Abstract
Objective:
To review the mechanism of action, mechanisms of resistance, in vitro activity, pharmacokinetics, pharmacodynamics, and clinical data for a novel aminoglycoside.
Data sources:
A PubMed search was performed from January 2006 to August 2019 using the following search terms: plazomicin and ACHN-490. Another search was conducted on clinicaltrials.gov for published clinical data. References from selected studies were also used to find additional literature.
Study selection and data extraction:
All English-language studies presenting original research (in vitro, in vivo, pharmacokinetic, and clinical) were evaluated.
Data synthesis:
Plazomicin has in vitro activity against several multi-drug-resistant organisms, including carbapenem-resistant Enterobacteriaceae. It was Food and Drug Administration (FDA) approved to treat complicated urinary tract infections (cUTIs), including acute pyelonephritis, following phase II and III trials compared with levofloxacin and meropenem, respectively. Despite the FDA Black Box Warning for aminoglycoside class effects (nephrotoxicity, ototoxicity, neuromuscular blockade, and pregnancy risk), it exhibited a favorable safety profile with the most common adverse effects being decreased renal function (3.7%), diarrhea (2.3%), hypertension (2.3%), headache (1.3%), nausea (1.3%), vomiting (1.3%), and hypotension (1.0%) in the largest in-human trial.
Relevance to patient care and clinical practice:
Plazomicin will likely be used in the treatment of multi-drug-resistant cUTIs or in combination to treat serious carbapenem-resistant Enterobacteriaceae infections.
Conclusions:
Plazomicin appears poised to help fill the need for new agents to treat infections caused by multi-drug-resistant Enterobacteriaceae.
Keywords: aminoglycosides, aminoglycoside modifying enzymes (AME), carbapenem-resistant Enterobacteriales, extended-spectrum beta-lactamase, plazomicin
Introduction
Antimicrobial resistance has positioned itself as a serious threat to patient care with global reach. A recent projection from the World Health Organization stated that mortality due to antimicrobial-resistant infections could reach 10 million by 2050, up from ~700,000 currently.1 Some groups have suggested that this projection is a bit inflated,2,3 but regardless, our current situation remains dire. In an effort to raise awareness of this growing crisis, the Centers for Disease Control and Prevention released the Antibiotic Resistance Threats in the United States, 2013.4 Many of the more serious threats listed were multi-drug-resistant (MDR) Gram-negative organisms, including carbapenem-resistant Enterobacteriaceae (CRE), which was assigned the highest level of concern. This remains true in the most recent threats report from 2019, which indicates more work is needed to curb this public health issue.5
The signing of the 21st Century Cures Act and the GAIN (Generating Antibiotic Incentives Now) ACT, which created the qualified infectious disease product (QIDP) indication, has helped to rejuvenate innovation to address antibiotic resistance. Examples of successful QIDP antimicrobials are ceftazidime/avibactam (Avycaz®), meropenem/vaborbactam (Vabomere®), imipenem/cilastatin/relebactam (Recarbrio®), eravacycline (Xerava®). All of these have documented activity against organisms possessing many different resistance phenotypes, including CRE. Another success of the QIDP indication, plazomicin (Zemdri®), looks to add yet another viable option. This article will review the pharmacokinetics/pharmacodynamics, available pre-clinical data, and clinical trials for plazomicin, and discuss its role in therapy.
Search methods
A PubMed search was completed from January 2006 to August 2019 using the search “ACHN-490” or “plazomicin”. All English-speaking studies were collected and evaluated for inclusion in the review. The addition of other search terms, namely resistance phenotypes like “extended-spectrum beta-lactamase”, “carbapenem-resistant Enterobacteriaceae”, or “aminoglycoside modifying enzyme”, did not broaden the search beyond the original search. In addition, a search of clinicaltrials.gov using the search term “plazomicin” was completed to include all available clinical trial data. References cited in published literature were used to identify additional information not included in either of these databases. Also helpful were documentation provided by the FDA website, specifically the package insert and the NDA documentation. Relevant posters and unpublished conference data were also used; however, an exhaustive search for this data was not performed.
Chemistry and mechanism of action
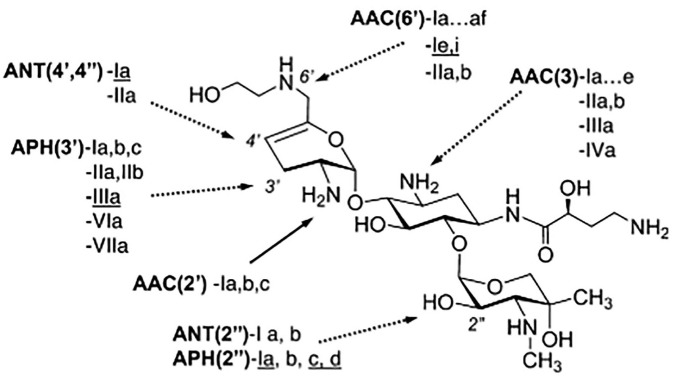
As the name suggests, aminoglycosides are amine-containing sugars linked together by glycosidic bonds. The most clinically relevant aminoglycosides (gentamicin, tobramycin, and amikacin) contain three sugars. Plazomicin is a semi-synthetic aminoglycoside, created in an eight-step synthesis from sisomicin. During this synthesis, a hydroxy-aminobutyric acid (HABA) group is added to the amine at C-1, similarly to amikacin. Uniquely, an additional hydroxyethyl group is added to the amine at C-6′.6 These structural features allow plazomicin to evade almost all clinically relevant aminoglycoside-modifying enzymes (AMEs), as demonstrated in Figure 1.
Figure 1.
Plazomicin structure shown with clinically relevant aminoglycoside-modifying enzyme (AMEs) from both Gram-negative and positive (underlined) organisms. AMEs with a dotted line cannot modify plazomicin. Reproduced from Aggen et al with permission from the American Society of Microbiology.6
Aminoglycosides bind to the aminoacyl-tRNA recognition site (A-site) of the 16S rRNA, which is a component of the 30S ribosomal subunit. This interrupts the elongation of the nascent protein sequence during the translation phase and therefore inhibits ribosomal protein synthesis.7 Since they are cationic, hydrophilic molecules, aminoglycosides are thought to enter into Gram-negative bacterial cells via porin channels; however, it is believed that they may also enter cells via disruption of the lipopolysaccharide outer membrane.8 Passage into the cell across the inner membrane is reliant on electron transport. Because this is an aerobic process, aminoglycosides exhibit poor activity in anaerobic environments. Low pH also affects this transport and appears to explain the compromised aminoglycoside activity in these conditions.9
In vitro studies
Plazomicin has been assigned susceptibility breakpoints from the U.S. Food and Drug Administration (FDA) and U.S. Committee on Antimicrobial Susceptibility Testing for Enterobacteriaceae: ⩽2 µg/mL10 and ⩽4 µg/mL,11 respectively. Susceptibility data for plazomicin from selected studies are displayed in Table 1. Plazomicin has demonstrated excellent activity against Enterobacteriaceae. In the two largest studies, the minimum inhibitory concentration of plazomicin needed to inhibit 50% and 90% of the tested isolates, respectively (MIC50/90) = 0.5 µg/mL / 2 µg/mL with % susceptibility of >95% in both studies. Against Klebsiella, Escherichia, Enterobacter, Serratia, and Citrobacter species, plazomicin exhibited MIC50/90 = 0.25–0.5 µg/mL / 0.5–1 µg/mL. Plazomicin activity against Proteus, Morganella, and Providencia species was considerably lower, MIC50/90 = 1–4 µg/mL / 2–8 µg/mL. In several of these large surveillance studies, all the other aminoglycosides tested demonstrated activity similar to plazomicin against Enterobacteriaceae. What separated plazomicin from the other aminoglycosides was its activity against isolates considered to be MDR and/or carbapenem-resistant. All other aminoglycosides demonstrated significantly lower activity against these isolates with the exception of Enterobacteriaceae expressing 16S rRNA methyltransferases, which conferred resistance to all aminoglycosides as discussed.12–15 These MDR isolates are known to carry numerous determinants of resistance against aminoglycosides, namely AMEs, which explain this sharp decline in activity.
