Abstract
The title compounds were prepared, and their reactivity was studied upon sensitized irradiation at λ = 420 nm. Thioxanthen-9-one was employed as the sensitizer at a loading of 10 mol % in small-scale reactions and of 2.5 mol % on a larger scale. Cyclohex-2-enones substituted by a 2′-propenyloxy, 2′-butenyloxy, 2′-pentenyloxy, or 2′-methyl-2′-propenyloxy group in the 2-position gave the products of an intramolecular [2 + 2] photocycloadditon. The reaction proceeded with high regioselectivity (crossed product) and perfect diastereoselectivity (nine examples, 34–99% yield). If the olefin in the tether was trisubstituted (3′-methyl-2′-butenyloxy), no cycloaddition was observed. Rather, a cyclization with subsequent hydrogen abstraction occurred (three examples, 65–86% yield). The results are consistent with a reaction course via a triplet enone intermediate and the formation of a 1,4-diradical by an initial cyclization. The analogous cyclopent-2-enones were less prone to an intramolecular reaction. Instead, decomposition or intermolecular [2 + 2] photocycloaddition reactions prevailed. In the latter event, two main products were identified (three examples, 30–43% yield), resulting either from a head-to-head [2 + 2]-photodimerization or from a twofold [2 + 2] photocycloaddition of the enone to the olefin. The latter reaction sequence generated pentacyclic products with a central [1,5]dioxocane ring. The structure assignment of the two product types was corroborated by a single-crystal X-ray analysis.
Introduction
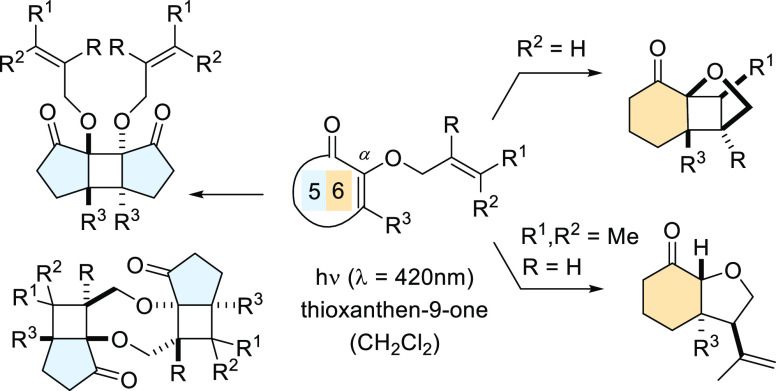
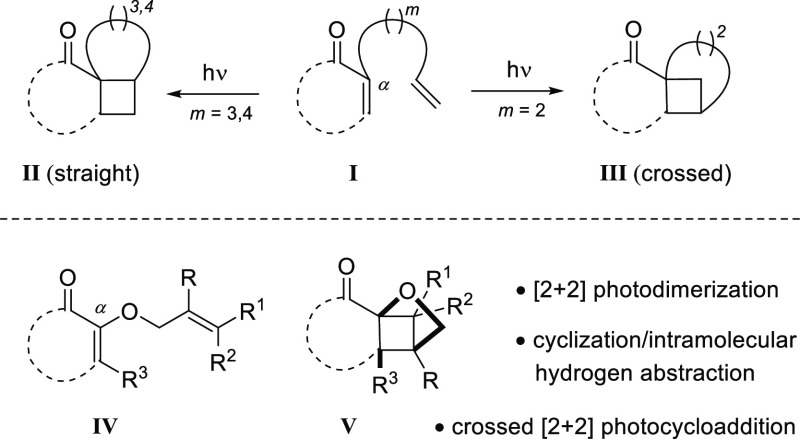
Like most intramolecular cycloadditions, the [2 + 2] photocycloaddition1 benefits from an improved regioselectivity if the two reaction partners are linked by a suitable tether. Cyclic α,β-unsaturated ketones represent the most frequently used chromophores in these reactions2 and the preferred position for attachment of a tether is the α- or the β-position.3 For an α-substituted enone I (Scheme 1), the number m of atoms in the tether governs the regioselectivity. A preference toward a parallel approach of the two olefins is observed if three or four atoms link the two olefinic groups (m = 3, 4), and the photocycloaddition products II are frequently referred to as straight products. If the tether is short (m = 2) photocycloaddition products III prevail in which the two olefins approach each other in a crossed fashion. The regioselectivity can be understood by the mechanistic course of the reaction4 which involves an initial attack of the olefin on the photoexcited enone to a 1,4-diradical intermediate (vide infra). The first step of the cycloaddition is consequently a cyclization in which the formation of five-membered rings5 is preferred.
Scheme 1. Regioselectivity in the Intramolecular [2 + 2] Photocycloaddition of α-Substituted Enones I and Possible Reaction Pathways of α-Allyloxy-Substituted Enones IV.
2-(2′-Alkenyloxy)cycloalk-2-enones with the general structure IV represent a relatively unexplored compound class in [2 + 2] photocycloaddition chemistry. Ikeda et al. performed experiments with three 2-allyloxycyclohex-2-enones as substrates, employing a high-pressure mercury lamp as the light source and acetone as the solvent.6 Crossed products (general structure V) were obtained in 53–63% yield and subsequent transformations of these products were intensively studied. We have now looked at an expanded set of substrates with two main objectives: (a) Instead of UV light it was attempted to perform the photochemical reactions with visible light. To this end, a suitable sensitizer was required which absorbs in the visible range and does not lead to any side reactions. While visible light-mediated, sensitized reactions of styrenes and related compounds have recently received broad attention,7 studies with cyclic enones have remained scarce.8 (b) It was expected that other reaction pathways might be feasible depending on the structure of the chromophore and the substitution pattern of the olefin. Most importantly, [2 + 2] photodimerization and hydrogen abstraction9 were foreseen as competing reactions which would in turn lead to intriguing new structures. The results of our experimental work are summarized in this account.
Results and Discussion
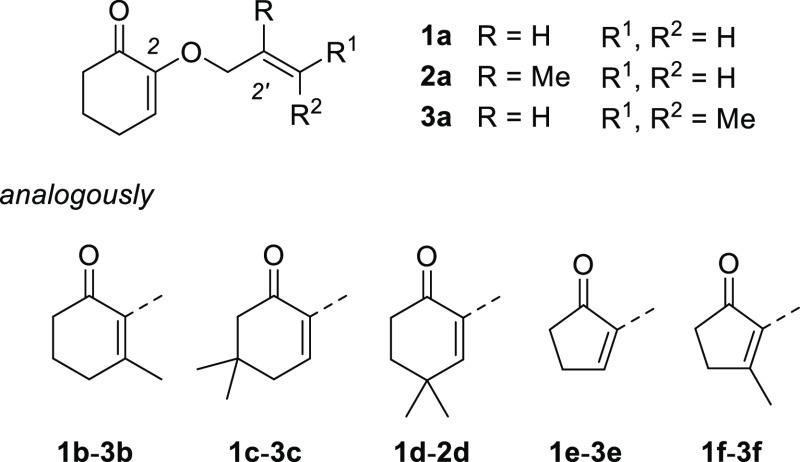
Figure 1 provides an overview about the compounds we employed in the present study. With regard to the olefin component, three 2′-alkenyloxy groups were employed, that is, a 2′-propenyloxy (allyloxy) group (substrates 1), a 2′-methyl-2′-propenyloxy (methallyloxy) group (substrates 2), and a 3′-methyl-2′-butenyloxy (prenyloxy) group (substrates 3). With regard to the enone component, some substituted cyclic enones (substrates b, c, d, f) were probed as starting materials apart from the unsubstituted cyclohex-2-enones (substrates a) and cyclopent-2-enones (substrates e). Cyclohex-2-enones with (Z)- and (E)-substituted 2′-alkenyloxy groups were also prepared but will be discussed in a later section.
Figure 1.
Overview about the 2-(2′-alkenyloxy)cycloalk-2-enones 1–3 employed in this study.
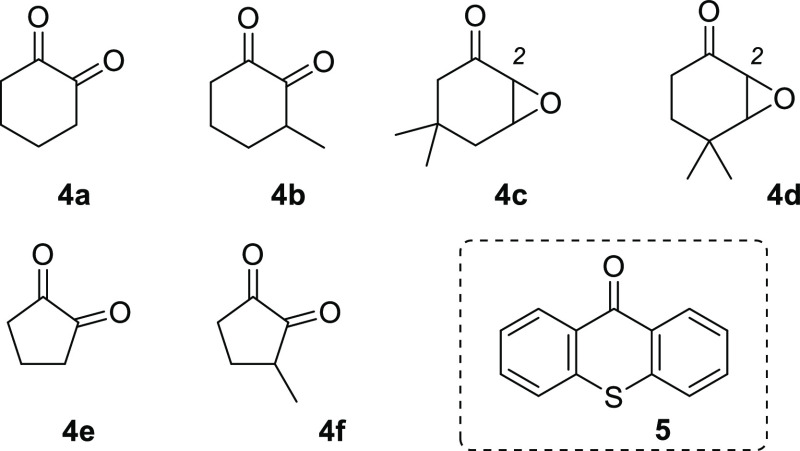
Because the synthesis of 2-(2′-alkenyloxy)-4,4-dimethylcyclohex-2-enones was low yielding only substrates 1d and 2d were synthesized but not 3d. In general, three approaches toward the [2 + 2] photocycloaddition precursors were taken. Starting from cyclic 1,2-diketones the alkenyloxy group was introduced either by condensation with the respective allylic alcohol under acidic conditions (method A: TsOH in cyclohexane, reflux)6,10 or by nucleophilic substitution of an allyl bromide via the enolate (method B: K2CO3 in DMF, room temperature).11 Along these lines, 1,2-diketones 4a, 4b, 4e, and 4f (Figure 2) served as precursors for the respective 2-(2′-alkenyloxy)cycloalk-2-enones 1a–3a, 1b–3b, 1e–3e, and 1f–3f. For the synthesis of substituted cyclohexenones 1c–3c and 1d, 2d epoxides 4c and 4d served as starting materials, which underwent nucleophilic substitution in position C2 when treated with an excess of allylic alcohol under basic conditions (method C: NaH or KOH as base).12 As mentioned above the reaction gave low yields (6–12%) when applied to epoxide 4d which limited the supply of the starting material. Reasons for the inefficiency of the procedure were difficulties in removing excess allylic alcohol and formation of a side product (see the Supporting Information for further details) by dimerization of the epoxide. Because the emphasis of the study was on the [2 + 2] photocycloaddition chemistry, alternative routes to the substituted enones were not pursued. With the exception of compounds 1a, 2a, and 1c, 2-(2′-alkenyloxy)cycloalk-2-enones have not been previously employed in photochemical reactions.
Figure 2.
Structure of starting materials 4 for the synthesis of 2-(2′-alkenyloxy)cycloalk-2-enones and structure of thioxanthen-9-one (5).
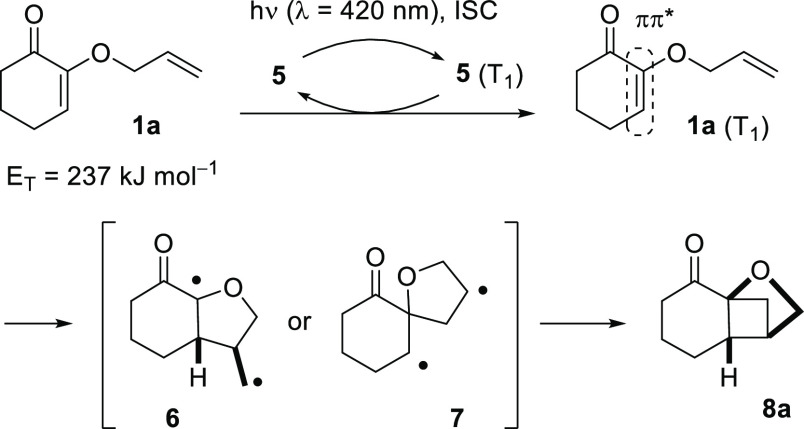
Preliminary photochemical studies were performed with substrate 1a. Its triplet energy was determined from phosphorescence experiments (77 K, CH2Cl2) as ET = 235 kJ mol–1. Because a wavelength of λ = 400 nm corresponds to an energy of ca. 300 kJ Es–1 (1 Es = 6.022 × 1023 photons), it seemed feasible to promote the [2 + 2] photocycloaddition with visible light upon a judicious choice of a sensitizer. In this regard, parent thioxanthen-9-one (5) and its derivatives represent an excellent option and have been nicely exploited by Booker-Milburn and co-workers in [2 + 2] photocycloaddition reactions on a large scale.8b In recent works on visible light-mediated reactions, we have also seen that catalyst loadings can be as low as 1 mol % for a thioxanthone sensitizer.13 Experiments with a selection of potential sensitizers (see the Supporting Information for details) confirmed the suitability of thioxanthen-9-one (5) as the catalyst in the planned reaction (ET = 268 kJ mol–1).14 Dichloromethane was established as the preferred solvent and there was no improvement in selectivity if the reaction was performed at low temperatures. The reactions were run on a small scale (50–200 μmol substrate, c = 10 mM) at a wavelength of λ = 420 nm (16 fluorescent lamps with an emission maximum at λ = 420 nm, for the emission spectrum, see the Supporting Information) and yields of product 8a (Scheme 2) were consistently around 70%, irrespective of whether 2.5, 5, or 10 mol % of 5 were used. Indeed, the low-molecular weight (212 Da) of thioxanthen-9-one (5) made it relatively difficult to measure the exact quantity. Iridium catalysts (2.5 mol %) with a much higher molecular weight performed similar to 5 and there was a correlation between the reaction time and the triplet energy of the catalyst. Sensitizers with a triplet energy ET > 250 kJ mol–1 led to a complete conversion after 2.5 h. The reaction time increased with a decrease in triplet energy. The reaction with Ir(ppy)3 (ET = 231 kJ mol–1),15 for example, required 5 h before no substrate was detected by TLC. The ruthenium complex Ru(bpy)3(PF6)2 (ET = 193 kJ mol–1)16 did not promote the reaction and there was also no reaction in the absence of a sensitizer.
