresholds compared with NMRs after SNI (Figure 1(c); t14 = 3.37, p < 0.01). Body weights were similar between species indicating that threshold differences were not due to size disparities between NMRs and mice (mice: 43.29 ± 1.15 g; NMRs: 45.82 ± 1.25 g; t37 = 1.074, p = 0.29). Sex or social status of the NMR also did not appear to significantly influence the development of hypersensitivity following SNI as the percent decrease from baseline for von Frey thresholds between NMRs was not statistically different between sexes or different sub-castes (i.e. soldier or worker) (Figure 1(d), two-way ANOVA, main effect of sex: F1,11 = 0.07, p = 0.802; main effect of status: F1,11 = 0.11, p = 0.40; sex × status interaction: F1,11 = 0.75, p = 0.404). Sample size was small for these comparisons; however, we decided not to investigate this further as it was secondary to our main line of investigation.
Figure 1.
Effects of stimuli after nerve injury in mice and naked mole-rats. (a) Mice (n = 8) developed hypersensitivity to innocuous mechanical stimuli within seven days of spared nerve injury compared to BL, which was maintained for at least four weeks, ***p < 0.001. (b) Like mice, NMRs developed sensitivity to innocuous mechanical touch after nerve injury compared to BL threshold. Threshold measurements differed between sham (n = 16) and SNI (n = 15) animals at every time point beyond baseline, ***p < 0.001. (c) Mice (n = 8) exhibited a larger percent change from BL after nerve injury compared to NMRs (n = 15), **p < 0.01. (d) Neither sex nor status had an effect on the development of hypersensitivity to innocuous mechanical touch in NMRs (male soldier n = 2, female soldier n = 3, male worker n = 5, female worker n = 5; p = 0.404). (e) Both species developed similar reaction patterns to noxious mechanical pinprick over time, p = 0.117. Independent t-tests revealed statistical difference between mice and NMR reaction time at WK4, *p < 0.05. (f) Reaction to an acetone droplet differed between NMR and mice across time points. Independent t-tests showed statistical difference between mice and NMR both at baseline and at every time point after surgery, **p < 0.01. Data plotted as mean ± SEM. BL: baseline; NMR: naked mole-rat; SNI: spared nerve injury; WK: week.
Effect of noxious mechanical stimuli after nerve injury
To further characterize pain behavior, we used the pinprick assay as a metric for noxious mechanical stimuli. Following SNI, no statistical difference in response time between NMRs and mice was observed in the pinprick assay (Figure 1(e); two-way ANOVA, main effect of time: F2,36 = 2.28, p = 0.123; species × time interaction: F2,36 = 2.34, p = 0.117). However, subsequent independent t-tests revealed that NMRs exhibited significantly less reaction to the pinprick compared with mice four weeks following nerve injury (week 4, t7 = 2.871, p < 0.05). Mice showed different reaction times to pinprick following SNI, but only at four weeks were these times increased compared with baseline (paired samples t-test, t7 = 2.95, p = 0.021). Response times of NMRs were not significantly elevated at any of the time points following injury compared with baseline responses (paired samples t-test, all t values < 1.17, p > 0.05).
Effect of mild cold stimuli after nerve injury
Following SNI, mice displayed an increase in the amount of time spent attending to the hind paw after the application of acetone, with response times in NMRs remaining virtually unchanged (Figure 1(f); two-way ANOVA, species × time interaction: F2,40 = 20.205, p < 0.001). There was a consistent increase in time spent attending in mice that was not observed in NMRs, where a complete lack of behavioral responding was observed. At all time points, the amount of time attending to the hind paw after acetone application was significantly greater in mice compared with NMRs (baseline: t7 = 3.58, week 1: t7 = 3.936, week 2: t7 = 5.921, week 3: t7 = 4.851, week 4: t7 = 4.522, all p values < 0.01).