Table 1.
In vitro activity of plazomicin in Gram-negative organisms.
| Organism | % S | MIC50/90 (µg/mL) | Range (µg/mL) |
|---|---|---|---|
| Gram negative | |||
| Enterobacteriaceae | |||
| Enterobacteriaceae (n = 254)6 | 80 | 1/4 | ⩽0.25–>64 |
| Enterobacteriaceae (n = 4217)12 | 95.8 | 0.5/2 | ⩽0.06–>128 |
| blaKPC (n = 113) | 92.9 | 0.25/2 | ⩽0.25–>128 |
| MBL (n = 37) | 40.5 | 128/>128 | ⩽0.25–>128 |
| blaOXA-48-like (n = 54) | 87 | 0.25/16 | ⩽0.25–>128 |
| Carbapenemase-negative (n = 59) | 94.9 | 0.25/1 | ⩽0.25–>128 |
| AME genes (n = 728) | 99 | 0.25/1 | ⩽0.25–16 |
| aac(6′)-Ib (n = 585) | 99.3 | 0.25/1 | ⩽0.25–16 |
| aac(3)-IIa (n = 453) | 98.9 | 0.25/1 | ⩽0.25–16 |
| 16S rRNA methyltransferase (n = 60) | 0 | >128/>128 | 128–>128 |
| Enterobacteriaceae (n = 499)16 | NA | 0.5/64 | ⩽0.125–>64 |
| KPC-2 (n = 389) | 85 | 0.5/>64 | ⩽0.125–>64 |
| NDM-1 (n = 81) | 80 | 0.5/16 | ⩽0.125–>64 |
| Enterobacteriaceae (n = 4362)14 | 96.4 | 0.5/2 | ⩽0.06–>128 |
| CRE (n = 97) | 99a | 0.5/1 | ⩽0.06–>128 |
| blaKPC (n = 87) | 98.9a | 0.25/1 | ⩽0.06–>128 |
| MDR Enterobacteriaceae (n = 300)15 | 96 | 1/2 | ⩽0.25–4 |
| MBL (n = 488)17 | 76.4 | 1/>64 | ⩽0.12–>64 |
| Klebsiella species | |||
| K. pneumoniae (n = 1429)12 | 95.8 | 0.25/0.5 | ⩽0.06–>128 |
| K. oxytoca (n = 317)12 | 100 | 0.5/0.5 | 0.12–2 |
| K. aerogenes (n = 129)12 | 100 | 0.5/1 | ⩽0.06–2 |
| K. aerogenes (n = 120)14 | 99.2 | 0.5/1 | ⩽0.06–4 |
| K. pneumoniae (n = 1506)14 | 99.8 | 0.25/0.5 | ⩽0.06–>128 |
| K. oxytoca (n = 359)14 | 99.2 | 0.5/0.5 | ⩽0.06–>128 |
| K. pneumoniae (n = 241)15 | 95 | 1/2 | ⩽0.5–4 |
| K. pneumoniae (n = 395)18 | NA | 0.25/0.5 | ⩽0.12–>64 |
| K. pneumoniae (n = 1039)19 | 99.8 | 0.25/0.5 | ⩽0.12–>64 |
| K. oxytoca (n = 279)19 | 100 | 0.25/0.5 | ⩽0.12–2 |
| K. pneumoniae (n = 1155)20 | NA | 0.5/1 | 0.12–>8 |
| Escherichia coli | |||
| E. coli (n = 1399)12 | 99.4 | 0.5/1 | 0.12–16 |
| E. coli (n = 1346)14 | 99.4 | 0.5/1 | ⩽0.06–>128 |
| E. coli (n = 1146)18 | NA | 0.5/1 | ⩽0.12–4 |
| E. coli (n = 3094)19 | 99.5 | 0.5/1 | ⩽0.12–>64 |
| MDR (n = 358) | 99.4 | 0.5/1 | ⩽0.12–>64 |
| E. coli (n = 3050)20 | NA | 0.5/1 | ⩽0.06–>8 |
| Enterobacter species | |||
| E. cloacae (n = 104)14 | 100 | 0.5/0.5 | 0.12–2 |
| E. cloacae (n = 470)19 | 100 | 0.25/0.5 | ⩽0.12–2 |
| Serratia marcescens | |||
| S. marcescens (n = 105)12 | 99 | 1/1 | 0.25–8 |
| S. marcescens (n = 107)14 | 97.2 | 1/2 | 0.12–4 |
| S. marcescens (n = 255)19 | 97.6 | 0.5/1 | ⩽0.12–8 |
| Citrobacter species | |||
| C. freundii (n = 131)12 | 99.2 | 0.5/1 | 0.12–4 |
| C. koseri (n = 145)12 | 100 | 0.25/0.5 | ⩽0.06–1 |
| C. freundii (n = 159)14 | 99.4 | 0.5/1 | 0.12–4 |
| C. koseri (n = 145)14 | 99.3 | 0.25/0.5 | ⩽0.06–4 |
| Proteus species | |||
| P. mirabilis (n = 119)12 | 74.8 | 2/4 | 0.5–>128 |
| P. vulgaris group (n = 109)12 | 91.7 | 1/2 | 0.25–8 |
| P. mirabilis (n = 124)14 | 82.3 | 2/4 | 0.25–8 |
| P. vulgaris group (n = 116)14 | 88.8 | 2/4 | 0.5–16 |
| P. mirabilis (n = 235)19 | 44.3 | 4/4 | 0.5–32 |
| Other Enterobacteriaceae | |||
| Morganella morganii (n = 131)12 | 68.7 | 2/4 | 0.25–16 |
| Providencia spp. (n = 84)12 | 67.9 | 2/8 | 0.5–>128 |
| Morganella morganii (n = 118)14 | 64.4 | 2/4 | 0.5–64 |
| Providencia spp. (n = 158)14 | 63.3 | 2/4 | 0.12–64 |
| Morganella morganii (n = 54)19 | 66.7 | 2/4 | 0.25–8 |
| Pseudomonas aeruginosa | |||
| P. aeruginosa (n = 593)18 | NA | 4/16 | ⩽0.12–>64 |
| P. aeruginosa (n = 1789)19 | NA | 4/16 | ⩽0.12–>64 |
| MDR (n = 256) | NA | 8/64 | ⩽0.12–>64 |
| P. aeruginosa (n = 679)21 | NA | 8/32 | 0.12–>64 |
| Acinetobacter species | |||
| Acinetobacter spp. (n = 82)6 | NA | 8/32 | 0.5–>64 |
| Acinetobacter spp. (n = 99)12 | NA | 8/>128 | ⩽0.06–>128 |
| Acinetobacter spp. (n = 95)14 | NA | 2/16 | ⩽0.06–>128 |
| A. baumannii (n = 68)19 | NA | 1/8 | 0.25–>64 |
| A. baumannii (n = 407)21 | NA | 8/16 | 0.12–>64 |
Food and Drug Administration susceptibility breakpoint is used for plazomicin (⩽2 µg/mL).10
Study reported % susceptibility using a susceptibility breakpoint of ⩽4 µg/mL.
