Dear Editor,
Since December 2019, A new type of coronavirus pneumonia (coronavirus disease 2019, COVID-19) has become endemic in Wuhan, China. So far, COVID-19 has developed into a global epidemic. The body’s immune system plays an important role in the fight against COVID-19. Here, we followed up the clinical data and treatment of two COVID-19 patients diagnosed with acquired immunodeficiency syndrome (AIDS), hoping to be helpful for the subsequent diagnosis and treatment of patients with related diseases.
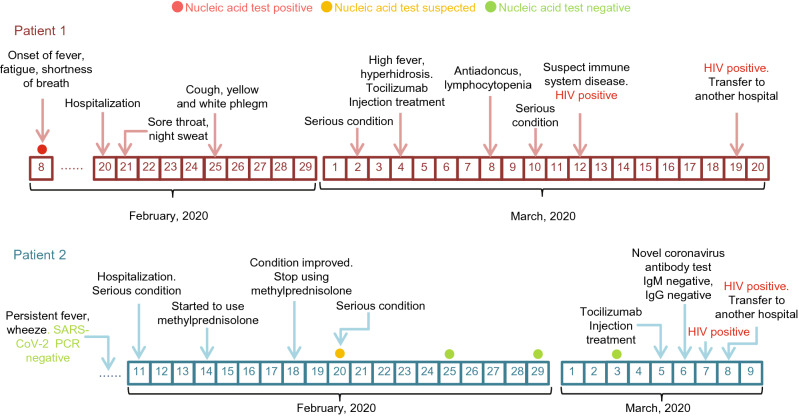
Patient 1, male, 24 years old, unmarried, lived in Hankou Jiangan District, Wuhan City before the onset of the disease, presented with fever symptoms up to 40 °C on February 8, accompanied by fatigue, anorexia, dizziness, and apparent chest tightness and shortness of breath after the exercise. Then he came to our hospital for treatment on the evening of February 20, 2020. The CT scan indicated that double lungs were compromised, suggesting the possibility of viral pneumonia (Supplementary Table S1). He also tested positive for SARS-CoV-2 in his throat’s swab. Patient 1 was admitted to the COVID-19 designated hospital on February 20, with no significant remission from previous treatment. One day after admission (February 21), the patient developed symptoms of sore throat and night sweats, suggesting possible secondary bacterial infections. CT scan on March 2 suggested an increase in double-lung lesions and a risk of disease aggravation. Subsequently, patient 1 developed a high fever during the night on March 3. One day later, on March 4, tocilizumab injection (an IL-6 inhibitor) was started. The hospital began to treat patient 1 with antibiotics due to his antiadoncus on March 8, suggesting nosocomial infection. Because a variety of treatments did not work, three CT scan results of the patient also showed no improvement, the hospital began to consider the impact of immune-related diseases. The blood routine and biochemical results suggested a lymphocytic albumin decrease (Tables 1 and 2). The patient was tested for HIV antibodies and obtain a positive result, which was reported to the Wuhan Center for Disease Control and Prevention for reexamination on March 12. The patient was transferred to a specialized hospital for treatment on March 19, and subsequent reexamination confirmed HIV infection. Patient 1 underwent the SARS-CoV-2 antibody test on March 4 and tested positive for IgM and IgG antibodies. However, when the patient was re-examined on March 14, the result of the SARS-CoV-2 antibody test was negative (Table 1). Meanwhile, the patient’s condition did not improve (Fig. 1).
Table 1.