Effect of chemical transient receptor potential agonists on surgically naïve animals
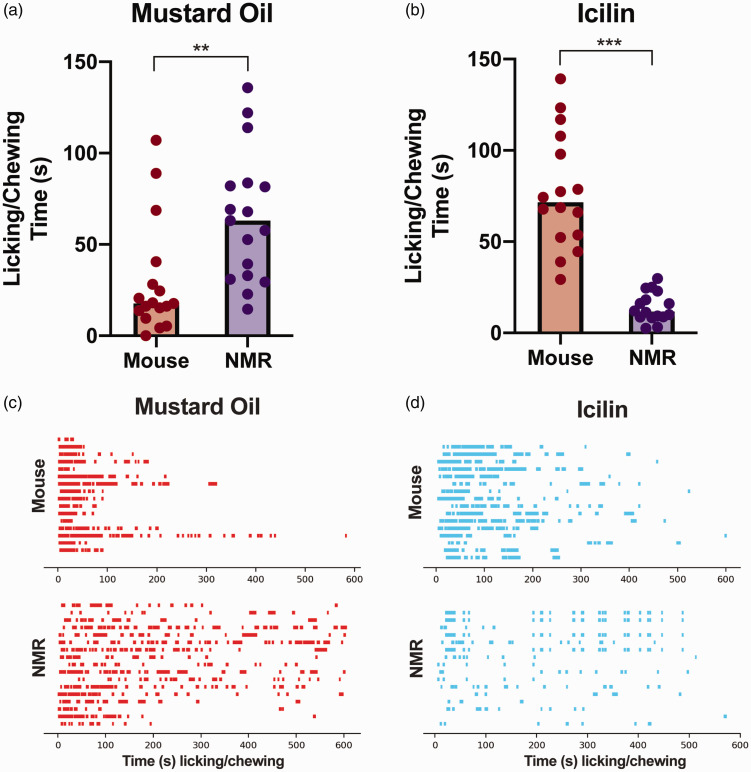
We next sought to determine whether the lack of response to acetone in NMRs generalized to different cold stimuli in the absence of nerve injury. Accordingly, we used surgically naïve animals and compared response time in both NMRs and mice to intraplantar injections of two algogens known to activate TRP receptors involved in the response of cold stimuli. In particular, we tested intraplantar injections of mustard oil, which activates TRPA1, and icilin, a strong activator of TRPM8. Mice displayed a lower amount of licking/chewing of the hind paw when injected with mustard oil, compared with responses in NMRs (Figure 2(a) and (c), t32 = 3.13, p < 0.01). Conversely, NMRs displayed less licking/chewing behavior compared to mice following intraplantar injection of icilin (Figure 2(b) and (d), t17 = 7.69, p < 0.001), indicating that an obvious reaction to icilin is lacking in the NMR. Ethograms displaying individual licking episodes over the entire 10 min observation period are shown for mustard oil (Figure 2(c)) and icilin (Figure 2(d)) behaviors for mice and NMRs.
Figure 2.
Behavioral reaction to chemical activators of cold receptors in surgically naïve animals. (a) Mice (n = 17) exhibited less licking/chewing in the 10 min after intraplantar injection of mustard oil, activator of TRPA1, compared to NMRs (NMR; n = 17), **p < 0.01. (b) In contrast, NMRs (n = 16) displayed little licking/chewing behavior compared to mice (n = 16) in the 10 min after an intraplantar injection of icilin, strong activator of TRPM8, ***p < 0.001. (c and d) Raster plots of time spent licking/chewing (s) after intraplantar mustard oil (c) and icilin (d). NMR: naked mole-rat.