MIC50/90 – minimum inhibitory concentration needed to inhibit 50% and 90% of the included isolates, respectively aac, n-acetyltransferase; AME, aminoglycoside modifying enzyme; bla, beta-lactamase gene; CRE, carbapenem-resistant Enterobacteriaceae; KPC, Klebsiella pneumoniae carbapenemase; MBL, metallo-beta-lactamase; MDR, multi-drug resistant; NA, data not included in study; NDM, New Delhi metallo-beta-lactamase; OXA, oxacillinase; S, susceptible; spp., species
Although plazomicin only has susceptibility breakpoints assigned for Enterobacteriaceae, it has demonstrated in vitro activity against other organisms. In several surveillance studies, plazomicin exhibited MIC50/90 against Pseudomonas aeruginosa between 4–8 and 8–32 µg/mL.12,14,18,19,21 This was similar to the amikacin activity in all of these studies. Similarly, in the studies in which these data were published, the plazomicin MIC50/90 in isolates resistant to amikacin jumped to 64/>64 µg/mL.18,19,21 Plazomicin also exhibited similar activity to amikacin against Acinetobacter species (mostly of the baumannii species); however, the activity was much more variable having MIC50/90 between 1–8 and 8–>128 µg/mL.12,14,19,21 Against Staphylococcus species, plazomicin displayed superior MIC50/90 to amikacin; however, gentamicin and tobramycin displayed MIC50/90 superior to either plazomicin or amikacin. Plazomicin, like other aminoglycosides, was not effective against Enterococcus, Streptococcus, and Stenotrophomonas species.12,13,18,19
Aminoglycoside resistance
Aminoglycoside resistance can be mediated by three types of mechanisms: enzymatic modification, target site modification, and porin channel/efflux pump expression changes. The most common mechanism in Enterobacteriaceae species is enzymatic modification mostly via three AME classes: n-acetyltransferases (AACs), o-adenyltransferases (ANTs), and o-phosphotransferases (APHs). Two recent publications from the Antimicrobial Longitudinal Evaluation and Resistance Trends global surveillance program identified AAC(6′)-Ib and AAC(3)-IIa as the enzymes most responsible for aminoglycoside resistance in the U.S., Europe, and select countries in Asia.12,13 While uncommon in Enterobacteriaceae, down-regulation of porin channels and/or increase in efflux pump expression can be seen in P. aeruginosa, and Acinetobacter baumannii in addition to AME expression, which explains the higher MICs often seen in these organisms.6 Target site modification is carried out by 16S rRNA methyltransferases and completely nullifies the activity of all 4, 6 disubstituted aminoglycosides, which includes gentamicin, tobramycin, and amikacin. ArmA and RmtB are the most commonly expressed of these enzymes;7 however, they are rarely expressed in clinical isolates, with only 0.14%, 1.28%, and 0.05% isolates identified from the U.S., Europe and parts of Asia, and Canada in recent surveillance studies.12,13,22 Recent concern has been raised around these enzymes due to their increasing co-expression with New Delhi metallo-beta-lactamase producing isolates.16,23,24
As mentioned before, plazomicin is protected from nearly all clinically relevant AMEs due to structural differences. The lack of -OH groups at the C-3′ and 4′ positions prevents activity from APH(3′) and ANT(4′). The addition of a HABA at the C-1 position protects against AAC(3), ANT(2′′), and APH(2′′). Furthermore, the addition of the hydroxyethyl group to the amine at the C-6′ position protects against AAC(6′). The only AME currently identified amongst Gram-negative organisms with activity against plazomicin is AAC(2′)-I, which is chromosomally expressed in some Providencia stuartii isolates.6 Another known AME with activity against plazomicin is APH(2′′)-Iva; however, this enzyme has only been identified in Enterococcus species in which plazomicin would not be considered a treatment option.25 Plazomicin remains susceptible to outer membrane changes, which have been noted in some Proteae species, and alterations of porin channel and efflux pump expression. In addition, 16S rRNA methyltransferases prevent plazomicin activity as with all other clinically utilized aminoglycosides.6
Dosing and administration
Plazomicin is administered as a 15 mg/kg intravenous (IV) infusion over 30 min once daily and is dosed using total body weight (TBW) for patients with TBW <125% ideal body weight (IBW). For patients with TBW >125%, adjusted body weight (ABW) should be utilized and is calculated using the following equation: ABW = IBW + 0.4 (TBW - IBW). Plazomicin is supplied as 10 mL, 50 mg/mL vials. For administration, the desired dose of plazomicin should be diluted to a final volume of 50 mL in either 0.9% sodium chloride, USP, or lactated Ringers, USP. Sterilely compounded products between 2.5 and 45 mg/mL are stable at room temperature for 24 h.26
Pharmacokinetics/pharmacodynamics
Clinically relevant pharmacokinetic parameters from phase I clinical trials may be found in Table 2. Plazomicin displayed linear and dose-proportional pharmacokinetics following a single dose or multiple doses across a range of doses. These relationships can be seen when comparing the results of P1-01, which used half the normal dose of plazomicin (plazomicin 7.5 mg/kg), with other studies in Table 2. The maximum concentration (Cmax) and area under the curve 0–∞ (AUC0–∞) for P1-01 are approximately half of those seen in other studies. Overall, Cmax ranged from 161 ± 31 to 76.0 ± 19.6 mg/L and was reached immediately following the infusion in most studies. The wide range of measured values stems from the use of two different infusion times across phase I studies (30 and 10 min). Not surprisingly, the studies using a 10-min infusion measured higher Cmax and had a lower time to max (Tmax). AUC0–∞ ranged from 246 ± 39 mg*h/L to 309 ± 45 mg*h/L. The volume of distribution (Vd) of plazomicin ranged from 36.0 ± 7.8 to 11.3 ± 1.4 L across phase I studies and approximated total body water volume, similar to other aminoglycosides.27–32 Two studies calculated Vss using non-compartmental models, which resulted in higher reported values, as seen in Table 2.27,30 Protein binding appears to be relatively low in plazomicin at 16% ± 5.31 Plazomicin was also found to penetrate the lungs to a similar degree as amikacin in non-inflamed lungs (ELF: plasma AUC 13% and 14% for plazomicin and amikacin, respectively).32 Plazomicin is almost exclusively renally excreted. Following a single dose of plazomicin 15 mg/kg, 97.5% of the administered dose was recovered unchanged in the urine (56% within the first 4 h), while <0.2% was recovered from feces.30
Table 2.
