Abstract
Background:
As a debilitating neurodegenerative disease, neovascular age-related macular degeneration (nAMD) accounts for more than 90% of severe visual loss or legal blindness among AMD patients. Anti-vascular endothelial growth factor (VEGF) had been applied widely in nAMD treatment. To date, debate regarding efficacy and safety still exists among different anti-VEGF regimens as management of nAMD. To provide substantial evidence for clinical nAMD treatment, this study ranks the priority of anti-VEGF regimens via Bayesian network meta-analysis (NMA), comparing data collected from randomized controlled trials (RCTs).
Methods:
We searched PubMed Central, MEDLINE Ovid, Embase Ovid, ISRCTN, ICTRP and ClinicalTrials. gov from a database established until 1 April 2019 systematically for anti-VEGF regimens. Bayesian NMA with random-effect was conducted to compare efficacy and safety and rank priority of anti-VEGF regimens. The primary efficacy and safety outcomes were the proportion of patients gaining 15 or more letters, and the incidence of arterial thromboembolic (ATC) events. The effect measure is the standard mean difference (SMD), or the odds ratio (OR) with their 95% confidence interval (CI). The study protocol is registered with PROSPERO, number CRD42019132243.
Results:
We obtained 6467 citations and identified 29 RCTs including 13,596 participants; 86% of these trials were low risk or of uncertain risk bias. In NMA, ORs compared with sham injection for the proportion of patients gaining 15 or more letters (12,699 participants from 23 trials) ranged from 4.05 [95% Bayesian credible interval (CrI) 1.62–10.11] for ranibizumab quarterly regimen to 8.57 (95% CrI 4.66–15.73) for a ranibizumab treat-and-extend regimen. No difference was found between sham injection and anti-VEGF regimens for ATC events (11,500 participants from 18 trials). Results for the primary outcome did not substantially change in sensitivity analyses after removing studies at high risk of bias and small sample size (n < 100), respectively.
Conclusion:
The treat-and-extend regimen of ranibizumab and aflibercept are the preferred anti-VEGF regimens for nAMD. Bevacizumab treat-and-extend regimens need more head-to-head comparisons with other regimens or sham injection for advanced application. The treat-and-extend regimen proved to be the most effective regimen for each anti-VEGF drug in the NMA. Pegaptanib every 6 weeks and Conbercept quarterly are unable to satisfy the best corrected visual acuity (BCVA) improvement requirement of nAMD patients.
Keywords: anti-VEGF, regimen, Bayesian meta-analysis, neovascular macular degeneration
Introduction
Age-related macular degeneration (AMD) – a debilitating neurodegenerative disease – causes progressive compromise of the macular region.1 With a global prevalence of 8.7%, projected cases of any AMD are 196 million globally in 2020,2 with an expected increase to 288 million in 2040, with most cases occurring in Asia (113 million in 2040).3 At the early stages of AMD, drusen can be observed in the macular region, and advanced stages of AMD may manifest as non-neovascular or neovascular. Neovascular age-related macular degeneration (nAMD), characterized by choroidal neovascularization, accounts for only 4–7% of the overall prevalence of AMD,2 but is responsible for more than 90% of severe visual loss or legal blindness caused by AMD.4–6 Choroidal neovascularization (CNV) deriving from the choriocapillaris toward the retinal neurosensory layer underneath the macula can cause retinal pigment epithelium (RPE) and Bruch’s membrane breakdown, and subretinal bleb detachment bleeding.7 During pathogenesis of CNV, ischemia and hy-poxia induce the expression of hypoxia-inducible factor-1α (HIF-1α), which transcribes vascular endothelial growth factor (VEGF).7,8 Overexpres-sion of VEGF plays a central role in CNV growth. Before anti-VEGF drug clinical application, Verteporfin photodynamic therapy (PDT) was the main approach for CNV treatment.9 Substantial studies have showed the efficacy of Verteporfin PDT in averting significant visual acuity (VA) loss.10,11 However, studies also demonstrate the incompetence of PDT for VA improvement.10 Targeting VEGF therapy, which can control or slow down VA loss and even restore or improve VA, has also been a landmark for nAMD treatment.4,12–15
Anti-VEGF drugs licensed by the United States Food and Drug Administration (FDA) or that have undergone phase III trials are classified into three main forms12,16–20: ribonucleic acid aptamers, such as Pegaptanib (Macugen; Eyetech Pharmaceuticals, New York); humanized monoclonal antibodies, such as ranibizumab (Lucentis; Genentech, South San Francisco, California, USA), bevacizumab (Avastin; Genentech, South San Francisco, California, USA), and brolucizumab (ESBA1008 and RTH258, Novartis Pharmaceuticals Corporation; Basel, Switzerland); and soluble decoy receptor fusion proteins, such as aflibercept (Eylea; Regeneron, Tarrytown, New York, USA) and conbercept (Lumitin; Chengdu Kanghong Biotech Co, Ltd, Chengdu, China). However, limited by rare but severe adverse effects and burden of frequent visits, pharmacotherapy seems to be unsustainable for some patients. Research priorities for anti-VEGF for nAMD treatment are to maintain efficacy while reducing the frequency of clinic injections and visits.
Beside the common monthly protocol, pro re nata (PRN) consists mainly of three consecutive monthly injections with subsequent monthly visits, with re-treatment not being applied until disease activity was detected.21–23 For aflibercept and brolucizumab, a bimonthly protocol (every 8 weeks) is mainly applied.24,25 As for trend-and-extend (treat-and-extend regimen) protocol, after monthly loading doses PRN, the interval between each subsequent visit was extended by 2 weeks (intervals of 6 weeks, 8 weeks, 10 weeks, and a maximum of 12 weeks) until disease instability, whereupon injection was applied every visit.26–28 A quarterly protocol proved to be maintainable for treatment efficacy and was used to significantly decrease treatment frequency.29,30 With the increased prevalence of nAMD, an advance understanding of the benefits and potential risks of different regimens is essential for clinical decision making. Ranibizumab and aflibercept, whose commencement, switching, and stopping circumstances have also been stated in detail, have been recommended as first options for the treatment of nAMD by the National Institute for Health and Clinical Excellence.31 However, this guidance did not set out the frequency and rank of anti-VEGF drugs. Due to the lack of direct comparison, differences in efficacy and safety between anti-VEGF regimens cannot be estimated. Based on their consistency and transitivity, network meta-analysis (NMA) provide a means to synthesize direct (comparison of regimens assessed within the same trials) and indirect (comparisons of regimens across different trials with a common comparator) evidence to allow comparison of different regimen options.32
We compared and ranked anti-VEGF regimens for nAMD by quantifying data from randomized controlled trials (RCTs) and ranked the priority of anti-VEGF regimens to provide statistical evidence for clinical practice.
Methods
A study protocol was designed according to PRISMA guidelines for network meta-analysis,33 and the final version of this protocol was registered with PROSPERO, number CRD42019132243. The full dataset is available online.
Search strategy
PubMed Central, MEDLINE Ovid, Embase Ovid, ISRCTN, ICTRP and ClinicalTrials.gov were searched from database inception to 1 April 2019 with no language restriction. The detailed search strategy of each database is described in the Supplemental Material, section 1.