Scheme 2. Mechanistic Proposal for the Course of the Sensitized Intermolecular [2 + 2] Photocycloaddition of Cyclohex-2-enone 1a.
The formation of crossed product 8a can be easily understood by assuming an energy transfer from the triplet state T1 of thioxanthone 5 to substrate 1a. Intermediate 5 (T1) is populated with high efficiency by direct excitation and rapid17 intersystem crossing (ISC). An exothermic triplet energy transfer occurs by a spin-allowed, mutual electron–electron exchange process, often referred to as sensitization or triplet sensitization.18 Upon energy transfer, the reactive triplet state 1a (T1) is generated which is ππ* in character and prone to undergo double bond addition.4 Subsequently, 1,4-diradicals 6 and 7 are formed by the five-membered ring closure5 and both represent potential precursors to product 8a. The low reduction potentials of α,β-unsaturated enones (E1/2red ≤ −2.0 V)19 render any single electron transfer (photoredox) processes not viable.
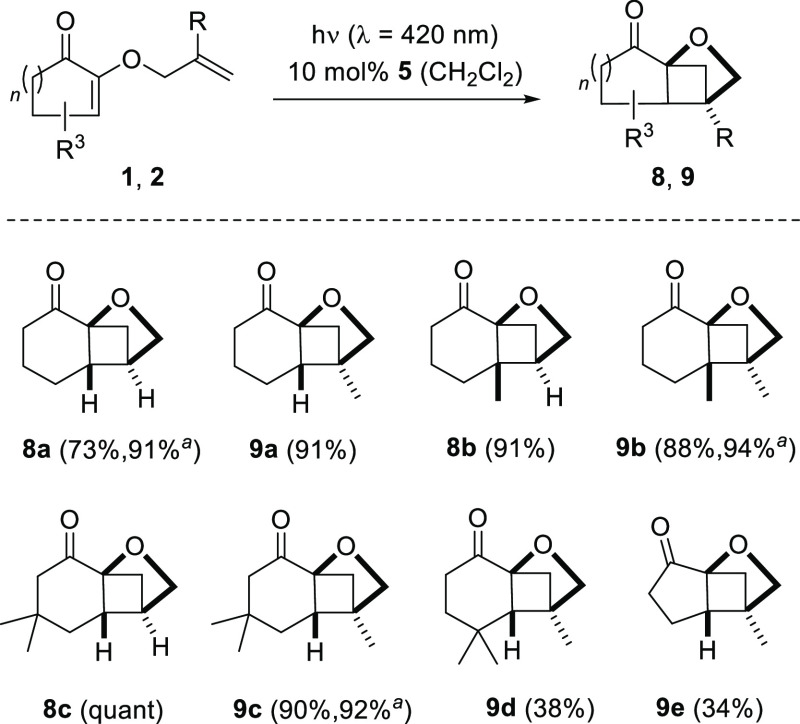
Several allyloxy- and methallyloxy-substituted cycloalk-2-enones reacted similar to substrate 1a, and the results are summarized in Scheme 3. In order to secure a reproducible reaction course, 10 mol % of the sensitizer were used for small scale reactions. Some of the reactions were also performed on a larger scale (1 mmol) and at a higher concentration (c = 25 mM). In this case, only 2.5 mol % of the sensitizer was employed. Despite a longer reaction time, chemoselectivity did not suffer and the yields were as good as on a small scale or even higher.
Scheme 3. Intramolecular [2 + 2] Photocycloaddition of Various 2-(2′-Alkenyloxy)cycloalk-2-enones upon Irradiation with Visible Light.
Yield if the reaction was performed on a 1 mmol scale.
Crossed photocycloadditon products 8a–8c and 9a–9c were isolated cleanly without any indication for the formation of other side products. Among the 4,4-dimethylcyclohexenones 1d and 2d, only the latter substrate showed a photochemical reaction. The former substrate did not react and the starting material was recovered. Because quantities were limited, the low yield of product 9d might be associated with the loss of the product during chromatographic purification. Both compounds 9d and 9e were difficult to detect on TLC which further impaired a quantitative isolation. Cyclopentenone 1e was converted upon sensitized irradiation (cf. Scheme 3) but no defined products could be isolated. The same observation was made when the compound was irradiated directly at λ = 350 nm (c = 10 mM, CH2Cl2).
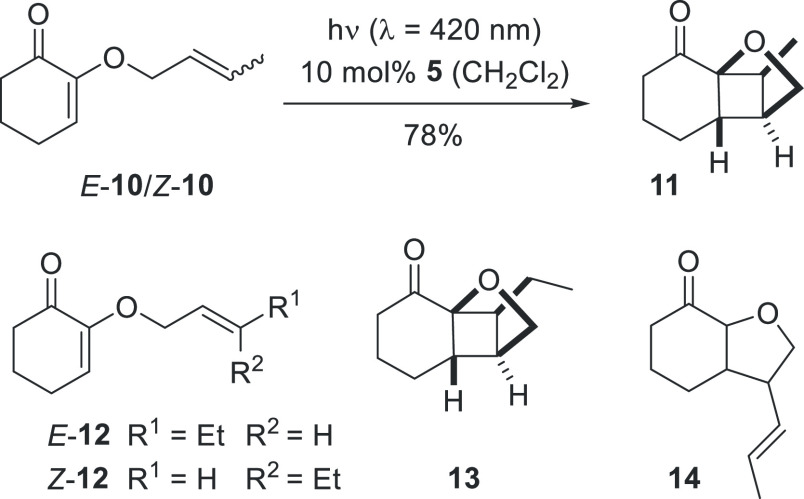
The suspected intermediacy of 1,4-diradicals in the course of the [2 + 2] photocycloaddition implied the possibility to perform stereoconvergent reactions20 with 1,2-disubstituted olefins in the tether. Substrates E-10/Z-10 (Scheme 4) were obtained from an E/Z-mixture (d.r. = 50/50) of crotyl alcohol (2-butenol) by condensation with 1,2-diketone 4a (method A). Sensitized irradiation delivered photocycloaddition product 11 as a single diastereoisomer accompanied by an olefinic impurity which was removed by treatment with 3,6-bis(methoxycarbonyl)-1,2,4,5-tetrazine.21 The yield of the clean isolated product was 78% and its relative configuration was assigned based on NOESY spectra (see the Supporting Information for further details).
Scheme 4. Stereoconvergent Formation of [2 + 2] Photocycloaddition Products 11 and 13.
The ethyl-substituted substrates 12 were obtained in a diastereomerically pure form from (E)- and (Z)-3-penten-1-ol and diketone 4a. The compounds were individually subjected to the irradiation conditions and delivered the same product 13. In this case, the product was not completely homogenous but was contaminated by compound 14 (yield from E-12: quant., 13/14 = 82/18; yield from Z-12: 90%, 13/14 = 83/17). Byproduct formation is likely to occur from the intermediate 1,4-diradical by intramolecular hydrogen abstraction (vide infra). It was possible to remove the undesired compound by oxidation and the desired product 13 was obtained in 69% yield. The outcome of the reactions suggests that 1,4-diradicals related to 6 are the major intermediates in the [2 + 2] photocycloaddition to products 11 and 13. Not only do they explain the observed stereoconvergence of the reaction but they also account for the formation of the olefinic side products.
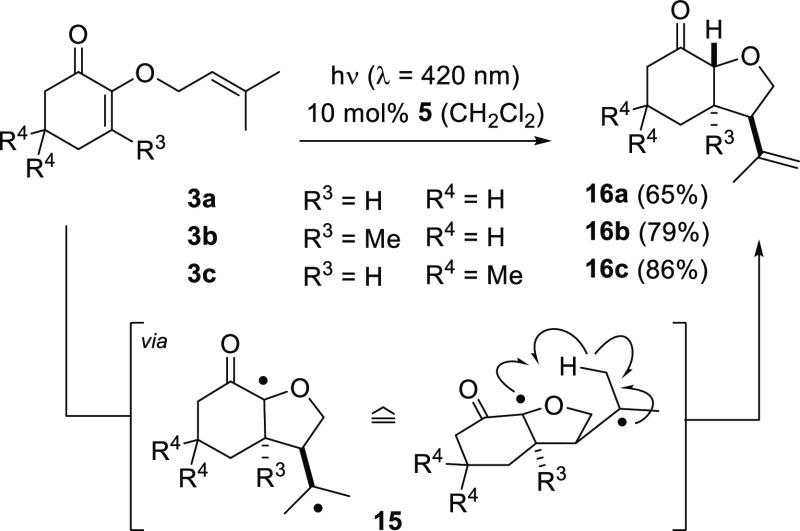
When cyclohexenones 3a–3c with a trisubstituted tethered olefin were employed as starting materials, a cyclization/reduction pathway became dominant and [2 + 2] photocycloaddition products were not detected. The high yields achieved for products 16a–16c (Scheme 5) render some synthetic utility to this method, which represents formally a conjugate addition to the β-carbon atom of the α,β-unsaturated enone. The assignment of the relative product configuration was suggested by 1H NMR coupling constants and NOESY experiments (see the Supporting Information for details) and the selectivity of the process is remarkable as it allows to construct three contiguous stereogenic centers with a defined configuration.
Scheme 5. Formation of Cyclization Products 16 from 2-Dimethylallyloxy-Substituted Enones 3 via 1,4-Diradicals 15.
Mechanistically, the first step of the sequence occurs presumably in analogy to the C–C bond forming event previously mentioned (1a → 6), that is, the triplet of 3 undergoes intramolecular addition to the tethered olefin by a five-membered ring cyclization. However, because of steric hindrance of the substituents (R3 and CMe2), bond formation occurs in a trans fashion. In the intermediate bicyclic 1,4-diradical 15 cyclization to a cyclobutane by C–C bond formation is not feasible, but an intramolecular hydrogen abstraction9 can readily occur which generates products 16.
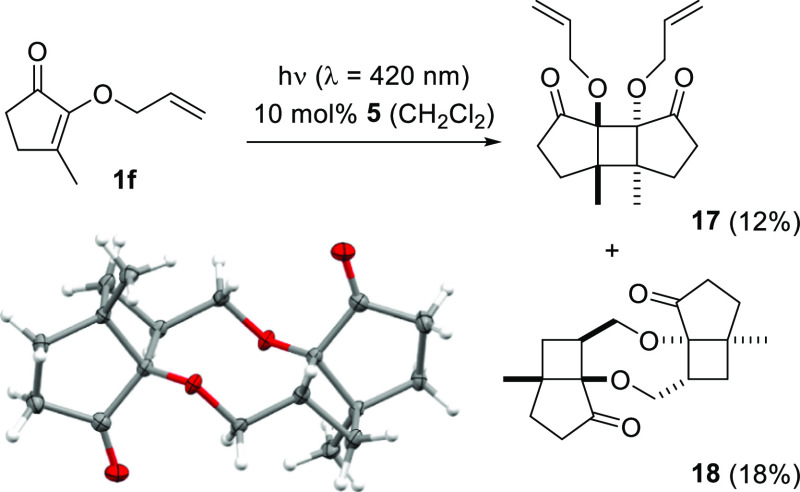
Like cyclopentenone 1e in the [2 + 2] photocycloaddition study, the cyclopentenone with a prenyloxy substituent (3e) showed only decomposition under the conditions successfully employed for substrate 3a. Also the cyclopentenones 1f–3f with an additional methyl group in the β-position displayed a reactivity that did not parallel the reactivity pattern of the comparable six-membered cyclic enones 1b–3b. In this instance, however, defined products could be isolated albeit in only low to moderate yields. The preferred reaction pathway of the cyclopentenones 1f–3f was a [2 + 2] photodimerization with the interesting twist that there are two double bonds available in each component. Along these lines, 2-(2′-propenyloxy)-3-methylcycloalk-2-enone (1f) produced a set of [2 + 2] photocycloaddition products from which two products 17 and 18 could be isolated (Scheme 6). Product 17 is the [2 + 2] photodimerization product resulting from the addition of the two enone double bonds in what is commonly referred to as a cis-anti-cis addition.1,2 The cis fusion between the five-membered rings and the four-membered ring is to be expected because of the rigidity of the skeleton, while the anti (trans) configuration of the cyclopentanone rings minimizes their steric repulsion. Based on its NMR data, second product 18 was also symmetric and—as confirmed by its crystal structure—it displays an inversion center (S2 symmetry).22 The crystal structure revealed that the bicyclo[3.2.0]heptane core is cis connected and all substituents within the cyclobutane are positioned in a cis fashion. The two constitutionally identical bicyclo[3.2.0]heptane fragments possess an opposite absolute configuration. Given the low combined yield for compounds 17 and 18, it is likely that other dimerization products were formed but could not be isolated in a pure form. [2 + 2] Photodimerization, in a head-to-head fashion, is common for cyclopentenones,23 while the regioselectivity of the intermolecular head-to-head double bond addition of a monosubstituted olefin to an enone has less precedence.9d,23d,24
Scheme 6. [2 + 2] Photodimerization of Cyclopent-2-enone 1f and Crystal Structure of Product 18.
Ellipsoids in the ORTEP structure are displayed at the 50% probability level.
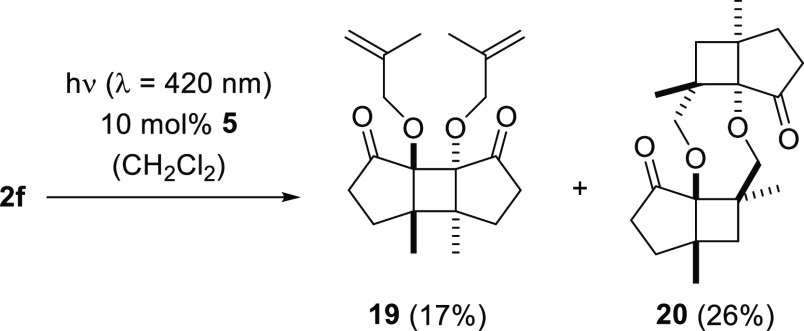
The overall product yield achieved in the [2 + 2] photodimerization of compound 2f was slightly higher (44%) than observed for 1f. Again two regioisomers were isolated, one of which was the enone dimerization product 19 and the other the product of an enone/olefin addition. The configuration assignment for the latter product 20 was based on an analogy to product 18 (Scheme 7).
