Abstract
Background: Radiation-induced enteritis and proctitis are common side effects of abdominopelvic cancers among patients that undergo radiotherapy for prostate, colorectal or urinary cancers. Exposure of these tissues to high doses of radiation leads to damage to villous, inflammation, pain, ulcer and bleeding, which may cause malabsorption and gastrointestinal disorders. To date, several procedures such as pharmaceutical treatment have been proposed for protection and mitigation of gastrointestinal toxicity following radiotherapy.
Aims: In the current study, we aimed to investigate the possible radioprotection of ileum and colon in rats using a combination of melatonin and metformin.
Methods: In this experimental study, 30 male Wistar rats were randomly assigned to six groups: control, melatonin (100 mg/kg) treatment, melatonin (100 mg/kg) plus metformin (100 mg/kg) treatment, radiation (10 Gy to whole body) group, radiation + melatonin (100 mg/kg) treatment, and radiation + melatonin (100 mg/kg) plus metformin (100 mg/kg) treatment. After 3.5 days, rats were sacrificed and their ileum and colon tissues carefully removed. Histopathological evaluations were conducted on these tissue samples.
Results: Histological evaluations reported moderate to severe damages to ileum and colon following whole body irradiation. Melatonin administration was able to protect the ileum remarkably, while the combination of melatonin and metformin was less effective. Interestingly, for the colon, melatonin was less effective while its combination with metformin was able to protect against radiation toxicity completely.
Conclusion: For the ileum, melatonin was a more effective radioprotector compared to its combination with metformin. However, the combination of melatonin and metformin can be proposed as an ideal radioprotector for the colon.
Keywords: Colon, ileum, melatonin, metformin, radiation, radiation-induced enteritis
1. INTRODUCTION
Abdominopelvic cancers are among the most common malignancies in human [1]. Radiation enteritis and proctitis are the most serious side effects of abdominopelvic cancers in the gastrointestinal system [2]. In addition to these cancers, the lower parts of the gastrointestinal system are affected by exposure to radiation by radiotherapy for other tumors such as prostate, colorectal and urinary cancers [3, 4]. Exposure of the lower parts of the intestine including ileum and colon, to high doses of ionizing radiation may lead to acute and late effects such as damage to villous, inflammation, pain, ulcer and bleeding. These consequences of radiotherapy can affect the quality of patients potently. Damage to villous and chronic inflammation may cause malabsorption and also increase the risk of second primary malignancies [5]. The main effect of ionizing radiation on the vital cells is mediated via generation of reactive oxygen species (ROS), following radiolysis of water molecules. The produced ROS can attack DNA and other organelles, leading to apoptosis, mitotic catastrophe and necrosis. Massive apoptosis and necrosis cause stimulation of several immune signaling pathways, leading to the release of several chemokines and cytokines. These changes result in infiltration of macrophages and lymphocytes as well as the appearance of inflammation, ulcer and bleeding [6].
Protection of normal tissues by pharmaceutical or natural agents is one of the most interesting topics in radiobiology. So far, several agents such as curcumin, glucagon-like peptide-1, zinc and quercetin have been used for amelioration of radiation toxicity in gastrointestinal system including ileum and colon [7-10]. However, studies for exploring lower toxic, more effective and low cost radioprotectors are ongoing. Furthermore, radioprotectors should not cause tumor protection during radiotherapy. Melatonin is a natural pineal hormone that has promising properties for use as an anti-inflammation and antioxidant drug. Melatonin has been shown to scavenge ROS via direct interaction and also by stimulating antioxidant defense mediators such as NRF-2, glutathione peroxidase (GPx), glutathione (GSH), and superoxide dismutase (SOD). Melatonin is also able to trigger DNA damage responses (DDRs) and reduce apoptosis, leading to a reduction in cell death and subsequent consequences like inflammation. Melatonin also has the ability to inhibit inflammatory mediators directly and inhibits endogenous production of ROS and nitric oxide (NO) [11, 12].