Pharmacokinetics in healthy volunteers from phase I clinical trials of plazomicin.
| Single 7.5 mg/kg dose (30 min infusion) |
Single 15 mg/kg dose (30 min infusion) |
Single 15 mg/kg dose (10 min infusion) |
||||
|---|---|---|---|---|---|---|
| P1-01a
n = 6 |
P1-02b
n = 16 |
P1-03c
n = 54 |
P1-04d
n = 6 |
P1-05e
n = 6 |
P1-06f
n = 15 |
|
| AUC0–∞
(mg*h/L) |
136 ± 17.2 | 246 ± 30.8 | 265 ± 66.5 | 269 (11.4) | 246 ± 39 | 309 ± 45 |
| Cmax
(mg/L) |
37.9 ± 5.01 | 85.2 ± 11.2 | 76.0 ± 19.6 | 92.1 (8.4) | 144 ± 45 | 161 ± 31 |
| Vd
(L/kg) |
0.43 ± 0.09 | 0.23 ± 0.03 | 0.24 ± 0.06 | 0.42 (21.0) | 0.20 ± 0.03 | 0.161 ± 0.0203g |
| CLT
(mL/min per kg) |
0.93 ± 0.23 | 1.03 ± 0.10 | 0.996 ± 0.195 | 1.00 (17.1) | 1.04 ± 0.17 | 0.824 ± 0.116 |
| T1/2
(h) |
NR | 3.82 ± 0.35 | 3.5 ± 0.5 | NR | 3.4 ± 0.8 | 2.8 ± 0.6 |
Values reported are mean ± SD, except P1-06, which is geometric mean (CV%).
P1-01: [ClinicalTrials.gov identifier: NCT01462136], Komirenko et al.27
P1-02: [ClinicalTrials.gov identifier: NCT03270553], Choi et al.28
P1-03: [ClinicalTrials.gov identifier: NCT01514929], Gall et al.29
P1-04: [ClinicalTrials.gov identifier: NCT03177278], Choi et al.30
P1-05: [ClinicalTrials.gov identifier: NCT00822978], Cass et al.31
P1-06: [ClinicalTrials.gov identifier: NCT01034774], Cass et al.32
Vss = 0.248 L ± 0.0398 L after 5 days of 15 mg/kg.
AUC0–∞, area under the curve 0–∞ ; Cmax, maximum concentration; CLT, clearance (total); NR, not reported; T1/2, half-life; Vd, volume of distribution; Vss, volume of distribution at steady state
In vitro studies showed that plazomicin selectively inhibited multidrug and toxin extrusion 2-K (MATE2-K) and to a lesser extent multidrug and toxin extrusion 1 (MATE1), and organic cation transporter 2 (OCT2), which are important transporters involved with tubular secretion. However, in a phase I randomized, crossover study in which patients were given a single dose of metformin 850 mg alone or in combination with a single dose of plazomicin 15 mg/kg, all measured pharmacokinetic parameters of metformin were similar between groups, even though metformin is a known substrate of these transporters and is 90% eliminated via tubular secretion. This suggests that plazomicin will not interact with drugs secreted via this mechanism.28
In a study that recruited patients with various degrees of renal dysfunction at baseline, plazomicin AUC0–∞ was higher in patients with lower creatinine clearance (CLCr) as expected. AUC0–∞ in patients with normal (CLCr ⩾90 mL/min) and mild renal impairment (CLCr <90 and ⩾60 mL/min) were negligible, but were 1.98- and 4.42-fold higher on average in patients with moderate (CLCr <60 and ⩾30 mL/min) and severe renal impairment (CLCr <30 and ⩾15 mL/min), respectively.27 In order to maintain similar exposures in patients with normal and impaired renal function, which may occur during complicated urinary tract infection (cUTI), the FDA package insert recommends alternate dosing regimens of 10 mg/kg once daily in patients with CLCr ⩽30 and <60 mL/min and 10 mg/kg every 48 h in patients with CLCr ⩽15 and <30 mL/min. Following the initial dose, the dosing interval may be adjusted 1.5-fold based on therapeutic drug monitoring (TDM) to maintain plasma trough concentrations <3 µg/mL. At the adjusted dosages, the AUC0–24h for patients with cUTI and mild or moderate renal impairment was 261 ± 102 mg*h/L and 224 ± 147 mg*h/L, respectively.26 In an attempt to protocolize TDM for plazomicin, the group from Hartford Hospital adapted their aminoglycoside dosing nomogram for dosing interval selection to better assess patients in need of renal adjustment.33,34 Importantly, patients with CLCr <15 mL/min or who are on renal replacement therapy were excluded from phase I studies, so recommendations for dosing adjustments in these patients are currently unavailable.
There are currently three pharmacodynamic parameters that determine efficacy of antimicrobial agents: ƒ%T > MIC, ƒAUC:MIC, and ƒPeak:MIC. These parameters are often elucidated in dose fractionation studies conducted in murine models of infection. The groups of infected rodents are exposed to increasing doses of a drug using multiple dosing intervals. Non-linear regression analyses are performed between each pharmacodynamic parameter and the bacterial concentrations, colony-forming units (CFU)/mL, with the best fitting parameter being chosen.35 Aminoglycosides have been shown to display optimal activity when the ratio of AUC:MIC is maximized.36 In addition, studies have shown an independent benefit gained by maximizing the ratio of Cmax:MIC.37–39 For plazomicin, AUC:MIC was the parameter of best fit (r2 = 0.876) as opposed to Peak:MIC (r2 = 0.783) and Time > MIC (r2 = 0.712). Furthermore, the median AUC:MIC ratios that corresponded to stasis (exposure necessary to prevent growth of bacteria) and one log killing were 24 and 89, respectively.40 Probability of target attainment analysis performed by the FDA using these targets, in addition to the in vitro and clinical data, led to the assignment of a susceptibility breakpoint of ⩽2 mg/L, intermediate category of 4 mg/L, and resistant category of ⩾8 mg/L for the treatment of Enterobacteriaceae.41 The approved dosing regimen of 15 mg/kg IV once daily ensures that a higher Cmax and AUC are achieved relative to the MIC during a multiple daily dosing regimen. This extended interval scheme for aminoglycosides has also been shown to limit nephrotoxicity.42
Clinical trials
Highlights from the phase II and III trials for plazomicin can be found in Table 3. Two indications have been pursued for plazomicin: cUTI in a phase II trial and the EPIC trial and serious CRE infection (included blood stream infections (BSIs), hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP)) in the CARE trial.
Table 3.