Criteria for considering studies for this review
Types of participants
Adults (⩾50 years) were treatment-naive patients with a primary diagnosis of nAMD, whose baseline BCVA was generally better than 20/500 (Snellen equivalent) assessed using Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity charts.
Types of interventions
The following 15 types of anti-VEGF regimens were included: pegaptanib every 6 weeks, ranibizumab monthly, ranibizumab quarterly, ranibizumab PRN, ranibizumab treat-and-extend regimen, bevacizumab monthly, bevacizumab PRN, bevacizumab treat-and-extend regimen, aflibercept monthly, aflibercept bimonthly, aflibercept treat-and-extend regimen, conbercept monthly, conbercept PRN, conbercept quarterly, brolucizumab bimonthly, brolucizumab quarterly, and PDT monotherapy. There was no dosage restriction on different drugs, i.e., the 0.3 mg and 0.5 mg ranibizumab dose were merged into one group, and the 0.5 mg and 2.0 mg doses of aflibercept were also combined. The course of each trial should last more than 12 months. All the anti-VEGF drugs were licensed by the FDA or had undergone phase III trials. Trials that used any other investigational drugs that had not entered phase III clinical trials, or were accompanied with steroids at the time of screening or replacement of the original anti-VEGF regimen during follow up, were excluded.
Types of outcomes
We took the proportion of patients gaining 15 (three ETDRS lines or 0.3 logMAR) or more letters, and the incidence of arterial thromboembolic (ATC) events as our primary efficacy and safety outcomes, respectively, from baseline to month 12. ATC events involve non-fatal myocardial infarction, non-fatal stroke, or death from a vascular cause and including any death from an unknown cause because most deaths in high-risk patients are likely to be due to vascular causes.34
Secondary efficacy outcomes comprised mean change in BCVA from baseline to 12 months, the change in anatomical measurements from baseline to 12 months, including reductions in central retinal thickness (CRT) measured using optical coherence tomography (OCT) and mean change in area of CNV based on fluorescein angiography (FA). In addition, secondary safety outcomes represented by the incidence of severe ocular adverse events (SOAEs) such as endophthalmitis, traumatic cataract, retinal detachment, and vitreous hemorrhage, from baseline to 12 months were recorded.
The end point for evaluation of the previously mentioned outcomes was 54 weeks after first treatment.
Types of study design
Included studies were published or unpublished RCTs with parallel group or crossover designs. Conference abstracts, editorials, reviews, meta-analyses, and case reports or case series were excluded.
Study screening process
Studies were selected by two review authors (LY and ZJQ) independently for inclusion to ensure reliability. Disagreements between the two review authors were resolved by a third review author (ZXH).
Data extraction and management
Based on a prepared extraction form, two review authors independently extracted the data from the main reports and supplemental materials. Discrepancies were settled by discussion.
The extracted data included study characteristics (including study duration and masking of treatment allocation), patient characteristics (mean age, sex, race distribution, mean baseline BCVA, mean baseline CRT, mean baseline CNV), interventions (anti-VEGF drug groups, and intervention intervals), and outcomes (proportion gaining BCVA more than 15 letters, proportion occurring ATC events, change in BCVA, change in CRT and proportion of SOAEs events occurring). All data extracted were entered Microsoft Excel 2019 by one review author and verified by a second reviewer.
Risk of bias assessment
Two review authors (LY and ZJQ) independently assessed risk of bias in randomized trials in accordance with the Cochrane Collaboration’s risk of bias tool.35 Potential risks of bias come from random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. We classified each domain as low risk of bias, high risk of bias, or unclear. Based on the latter domains, each trial also received an overall study-level score bias assessment. In case of doubt, a discussion was involved.
Reporting bias assessment
We plotted the comparison-adjusted funnel plot to investigate small-study bias at the network level.
Statistical analysis
Pairwise meta-analysis was carried out when at least two studies assessed the same intervention and comparator for a particular outcome. Odds ratio (OR) and 95% confidence intervals (CIs) were used for categorical outcomes, while standardized mean differences (SMD) and 95% CIs represented continuous outcomes. We carried out pairwise meta-analyses by STATA (version 15.0). Heterogeneity in each standard pairwise meta-analysis was conducted by I2 and the between-studies variance estimate obtained by τ2 (Profile likelihood estimator).35 The results were pooled using a random-effects model. A Bayesian framework was performed to conduct network meta-analyses with OpenBUGS (version 3.2.3).36 We calculated summary OR and 95% credible intervals (CrI) for categorical outcomes, along with SMD and 95% CrI for continuous outcomes to estimate the regimens effect size, respectively. Details about the OpenBUGS codes used can be seen in the Supplemental material, section 2. The summarized estimates were calculated using Markov Chains Monte Carlo (MCMC) methods.36 Three Markov chains were run simultaneously with different initial values. To ensure convergence, trace plots were observed. A heterogeneity variance σ – an estimated between-studies standard deviation (SD) in NMA – was used for each outcome.37 Inconsistency between direct and indirect sources of evidence was statistically assessed by globally and locally (by computing difference between direct and indirect estimates in each closed loop in the network). In order to separate the evidence of each comparison into indirect and direct evidence, a node splitting method was used. The regimens were ranked basing on the surface under the cumulative ranking curve (SUCRA).38 To summarize the efficacy and safety of all regimens, the resultant rankings are presented by clustered ranking plot.
Sensitivity analysis
We performed sensitivity analyses to assess the robustness of each result by removing small size trials (less than 100) or trials with high overall risk of bias.
Results
Literature search
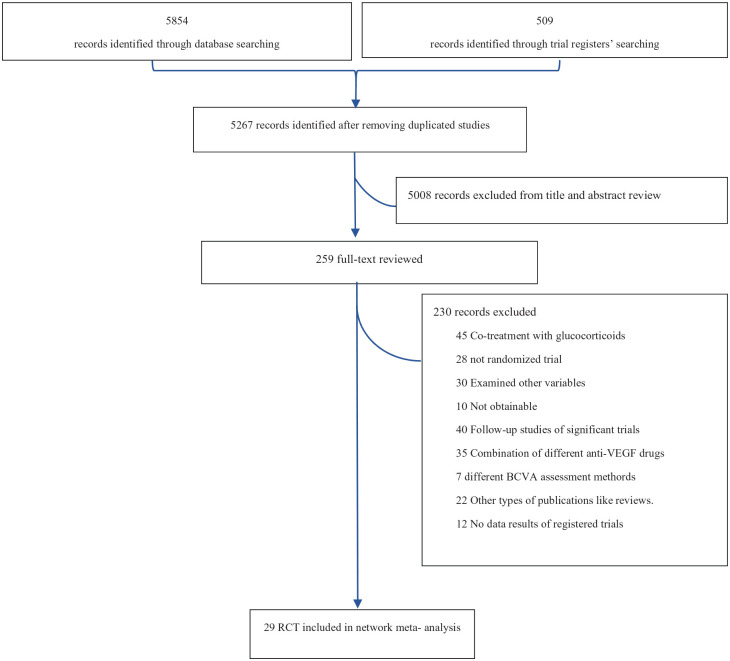
Overall, a total of 6467 records were identified by the database search, and 259 potentially eligible citations were selected for full-text review. After excluding 230 reports, 29 trials with 13,596 participants (references for all included trials are given in the Supplemental Material, section 3) fulfilled our inclusion criteria and were included in our NMA. The process is illustrated by a flow diagram (Figure 1).