Metformin, approved by the Food and Drug Administration (FDA), is mostly used as an anti-diabetic drug. It has an antioxidant effect due to its interactions with free radicals and stimulates antioxidant defense within cells [13]. Metformin has potent stimulatory effect on DNA repair mechanisms which is mediated following upregulation of AMP-activated protein kinase (AMPK) [14]. AMPK is involved in glucose metabolism, while it is able to induce regulation of p53 and p21 and help repair damaged DNA [15]. Furthermore, metformin has potent inhibitory effect on the mitochondria electron transport chain (ETC)-1. This property of metformin can help prevent continuous production of hydrogen peroxide by mitochondria that play a key role in chronic oxidative injury after exposure to radiation [13].
In present study, we evaluated the radioprotective role of the combination of metformin and melatonin against radiation-induced injury in ileum and colon. Furthermore, we compared the radioprotective effect of this combination to melatonin alone.
2. MATERIALS AND METHODS
2.1. Drugs
Melatonin, purchased from Merck Company (Germany), was first dissolved in 70% ethanol completely and then diluted with distilled water. For preparing melatonin solution, the final concentration of melatonin was 20 mg/mL of water, while that of ethanol was 15%. Metformin was obtained from Tehran Chemie Pharmaceutical Company (Tehran, Iran). For preparing the combined solution of metformin plus melatonin, melatonin solution was first provided as previously done. Afterwards, metformin was added in 20 mg/mL. For daily treat- ment of rats, each solution was freshly prepared. Drug solutions administrated as orally gavage.
2.2. Irradiation
Prior to exposure, rats were anesthetized using ketamine (20 mg/kg) and xylazine (5 mg/kg). Afterwards, they were positioned as supine on a treatment table. Each rat was administered a single dose of 10 Gy ionizing radiation from a cobalt-60 (60Co) gamma ray source (1.25 MeV energy), at a source to skin distance (SSD) of 60 cm and dose rate of 104 cGy/min.
2.3. Experimental Design
A total of 30 male Wistar rats (200 g body weight) were used throughout the study. All of them were kept in the same room under a constant temperature (22 ± 2°C), humidity (55-60%) and illuminated 7:00 a.m. to 7:00 p.m. with free access to food pellets and water. All study protocols were approved by Institutional Animal Ethical Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1396.683). Also, this animal study followed national guide for the care and use of laboratory animals. Animals were randomly divided into six groups (of five members): control, melatonin treatment, melatonin plus metformin treatment, radiation, radiation plus melatonin, and radiation plus both melatonin and metformin. In control group, rats received no radiation or drugs. In melatonin treatment group, rats received 100 mg/kg melatonin (1 ml melatonin solution) for 5 days. In melatonin plus metformin group, rats received 1 ml solution containing 20 mg melatonin and 20 mg metformin (100 mg/kg for each drug). In a radiation group, rats did not receive any drug, but were irradiated with 10 Gy gamma rays to whole body. In radiation plus melatonin group, rats received 100 mg/kg melatonin 24 h and 30 min before exposure to 10 Gy gamma rays to whole body. Treatment with melatonin in this group continued for 3 days after irradiation. In radiation plus melatonin and metformin group, rats received 100 mg/kg melatonin and metformin 24 h and 30 min before exposure to 10 Gy gamma rays to whole body. Similar to other groups, treatment in this group continued for 3 days after irradiation (totaling 5 days). 3.5 days after irradiation, all rats were euthanized and sacrificed. After abdominal opening, the gastrointestinal system parts including duodenum, jejunum, ileum and colon, were carefully removed and moved into 10% normal buffer formalin (Sigma Aldrich, St. Louis, MO, USA).
2.4. Histopathology
After complete fixation, all tissue samples including duodenum, jejunum, ileum and colon, were embedded into paraffin blocks and 5 µm thickness sections provided following cutting by microtome (Leica, RM2125RT, Germany). These tissue sections were stained using hematoxylin and eosin (H&E). Morphological changes were evaluated using a light microscope (Olympus BX51, Olympus Corporation, Tokyo, Japan) and scored as: 0=normal, 1=mild changes, 2=moderate changes, and 3=severe changes. Also, villi length in ileum was measured and results reported.