Summary table of phase II and III clinical trials of plazomicin.
| Trial |
Phase |
Indication |
Primary outcome |
Results No. patients (%) |
|
|---|---|---|---|---|---|
| P2-01 | II | cUTI | Microbiological eradication at TOC | PLZ | LVX |
| MITT population: | 31 (60.8) | 17 (58.6) | |||
| Difference: | 2.2% (95% CI: –22.9 to 27.2%) | ||||
| ME population: | 31 (88.6) | 17 (81.0) | |||
| Difference: | 7.6% (95% CI: –16.0 to 31.3%) | ||||
| EPIC | III | cUTI | Composite cureb | PLZ | MEM |
| Treatment day 5: | 168 (88.0) | 180 (91.4) | |||
| Difference: | –3.4% (95% CI: –10.0 to 3.1%) | ||||
| TOC visit: | 156 (81.7) | 138 (70.1) | |||
| Difference: | 11.6% (95% CI: 2.7 to 20.3%) | ||||
| CARE | III | CRE infectiona | Composite day 28 all-cause mortality and disease related complications | PLZc | CSTc |
| MMITT population: | 4 (24) | 10 (50) | |||
| Difference: | –26% (95% CI: –55 to 6%) | ||||
Dosages for trial drugs were: plazomicin 15 mg/kg intravenously (IV) once daily with therapeutic drug monitoring for maintenance dosing, levofloxacin 750 mg IV once daily, meropenem 1 g IV q 8 h, and colistin 5 mg/kg IV loading dose with 5 mg/kg per day IV divided into 8–12 h dosing intervals maintenance dosing.
P2-01: [ClinicalTrials.gov identifier: NCT01096849], Connolly et al.,43 EPIC44, CARE.45
Included blood stream infection, hospital-acquired pneumonia, and ventilator-associated pneumonia.
Composite cure defined as both clinical cure and microbiological cure. Clinical cure was defined as reduced symptom severity at day 5/end of the infusion, complete symptom resolution at the TOC visit, or return to patient baseline prior to urinary tract infection. Microbiological eradication was defined as reduction in causative pathogen to <104 CFU/mL.
Given in combination with either meropenem 2 g IV q 8h (3 h extended-interval infusion) or tigecycline 100–200 mg IV loading dose with 50–100 mg IV q 12 h maintenance dosing.
CFU, colony-forming units; CI, confidence interval; CRE, carbapenem-resistant Enterobacteriaceae; CST, colistin; cUTI, complicated urinary tract infection; LVX, levofloxacin; ME, microbiologically evaluable; MEM, meropenem; MITT, modified-intent-to-treat; MMITT, microbiologic MITT; PLZ, plazomicin; TOC, test-of-cure.
cUTI
The FDA approved plazomicin for the treatment of cUTIs in July 2018 following the success of Study P2-01 and the EPIC trial. Study P2-01 was a phase II, multicenter, double-blind, randomized, comparator-controlled clinical trial. Patients were randomized 1:1:1 to receive either plazomicin 15 mg/kg IV once daily, plazomicin 10 mg/kg IV once daily, or levofloxacin 750 mg IV once daily. Preference was later given to the plazomicin 15 mg/kg IV once daily and subsequent randomization proceeded 2:1 to receive plazomicin 15 mg/kg IV once daily or levofloxacin 750 mg IV once daily. Patients enrolled in the study were between 18 and 85 years of age, ⩽100 kg, and had a CLCr ⩾60 mL/min based on Cockcroft and Gault. The primary efficacy endpoints in this trial were microbiological eradication (<104 CFU/mL of causative pathogen) in both the modified-intent-to-treat (MITT) and microbiologically evaluable (ME) populations at the test-of-cure (TOC) visit (5 and 12 days post-treatment).
The differences in percent microbiological eradication rate in the MITT and ME populations between groups was 2.2 (95% confidence interval (CI): –22.9 to 27.2) and 7.6 (95% CI: –16.0 to 31.3), respectively, both in favor of plazomicin, though neither result was considered statistically significant. The number of patients experiencing any adverse effect (AE) was similar between groups, with the most common AEs in either plazomicin group being headache (8.3%), dizziness (4.2%), nausea (4.2%), vomiting (4.2%), and diarrhea (4.2%), which is similar to phase I trial data. In addition, an increase in serum creatinine ⩾0.5 mg/dL occurred in 3.2% of patients receiving plazomicin, which did not occur in any patient receiving levofloxacin.43
The EPIC trial was a phase III multicenter, multinational, double-blind, randomized clinical trial. Patients were randomized 1:1 to receive either plazomicin 15 mg/kg IV once daily or meropenem 1 g IV every 8 h for 7–10 days. The eligibility criteria in the EPIC trial were similar to the previous trial; although, patients with a CLCr ⩽30 mL/min were included in the EPIC trial. The primary efficacy endpoints were composite cure (both clinical cure and microbiological eradication) at day 5 of therapy and at the TOC visit (15–19 days following initiation of IV therapy) in the MITT population. Clinical cure was defined as reduced symptom severity at day 5 or end of the infusion, complete symptom resolution at the TOC visit, or return to patient baseline prior to urinary tract infection and microbiological eradication as reduction in causative pathogen to <104 CFU/mL.
At day 5 of therapy, the difference in percent composite cure between groups was not statistically significantly different; however, at both the TOC visit and the late follow-up visit (days 24–32), the difference was 11.6 (95% CI: 2.7–20.3) and 16.6 (95% CI: 7.0–25.7), respectively, both in favor of plazomicin. In addition, sub-group analysis of composite cure numerically favored plazomicin in every group tested. The frequency of AEs was similar between groups, with 19.5% and 21.6% of patients reporting any event in the plazomicin and meropenem groups, respectively. The most common AEs reported for plazomicin were similar to Study P2-01. Similar numbers of patients experienced a ⩾0.5 mg/dL increase in serum creatinine in the plazomicin (3.7%) and meropenem (3.0%) groups while receiving IV therapy, with full renal recovery occurring in 81.8% and 100% of patients receiving plazomicin and meropenem, respectively.44
Plazomicin achieved non-inferiority for all primary efficacy endpoints in both trials when compared with standard of care therapy for cUTI. Moreover, it exhibited excellent activity against numerous resistance phenotypes between the two studies, including aminoglycoside and fluoroquinolone resistance, extended-spectrum beta-lactamase and CRE, and MDR isolates (resistant to at least one agent in three different antibiotic classes). Of the nine isolates in the plazomicin group that were CRE, 77.8% were eradicated at the TOC visit in the EPIC trial.
Serious CRE infection
The CARE trial was a phase III, multicenter, randomized, open-label trial. Patients were randomized 1:1 to receive either plazomicin 15 mg/kg IV once daily or colistin 5 mg/kg IV loading dose (300 mg maximum) colistin base activity followed by 5 mg/kg per day IV maintenance dose q 8–12 h for 7–14 days. Plazomicin was adjusted based on TDM in patients with renal impairment to target an AUC0–24h of 262 mg*h/L. Both agents were administered in combination with either meropenem 2 g IV (3-h extended infusion) every 8 h or tigecycline 100–200 mg IV loading dose followed by 50–100 mg IV maintenance dose q 12 h. Patients enrolled in the study were between 18 and 85 years of age, had an Acute Physiology and Chronic Health Evaluation II score between 15 and 30, and had either a BSI, HAP, or VAP suspected/confirmed to be caused by a CRE. The primary efficacy endpoint was a composite of all-cause mortality at 28 days or clinically significant disease-related complications in the microbiologic MITT (MMITT) population (confirmed CRE infection who received ⩾1 dose of trial drug).