Figure 1.
Flow diagram of study selection.
BCVA, best corrected visual acuity; RCT, randomized controlled trial; VEGF, vascular endothelial growth factor.
A total of 27 records published between 2004 and 2019 were included; 13 trials (45%) were conducted mainly in the United States (US), 9 (31%) in Europe, 6 (21%) in Asia, and 1 (3%) in Australia. A total of 18 multicenter RCTs recruited patients from the US or Europe. Six studies (20%) contained participants of predominantly Mongolian race, whereas the rest had mostly Caucasian patients. The mean sample size of the included studies was 159 participants (range 22–2457).
Regarding participants, the included records recruited 13,596 patients (mean age 74 years) and 56% (n = 7679) were female. The median baseline BCVA across studies was 56.7 letters [interquartile range (IQR) = 52.5–60.6]. Female proportion (p = 0.99), baseline BCVA (p = 0.98), and mean age (p = 0.99) were similar across included trials. Participants with polypoidal choroidal vasculopathy (PCV) were involved in 17 trials.
These studies covered PDT and 15 different regimens for six anti-VEGF drugs. Of 153 possible comparisons between included treatments, 24 were compared directly in the identified studies. The follow-up time of all included trials was more than 1 year, and we choose month 12 as the end point for evaluation. Re-treatment criteria for PRN or treat-and-extend regimens were quite similar among different studies, such as BCVA decreased more than five letters compared with the preceding examination; intraretinal/subretinal fluid or new hemorrhage on OCT; dye leakage, or increased lesion size on FA; increase in OCT central retinal thickness of at least 50 μm (detailed information for each trial is presented in the Supplemental Material, section 4).
The percentage of studies with high risk of bias for each individual domain was: 17.2% for allocation concealment, 27% for blinding of participants and personnel, 10.7% for blinding of outcome assessment, and 7% for missing information. Supplemental Material sections 5 and 6 present detail assessment of risk of bias items results and their supporting statements. As for overall risk of bias, 86% of these trials were rated as low risk or uncertain risk bias.
Pairwise meta-analysis
Supplemental material, section 7 presents the detailed results of the pairwise meta-analysis and heterogeneity estimates.
In brief, pegaptanib every 6 weeks was more efficacious than sham injections in terms of primary efficacy outcome (proportions of patients with gain of three or more BCVA lines: OR 2.68, 95% CI 1.17–6.085; τ2 = 0; I2 = 0%, p = 0.021). Ranibizumab monthly showed greater BCVA improvement compared with ranibizumab PRN (SMD 0.118, 95% CI 0.031–0.208, τ2 = 0; I2 = 0%, p = 0.008). For mean change in CNV, ranibizumab monthly was better than ranibizumab PRN (SMD –0.191, 95% CI –0.288 to –0.095, τ2 = 0; I2 = 0%, p = 0.000). And no differences were found between active treatments and sham therapy for ATC and SOAEs events. Furthermore, significant heterogeneity (τ2 = 0.021; I2 = 64.4%) was found in ranibizumab monthly versus a ranibizumab treat-and-extend regimen in terms of BCVA change.
Network meta-analysis
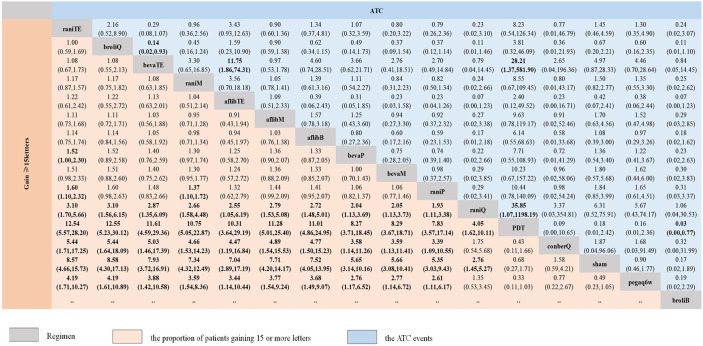
Figure 2 presents the results of the NMA for the primary outcome of efficacy (the proportion of patients gaining 15 or more BCVA letters) and safety (incidence of ATC events). The primary outcome of efficacy results contains 105 treatment arms made up of 51 data points (Figure 3).
Figure 2.
Network meta-analysis of primary efficacy and safety outcomes.
Regimens are reported in order of patients’ proportion gaining 15 or more letters ranking according to SUCRAs. Summary OR and 95% CrI for categorical outcomes to estimate the treatment effect size.
afflibB, aflibercept Bimonthly; aflibM, aflibercept Monthly; aflibTE, aflibercept treat-and-extend regimen; ATC, arterial thromboembolic; bevaM, bevacizumab Monthly; bevaP, bevacizumab PRN; bevaTE, bevacizumab treat-and-extend regimen; broliB, brolucizumab Bimonthly; broliQ, brolucizumab Quarterly; conberM, conbercept Monthly; conberP, conbercept PRN; conberQ, conbercept Quarterly; CrI, credible intervals; OR, odds ratio; Pegaq6w, pegaptanib every 6 weeks; raniM, ranibizumab Monthly; raniP, ranibizumab PRN; raniQ, ranibizumab Quarterly; raniTE, ranibizumab treat-and-extend regimen; SUCRA, surface under the cumulative ranking curve.
Figure 3.
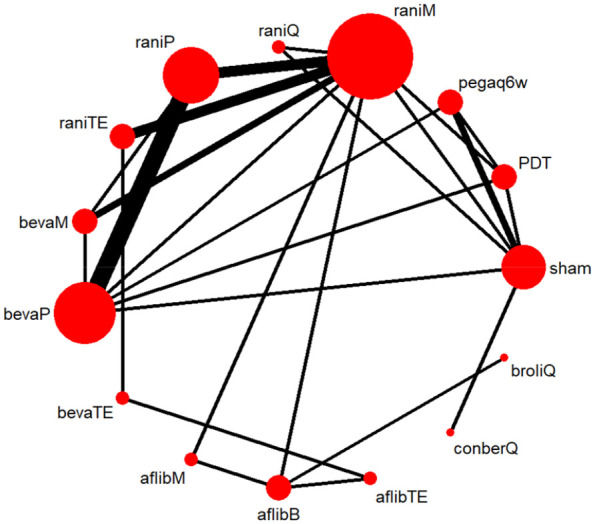
Network plot of available treatment comparisons for primary efficacy outcome.
Size of node represent the number of patients randomized to each regimen. Line width represent the number of RCTs comparing each pair of regimens directly.
afflibB, aflibercept Bimonthly; aflibM, aflibercept Monthly; aflibTE, aflibercept treat-and-extend regimen; bevaM, bevacizumab Monthly; bevaP, bevacizumab PRN; bevaTE, bevacizumab treat-and-extend regimen; broliQ, brolucizumab Quarterly; conberQ, conbercept Quarterly; Pegaq6w, pegaptanib every 6 weeks; raniM, ranibizumab Monthly; raniP, ranibizumab PRN; raniQ, ranibizumab Quarterly; raniTE, ranibizumab treat-and-extend regimen; RCT, randomized controlled trial.