Species expression of TRPA1 and TRPM8 receptor mRNA
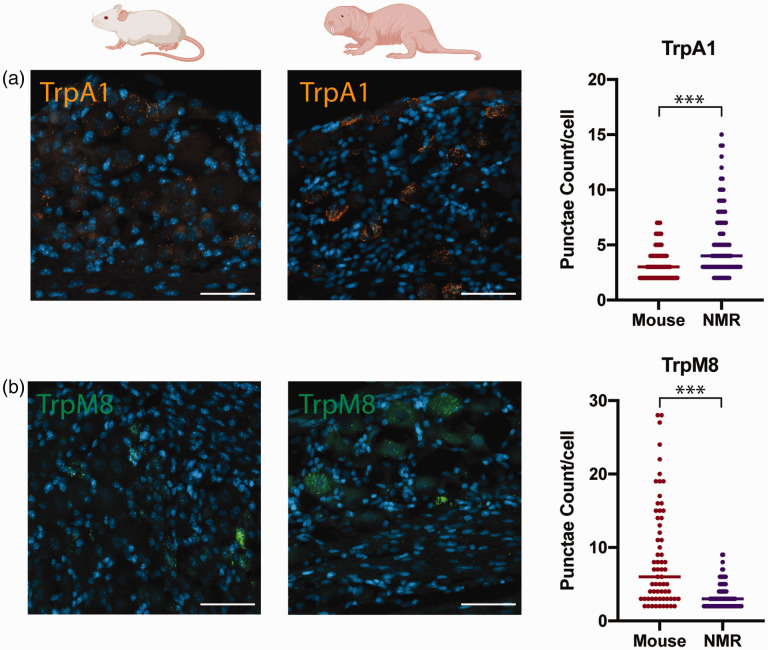
In order to determine whether paw attending in surgically naïve NMRs following mustard oil or icilin injections was associated with differences in the expression of TRPA1 or TRPM8, we used RNAscope, an in situ hybridization stain. Specifically, we quantified the average number of punctae in TRPA1 and TRPM8 positive cells in DRG tissue between surgically naïve animals of both species. The average number of TRPA1 was significantly higher (Figure 3(a), U = 3052, p < 0.0001), while TRPM8 was lower (Figure 3(b), U = 1564, p < 0.001) in NMRs compared with mice when mRNA puncta per cell was analyzed. We also probed for TRPV1 mRNA transcripts as a further TRP channel comparison and found that the average number of punctae per cell was similar between the species (data not shown; U = 4133, p = 0.14).
Figure 3.
Expression of TRPA1 and TRPM8 mRNA in DRG of surgically naïve animals. (a) Representative images for mouse (left panel) and NMR (middle panel) showing TRPA1 mRNA punctae (orange). Significantly higher punctae per cell (right panel) in NMR DRG (n = 95 cells) compared with mice (n = 157 cells), ***p < 0.001. (b) Representative images for mouse (left panel) and NMR (middle panel) showing TRPM8 mRNA punctae (green). Significantly lower puncta per cell (right panel) in NMR DRG (n = 137 cells) compared with mice (n = 70 cells). Scale bars = 50 µM. NMR: naked mole-rat.
Discussion
The African NMR (Heterocephalus glaber) was chosen for the current study due to several modifications to the nociceptive system that have evolved to help it navigate a challenging subterranean environment. Initially, we set out to assess whether unique features of the NMR somatosensory system may extend to neuropathic pain phenotypes, specifically the development of sensitivity to mechanical and cold stimuli following nerve injury. NMRs displayed a lack of cold allodynia following nerve injury when compared with mice, which led us to assess behavior responses in surgically naïve animals to TRP channel activators associated with cold stimuli. Nocifensive responses to mustard oil, a TRPA1 activator, were enhanced when compared with mice, while icilin, a strong activator of TRMP819,20 did not evoke detectable behaviors in NMRs. We also observed abundant expression of TRPA1, but lower expression of TRPM8, mRNA puncta in the DRG of NMRs when compared with mice. NMRs and mice were compared because prior investigations into the somatosensory system of the NMR have been made with mice and not rats.8–11 In addition, RNAseq profiling has revealed that NMR sensory neurons can be classified at the molecular level in a similar way to those of mice.21,22
After SNI surgery, NMRs and mice developed similar patterns of sensitivity to innocuous touch represented by a sustained decrease in von Frey threshold following injury (Figure 1(a) and (b)). This result is comparable with previous studies that have reported similar mechanical sensitivity between the two species following inflammatory injury.8 Since the absolute threshold values for mice and NMRs were quite different, we standardized the change in sensitivity as a percent of baseline values and found greater hypersensitivity in mice compared with NMRs following surgery (Figure 1(c)). While this observation may indicate that NMRs develop less hypersensitivity following nerve injury, these data may reflect the constant tunneling and digging movement of NMRs, which make the measurement of mechanical thresholds difficult. There are reported behavioral differences in subordinate colony members with some individuals being more aggressive and others spending more time digging and moving food.23 However, when separated from the colony and placed into testing cubicles, all subordinate NMRs constantly bite at the cubicle and push on the sides in an attempt to tunnel out with occasional breaks of typically no more than 1 min. This behavior is in stark contrast to mice, which habituate and remain relatively stationary within the testing cubicles after as little as 30 min. In a species such as the NMR, which spends much of the day tunneling and digging, cognitive attention may be pulled more toward digging as opposed to responding to the evoked mechanical stimulation. This would be similar to increased pain thresholds of human participants in response to distraction24–26 and reduced pain behavior during grooming and movement in mice.27