Laboratory findings of patient 1 co-infected with SARS-CoV-2 and HIV
| Laboratory findings (Patient 1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detecting Item (Normal range) | Feb 8 | Feb 21 | Feb 26 | Mar 2 | Mar 3 | Mar 4 | Mar 7 | Mar 9 | Mar 11 | Mar 14 |
| White blood cell count (×109/L) (3.50–9.50) | ND | 6.3 | 5.0 | ND | 6.9 | ND | 4.3 | ND | ND | 7.8 |
| Neutrophil count (×109/L) (1.80–6.30) | ND | 4.47 | 2.98 | ND | 4.70 | ND | 2.78 | ND | ND | 5.42 |
| Lymphocyte count (×109/L) (1.10–3.20) | ND | 1.08 | 1.16 | ND | 1.20 | ND | 0.70 | ND | ND | 1.31 |
| C-reactive protein (mg/L) (0.00–5.00) | ND | 39.71 | ND | ND | ND | ND | 1.56 | ND | ND | 0.29 |
| Red blood cell count (×1012/L) (4.3–5.8) | ND | 4.07 | 3.98 | ND | 3.77 | ND | 4.01 | ND | ND | 4.02 |
| Urea (mmol/L) (1.8–7.3) | ND | 1.97 | 1.55 | ND | ND | ND | 1.95 | ND | 2.94 | 3.61 |
| Creatinine (µmol/L) (44–97) | ND | 70.7 | 59.2 | ND | ND | ND | 63.0 | ND | 74.3 | 31.7 |
| Uric acid (mmol/L) (3.1–8) | ND | 217 | 164 | ND | ND | ND | 230 | ND | 285 | 231 |
| IL-6 (pg/mL) (< 7) | ND | ND | ND | 30.54 | ND | ND | ND | 688.40 | ND | 521 |
| SARS-CoV-2 IgM (< 10) | ND | ND | ND | ND | ND | 30.12 | ND | ND | ND | 0.96 |
| SARS-CoV-2 IgG (< 10) | ND | ND | ND | ND | ND | 63.52 | ND | ND | ND | 3.87 |
| SARS-CoV-2 nucleic acid test | + | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Co-infection detection | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV(0-1) | ND | ND | ND | ND | ND | ND | ND | ND | 69.63 | ND |
| Syphilis(0-1) | ND | ND | ND | ND | ND | ND | ND | ND | 8.74 | ND |
| Hepatitis B | ND | ND | ND | ND | ND | ND | ND | ND | − | ND |
| Hepatitis C | ND | ND | ND | ND | ND | ND | ND | ND | − | ND |
Notes: ND, no data; +, positive; −, negative
Table 2.
Leukocyte differential count and Lymphocyte subpopulation
| Detecting Item (Normal range) | Result (%) |
|---|---|
| Leukocyte differential count (Parient 1) | |
| Lymphocyte count (20%–40%) | 13.1 |
| Monocyte count (3%–8%) | 7.2 |
| Granulocyte count (50%–70%) | 79.6 |
| CD3+ (59%–85%) | 77.6 |
| CD3+CD4+ (Male, 29%–57%) | 1.0 |
| CD3+CD8+ (11%–38%) | 72.7 |
| CD3+CD4+/CD3+CD8+ (0.9–3.6) | 0.01 |
| CD3-CD19+ (6.4%–23%) | 12.9 |
| CD3-(CD16+/CD56+) (5.6%–31%) | 4.7 |
Fig. 1.
The clinical courses of two cases co-infected with SARS-CoV-2 and HIV.
Patient 2, male, 37 years old, unmarried, lived in Wuchang District, Wuhan City before onset of the disease, developed fever without any cause in early January, when chest pain was intermittent. CT scan results in early February indicated lesions in bilateral lungs (Supplementary Table S1), but the result of the SARS-CoV-2 nucleic acid test was negative. Patient 2 was severely ill and admitted on February 11, with significant wheezing symptoms. Low-dose hormonal anti-inflammatory therapy began on February 14 and continued until patient 2 became relieved on February 18. Patient 2 was also treated with antiviral, anti-infective, and other symptomatic treatments. However, the patient’s condition deteriorated again on February 20, and the nucleic acid test results were single positive for COVID-19 SARS-CoV-2. After that, three more SARS-CoV-2 nucleic acid test results were negative. The patient was treated with tocilizumab injection on March 5 due to his critical condition. However, two SARS-CoV-2 antibody tests of patient 2 obtained negative results on March 5 and 6. The patient was tested for HIV antibodies on March 7 and 8, respectively, and the results were both positive (Table 3). After that, the test results were reported to the Wuhan Center for Disease Control and Prevention for review, the final reexamination result is still positive. On March 8, the patient was transferred to a specialized hospital for further treatment.
Table 3.