Scoring of histological evaluation was based on pathological scoring for each parameter. For mucositis, grade 1 is including mild inflamed mucosa, grade 2 is associated with moderate erythema and ulceration, and grade 3 is associated with severe erythema and ulceration [16]. Congestion can score as normal= no congestion, grade 1= up to 3 congestive vessels, 2= 3 to 5 congestive vessels, and 3= more than 5 congestive vessels [17]. Degenerative changes scoring is as normal = 0. In grade 1, cells have normal nuclei, cytoplasmic granules are normal and only a slight enlarged cells are observable. In grade 2, more than 50% cells show cytoplasm vacuolization, and finally in grade 3, all cells show pronounced cytoplasm vacuolization. Infiltration of macrophages and lymphocytes scored as normal, mild, moderate and severe infiltration.
2.5. Statistical Analysis
Results (reported as mean ± standard deviation) of histopathological evaluation for each group were scored and analyzed using SPSS software version 24 (IBM, Chicago, USA). Mann-Whitney test was used for determining significant differences between groups. Statistical significance was set at p values <0.05.
3. RESULTS
3.1. Ileum
Histopathological evaluation of the radioprotective effect of melatonin or melatonin plus metformin in ileum was shown in Fig. (1) and Table 1.
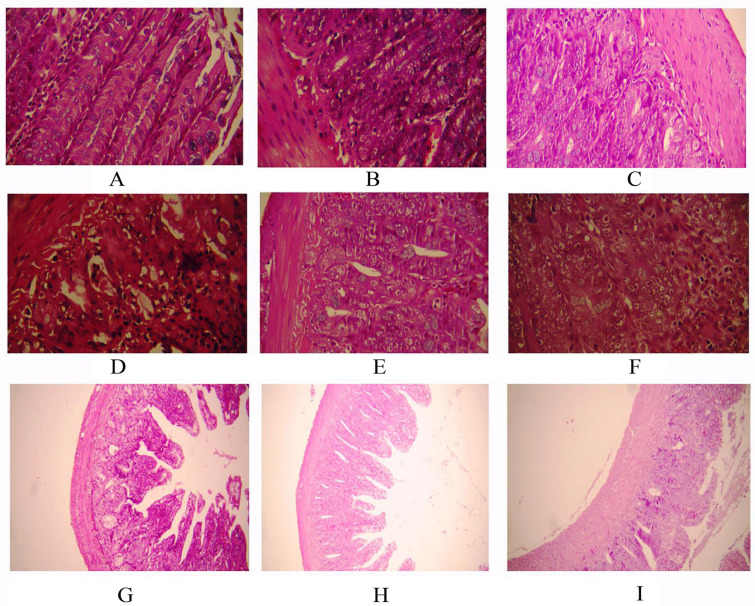
Fig. (1).
Histopathological evaluation of the radioprotective effect of melatonin or melatonin plus metformin in ileum. A: control; B: melatonin treatment; C: melatonin plus metformin treatment; D: radiation (×400); E: radiation + melatonin (×400); F: radiation + melatonin plus metformin (×400); G: radiation (×100); H: radiation + melatonin (×100); I: radiation + melatonin plus metformin (×100).
Table 1.
Results of histopathological evaluation of ileum following whole body irradiation with 10 Gy gamma rays and treatment with melatonin or melatonin plus metformin. Histopathological results were scored as 0= normal; 1= mild changes; 2= moderate changes; and 3= severe changes.