Scheme 7. [2 + 2] Photodimerization of Cyclopent-2-enone 2f to Products 19 and 20.
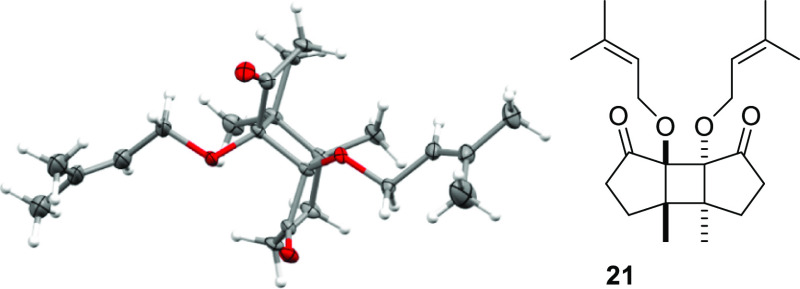
Remarkably, even prenyloxy-substituted enone 3f did not display the cyclization tendency observed with cyclohexenones 3a–3c. Instead, the only product isolated from its sensitized irradiation was the head-to-head regioisomer 21 of a [2 + 2] photodimerization that was obtained in 34% yield. The C2-symmetric compound displayed the same relative configuration as the previously discussed enone dimers 17 and 19, and the structure assignment could in this specific case be secured by a single-crystal structure analysis (Figure 3).
Figure 3.
Structure of [2 + 2] photodimerization product 21 (ellipsoids in the ORTEP structure are displayed at the 50% probability level) obtained by sensitized irradiation of substrate 3f (34% yield).
The difference between the cyclopentenone and the cyclohexenone chromophore in the photochemical reactivity of their 2-(2′-alkenyloxy)-substituted derivatives is remarkable. Only in a single case (product 9e), an intramolecular attack of the tethered olefin occurred while with all other substrates there was no indication for such an attack, not even for a cyclization to a 1,4-diradical (in analogy to the formation of intermediate 15). The cyclization seems retarded and intermolecular [2 + 2] photocycloaddition reactions become competitive. The observation is important because it allows to access cyclopentenone dimers without protecting the olefinic side chains.
In summary, an array of structurally diverse products has been obtained by irradiation of the title compounds in the presence of a sensitizer. The photochemical reactions of all cyclohexenone derivatives proceeded in moderate to excellent yields and with a high degree of diastereoselectivity. Intramolecular reactions were observed exclusively either as [2 + 2] photocycloaddition or as a cyclization/hydrogen abstraction cascade. Up to four stereogenic centers can be established in a single reaction and the products hold promise as scaffolds which enable further functionalization at several positions. The cyclopentenone derivatives were less prone to intramolecular reactions but underwent preferentially an intermolecular reaction leading to [2 + 2] photodimerization products.
Experimental Section
General Methods
All air and moisture sensitive reactions were carried out in flame-dried glassware under an argon atmosphere using standard Schlenk techniques. For moisture sensitive reactions, tetrahydrofuran (THF), diethylether (Et2O), and dichloromethane (CH2Cl2) were dried using a solvent purification system. Cyclohexane was dried over neutral aluminum oxide and stored over 4 Å molecular sieves. Dry N,N-dimethylformamide (DMF) was obtained in the highest available purity stored over molecular sieves and used without further purification. For photochemical reactions, dry dichloromethane was degassed by three freeze–pump–thaw cycles. Cooling baths used were ice/water (0 °C) and dry ice/ethanol (−78 °C). Technical solvents [1,2-dichloroethane (DCE), pentane (P), Et2O, CH2Cl2, methanol (MeOH), ethyl acetate (EtOAc), cyclohexane] were distilled prior to use. Flash column chromatography was performed on silica 60 (230–400 mesh) and thin-layer chromatography (TLC) was performed on silica-coated glass plates (silica 60 F254) with detection by UV-light (λ = 254 nm) and/or by staining with a KMnO4 solution followed by heat treatment. Infrared spectra (IR) were recorded by the attenuated total reflection (ATR) technique and are reported as wave numbers ν̃ (cm–1). The following abbreviations for intensities were used: vs (very strong), s (strong), m (medium), w (weak). NMR spectra were recorded at room temperature on either a 300, 400, or 500 MHz nuclear magnetic resonance spectrometer. The 1H NMR spectra were referenced to the residual solvent peak of either chloroform (7.26 ppm) or C6D6 (7.16 ppm), and the 13C{1H} NMR spectra were referenced either against the central peak of CDCl3 (77.16 ppm) or the residual solvent peak of C6D6 (128.06 ppm). 1H NMR spectra were reported as follows: chemical shift in parts per million (ppm), peak shape (s - singlet, d - dublet, t - triplet, q - quartet, quint. - quintet, sept. - septet, m - multiplet), coupling constant in Hertz (Hz), and integration. Apparent multiplets, which occur as a result of the coupling constant equality between magnetically nonequivalent protons, are marked as virtual (virt.). Mass spectra were measured with a mass selective quadrupole detector (EI, 70 eV). HRMS data were determined at a double-focussing magnetic sector instrument (EI, 70 eV). UV/Vis Spectra were recorded using a precision cell made of quartz with a pathway of 1 mm.
General Procedure 1 (Method A)
To a solution of the respective 1,2-cyclohexadione (1.0 equiv) in dry cyclohexane (40 mL/g starting material) was added p-TsOH·H2O (3.0 mol %) and the corresponding allylic alcohol (4.0 equiv). The reaction mixture was heated at reflux in a Dean–Stark apparatus for 15–17 h. The solution was allowed to cool to room temperature, washed with brine (2 × 50 mL/g), dried over Na2SO4, and filtered. The solvent was removed under reduced pressure and the crude product was purified using flash column chromatography (P/Et2O).
General Procedure 2 (Method B)
To a solution of the respective 1,2-cyclohexadione (1.0 equiv) in dry DMF (40 mL/g starting material) was added K2CO3 (1.2 equiv) and the respective allylbromide (1.2 equiv). The reaction mixture was stirred at room temperature for 4.5–21 h. The reaction was quenched by the addition of H2O (20 mL/g). Et2O (40 mL/g) was added and the layers were separated. The organic layer was washed with H2O (4 × 40 mL/g) and brine (1 × 60 mL/g), dried over Na2SO4, and filtered. The solvent was removed under reduced pressure and the crude product was purified using flash column chromatography (P/Et2O).
General Procedure 3 (Method C)
A round bottom flask charged with NaH (60 wt %, 1.2 equiv) was cooled to 0 °C and the respective allylic alcohol (30 equiv) was added dropwise. The reaction mixture was stirred for 10 min and subsequently the corresponding epoxide (1.0 equiv) was added dropwise. The reaction mixture was allowed to warm to room temperature and stirred for 20–24 h. The mixture was diluted with Et2O (75 mL/g) and quenched by the addition of H2O (35 mL/g). The layers were separated and the organic layer was washed with H2O (1 × 35 mL/g) and brine (1 × 75 mL/g), dried over Na2SO4, and filtered. The solvent was removed under reduced pressure and the crude product was purified by flash column chromatography (P/Et2O).
General Procedure 4 (Method B)
To a solution of the respective 1,2-cyclopentadione (1.0 equiv) in dry DMF (40 mL/g starting material) was added K2CO3 (1.2 equiv) and the respective allylbromide (1.2 equiv). The reaction mixture was stirred at room temperature for 17–22 h. The reaction was quenched by the addition of H2O (20 mL/g). Et2O (40 mL/g) was added and the layers were separated. The organic layer was washed with H2O (4 × 40 mL/g) and brine (1 × 60 mL/g), dried over Na2SO4, and filtered. The solvent was removed under reduced pressure and the crude product was purified using flash column chromatography (P/Et2O).
2-(Allyloxy)cyclohex-2-en-1-one (1a)
According to general procedure 1, compound 1a was synthesized starting from 4a (1.00 g, 8.92 mmol, 1.0 equiv), allylic alcohol (2.43 mL, 2.07 g, 35.7 mmol, 4.0 equiv), and p-TsOH·H2O (51.3 mg, 270 μmol, 3.0 mol %). Purification by flash column chromatography (SiO2, P/Et2O = 5/1, UV) afforded the product (667 mg, 4.38 mmol, 49%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.18 [UV, KMnO4]. 1H NMR (400 MHz, CDCl3, 300 K): δ 1.97 (virt. quint. 3J ≅ 3J = 6.2 Hz, 2H), 2.41 (td, 3J = 6.0, 4.7 Hz, 2H), 2.48–2.53 (m, 2H), 4.31 (virt. dt 3J = 5.5 Hz, 4J ≅ 4J = 1.5 Hz, 2H), 5.25 (ddt, 2J = 1.5 Hz, 3J = 10.6 Hz, 4J = 1.5 Hz, 1H), 5.33 (ddt, 2J = 1.5 Hz, 3J = 17.4 Hz, 4J = 1.5 Hz, 1H), 5.89 (t, 3J = 4.7 Hz, 1H), 5.98 (ddt, 3J = 17.4, 10.6, 5.5 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ 23.1 (t), 24.7 (t), 39.0 (t), 68.8 (t), 118.1 (t), 118.7 (d), 133.1 (d), 150.5 (s), 194.5 (s). UV/vis (CH2Cl2, c = 0.5 mm): λ = 259 nm (ε = 3736 cm–1 M–1). Data of this compound were in accordance with the literature.6b
2-(Allyloxy)-3-methylcyclohex-2-en-1-one (1b)
According to general procedure 2, compound 1b(12a,25) was synthesized starting from 4a (500 mg, 3.96 mmol, 1.0 equiv), allyl bromide (410 μL, 580 mg, 4.76 mmol, 1.2 equiv), and K2CO3 (670 mg, 4.85 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 9/1, UV) afforded the product (259 mg, 1.56 mmol, 39%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.30 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.92 (s, 3H), 1.92–1.97 (m, 2H), 2.38 (t, 3J = 6.1 Hz, 2H), 2.41–2.47 (m, 2H), 4.34 (virt. dt, 3J = 6.0 Hz, 2J ≅ 4J = 1.4 Hz, 2H), 5.18 (virt. dt, 3J = 10.3 Hz, 2J ≅ 4J = 1.4 Hz, 1H), 5.28 (virt. dtd, 3J = 17.2 Hz, 2J ≅ 4J = 1.6 Hz, 4J = 1.5 Hz, 1H), 5.99 (ddt, 3J = 17.0, 10.3, 6.0 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ 18.1 (q), 22.3 (t), 31.7 (t), 38.9 (t), 73.0 (t), 117.8 (t), 134.5 (d), 146.4 (s), 147.9 (s), 194.9 (s). IR (ATR) ν̃: 2928 (w), 1672 (vs), 1631 (m), 1431 (w), 1379 (w), 1304 (w), 1193 (s), 1153 (s), 985 (s), 926 (s). MS (EI, 70 eV) m/z (%): 166 (80), 151 (100), 137 (13), 125 (17), 113 (38), 110 (93), 95 (82), 82 (78). HRMS (EI) m/z: [M]+ calcd for C10H14O2, 166.0982; found, 166.0988. UV/vis (CH2Cl2, c = 0.5 mm): λ = 247 nm (ε = 8212 cm–1 M–1).
2-(Allyloxy)-5,5-dimethylcyclohex-2-en-1-one (1c)
According to general procedure 3, compound 1c(12a) was synthesized starting from epoxide 4c (200 mg, 1.43 mmol, 1.0 equiv), allylic alcohol (3.00 mL, 2.55 g, 43.9 mmol, 31 equiv), and NaH (60 wt %, 72.0 mg, 1.80 mmol, 1.3 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 9/1 → 6/1, UV) afforded the product (115 mg, 640 μmol, 45%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.31 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.06 (s, 6H), 2.31 (d, 3J = 4.6 Hz, 2H), 2.37 (s, 2H), 4.32 (virt. dt, 3J = 5.8 Hz, 2J ≅ 4J = 1.4 Hz, 2H), 5.25 (virt. dq, 3J = 10.6 Hz, 2J ≅ 4J = 1.3 Hz, 1H), 5.33 (virt. dq, 3J = 17.3 Hz, 2J ≅ 4J = 1.5 Hz, 1H), 5.73 (t, 3J = 4.6 Hz, 1H), 5.98 (ddt, 3J = 17.2, 10.8, 5.5 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 28.3 (q), 34.1 (s), 38.6 (t), 52.4 (t), 68.8 (t), 116.1 (d), 118.2 (t), 133.0 (d), 149.8 (s), 194.5 (s). IR (ATR) ν̃: 2959 (m), 1690 (vs), 1629 (m), 1467 (w), 1368 (w), 1165 (m), 999 (m). MS (EI, 70 eV) m/z (%): 180 (100), 165 (10), 151 (10), 127 (10), 124 (51), 109 (49), 95 (52), 83 (19). HRMS (EI) m/z: [M]+ calcd for C11H16O2, 180.1145; found, 180.1145. UV/vis (CH2Cl2, c = 0.5 mm): λ = 261 nm (ε = 5484 cm–1 M–1).