Laboratory findings of patient 2 co-infected with SARS-CoV-2 and HIV
| Laboratory findings (Patient 2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detecting Item (Normal range) | Feb 11 | Feb 16 | Feb 20 | Feb 22 | Feb 25 | Feb 26 | Feb 29 | Mar 1 | Mar 2 | Mar 3 | Mar 5 | Mar 6 |
| White blood cell count (×109/L) (3.50–9.50) | 4.2 | 3.2 | 4.6 | 3.8 | ND | 4.6 | 4.8 | 3.3 | ND | ND | ND | ND |
|
Neutrophil count (×109/L) (1.80–6.30) |
1.97 | 2.18 | 3.16 | 2.38 | ND | 2.70 | 2.80 | 2.23 | ND | ND | ND | ND |
| Lymphocyte count (×109/L) (1.10–3.20) | 1.55 | 0.60 | 0.91 | 0.84 | ND | 1.30 | 1.34 | 0.56 | ND | ND | ND | ND |
| C-reactive protein (mg/L) (0.00–5.00) | 96.51 | 42.70 | 26.06 | 11.14 | ND | 6.89 | 6.78 | 11.65 | ND | ND | ND | ND |
| Red blood cell count (×1012/L) (4.3–5.8) | 4.99 | 4.85 | 5.28 | 4.67 | ND | 5.06 | 5.04 | 4.52 | ND | ND | ND | ND |
| Urea (mmol/L) (1.8–7.3) | 6.21 | ND | 4.63 | 6.00 | ND | ND | 4.91 | 3.56 | ND | ND | ND | ND |
| Creatinine (µmol/L) (44–97) | 92.0 | ND | 64.6 | 71.7 | ND | ND | 64.4 | 63.2 | ND | ND | ND | ND |
| Uric acid (mmol/L) (3.1–8) | 286 | ND | 236 | 292 | ND | ND | 321 | 299 | ND | ND | ND | ND |
| IL-6 (pg/mL) (< 7) | ND | ND | ND | ND | ND | ND | ND | ND | 9.87 | ND | ND | 141.40 |
| SARS-CoV-2 IgM (< 10) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.79 | 0.86 |
| SARS-CoV-2 IgG (< 10) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.63 | 0.57 |
| SARS-CoV-2 nucleic acid test | − | ND | +* | ND | − | ND | − | ND | ND | − | ND | ND |
| Co-infection detection | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV (0-1) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 58.3 |
| Syphilis (0-1) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 11.67 |
| Hepatitis B | ND | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND |
| Hepatitis C | ND | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND |
Notes: ND, no data; +*, single positive; -, negative
We report the early clinical treatment of two COVID-19 patients diagnosed with AIDS. Both patients showed typical symptoms of SARS-CoV-2 infection throughout the disease, such as fever, chest tightness, and shortness of breath. CT scans of both patients can be used as imaging evidence for COVID-19 infection. Meanwhile, CT scans of both patients during treatment showed disease deterioration.