| - | Control | MLT | MLT+MET | RAD | RAD+MLT |
RAD+MLT+
MET |
|---|---|---|---|---|---|---|
| Congestion of blood vessels | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.80 ± 0.45a | 1.50 ± 0.55b | 2.50 ± 0.57 |
| Degenerative changes and necrosis of lamina propria | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.80 ± 0.45a | 0.60 ± 0.55b | 1.50 ± 0.58b |
| Intestinal mucositis changes | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.40 ± 0.55a | 0.00 ± 00b | 0.00 ± 00b |
| Inflammatory cells’ infiltration | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.80 ± 0.45a | 0.00 ± 00b | 0.00 ± 00b |
| Average number of goblet cells | 63.00 ± 1.87 | 64.80 ± 0.75 | 63.20 ± 0.98 | 21.00 ± 1.58a | 42.20 ± 0.80b | 35.75 ± 1.50b |
| Length of villi | 826 ± 64μm | 882 ± 66μm | 835 ± 46μm | 561 ± 42μma | 854 ± 70μmb | 615 ± 39 |
3.1.1. Congestion of Blood Vessels
Results of histological evaluation showed that neither melatonin nor melatonin plus metformin could cause damage to ileum blood vessels. Exposure to radiation to whole body led to severe vascular injury (p=0.004). Treatment with melatonin before and after exposure to radiation led to significant reduction of vascular injury (p=0.009). This group showed mild to moderate congestion of blood vessels. In contrast to melatonin, treatment with the combined form of melatonin and metformin could not reduce blood vessel congestion significantly (p=0.371). Comparison between the groups of rats that received melatonin with those that received the combined form of these drugs showed that single administration of melatonin was more efficient for protection of blood vessels against ionizing radiation (p=0.037).
3.1.2. Degenerative Changes and Necrosis of Lamina Propria
Single administration of melatonin or its combination with metformin did not cause any degenerative effect or damage to the lamina propria. When rats were irradiated to whole body with 10 Gy gamma rays, severe necrosis of lamina propria and degenerative changes were observed (p=0.004). Administration of melatonin to rats led to significant reduction in necrosis of lamina propria (p=0.006). These rats showed only a mild damage to lamina propria compared to severe changes in irradiated rats. Similar to melatonin, the combination of these drugs showed abilities to alleviate necrosis of lamina propria and degenerative changes (p=0.018). Comparison between rats that received melatonin with those that received the combined drugs showed no significant difference between these groups.
3.1.3. Intestinal Mucositis Changes
Whole body irradiation led to moderate to severe mucositis in the ileum (p=0.005). In contrast, rats treated with melatonin before and after whole body irradiation did not show any sign of mucositis (p=0.005). Similarly, administering the combination of these drugs was able to reverse mucositis (p=0.009). No significant difference was observed for rats who received melatonin, either alone or in combination with metformin. Neither melatonin nor melatonin plus metformin treatment did not cause any significant mucositis to the ileum.
3.1.4. Inflammatory Cells Infiltration
A severe infiltration of macrophages and leukocytes were reported for rats exposed to whole body with 10 Gy gamma rays (p=0.004). Administering melatonin to irradiated rats led to complete reversal of macrophages and lymphocyte infiltration (p=0.004). Similar to melatonin, the combined form of these drugs completely reversed macrophages and lymphocyte infiltration (p=0.007). Treatment with melatonin alone did not show any infiltration of inflammatory cells such as macrophages or leukocytes.
3.1.5. Average Numbers of Goblet Cells
The average numbers of goblet cells in control group was near to 63 cells in villi. Results of histological evaluation showed that whole body exposure to 10 Gy gamma rays led to severe reduction of goblet cells by more than 60% (p=0.009). Treatment of melatonin for rats that received gamma rays led to significant increase in the number of goblet cells (42.20 ± 0.80) compared to irradiated rats that did not receive any drug (p=0.009). Administering the combined form of these drugs could augment the number of goblet cells compared to radiation group (p=0.014). However, comparison showed that melatonin, when administered alone, was more effective for protecting goblet cells against ionizing radiation (p=0.013).
3.2. Length of Villi
Results showed that neither melatonin nor melatonin plus metformin caused damage to villi and reduction of villi length. Results also showed that whole body irradiation with 10 Gy gamma rays caused significant reduction of villi length (561 ± 42 μm) compared to control group (826 ± 64 μm) (p=0.004). Treatment with melatonin led to significant amelioration of villi shortening and improved villi length (854 ± 70 μm) compared to radiation group (p=0.004). Interestingly, treatment with the combined drugs could not improve villi length (615 ± 39) compared to radiation group. Comparison showed that rats which received melatonin before and after irradiation had higher length of villi compared to those who received the combination of melatonin and metformin (p=0.003).
3.3. Colon
Histopathological evaluation of the radioprotective effect of melatonin or melatonin plus metformin in colon was shown in Fig. (2) and Table 2.
