Abstract
Brain metastases are a major cause of death in breast cancer patients. A key event in the metastatic progression of breast cancer in the brain is the migration of cancer cells across the blood-brain barrier (BBB). The BBB is a natural barrier with specialized functions that protect the brain from harmful substances, including anti-tumor drugs. Extracellular vesicles (EVs) sequestered by cells are mediators of cell-cell communication. EVs carry cellular components, including microRNAs that affect the cellular processes of target cells. Here, we summarize the knowledge about microRNAs known to play a significant role in breast cancer and/or in the BBB function. In addition, we describe previously established in vitro BBB models, which are a useful tool for studying molecular mechanisms involved in the formation of brain metastases.
Keywords: Metastatic breast cancer, blood-brain barrier, in vitro models, microRNA, extracellular vesicles (EVs), brain metastases
1. INTRODUCTION
Breast cancer is the most common malignant tumor that causes the highest cancer-associated death of women from industrial nations. Cancer metastases have a significant impact on mortality and overall survival of these patients. Importantly, the average mortality has been significantly reduced in recent years due to differential early detection and screening measures as well as advanced preventive examinations. However, despite all this, many patients die prematurely due to a pronounced tumor affliction.
MicroRNAs (miRs) are short, non-coding RNAs that are approximately 20 nucleotides in length. They regulate gene expression post-transcriptionally by degrading mRNA or blocking its translation [1]. Only 2% of the genome consists of protein-coding sequences, while the non-coding sequences predominate [2]. These are the least researched parts of the genome so far and their effects on biological processes in the human body, but also in tumorigenesis, are not yet well explained. In recent years, various research groups have demonstrated that miRs can not only be detected in tissue, but also circulate in cell-free body fluids such as plasma or serum [3]. More importantly, it has been demonstrated that miRs play a prognostic role in cancer of various entities [4-6].
Eukaryotic cells sequester extracellular vesicles (EVs) which, depending on their size, are divided into exosomes, activation or apoptosis-induced microvesicles and apoptotic bodies. Microvesicles are cell membrane vesicles with a diameter of 100-1000 nm, apoptotic bodies are vesicles with a diameter of 1-5 µm, while exosomes have a diameter of 30-100 nm [7-9]. Because of their small size, exosomes have emerged as a novel approach to drug delivery and biomarker research. Exosomes can transfer proteins and genetic material [10]. They circulate in body fluids carrying active molecules to distant cells in the body where they canincorporate and release their constituents. Exosomes are considered to be powerful non-invasive biomarkers because of their high stability and well-optimized methods for their isolation and characterization. Tumor cells (TCs) secrete more exosomes than normal cells [11, 12]. Due to this fact, expression profiles of exosomes isolated from serum/plasma of cancer patients showed different levels of numerous miRs as compared to healthy individuals [13]. In the advanced stage of the disease, the tumor can spread from the primary tissue and form metastases. Besides lung cancer (small cell and non-small cell), melanoma and renal cancer, the highest incidence to metastasize in the central nervous system (CNS) has been described for breast cancer. Exosomes isolated from patients with metastases show a different miR expression pattern compared to healthy individuals or patients with primary neoplasms [14]. An efficient characterization of these tumor signatures may enable the development of new classification criteria and novel therapies for these pathologies in the future. In particular, cerebral metastases of breast cancer have recently been dynamically studied [15]. TCs must pass through a highly selective and tight barrier, the blood-brain barrier (BBB), to form brain metastases. In the brain, TCs are “protected” by the BBB, as most effective anticancer drugs do not cross the BBB or are pumped out of endothelial cells (ECs) by active efflux transporters [16]. This review focuses on miRs differentially expressed in exosomes of breast cancer patients, and describes miRs that have been shown to affect the BBB. In this context, in vitro BBB models have become a powerful tool to study the molecular mechanisms involved in CNS disorders. We summarize recent developments in the modeling of the BBB, including a promising advancement in the use of human cell-based models.
2. BREAST CANCER
2.1. Molecular Characteristics
The common classification of the subtypes of breast cancer in the St. Gallen Classification divides it into four types. The classification is based on the analysis of biological markers in the primary tumor including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2/neu) and proliferation marker Ki67, together with age, tumor size, histological grade and lymph node engagement [17, 18]. Four-type-classification divides breast cancer into luminal A (ER + and/or PR+, Ki67 low and HER2/neu-), luminal B (ER + and/or PR+, Ki67 high and/or HER2/neu+), HER2/neu-positive (ER+/-, PR+/- and HER2/neu+) and triple-negative (ER-, PR-and HER2/neu-). This classification gives a prediction of disease features, recurrence pattern and disease-free survival.
2.2. Current Therapies of Primary Breast Cancer
The different oncological societies worldwide regularly renew the recommended gold standard of care after the newest published state-of-art and clinical trials. There are active discussions about the best and valid treatments of breast cancer subtypes [19]. The treatment of breast cancer usually includes surgery, mostly radiation, and subtypes of higher risk systemic chemotherapy (adjuvant, neo-adjuvant, or even both) or anti-hormone therapy with subtypes of endocrine origin [20]. The main portion (approximately 75%) of the breast cancer types belong to estrogen receptor (ER) positive breast cancer and are classified into the luminal A and luminal B subtypes. The division into one or the other luminal-like group has major consequences for the treatment of the patient. The luminal A subtype is usually characterized by a favorable prognosis compared to the luminal B subtype. Systemic therapy is therefore limited to endocrine therapy for at least 5 years after the initial treatment (one or another kind of surgery and eventually radiation). In contrast, the luminal B subtype is distinguished by a high proliferation rate and/or a high histological grade, therefore it is suggested to treat those patients after surgery and mostly radiation with systemic chemotherapy followed by endocrine therapy for at least 5 years [21]. Endocrine therapy differs between the premenopausal and postmenopausal patients. For premenopausal patients, tamoxifen is recommended for about 5-10 years after the treatment, whereas postmenopausal patients should be treated with aromatase inhibitors [22]. Luminal B, HER2/neu and triple-negative tumor patients are recommended to get systemic chemotherapy. The regimes differ in the kind of applied chemotherapy or antibodies and the time when it is applied (adjuvant or neo-adjuvant) and in which dosing [22]. There is a wide range of different regimes. In general, it is recommended to treat luminal B tumors (tumors ER + with high risk) after surgery and mostly radiation with adjuvant chemotherapy based on anthracycline and taxane or a dose-intensified therapy [20]. The HER2/neu positive subtype of breast cancer is therefore mostly treated with a neo-adjuvant chemotherapy, which includes at least one systemic chemotherapy compound and either one anti-HER2/neu- antibody or even two (subtype with higher risk). After neo-adjuvant treatment and surgery plus radiation, the antibody (one or two) is given adjuvant for one year. Triple-negative breast cancers (abbreviated TNBC) are those cancer subtypes, which do not express ER, PR or HER2/neu [23]. TNBC is a molecularly very heterogeneous cancer, which is considered to be hard to treat, because of its aggressive characteristics. The risk of a secondary tumor spread and metastasis is higher in comparison to the other subtypes. The treatment in the curative situation of surgery and radiation plus systemic chemotherapy (neo-adjuvant or adjuvant treatment) with anthracyclines and/or taxanes are used in general as the first-line therapy [24]. Because of its heterogeneity and aggressiveness, major efforts were made in the past to subclassify the TNBC and find a targeted therapy [25].
2.3. Current Therapies of Metastatic Breast Cancer
The recurrence of TCs not locoregional but in other organs characterizes a systemic tumor disease. The consequence for patients is a life-long therapy of one or other kind. One of the first organs to suffer from a metastatic disease of breast cancer is the bones. A bone stabilization therapy with bisphosphonates or RANKL-antibody is recommended. There are different subtypes of therapy which are either applied intravenously, subcutaneously or orally. These have a bone stabilization effect, as they inhibit bones resorbing osteoclasts. Bone metastases with fracture risk, functional impairment, bone pain or neuropathic bone pain should be treated by radiotherapy [26]. Other organs, which are often affected by metastatic spread, are distant nodal metastasis, liver, bone, brain and lungs [27]. The metastatic breast cancer is again subdivided into the classification of luminal, HER2/neu and TNBC. Therefore it is necessary to regain a new histological sample, as there is always the possibility of a receptor switch. Afterwards, the decision of systemic therapy has to be made. The treatment of metastatic breast cancer is discussed intensively and regularly by the different oncological societies worldwide. New specific substances and medications regularly capture the market of oncological therapy [28]. Metastatic luminal-like tumors are treated with endocrine therapy or extended endocrine therapy (CDK4/6 inhibitors). At the state of a so-called “visceral crisis”, a systemic therapy with taxane, but also VEGF- antibodies is given. The so-calledd second or further chemotherapy lines include substances like anthracylines or microtubule-inhibitor. There is the possibility of mono- or poly-chemotherapy regime. It is always necessary to consider the pretreated adjuvant therapies, the side-effects and conditions for choosing a therapy regime [24]. Patients who suffer from a breast cancer early onset (BRCA) gene mutation and a TNBC metastatic breast cancer can achieve an oral treatment with poly(ADP-ribose)-polymerase (PARP)-Inhibitor. Metastatic HER2/neu breast cancers are treated with two HER2/neu- antibody compounds and a systemic chemo-compound of the taxane group. After reducing tumor progression, the antibodies should be further applied until the progression of the disease. For the second-line therapy and further therapy lines, trastuzumab-emtansin (T-DM1) is often used, a combined chemotherapeutic molecule of HER2/neu-antibody and a systemic chemotherapy compound, capecitabine, a prodrug of 5-fluorouracil or tyrosin-kinase inhibitor (Lapatinib). It is very important to consider the pretreated adjuvant therapies and the side-effects of the therapy. The metastatic TNBC (mTNBC) tumor is a very challenging kind of cancer. Next to very innovative new therapies of phase-1 study trials, systemic chemotherapy compounds in mono- or poly-therapy regimes, there is a new way of medication with immune-checkpoint inhibitors given next to systemic chemotherapeutic compound [29]. Brain metastasis is associated with a poor prognosis, as treatment options are limited. Options of treatment involve multimodality approaches including surgery, radiotherapy, radiosurgery and sometimes systemic therapy [30]. Overall, upcoming therapies, especially targeted therapies and/or immune modulation, must show whether they suppress further metastatic progress, thus extending the overall survival of the patient.