In our opinion, the most intriguing finding from the present study is that NMRs showed an unusual lack of reflexive behavior to a mild cold stimulus both before and after nerve injury, and surgically naïve animals did not display behavioral responses to icilin. Mice respond to acetone with a brief flick of the foot and the duration of foot flicking increases over time following nerve injury. In contrast, NMRs did not respond to a droplet of acetone before or after nerve injury, indicating that the stimulus may not be transmitted properly, or the signals are not processed as in other rodent species.28 In addition, electrophysiological recordings have shown that high concentrations of icilin activate TRPA1, but behavioral responses have been shown to rely on TRPM8 and not TRPA1 activation.19,20,29
Based on the lack of icilin responsiveness in NMRs, we reasoned that they might have a lower expression of TRPM8 in sensory neurons. Thus, we used RNAscope in situ hybridization to investigate this hypothesis because commercially available TRPM8 antibodies—even for mouse and rat—are limited, and RNAscope allowed us to design probes specific for the NMR. We observed a lower number of TRPM8 mRNA puncta between in NMR when compared with surgically naïve mice and analyzed per cell. Further to this point, each cell was considered independent because each RNAscope dot is derived from a single mRNA molecule.18 However, caution must be exercised when interpreting the RNAscope data because of the small sample size. These data are a limitation of the current study, but we would like to reinforce that the primary reason for including RNAscope was not to give an in-depth analysis of TRPM8 (or even TRPA1), but rather to show the presence of these transcripts in both species.
It is reasonable to expect that TRPM8 or TRPA1 are expressed by different subpopulations of neurons in NMRs because differences in TRP channel expression have been previously reported among other species. For example TRPV1 is almost exclusively found in heat-specific peptidergic sensory neurons in mice,30,31 while it is found in both peptidergic and nonpeptidergic C-fibers in rats.30,32,33 A recent RNAscope study revealed that TRPV1 mRNA is expressed in the entire nociceptor population in humans (77.7% of all neurons) compared to only 32.4% in mouse. Contrastingly, this same study found less TRPA1 mRNA expressing neurons in human DRG (16.3%) than mouse (55.2%).34 TRPM8 and TRPA1 mRNA are expressed in different subsets of rat DRG cells, with TRPM8 found in both C fibers and A fibers, while TRPA1 is found almost exclusively in a different subset of C fibers.35Follow-up studies will be imperative toward understanding the cellular distribution of TRPM8 (and TRPA1) in NMRs, while also examining their functional sensitivity to activators of these ion channels. However, in both rats and mice, TRPM8 and TRPA1 are downregulated following nerve injury with distribution patterns remaining intact.36
It is challenging to draw conclusions without conducting a functional experiment. However, motivated behavioral responses do not always correspond with fiber and receptor activation.37 In NMRs, the TRPV1 receptor is expressed at normal levels in DRG and functions despite a lack of behavioral response to capsaicin10 and heat hypersensitivity.8 Explanations for the lack of response from capsaicin in the NMR have been proposed, including deeper superficial rooting of TRPV1 receptor-expressing C fibers into the dorsal horn8 and modification of receptor function either on the receptor itself or through interaction with other molecules.10 Given that monosynaptic input from a subset of unmyelinated primary afferents converge on capsaicin- and icilin-responsive spinal interneurons, interactions between distinct afferent messages may be dependent on similar mechanisms.38 However, eradicating CGRP-expressing neurons in mice enhances both cold sensitivity and icilin-evoked spinal neuron responses.37 These results conflict with the lack of cold sensitivity and icilin-responsiveness in NMRs as they naturally do not express CGRP in peripheral afferents.11 Another possibility is that NMRs present lower expression of TRPM8 at central terminals compared to the expression of these receptors in mice. We attempted to investigate this possibility, but punctate staining was sparse in both species making the interpretation of the data difficult (data not shown). Enhanced TRPM8 signaling has also been implicated in cellular proliferation39 and decreased TRPM8 signaling in NMRs would coincide with their resilience to cancer.40