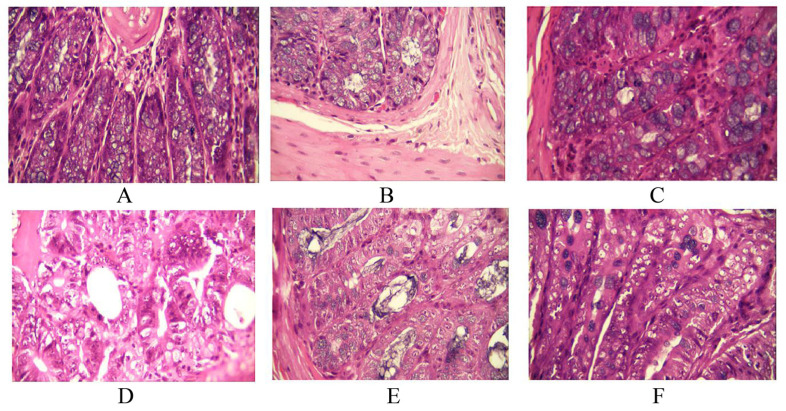
Fig. (2).
Histopathological evaluation of the radioprotective effect of melatonin or melatonin plus metformin in colon. A: control; B: melatonin treatment; C: melatonin plus metformin treatment; D: radiation (×400); E: radiation + melatonin (×400); F: radiation + melatonin plus metformin (×400).
Table 2.
Results of histopathological evaluation of colon following whole body irradiation with 10 Gy gamma rays and treatment with melatonin or melatonin plus metformin. Histopathological results were scored as 0= normal; 1= mild changes; 2= moderate changes; and 3= severe changes.
| - | Control | MLT | MLT+MET | RAD | RAD+MLT |
RAD+MLT+
MET |
|---|---|---|---|---|---|---|
| Congestion | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.80 ± 0.55 | 0.80 ± 0.55b | 0.00 ± 00b |
| Degenerative changes | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.80 ± 0.45 | 2.40 ± 0.55 | 0.00 ± 00b |
| Blood vessel changes | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.20 ± 0.45 | 2.60 ± 0.55b | 0.00 ± 00b |
|
Inflammatory cells (leucocyte & macrophage) |
0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.60 ± 0.55 | 0.80 ± 0.45b | 0.00 ± 00b |
| Muscular layer changes | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.60 ± 0.55 | 0.60 ± 0.55b | 0.00 ± 00b |
| Goblet cell damage | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.60 ± 0.55 | 0.80 ± 0.55b | 0.00 ± 00b |
| Lieberkühn gland damage | 0.00 ± 00 | 0.00 ± 00 | 0.00 ± 00 | 2.60 ± 0.55 | 0.60 ± 0.55b | 0.00 ± 00b |
3.3.1. Congestion
Histological observations showed no congestion in the colon following administration of melatonin or its combination with metformin. Whole body irradiation led to severe congestion in the colon (p=0.004). Results also showed that administering melatonin significantly reduced congestion (p=0.005). Treatment using melatonin plus metformin completely reversed congestion in the colon (p=0.004). Furthermore, administering melatonin plus metformin was more effective for amelioration of congestion compared to melatonin alone (p=0.014).
3.3.2. Degenerative Changes
Results showed that exposure to radiation caused severe degenerative changes in the colon (p=0.0). Pre-and post-treatment with melatonin was not able to alleviate these changes. However, when rats were treated with melatonin plus metformin, degenerative changes were completely reversed (p=0.004). Comparison between groups showed that treatment with the combined form of these drugs had significant effective compared to melatonin alone (p=0.005). No degenerative change was observed in rats treated with drugs without radiation.
3.3.3. Blood Vessel Changes
Histological observations showed that whole body irradiation caused moderate to severe damages to blood vessels (p=0.004). Rats which received melatonin pre and post-irradiation showed no improvement in vascular injury. However, when rats received the combined form of these drugs, blood vascular injury was improved completely (p=0.004). Comparison between groups showed significant difference between rats treated with the combined drugs to those which received melatonin alone, before and after whole body irradiation (p=0.005).