3. CIRCULATING EXOSOMES AND MICRORNAS IN BREAST CANCER
3.1. Exosomes in Breast Cancer
Exosomes are nanovesicles, which are secreted and produced by almost all cell types. The approximate size is 30 to 100 nm in diameter and they play a role in the endosomal pathway by paracrine and autocrine cell communication [31]. Exosomes are loaded with a lot of different charges like proteins, lipids, mRNAs, miRs, long-noncoding RNA and DNA [9, 32]. Analyzing the content of exosomes might further affect the study of diseases and play a role in the understanding of those related to cancer research [33]. Exosomes are playing a role in tumor escape and also cancer immune surveillance through communication between immune cells and cancer cells [34]. Next to that, exosomes play a role in the signaling cascade from cancer cells to other cancer cells in order to induce cell growth, transformation, and survival signals. This could be shown through the use of exosomes from patients and breast cancer cell lines that induced transformation and tumor formation in non-tumorigenic mammary cells [35]. Exosomes travel in order to fulfil their metastatic spread to healthy organic sites to prime the environment as future metastatic niche [36]. They interfere in the glucose metabolism of normal healthy cells in order to influence the precancerous environment [36]. Exosomes can fuse preferentially with resident cells at their predicted destination using specific integrins at their surface. Integrins in tumor exosomes differ and determine their organotropic metastasis and could be used to predict organ-specific metastasis. In addition, exosomes can activate specific signaling pathways in resident cells in order to establish a favorable microenvironment that promotes the growth of disseminated TCs [37]. Riches et al. could show in an in vitro model, that a breast cancer cell line secretes higher amounts of exosomes than non-tumorous cell lines and that exosomes from normal mammary epithelial cells also inhibit exosome secretion by breast cancer cells in a tissue specific manner [38]. Exosomes derived from highly metastatic breast cancer can transfer increased metastatic capacity to a poorly metastatic tumor [39].
Exosomes can cross the BBB and influence signaling in TCs as well as in brain microvascular ECs of brain vessels. Circulating cancer cells can traverse the BBB and colonialize the brain [40]. Brain metastatic cells express protocadherin 7 (PCDH7), which promotes the assembly of carcinoma–astrocyte gap junctions allowing for passage of cGAMP from cancer cells to astrocytes [41]. This activates signaling pathways in astrocytes leading to a production of inflammatory cytokines that support growth and chemoresistance in brain-metastatic cells [41]. Also brain microvascular ECs express multiple protocadherin genes, which might be involved in the interaction of ECs with TCs [42]. The mechanism of breaching the BBB by exosomes is mainly unknown. Morad et al. postulated that exosomes breach the intact BBB by transcytosis [43]. Exosomes circumvent the low physiological rate of transcytosis in the BBB by decreasing the expression of the endosomal GTPase Rab7 that controls endosomal trafficking [43]. Other authors published that exosomes can cross the BBB only under inflamed conditions but not under normal conditions [44]. Exosomes promote cancer cell colonization in brain metastasis by upregulation of pro-inflammatory cytokines, which promote brain vascular remodeling [45]. Circulating exosomes carry miRs with potential regulatory functions at the BBB. For example, cancer-derived EVs containing miR-181c promote the destruction of the BBB through the abnormal localization of actin. This is achieved by downregulating the miR-181c target gene PDPK1 and then downregulating phosphorylated cofilin, which leads to a modulation of actin dynamics induced by cofilin [46]. A number of different miRs have been implicated to directly regulate targets in brain ECs. Expression of miR-155 was strongly upregulated by inflammatory cytokines in brain microvascular ECs and led to an increase in permeability. Genes involved in cell contact organization such as claudin-1 (CLDN1), annexin-2 (ANXA-2), DOCK-1 and syntenin-1 have been identified as direct targets of miR-155 in brain microvascular ECs [47]. Similarly, in addition to CLDN1, miR-212/132 also targets other endothelial junction complex genes, such as junctional adhesion molecule 3 (JAM3) and tight junction-associated protein 1 (TJAP1) leading to increased endothelial permeability [48]. MiR-150 directly targets angiopoietin receptor Tie-2 at BBB and its overexpression leads to increased endothelial permeability, while its inhibition contributes to BBB protection [49]. BBB-stabilizing effects also inhibit miR-143, which targets p53 upregulated modulator of apoptosis (PUMA) [50]. Overexpression of miR-210 results in increased endothelial permeability by downregulation of miR-210 targets within the junctional complex, occludin (OCLN) and β-catenin (CTNB1) [51]. MiR-34a regulates BBB by targeting several mitochondria-associated genes such as cytochrome c [52]. All miRs that can target genes from brain ECs can be used by TCs to cross the BBB. However, more studies on tumor and endothelial miRs are required to use miRs profiles as prognostic markers.
3.2. MicroRNAs in Breast Cancer as Potential Biomarkers
MiRs are single-stranded short (about 19 to 25 nucleotides) RNA molecules, negatively regulating gene expression. They bind to the 3’-untranslated region (3’-UTR) of their specific target mRNA and repress the initiation of translation or destabilize the target mRNA leading to its degradation [53].
Breast cancer is a heterogeneous disease in which each patients’ tumor has specific and different genetic characteristics [54, 55]. Those already known characteristics nowadays play a main role in the treatment, prognosis, and handling of the disease. In spite of all the existing knowledge and treatments, breast cancer becomes a life-threatening disease when cancer spreads from the origin breast tissue to other places and organs of the body (metastasis) [56]. At primary diagnosis, specific molecular characteristics are used to identify if the cancer has already spread and transformed, therefore in a systemic, mostly incurable disease. This raises the question, whether in addition to already established diagnostics and tumor characteristics (mammography, mammary Magnetic Resonance Imaging, tumor marker CA 15-3 / CEA, histological receptors and growth factors, gene analysis), if there are other markers for predicting the aggressiveness and metastasis potential of the tumor. MiRs might be a new and relevant tool to find a better way of prognosis prediction and understanding of metastasis development. In the following, we review the up to date published knowledge of miR peculiarities in different stages of breast cancer expansion.
3.2.1. Primary Tumors
Different studies show that various breast cancer subtypes present different kinds of miR expression [57-60]. Van Schooneveld and colleagues described various subtype-specific miRs in a study and meta-analyses. The miR-let-7c, miR-10a and let-7f were found to be associated with the luminal A type; miR-18a, miR-135b, miR-93 and miR-155 were shown to be associated with the basal type; miR-142-3p and miR-150 were associated with the HER2/neu type [58, 60]. Lowery and colleagues postulated in their study that with miR signatures ER, PR and HER2/neu, receptor status is predictable. Especially miR-342 should have a high expression in the ER-positive/HER2-positive tumors [3]. In in vitro cell culture investigations, Fkih M’hamed and colleagues showed that miR-10b, miR-26a, miR-146a and miR-153 could be possible TNBC biomarkers in the future [61]. In a very detailed overall systematic review, Adhami and colleagues postulated that especially two miRs (miR-21 and miR-210) were upregulated consistently. MiR-21 was upregulated in six profiling studies. In contrast, six miRs (miR-145, miR-139-5p, miR-195, miR-99a, miR-497 and miR-205) were downregulated consistently in at least three studies of the eleven regarded [62]. In a small clinical study, the authors used miR as a complementary tool in the diagnosis and prediction of treatment response under neoadjuvant chemotherapy and showed that especially before neoadjuvant therapy, exosomal miR-21 and miR-105 expression levels were higher in metastatic versus non-metastatic patients and healthy probands [63]. It could also be shown that higher levels of exosomal miR-21, miR-222, and miR-155 were significantly associated with the presence of circulating TCs [63]. There are also reports showing that a downregulation of miR-221/222 corresponds to an enhancement of tamoxifen sensitivity in TCs [64, 65].
3.2.2. Bone Metastatic Tumors
In advanced stages of breast cancer, patients suffer often from bone metastases, which also describe the incurability of the disease [66, 67]. Bone-only disease with bone as a single metastatic site has a better prognosis than those with visceral or both bone and visceral disease [68]. In the recent years, miR has become a subject of investigations analyzing the role of miR in the development of bone metastasis [69-72]. In in vitro studies, Cai et al. postulate that in mice models, especially miR-124 inhibits bone metastasis of breast
cancer by repressing interleukin-11 [73]. MiR-124 is described as a tumor suppressor in the development of bone metastasis [74, 75]. Next to miR-124, also miR-214 is strongly increased in the bone specimen of breast cancer patients with osteolytic bone metastases [76, 77]. In another study, five types of miRs (miR-33a, miR-133a, miR-141, miR-190, and miR-219) were shown to regulate tumor-induced osteoclast differentiation [78]. MiR-135 and miR-203 interact with the protein RUNX2, which plays a role in normal bone formation but is often dysregulated in bone-metastatic breast cancer cells [79, 80]. In another clinical trial, Zhao and colleagues showed that especially miR10b shows an overexpression in contrast to patients with no bone metastasis. They postulate therefore that miR-10b could be a useful biomarker in the future [81].
3.2.3. Visceral Metastatic Tumors
The dispersal of TCs in organs further manifests the systemic character of the disease. Also here, miR-10b is discussed as a potential marker to play a major role in the spread of metastasis, as it promotes metastasis in otherwise non-metastatic breast cancer cells [82]. This was shown by Ma et al. in in vitro models of metastatic breast cancer. He and his colleagues also postulate that the level of miR-10b expression in primary breast carcinomas correlates with the clinical progression of the disease. Ell and colleagues showed in in vitro models that the miR-23b/27b/24 cluster promotes breast cancer lung metastasis by targeting metastasis-suppressive gene prosaposin [83]. Huang et al. postulated that miR-373 and miR-520c are metastasis-promoting miRs, which are involved in tumor migration and invasion [84]. The infiltration of lymph nodes is often the first sign of metastatic spread. Chen and his colleagues investigated nodal positive patients and found a signature of four miRs, consisting of miR-191-5p, miR-214-3p, miR-451a, and miR-489, which seem to play a role in cell proliferation, migration, and invasion abilities [85]. MiR-21 is also discussed to be associated with advanced clinical stage, lymph node metastasis and patient poor prognosis [86].