Compared with sham injection for efficacy (12,699 participants from 23 trials) ORs ranged from 4.05 (95% CrI 1.62–10.11) for ranibizumab quarterly to 8.57 (95% CrI 4.66–15.73) for ranibizumab treat-and-extend regimen. In pair-wise comparisons between two anti-VEGF regimens, the response rates for primary efficacy outcome compared with conbercept quarterly in 10 of 14 regimens (71%) were found to be heightened significantly, ranging from 3.39 (95% CrI 1.09–10.55) for ranibizumab PRN to 5.44 (95% CrI 1.71–17.25) for ranibizumab treat-and-extend regimen. Compared with a pegaptanib every 6 weeks regimen, the same 10 of 14 regimens (71%) were significantly more effective, with ORs ranging from 2.61 (95% CrI 1.11–6.17) for ranibizumab PRN to 4.19 (95% CrI 1.71–10.27) for ranibizumab treat-and-extend regimen. The OR for the 10 regimens associated with a higher proportion of patients gaining 15 or more BCVA letters compared with ranibizumab quarterly ranged from 1.93 (95% CrI 1.11–3.38) for ranibizumab PRN regimen to 3.10 (95% CrI 1.70–5.66) for ranibizumab treat-and-extend regimen. In addition, both ranibizumab treat-and-extend regimen (OR 1.60, 95% CrI 1.10–2.32) and ranibizumab monthly (OR 1.37, 95% CrI 1.10–1.72) were superior to ranibizumab PRN regimen in efficacy. Further, 11 of the 14 regimens (79%) had significantly higher rates than the PDT group concerning the proportion of patients gaining 15 or more letters, with ORs ranging from 4.05 (95% CrI 1.62–10.11) for ranibizumab quarterly to 12.5 (95% CrI 5.57–28.20) for the ranibizumab treat-and-extend regimen. The highest probability of being most efficacious in terms of primary efficacy outcome was the ranibizumab treat-and-extend regimen (SUCRA 86.7%), whereas pegaptanib every 6 weeks (SUCRA 3.2%) was lowest (Supplemental Material, section 10.1).
A total of 18 studies with 11,500 participants reported usable data concerning the primary outcome of safety results (incidence of ATC events), with 120 treatment arms containing 16 regimens available (Figure 3). None of the anti-VEGF regimens were significantly different from sham injections in safety, whereas the bevacizumab treat-and-extend regimen had a significantly lower incidence of ATC events than that of brolucizumab quarterly (OR 0.14, 95% CrI 0.02–0.93) or the aflibercept treat-and-extend regimen (OR 11.75, 95% CrI 1.86–74.31). With respect to ranking probabilities, the bevacizumab treat-and-extend regimen (SUCRA 87.5%) had the highest mean ranks, whereas brolucizumab quarterly (SUCRA 12.7%) possessed the lowest ranks (Supplemental Material, section 10.2).
Supplemental Material, section 9 presents secondary outcomes results.
A total of 10,588 participants from 22 studies presented usable mean BCVA change data. Compared with sham injection, the SMDs for 13 regimens were associated with significant BCVA improvement, ranging from 0.82 (95% CrI 0.38–1.26) for ranibizumab quarterly to 1.16 (95% CrI 0.58–1.74) for ranibizumab treat-and-extend regimen. The effect sizes of 12 regimens that significantly improved BCVA compared with conbercept quarterly ranged from 0.73 (95% CrI 0.11–1.35) for ranibizumab quarterly to 1.07 (95% CrI 0.34–1.80) for ranibizumab treat-and-extend regimen. Moreover, 13 (76.4%) anti-VEGF regimens increased BCVA significantly compared with PDT, ranging between 0.82 (95% CrI 0.38–1.26) for ranibizumab quarterly to 1.11 (95% CrI 0.77–1.45) for the ranibizumab treat-and-extend regimen. Based on SUCRA plots, the ranibizumab treat-and-extend regimen (SUCRA 77.7%) had the highest mean ranks, whereas the conbercept quarterly regimen (SUCRA 11.8%) and PDT (SUCRA 5.3%) had the lowest ranks.
A total of 18 studies with 9223 participants presented data of mean CRT change. A brolucizumab quarterly regimen significantly reduced CRT compared with a conbercept PRN regimen (SMD –0.31, 95% CrI –0.41 to –0.20). In addition, a bevacizumab PRN regimen was inferior to ranibizumab monthly (SMD –0.17, 95% CrI –0.29 to –0.05), aflibercept bimonthly (SMD –0.17, 95% CrI –0.33 to –0.01), and a ranibizumab PRN regimen (SMD –0.12, 95% CrI –0.22 to –0.01). Brolucizumab quarterly (SUCRA 75.1%) had the highest mean ranks, whereas bevacizumab PRN regimen (SUCRA 20.5%) had the lowest ranks.
Only 8 studies with 6117 participants reported usable result for mean change in CNV area. The SMDs for the eight (80%) anti-VEGF regimens that significantly reduced CNV area ranged from –0.90 (95% CrI –1.30 to –0.50) for aflibercept monthly to –0.44 (–0.81 to –0.06) to a conbercept quarterly regimen. Aflibercept monthly regimen (SUCRA 81.6%) had the highest mean ranks, whereas conbercept quarterly regimen (SUCRA 34%) and PDT (SUCRA 8.9%) had the lowest ranks.
A total of 11,500 participants from 17 trials reported usable result for the rates of SOAEs. No significant difference was found between active regimens or sham injection.
The findings of SUCRA for the secondary outcomes are presented in Supplemental Material, section 10.3-6.
Efficacy versus safety in network analysis
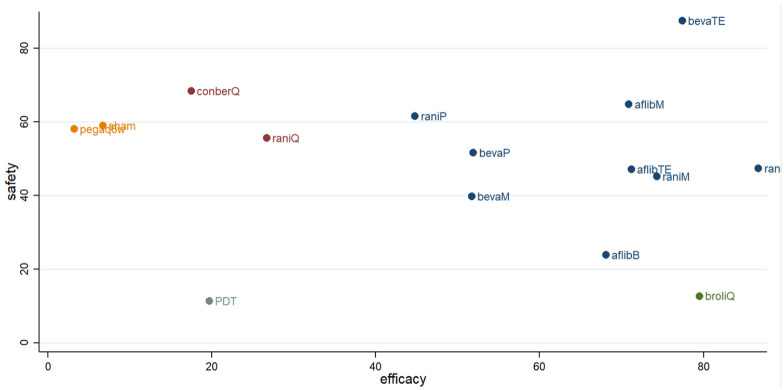
A clustered ranking plot for both primary efficacy and safety results indicated that the higher frequency injection regimens were better for efficacy and worse for safety, as most of them lay in the lower right corner. Among included anti-VEGF regimens, the bevacizumab treat-and-extend regimen was the most efficacious and safest regimen in this analysis (Figure 4).
Figure 4.
Clustered ranking plot of nAMD regimens network based on primary efficacy and safety outcomes.