2-(Allyloxy)-4,4-dimethylcyclohex-2-en-1-one (1d)
KOH (85 wt %, 94.2 mg, 1.43 mmol, 1.0 equiv) was dissolved in allylic alcohol (1.00 mL, 854 mg, 14.7 mmol, 10 equiv). A solution of epoxide 4d (200 mg, 1.43 mmol, 1.0 equiv) in allylic alcohol (1.5 mL, 1.28 g, 22.1 mmol, 15 equiv) was then added dropwise, and the reaction mixture was stirred at room temperature overnight. Subsequently, the reaction mixture was diluted with 15 mL of Et2O and quenched by the addition of 15 mL of H2O. The layers were separated and the aqueous layer was extracted with Et2O (2 × 15 mL). The combined organic layers were washed with brine (1 × 50 mL), dried over Na2SO4, filtered, and the solvent as well as the remaining allylic alcohol were removed under reduced pressure. After purification by flash column chromatography (SiO2, P/Et2O = 20/1 → 6/1, UV), product 1d (30.5 mg, 169 μmol, 12%) was obtained as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.28 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.18 (s, 6H), 1.82 (t, 3J = 6.5 Hz, 2H), 2.55 (t, 3J = 6.5 Hz, 2H), 4.27 (virt. dt, 3J = 5.6 Hz, 4J ≅ 4J = 1.5 Hz, 2H), 5.24 (virt. dq, 3J = 10.4 Hz, 2J ≅ 4J = 1.4 Hz, 1H), 5.32 (virt. dq, 3J = 17.2 Hz, 2J ≅ 4J = 1.6 Hz, 1H), 5.59 (s, 1H), 5.97 (ddt, 3J = 17.3, 10.8, 5.6 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 29.2 (q), 33.0 (s), 35.0 (t), 36.0 (t), 68.7 (t), 118.2 (t), 128.7 (d), 132.9 (d), 148.1 (s), 194.1 (s). IR (ATR) ν̃: 2958 (m), 1690 (vs), 1619 (s), 1458 (w), 1362 (m), 1261 (m), 1200 (s), 1130 (s), 1107 (s), 1014 (m), 925 (w). MS (EI, 70 eV) m/z (%): 180 (24), 165 (24), 137 (4), 124 (100), 109 (23), 96 (15), 83 (11). HRMS (EI) m/z: [M]+ calcd for C11H16O2, 180.1145; found, 180.1144. UV/vis (CH2Cl2, c = 0.5 mm): λ = 260 nm (ε = 7584 cm–1 M–1).
2-(Allyloxy)cyclopent-2-en-1-one (1e)
To a solution of 1,2-cyclopentadione (4e) (95.0 mg, 968 μmol, 1.0 equiv) in 5.0 mL of dry cyclohexane was added p-TsOH·H2O (6.10 mg, 32.1 μmol, 3.0 mol %) and allylic alcohol (210 μL, 178 mg, 3.06 mmol, 3.2 equiv). The reaction mixture was heated at reflux using a Dean–Stark apparatus for 23 h. The solution was allowed to cool to room temperature and the solvent was removed under reduced pressure. After purification by flash column chromatography (SiO2, P/Et2O = 3/1, UV), product 1e(25) (100 mg, 720 μmol, 74%) was obtained as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.14 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 2.38–2.48 (m, 2H), 2.52 (ddd, 3J = 6.4, 4.2, 2.9 Hz, 2H), 4.43 (virt. dt, 3J = 5.6 Hz, 4J ≅ 4J = 1.5 Hz, 2H), 5.28 (virt. dq, 3J = 10.6 Hz, 2J ≅ 4J = 1.4 Hz, 1H), 5.36 (virt. dq, 3J = 17.3 Hz, 2J ≅ 4J = 1.6 Hz, 1H), 5.98 (ddt, 3J = 17.3, 10.9, 5.6 Hz, 1H), 6.39 (t, 3J = 2.9 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 22.1 (t), 33.2 (t), 70.8 (t), 118.7 (t), 128.3 (d), 132.4 (d), 156.4 (s), 202.7 (s). IR (ATR) ν̃: 2925 (w), 1715 (vs), 1624 (m), 1337 (w), 1276 (w), 1119 (m), 1027 (w), 934 (w). MS (EI, 70 eV) m/z (%): 138 (100), 109 (15), 95 (11), 82 (52). HRMS (EI) m/z: [M]+ calcd for C8H10O2, 138.0675; found, 138.0677, [M]+ calcd for C713C1H10O2, 139.0709; found, 139.0712. UV/vis (CH2Cl2, c = 0.5 mm): λ = 248 nm (ε = 5180 cm–1 M–1).
2-(Allyloxy)-3-methylcyclopent-2-en-2-one (1f)
According to general procedure 4, compound 1f(12a) was synthesized starting from 4f (500 mg, 4.46 mmol, 1.0 equiv), allyl bromide (460 μL, 650 mg, 5.35 mmol, 1.2 equiv), and K2CO3 (740 mg, 5.35 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 3/1, UV) afforded the product (387 mg, 2.54 mmol, 55%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.24 [UV, KMnO4]. 1H NMR (400 MHz, CDCl3, 300 K): δ 1.98 (s, 3H), 2.30–2.39 (m, 2H), 2.43 (virt. dtd, 2J = 7.1 Hz, 3J ≅ 3J = 2.5, 3J = 1.2 Hz, 2H), 4.67 (virt. dt, 3J = 5.9 Hz, 2J ≅ 2J = 1.4 Hz, 2H), 5.18 (virt. dq, 3J = 10.4 Hz, 2J ≅ 4J = 1.3 Hz, 1H), 5.29 (virt. dq, 3J = 17.2 Hz, 2J ≅ 4J = 1.6 Hz, 1H), 5.94 (ddt, 3J = 17.2, 10.4, 5.8 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ 15.1 (q), 27.6 (t), 33.1 (t), 70.9 (t), 117.9 (t), 134.3 (d), 151.7 (s), 155.6 (s), 203.3 (s). IR (ATR) ν̃: 2917 (w), 1699 (vs), 1643 (m), 1442 (w), 1389 (w), 1333 (m), 1204 (m), 1094 (s), 984 (m), 928 (m). MS (EI, 70 eV) m/z (%): 152 (49), 137 (76), 123 (19), 112 (9), 96 (22), 84 (16), 81 (8), 69 (26), 67 (15), 55 (19), 41 (100). HRMS (EI) m/z: [M]+ calcd for C9H12O2, 152.0827; found, 152.0832. UV/vis (CH2Cl2, c = 0.5 mm): λ = 246 nm (ε = 10,132 cm–1 M–1).
2-[(2-Methylallyl)oxy]cyclohex-2-en-1-one (2a)
According to general procedure 1, compound 2a was synthesized starting from 4a (1.00 g, 8.92 mmol, 1.0 equiv), methallylic alcohol (3.00 mL, 2.57 g, 35.7 mmol, 4.0 equiv), and p-TsOH·H2O (52.3 mg, 275 μmol, 3.0 mol %). Purification by flash column chromatography (SiO2, P/Et2O = 9/1 → 4/1, UV) afforded the product (682 mg, 4.10 mmol, 46%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.19 [UV, KMnO4]. 1H NMR (500 MHz, C6D6, 300 K): δ 1.36 (virt. quint. 3J ≅ 3J = 6.1 Hz, 2H), 1.66 (s, 3H), 1.74 (td, 3J = 6.0, 4.6 Hz, 2H), 2.05–2.21 (m, 2H), 3.90 (s, 2H), 4.86 (s, 1H), 5.11 (s, 1H), 5.30 (t, 3J = 4.6 Hz, 1H). 13C{1H} NMR (75 MHz, C6D6, 300 K): δ 19.8 (q), 23.5 (t), 24.8 (t), 39.6 (t), 71.9 (t), 113.0 (t), 118.1 (d), 141.6 (s), 151.5 (s), 190.4 (s). UV/vis (CH2Cl2, c = 0.5 mm): λ = 259 (ε = 4518 cm–1 M–1). Data of this compound were in accordance with the literature.6b
3-Methyl-2-[(2-methylallyl)oxy]cyclohex-2-en-1-one (2b)
According to general procedure 2, compound 2b was synthesized starting from 4b (500 mg, 3.96 mmol, 1.0 equiv), methallyl bromide (480 μL, 640 mg, 4.76 mmol, 1.2 equiv), and K2CO3 (0.660 mg, 4.76 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 9/1, UV) afforded the product (414 mg, 2.29 mmol, 58%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.29 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.82 (s, 3H), 1.90–1.98 (m, 5H), 2.38 (t, 3J = 6.1 Hz, 2H), 2.44 (t, 3J = 6.7 Hz, 2H), 4.22 (s, 2H), 4.90 (virt. t, 2J ≅ 4J = 2.0 Hz, 1H), 5.02 (s, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 18.0 (q), 19.9 (q), 22.3 (t), 31.7 (t), 38.9 (t), 75.7 (t), 112.8 (t), 142.0 (s), 146.1 (s), 148.2 (s), 194.9 (s). IR (ATR) ν̃: 2920 (w), 1673 (vs), 1632 (m), 1433 (w), 1378 (m), 1304 (m), 1193 (s), 1151 (vs), 993 (m), 895 (m). MS (EI, 70 eV) m/z (%): 180 (100), 165 (37), 137 (11), 123 (21), 110 (94), 95 (74). HRMS (EI) m/z: [M]+ calcd for C11H16O2, 180.1145; found, 180.1145, [M]+ calcd for C1013C1H16O2, 181.1185; found, 181.1178. UV/vis (CH2Cl2, c = 0.5 mm): λ = 248 nm (ε = 7196 cm–1 M–1).
5,5-Dimethyl-2-[(2-methylallyl)oxy]cyclohex-2-en-1-one (2c)
According to general procedure 3, compound 2c was synthesized starting from epoxide 4c (200 mg, 1.43 mmol, 1.0 equiv), methallylic alcohol (2.40 mL, 2.06 g, 28.6 mmol, 20 equiv), and NaH (60 wt %, 70.0 mg, 1.75 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 9/1, UV) afforded the product (102 mg, 527 μmol, 37%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.40 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.06 (s, 6H), 1.76 (s, 3H), 2.29 (d, 3J = 4.6 Hz, 2H), 2.36 (s, 2H), 4.25 (s, 2H), 4.95 (s, 1H), 5.00 (s, 1H), 5.73 (t, 3J = 4.6 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 19.4 (q), 28.3 (q), 34.2 (s), 38.5 (t), 52.4 (t), 71.6 (t), 112.9 (t), 116.3 (d), 140.5 (s), 149.9 (s), 194.3 (s). IR (ATR) ν̃: 2958 (m), 1691 (vs), 1629 (m), 1455 (w), 1368 (w), 1163 (m), 902 (w). MS (EI, 70 eV) m/z (%): 194 (57), 179 (8), 151 (9), 138 (22), 127 (14), 109 (100), 95 (9), 83 (14). HRMS (EI) m/z: [M]+ calcd for C12H18O2, 194.1301; found, 194.1302. UV/vis (CH2Cl2, c = 0.5 mm): λ = 261 nm (ε = 4374 cm–1 M–1).
4,4-Dimethyl-2-[(2-methylallyl)oxy]cyclohex-2-en-1-one (2d)
KOH (85 wt %, 59.9 mg, 908 μmol, 1.0 equiv) was dissolved in methallylic alcohol (1.00 mL, 851 mg, 11.8 mmol, 13 equiv). A solution of epoxide 4d (127 mg, 908 μmol, 1.0 equiv) in methallylic alcohol (1.0 mL, 0.851 mg, 11.8 mmol, 13 equiv) was then added dropwise, and the reaction mixture was stirred at room temperature overnight. Subsequently, the reaction mixture was diluted with 15 mL of Et2O and quenched by the addition of 15 mL of H2O. The layers were separated and the aqueous layer was extracted with Et2O (2 × 15 mL). The combined organic layers were washed with brine (1 × 50 mL), dried over Na2SO4, filtered, and the solvent as well as the remaining volatiles were removed under reduced pressure. After purification by flash column chromatography (SiO2, P/Et2O = 6/1), product 2d (11.0 mg, 56.6 μmol, 6%) was obtained as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.37 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.17 (s, 6H), 1.76 (t, 4J = 1.2 Hz, 3H), 1.80–1.83 (m, 2H), 2.52–2.60 (m, 2H), 4.18 (s, 2H), 4.94 (virt. quint., 2J ≅ 4J = 1.3 Hz, 1H), 5.00 (virt. dq, 2J = 2.1 Hz, 4J ≅ 4J = 1.2 Hz, 1H), 5.60 (s, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 19.5 (q), 29.2 (q), 33.0 (s), 35.1 (t), 36.0 (t), 71.5 (t), 113.1 (t), 128.9 (d), 140.3 (s), 148.2 (s), 194.1 (s). IR (ATR) ν̃: 2958 (m), 1692 (vs), 1619 (s), 1455 (w), 1363 (m), 1201 (s), 1130 (s), 1109 (s), 1014 (m), 901 (w). MS (EI, 70 eV) m/z (%): 194 (44), 179 (31), 151 (14), 138 (100), 125 (20), 109 (37), 97 (22), 83 (10). HRMS (EI) m/z: [M]+ calcd for C12H18O2, 194.1301; found, 194.1301, [M]+ calcd for C1113C1H18O2, 195.1335; found, 195.1350. UV/vis (CH2Cl2, c = 0.5 mm): λ = 260 nm (ε = 6300 cm–1 M–1), 338 nm (ε = 912 cm–1 M–1).
2-[(2-Methylallyl)oxy]cyclopent-2-en-1-one (2e)
In analogy to general procedure 4, compound 2e(23) was synthesized starting from 4e (100 mg, 1.02 mmol, 1.0 equiv), methallyl bromide (150 μL, 207 mg, 1.53 mmol, 1.5 equiv), and K2CO3 (211 mg, 1.53 mmol, 1.5 equiv) in 4.0 mL of dry DMF. The reaction was quenched by the addition of 5 mL of H2O and 10 mL of Et2O. The organic layer was washed with H2O (4 × 10 mL) and brine (1 × 20 mL), dried over Na2SO4, filtered, and the solvent was removed under reduced pressure. Purification by flash column chromatography (SiO2, P/Et2O = 3/1, UV) afforded the product (55.1 mg, 360 μmol, 35%) as colorless oil. TLC (P/Et2O = 1/1): Rf = 0.30 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.78 (s, 3H), 2.40–2.44 (m, 2H), 2.50 (dt, 3J = 6.1, 2.8 Hz, 2H), 4.34 (s, 2H), 4.98 (s, 1H), 5.03 (s, 1H), 6.40 (t, 3J = 2.8 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ 19.4 (q), 22.1 (t), 33.2 (t), 73.8 (t), 113.7 (t), 128.5 (d, C-3), 140.0 (s), 156.5 (s), 202.6 (s). IR (ATR) ν̃: 2922 (w), 1708 (vs), 1623 (s), 1407 (w), 1336 (w), 1273 (m), 1104 (vs), 1025 (m), 902 (m), 782 (m). MS (EI, 70 eV) m/z (%): 152 (100), 137 (16), 124 (10), 109 (21), 96 (90), 82 (19). HRMS (EI) m/z: [M]+ calcd for C9H12O2, 152.0832; found, 152.0831. UV/vis (CH2Cl2, c = 0.5 mm): λ = 248 nm (ε = 6794 cm–1 M–1).