3.3.4. Inflammatory Cells (Leukocyte and Macrophage)
Irradiation caused moderate to severe infiltration of leukocytes and macrophages in the colon (p=0.005). Melatonin administration led to their significant reduction (p=0.006), with colon tissues showing only a mild infiltration of leukocytes and macrophages. Administration of both melatonin and metformin led to remarkable alleviation of infiltration of immune cells, with colon samples showing no infiltration of macrophages or lymphocytes (p=0.005). Furthermore, the combination of these drugs was more effective compared to melatonin alone (p=0.014). No cytotoxicity was reported after administering drugs.
3.3.5. Muscular Layer Changes
Whole body irradiation led to moderate to severe damage to muscular layers in the colon (p=0.005). Treatment with melatonin was able to ameliorate muscle injury (p=0.007). Rats which received melatonin before and after irradiation showed only a mild change in muscular layers. Treatment with melatonin plus metformin pre and post-irradiation led to complete protection against radiation-induced muscle injury (p=0.005). No significant difference was reported for rats which received melatonin compared to those which received the combined drugs pre and post-irradiation.
3.3.6. Goblet Cell Damage
Irradiation led to severe damage to goblet cells (p=0.005). Melatonin administration caused amelioration of radiation toxicity in goblet cells (p=0.006). Similarly, administering melatonin plus metformin suppressed radiation damage in goblet cells (p=0.005). However, this combination was more effective compared to melatonin alone (p=0.014). No toxicity was observed for rats that received melatonin or melatonin plus metformin without exposure to radiation.
3.3.7. Lieberkühn Glands Damage
Results of histological evaluation showed no damage to Lieberkühn glands for rats that received drugs. However, exposure to radiation led to moderate to severe injury in Lieberkühn glands (p=0.005). Melatonin treatment pre and post-irradiation led to alleviation of damages to these glands (p=0.007), however, the combination of these drugs was able to completely overcome damages to Lieberkühn glands (p=0.005). There was no significant difference between the rats which received melatonin or melatonin plus metformin pre and post-irradiation.
4. DISCUSSION
The aim of the present study was to investigate the possible radioprotection of rat’s ileum and colon using a combined form of melatonin and metformin. The radioprotective effect of melatonin in the ileum was previously reported [18], while so far, no study has reported radioprotection of ileum and colon using metformin or its combination with melatonin. In this study, we detected the main consequences of radiation toxicity in the ileum and colon such as villi shortening, inflammation and vascular injury in ileum, as well as damages to vessels, glands, muscles and goblet cells, inflammation and congestion in colon. Results of histopathological evaluation showed that whole body irradiation caused severe damages to both ileum and colon. Reduction of goblet cells in the ileum was very obvious. Also, irradiation led to remarkable reduction of villi length in the ileum. These changes are common signs of radiation-induced enteritis for patients that undergo radiotherapy or people accidentally exposed to a high dose of radiation. In these people, shortening of villi can cause malabsorption. Also, infiltration of inflammatory cells and mucositis lead to chronic inflammation and continuous production of free radicals, which cause damage to epithelium, ulcer, bleeding and abdominal pain.
Results indicated that melatonin was able to protect ileum against radiation toxicity remarkably. Rats in this group showed a mild damage to vessels and goblet cells, without mucositis or infiltration of macrophages and lymphocytes. Furthermore, length of villi in this group was similar to control group. This showed the potent inhibitory effect of melatonin on radiation-induced apoptosis in ileum stem cells. The combination of melatonin with metformin reduced the radioprotective effect of melatonin in the ileum. However, for the colon, the combined drugs had potent radioprotective effect and was able to completely prevent radiation injury. It seems that melatonin was less effective for colon compared to ileum. The exact mechanisms of radioprotection of ileum and colon using this combination of drugs need to be elucidated using biochemical and molecular studies.