NMRs possessed intact nocifensive behaviors in response to mustard oil, a TRPA1 agonist, suggesting that unique rather than general pain adaptations have occurred in this species. Interestingly, mustard oil does not evoke noxious pain responses in the highveld mole-rat, a closely related species to the NMR.22 TRPA1 mRNA was elevated in NMRs, supporting previously published sequencing work22 and the mustard oil behavioral data reported herein. In addition to responding to mustard oil and other chemical signals, TRPA1 responds to cold and mechanical stimuli.41–43 Focusing on cold signals, the debate on the role of TRPA1 and TRPM8 in cold sensation has been lively since the discovery of these receptors.44–46 While the involvement of TRPM8 in cold stimuli has been relatively consistent,46–48 some studies found a role for TRPA1 in strong cold perception,49,50 mild cold perception,51,52 and no role for either TRPA1 or TRPM8 in cold sensitivity after nerve injury.36,53 However, recent studies using calcium imaging observed large regions of neurons in the spinal cord and trigeminal ganglia, revealed TRPM8 to be involved in the encoding of both mild and strong cold stimuli, and found no role for TRPA1 in the encoding of cold stimuli.54,55 Instead, TRPA1 has been implicated in the encoding for heat stimuli, strengthened by the co-expression of TRPA1 with TRPV1.55
Together, these data have broadened the overall picture of the pain system in this fossorial species by exploring neuropathic pain. Continued investigation should reveal the mechanism behind the lack of mild cold response in this unique rodent and will potentially help to understand the role of TRP receptors in cold allodynia in neuropathic pain and the interaction of receptors in sensory signal transmission. Concerning symptoms in nociceptive and chronic pain, continued research into potential TRP targets may help alleviate sensations that often fail to respond to NSAIDs or opiate therapies widely used today.56 It should also be noted that TRP channel antagonists have been tested in drug development studies with side effects of hyper- and hypothermia.57 Thus, targeting local TRP channels at the site of injury may be essential to avoid systemic side effects from TRP modulators in sensitized nociceptive fibers.
Acknowledgments
The authors thank Amy Tu and Dr. Milind Muley at The Hospital for Sick Children, Toronto ON, for sharing their expertise in neural tissue dissection.
Author Contributions
LJM, MMH, and SJP conceived of the project. SJP conducted all experiments. AA, ND, and RR analyzed behavioral videos. AA, IIA, and KM quantified RNAscope punctae. SJP and LJM analyzed data and wrote the paper. All authors edited and commented on the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Canada Research Chairs Program (LJM), Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2016–06284 to LJM and RGPIN 2018–04780 to MMH), an NSERC Discovery Accelerator Supplement (RGPAS2018-522465 to MMH) and Ontario Early Researcher Awards (LJM and MMH).
ORCID iD
Sandra J Poulson https://orcid.org/0000-0002-5533-4599
References
- 1.Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol 2011; 24: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore R, Chi C, Wiffen P, Derry S, Rice A. Oral nonsteroidal anti‐inflammatory drugs for neuropathic pain. Cochrane Database Syst Rev 2015; 2015: CD010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNicol E, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev 2013; 2013: CD006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drissi I, Woods WA, Woods CG. Understanding the genetic basis of congenital insensitivity to pain. Br Med Bull 2020; 133: 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet 1996; 13: 485–488. [DOI] [PubMed] [Google Scholar]
- 6.Shatzky S, Moses S, Levy J, Pinsk V, Hershkovitz E, Herzog L, Shorer Z, Luder A, Parvari R. Congenital insensitivity to pain with anhidrosis (CIPA) in Israeli-Bedouins: Genetic heterogeneity, novel mutations in the TRKA/NGF receptor gene, clinical findings, and results of nerve conduction studies. Am J Med Genet 2000; 92: 353–360. [DOI] [PubMed] [Google Scholar]
- 7.Chen LC, Nishidate K, Saito Y, Mori K, Asakawa D, Takeda S, Kubota T, Terada N, Hashimoto Y, Hori H, Hiraoka K. Application of probe electrospray to direct ambient analysis of biological samples. Rapid Commun Mass Spectrom 2008; 22: 2366–2374. [DOI] [PubMed] [Google Scholar]