3-Methyl-2-[(2-methylallyl)oxy]cyclopent-2-en-1-one (2f)
According to general procedure 4, compound 2f was synthesized starting from 4f (500 mg, 4.46 mmol, 1.0 equiv), methallyl bromide (550 μL, 736 mg, 5.46 mmol, 1.2 equiv), and K2CO3 (740 mg, 5.35 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 9/1, UV) afforded the product (621 mg, 3.74 mmol, 84%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.28 [UV, KMnO4]. 1H NMR (300 MHz, C6D6, 300 K): δ 1.57–1.59 (m, 3H), 1.56–1.67 (m, 2H), 1.64–1.71 (m, 3H), 1.85–1.90 (m, 2H), 4.79–4.86 (m, 2H), 4.86 (virt. ddq, 2J = 2.3 Hz, 4J = 1.6, 4J ≅ 4J = 0.8 Hz, 1H), 5.11 (virt. dq, 2J = 2.3 Hz, 4J ≅ 4J = 1.1 Hz, 1H). 13C{1H} NMR (75 MHz, C6D6, 300 K): δ 14.4 (q), 19.4 (q), 26.8 (t), 33.1 (t), 73.0 (t), 112.7 (t), 142.5 (s), 151.8 (s), 152.1 (s), 201.4 (s). IR (ATR) ν̃: 2917 (w), 1700 (vs), 1646 (m), 1448 (w), 1389 (m), 1335 (m), 1204 (m), 1096 (s), 990 (m), 903 (m). MS (EI, 70 eV) m/z (%): 166 (56), 151 (31), 137 (10), 110 (9), 96 (12), 84 (15), 69 (16), 55 (100), 41 (24). HRMS (EI) m/z: [M]+ calcd for C10H14O2, 166.0988; found, 166.0985, [M]+ calcd for C913C1H14O2, 167.1022; found, 167.1026. UV/vis (CH2Cl2, c = 0.5 mm): λ = 248 nm (ε = 9284 cm–1 M–1).
2-[(3-Methylbut-2-en-1-yl)oxy]cyclohex-2-en-1-one (3a)
According to general procedure 2, compound 3a was synthesized starting from 4a (1.00 g, 8.92 mmol, 1.0 equiv), 3,3-dimethylallyl bromide (1.24 mL, 1.60 g, 10.7 mmol, 1.2 equiv), and K2CO3 (1.48 g, 10.7 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 9/1 → 4/1 → 3/1, UV) afforded the product (227 mg, 1.26 mmol, 14%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.15 [UV, KMnO4]. 1H NMR (400 MHz, CDCl3, 300 K): δ 1.64 (s, 3H), 1.71 (s, 3H), 1.86–1.99 (m, 2H), 2.39 (td, 3J = 6.1, 4.7 Hz, 2H), 2.47 (dd, 3J = 7.4, 6.0 Hz, 2H), 4.23 (d, 3J = 5.9 Hz, 2H), 5.38 (virt. ddq, 3J = 8.2, 5.9 Hz, 4J ≅ 4J = 1.5 Hz, 1H), 5.85 (t, 3J = 4.7 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ 18.2 (q), 23.0 (t), 24.6 (t), 25.8 (q), 38.9 (t), 64.6 (t), 117.9 (d), 119.6 (d), 137.6 (s), 150.7 (s), 194.5 (s). IR (ATR) ν̃: 2926 (w), 1686 (vs), 1622 (m), 1444 (w), 1375 (w), 1259 (m), 1184 (s), 1148 (vs), 1003 (m), 874 (w). MS (EI, 70 eV) m/z (%): 180 (27), 147 (11), 134 (8), 118 (57), 112 (100), 99 (12), 95 (7), 84 (27). HRMS (EI) m/z: [M]+ calcd for C11H16O2, 180.1148; found, 180.1145, [M]+ calcd for C1013C1H16O2, 181.1185; found, 181.1178. UV/vis (CH2Cl2, c = 0.5 mm): λ = 261 nm (ε = 4126 cm–1 M–1).
3-Methyl-2-[(3-methylbut-2-en-1-yl)oxy]cyclohex-2-en-1-one (3b)
According to general procedure 2, compound 3b(26) was synthesized starting from 4b (500 mg, 3.96 mmol, 1.0 equiv), 3,3-dimethylallyl bromide (550 μL, 710 mg, 4.76 mmol, 1.2 equiv), and K2CO3 (0.660 mg, 4.76 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 9/1 → 3/1, UV) afforded the product (197 mg, 1.01 mmol, 26%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.24 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.68 (s, 3H), 1.75 (s, 3H), 1.86–2.03 (m, 5H), 2.38 (t, 3J = 6.1 Hz, 2H), 2.41–2.45 (m, 2H), 4.32 (d, 3J = 7.4 Hz, 2H), 5.44 (virt. ddq, 3J = 7.4, 5.9 Hz, 4J ≅ 4J = 1.4 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 18.1 (q), 22.3 (t), 26.0 (q), 31.6 (t), 38.9 (t), 68.3 (t), 120.7 (d), 138.2 (s), 146.5 (s), 148.0 (s), 195.1 (s). IR (ATR) ν̃: 2917 (w), 1673 (vs), 1630 (m), 1433 (w), 1381 (m), 1301 (m), 1191 (s), 1148 (s), 970 (m). MS (EI, 70 eV) m/z (%): 126 (100), 98 (11), 84 (23). HRMS (EI) m/z: [M]+ calcd for C12H18O2, 194.1300; found, 194.1301. UV/vis (CH2Cl2, c = 0.5 mm): λ = 248 nm (ε = 6640 cm–1 M–1).
5,5-Dimethyl-2-[(3-methylbut-2-en-1-yl)oxy]cyclohex-2-en-1-one (3c)
According to general procedure 3, compound 3c was synthesized starting from epoxide 4c (200 mg, 1.43 mmol, 1.0 equiv), 3,3-dimethylallylic alcohol (4.50 mL, 3.83 g, 44.4 mmol, 31 equiv), and NaH (60 wt %, 69.0 mg, 1.73 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 9/1, P/Et2O = 9/1 → 6/1, UV) afforded the product (45.2 mg, 217 μmol, 15%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.28 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.06 (s, 6H), 1.68 (s, 3H), 1.75 (s, 3H), 2.31 (d, 3J = 4.6 Hz, 2H), 2.36 (s, 2H), 4.28 (d, 3J = 7.3 Hz, 2H), 5.42 (ddd, 3J = 7.3 Hz, 4J = 4.1, 1.5 Hz, 1H), 5.71 (t, 3J = 4.6 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 18.3 (q), 25.9 (q), 28.3 (q), 34.1 (s), 38.6 (t), 52.4 (t), 64.6 (t), 115.3 (d), 119.6 (d), 137.7 (s), 150.1 (s), 194.5 (s). IR (ATR) ν̃: 2958 (m), 1691 (vs), 1627 (m), 1452 (w), 1368 (w), 1162 (s), 1108 (m), 1003 (w). MS (EI, 70 eV) m/z (%): 208 (28), 175 (12), 146 (30), 140 (100), 125 (49), 112 (10), 98 (71), 84 (57). HRMS (EI) m/z: [M]+ calcd for C13H20O2, 208.1458; found, 208.1445, [M]+ calcd for C1213C1H20O2, 209.1491; found, 209.1482. UV/vis (CH2Cl2, c = 0.5 mm): λ = 263 nm (ε = 4500 cm–1 M–1).
2-[(3-Methylbut-2-en-1-yl)oxy]cyclopent-2-en-1-one (3e)
In analogy to general procedure 4, compound 3e was synthesized starting from 4e (170 mg, 1.73 mmol, 1.0 equiv), 3,3-dimethylallyl bromide (310 μL, 340 mg, 2.68 mmol, 1.5 equiv), and K2CO3 (377 mg, 2.73 mmol, 1.6 equiv) in 7.0 mL of dry DMF. The reaction was quenched by the addition of 7 mL of H2O and 15 mL of Et2O. The organic layer was washed with H2O (4 × 15 mL) and brine (1 × 30 mL), dried over Na2SO4, filtered, and the solvent was removed under reduced pressure. Purification by flash column chromatography (SiO2, P/Et2O = 3/1, UV) afforded the product (60.0 mg, 360 μmol, 21%) as a colorless solid. mp 42–43 °C. TLC (P/Et2O = 1/1): Rf = 0.35 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.70 (s, 3H), 1.76 (s, 3H), 2.41–2.44 (m, 2H), 2.48–2.60 (m, 2H), 4.40 (d, 3J = 6.8 Hz, 2H), 5.43 (t, 3J = 6.8 Hz, 1H), 6.36 (virt. q, 3J ≅ 4J = 2.5 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 18.3 (q), 22.2 (t), 25.9 (q), 33.2 (t), 66.7 (t), 119.1 (d), 127.6 (d), 138.7 (s), 156.6 (s), 202.9 (s). IR (ATR) ν̃: 2924 (w), 1715 (vs), 1623 (s), 1449 (w), 1342 (w), 1275 (w), 1110 (s), 1026 (w), 964 (w), 782 (m). MS (EI, 70 eV) m/z (%): 166 (59), 148 (21), 120 (11), 110 (23), 106 (28), 99 (100). HRMS (EI) m/z: [M]+ calcd for C10H14O2, 166.0988; found, 166.0983, [M]+ calcd for C913C1H14O2, 167.1022; found, 167.1021. UV/vis (CH2Cl2, c = 0.5 mm): λ = 250 nm (ε = 5976 cm–1 M–1).
3-Methyl-2-[(3-methylbut-2-en-1-yl)oxy]cyclopent-2-en-1-one (3f)
According to general procedure 4, compound 3f(26) was synthesized starting from 4f (500 mg, 4.46 mmol, 1.0 equiv), 3,3-dimethylallyl bromide (620 μL, 800 mg, 5.35 mmol, 1.2 equiv), and K2CO3 (750 mg, 5.42 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 9/1, UV) afforded the product (558 mg, 3.09 mmol, 69%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.26 [UV, KMnO4]. 1H NMR (300 MHz, C6D6, 300 K): δ 1.55 (dd, 4J = 1.4, 0.8 Hz, 6H), 1.60–1.62 (m, 3H), 1.63–1.67 (m, 2H), 1.82–1.99 (m, 2H), 4.97 (virt. dquint., 3J = 7.1 Hz, 4J ≅ 4J = 0.9 Hz, 2H), 5.52 (virt. ddquint., 3J = 8.4, 5.6 Hz, 4J ≅ 4J = 1.4 Hz, 1H). 13C{1H} NMR (75 MHz, C6D6, 300 K): δ 14.5 (q), 18.0 (q), 25.8 (q), 33.1 (t), 66.4 (t), 121.9 (d), 137.4 (s), 152.2 (s), 152.7 (s), 201.8 (s). IR (ATR) ν̃: 2915 (w), 1700 (vs), 1645 (m), 1443 (w), 1390 (m), 1338 (m), 1205 (m), 1092 (s), 969 (m), 905 (m). MS (EI, 70 eV) m/z (%): 112 (90), 84 (32), 69 (100), 53 (9), 41 (74). HRMS (EI) m/z: [M]+ calcd for C11H16O2, 180.1145; found, 180.1143. UV/vis (CH2Cl2, c = 0.5 mm): λ = 247 nm (ε = 9532 cm–1 M–1).