Each color represents a group of regimens that belong to the same cluster. Regimens lying in the upper right corner are more effective and acceptable than the other regimens.
afflibB, aflibercept Bimonthly; aflibM, aflibercept Monthly; aflibTE, aflibercept treat-and-extend regimen; bevaM, bevacizumab Monthly; bevaP, bevacizumab PRN; bevaTE, bevacizumab treat-and-extend regimen; broliQ, brolucizumab Quarterly; conberQ, conbercept Quarterly; nAMD, neovascular age-related macular degeneration; Pegaq6w, pegaptanib every 6 weeks; raniM, ranibizumab Monthly; raniP, ranibizumab PRN; raniQ, ranibizumab Quarterly; raniTE, ranibizumab treat-and-extend regimen.
Inconsistency
The common heterogeneity σ was 0.15 (95% CI 0.006–0.424) for the proportion of patients gaining 15 or more BCVA letters, 0.35 (95% CI 0.014–1.207) for the incidence of ATC events, 0.16 (95% CI 0.056–0.332) for BCVA change, 0.09 (95% CI 0.003–0.330) for reductions in CRT, 0.16 (95% CI 0.003–0.739) for area of CNV change, and 1.6 (95% CI 0.086–4.306) for SOAEs.
The test of global inconsistency (Supplemental Material, section 11.1) did not detect any evidence of statistically significant inconsistency for primary and secondary outcomes (global inconsistency: p = 0.2–0.63). Tests of local inconsistency reported that inconsistency was found only in the triangle-loop aflibercept bimonthly-ranibizumab monthly-PDT (IF 0.43 95% CI 0.05–0.82) for mean BCVA change (Supplemental Material, section 11.2). The test of inconsistency from the node-splitting model presented significant differences between five comparisons (19.2%) in primary efficacy (p < 0.1), but not for safety (Supplemental Material, section 11.3).
No publication bias was found in comparison-adjusted funnel plots of the NMA for any outcomes (Supplemental Material, section 12).
Sensitivity analysis
Supplemental Material, section 13 presents the results of the sensitivity analyses for primary and secondary outcomes. For primary efficacy outcomes, excluding studies at high risk of bias and small sample size (n < 100) studies results in the aflibercept treat-and-extend regimen no longer being associated with higher efficaciousness compared with ranibizumab quarterly. Aflibercept monthly increased the rates of occurrence of ATC events compared with brolucizumab quarterly regimen (0.37, 95% CrI 0.17–0.82) after removing studies at high risk of bias. Similarly, when we excluded small sample size studies, aflibercept monthly was associated with a lower likelihood of ATC events than aflibercept bimonthly (OR 1.68, 95% CrI 1.01–2.8), while significant differences between the bevacizumab treat-and-extend and aflibercept treat-and-extend regimens no longer exist.
Discussion
Based on 29 clinical trials involving 13,596 participants with nAMD randomly assigned to PDT, sham injection, or anti-VEGF regimens, this analysis is the largest NMA of pharmacological regimens in the field of nAMD treatment. The quality of the evidence was typically of low or unclear risk of bias (25 out of 29 trials; 86%). Most results for primary outcomes did not change substantially in sensitivity analyses, supporting the robustness of the findings.
Ranibizumab monthly was the first FDA-approved anti-VEGF regimen used widely in clinical practice for nAMD. Hence, it has often been used as a noninferior control group for new anti-VEGF regimens.26,31,39,40 In this NMA, the effects of ranibizumab monthly were also shown to be beneficial for improving BCVA gains of nAMD. Yet, rare but severe adverse effects and the burden of frequent visits have limited its use in clinical practice. Balancing benefits and relative risks for each regimen is a crucial issue in choosing nAMD regimen options in clinical practice.
Given the shortage of head-to-head research, direct efficacy comparisons were hard to apply between quite a few anti-VEGF regimens. This analysis provided indirect evidence via transitivity of one of the basal characteristics for NMA, i.e., the comparison between ranibizumab treat-and-extend regimen and sham injection.
Based on five RCTs with 2012 participants, the ranibizumab treat-and-extend regimen topped the hierarchy in efficacy among included anti-VEGF regimens. Compared with ranibizumab monthly, the ranibizumab treat-and-extend regimen with lower treatment frequency was superior in efficacy, giving it an even higher ranking.
These results also filled the gaps of meta-analysis between the two regimes previously mentioned. Further, the ranibizumab treat-and-extend regimen, monthly regimen, or PRN regimen were significantly more effective than the quarterly regimen, which may indicate the existence of a certain optimal treatment frequency threshold. Once treatment frequency reaches suprathreshold, the ability to treat nAMD would significantly decrease or even become invalid. The quarterly regimen interval was too long to maintain stable nAMD treatment. Although the monthly ranibizumab regimen was significantly more efficacious than the PRN regimen in our NMA, pairwise meta-analysis found no substantial difference between these two regimens.41 This evidence suggests not only that the difference between the two ranibizumab regimens may be masked by the effects of small sample size studies, but also that proactive regimens are preferred over reactive regimens in improving BCVA. Regarding mean BCVA change from baseline to month 12, the ranibizumab treat-and-extend regimen showed significant BCVA improvement and had the highest priority among anti-VEGF regimens, which was consistent with the primary efficacy outcome. However, no difference in BCVA change was found between treat-and-extend regimen and PRN of ranibizumab. Besides, ranibizumab treat-and-extend regimen was not found to be superior to a quarterly regimen. The distinction between BCVA change and primary efficacy outcome might be attributable to different information content caused by variable type.
The bevacizumab treat-and-extend regimen tended to have lower hierarchy than the ranibizumab treat-and-extend regimen for primary effective outcome, while having higher hierarchy than ranibizumab monthly and most other regimens in primary efficacy outcome. Our analysis found that the higher efficacy bevacizumab treat-and-extend regimen possessed the highest safety rank of all regimens. Nevertheless, these findings originated from only one study involving 221 participants, and was limited in clinical application owing to small sample sizes. Larger placebo-controlled and head-to-head studies with other regimens are still desperately needed to confirm these findings. Moreover, no evidence suggested any difference between treat-and-extend, monthly and PRN regimens of bevacizumab, and the priority of the first two regimens in improving the ratio of BCVA gains of more than 15 letters is higher than the monthly regimen of bevacizumab. Unlike the work of the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) research group,42–44 this evidence implied that properly extending the interval of bevacizumab injection may lead to better BCVA results. Furthermore, similar evidence was also found in mean BCVA change.
In accordance with the HAWK and HARRIER studies,45,46 brolucizumab quarterly, a novel anti-VEGF regimen, was far more efficacious than the sham injection group, with a relative priority of primary efficacy outcome second only to the ranibizumab treat-and-extend regimen. As a single-chain antibody fragment (scFv), the molecular weight of brolucizumab is 26 kDa, which is far smaller than ranibizumab (48 kDa) or bevacizumab (149 kDa). The lower molecular weight, accompanied by a higher concentration gradient between the vitreous and retina, increases the distribution of the drug to the target site, resulting in more effective control of lesions. Even so, rates of ATE events were high with the brolucizumab quarterly regimen, which suggests that a longer injection interval would be appropriate, and would reduce adverse effects while maintaining efficacy. In addition, clinical experience with brolucizumab was potentially limited to short head-to-head comparisons with other anti-VEGF drugs. More multicenter RCTs are needed to provide more direct evidence. Concerning BCVA change, both bimonthly and quarterly regimen of brolucizumab were also rated significantly higher than sham injection.