3.2.4. Cerebral Metastatic Tumors
At least 10-30% of the breast cancer patients develop brain metastases, which is associated with a very poor prognosis with a one-year survival of 20% [87, 88]. Therefore, the need for specific biomarkers like miRs to identify potentially metastatic tumors in the early stage of the disease is very high, especially for high-risk patients such as HER2/neu positive and triple-negative patients. As a result of improved therapy opportunities, the development of brain metastases has become the most limiting factor in relation to time and quality [89]. During the formation of cerebral metastasis, there is an exceptional situation where single circulating tumor cells have to pass the BBB. There are many recent studies on up- or downregulated miRs in the metastatic tissues because of the importance of early detection and development of new therapies for cerebral metastasis. Analysis of a data-set with carcinoma patients and patients with cerebral metastasis showed that miR-17-5p and miR-16-5p have the highest association with targeted mRNAs (such as B-cell lymphoma 2 (BLC-2), SMAD3 and SDOSCS1) and regulate processes of metastatic progression [88]. Moreover, there was a negative correlation between miR-17-5p and the total observed viability of patients [88]. Heparanase (HPSE), which is considered to be an important enzyme in tumor-formation and metastatic progression, is overexpressed in brain metastatic tissue. It was shown that miR-1258 inhibits HPSE and therefore, a low amount of it correlates with highly aggressive brain metastatic breast cancer [90].
Furthermore, in a report about the miR profile of cancer stem cells, which are considered to be a key player in metastatic tumors, a significantly lower level of miR-7 was shown. MiR-7 modulates the activity of KLF4, which is an important stem-cell gene. In addition to that, a high level of miR-7 inhibits the development of brain metastasis in animal models [91]. Plasma level of specific miRs could be a predictive biomarker of chemotherapy resistance in metastatic breast cancer. As shown by Shao et al., plasma levels of miR-200a and miR-210 showed high diagnostic accuracy for distinguishing chemotherapy-sensitive from chemotherapy-resistant patients [92]. MiR-20b showed increased expression in brain metastases of breast cancer patients, compared to primary breast tumors and patients without brain metastasis [93]. More research is needed to identify more potential biomarkers of brain metastatic breast cancer as well as therapeutic targets.
4. BLOOD-BRAIN-BARRIER
4.1. BBB Characteristics
The BBB is a physical and metabolic barrier formed by specialized brain microvascular ECs, together with other components of the neurovascular unit (NVU) such as pericytes, astrocytes, microglia, neurons and extracellular matrix (ECM). Brain microvascular ECs express high levels of tight junction (TJ) proteins, efflux and influx transporters that selectively regulate the movement of molecules through the BBB [94]. TCs interact with brain ECs affecting them in different ways [95, 96]. For example, breast cancer cells expressing low levels of claudin-3, -4 and -7 metastasize with a high probability to the brain [97]. Other molecules, such as heparin-binding EGF-like growth factor (HB-EGF) and cyclooxygenase 2 highly expressed by TCs facilitate brain metastasis process [40]. The key players of metastasis forming can be identified at a molecular level in animal models or in vitro by using isolated cells of NVU.
4.2. In vitro Models for Studying Mechanisms of Breast Cancer Metastasis
During the last two decades, many in vitro BBB models have been developed due to the increasing need to facilitate cerebrovascular research and drug development [98]. However, none of these cell culture models fully reflect the complexity of the BBB structure and its dynamics due to the limitations of an in vitro system [99]. Therefore, data acquired in studies where such models have been applied might be considered with respect to the chosen model. The most important cell type of all BBB in vitro models is brain microvascular ECs building a highly selective monolayer. Besides brain microvascular ECs, other cell types associated or interacting with the BBB can be involved in such models. The advantage of co-cultures comprising two or three different cell types allows breaking down the complexity of the BBB into the cell types of interest and enables the analysis of cellular processes with regard to a limited number of parameters. Here, we outline the most widely used in vitro BBB models with a special focus on opportunities to study the interaction of brain microvascular ECs with TCs.
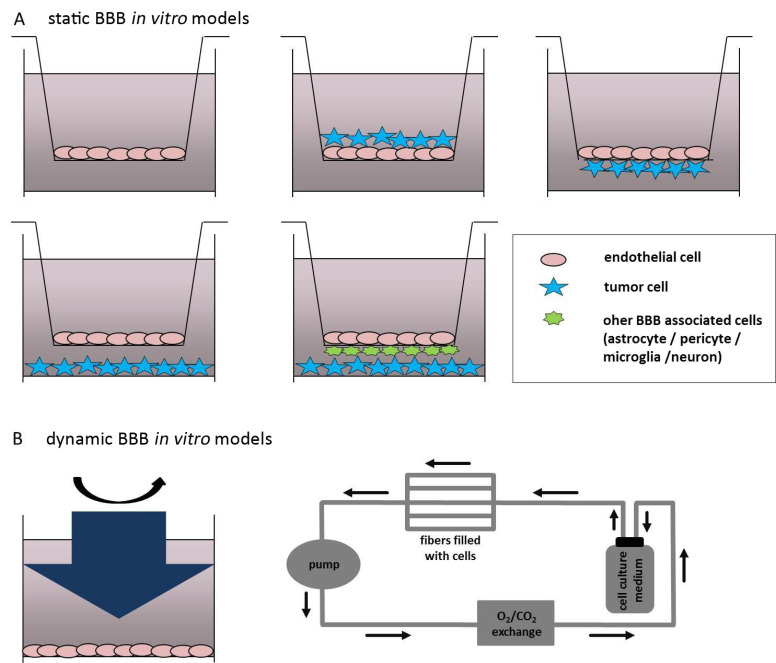
BBB in vitro models can be subdivided into two categories, static and dynamic, as schematically depicted in Fig. (1A & B).
Fig. (1).
In vitro models to study the blood-brain barrier-tumor cell interactions
(A) Static Blood-Brain Barrier (BBB) in vitro models involving a single monoculture of brain microvascular ECs in a transwell insert (upper left transwell insert); a co-culture system of brain endothelial cells (ECs) grown in the presence of tumor cells (TCs) at the luminal side of the insert; co-culture of brain ECs on the luminal indirectly contacting TCs on the abluminal side of the transwell insert (upper right transwell insert); brain ECs grown in the upper compartment of the transwell insert and TCs seeded on the bottom of the cell culture vessel in which the insert is placed (lower left transwell insert); luminally grown cerebrovascular ECs indirectly contacting other BBB associated cell types (astrocytes, pericytes, neurons or microglia cells) and TCs seeded on the bottom of the cell culture vessel. (B) Dynamic BBB in vitro models represented by the cone-plate apparatus generating shear stress to brain ECs (left picture) and by the microporous hollow fibers connected with a speed pump generating shear stress and with a gas-permeable tubing system allowing O2/CO2 exchange of the system (right picture).
4.2.1. Static In Vitro Blood-Brain Barrier Models
Depending on the number of cell types involved, static BBB models can be subcategorized into monocultures or co-cultures. Moreover, there are primary cell cultures and immortalized cell lines used to study the BBB. Besides rodents, which are still the most widely used animals in the BBB research, brain ECs have been isolated from larger animals, such as bovine, porcine and non-human primate in order to increase the EC yield [100-104]. However, problem appearing with the use of brain microvascular ECs derived from larger animals is that there are no or only a few respective antibodies available for these species and further, there are no transgenic animals available.
There are some important requirements that generally applicable BBB in vitro models have to incorporate [105, 106]. The polarized localization of transporters, receptors and enzymes is necessarily required in brain ECs used for BBB studies, but the suitable expression of BBB-specific tight junction (TJ) proteins responsible for the characteristically high TEER of brain ECs displays a serious problem in the development of suitable BBB in vitro systems [105, 107-109]. Primarily, low passage brain microvascular ECs were identified to display a differentiated phenotype and physiological and biochemical properties, that are characteristic for the BBB in vivo [102, 110]. Nevertheless, there are some disadvantages of the usage of primary brain ECs, which have to be mastered. First, isolation and cultivation of primary brain ECs cultures are limited due to the cell number within the brain. Brain microvascular ECs account only for 0.1% (v/v) of the brain, meaning that a large number of animals might be necessary to generate enough cells for the respective experiment. Moreover, there is a high probability to contaminate the brain microvascular EC culture with other cell types. A temporary application of puromycin into the cell culture medium has been reported to improve the purity of primary brain ECs [111].
In order to solve the problem of yield and purity, the immortalized brain microvascular EC cell cultures were developed, which however, often resulted in a more de-differentiated phenotype [98]. Moreover, not all immortalized systems achieve the high TEER of brain microvascular ECs in vivo due to the minor expression of TJ proteins [112]. However, some immortalized BBB systems reflect BBB properties known from primary brain EC cultures, and because of the lower effort in cultivation, they are widely used in the research of cerebrovascular diseases [113-118].