The laboratory test of SARS-CoV-2 mainly consists of SARS-CoV-2 virus nucleic acid test and serological test. Specific antibodies are generally produced 3–5 days after the onset of COVID-19. An increased IgM antibody indicates recent acute infection, while an increased IgG antibody indicates a previous infection. In this study, we found unnormal changes of viral nucleic acid and antibodies in two patients. For patient 1, at the beginning of the illness, the viral nucleic acid test result was positive, and the viral antibody test result was also positive. However, while the patient’s condition worsened, which led to his transfer, the viral antibody test result became negative. Compared with patient 1, patient 2 was in a more severe condition. Several SARS-CoV-2 nucleic acid tests were performed while the patient was in the hospital. Curiously, except for a single positive SARS-CoV-2 nucleic acid test result on February 20, other test results on February 25, February 29, and March 3 were all negative. Both patients were diagnosed with AIDS and developed nosocomial infections and poor treatment response. We assumed that HIV infection had damaged their immune systems; this could also explain why the patient tested negative for SARS-CoV-2 antibodies in the late stages of treatment when the disease became worse. The destruction of the immune system by HIV may have affected the normal humoral immune process. Meanwhile, considering the latent period of HIV is usually quite long, 8–9 years, on average, we suspect that co-infection with SARS-CoV-2 may increase the pathogenicity of both and lead to the destruction of the immune system. The patient’s symptoms seemed to ease in the early stages of treatment, but as AIDS progressed, the immune system was destroyed, making COVID-19 condition worse and the treatment less effective. It is also worth noting that the patient was admitted to a designated hospital for COVID-19 treatment, where an IL-6 inhibitor (tocilizumab injection) was used to relieve severe inflammation. SARS-CoV-2 infected patients may develop an uncontrolled immune response known as a cytokine storm, which leads to the massive production of proinflammatory cytokines and other inflammatory proteins, such as IL-6, TNF-α, and GM-CSF, causing severe lung tissue damage, respiratory failure, and even death (Vaninov 2020; Mehta et al. 2020). Interleukin 6 (IL-6) is a type of cytokine produced by activated T cells and fibroblasts. It turns the precursor of B cells into antibody-producing cells. Synergizing with colony-stimulating factor, it can promote the growth and differentiation of original bone marrow-derived cells and enhance the lysis function of natural killer cells (Smits et al. 2011; Tanaka et al. 2014). IL-6 appears to be a significant contributor to coronavirus-mediated respiratory failure (Herold et al. 2020; Liu et al. 2020). In this study, both severely ill patients presented with abnormally high levels of IL-6. The patient 1 began treatment with tocilizumab on March 4. The data indicated that the patient’s symptoms were relieved, and there was no fever after 3 days of treatment with tocilizumab. The clinical manifestations of patient 1 after the administration of IL-6 inhibitors indicated that IL-6 could be a key target for inhibiting COVID-19 inflammation. Although the patient’s condition deteriorated again later, we suspect that this was related to the destruction of the immune system in patient 1 with AIDS. Patient 2 was also treated with tocilizumab on March 5. Because 1 day after the start of the medication, the patient was transferred to a specialized hospital for further treatment, the treatment effect still needs to be evaluated by the following study. In general, the blocking of the IL-6 receptor with tocilizumab has a particular effect on the treatment of COVID-19 patients with severe disease, but it may have little effect on patients with autoimmune diseases, which should be considered comprehensively in clinical practice.
COVID-19 patients with immunodeficiency disease may cause more severe illness and poor treatment response due to the destruction of the immune system. However, the current screening work for patients with immunological diseases is still not very adequate; the therapeutic effect for an immunocompromised patient without the corresponding targeted treatment may not be evident (Castro and Gourley 2010). Therefore, we suggest that patients with COVID-19 should be tested for immune diseases to achieve targeted treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
This study was supported by the Special Fund for COVID-19 Research of Wuhan University. We are grateful to Taikang Insurance Group Co., Ltd, Beijing Taikang Yicai Foundation. This study was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention (No. 2017ZX10103005), and National Key Research and Development Program of China (No. 2018YFE0204500).
Compliance with Ethical Standards
Conflict of interest
All authors report no competing interests.
Animal and Human Rights Statement
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants enrolled in the study.
Footnotes
Rong Zhang, Xiaohua Chen and Yuqing Huang contributed equally to this work.
Contributor Information
Fang Liu, Email: liufung@whu.edu.cn.
Yingle Liu, Email: mvlwu@whu.edu.cn.
Ke Lan, Email: klan@whu.edu.cn.
References
- Castro C, Gourley M. Diagnostic testing and interpretation of tests for autoimmunity. J Allergy Clin Immunol. 2010;125:S238–S247. doi: 10.1016/j.jaci.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, Klein M, Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136.e41. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang J, Yang Y, Ma H, Li Z, Zhang J, Cheng J, Zhang X, Zhao Y, Xia Z, Zhang L, Wu G, Yi J. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12:e12421. doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits SL, van den Brand JM, de Lang A, Leijten LM, van Ijcken WF, van Amerongen G, Osterhaus AD, Andeweg AC, Haagmans BL. Distinct severe acute respiratory syndrome coronavirus-induced acute lung injury pathways in two different nonhuman primate species. J Virol. 2011;85:4234–4245. doi: 10.1128/JVI.02395-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. 2020;20:277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.