On the basis of our research, no difference in efficacy was found among treat-and-extend, monthly and bimonthly regimens of aflibercept, whereas the aflibercept treat-and-extend regimen ranked higher in efficacy over the latter two. Furthermore, the aflibercept treat-and-extend regimen is not inferior to ranibizumab monthly either, and the efficacy of conbercept quarterly cannot be distinguished from sham injection, with data from only 162 participants. Previous meta-analyses in nAMD had stated that conbercept was more efficacious and safer compared with ranibizumab when neglecting treatment frequency.4,47,48 Whereas conbercept quarterly was significantly inferior in terms of efficacy to all types of ranibizumab regimen except ranibizumab quarterly regimen in our NMA. The evidence mentioned previously can also be found in mean BCVA change. These findings indicate that our NMA strengthens the evidence base for treatment frequency influence on nAMD. Analyzing SUCRA results of humanized monoclonal antibody and soluble decoy receptor fusion protein regimens, respectively, showed that, on average, humanized monoclonal antibody regimens (61.6%) are generally more preferred than soluble decoy receptor fusion protein regimens (56.9%) for nAMD treatment, which might be linked to the more head-to-head nature of research into most humanized monoclonal antibody drugs, which may correct errors in a network frame context.
With regard to anatomical measurements, the ranking of different regimens for CRT reduction was different from the primary efficacy outcome. This finding may be attributed to the absence of studies concerning anatomical data for the sham injection group in this review. Few data directly address CRT morphological correlates to VA decline, which matching several previous studies.13,41,49,50 Only ranibizumab monthly, ranibizumab PRN, and aflibercept bimonthly regimen were significantly more effective than sham injection for mean CRT change. The corresponding SMDs were <0.2 (considered to be a small effect size),51–53 which is close to the point of no difference. CNV plays an essential role in nAMD generation, but CNV-related data were insufficient in many studies. If reported, anti-VEGF regimens, especially aflibercept monthly, aflibercept bimonthly and ranibizumab monthly, whose SMDs were >0.8 (considered to be large effect sizes), showed significant differences from sham injection. As a result, anti-VEGF regimens play a different role in change of CNV area and CRT, which suggests that CRT in nAMD is not determined entirely by the area of CNV leakage. CRT change may also implicate the morphological disruption and dysfunction of the inner or outer blood-retinal barrier during the development of disease.54–58
There was no significant difference between pegaptanib every 6 weeks and the sham injection group in terms of efficacy and safety, which differs from the results of the VISION trials.17,59 This finding, together with National Institute for Health and Care Excellence (NICE) guidance, indicated that pegaptanib is not recommended for the treatment of nAMD.31
With frequent injection visits together with potential serious ocular side-effects, patient compliance may be lowered. Theoretically, the incidence of SOAEs with anti-VEGF regimens always surpassed that of the sham injection group, whereas in this study no significant difference was observed between them.
Further, the burden of different drugs also accounts for loss of follow up. Per-dose cost is approximately US $772.94 per syringe, per pill, or unit, for pegaptanib; US $1216.65 per syringe, per pill, or unit for ranibizumab; US $1920.04 per vial, per pill, or unit, for aflibercept; and US $50 for bevacizumab.60–62 According to the model developed by Kuopio University Hospital,63 the direct medical costs include: (a) the costs of diagnosis including medical visit, FA, and OCT; (b) the cost of intravitreal injection of the medication; (c) the costs of follow up, including a medical visit and OCT; and (d) rehabilitation of legally blind persons. For one patient, a 6-week regimen with pegaptanib costs US $7729.40 per year, while bevacizumab PRN costs US $1610.40, ranibizumab PRN costs US $16,687.70, bevacizumab monthly costs US $2924.60, ranibizumab monthly US $25,759.20, aflibercept monthly costs US $13,954.70, aflibercept bimonthly costs US $6977.3, bevacizumab treat-and-extend regimen costs US $2167.9, ranibizumab treat-and-extend regimen costs US $18,678.9, and afliber-cept treat-and-extend regimen costs US $11,280.1.
As for efficacy versus safety outcomes, the bevacizumab treat-and-extend regimen was efficacious and well tolerated compared with other regimens, at a cost that can be affordable for most people in low- and middle-income countries. In addition, the treat-and-extend regimen proved to be the most effective regimen for each anti-VEGF drugs in NMA, and strengthens the basis of a previous pair-wise meta-analysis, which stated that the treat-and-extend regimen is superior to monthly or PRN regimen in both efficacy and safety outcomes. This evidence strengthened the view that proactive therapy is preferred over reactive therapy.
Verteporfin PDT cannot improve BCVA in our analysis. A conceivable explanation is that PDT impacts the choriocapillaris bed surrounding the CNV lesion, which may cause hypoxia, leading to upregulation of VEGF and stimulation of further CNV growth.10,64 Likewise, the evidence that the effect of PDT and anti-VEGF combination therapy, which can potentially decrease the number of anti-VEGF injections needed to be effective for achieving BCVA gain and CRT reduction, is comparable with that of anti-VEGF monotherapy had been indicated in several studies.9,11,65,66 Our analysis includes several unpublished studies, which reduced publication bias.
Our study has some limitations. It was impossible to run subgroup and network meta-regression analyses to address whether age, publication year, baseline BCVA, sex, or race issues affected the results due to the limited number of studies for each subgroup. Some evidence of statistical heterogeneity (I2 64%, τ² 0.021) within pairwise comparisons was detected, while one of the corresponding loops in our NMA for BCVA change was inconsistent, suggesting that inconsistency between direct and indirect comparisons might be related to heterogeneity as indicated in a previous study. Other key information, such as the incidence of macular atrophy or fibrosis, were generally not well reported in these studies. Such data represents the potential long-term influence on nAMD prognosis.
Conclusion
This comprehensive Bayesian NMA provides substantial evidence for the clinical application of anti-VEGF drug regimens for nAMD. The treat-and-extend regimen of ranibizumab and aflibercept are the preferred anti-VEGF regimens for nAMD. The bevacizumab treat-and-extend regimen needs more head-to-head comparisons with other regimens or sham injection for advanced application. The treat-and-extend regimen proved to be the most effective for all the anti-VEGF drugs in this NMA. Pegaptanib every 6 weeks and Conbercept quarterly are unable to satisfy the BCVA improvement required by nAMD patients.
Supplemental Material
Supplemental material, supplementary for Comparative efficacy and safety of anti-vascular endothelial growth factor regimens for neovascular age-related macular degeneration: systematic review and Bayesian network meta-analysis by Lu Ye, Zhao Jiaqi, Wang Jianchao, Feng Zhaohui, Yao Liang and Zhang Xiaohui in Therapeutic Advances in Chronic Disease
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Contributors: LY conceived the research, participated in pilot testing, screening, and the data abstraction forms, helped conceptualize the analysis, interpreted the data, and wrote the first draſt of the manuscript. LY and ZJQ independently performed the literature search and conducted the risk of bias assessment, and prepared the tables and figures. LY analyzed and interpreted the data with input from FZH, WJC, and YL. ZXH coordinated the study, resolved discrepancies, verified data accuracy and analysis. ZXH and FZH reviewed the manuscript. LY is guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zhang Xiaohui  https://orcid.org/0000-0001-9020-5410
https://orcid.org/0000-0001-9020-5410
Registration number: Prospero CRD42019132243.