As this study has shown both melatonin or melatonin plus metformin combination have potent anti-inflammatory effects. Studies have proposed that both melatonin and metformin can scavenge free radicals, trigger DNA damage response, and stimulate antioxidant defense in cells [19, 20]. Melatonin is able to protect against radiation-induced apoptosis in normal cells via downregulation of pro-apoptosis genes such as Bax, and upregulation of anti-apoptosis mediators. The anti-apoptosis effect of melatonin can help protect radiosensitive tissues with high expression of pro-apoptosis genes [21]. This mechanism may be involved in preserving villi in the intestine, as we have investigated in our study. On the other hand, metformin is a potent inhibitor of mitochondrial derived ROS as well as some other pro-oxidants that may be involved in radiation toxicity [22, 23]. The combination of these drugs may be useful for radioprotection of some tissues including colon, whose injuries due to pelvic radiotherapy may lead to severe problems for patients.
CONCLUSION
This study has been conducted to investigate the radioprotective effect of melatonin alone or its combination with metformin, on ileum and colon. Results showed that administering melatonin alone was a more effective radioprotector for the ileum. It also preserved villi length and inhibited inflammation. However, the combination of melatonin and metformin had less radioprotection and could not improve villi length, which is necessary for protection against malabsorption. However, the combined form of melatonin and metformin had potent radioprotective effect on the colon. This combination could completely prevent the appearance of radiation toxicity in the colon.
ACKNOWLEDGEMENTS
Declared none.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All study protocols were approved by the Institutional Animal Ethical Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, (IR.AJUMS.REC.1396.683).
HUMAN AND ANIMAL RIGHTS
No humans were used in this study. All animal research procedures followed were in accordance with the standards of Guide for the Care and Use of Laboratory Animals.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This research was financially supported by Cancer Research Center (Grant number: CRC-9608) from the vice chancellor of research at Ahvaz Jundishapur University of Medical Sciences (Iran).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Czito B.G., Meyer J.J., Willett C.G. Overview of gastrointestinal toxicity of radiation therapy. Uptodate; 2017. [Google Scholar]
- 3.Peach M.S., Showalter T.N., Ohri N. Systematic review of the relationship between acute and late gastrointestinal toxicity after radiotherapy for prostate cancer. Prostate Cancer. 2015;2015: 624736. doi: 10.1155/2015/624736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gestaut M.M., Swanson G.P. Long term clinical toxicity of radiation therapy in prostate cancer patients with Inflammatory Bowel Disease. Rep. Pract. Oncol. Radiother. 2016;22(1):77–82. doi: 10.1016/j.rpor.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stansborough R.L., Bateman E.H., Al-Dasooqi N., Bowen J.M., Keefe D.M.K., Yeoh A.S.J., Logan R.M., Yeoh E.E.K., Stringer A.M., Gibson R.J. Fractionated abdominal irradiation induces intestinal microvascular changes in an in vivo model of radiotherapy-induced gut toxicity. Support. Care Cancer. 2017;25(6):1973–1983. doi: 10.1007/s00520-017-3601-3. [DOI] [PubMed] [Google Scholar]
- 6.Azzam E.I., Jay-Gerin J-P., Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1-2):48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbulut A., Sadic M., Yumusak N., Aydinbelge F., Koca G., Korkmaz M. The effects of zinc in the gastrointestinal system as a radioprotective agent. Int. J. Radiation Res. 2018;16(3):333–339. [Google Scholar]
- 8.Deniz M., Atasoy B.M., Dane F., Can G., Erzik C., Çetinel Ş., Yeğen B.Ç. Radiation-induced oxidative injury of the ileum and colon is alleviated by glucagon-like peptide-1 and-2. J. Radiation Res. Appl. Sci. 2015;8(2):234–242. doi: 10.1016/j.jrras.2015.01.010. [DOI] [Google Scholar]