- 8.Park TJ, Lu Y, Jüttner R, Smith ESJ, Hu J, Brand A, Wetzel C, Milenkovic N, Erdmann B, Heppenstall PA, Laurito CE, Wilson SP, Lewin GR. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber). PLoS Biol 2008; 6: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith E S J, Omerbašić D, Lechner S G, Anirudhan G, Lapatsina L, Lewin G R. The molecular basis of acid insensitivity in the African naked mole-rat. Science 2011; 334: 1557–1560. [DOI] [PubMed] [Google Scholar]
- 10.Omerbašić D, Smith ESJ, Moroni M, Homfeld J, Eigenbrod O, Bennett NC, Reznick J, Faulkes CG, Selbach M, Lewin GR. Hypofunctional TrkA accounts for the absence of pain sensitization in the African naked mole-rat. Cell Rep 2016; 17: 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park T J, Comer C, Carol A, Lu Y, Hong H-S, Rice F L. Somatosensory organization and behavior in naked mole-rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. J Comp Neurol 2003; 465: 104–120. [DOI] [PubMed] [Google Scholar]
- 12.Smith ES, Purfürst B, Grigoryan T, Park TJ, Bennett NC, Lewin GR. Specific paucity of unmyelinated C-fibers in cutaneous peripheral nerves of the African naked-mole rat: comparative analysis using six species of Bathyergidae. J Comp Neurol 2012; 520: 2785–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. [DOI] [PubMed] [Google Scholar]
- 14.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 15.Yoon C, Wook YY, Sik NH, Ho KS, Mo CJ. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994; 59: 369–376. [DOI] [PubMed] [Google Scholar]
- 16.Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain 1994; 57: 375–382. [DOI] [PubMed] [Google Scholar]
- 17.Hunter JD. Matplotlib: A 2D graphics environment. Comput Sci Eng 2007; 9: 90–95. [Google Scholar]
- 18.Wang F, Flanagan J, Su N, Wang L-C, Bui S, Nielson A, Wu X, Vo H-T, Ma X-J, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2012; 14: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowlton WM, Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010; 150: 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentry C, Stoakley N, Andersson DA, Bevan S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol Pain 2010; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015; 18: 145–153. [DOI] [PubMed] [Google Scholar]
- 22.Eigenbrod O, Debus KY, Reznick J, Bennett NC, Sánchez-Carranza O, Omerbašić D, Hart DW, Barker AJ, Zhong W, Lutermann H, Katandukila JV, Mgode G, Park TJ, Lewin GR. Rapid molecular evolution of pain insensitivity in multiple African rodents. Science 2019; 364: 852–859. [DOI] [PubMed] [Google Scholar]
- 23.Mooney SJ, Filice DCS, Douglas NR, Holmes MM. Task specialization and task switching in eusocial mammals. Anim Behav 2015; 109: 227–233. [Google Scholar]
- 24.Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain 2010; 149: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston NE, Atlas LY, Wager TD. Opposing effects of expectancy and somatic focus on pain. PLoS One 2012; 7: e38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain 2004; 109: 399–408. [DOI] [PubMed] [Google Scholar]
- 27.Callahan BL, Gil ASC, Levesque A, Mogil JS. Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J Pain 2008; 9: 174–184. [DOI] [PubMed] [Google Scholar]
- 28.Saadé NE, Baliki M, El-Khoury C, Hawwa N, Atweh SF, Apkarian AV, Jabbur SJ. The role of the dorsal columns in neuropathic behavior: evidence for plasticity and non-specificity. Neuroscience 2002; 115: 403–413. [DOI] [PubMed] [Google Scholar]
- 29.Rawls SM, Gomez T, Ding Z, Raffa RB. Differential behavioral effect of the TRPM8/TRPA1 channel agonist icilin (AG-3-5). Eur J Pharmacol . 2007; 575: 103–104. [DOI] [PubMed] [Google Scholar]
- 30.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci 2004; 24: 6410–6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavanaugh DJ, Chesler AT, Bráz JM, Shah NM, Julius D, Basbaum AI. Restriction of TRPV1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci 2011; 31: 10119–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience 1996; 71: 265–275. [DOI] [PubMed] [Google Scholar]
- 33.Yu L, Yang F, Luo H, Liu F-Y, Han J-S, Xing G-G, Wan Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain 2008; 4: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiers S, Klein RM, Price TJ. Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain Epub ahead of print 24 June 2020. DOI: 10.1097/j.pain.0000000000001973. https://journals.lww.com/pain/Fulltext/9000/Quantitative_differences_in_neuronal.98358.aspx [DOI] [PMC free article] [PubMed]