3-Ethoxy-5,5-dimethylcyclohex-2-en-1-one
According to a modified literature procedure:21 To a solution of dimedone (2.10 g, 15.0 mmol, 1.0 equiv) in 46 mL of dry toluene were added EtOH (3.56 mL, 2.80 g, 61.0 mmol, 4.0 equiv) and p-TsOH·H2O (139 mg, 0.73 mmol, 5.0 mol %). The reaction mixture was heated at reflux using a water separator for 90 min at which point EtOH (3.00 mL, 2.37 g, 50.3 mmol, 3.4 equiv) was added. Subsequently, the solution was refluxed for additional 14 h. It was allowed to cool to room temperature and the solvent was removed under reduced pressure. The crude product was then filtered over a short plug of Al2O3 (Et2O, UV) and the product (2.52 g, 15.0 mmol, 100%) was obtained as pale yellow oil. TLC (P/Et2O = 3/1): Rf = 0.17 [UV, KMnO4]. 1H NMR (300 MHz, CDCl3, 300 K): δ 1.07 (s, 6H), 1.36 (t, 3J = 7.0 Hz, 3H), 2.21 (s, 2H), 2.27 (s, 2H), 3.90 (q, 3J = 7.0 Hz, 2H), 5.35 (s, 1H). 13C{1H} NMR (75 MHz, CDCl3, 300 K): δ 14.3 (q), 28.4 (q), 32.6 (t), 43.1 (t), 50.8 (t), 64.4 (t), 101.6 (d), 176.5 (s), 199.8 (s). Data of this compound were in accordance to the literature.21
5,5-Dimethylcyclohex-2-en-1-one
According to a modified literature procedure:21 To an ice-cooled suspension of LiAlH4 (138 mg, 3.63 mmol, 0.4 equiv) in 4.0 mL of dry THF, a solution of 3-ethoxy-5,5-dimethylcyclohex-2-en-1-one (1.50 g, 8.92 mmol, 1.0 equiv) in 7.5 mL of dry THF was added dropwise. Subsequently, the reaction mixture was allowed to warm to room temperature and stirred for 3 h. The reaction mixture was then cooled to 0 °C and quenched by the addition of MeOH (approx. 10 mL) until no further gas evolution was observed. After addition of 14 mL of 1 m HCl, the reaction mixture was stirred for 30 min, the layers were separated and the aqueous layer was extracted with Et2O (3 × 30 mL). The combined organic layers were washed with brine (2 × 60 mL), dried over Na2SO4, filtered, and the solvent was removed under reduced pressure. After purification by flash column chromatography (SiO2, P/Et2O = 8/1, UV), the product (663 mg, 5.34 mmol, 60%) was obtained as colorless oil. TLC (P/Et2O = 1/1): Rf = 0.29 [UV, KMnO4]. 1H NMR (300 MHz, CDCl3, 300 K): δ 1.05 (s, 6H, CH3), 2.24 (dd, 3J = 4.1 Hz, 4J = 2.1 Hz, 2H, H-4), 2.27 (s, 2H, H-6), 6.02 (dt, 3J = 10.1 Hz, 4J = 2.1 Hz, 1H, H-2), 6.86 (dt, 3J = 10.1, 4.1 Hz, 1H, H3). 13C{1H} NMR (75 MHz, CDCl3, 300 K): δ 28.5 (q), 34.0 (s), 40.0 (t), 51.9 (t), 129.1 (d), 148.5 (d), 200.1 (s). Data of this compound were in accordance to the literature.21
(1SR,6SR)-4,4-Dimethyl-7-oxabicyclo[4.1.0]heptan-2-one (4c)
According to a modified literature procedure:27 To an ice-cooled solution of 5,5-dimethylcyclohex-2-en-1-one21 (990 mg, 7.97 mmol, 1.0 equiv) in 16 mL of MeOH were dropwise added H2O2 (30 wt %, 3.30 mL, 1.10 g, 32.3 mmol, 4.1 equiv) and 6 m NaOH (0.64 mL, 154 mg, 3.84 mmol, 0.5 equiv). The reaction mixture was allowed to warm to room temperature and stirred for 2 h. Subsequently, the reaction mixture was diluted with 32 mL of CH2Cl2 and H2O was added until proper phase separation was achieved. The layers were separated and the aqueous layer was extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were washed with brine (2 × 80 mL), dried over Na2SO4, filtered, and the solvent was removed under reduced pressure. After purification by flash column chromatography (SiO2, P/Et2O = 2/1, KMnO4), the product (901 mg, 6.43 mmol, 80%) was obtained as colorless oil. TLC (P/Et2O = 2/1): Rf = 0.56 [KMnO4]. 1H NMR (400 MHz, CDCl3, 300 K): δ 0.92 (s, 3H), 1.06 (s, 3H), 1.74–1.89 (m, 2H), 2.04 (d, 2J = 14.3 Hz, 1H), 2.66 (d, 2J = 14.3 Hz, 1H), 3.21 (virt. dt 3J = 3.8 Hz, 3J ≅ 3J = 1.0 Hz, 1H), 3.51 (dd, 3J = 3.8, 4.7 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ 28.2 (q), 31.2 (q), 37.4 (t), 37.5 (t), 48.8 (s), 54.9 (d), 57.2 (d), 207.6 (s). Data of this compound were in accordance with the literature.27
(1SR,6SR)-5,5-Dimethyl-7-oxabicyclo[4.1.0]heptan-2-one (4d)
According to a modified literature procedure:27 To an ice-cooled solution of 4,4-dimethylcyclohex-2-en-1-one (2.00 g, 16.1 mmol, 1.0 equiv) in 32 mL of MeOH were dropwise added H2O2 (30 wt %, 8.23 mL, 80.5 mmol, 5.0 equiv) and 6 m NaOH (1.33 mL, 8.05 mmol, 0.5 equiv). The reaction mixture was allowed to warm to room temperature and stirred for 3.5 h. Subsequently, the reaction mixture was diluted with 60 mL of CH2Cl2 and H2O was added until proper phase separation was achieved. The layers were separated and the aqueous layer was extracted with CH2Cl2 (3 × 60 mL). The combined organic layers were washed with brine (1 ×120 mL), dried over Na2SO4, filtered, and the solvent was removed under reduced pressure. After purification by flash column chromatography (SiO2, P/Et2O = 6/1, KMnO4), the product (1.67 g, 11.9 mmol, 74%) was obtained as colorless oil. TLC (P/Et2O = 2/1): Rf = 0.68 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.06 (s, 3H), 1.22 (s, 3H), 1.34 (dddd, 2J = 13.7 Hz, 3J = 7.1, 3.0 Hz, 4J = 1.3 Hz, 1H), 1.90 (ddd, 2J = 13.7 Hz, 3J = 11.7, 6.4 Hz, 1H), 2.19 (ddd, 2J = 18.9 Hz, 3J = 11.7, 7.0 Hz, 1H), 2.40 (ddd, 2J = 18.9 Hz, 3J = 6.4, 3.0 Hz, 1H), 3.18 (dd, 3J = 4.0 Hz, 4J = 1.3 Hz, 1H), 3.23 (d, 3J = 4.0 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 23.1 (q), 27.6 (q), 29.9 (t), 30.9 (s), 33.3 (t), 56.1 (d), 64.3 (d), 206.2 (s). Data of this compound were in accordance with the literature.28
General Procedure 5 (Irradiation)
Thioxanthone 5 (10 mol %) was dissolved in 1.0 mL of dry degassed CH2Cl2 and transferred to a flame dried Duran phototube. The respective 2-(2′-alkenyloxy)cycloalk-2-enone (1.0 equiv) dissolved in 1.0 mL of dry degassed CH2Cl2 was then added, and the reaction mixture was diluted with dry degassed CH2Cl2 until a concentration of 10 mm (relative to the substrate) was reached. The reaction mixture was irradiated at room temperature in a previously described irradiation setup21 (λ = 420 nm, for details about the light source, see the Supporting Information). Irradiation was continued until full conversion was reached and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography (P/Et2O).
(3RS,3aSR,7aSR)-Hexahydro-7H-3,7a-methanobenzofuran-7-one (8a)
According to general procedure 5, compound 8a was synthesized starting from 1a (15.6 mg, 103 μmol, 1.0 equiv) and thioxanthone 5 (2.21 mg, 10.4 μmol, 10 mol %) over 2.5 h. Purification by flash column chromatography (SiO2, P/Et2O = 1/2, KMnO4) afforded the product (11.2 mg, 73.6 μmol, 73%) as a colorless solid. The reaction on a 1 mmol scale was performed with 152 mg of 1a (1.00 mmol, 1.0 equiv) and 5.30 mg of 5 (25.0 μmol, 2.5 mol %) in 40 mL of CH2Cl2 (25 mm, irradiation time: 5 h). The yield was 138 mg (0.91 mmol, 91%). Mp 59–65 °C. TLC (P/Et2O = 1/2): Rf = 0.11 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.64–1.78 (m, 1H), 1.81–1.96 (m, 2H), 2.03–2.18 (m, 2H), 2.30 (ddd, 3J = 11.0, 7.8, 6.4 Hz, 1H), 2.35–2.44 (m, 2H), 2.84 (dd, 2J = 8.1 Hz, 3J = 3.0 Hz, 1H), 2.88 (d, 3J = 3.0 Hz, 1H), 3.85 (d, 3J = 6.0 Hz, 1H), 3.94 (d, 3J = 6.0 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ 25.4 (t), 27.3 (t), 40.1 (t), 41.6 (t), 42.0 (d), 55.4 (d), 70.8 (t), 88.6 (s), 203.6 (s). Data of this compound were in accordance with the literature.6b
(3RS,3aSR,7aSR)-3a-Methylhexahydro-7H-3,7a-methanobenzofuran-7-one (8b)
According to general procedure 5, compound 8b was synthesized starting from 1b (16.8 mg, 101 μmol, 1.0 equiv) and thioxanthone 5 (2.19 mg, 10.3 μmol, 10 mol %) over 2 h. Purification by flash column chromatography (SiO2, P/Et2O = 1/2, KMnO4) afforded the product (15.2 mg, 91.2 μmol, 91%) as a colorless solid. Mp 38–47 °C. TLC (P/Et2O = 1/2): Rf = 0.04 [KMnO4]. 1H NMR (400 MHz, CDCl3, 300 K): δ 0.99 (s, 3H), 1.76 (d, 2J = 8.2 Hz), 1.84 (ddt, 2J = 14.1 Hz, 3J = 4.2 Hz, 4J = 1.9 Hz, 1H), 2.00 (virt. qt, 2J ≅ 3J = 13.9 Hz, 3J = 4.2 Hz, 1H), 2.08–2.18 (m, 1H), 2.28 (virt. td, 2J ≅ 3J = 14.1 Hz, 3J = 4.3 Hz, 1H), 2.36 (ddt, 2J = 14.5 Hz, 3J = 4.1 Hz, 4J = 1.9 Hz, 1H), 2.49 (td, 2J = 14.5 Hz, 3J = 5.8 Hz, 1H), 2.72 (d, 3J = 3.0 Hz, 1H), 2.84 (dd, 2J = 8.2 Hz, 3J = 3.0 Hz, 1H), 3.86 (d, 2J = 6.7 Hz, 1H), 3.92 (d, 2J = 6.7 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 15.8 (q), 25.7 (t), 31.8 (t), 40.1 (t), 40.8 (t), 44.6 (d), 56.0 (s), 68.5 (t), 89.9 (s), 204.0 (s). IR (ATR) ν̃: 2925 (m), 1713 (s), 1458 (w), 1123 (w), 1043 (w), 945 (w), 862 (w). MS (EI, 70 eV) m/z (%): 166 (100), 163 (21), 149 (39), 123 (35), 109 (62), 95 (44), 81 (100). HRMS (EI) m/z: [M]+ calcd for C10H14O2, 166.0988; found, 166.0982.
(3RS,3aSR,7aSR)-5,5-Dimethylhexahydro-7H-3,7a-methanobenzofuran-7-one (8c)
According to general procedure 5, compound 8c was synthesized starting from 1c (36.2 mg, 201 μmol, 1.0 equiv) and thioxanthone 5 (4.34 mg, 20.4 μmol, 10 mol %) over 2.5 h. Purification by flash column chromatography (SiO2, P/Et2O = 3/1 → 1/1, KMnO4) afforded the product (36.2 mg, 201 μmol, 100%) as a colorless solid. Mp 73–74 °C. TLC (P/Et2O = 1/1): Rf = 0.14 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 0.89 (s, 3H), 1.11 (s, 3H), 1.76–1.91 (m, 3H), 2.06 (dd, 2J = 14.2 Hz, 4J = 2.3 Hz, 1H), 2.34–2.45 (m, 2H), 2.81 (ddd, 2J = 8.2 Hz, 3J = 3.1 Hz, 4J = 1.1 Hz, 1H), 2.84 (d, 3J = 3.1 Hz, 1H), 3.90 (d, 2J = 6.0 Hz, 1H), 3.97 (d, 2J = 6.0 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 25.5 (q), 31.4 (q), 38.2 (t), 39.9 (s), 41.0 (t), 41.3 (d), 52.8 (d), 53.1 (t), 71.6 (t), 88.5 (s), 203.4 (s). Data of this compound were in accordance with the literature.6b
(3RS,3aSR,7aSR)-3-Methylhexahydro-7H-3,7a-methanobenzofuran-7-one (9a)
According to general procedure 5, compound 9a was synthesized starting from 2a (16.8 mg, 100 μmol, 1.0 equiv) and thioxanthone 5 (2.24 mg, 10.6 μmol, 10 mol %) over 3 h. Purification by flash column chromatography (SiO2, P/Et2O = 1/2, KMnO4) afforded the product (15.3 mg, 92.0 μmol, 91%) as colorless oil. TLC (P/Et2O = 1/2): Rf = 0.15 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ [ppm] = 1.25 (s, 3H), 1.66–1.82 (m, 2H), 1.88 (virt. t, 2J ≅ 4J = 7.9 Hz, 1H), 1.95–2.04 (m, 1H), 2.14 (ddt, 2J = 10.7 Hz, 3J = 6.0, 2.9 Hz, 1H), 2.24 (virt. qd, 3J ≅ 3J = 7.7 Hz, 4J = 3.7 Hz, 1H), 2.31–2.44 (m, 2H), 2.59 (d, 2J = 7.9 Hz, 1H), 3.64 (dd, 2J = 5.9 Hz, 4J = 1.2 Hz, 1H), 3.74 (d, 2J = 5.9 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ [ppm] = 12.8 (q), 23.6 (t), 27.1 (t), 40.1 (t), 45.4 (t), 49.1 (s), 57.4 (d), 75.4 (t), 87.2 (s), 204.2 (s). Data of this compound were in accordance with the literature.6b
(3RS,3aSR,7aSR)-3,3a-Dimethylhexahydro-7H-3,7a-methanobenzofuran-7-one (9b)
According to general procedure 5, compound 9b was synthesized starting from 2b (18.2 mg, 101 μmol, 1.0 equiv) and thioxanthone 5 (2.14 mg, 10.1 μmol, 10 mol %) over 5 h. Purification by flash column chromatography (SiO2, P/Et2O = 1/2, KMnO4) afforded the product (15.9 mg, 88.3 μmol, 88%) as a colorless solid. The reaction on a 1 mmol scale was performed with 180 mg of 2b (1.00 mmol, 1.0 equiv) and 5.40 mg of 5 (25.0 μmol, 2.5 mol %) in 40 mL of CH2Cl2 (25 mm, irradiation time: 9 h). The yield was 169 mg (0.94 mmol, 94%). Mp 67–70 °C. TLC (P/Et2O = 1/2): Rf = 0.08 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 0.90 (s, 3H), 1.14 (s, 3H), 1.65 (ddt, 2J = 13.5 Hz, 3J = 3.9 Hz, 4J = 1.8 Hz, 1H), 1.78 (d, 2J = 8.2 Hz, 1H), 1.92–2.06 (m, 1H), 2.07–2.20 (m, 2H), 2.28–2.36 (m, 1H), 2.38–2.51 (m, 1H), 2.62 (d, 2J = 8.2 Hz, 1H), 3.61 (d, 2J = 6.5 Hz, 1H), 3.66 (dd, 2J = 6.5 Hz, 4J = 1.4 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 10.5 (q), 13.8 (q), 25.6 (t), 29.9 (t), 40.0 (t), 45.4 (t), 50.6 (s), 57.0 (s), 72.9 (t), 89.4 (s), 204.5 (s). IR (ATR) ν̃: 2922 (s), 1714 (vs), 1456 (w), 1385 (w), 1245 (w), 949 (w). MS (EI, 70 eV) m/z (%): 180 (6), 165 (48), 135 (54), 125 (78), 110 (49), 97 (100), 83 (71). HRMS (EI) m/z: [M]+ calcd for C11H16O2, 180.1145; found, 180.1145.