A transwell insert mimicking the blood (luminal) and the well in which the insert is placed, simulating the parenchymal (abluminal) side of a vessel can be used as a simple BBB in vitro system (Fig. 1A). The pore size of the transwell insert should be 0.4 µm in order to allow the exchange of small molecules and growth factors secreted by the cells. Furthermore, the relatively small pore size functions as a migration barrier for the cells from one compartment to the other. The application of transwell inserts allows the co-cultivation of two or more different cell types together with brain microvascular ECs in one system, thereby allowing a more realistic simulation of the BBB [119-121]. Besides the interaction of brain ECs with other types of the NVU, which has in part been described in this section, transwell insert can be used for co-culturing ECs in the presence of tumor-secreted factors or brain TCs/TCs isolated from brain metastases, which allows the interaction between both cell types and investigation of the signaling pathways. As depicted in Fig. 1A, TCs can be grown on the top of the brain EC culture at the luminal side of the transwell. This co-culture system might be used in order to study the interaction of TCs with ECs during the period prior to extravasation from the bloodstream into the CNS. The disadvantage of this culture is that there is no possibility to separate the cultures from each other. Therefore gene or protein expression analysis cannot be performed. However, electrophysiological TEER measurements are still feasible applying a respective control culture of brain ECs grown alone on the transwell insert. Comparing with that model, the interaction of TCs with the cerebrovascular endothelium on molecular level would be possible by seeding ECs luminally and TCs abluminally, directly on the transwell (Fig. 1A). The advantage of these models is that either electrophysiological measurements or gene and protein expression analyses of each culture are feasible. This co-culture model might mimic the co-option phase of TCs with brain ECs after invasion of the brain parenchyma, since the TCs are in indirect contact with the abluminal side of the EC culture. A report where this kind of co-culture system of immortalized human brain ECs (hCMEC/D3 cells) with human medulloblastoma cell line (VC312R cells) has been applied to study cellular interactions between EC and TCs revealed an impact of TCs on permeability and transport properties of brain ECs [122]. Similarly to this model, TCs might also be seeded at the bottom of the cell culture vessel, as it is demonstrated in the left lower transwell Fig. 1A. This technique is even easily applicable when compared to the co-culture system described above. Following this co-culture system, the impact of mouse glioma cell line GL261 on the expression of relevant genes involved in Hedgehog pathway, as well as on the proliferation and migration of the brain EC (b.END3 cell line) has been tested [123]. Finally, a co-culture model is prepared with luminally grown brain ECs, other BBB associated cells, i.e. astrocytes or pericytes seeded at the abluminal side of the transwell insert, and TCs seeded on the bottom of the cell culture vessel where the transwell insert is placed. This co-culture system might allow analyzing a relatively physiological BBB in vitro model that interacts with TCs. All cell types involved in this model can be analyzed exclusively on a molecular level. A recent study of Anfuso and colleagues established a similar triple-culture model applying primary ECs with abluminal cultured pericytes and C6 glioma cells grown on the bottom of the culture plate [124]. As controls, brain ECs were also cultured with either C6 cells or pericytes alone (double co-cultures). Following this technique, the authors of the study investigated the role of pericytes in the interaction with brain microvascular ECs and glioma cells, the influence of TCs in the presence of pericytes on EC permeability, TJ expression, and prostaglandin production. Moreover, pericytes used in this culture system exhibited an important modulating role in the initial stages of angiogenesis driven by brain TCs [124]. Brain microvascular ECs in co-cultures with TCs and pericytes or astrocytes are characterized by a different permeability and are therefore ideal for cell-cell interaction through growth factors released by the cell types that are involved in the system and have an impact on the identification and optimization of drugs. The most significant disadvantage of these co-culture models is the lack of shear stress, being critical for the induction and maintenance of the BBB phenotype [125, 126].
4.2.2. Dynamic In Vitro Blood-Brain Barrier Models
A further parameter reflecting the BBB under physiological conditions and having an important impact on brain microvascular EC properties is the presence of shear stress. Shear stress affects the expression of transporters and cellular contacts directly influencing the brain microvascular EC monolayer permeability [127]. There are different kinds of dynamic BBB models in vitro, including cone-plates and microfluidic in vitro models. Cone-plates transmit shear forces on ECs through rotating inside of the cell culture medium (Fig. 1B). The limiting factor in this dynamic model is that besides brain microvascular ECs, no other cell types can be applied to the experiment, therefore diminishing the significance of data it generates and the application for brain metastasis research in vitro.
To allow the application of shear stress and incorporate two different cell types, brain microvascular ECs and TCs (or other BBB-associated cell types), the microporous hollow fibers are used [128-131]. In this system, brain microvascular ECs are grown in the luminal, the inner porous of the hollow fiber, whereas the TCs are seeded on the abluminal, the outer side of the fiber. A variable-speed pump pumps the culture medium into this culture system and generates shear stress that can vary from 5-23 dynes/cm2 [132, 133]. Further, a gas-permeable tubing system maintains a stable microenvironment by exchanging O2 and CO2. Cucullo and colleagues used human aortic ECs and the C6 glioma cell line in order to determine the impact of TCs on ECs in the presence of shear stress [134].
4.2.3. Human Stem-cell Based In Vitro BBB Models
The generation of human brain capillary-like EC cultures from pluripotent stem cells constitutes the new approach allowing purification of a large number of cells of interest as well as characterization of BBB function in the direct context of different pathologies including brain tumors or metastasis compared to normal individuals. So far, there are varying protocols for isolation of brain capillary-like ECs from induced pluripotent stem cells (iPSCs) that relied on non-defined (i.e. containing serum) [135-139] or chemically defined media without prior purification of endothelial progenitor cells (EPCs) [140, 141]. These methodologies focus on selective isolation of brain capillary-like ECs from a heterogeneous cell mixture containing neural progenitor cells using selective media and ECM components. Due to the heterogeneity of the cell suspensions, serum or ECM mixtures, specification of iPSCs into brain capillary-like ECs and the cost efficiency remained unsatisfactory and insufficient. Moreover, the yield and the phenotypic features of the brain capillary-like ECs were strongly dependent on the original iPSC line applied [135]. In contrast, chemically defined media allow better reproducibility, control of the basic experimental conditions and the framework of the cell purification but remain cost-intensive.
Qian et al. described the derivation of brain capillary-like ECs using an intermediary EPC population and chemically defined media [142]. The differentiation of iPSCs into EPCs was defined by the expression of VEGFR2 and CD31. The specification of EPCs into brain capillary-like ECs was assured by retinoic acid. The influencing role of other soluble or non-soluble signaling and small molecules, and growth factors involved in the development of specific BBB characteristics was however neglected.
A recent work of Praça et al. described a complex multifactorial experimental setup to derive brain capillary-like ECs followed by a molecular and functional characterization of differential BBB properties [143]. The initial isolation was performed by differentiating brain capillary-like ECs from an intermediary EPC population followed by modulation of three different signaling pathways: VEGF, retinoic acid and WNT and applying chemically defined endothelial cell medium. Compared with purification protocols shown by others, Praça and colleagues described a method to isolate brain capillary-like ECs with relatively high TEER. Moreover, co-culture experiments with pericytes showed a substantial maturation of the brain capillary-like ECs through an increase of TEER values and monolayer organization by improving the expression of cell-cell contact molecules.
As experiments of Praça, Lippmann and Katt have demonstrated, the control of the Wnt3a, VEGF and retinoic acid signaling pathways plays a decisive role in the differentiation of EPCs into brain capillary-like ECs and their maturation. Moreover, a defined ECM is important to provide physical stabilization and provides molecules essential to develop BBB characteristics. Platforms using channels structured in thick three-dimensional hydrogels could provide a useful tool for multicellular approaches in vitro by mimicking tissue structures that play a role in differentiating EPCs [144]. On one hand, defined ECM can be achieved by chemically applied substrates or by cultivating brain capillary-like ECs with other types of cells building the NVU. The decellularized ECM components are derived from different animal sources. The impact of cell-free ECMs of human origin should be investigated in future studies. In this context, iPSCs also play an important role. Patient-derived brain capillary-like ECs differentiated from iPSCs of breast cancer patients versus healthy individuals would be a useful tool for studying cellular mechanisms of metastasis forming.
Another useful human brain capillary-like ECs model has been established from human cord blood-derived hematopoietic stem cells [145-147]. The cells were first differentiated into ECs, then the BBB properties were induced by co-culture with pericytes. Such a model appeared to be very reproducible and easy to establish.