Supplemental material: Supplemental material for this article is available online.
Transparency declaration: The lead author (LY) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Contributor Information
Lu Ye, Ophthalmology Department, The Second Affiliated Hospital, Medical College, Xi’an Jiaotong University, Xi’an, PR China.
Zhao Jiaqi, Ophthalmology Department, The Second Affiliated Hospital, Medical College, Xi’an Jiaotong University, Xi’an, PR China.
Wang Jianchao, Ophthalmology Department, The Second Affiliated Hospital, Medical College, Xi’an Jiaotong University, Xi’an, PR China.
Feng Zhaohui, Ophthalmology Department, The Second Affiliated Hospital, Medical College, Xi’an Jiaotong University, Xi’an, PR China.
Yao Liang, Ophthalmology Department, The Second Affiliated Hospital, Medical College, Xi’an Jiaotong University, Xi’an, PR China.
Zhang Xiaohui, Ophthalmology Department, The Second Affiliated Hospital, Medical College, Xi’an Jiaotong University, No. 157 Xiwu Road, Xi’an, 710004, PR China.
References
- 1. Maugeri A, Barchitta M, Fallico M, et al. Characterization of SIRT1/DNMTs functions and LINE-1 methylation in patients with age-related macular degeneration. J Clin Med 2019; 8: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong WL, Su XY, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014; 2: 106–116. [DOI] [PubMed] [Google Scholar]
- 3. Ng ALK, Leung HH, Kawasaki R, et al. Dietary habits, fatty acids and carotenoid levels are associated with neovascular age-related macular degeneration in Chinese. Nutrients 2019; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L, Zhang C, Hua R. Clinical effectiveness of ranibizumab and conbercept for neovascular age-related macular degeneration: a meta-analysis. Drug Des Devel Ther 2018; 12: 3625–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moschos MM, Nitoda E. The role of mf-ERG in the diagnosis and treatment of age-related macular degeneration: electrophysiological features of AMD. Semin Ophthalmol 2018; 33: 461–469. [DOI] [PubMed] [Google Scholar]
- 6. Colijn JM, Buitendijk GHS, Prokofyeva E, et al. Prevalence of age-related macular degeneration in Europe. Ophthalmology 2017; 124: 1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao M, Mantel I, Gelize E, et al. Mineralocorticoid receptor antagonism limits experimental choroidal neovascularization and structural changes associated with neovascular age-related macular degeneration. Nat Commun 2019; 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J, Tu Y, Wang Y, et al. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1alpha/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomed Pharmacother 2020; 121: 1–9. [DOI] [PubMed] [Google Scholar]
- 9. Gallemore RP, Wallsh J, Hudson HL, et al. Combination verteporfin photodynamic therapy ranibizumab-dexamethasone in choroidal neovascularization due to age-related macular degeneration: results of a phase II randomized trial. Clin Ophthalmol 2017; 11: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao Y, Yu T, Zhang Y, et al. Anti-VEGF monotherapy versus photodynamic therapy and anti-VEGF combination treatment for neovascular age-related macular degeneration: a meta-analysis. Invest Ophthalmol Vis Sci 2018; 59: 4307–4317. [DOI] [PubMed] [Google Scholar]
- 11. Hatz K, Schneider U, Henrich B, et al. Comparing ranibizumab monotherapy and combination with single photodynamic therapy in wet AMD: retreatment and morphologic results. Eur J Ophthalmol 2017; 27: 470–475. [DOI] [PubMed] [Google Scholar]
- 12. Guymer RH, Markey CM, McAllister IL, et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology 2019; 126: 723–734. [DOI] [PubMed] [Google Scholar]
- 13. Bailey C, Scott LJ, Rogers CA, et al. Intralesional macular atrophy in anti-vascular endothelial growth factor therapy for age-related macular degeneration in the IVAN trial. Ophthalmology 2019; 126: 75–86. [DOI] [PubMed] [Google Scholar]
- 14. Jaffe GJ, Ying GS, Toth CA, et al. Macular morphology and visual acuity in year five of the comparison of age-related macular degeneration treatments trials. Ophthalmology 2019; 126: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Supuran CT. Agents for the prevention and treatment of age-related macular degeneration and macular edema: a literature and patent review. Expert Opin Ther Pat 2019; 29: 761–767. [DOI] [PubMed] [Google Scholar]
- 16. Virgili G, Parravano M, Evans JR, et al. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev 2018; 10: CD007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyer DS, Goldbaum M, Leys AM, et al. Effect of pegaptanib sodium 0.3 mg intravitreal injections (Macugen) in intraocular pressure: posthoc analysis from V.I.S.I.O.N. study. Brit J Ophthalmol 2014; 98: 1543–1546. [DOI] [PubMed] [Google Scholar]
- 18. Russo A, Scaroni N, Gambicorti E, et al. Combination of ranibizumab and indomethacin for neovascular age-related macular degeneration: randomized controlled trial. Clin Ophthalmol 2018; 12: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Motarjemizadeh Q, Aidenloo NS, Abbaszadeh M, et al. Intravitreal bevacizumab with or without triamcinolone for wet age-related macular degeneration: twelve-month results of a prospective, randomized investigation. Middle East Afr J Ophthalmol 2018; 25: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013; 120: 1046–1056. [DOI] [PubMed] [Google Scholar]
- 21. Kodjikian L, Souied EH, Mimoun G, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology 2013; 120: 2300–2309. [DOI] [PubMed] [Google Scholar]
- 22. Tufail A, Patel PJ, Egan C, et al. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ 2010; 340: 24–59. [DOI] [PubMed] [Google Scholar]
- 23. Muftuoglu IK, Alam M, You QS, et al. Long-term remission of neovascular age-related macular degeneration with as-needed anti-vascular endothelial growth factor therapy. Retina 2018; 38: 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khurana RN, Rahimy E, Joseph WA, et al. 21 Extended (every 12 weeks or longer) dosing interval with intravitreal aflibercept and ranibizumab in neovascular age-related macular degeneration: post hoc analysis of VIEW trials. Am J Ophthalmol 2019; 200: 161–168. [DOI] [PubMed] [Google Scholar]
- 25. Chang A, Warburton J, Weichselberger A, et al. Poster abstracts-Phase III studies comparing the efficacy and safety of brolucizumab vs aflibercept in subjects with neovascular age-related macular degeneration: testing an alternative treatment regimen. Clin Exp Ophthalmol 2016; 44: 123–124. [Google Scholar]
- 26. Silva R, Berta A, Larsen M, et al. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology 2018; 125: 57–65. [DOI] [PubMed] [Google Scholar]
- 27. Kertes PJ, Galic IJ, Greve M, et al. Canadian treat-and-extend analysis trial with ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized Canadian treat-and-extend analysis trial with ranibizumab study. Ophthalmology 2019; 126: 841–848. [DOI] [PubMed] [Google Scholar]
- 28. Berg K, Pedersen TR, Sandvik L, et al. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 2015; 122: 146–152. [DOI] [PubMed] [Google Scholar]
- 29. Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 2011; 118: 831–839. [DOI] [PubMed] [Google Scholar]