(3RS,3aSR,7aSR)-3,5,5-Trimethylhexahydro-7H-3,7a-methanobenzofuran-7-one (9c)
According to general procedure 5, compound 9c was synthesized starting from 2c (38.9 mg, 200 μmol, 1.0 equiv) and thioxanthone 5 (4.36 mg, 20.5 μmol, 10 mol %) over 2.5 h. Purification by flash column chromatography (SiO2, P/Et2O = 1/1, KMnO4) afforded the product (35.1 mg, 181 μmol, 90%) as a colorless solid. The reaction on a 1 mmol scale was performed with 194 mg of 2c (1.00 mmol, 1.0 equiv) and 5.30 mg of 5 (25.0 μmol, 2.5 mol %) in 40 mL of CH2Cl2 (25 mm, irradiation time: 4.5 h). The yield was 178 mg (0.92 mmol, 92%). Mp 65–67 °C. TLC (P/Et2O = 1/1): Rf = 0.19 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 0.90 (s, 3H), 1.12 (s, 3H), 1.23 (s, 3H), 1.68 (ddd, 2J = 14.0 Hz, 3J = 7.0 Hz, 4J = 2.3 Hz, 1H), 1.75 (dd, 2J = 14.0 Hz, 3J = 11.1 Hz, 1H), 1.88 (virt. t, 2J ≅ 4J = 7.9 Hz, 1H), 2.05 (dd, 2J = 14.2 Hz, 4J = 2.3 Hz, 1H), 2.28–2.41 (m, 2H), 2.56 (dd, 2J = 8.0 Hz, 4J = 1.2 Hz, 1H), 3.68 (dd, 2J = 5.9 Hz, 4J = 1.2 Hz, 1H), 3.76 (d, 2J = 5.9 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 12.8 (q), 25.7 (q), 31.4 (q), 36.4 (t), 39.9 (s), 44.7 (t), 48.4 (s), 53.0 (t), 54.7 (d), 76.1 (t), 87.1 (s), 204.0 (s). IR (ATR) ν̃: 2954 (m), 1714 (vs), 1468 (w), 1245 (w), 1163 (w), 1082 (w), 964 (s). MS (EI, 70 eV) m/z (%): 194 (25), 179 (9), 151 (16), 124 (10), 109 (100), 95 (16), 83 (15). HRMS (EI) m/z: [M]+ calcd for C12H18O2, 194.1301; found, 194.1301.
(3RS,3aSR,7aSR)-3,4,4-Trimethylhexahydro-7H-3,7a-methanobenzofuran-7-one (9d)
According to general procedure 5, compound 9d was synthesized starting from 2d (11.0 mg, 56.6 μmol, 1.0 equiv) and thioxanthone 5 (1.20 mg, 5.66 μmol, 10 mol %) over 7.5 h. Purification by flash column chromatography (SiO2, P/Et2O = 3/1 → 1/1, KMnO4) afforded the product (4.20 mg, 21.6 μmol, 38%) as colorless oil. TLC (P/Et2O = 1/1): Rf = 0.14 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.17 (s, 3H), 1.32–1.41 (m, 6H), 1.66–1.75 (m, 2H), 1.88 (virt. t, 2J ≅ 4J = 8.4 Hz, 1H), 2.01 (d, 4J = 8.5 Hz, 1H), 2.31 (dt, 2J = 16.2 Hz, 3J = 4.2 Hz, 1H), 2.50 (ddd, 2J = 16.2 Hz, 3J = 10.2, 7.4 Hz, 1H), 2.76 (dd, 2J = 8.1 Hz, 4J = 1.2 Hz, 1H), 3.61 (dd, 2J = 5.9 Hz, 4J = 1.2 Hz, 1H), 3.64 (d, 2J = 5.9 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 13.6 (q), 24.0 (q), 32.9 (s), 33.7 (q), 37.0 (t), 41.4 (t), 45.9 (t), 49.7 (s), 65.0 (d), 76.8 (t), 86.3 (s), 205.1 (s). IR (ATR) ν̃: 2954 (m), 1719 (vs), 1458 (w), 1062 (m), 948 (w). MS (EI, 70 eV) m/z (%): 194 (8), 179 (33), 164 (19), 149 (34), 138 (100), 125 (38), 109 (36), 97 (34), 81 (43). HRMS (EI) m/z: [M]+ calcd for C12H18O2, 194.1301; found, 194.1298.
(3RS,3aSR,6aSR)-3-Methyltetrahydro-3,6a-methanocyclopenta[b]furan-6(2H)-one (9e)
According to general procedure 5, compound 9e was synthesized starting from 2e (30.7 mg, 202 μmol, 1.0 equiv) and thioxanthone 5 (4.26 mg, 20.1 μmol, 10 mol %) over 19 h. Purification by flash column chromatography (SiO2, P/Et2O = 3/1, KMnO4) afforded the product (10.4 mg, 68.3 μmol, 34%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.07 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.29 (s, 3H), 1.95 (virt. t, 2J ≅ 4J = 8.2 Hz, 1H), 2.02 (ddd, 3J = 9.2, 7.7, 5.8 Hz, 2H), 2.48 (virt. q, 3J ≅ 3J = 8.3 Hz, 1H), 2.57 (dt, 2J = 19.2 Hz, 3J = 9.2 Hz, 1H), 2.62–2.73 (m, 2H), 4.04 (dd, 2J = 6.2 Hz, 4J = 1.3 Hz, 1H), 4.08 (d, 2J = 6.2 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 13.8 (q), 16.9 (t), 41.3 (t), 44.2 (t), 49.0 (s), 51.3 (d), 83.1 (t), 93.0 (s), 206.3 (s). IR (ATR) ν̃: 2956 (w), 1737 (vs), 1451 (w), 1238 (w), 1038 (m), 1006 (m), 975 (s), 919 (m), 894 (m). MS (EI, 70 eV) m/z (%): 151 (6), 137 (36), 123 (13), 110 (100), 96 (70), 81 (92). HRMS (EI) m/z: [M]+ calcd for C9H12O2, 152.0832; found, 152.0828.
(E/Z)-2-(But-2-en-1-yloxy)cyclohex-2-en-1-one (E-10/Z-10)
According to general procedure 1, compound E-10/Z-1029 was synthesized starting from 4a (1.00 g, 8.92 mmol, 1.0 equiv), crotyl alcohol (E/Z = 1/1) (3.00 mL, 2.55 g, 35.4 mmol, 4.0 equiv), and p-TsOH·H2O (51.7 mg, 272 μmol, 3.0 mol %). Purification by flash column chromatography (SiO2, P/Et2O = 3/1, UV) afforded the product (510 mg, 3.07 mmol, 34%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.14 [UV, KMnO4]. 1H NMR (400 MHz, CDCl3, 300 K): δ 1.72 (dd, 3J = 6.3 Hz, 4J = 1.1 Hz, 3H), 1.96 (virt. quint. 3J ≅ 3J = 6.2 Hz, 2H), 2.41 (virt. td, 3J ≅ 3J = 5.9 Hz, 3J = 4.6 Hz, 2H), 2.50 (dd, 3J = 7.4, 6.0 Hz, 2H), 4.20 (d, 3J = 6.3 Hz, 2H), 5.62–5.71 (m, 1H), 5.71–5.83 (m, 1H), 5.88 (t, 3J = 4.6 Hz, 1H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ 17.9 (q), 23.1 (t), 24.7 (t), 39.0 (t), 68.6 (t), 118.1 (d), 125.9 (d), 130.9 (d), 150.6 (s), 194.5 (s). IR (ATR) ν̃: 2939 (w), 1686 (vs), 1622 (m), 1439 (w), 1365 (w), 1259 (m), 1185 (s), 1149 (vs), 1006 (m), 965 (s), 872 (w). MS (EI, 70 eV) m/z (%): 166 (21), 112 (100), 95 (6), 84 (32). HRMS (EI) m/z: [M]+ calcd for C10H14O2, 166.0988; found: 166.0988, [M]+ calcd for C913C1H14O2, 167.1022; found, 167.1025. UV/vis (CH2Cl2, c = 0.5 mm): λ = 260 nm (ε = 4882 cm–1 M–1).
(3RS,3aSR,7aSR,8SR)-8-Methylhexahydro-7H-3,7a-methanobenzofuran-7-one (11)
In analogy to general procedure 5, compound 11 was synthesized starting from E-10/Z-10 (16.6 mg, 100 μmol, 1.0 equiv) and thioxanthone 5 (2.15 mg, 10.1 μmol, 10 mol %) over 4.5 h. The solvent was removed under reduced pressure and the residue redissolved in 1.0 mL of CH2Cl2 and dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate (5.30 mg, 26.7 μmol, 0.27 equiv) was added.21 The reaction mixture was stirred at room temperature for 4.5 h and the solvent was removed under reduced pressure. Purification by flash column chromatography (SiO2, P/Et2O = 1/2, KMnO4) afforded the product (13.0 mg, 78.2 μmol, 78%) as a colorless solid. Mp 48–53 °C. TLC (P/Et2O = 1/2): Rf = 0.21 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 0.91 (d, 3J = 6.4 Hz, 3H), 1.62–1.76 (m, 1H), 1.91 (virt. tdd, 2J ≅ 3J = 14.2 Hz, 3J = 11.0 Hz, 3J = 3.7 Hz, 1H), 2.00–2.07 (m, 1H), 2.11 (ddd, 2J = 13.4 Hz, 3J = 6.7, 3.6 Hz, 1H), 2.21 (dd, 3J = 11.0, 6.7 Hz, 1H), 2.28–2.41 (m, 2H), 2.65 (d, 3J = 2.9 Hz, 1H), 3.06 (qd, 3J = 6.4 Hz, 2.9 Hz, 1H), 3.76 (d, 2J = 6.5 Hz, 1H), 3.88 (d, 2J = 6.5 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 9.7 (q), 24.4 (t), 27.1 (t), 40.1 (t), 44.4 (d), 45.0 (d), 52.7 (d), 67.5 (t), 89.1 (s), 203.8 (s). IR (ATR) ν̃: 2946 (m), 1710 (vs), 1451 (w), 1320 (w), 1094 (m), 994 (m), 930 (m), 848 (m). MS (EI, 70 eV) m/z (%): 166 (20), 151 (4), 138 (5), 123 (5), 112 (100), 109 (15), 95 (11), 84 (41). HRMS (EI) m/z: [M]+ calcd for C10H14O2, 166.0988; found, 166.0982.
(E)-2-(Pent-2-en-1-yloxy)cyclohex-2-en-1-one (E-12)
According to general procedure 1, compound E-12 was synthesized starting from 4a (250 mg, 2.23 mmol, 1.0 equiv), (E)-pent-2-en-1-ol (0.90 mL, 770 mg, 8.92 mmol, 4.0 equiv), and p-TsOH·H2O (12.7 mg, 70.0 μmol, 3.0 mol %). Purification by flash column chromatography (SiO2, P/Et2O = 6/1 → 3/1, P/Et2O = 6/1, UV) afforded the product (152 mg, 840 μmol, 38%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.18 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.00 (t, 3J = 7.5 Hz, 3H), 1.96 (dt, 2J = 12.4 Hz, 3J = 6.0 Hz, 2H), 2.02–2.13 (m, 2H), 2.41 (virt. td, 3J ≅ 3J = 6.0 Hz, 3J = 4.6 Hz, 2H), 2.50 (dd, 3J = 7.4, 6.0 Hz, 2H), 4.22 (dd, 3J = 6.3 Hz, 4J = 1.4 Hz, 2H), 5.64 (dtt, 3J = 15.5, 6.3 Hz, 4J = 1.6 Hz, 1H), 5.75–5.85 (m, 1H), 5.88 (t, 3J = 4.6 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 13.3 (q), 23.1 (t), 24.7 (t), 25.5 (t), 39.0 (t), 68.7 (t), 118.2 (d), 123.5 (d), 137.6 (d), 150.5 (s), 194.6 (s). IR (ATR) ν̃: 2933 (w), 1686 (vs), 1623 (m), 1458 (w), 1365 (w), 1259 (m), 1185 (s), 1149 (vs), 1123 (s), 1007 (m), 967 (s), 880 (w). MS (EI, 70 eV) m/z (%): 180 (11), 145 (93), 129 (11), 113 (16), 99 (100), 83 (23). HRMS (EI) m/z: [M]+ calcd for C11H16O2, 180.1145; found, 180.1143. UV/vis (CH2Cl2, c = 0.5 mm): λ = 260 nm (ε = 4760 cm–1 M–1).
(Z)-1-Bromopent-2-ene30
(Z)-Pent-2-en-1-ol (0.81 mL, 689 mg, 8.00 mmol, 1.0 equiv) was dissolved in 16 mL of dry Et2O and cooled to 0 °C. PBr3 (0.90 mL, 2.60 g, 9.60 mmol, 1.2 equiv) was added dropwise, the reaction mixture was allowed to warm to room temperature and stirred for 4 h. The reaction was quenched by the addition of 20 mL of H2O and the layers were separated. The organic layer was washed with NaHCO3 (1 × 20 mL), H2O (1 × 20 mL) and brine (1 × 20 mL), dried over Na2SO4, filtered, and the solvent was removed under reduced pressure. After purification by flash column chromatography (SiO2, P), the product (119 mg, 0.80 mmol, 10%) was obtained as colorless oil. TLC (P): Rf = 0.67 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.03 (t, 3J = 7.5 Hz, 3H), 2.16 (virt. quint.d 3J ≅ 3J = 7.5 Hz, 4J = 1.5 Hz, 2H), 4.00 (dd, 3J = 8.3 Hz, 4J = 0.7 Hz, 2H), 5.60 (dt, 3J = 10.6, 7.4 Hz, 1H), 5.70 (m, 1H). 13C{1H} NMR (75 MHz, CDCl3, 300 K): δ 13.9 (q), 20.4 (t), 27.4 (t), 124.8 (d), 137.7 (d). Data of this compound were in accordance to the literature.30
(Z)-2-(Pent-2-en-1-yloxy)cyclohex-2-en-1-one (Z-12)
According to general procedure 2, compound Z-12 was synthesized starting from 4a (520 mg, 4.64 mmol, 1.0 equiv), (Z)-1-bromopent-2-ene (830 mg, 5.57 mmol, 1.2 equiv) and K2CO3 (0.770 mg, 5.57 mmol, 1.2 equiv). Purification by flash column chromatography (SiO2, P/Et2O = 3/1, H/EtOAc = 24/1 → 7/3 → 0/1 UV) afforded the product (133 mg, 737 μmol, 17%) as colorless oil. TLC (P/Et2O = 3/1): Rf = 0.19 [UV, KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 0.99 (t, 3J = 7.5 Hz, 3H), 1.89–2.03 (m, 2H), 2.03–2.15 (m, 2H), 2.42 (virt. td, 3J ≅ 3J = 6.0 Hz, 3J = 4.6 Hz, 2H), 2.51 (dd, 3J = 7.5, 6.0 Hz, 2H), 4.36 (d, 3J = 5.1 Hz, 2H), 5.43–5.67 (m, 2H), 5.87 (t, 3J = 4.6 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 14.2 (q), 21.3 (t), 23.1 (t), 24.7 (t), 39.0 (t), 63.8 (t), 118.2 (d), 124.0 (d), 135.7 (d), 150.5 (s), 194.6 (s). IR (ATR) ν̃: 2933 (w), 1687 (vs), 1622 (m), 1457 (w), 1374 (w), 1259 (m), 1185 (m), 1149 (vs), 1123 (s), 1007 (m), 873 (w). MS (EI, 70 eV) m/z (%): 180 (17), 145 (58), 129 (11), 113 (100), 99 (87), 84 (40). HRMS (EI) m/z: [M]+ calcd for C11H16O2, 180.1145; found, 180.1145, [M]+ calcd for C1013C1H16O2, 181.1178; found, 181.1183. UV/vis (CH2Cl2, c = 0.5 mm): λ = 259 nm (ε = 3754 cm–1 M–1).