In summary, BBB in vitro models play an important role in data collection regarding the BBB function under physiological and pathological conditions. In addition, the use of in vitro models allows the study of drugs used in various neurological disorders, including brain tumors. Knowing the advantages and disadvantages of the BBB models presented here, the appropriate cell culture system relevant to the study must be selected and the generated data might be adequately interpreted.
CONCLUSION AND FUTURE PERSPECTIVES
Treatment for brain metastasis is the most challenging. Finding novel therapies to prevent the metastatic progression of breast cancer and treat brain metastases require a better understanding of the biology and molecular mechanisms behind this process. The in vitro models, especially those based on cells from human origin, may contribute to gaining more knowledge and finding novel biomarkers of metastatic brain disease.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The study was funded by the Universitätsbund Würzburg (grant number AZ 19-16) and the institutional funds, Germany.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Lee R., Feinbaum R., Ambros V. A short history of a short RNA. Cell. 2004;116(2) Suppl.:S89–S92,. doi: 10.1016/S0092-8674(04)00035-2. [DOI] [PubMed] [Google Scholar]
- 2.Consortium E.P. ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowery A.J., Miller N., Devaney A., McNeill R.E., Davoren P.A., Lemetre C., Benes V., Schmidt S., Blake J., Ball G., Kerin M.J. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res. 2009;11(3):R27. doi: 10.1186/bcr2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Z., Huang D., Ni S., Peng Z., Sheng W., Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int. J. Cancer. 2010;127(1):118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 5.Maierthaler M., Benner A., Hoffmeister M., Surowy H., Jansen L., Knebel P., Chang-Claude J., Brenner H., Burwinkel B. Plasma miR-122 and miR-200 family are prognostic markers in colorectal cancer. Int. J. Cancer. 2017;140(1):176–187. doi: 10.1002/ijc.30433. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C., Wang C., Chen X., Yang C., Li K., Wang J., Dai J., Hu Z., Zhou X., Chen L., Zhang Y., Li Y., Qiu H., Xing J., Liang Z., Ren B., Yang C., Zen K., Zhang C.Y. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin. Chem. 2010;56(12):1871–1879. doi: 10.1373/clinchem.2010.147553. [DOI] [PubMed] [Google Scholar]
- 7.Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 8.András I.E., Toborek M. Extracellular vesicles of the blood-brain barrier. Tissue Barriers. 2015;4(1):: e1131804. doi: 10.1080/21688370.2015.1131804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.György B., Szabó T.G., Pásztói M., Pál Z., Misják P., Aradi B., László V., Pállinger E., Pap E., Kittel A., Nagy G., Falus A., Buzás E.I. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011;68(16):2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu R., Rai A., Chen M., Suwakulsiri W., Greening D.W., Simpson R.J. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018;15(10):617–638. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang M., Yu F., Ding H., Wang Y., Li P., Wang K. Emerging function and clinical values of exosomal MicroRNAs in cancer. Mol. Ther. Nucleic Acids. 2019;16:791–804. doi: 10.1016/j.omtn.2019.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sempere L.F., Keto J., Fabbri M. Exosomal MicroRNAs in breast cancer towards diagnostic and therapeutic applications. Cancers (Basel) 2017;9(7):: E71. doi: 10.3390/cancers9070071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahrami A., Aledavood A., Anvari K., Hassanian S.M., Maftouh M., Yaghobzade A., Salarzaee O., ShahidSales S., Avan A. The prognostic and therapeutic application of microRNAs in breast cancer: Tissue and circulating microRNAs. J. Cell. Physiol. 2018;233(2):774–786. doi: 10.1002/jcp.25813. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G., Zhang W., Li B., Stringer-Reasor E., Chu C., Sun L., Bae S., Chen D., Wei S., Jiao K., Yang W.H., Cui R., Liu R., Wang L. MicroRNA-200c and microRNA- 141 are regulated by a FOXP3-KAT2B axis and associated with tumor metastasis in breast cancer. Breast Cancer Res. 2017;19(1):73. doi: 10.1186/s13058-017-0858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blecharz K.G., Colla R., Rohde V., Vajkoczy P. Control of the blood-brain barrier function in cancer cell metastasis. Biol. Cell. 2015;107(10):342–371. doi: 10.1111/boc.201500011. [DOI] [PubMed] [Google Scholar]
- 16.Weidle U.H., Niewöhner J., Tiefenthaler G. The blood-brain barrier challenge for the treatment of brain cancer, secondary brain metastases, and neurological diseases. Cancer Genomics Proteomics. 2015;12(4):167–177. [PubMed] [Google Scholar]
- 17.Vasconcelos I., Hussainzada A., Berger S., Fietze E., Linke J., Siedentopf F., Schoenegg W. The St. Gallen surrogate classification for breast cancer subtypes successfully predicts tumor presenting features, nodal involvement, recurrence patterns and disease free survival. Breast. 2016;29:181–185. doi: 10.1016/j.breast.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Goldhirsch A., Ingle J.N., Gelber R.D., Coates A.S., Thürlimann B., Senn H.J. ENCODE project consortium. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann. Oncol. 2009;20(8):1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gluz O., Hartkopf A., Kümmel S., Marmé F. ASCO 2019: new results in breast cancer. Breast Care (Basel) 2019;14(4):256–258. doi: 10.1159/000501874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Untch M., Thomssen C., Bauerfeind I., Braun M., Brucker S.Y., Felberbaum R., Hagemann F., Haidinger R., Hönig A., Huober J., Jackisch C., Kolberg H.C., Kolberg-Liedtke C., Kühn T., Lüftner D., Maass N., Reimer T., Schneeweiss A., Schumacher-Wulf E., Schütz F., Thill M., Wuerstlein R., Fasching P.A., Harbeck N. Primary therapy of early breast cancer: evidence, controversies, consensus: spectrum of opinion of german specialists on the 16th st. gallen international breast cancer conference (vienna 2019). Geburtshilfe Frauenheilkd. 2019;79(6):591–604. doi: 10.1055/a-0897-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldhirsch A., Wood W.C., Coates A.S., Gelber R.D., Thürlimann B., Senn H.J. Panel members. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liedtke C., Thill M., Jackisch C., Thomssen C., Müller V., Janni W., Janni W., AGO Breast Committee* AGO breast committee*. AGO recommendations for the diagnosis and treatment of patients with early breast cancer: update 2017. Breast Care (Basel) 2017;12(3):172–183. doi: 10.1159/000477575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneeweiss A., Denkert C., Fasching P.A., Fremd C., Gluz O., Kolberg-Liedtke C., Loibl S., Lück H.J. Diagnosis and therapy of triple-negative breast cancer (TNBC) - recommendations for daily routine practice. Geburtshilfe Frauenheilkd. 2019;79(6):605–617. doi: 10.1055/a-0887-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wöckel A., Festl J., Stüber T., Brust K., Krockenberger M., Heuschmann P.U., Jírů-Hillmann S., Albert U.S., Budach W., Follmann M., Janni W., Kopp I., Kreienberg R., Kühn T., Langer T., Nothacker M., Scharl A., Schreer I., Link H., Engel J., Fehm T., Weis J., Welt A., Steckelberg A., Feyer P., König K., Hahne A., Baumgartner T., Kreipe H.H., Knoefel W.T., Denkinger M., Brucker S., Lüftner D., Kubisch C., Gerlach C., Lebeau A., Siedentopf F., Petersen C., Bartsch H.H., Schulz-Wendtland R., Hahn M., Hanf V., Müller-Schimpfle M., Henscher U., Roncarati R., Katalinic A., Heitmann C., Honegger C., Paradies K., Bjelic-Radisic V., Degenhardt F., Wenz F., Rick O., Hölzel D., Zaiss M., Kemper G., Budach V., Denkert C., Gerber B., Tesch H., Hirsmüller S., Sinn H.P., Dunst J., Münstedt K., Bick U., Fallenberg E., Tholen R., Hung R., Baumann F., Beckmann M.W., Blohmer J., Fasching P., Lux M.P., Harbeck N., Hadji P., Hauner H., Heywang-Köbrunner S., Huober J., Hübner J., Jackisch C., Loibl S., Lück H.J., von Minckwitz G., Möbus V., Müller V., Nöthlings U., Schmidt M., Schmutzler R., Schneeweiss A., Schütz F., Stickeler E., Thomssen C., Untch M., Wesselmann S., Bücker A., Buck A., Stangl S. Interdisciplinary screening, diagnosis, therapy and follow-up of breast cancer. Guideline of the DGGG and the DKG (S3-Level, AWMF Registry Number 032/045OL, December 2017) - Part 2 with recommendations for the therapy of primary, recurrent and advanced breast cancer. Geburtshilfe Frauenheilkd. 2018;78(11):1056–1088. doi: 10.1055/a-0646-4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehmann B.D., Jovanović B., Chen X., Estrada M.V., Johnson K.N., Shyr Y., Moses H.L., Sanders M.E., Pietenpol J.A. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11(6):: e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thill M., Jackisch C., Janni W., Müller V., Albert U.S., Bauerfeind I., Blohmer J., Budach W., Dall P., Diel I., Fasching P.A., Fehm T., Friedrich M., Gerber B., Hanf V., Harbeck N., Huober J., Kolberg-Liedtke C., Kreipe H.H., Krug D., Kühn T., Kümmel S., Loibl S., Lüftner D., Lux M.P., Maass N., Möbus V., Müller-Schimpfle M., Mundhenke C., Nitz U., Rhiem K., Rody A., Schmidt M., Schneeweiss A., Schütz F., Sinn H.P., Solbach C., Solomayer E.F., Stickeler E., Thomssen C., Untch M., Wenz F., Witzel I., Wöckel A., Ditsch N. AGO recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer: update 2019. Breast Care (Basel) 2019;14(4):247–255. doi: 10.1159/000500999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann M., Maass N., Costa S.D., Schneeweiss A., Loibl S., Sütterlin M.W., Schrader I., Gerber B., Bauer W., Wiest W., Tomé O., Distelrath A., Hagen V., Kleine-Tebbe A., Ruckhaeberle E., Mehta K., von Minckwitz G. GBG-39 Trialists. First-line therapy with moderate dose capecitabine in metastatic breast cancer is safe and active: results of the MONICA trial. Eur. J. Cancer. 2010;46(18):3184–3191. doi: 10.1016/j.ejca.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Thill M., Liedtke C., Müller V., Janni W., Schmidt M., Committee A.G.O.B., AGO Breast Committee AGO breast committee. AGO recommendations for the diagnosis and treatment of patients with advanced and metastatic breast cancer: update 2018. Breast Care (Basel) 2018;13(3):209–215. doi: 10.1159/000489331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Hegg R., Im S.A., Shaw Wright G., Henschel V., Molinero L., Chui S.Y., Funke R., Husain A., Winer E.P., Loi S., Emens L.A., IMpassion130 Trial Investigators IMpassion130 trial investigators. Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 30.Kotecki N., Lefranc F., Devriendt D., Awada A. Therapy of breast cancer brain metastases: challenges, emerging treatments and perspectives. Ther. Adv. Med. Oncol. 2018;10:: 1758835918780312. doi: 10.1177/1758835918780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl P.D., Barbieri M.A. Multivesicular bodies and multivesicular endosomes: the “ins and outs” of endosomal traffic. Sci. STKE. 2002;2002(141):pe32. doi: 10.1126/stke.2002.141.pe32. [DOI] [PubMed] [Google Scholar]