- 30. Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol 2010; 150: 315–324. [DOI] [PubMed] [Google Scholar]
- 31. National Institute for Health and Clinical Excellence. Age-related macular degeneration[NG82], NICE guideline, https://www.nice.org.uk/guidance/NG82 (2018). (accessed 13 May 2019). [PubMed] [Google Scholar]
- 32. Slee A, Nazareth I, Bondaronek P, et al. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet 2019; 393: 768–777. [DOI] [PubMed] [Google Scholar]
- 33. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 34. Katz D, Gavin MC. Stable Ischemic heart disease. Ann Intern Med 2019; 171: ITC17–ITC32. [DOI] [PubMed] [Google Scholar]
- 35. Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0. 2017; 8: 3–23. [Google Scholar]
- 36. Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomized controlled trials. National Institute for Health and Care Excellence. Updated September 2016. National Institute for Health and Care Excellence. 2016; 256: 450–518. [PubMed] [Google Scholar]
- 37. Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 2: heterogeneity: subgroups, meta-regression, bias and bias-adjustment. National Institute for Health and Care Excellence. Updated April 2012. National Institute for Health and Care Excellence. 2012; 94: 1–140. [Google Scholar]
- 38. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. Plos One 2013; 8: 54–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abdelfattah NS, Al-Sheikh M, Pitetta S, et al. Macular atrophy in neovascular age-related macular degeneration with monthly versus treat-and-extend ranibizumab: findings from the TREX-AMD trial. Ophthalmology 2017; 124: 215–223. [DOI] [PubMed] [Google Scholar]
- 40. Li X, Zhu A, Egger A, et al. Ranibizumab 0.5 mg for neovascular age-related macular degeneration: monthly versus as needed dosing in the dragon study. Ophthalmologica 2016; 236: 20–21. [Google Scholar]
- 41. Solomon SD, Lindsley K, Vedula SS, et al. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2014; 8: CD005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration the CATT research group. New Engl J Med 2011; 364: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology 2012; 119: 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Comparison of Age-related Macular Degeneration Treatments Trials Research Group, Maguire MG, Martin DF, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology 2016; 123: 1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020; 127: 72–84. [DOI] [PubMed] [Google Scholar]
- 46. Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology 2017; 124: 1296–1304. [DOI] [PubMed] [Google Scholar]
- 47. Cui C, Lu H. Clinical observations on the use of new anti-VEGF drug, conbercept, in age-related macular degeneration therapy: a meta-analysis. Clin Interv Aging 2018; 13: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang J, Liang Y, Xie J, et al. Conbercept for patients with age-related macular degeneration: a systematic review. BMC Ophthalmol 2018; 18: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lass JH, Benetz BA, Menegay HJ, et al. 40 effects of repeated intravitreal aflibercept injection on the corneal endothelium in patients with age-related macular degeneration: outcomes from the RE-VIEW study. Cornea 2018; 37: 596–601. [DOI] [PubMed] [Google Scholar]
- 50. Amarakoon S, Martinez-Ciriano JP, van den Born LI, et al. Bevacizumab in age-related macular degeneration: a randomized controlled trial on the effect of on-demand therapy every 4 or 8 weeks. Acta Ophthalmol 2018; 97: 107–112. [DOI] [PubMed] [Google Scholar]
- 51. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet 2016; 388: 881–890. [DOI] [PubMed] [Google Scholar]
- 52. Leppink J, O’Sullivan P, Winston K. Effect size - large, medium, and small. Perspect Med Educ 2016; 5: 347–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grzybowski A, Mianowany M. Statistics in ophthalmology revisited: the (effect) size matters. Acta Ophthalmol 2018; 96: 885–888. [DOI] [PubMed] [Google Scholar]
- 54. Xia T, Rizzolo LJ. Effects of diabetic retinopathy on the barrier functions of the retinal pigment epithelium. Vision Res 2017; 139: 72–81. [DOI] [PubMed] [Google Scholar]
- 55. Chen P, Miao Y, Yan PJ, et al. MiR-455-5p ameliorates HG-induced apoptosis, oxidative stress and inflammatory via targeting SOCS3 in retinal pigment epithelial cells. J Cell Physiol 2019; 234: 21915–21924. [DOI] [PubMed] [Google Scholar]
- 56. Wu Y, Wei QQ, Yu J. The cGAS/STING pathway: a sensor of senescence-associated DNA damage and trigger of inflammation in early age-related macular degeneration. Clin Interv Aging 2019; 14: 1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Garczorz W, Gallego-Colon E, Kosowska A, et al. Exenatide modulates expression of metalloproteinases and their tissue inhibitors in TNF-alpha stimulated human retinal pigment epithelial cells. Pharmacol Rep 2019; 71: 175–182. [DOI] [PubMed] [Google Scholar]
- 58. Daruich A, Matet A, Moulin A, et al. Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res 2018; 63: 20–68. [DOI] [PubMed] [Google Scholar]
- 59. Gragoudas ES, Adamis AP, Cunningham ET, et al. Pegaptanib for neovascular age-related macular degeneration. New Engl J Med 2004; 351: 2805-2816. [DOI] [PubMed] [Google Scholar]
- 60. The Lancet. Age-related macular degeneration: treatment at what cost? Lancet 2018; 392: 1090. [DOI] [PubMed] [Google Scholar]
- 61. Hernandez L, Lanitis T, Cele C, et al. Intravitreal aflibercept versus ranibizumab for wet age-related macular degeneration: a cost-effectiveness analysis. J Manag Care Spec Ph 2018; 24: 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carrasco J, Pietsch GA, Nicolas MP, et al. Real-world effectiveness and real-world cost-effectiveness of intravitreal aflibercept and intravitreal ranibizumab in neovascular age-related macular degeneration: systematic review and meta-analysis of real-world studies. Adv Ther 2020; 37: 300–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vottonen P. Anti-vascular endothelial growth factors treatment of wet age-related macular degeneration: from neurophysiology to cost-effectiveness. Acta Opthalmologica 2018; 96: 1–46. [DOI] [PubMed] [Google Scholar]
- 64. Ao J, Wood JPM, Chidlow G, et al. Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin Exp Ophthalmol 2018; 46: 670–686. [DOI] [PubMed] [Google Scholar]
- 65. Li X, Chen Y, Zhang J, et al. Intravitreal aflibercept versus photodynamic therapy in Chinese patients with neovascular age-related macular degeneration: outcomes of the sight study. J Ocul Pharmacol Ther 2017; 33: 435–444. [Google Scholar]
- 66. Dong Y, Wan G, Yan P, et al. Effect of anti-VEGF drugs combined with photodynamic therapy in the treatment of age-related macular degeneration. Exp Ther Med 2016; 12: 3923–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplementary for Comparative efficacy and safety of anti-vascular endothelial growth factor regimens for neovascular age-related macular degeneration: systematic review and Bayesian network meta-analysis by Lu Ye, Zhao Jiaqi, Wang Jianchao, Feng Zhaohui, Yao Liang and Zhang Xiaohui in Therapeutic Advances in Chronic Disease