Unfortunately, the trial was ended prematurely due to slow enrollment. The difference in percent occurrence of a primary endpoint event in the MMITT population was 26 (95% CI: −55 to 6) in favor of plazomicin. Sub-group analysis by infection type revealed this difference to be 39 (95% CI: −69 to −4) in favor of plazomicin in the BSI group and 27 (95% CI: −48 to 82) in favor of colistin in the HAP or VAP group. Kaplan–Meier estimates in the MMITT groups showed the hazard ratio for all-cause mortality to day 28 [0.25 (95% CI: 0.05–1.19)] and day 60 [0.47 (95% CI: 0.19–1.19)] both favored plazomicin combinations. Plazomicin combinations also had a more favorable AE profile, with fewer serious adverse events (50%) and ⩾0.5 mg/dL serum creatinine increases (16.7%) occurring in the safety population than in the colistin group; 81% and 50%, respectively.45
Due to the small sample size of the study, the FDA did not grant the CRE indication to plazomicin. Regardless, given the current lack of data in treating CRE infections in randomized controlled trials, these data, taken with in vitro and in vivo data, are suggestive of a role for plazomicin in the treatment of CRE infections. In addition, plazomicin has demonstrated an improved AE profile when compared with other commonly used adjunctive agents for the treatment of CRE infections, especially colistin.
Clinical resistance
Pathogens demonstrating resistance to plazomicin were rarely encountered across these clinical trials. Due to concerns for balancing the intervention groups, isolates having baseline MICs considered to be non-susceptible to either meropenem or plazomicin were not included in the primary analysis of the EPIC trial; however, six isolates cultured in the CARE trial (two from the plazomicin arm and four from the colistin arm) had baseline MICs resistant to plazomicin. All of these isolates had MICs >128 µg/mL and were confirmed to express 16S rRNA methyltransferases.45,46
Treatment emergent resistance to plazomicin was also noted in phase III clinical trials, though this too was a rare occurrence. In total, seven isolates cultured from six patients met the criteria for resistance emergence (an isolate having a ⩾4-fold increase in MIC and whose baseline MIC changed from ⩽4 µg/mL to > 4 µg/mL after treatment). All of these patients were enrolled in the EPIC trial. Of these six patients, four achieved clinical cure at the TOC visit regardless, and only one of the other two patients required additional antimicrobial therapy following the initial administration of study drug.46
Whole genome sequencing revealed that five of the seven isolates shared the genetic profile of the baseline pathogen with the addition of a plasmid-encoded 16S rRNA methyltransferase. No definitive resistance mechanism was determined for the other two isolates, but genetic changes consistent with increased aminoglycoside MICs (cydA, cpxA and cpxR, and sbmA) were posited as an explanation. Interestingly, five of the isolates were cultured prior to the end of intravenous therapy, prompting the investigators to suggest that these emergent events were likely the result of a resistant subpopulation flourishing under selective pressure due to the rapidity with which the phenotypes appeared. Overall, the low frequency of emerging resistance and the resistance phenotypes isolated in these studies are consistent with epidemiological data of the regions from which the patients were enrolled and in vitro and in vivo data published for plazomicin.46
Safety
Plazomicin was evaluated in six phase I clinical trials, one phase II clinical trial in patients with cUTI, and in two phase III clinical trials (one in patients diagnosed with severe CRE infections and one in patients diagnosed with cUTI). It should be noted that the FDA approved plazomicin with a Black Box Warning for aminoglycoside class effects (nephrotoxicity, ototoxicity, neuromuscular blockade, and pregnancy risk) as it has for other aminoglycosides; however, plazomicin demonstrated a safe AE profile across all clinical trials. In the EPIC trial, which enrolled the most patients of any other trial (303 received plazomicin), the most common AEs reported were decreased renal function (3.7%), diarrhea (2.3%), hypertension (2.3%), headache (1.3%), nausea (1.3%), vomiting (1.3%), and hypotension (1.0%). Given that plazomicin is an aminoglycoside, decreases in renal function are expected as a class effect; however, a similar frequency of clinically significant renal function decreases (increase ⩾0.5 mg/dL serum creatinine from baseline) occurred in patients receiving meropenem (3.0%). Furthermore, most patients in the plazomicin group had full renal recovery by the final follow-up visit (81.0%).44 Patients experiencing any severe AE were similar between groups (1.7%). Although ototoxicity events were rare in clinical trials, patients should be monitored for these events especially if they have structural abnormalities or a history of otologic disease as these patients were excluded from participation. While the safety data provided here are promising, conditions in clinical trials often differ from clinical practice, especially in the duration of therapy. This should be considered when using plazomicin in practice as these percentages may not extend to patients being treated due to the differing contexts.
Relevance to patient care and clinical practice
Plazomicin has received an FDA indication for the treatment of cUTIs, including acute pyelonephritis, following positive results in a phase II and III clinical trial against the current standard of care. The majority of cUTIs are not caused by MDR organisms and can be effectively treated with more targeted therapy. Given its exceptional in vitro profile and success against cUTIs caused by antimicrobial-resistant isolates, plazomicin will most likely be reserved to treat more resistant cUTIs.
Newer beta-lactam/beta-lactamase inhibitors have demonstrated excellent activity against most major carbapenem-resistant phenotypes; yet, emergence of resistance to ceftazidime/avibactam (the first novel beta-lactam/beta-lactamase combination commercially available) has already been reported, occurring both prior to exposure to the antibiotic and during active treatment.47 Aminoglycosides have been utilized as add-on therapy with beta-lactams for serious infections for decades due to their synergistic mechanisms of action. However, recent spread of resistance determinants against aminoglycosides has threatened this antimicrobial class. This is especially true in CRE isolates, which have been shown to harbor numerous AME phenotypes. This could be another avenue for plazomicin to enter routine clinical use. It bears repeating that plazomicin did not receive an FDA indication for treating severe CRE infections; however, several data, both in vitro and in vivo, currently support its use in combination regimens for this indication.48–51 Again, prior to prematurely closing the CARE trial due to slow enrollment, combinations using plazomicin and meropenem or tigecycline appeared to be both more effective and safer than those using colistin.