- 35.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with Aδ/C fibers and colocalization with TRK receptors. J Comp Neurol 2005; 493: 596–606. [DOI] [PubMed] [Google Scholar]
- 36.Caspani O, Zurborg S, Labuz D, Heppenstall PA. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One 2009; 4: e7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPα primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 2013; 78: 138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng J, Lu Y, Perl ER. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol (Lond). 2010; 588: 2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grolez GP, Gordiendko DV, Clarisse M, Hammadi M, Desruelles E, Fromont G, Prevarskaya N, Slomianny C, Gkika D. TRPM8-androgen receptor association within lipid rafts promotes prostate cancer cell migration. Cell Death Dis 2019; 10: 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013; 499: 346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mickle DA, Shepherd JA, Mohapatra PD. Nociceptive TRP channels: Sensory detectors and transducers in multiple pain pathologies. J Pharm 2016; 9: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lennertz RC, Kossyreva EA, Smith AK, Stucky CL. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS One 2012; 7: e43597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA 2009; 106: 1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laursen WJ, Anderson EO, Hoffstaetter LJ, Bagriantsev SN, Gracheva EO. Species-specific temperature sensitivity of TRPA1. Temperature (Austin) 2015; 2: 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guimaraes MZP, Jordt S-E. TRPA1: a sensory channel of many talents. Chapter 11 In: WB Liedtke, S Heller. (eds) TRP ion channel function in sensory transduction and cellular signaling cascades. Boca Raton: CRC Press/Taylor & Francis, 2007. [Google Scholar]
- 46.Tominaga M. The role of TRP channels in thermosensation. Chapter 20 In: WB Liedtke, S Heller. (eds) TRP ion channel function in sensory transduction and cellular signaling cascades. Boca Raton: CRC Press/Taylor & Francis, 2007. [Google Scholar]
- 47.McKemy DD. Trpm8: the cold and menthol receptor Chapter 13 In: WB Liedtke, S Heller. (eds) TRP ion channel function in sensory transduction and cellular signaling cascades. Boca Raton: CRC Press/Taylor & Francis, 2007. [PubMed] [Google Scholar]
- 48.Pan Y, Thapa D, Baldissera L, Argunhan F, Aubdool AA, Brain SD. Relevance of TRPA1 and TRPM8 channels as vascular sensors of cold in the cutaneous microvasculature. Pflügers Arch 2018; 470: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006; 50: 277–289. [DOI] [PubMed] [Google Scholar]
- 50.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003; 112: 819–829. [DOI] [PubMed] [Google Scholar]
- 51.Aubdool AA, Graepel R, Kodji X, Alawi KM, Bodkin JV, Srivastava S, Gentry C, Heads R, Grant AD, Fernandes ES, Bevan S, Brain SD. TRPA1 is essential for the vascular response to environmental cold exposure. Nat Commun 2014; 5: 5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D’Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM. TRPA1 contributes to cold hypersensitivity. J Neurosci 2010; 30: 15165–15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Oliveira C, Garami A, Lehto SG, Pakai E, Tekus V, Pohoczky K, Youngblood BD, Wang W, Kort ME, Kym PR, Pinter E, Gavva NR, Romanovsky AA. Transient receptor potential channel Ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J Neurosci 2014; 34: 4445–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ran C, Hoon MA, Chen X. The coding of cutaneous temperature in the spinal cord. Nat Neurosci 2016; 19: 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yarmolinsky DA, Peng Y, Pogorzala LA, Rutlin M, Hoon MA, Zuker CS. Coding and plasticity in the mammalian thermosensory system. Neuron 2016; 92: 1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moran MM, Szallasi A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol 2018; 175: 2185–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garami A, Pakai E, McDonald HA, Reilly RM, Gomtsyan A, Corrigan JJ, Pinter E, Zhu DXD, Lehto SG, Gavva NR, Kym PR, Romanovsky AA. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol 2018; 223: e13088. [DOI] [PMC free article] [PubMed] [Google Scholar]