- 32.Mathivanan S., Ji H., Simpson R.J. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Filipazzi P., Bürdek M., Villa A., Rivoltini L., Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin. Cancer Biol. 2012;22(4):342–349. doi: 10.1016/j.semcancer.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Barros F.M., Carneiro F., Machado J.C., Melo S.A. Exosomes and immune response in cancer: friends or foes? Front. Immunol. 2018;9:730. doi: 10.3389/fimmu.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melo S.A., Sugimoto H., O’Connell J.T., Kato N., Villanueva A., Vidal A., Qiu L., Vitkin E., Perelman L.T., Melo C.A., Lucci A., Ivan C., Calin G.A., Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fong M.Y., Zhou W., Liu L., Alontaga A.Y., Chandra M., Ashby J., Chow A., O’Connor S.T., Li S., Chin A.R., Somlo G., Palomares M., Li Z., Tremblay J.R., Tsuyada A., Sun G., Reid M.A., Wu X., Swiderski P., Ren X., Shi Y., Kong M., Zhong W., Chen Y., Wang S.E. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015;17(2):183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., Singh S., Williams C., Soplop N., Uryu K., Pharmer L., King T., Bojmar L., Davies A.E., Ararso Y., Zhang T., Zhang H., Hernandez J., Weiss J.M., Dumont-Cole V.D., Kramer K., Wexler L.H., Narendran A., Schwartz G.K., Healey J.H., Sandstrom P., Labori K.J., Kure E.H., Grandgenett P.M., Hollingsworth M.A., de Sousa M., Kaur S., Jain M., Mallya K., Batra S.K., Jarnagin W.R., Brady M.S., Fodstad O., Muller V., Pantel K., Minn A.J., Bissell M.J., Garcia B.A., Kang Y., Rajasekhar V.K., Ghajar C.M., Matei I., Peinado H., Bromberg J., Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riches A., Campbell E., Borger E., Powis S. Regulation of exosome release from mammary epithelial and breast cancer cells - a new regulatory pathway. Eur. J. Cancer. 2014;50(5):1025–1034. doi: 10.1016/j.ejca.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 39.Gorczynski R.M., Erin N., Zhu F. Serum-derived exosomes from mice with highly metastatic breast cancer transfer increased metastatic capacity to a poorly metastatic tumor. Cancer Med. 2016;5(2):325–336. doi: 10.1002/cam4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bos P.D., Zhang X.H., Nadal C., Shu W., Gomis R.R., Nguyen D.X., Minn A.J., van de Vijver M.J., Gerald W.L., Foekens J.A., Massagué J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Q., Boire A., Jin X., Valiente M., Er E.E., Lopez-Soto A., Jacob L., Patwa R., Shah H., Xu K., Cross J.R., Massagué J. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dilling C., Roewer N., Förster C.Y., Burek M. Multiple protocadherins are expressed in brain microvascular endothelial cells and might play a role in tight junction protein regulation. J. Cereb. Blood Flow Metab. 2017;37(10):3391–3400. doi: 10.1177/0271678X16688706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morad G., Carman C.V., Hagedorn E.J., Perlin J.R., Zon L.I., Mustafaoglu N., Park T.E., Ingber D.E., Daisy C.C., Moses M.A. Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano. 2019;13(12):13853–13865. doi: 10.1021/acsnano.9b04397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen C.C., Liu L., Ma F., Wong C.W., Guo X.E., Chacko J.V., Farhoodi H.P., Zhang S.X., Zimak J., Ségaliny A., Riazifar M., Pham V., Digman M.A., Pone E.J., Zhao W. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell. Mol. Bioeng. 2016;9(4):509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigues G., Hoshino A., Kenific C.M., Matei I.R., Steiner L., Freitas D., Kim H.S., Oxley P.R., Scandariato I., Casanova-Salas I., Dai J., Badwe C.R., Gril B., Tešić Mark M., Dill B.D., Molina H., Zhang H., Benito-Martin A., Bojmar L., Ararso Y., Offer K., LaPlant Q., Buehring W., Wang H., Jiang X., Lu T.M., Liu Y., Sabari J.K., Shin S.J., Narula N., Ginter P.S., Rajasekhar V.K., Healey J.H., Meylan E., Costa-Silva B., Wang S.E., Rafii S., Altorki N.K., Rudin C.M., Jones D.R., Steeg P.S., Peinado H., Ghajar C.M., Bromberg J., de Sousa M., Pisapia D., Lyden D. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat. Cell Biol. 2019;21(11):1403–1412. doi: 10.1038/s41556-019-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tominaga N., Kosaka N., Ono M., Katsuda T., Yoshioka Y., Tamura K., Lötvall J., Nakagama H., Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Ramirez M.A., Wu D., Pryce G., Simpson J.E., Reijerkerk A., King-Robson J., Kay O., de Vries H.E., Hirst M.C., Sharrack B., Baker D., Male D.K., Michael G.J., Romero I.A. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J. 2014;28(6):2551–2565. doi: 10.1096/fj.13-248880. [DOI] [PubMed] [Google Scholar]
- 48.Burek M., König A., Lang M., Fiedler J., Oerter S., Roewer N., Bohnert M., Thal S.C., Blecharz-Lang K.G., Woitzik J., Thum T., Förster C.Y. Hypoxia-induced MicroRNA-212/132 alter blood-brain barrier integrity through inhibition of tight junction-associated proteins in human and mouse brain microvascular endothelial cells. Transl. Stroke Res. 2019;10(6):672–683. doi: 10.1007/s12975-018-0683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang Z., He Q.W., Li Q., Chen X.L., Baral S., Jin H.J., Zhu Y.Y., Li M., Xia Y.P., Mao L., Hu B. MicroRNA-150 regulates blood-brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J. 2016;30(6):2097–2107. doi: 10.1096/fj.201500126. [DOI] [PubMed] [Google Scholar]
- 50.Bai Y., Zhang Y., Hua J., Yang X., Zhang X., Duan M., Zhu X., Huang W., Chao J., Zhou R., Hu G., Yao H. Silencing microRNA-143 protects the integrity of the blood-brain barrier: implications for methamphetamine abuse. Sci. Rep. 2016;6:35642. doi: 10.1038/srep35642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma Q., Dasgupta C., Li Y., Huang L., Zhang L. MicroRNA-210 suppresses junction proteins and disrupts blood-brain barrier integrity in neonatal rat hypoxic-ischemic brain injury. Int. J. Mol. Sci. 2017;18(7):: E1356. doi: 10.3390/ijms18071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bukeirat M., Sarkar S.N., Hu H., Quintana D.D., Simpkins J.W., Ren X. MiR-34a regulates blood-brain barrier permeability and mitochondrial function by targeting cytochrome c. J. Cereb. Blood Flow Metab. 2016;36(2):387–392. doi: 10.1177/0271678X15606147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z., Rana T.M. Therapeutic targeting of microRNAs: current status and future challenges. Nat. Rev. Drug Discov. 2014;13(8):622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 54.Perou C.M., Sørlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., Fluge O., Pergamenschikov A., Williams C., Zhu S.X., Lønning P.E., Børresen-Dale A.L., Brown P.O., Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 55.Sørlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Thorsen T., Quist H., Matese J.C., Brown P.O., Botstein D., Lønning P.E., Børresen-Dale A.L. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 57.Serpico D., Molino L., Di Cosimo S. microRNAs in breast cancer development and treatment. Cancer Treat. Rev. 2014;40(5):595–604. doi: 10.1016/j.ctrv.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 58.van Schooneveld E., Wildiers H., Vergote I., Vermeulen P.B., Dirix L.Y., Van Laere S.J. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015;17:21. doi: 10.1186/s13058-015-0526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blenkiron C., Goldstein L.D., Thorne N.P., Spiteri I., Chin S.F., Dunning M.J., Barbosa-Morais N.L., Teschendorff A.E., Green A.R., Ellis I.O., Tavaré S., Caldas C., Miska E.A. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8(10):R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurozumi S., Yamaguchi Y., Kurosumi M., Ohira M., Matsumoto H., Horiguchi J. Recent trends in microRNA research into breast cancer with particular focus on the associations between microRNAs and intrinsic subtypes. J. Hum. Genet. 2017;62(1):15–24. doi: 10.1038/jhg.2016.89. [DOI] [PubMed] [Google Scholar]
- 61.Fkih M’hamed I., Privat M., Ponelle F., Penault-Llorca F., Kenani A., Bignon Y.J. Identification of miR-10b, miR-26a, miR-146a and miR-153 as potential triple-negative breast cancer biomarkers. Cell Oncol. (Dordr.) 2015;38(6):433–442. doi: 10.1007/s13402-015-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adhami M., Haghdoost A.A., Sadeghi B., Malekpour Afshar R. Candidate miRNAs in human breast cancer biomarkers: a systematic review. Breast Cancer. 2018;25(2):198–205. doi: 10.1007/s12282-017-0814-8. [DOI] [PubMed] [Google Scholar]
- 63.Rodríguez-Martínez A., de Miguel-Pérez D., Ortega F.G., García-Puche J.L., Robles-Fernández I., Exposito J., Martorell-Marugan J., Carmona-Sáez P., Garrido-Navas M.D.C., Rolfo C., Ilyine H., Lorente J.A., Legueren M., Serrano M.J. Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy. Breast Cancer Res. 2019;21(1):21. doi: 10.1186/s13058-019-1109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gan R., Yang Y., Yang X., Zhao L., Lu J., Meng Q.H. Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. Cancer Gene Ther. 2014;21(7):290–296. doi: 10.1038/cgt.2014.29. [DOI] [PubMed] [Google Scholar]