Caution should be exercised when using plazomicin to treat New Delhi metallo-beta-lactamase (NDM)-producing Enterobacteriaceae. NDM-producing phenotypes have been sporadically noted in the U.S.; however, other countries, in Europe and Asia, have documented more endemic prevalence.52 Increasingly, co-expression of 16S rRNA methyltransferases has been reported in NDM-producing Enterobacteriaceae. In an analysis of metallo-beta-lactamase (MBL)-producing isolates identified in several studies performed across many countries, of the 488 isolates included, 282 (57.8%) expressed NDM, and 64 (22.7%) of these isolates also expressed a 16S rRNA methyltransferase.17 Another surveillance study of aminoglycoside-resistant isolates collected from the UK and Ireland reported that 592/762 (78%) of the isolates positive for a 16S rRNA methyltransferase co-expressed NDM. Interestingly, 169/762 (22%) of these isolates co-expressed OXA-48-like carbapenemases.24
This trend has not yet been associated with any other CRE phenotype, including other MBLs. Numerous commercially available rapid detection tests can identify the presence of carbapenemases, including NDM, which can guide empiric administration of plazomicin.53,54 A rapid in vitro test to more directly detect aminoglycoside resistance has recently been published; however, its implementation could be challenging as no commercial product is yet available.55 Therefore, empiric use of plazomicin in treating infections caused by NDM-producing Enterobacteriaceae may be questionable, especially in regions where co-expression with 16S rRNA methyltransferase is endemic. However, 16S rRNA methyltransferases may be expressed in any isolate regardless of carbapenem-resistant phenotype. Clinicians are advised, as always, to consult their local antibiograms prior to recommending any empiric therapy and to deescalate appropriately as new patient data are made available.
Conclusion
Because of the persistence of bacterial evolution, it seems unlikely that a single agent will emerge as a panacea against infection; rather, an armamentarium seems necessary to keep pace in the fight against antimicrobial resistance. Plazomicin appears poised to help fill the need for new agents to treat infections caused by MDR Enterobacteriaceae. Further research and reports following its use in the clinical setting will help crystalize its role in therapy for these serious infections.
Footnotes
Author contributions: Both JAC and DSB contributed significantly towards the conception of the project, the interpretation of the data, drafting/editing process, and ultimate approval of the published draft.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Justin A. Clark  https://orcid.org/0000-0002-3967-5350
https://orcid.org/0000-0002-3967-5350
Contributor Information
Justin A. Clark, University of Kentucky College of Pharmacy, Lexington, KY, USA
David S. Burgess, University of Kentucky College of Pharmacy, 292K TODD Building, 789 South Limestone St., Lexington, KY 40536-0596, USA.
References
- 1. O’Neil J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Health Wealth Nations. 2014. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiRheP57ZvrAhUSVs0KHdE5D3wQFjAAegQIAxAB&url=https%3A%2F%2Famr-review.org%2Fsites%2Fdefault%2Ffiles%2FAMR%2520Review%2520Paper%2520-%2520Tackling%2520a%2520crisis%2520for%2520the%2520health%2520and%2520wealth%2520of%2520nations_1.pdf&usg=AOvVaw2o4ZjnxVEehWL9MapFf5DH (accessed 7 September 2019). [Google Scholar]
- 2. Abat C, Rolain JM, Dubourg G, et al. Evaluating the clinical burden and mortality attributable to antibiotic resistance: the disparity of empirical data and simple model estimations. Clin Infect Dis 2017; 65(Suppl. 1): S58–S63. [DOI] [PubMed] [Google Scholar]
- 3. de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med 2016; 13: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. (2013, accessed 5 December 2015).
- 5. Centers for Disesae Control and Prevention. Antibiotic resistance threats in the United States, https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (2019, accessed 1 May 20).
- 6. Aggen JB, Armstrong ES, Goldblum AA, et al. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother 2010; 54: 4636–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doi Y, Wachino J, Arakawa Y. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin NA 2016; 30: 523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garneau-Tsodikova S, Labby KJ. Mechanisms of resistance to aminoglycoside antibiotics: overview and perspectives. Medchemcomm 2016; 7: 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother 1999; 43: 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Food and Drug Administration. Plazomicin–injection. FDA-identified interpretive criteria. Silver Spring, MD: U.S. Food and Drug Administration, https://www.fda.gov/drugs/development-resources/plazomicin-injection (2019, accessed August 2019). [Google Scholar]
- 11. The United States Committee on Antimicrobial Susceptibility Testing (USCAST). Breakpoint tables for interpretation of MIC and zone diameter results. Version 5.3, https://app.box.com/s/cru62wluz4qq64vd5izk41wle9ss172x (2019). (accessed August 2019).
- 12. Castanheira M, Deshpande LM, Woosley LN, et al. Activity of plazomicin compared with other aminoglycosides against isolates from European and adjacent countries, including Enterobacteriaceae molecularly characterized for aminoglycoside-modifying enzymes and other resistance mechanisms. J Antimicrob Chemother 2018; 73: 3346–3354. [DOI] [PubMed] [Google Scholar]
- 13. Castanheira M, Davis AP, Serio AW, et al. In vitro activity of plazomicin against Enterobacteriaceae isolates carrying genes encoding aminoglycoside-modifying enzymes most common in U.S. census divisions. Diagn Microbiol Infect Dis 2019; 94: 73–77. [DOI] [PubMed] [Google Scholar]
- 14. Castanheira M, Davis AP, Mendes RE, et al. In-vitro activity of plazomicin against Gram-negative and Gram-positive isolates collected from U.S. hospitals and comparative activities of aminoglycosides against carbapenem-resistant Enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrob Agents Chemother 2018; 62: e00313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galani I, Souli M, Daikos GL, et al. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. from Athens, Greece. J Chemother 2012; 24: 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martins AF, Bail L, Ito CAS, et al. Antimicrobial activity of plazomicin against Enterobacteriaceae producing carbapenemases from 50 Brazilian medical centers. Diagn Microbiol Infect Dis 2018; 90: 228–232. [DOI] [PubMed] [Google Scholar]
- 17. Serio AW, Keepers T, Krause KM. Plazomicin is active against metallo-beta-lactamase-producing Enterobacteriaceae. Open Forum Infect Dis 2019; 6: ofz123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walkty A, Adam H, Baxter M, et al. In vitro activity of plazomicin against 5,015 Gram-negative and Gram-positive clinical isolates obtained from patients in Canadian hospitals as part of the CANWARD study, 2011–2012. Antimicrob Agents Chemother 2014; 58: 2554–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walkty A, Karlowsky JA, Baxter MR, et al. In vitro activity of plazomicin against Gram-negative and Gram-positive bacterial pathogens isolated from patients in Canadian hospitals from 2013 to 2017 as part of the CANWARD surveillance study. Antimicrob Agents Chemother 2019; 63: e02068-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Landman D, Babu E, Shah N, et al. Activity of a novel aminoglycoside, ACHN-490, against clinical isolates of Escherichia coli and Klebsiella pneumoniae from New York City. J Antimicrob Chemother 2010; 65: 2123–2127. [DOI] [PubMed] [Google Scholar]