- 9.Pişkin Ö., Aydın B.G., Baş Y., Karakaya K., Can M., Elmas Ö., Büyükuysal M.Ç. Protective effects of quercetin on intestinal damage caused by ionizing radiation. Med. Bull. Haseki. 2018;56(1):14–21. [Google Scholar]
- 10.Akpolat M., Kanter M., Uzal M.C. Protective effects of curcumin against gamma radiation-induced ileal mucosal damage. Arch. Toxicol. 2009;83(6):609–617. doi: 10.1007/s00204-008-0352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haghi-Aminjan H., Asghari M.H., Farhood B., Rahimifard M., Hashemi Goradel N., Abdollahi M. The role of melatonin on chemotherapy-induced reproductive toxicity. J. Pharm. Pharmacol. 2018;70(3):291–306. doi: 10.1111/jphp.12855. [DOI] [PubMed] [Google Scholar]
- 12.Haghi-Aminjan H., Farhood B., Rahimifard M., Didari T., Baeeri M., Hassani S., Hosseini R., Abdollahi M. The protective role of melatonin in chemotherapy-induced nephrotoxicity: a systematic review of non-clinical studies. Expert Opin. Drug Metab. Toxicol. 2018;14(9):937–950. doi: 10.1080/17425255.2018.1513492. [DOI] [PubMed] [Google Scholar]
- 13.Mortezaee K., Shabeeb D., Musa A.E., Najafi M., Farhood B. Metformin as a radiation modifier; implications to normal tissue protection and tumor sensitization. Curr. Clin. Pharmacol. 2019;14(1):41–53. doi: 10.2174/1574884713666181025141559. [DOI] [PubMed] [Google Scholar]
- 14.Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2015;30(3):747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 15.Sanli T., Steinberg G.R., Singh G., Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol. Ther. 2014;15(2):156–169. doi: 10.4161/cbt.26726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacPherson B.R., Pfeiffer C.J. Experimental production of diffuse colitis in rats. Digestion. 1978;17(2):135–150. doi: 10.1159/000198104. [DOI] [PubMed] [Google Scholar]
- 17.Madani Z.S., Azarakhsh S., Shakib P.A., Karimi M. Histopathological changes in dental pulp of rats following radiotherapy. Dent Res J (Isfahan) 2017;14(1):19–24. doi: 10.4103/1735-3327.201139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guney Y., Hicsonmez A., Uluoglu C., Guney H.Z., Ozel Turkcu U., Take G., Yucel B., Caglar G., Bilgihan A., Erdogan D., Nalca Andrieu M., Kurtman C., Zengil H. Melatonin prevents inflammation and oxidative stress caused by abdominopelvic and total body irradiation of rat small intestine. Braz. J. Med. Biol. Res. 2007;40(10):1305–1314. doi: 10.1590/S0100-879X2006005000156. [DOI] [PubMed] [Google Scholar]
- 19.Dogan Turacli I., Candar T., Yuksel E.B., Kalay S., Oguz A.K., Demirtas S. Potential effects of metformin in DNA BER system based on oxidative status in type 2 diabetes. Biochimie. 2018;154:62–68. doi: 10.1016/j.biochi.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Asghari A., Akbari G., Meghdadi A., Mortazavi P. Effects of melatonin and metformin co-administration on testicular ischemia/reperfusion injury in rats. J. Pediatr. Urol. 2016;12(6):410.e411–410.e417. doi: 10.1016/j.jpurol.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Cheki M., Shirazi A., Mahmoudzadeh A., Bazzaz J.T., Hosseinimehr S.J. The radioprotective effect of metformin against cytotoxicity and genotoxicity induced by ionizing radiation in cultured human blood lymphocytes. Mutat. Res. 2016;809:24–32. doi: 10.1016/j.mrgentox.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Azmoonfar R., Amini P., Saffar H., Rezapoor S., Motevaseli E., Cheki M., Yahyapour R., Farhood B., Nouruzi F., Khodamoradi E., Shabeeb D., Eleojo Musa A., Najafi M. Metformin protects against radiation-induced pneumonitis and fibrosis and attenuates upregulation of dual oxidase genes expression. Adv. Pharm. Bull. 2018;8(4):697–704. doi: 10.15171/apb.2018.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yahyapour R., Amini P., Saffar H., Rezapoor S., Motevaseli E., Cheki M., Farhood B., Nouruzi F., Shabeeb D., Musa A.E, Najafi M. Metformin protects against radiation-induced heart injury and attenuates the up-regulation of dual oxidase genes following rat’s chest irradiation. Int. J. Mol. Cellular. Med. 2018;7(3):193–202. doi: 10.22088/IJMCM.BUMS.7.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.