- 65.Zhao J.J., Lin J., Yang H., Kong W., He L., Ma X., Coppola D., Cheng J.Q. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J. Biol. Chem. 2008;283(45):31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Pulido C., Vendrell I., Ferreira A.R., Casimiro S., Mansinho A., Alho I., Costa L. Bone metastasis risk factors in breast cancer. Ecancermedicalscience. 2017;11:715. doi: 10.3332/ecancer.2017.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 68.Ahn S.G., Lee H.M., Cho S.H., Lee S.A., Hwang S.H., Jeong J., Lee H.D. Prognostic factors for patients with bone-only metastasis in breast cancer. Yonsei Med. J. 2013;54(5):1168–1177. doi: 10.3349/ymj.2013.54.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Browne G., Taipaleenmäki H., Stein G.S., Stein J.L., Lian J.B. MicroRNAs in the control of metastatic bone disease. Trends Endocrinol. Metab. 2014;25(6):320–327. doi: 10.1016/j.tem.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vimalraj S., Miranda P.J., Ramyakrishna B., Selvamurugan N. Regulation of breast cancer and bone metastasis by microRNAs. Dis. Markers. 2013;35(5):369–387. doi: 10.1155/2013/451248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zoni E., van der Pluijm G. The role of microRNAs in bone metastasis. J. Bone Oncol. 2016;5(3):104–108. doi: 10.1016/j.jbo.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Croset M., Kan C., Clézardin P. Tumour-derived miRNAs and bone metastasis. Bonekey Rep. 2015;4:688. doi: 10.1038/bonekey.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai W.L., Huang W.D., Li B., Chen T.R., Li Z.X., Zhao C.L., Li H.Y., Wu Y.M., Yan W.J., Xiao J.R. microRNA-124 inhibits bone metastasis of breast cancer by repressing Interleukin-11. Mol. Cancer. 2018;17(1):9. doi: 10.1186/s12943-017-0746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong L.L., Chen L.M., Wang W.M., Zhang L.M. Decreased expression of microRNA-124 is an independent unfavorable prognostic factor for patients with breast cancer. Diagn. Pathol. 2015;10:45. doi: 10.1186/s13000-015-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang Y.J., Wang Q.Y., Zhou C.X., Yin Q.Q., He M., Yu X.T., Cao D.X., Chen G.Q., He J.R., Zhao Q. MiR-124 targets Slug to regulate epithelial-mesenchymal transition and metastasis of breast cancer. Carcinogenesis. 2013;34(3):713–722. doi: 10.1093/carcin/bgs383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J., Li D., Dang L., Liang C., Guo B., Lu C., He X., Cheung H.Y., He B., Liu B., Li F., Lu J., Wang L., Shaikh A.B., Jiang F., Lu C., Peng S., Zhang Z., Zhang B.T., Pan X., Xiao L., Lu A., Zhang G. Osteoclastic miR-214 targets TRAF3 to contribute to osteolytic bone metastasis of breast cancer. Sci. Rep. 2017;7:40487. doi: 10.1038/srep40487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X., Guo B., Li Q., Peng J., Yang Z., Wang A., Li D., Hou Z., Lv K., Kan G., Cao H., Wu H., Song J., Pan X., Sun Q., Ling S., Li Y., Zhu M., Zhang P., Peng S., Xie X., Tang T., Hong A., Bian Z., Bai Y., Lu A., Li Y., He F., Zhang G., Li Y. miR-214 targets ATF4 to inhibit bone formation. Nat. Med. 2013;19(1):93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- 78.Ell B., Mercatali L., Ibrahim T., Campbell N., Schwarzenbach H., Pantel K., Amadori D., Kang Y. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell. 2013;24(4):542–556. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taipaleenmäki H., Browne G., Akech J., Zustin J., van Wijnen A.J., Stein J.L., Hesse E., Stein G.S., Lian J.B. Targeting of Runx2 by miR-135 and miR-203 impairs progression of breast cancer and metastatic bone disease. Cancer Res. 2015;75(7):1433–1444. doi: 10.1158/0008-5472.CAN-14-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pratap J., Lian J.B., Javed A., Barnes G.L., van Wijnen A.J., Stein J.L., Stein G.S. Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev. 2006;25(4):589–600. doi: 10.1007/s10555-006-9032-0. [DOI] [PubMed] [Google Scholar]
- 81.Zhao F.L., Hu G.D., Wang X.F., Zhang X.H., Zhang Y.K., Yu Z.S. Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J. Int. Med. Res. 2012;40(3):859–866. doi: 10.1177/147323001204000304. [DOI] [PubMed] [Google Scholar]
- 82.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 83.Ell B., Qiu Q., Wei Y., Mercatali L., Ibrahim T., Amadori D., Kang Y. The microRNA-23b/27b/24 cluster promotes breast cancer lung metastasis by targeting metastasis-suppressive gene prosaposin. J. Biol. Chem. 2014;289(32):21888–21895. doi: 10.1074/jbc.M114.582866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang Q., Gumireddy K., Schrier M., le Sage C., Nagel R., Nair S., Egan D.A., Li A., Huang G., Klein-Szanto A.J., Gimotty P.A., Katsaros D., Coukos G., Zhang L., Puré E., Agami R. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol. 2008;10(2):202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 85.Chen X., Wang Y.W., Zhu W.J., Li Y., Liu L., Yin G., Gao P. A 4-microRNA signature predicts lymph node metastasis and prognosis in breast cancer. Hum. Pathol. 2018;76:122–132. doi: 10.1016/j.humpath.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 86.Yan L.X., Huang X.F., Shao Q., Huang M.Y., Deng L., Wu Q.L., Zeng Y.X., Shao J.Y. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14(11):2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rick J.W., Shahin M., Chandra A., Dalle Ore C., Yue J.K., Nguyen A., Yagnik G., Sagar S., Arfaie S., Aghi M.K. Systemic therapy for brain metastases. Crit. Rev. Oncol. Hematol. 2019;142:44–50. doi: 10.1016/j.critrevonc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z., Peng Z., Gu S., Zheng J., Feng D., Qin Q., He J. Global Analysis of miRNA-mRNA interaction network in breast cancer with brain metastasis. Anticancer Res. 2017;37(8):4455–4468. doi: 10.21873/anticanres.11841. [DOI] [PubMed] [Google Scholar]
- 89.Witzel I., Oliveira-Ferrer L., Pantel K., Müller V., Wikman H. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res. 2016;18(1):8. doi: 10.1186/s13058-015-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L., Sullivan P.S., Goodman J.C., Gunaratne P.H., Marchetti D. MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 2011;71(3):645–654. doi: 10.1158/0008-5472.CAN-10-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okuda H., Xing F., Pandey P.R., Sharma S., Watabe M., Pai S.K., Mo Y.Y., Iiizumi-Gairani M., Hirota S., Liu Y., Wu K., Pochampally R., Watabe K. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73(4):1434–1444. doi: 10.1158/0008-5472.CAN-12-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shao B., Wang X., Zhang L., Li D., Liu X., Song G., Cao H., Zhu J., Li H. Plasma microRNAs predict chemoresistance in patients with metastatic breast cancer. Technol. Cancer Res. Treat. 2019;18:: 1533033819828709. doi: 10.1177/1533033819828709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmad A., Ginnebaugh K.R., Sethi S., Chen W., Ali R., Mittal S., Sarkar F.H. miR-20b is up-regulated in brain metastases from primary breast cancers. Oncotarget. 2015;6(14):12188–12195. doi: 10.18632/oncotarget.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krizbai I.A., Nyúl-Tóth Á., Bauer H.C., Farkas A.E., Traweger A., Haskó J., Bauer H., Wilhelm I. Pharmaceutical targeting of the brain. Curr. Pharm. Des. 2016;22(35):5442–5462. doi: 10.2174/1381612822666160726144203. [DOI] [PubMed] [Google Scholar]
- 95.Wilhelm I., Fazakas C., Molnár K., Végh A.G., Haskó J., Krizbai I.A. Foe or friend? Janus-faces of the neurovascular unit in the formation of brain metastases. J. Cereb. Blood Flow Metab. 2018;38(4):563–587. doi: 10.1177/0271678X17732025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salvador E., Burek M., Förster C.Y. Tight junctions and the tumor microenvironment. Curr. Pathobiol. Rep. 2016;4:135–145. doi: 10.1007/s40139-016-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dias K., Dvorkin-Gheva A., Hallett R.M., Wu Y., Hassell J., Pond G.R., Levine M., Whelan T., Bane A.L. Claudin-low breast cancer; clinical & pathological characteristics. PLoS One. 2017;12(1):: e0168669. doi: 10.1371/journal.pone.0168669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Helms H.C., Abbott N.J., Burek M., Cecchelli R., Couraud P.O., Deli M.A., Förster C., Galla H.J., Romero I.A., Shusta E.V., Stebbins M.J., Vandenhaute E., Weksler B., Brodin B. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016;36(5):862–890. doi: 10.1177/0271678X16630991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He Y., Yao Y., Tsirka S.E., Cao Y. Cell-culture models of the blood-brain barrier. Stroke. 2014;45(8):2514–2526. doi: 10.1161/STROKEAHA.114.005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bowman P.D., Ennis S.R., Rarey K.E., Betz A.L., Goldstein G.W. Brain microvessel endothelial cells in tissue culture: a model for study of blood-brain barrier permeability. Ann. Neurol. 1983;14(4):396–402. doi: 10.1002/ana.410140403. [DOI] [PubMed] [Google Scholar]
- 101.Cecchelli R., Dehouck B., Descamps L., Fenart L., Buée-Scherrer V., Duhem C., Lundquist S., Rentfel M., Torpier G., Dehouck M.P. In vitro model for evaluating drug transport across the blood-brain barrier. Adv. Drug Deliv. Rev. 1999;36(2-3):165–178. doi: 10.1016/S0169-409X(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 102.Franke H., Galla H., Beuckmann C.T. Primary cultures of brain microvessel endothelial cells: a valid and flexible model to study drug transport through the blood-brain barrier in vitro. Brain Res. Brain Res. Protoc. 2000;5(3):248–256. doi: 10.1016/S1385-299X(00)00020-9. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y., Li C.S., Ye Y., Johnson K., Poe J., Johnson S., Bobrowski W., Garrido R., Madhu C. Porcine brain microvessel endothelial cells as an in vitro model to predict in vivo blood-brain barrier permeability. Drug Metab. Dispos. 2006;34(11):1935–1943. doi: 10.1124/dmd.105.006437. [DOI] [PubMed] [Google Scholar]
- 104.Patabendige A., Skinner R.A., Abbott N.J. Establishment of a simplified in vitro porcine blood-brain barrier model with high transendothelial electrical resistance. Brain Res. 2013;1521:1–15. doi: 10.1016/j.brainres.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garberg P., Ball M., Borg N., Cecchelli R., Fenart L., Hurst R.D., Lindmark T., Mabondzo A., Nilsson J.E., Raub T.J., Stanimirovic D., Terasaki T., Oberg J.O., Osterberg T. In vitro models for the blood-brain barrier. Toxicol. In Vitro. 2005;19(3):299–334. doi: 10.1016/j.tiv.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 106.Prieto P., Blaauboer B.J., de Boer A.G., Boveri M., Cecchelli R., Clemedson C., Coecke S., Forsby A., Galla H.J., Garberg P., Greenwood J., Price A., Tähti H. European Centre for the Validation of Alternative Methods. Blood-brain barrier in vitro models and their application in toxicology. The report and recommendations of ECVAM Workshop 49. Altern. Lab. Anim. 2004;32(1):37–50. doi: 10.1177/026119290403200107. [DOI] [PubMed] [Google Scholar]
- 107.Gumbleton M., Audus K.L. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J. Pharm. Sci. 2001;90(11):1681–1698. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- 108.Reichel A., Begley D.J., Abbott N.J. An overview of in vitro techniques for blood-brain barrier studies. Methods Mol. Med. 2003;89:307–324. doi: 10.1385/1-59259-419-0:307. [DOI] [PubMed] [Google Scholar]
- 109.Deli M.A., Abrahám C.S., Kataoka Y., Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell. Mol. Neurobiol. 2005;25(1):59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith M., Omidi Y., Gumbleton M. Primary porcine brain microvascular endothelial cells: biochemical and functional characterisation as a model for drug transport and targeting. J. Drug Target. 2007;15(4):253–268. doi: 10.1080/10611860701288539. [DOI] [PubMed] [Google Scholar]