- 21. Landman D, Kelly P, Bäcker M, et al. Antimicrobial activity of a novel aminoglycoside, ACHN-490, against Acinetobacter baumannii and Pseudomonas aeruginosa from New York city. J Antimicrob Chemother 2011; 66: 332–334. [DOI] [PubMed] [Google Scholar]
- 22. Walkty A, Karlowsky JA, Baxter MR, et al. Frequency of 16S ribosomal RNA methyltransferase detection among Escherichia coli and Klebsiella pneumoniae clinical isolates obtained from patients in Canadian hospitals (CANWARD, 2013–2017). Diagn Microbiol Infect Dis 2019; 94: 199–201. [DOI] [PubMed] [Google Scholar]
- 23. Livermore DM, Mushtaq S, Warner M, et al. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 2011; 66: 48–53. [DOI] [PubMed] [Google Scholar]
- 24. Taylor E, Sriskandan S, Woodford N, et al. High prevalence of 16S rRNA methyltransferases among carbapenemase-producing Enterobacteriaceae in the UK and Ireland. Int J Antimicrob Agents 2018; 52: 278–282. [DOI] [PubMed] [Google Scholar]
- 25. Cox G, Ejim L, Stogios PJ, et al. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis 2018; 4: 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Achaogen Inc. Zemdri (plazomicin) [package insert]. South San Francisco, CA: Achaogen, Inc, www.accessdata.fda.govdrugsatfda_docs/label/2018/210303Orig1s000lbl.pdf (2019, accessed 5 August 2019). [Google Scholar]
- 27. Komirenko AS, Riddle V, Gibbons JA, et al. A phase 1 study to assess the pharmacokinetics of intravenous plazomicin in adult subjects with varying degrees of renal function. Antimicrob Agents Chemother 2018; 62: e01128-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choi T, Komirenko AS, Riddle V, et al. No effect of plazomicin on the pharmacokinetics of metformin in healthy subjects. Clin Pharmacol Drug Dev 2019; 8: 818–826. [DOI] [PubMed] [Google Scholar]
- 29. Gall J, Choi T, Riddle V, et al. A phase 1 study of intravenous plazomicin in healthy adults to assess potential effects on the QT/QTc interval, safety, and pharmacokinetics. Clin Pharmacol Drug Dev. Epub ahead of print 16 January 2019. DOI: 10.1002/cpdd.653. [DOI] [PubMed] [Google Scholar]
- 30. Choi T, Seroogy JD, Sanghvi M, et al. Mass balance, metabolism, and excretion of [14C]-plazomicin in healthy human subjects. Open Forum Infect Dis 2018; 5(Suppl. 1): S431–S431. [Google Scholar]
- 31. Cass RT, Brooks CD, Havrilla NA, et al. Pharmacokinetics and safety of single and multiple doses of ACHN-490 injection administered intravenously in healthy subjects. Antimicrob Agents Chemother 2011; 55: 5874–5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cass R, Kostrub CF, Gotfried M, et al. A double-blind, randomized, placebo-controlled study to assess the safety, tolerability, plasma pharmacokinetics and lung penetration of intravenous plazomicin in healthy subjects. ECCMID 2013; 9: Abstract P1637. [Google Scholar]
- 33. Asempa TE, Kuti JL, Seroogy JD, et al. A simulated application of the hartford hospital aminoglycoside dosing nomogram for plazomicin dosing interval selection in patients with serious infections caused by carbapenem-resistant enterobacterales. Clin Ther 2019; 41: 1453–1462. [DOI] [PubMed] [Google Scholar]
- 34. Asempa TE, Kuti JL, Seroogy JD, et al. Application of the Hartford Hospital Nomogram for plazomicin dosing interval selection in patients with complicated urinary tract infection. Antimicrob Agents Chemother 2019; 63: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ambrose PG, Bhavnani SM, Rubino CM, et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis 2007; 44: 79–86. [DOI] [PubMed] [Google Scholar]
- 36. Bland CM, Pai MP, Lodise TP. Reappraisal of contemporary pharmacokinetic and pharmacodynamic principles for informing aminoglycoside dosing. Pharmacotherapy 2018; 38: 1229–1238. [DOI] [PubMed] [Google Scholar]
- 37. Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 1987; 155: 93–99. [DOI] [PubMed] [Google Scholar]
- 38. Bastone EB, Li SC, Ioannides-Demos LL, et al. Kill kinetics and regrowth patterns of Escherichia coli exposed to gentamicin concentration-time profiles simulating in vivo bolus and infusion dosing. Antimicrob Agents Chemother 1993; 37: 914–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blaser J, Stone BB, Groner MC, et al. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother 1987; 31: 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Louie A, Liu W, Nole J, et al. Pharmacokinetics-pharmacodynamics (PK-PD) of plazomicin (PLZ) against carbapenem-resistant Enterobacteriaceae (CRE) in neutropenic murine thigh Infection and pneumonia models. ESCMID/ASM 2018. Lisbon, Portugal, 2018. Poster 100. [Google Scholar]
- 41. U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Zemdri (plazomicin) 505(b)(1) Original NDA, Washington, DC, 2018. [Google Scholar]
- 42. Eliopoulos GM, Drusano GL, Ambrose PG, et al. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis 2007; 45: 753–760. [DOI] [PubMed] [Google Scholar]
- 43. Connolly LE, Riddle V, Cebrik D, et al. A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob Agents Chemother 2018; 62: e01989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wagenlehner FME, Cloutier DJ, Komirenko AS, et al. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med 2019; 380: 729–740. [DOI] [PubMed] [Google Scholar]
- 45. McKinnell JA, Dwyer JP, Talbot GH, et al. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med 2019; 380:791–793. [DOI] [PubMed] [Google Scholar]
- 46. Achaogen, Inc. Antimicrobial drugs advisory committee meeting briefing book, https://www.fda.gov/media/113190/download (2018). (accessed July 2020).
- 47. Shields RK, Nguyen MH, Press EG, et al. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K pneumoniae: a case report and review of literature. Open Forum Infect Dis 2017; 4: ofx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Almaghrabi R, Clancy CJ, Doi Y, et al. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 2014; 58: 4443–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thwaites M, Hall D, Shinabarger D, et al. Evaluation of the bactericidal activity of plazomicin and comparators against multidrug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abdelraouf K, Kim A, Krause KM, et al. In vivo efficacy of plazomicin alone or in combination with meropenem or tigecycline against Enterobacteriaceae isolates exhibiting various resistance mechanisms in an immunocompetent murine septicemia model. Antimicrob Agents Chemother 2018; 62: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuti JL, Kim A, Cloutier DJ, et al. Evaluation of plazomicin, tigecycline, and meropenem pharmacodynamic exposure against carbapenem-resistant Enterobacteriaceae in patients with bloodstream infection or hospital-acquired/ventilator-associated pneumonia from the CARE Study (ACHN-490-007). Infect Dis Ther 2019; 8: 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 2017; 215(Suppl. 1): S28–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mancini N, Infurnari L, Ghidoli N, et al. Potential impact of a microarray-based nucleic acid assay for rapid detection of gram-negative bacteria and resistance markers in positive blood cultures. J Clin Microbiol 2014; 52: 1242–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schmitz JE, Tang YW. The GenMark ePlex®: another weapon in the syndromic arsenal for infection diagnosis. Future Microbiol 2018; 13: 1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nordmann P, Dobias J, Poirel L. Rapid aminoglycoside NP test for rapid detection of multiple aminoglycoside resistance in Enterobacteriaceae. J Clin Microbiol 2017; 55: 1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]