- 111.Calabria A.R., Weidenfeller C., Jones A.R., de Vries H.E., Shusta E.V. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J. Neurochem. 2006;97(4):922–933. doi: 10.1111/j.1471-4159.2006.03793.x. [DOI] [PubMed] [Google Scholar]
- 112.Wijsman J.A., Shivers R.R. Immortalized mouse brain endothelial cells are ultrastructurally similar to endothelial cells and respond to astrocyte-conditioned medium. In Vitro Cell. Dev. Biol. Anim. 1998;34(10):777–784. doi: 10.1007/s11626-998-0032-y. [DOI] [PubMed] [Google Scholar]
- 113.Omidi Y., Campbell L., Barar J., Connell D., Akhtar S., Gumbleton M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res. 2003;990(1-2):95–112. doi: 10.1016/S0006-8993(03)03443-7. [DOI] [PubMed] [Google Scholar]
- 114.Grab D.J., Nikolskaia O., Kim Y.V., Lonsdale-Eccles J.D., Ito S., Hara T., Fukuma T., Nyarko E., Kim K.J., Stins M.F., Delannoy M.J., Rodgers J., Kim K.S. African trypanosome interactions with an in vitro model of the human blood-brain barrier. J. Parasitol. 2004;90(5):970–979. doi: 10.1645/GE-287R. [DOI] [PubMed] [Google Scholar]
- 115.Weksler B.B., Subileau E.A., Perrière N., Charneau P., Holloway K., Leveque M., Tricoire-Leignel H., Nicotra A., Bourdoulous S., Turowski P., Male D.K., Roux F., Greenwood J., Romero I.A., Couraud P.O. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19(13):1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 116.Sano Y., Shimizu F., Abe M., Maeda T., Kashiwamura Y., Ohtsuki S., Terasaki T., Obinata M., Kajiwara K., Fujii M., Suzuki M., Kanda T. Establishment of a new conditionally immortalized human brain microvascular endothelial cell line retaining an in vivo blood-brain barrier function. J. Cell. Physiol. 2010;225(2):519–528. doi: 10.1002/jcp.22232. [DOI] [PubMed] [Google Scholar]
- 117.Förster C., Silwedel C., Golenhofen N., Burek M., Kietz S., Mankertz J., Drenckhahn D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005;565(Pt 2):475–486. doi: 10.1113/jphysiol.2005.084038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Veszelka S., Meszaros M., Kiss L., Kota Z., Pali T., Hoyk Z., Bozso Z., Fulop L., Toth A., Rakhely G., Deli M.A. Biotin and glutathione targeting of solid nanoparticles to cross human brain endothelial cells. Curr. Pharm. Des. 2017;23(28):4198–4205. doi: 10.2174/1381612823666170727144450. [DOI] [PubMed] [Google Scholar]
- 119.Kido Y., Tamai I., Nakanishi T., Kagami T., Hirosawa I., Sai Y., Tsuji A. Evaluation of blood-brain barrier transporters by co-culture of brain capillary endothelial cells with astrocytes. Drug Metab. Pharmacokinet. 2002;17(1):34–41. doi: 10.2133/dmpk.17.34. [DOI] [PubMed] [Google Scholar]
- 120.Neuhaus W., Gaiser F., Mahringer A., Franz J., Riethmüller C., Förster C. The pivotal role of astrocytes in an in vitro stroke model of the blood-brain barrier. Front. Cell. Neurosci. 2014;8:352. doi: 10.3389/fncel.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Banks W.A., Kovac A., Morofuji Y. Neurovascular unit crosstalk: Pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells. J. Cereb. Blood Flow Metab. 2018;38(6):1104–1118. doi: 10.1177/0271678X17740793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Al-Shehri A., Favretto M.E., Ioannou P.V., Romero I.A., Couraud P.O., Weksler B.B., Parker T.L., Kallinteri P. Permeability of PEGylated immunoarsonoliposomes through in vitro blood brain barrier-medulloblastoma co-culture models for brain tumor therapy. Pharm. Res. 2015;32(3):1072–1083. doi: 10.1007/s11095-014-1519-8. [DOI] [PubMed] [Google Scholar]
- 123.Yan G.N., Lv Y.F., Yang L., Yao X.H., Cui Y.H., Guo D.Y. Glioma stem cells enhance endothelial cell migration and proliferation via the Hedgehog pathway. Oncol. Lett. 2013;6(5):1524–1530. doi: 10.3892/ol.2013.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anfuso C.D., Motta C., Giurdanella G., Arena V., Alberghina M., Lupo G. Endothelial PKCα-MAPK/ERK-phospholipase A2 pathway activation as a response of glioma in a triple culture model. A new role for pericytes? Biochimie. 2014;99:77–87. doi: 10.1016/j.biochi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 125.Siddharthan V., Kim Y.V., Liu S., Kim K.S. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tarbell J.M. Shear stress and the endothelial transport barrier. Cardiovasc. Res. 2010;87(2):320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davies P.F., Spaan J.A., Krams R. Shear stress biology of the endothelium. Ann. Biomed. Eng. 2005;33(12):1714–1718. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 128.Stanness K.A., Guatteo E., Janigro D. A dynamic model of the blood-brain barrier “in vitro”. Neurotoxicology. 1996;17(2):481–496. [PubMed] [Google Scholar]
- 129.Stanness K.A., Westrum L.E., Fornaciari E., Mascagni P., Nelson J.A., Stenglein S.G., Myers T., Janigro D. Morphological and functional characterization of an in vitro blood-brain barrier model. Brain Res. 1997;771(2):329–342. doi: 10.1016/S0006-8993(97)00829-9. [DOI] [PubMed] [Google Scholar]
- 130.Janigro D., Leaman S.M., Stanness K.A. Dynamic modeling of the blood-brain barrier: a novel tool for studies of drug delivery to the brain. Pharm. Sci. Technol. Today. 1999;2(1):7–12. doi: 10.1016/S1461-5347(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 131.Cucullo L., Couraud P.O., Weksler B., Romero I.A., Hossain M., Rapp E., Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J. Cereb. Blood Flow Metab. 2008;28(2):312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- 132.Dewey C.F., Jr, Bussolari S.R., Gimbrone M.A., Jr, Davies P.F. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 1981;103(3):177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 133.Koutsiaris A.G., Tachmitzi S.V., Batis N., Kotoula M.G., Karabatsas C.H., Tsironi E., Chatzoulis D.Z. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44(5-6):375–386. [PubMed] [Google Scholar]
- 134.Cucullo L., McAllister M.S., Kight K., Krizanac-Bengez L., Marroni M., Mayberg M.R., Stanness K.A., Janigro D. A new dynamic in vitro model for the multidimensional study of astrocyte-endothelial cell interactions at the blood-brain barrier. Brain Res. 2002;951(2):243–254. doi: 10.1016/S0006-8993(02)03167-0. [DOI] [PubMed] [Google Scholar]
- 135.Lippmann E.S., Azarin S.M., Kay J.E., Nessler R.A., Wilson H.K., Al-Ahmad A., Palecek S.P., Shusta E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012;30(8):783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Appelt-Menzel A., Cubukova A., Günther K., Edenhofer F., Piontek J., Krause G., Stüber T., Walles H., Neuhaus W., Metzger M. Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri- and multipotent stem cells. Stem Cell Reports. 2017;8(4):894–906. doi: 10.1016/j.stemcr.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Katt M.E., Xu Z.S., Gerecht S., Searson P.C. Human brain microvascular endothelial cells derived from the BC1 iPS cell line exhibit a blood-brain barrier phenotype. PLoS One. 2016;11(4):: e0152105. doi: 10.1371/journal.pone.0152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lim R.G., Quan C., Reyes-Ortiz A.M., Lutz S.E., Kedaigle A.J., Gipson T.A., Wu J., Vatine G.D., Stocksdale J., Casale M.S., Svendsen C.N., Fraenkel E., Housman D.E., Agalliu D., Thompson L.M. Huntington’s disease iPSC-derived brain microvascular endothelial cells reveal WNT-mediated angiogenic and blood-brain barrier deficits. Cell Rep. 2017;19(7):1365–1377. doi: 10.1016/j.celrep.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ribecco-Lutkiewicz M., Sodja C., Haukenfrers J., Haqqani A.S., Ly D., Zachar P., Baumann E., Ball M., Huang J., Rukhlova M., Martina M., Liu Q., Stanimirovic D., Jezierski A., Bani-Yaghoub M. A novel human induced pluripotent stem cell blood-brain barrier model: Applicability to study antibody-triggered receptor-mediated transcytosis. Sci. Rep. 2018;8(1):1873. doi: 10.1038/s41598-018-19522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hollmann E.K., Bailey A.K., Potharazu A.V., Neely M.D., Bowman A.B., Lippmann E.S. Accelerated differentiation of human induced pluripotent stem cells to blood-brain barrier endothelial cells. Fluids Barriers CNS. 2017;14(1):9. doi: 10.1186/s12987-017-0059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neal E.H., Marinelli N.A., Shi Y., McClatchey P.M., Balotin K.M., Gullett D.R., Hagerla K.A., Bowman A.B., Ess K.C., Wikswo J.P., Lippmann E.S. A simplified, fully defined differentiation scheme for producing blood-brain barrier endothelial cells from human iPSCs. Stem Cell Reports. 2019;12(6):1380–1388. doi: 10.1016/j.stemcr.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qian T., Maguire S.E., Canfield S.G., Bao X., Olson W.R., Shusta E.V., Palecek S.P. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci. Adv. 2017;3(11):: e1701679. doi: 10.1126/sciadv.1701679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Praça C., Rosa S.C., Sevin E., Cecchelli R., Dehouck M.P., Ferreira L.S. Derivation of brain capillary-like endothelial cells from human pluripotent stem cell-derived endothelial progenitor cells. Stem Cell Reports. 2019;13(4):599–611. doi: 10.1016/j.stemcr.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O’Grady B.J., Balikov D.A., Lippmann E.S., Bellan L.M. Spatiotemporal control of morphogen delivery to pattern stem cell differentiation in three-dimensional hydrogels. Curr. Protoc. Stem Cell Biol. 2019;51(1):: e97. doi: 10.1002/cpsc.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cecchelli R., Aday S., Sevin E., Almeida C., Culot M., Dehouck L., Coisne C., Engelhardt B., Dehouck M.P., Ferreira L. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS One. 2014;9(6):: e99733. doi: 10.1371/journal.pone.0099733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boyer-Di Ponio J., El-Ayoubi F., Glacial F., Ganeshamoorthy K., Driancourt C., Godet M., Perrière N., Guillevic O., Couraud P.O., Uzan G. Instruction of circulating endothelial progenitors in vitro towards specialized blood-brain barrier and arterial phenotypes. PLoS One. 2014;9(1):: e84179. doi: 10.1371/journal.pone.0084179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lyck R., Lécuyer M.A., Abadier M., Wyss C.B., Matti C., Rosito M., Enzmann G., Zeis T., Michel L., García Martín A.B., Sallusto F., Gosselet F., Deutsch U., Weiner J.A., Schaeren-Wiemers N., Prat A., Engelhardt B. ALCAM (CD166) is involved in extravasation of monocytes rather than T cells across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2017;37(8):2894–2909. doi: 10.1177/0271678X16678639. [DOI] [PMC free article] [PubMed] [Google Scholar]