(3RS,3aSR,7aSR,8SR)-8-Ethylhexahydro-7H-3,7a-methanobenzofuran-7-one (13)
According to general procedure 5, compound 13 was synthesized starting from E-12 (18.1 mg, 100 μmol, 1.0 equiv) and thioxanthone 5 (2.14 mg, 10.2 μmol, 10 mol %) over 2.5 h. Purification by flash column chromatography (SiO2, P/Et2O = 2/1 → 1/1, KMnO4) afforded a mixture of product 13 and olefinic byproduct 14 (18.1 mg, 100 μmol, 100%, 13/14 = 82/18) as a colorless solid.
According to general procedure 5, compound 13 was synthesized starting from Z-12 (18.1 mg, 100 μmol, 1.0 equiv) and thioxanthone 5 (2.17 mg, 10.1 μmol, 10 mol %) over 2.5 h. Purification by flash column chromatography (SiO2, P/Et2O = 1/1, KMnO4) afforded a mixture of product 13 and olefinic byproduct 14 (16.3 mg, 90.4 μmol, 90%, 13/14 = 83/17) as a colorless solid.
The product mixture (14.2 mg, 78.8 μmol) was dissolved in 500 μL of DCE and 400 μL of H2O. Subsequently, RuCl3·xH2O (1 small crystal, calc. 0.14 mg, 0.7 μmol, 3.5 mol % relative to byproduct) and NaIO4 (18.6 mg, 87.0 μmol, 4.3 equiv) were added and the reaction mixture was stirred at room temperature for 20 h. The reaction was quenched by addition of 2.0 mL of H2O, diluted with 2.0 mL Et2O and the layers were separated. The aqueous layer was extracted with Et2O (3 × 5 mL). The combined organic layers were then washed with H2O (1 × 10 mL) and brine (1 × 10 mL), dried over Na2SO4, filtered, and the solvent was removed under reduced pressure. Purification by flash column chromatography (SiO2, P/Et2O = 2/1, KMnO4) afforded the product (9.80 mg, 54.4 μmol, 69%) as a colorless solid. Mp 58–61 °C. TLC (P/Et2O = 1/2): Rf = 0.16 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 0.79 (t, 3J = 7.5 Hz, 3H), 1.21–1.33 (m, 1H), 1.37–1.51 (m, 1H), 1.63–1.78 (m, 1H), 1.93 (virt. tdd, 2J ≅ 3J = 14.2 Hz, 3J = 11.0 Hz, 3J = 3.8 Hz, 1H), 1.99–2.09 (m, 1H), 2.09–2.16 (m, 1H), 2.21 (dd, 3J = 11.0, 6.7 Hz, 1H), 2.31–2.43 (m, 2H), 2.71 (d, 3J = 2.9 Hz, 1H), 2.84 (ddd, 3J = 8.6, 6.0, 2.9 Hz, 1H), 3.75 (d, 2J = 6.5 Hz, 1H), 3.85 (d, 2J = 6.5 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 11.4 (q), 17.9 (t), 24.4 (t), 27.3 (t), 40.2 (t), 43.4 (d), 52.4 (d), 52.8 (d), 67.8 (t), 88.9 (s), 204.2 (s). IR (ATR) ν̃: 2956 (m), 1713 (vs), 1462 (w), 1327 (w), 1095 (m), 1013 (w), 947 (m), 851 (m). MS (EI, 70 eV) m/z (%): 180 (8), 151 (6), 123 (7), 113 (100), 109 (9), 95 (12), 84 (22). HRMS (EI) m/z: [M]+ calcd for C10H14O2, 180.1145; found, 180.1148.
(3RS,3aRS,7aRS)-3-(Prop-1-en-2-yl)hexahydrobenzofuran-7(4H)-one (16a)
According to general procedure 5, compound 16a was synthesized starting from 3a (18.4 mg, 102 μmol, 1.0 equiv) and thioxanthone 5 (2.21 mg, 10.4 μmol, 10 mol %) over 4.5 h. Purification by flash column chromatography (SiO2, P/Et2O = 1/2, KMnO4) afforded the product (11.9 mg, 66.0 μmol, 65%, d.r. >95/5) as colorless oil. TLC (P/Et2O = 1/2): Rf = 0.05 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.46 (virt. qd, 2J ≅ 3J = 12.4 Hz, 3J = 3.8 Hz, 1H), 1.64 (virt. qdd, 2J ≅ 3J = 12.6 Hz, 3J = 5.4, 3.8 Hz, 1H), 1.81 (s, 3H), 1.99–2.06 (m, 1H), 2.08–2.17 (m, 2H), 2.26 (virt. tdd, 2J ≅ 3J = 14.0 Hz, 3J = 5.4 Hz, 4J = 1.7 Hz, 1H), 2.36 (virt. ddt, 2J = 14.1 Hz, 3J = 5.1 Hz, 3J ≅ 4J = 1.8 Hz, 1H), 2.80–2.93 (m, 1H), 4.00 (dd, 2J = 9.1 Hz, 3J = 1.7 Hz, 1H), 4.10 (dd, 2J = 9.1 Hz, 3J = 6.3 Hz, 1H), 4.15 (dd, 3J = 12.4 Hz, 4J = 1.7 Hz, 1H), 4.79–4.81 (m, 1H), 5.03 (virt. quint., 2J ≅ 4J = 1.3 Hz, 1H). 13C{1H} NMR (75 MHz, CDCl3, 300 K): δ 24.7 (q), 25.9 (t), 27.3 (t), 39.8 (t), 47.5 (d), 50.7 (d), 73.7 (t), 84.0 (d), 112.7 (t), 144.3 (s), 207.8 (s). IR (ATR) ν̃: 2929 (m), 1730 (vs), 1449 (w), 1105 (m), 1045 (m), 900 (m). MS (EI, 70 eV) m/z (%): 180 (73), 165 (8), 136 (21), 123 (17), 107 (100), 93 (46), 81 (29). HRMS (EI) m/z: [M]+ calcd for C11H16O2, 180.1145; found, 180.1145, [M]+ calcd for C1013C1H16O2, 181.1178; found, 181.1183.
(3SR,3aRS,7aRS)-3a-Methyl-3-(prop-1-en-2-yl)hexahydrobenzofuran-7(4H)-one (16b)
According to general procedure 5, compound 16b was synthesized starting from 3b (19.6 mg, 101 μmol, 1.0 equiv) and thioxanthone 5 (2.16 mg, 10.2 μmol, 10 mol %) over 5 h. Purification by flash column chromatography (SiO2, P/Et2O = 2/1, KMnO4) afforded the product (15.4 mg, 79.1 μmol, 79%, d.r. >95/5) as a colorless solid. Mp 87–92 °C. TLC (P/Et2O = 1/2): Rf = 0.08 [KMnO4]. 1H NMR (400 MHz, CDCl3, 300 K): δ 0.97 (s, 3H), 1.66 (virt. td, 2J ≅ 3J = 13.0 Hz, 3J = 5.4 Hz, 1H), 1.73–1.79 (m, 1H), 1.81 (s, 3H), 1.87–1.98 (m, 1H), 1.98–2.06 (m, 1H), 2.20–2.34 (m, 2H), 2.52 (ddd, 3J = 7.0, 2.1 Hz, 4J = 0.9 Hz, 1H), 3.95 (dd, 2J = 9.6 Hz, 3J = 2.1 Hz, 1H), 4.19–4.26 (m, 2H), 4.79–4.81 (m, 1H), 5.07 (virt. quint., 2J ≅ 4J = 1.4 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 20.2 (q), 23.4 (t), 25.5 (q), 30.8 (t), 38.8 (t), 50.6 (s), 55.3 (d), 72.6 (t), 85.3 (d), 112.3 (t), 145.5 (s), 207.8 (s). IR (ATR) ν̃: 2924 (s), 1732 (vs), 1455 (w), 1377 (w), 1260 (w), 1104 (w), 1054 (m), 907 (w). MS (EI, 70 eV) m/z (%): 176 (6), 149 (7), 126 (97), 111 (100), 97 (67), 93 (45). HRMS (EI) m/z: [M]+ calcd for C12H18O2, 194.1301; found, 194.1299.
(3RS,3aRS,7aRS)-5,5-Dimethyl-3-(prop-1-en-2-yl)hexahydrobenzofuran-7(4H)-one (16c)
According to general procedure 5, compound 16c was synthesized starting from 3c (24.8 mg, 120 μmol, 1.0 equiv) and thioxanthone 5 (2.57 mg, 12.1 μmol, 10 mol %) over 1.5 h. Purification by flash column chromatography (SiO2, P/Et2O = 3/1, KMnO4) afforded the product (21.2 mg, 102 μmol, 86%, d.r. >95/5) as a colorless solid. Mp 95–101 °C. TLC (P/Et2O = 3/1): Rf = 0.09 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 0.99 (s, 3H), 1.13 (s, 3H), 1.46 (virt. t, 2J ≅ 3J = 13.0 Hz, 1H), 1.70 (ddd, 2J = 13.0 Hz, 3J = 3.2 Hz, 4J = 2.3 Hz, 1H), 1.81 (dd, 4J = 1.4, 0.8 Hz, 3H), 2.06 (dd, 2J = 13.5 Hz, 4J = 2.3 Hz, 1H), 2.25 (ddd, 2J = 13.5 Hz, 4J = 1.7, 0.9 Hz), 2.36 (virt. tdd, 3J ≅ 3J = 12.7 Hz, 3J = 6.8, 3.2 Hz, 1H), 2.84 (virt. t, 3J ≅ 3J = 6.6 Hz, 1H), 4.03 (dd, 2J = 9.2 Hz, 3J = 1.6 Hz, 1H), 4.10–4.21 (m, 2H), 4.79–4.81 (m, 1H), 5.06 (virt. quint., 2J ≅ 4J = 1.3 Hz, 1H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 24.9 (q), 27.2 (q), 32.1 (q), 38.5 (s), 39.0 (t), 46.3 (d), 47.4 (d), 52.9 (t), 74.4 (t), 83.7 (d), 112.7 (t), 144.4 (s), 207.2 (s). IR (ATR) ν̃: 2957 (m), 1733 (vs), 1459 (w), 1370 (w), 1126 (w), 1060 (m), 919 (w). MS (EI, 70 eV) m/z (%): 208 (100), 193 (8), 164 (28), 152 (6), 135 (44), 123 (87), 105 (26), 95 (56), 81 (10). HRMS (EI) m/z: [M]+ calcd for C13H20O2, 208.1458; found, 208.1458.
(3aSR,3bSR,6aRS,6bRS)-6a,6b-Bis(allyloxy)-3a,3b-dimethyloctahydrocyclo-buta[1,2:3,4]-di[5]annulene-1,6-dione (17)
According to general procedure 5, compound 17 was synthesized starting from 1f (61.7 mg, 405 μmol, 1.0 equiv) and thioxanthone 5 (8.50 mg, 40.0 μmol, 10 mol %) over 14 h. Purification by flash column chromatography (SiO2, P/Et2O = 3/1, KMnO4) afforded the product (7.10 mg, 23.3 μmol, 12%) as a colorless solid. Mp > 220 °C. TLC (P/Et2O = 3/1): Rf = 0.47 [KMnO4]. 1H NMR (400 MHz, CDCl3, 300 K): δ 1.25 (s, 6H), 1.70 (ddd, 2J = 14.9 Hz, 3J = 13.1, 8.8 Hz, 2H), 2.24–2.41 (m, 4H), 2.54–2.73 (m, 2H), 4.08 (virt. ddt, 2J = 12.8 Hz, 3J = 5.4 Hz, 4J ≅ 4J = 1.5 Hz, 2H), 4.18 (virt. ddt, 2J = 12.8 Hz, 3J = 5.0 Hz, 4J ≅ 4J = 1.6 Hz, 2H), 5.07 (virt. dq, 3J = 10.5 Hz, 2J ≅ 4J = 1.6 Hz, 2H), 5.18 (virt. dq, 3J = 17.2 Hz, 2J ≅ 4J = 1.6 Hz, 2H), 5.71–5.89 (m, 2H). 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ 19.1 (q), 32.7 (t), 37.2 (t), 44.2 (s), 68.4 (t), 86.1 (s), 116.1 (t), 134.8 (d), 214.2 (s). IR (ATR) ν̃: 2964 (w), 1748 (vs), 1451 (w), 1413 (w), 1186 (w), 1089 (m), 1060 (w), 988 (w), 923 (w). MS (EI, 70 eV) m/z (%): 152 (66), 137 (100), 123 (31), 96 (25), 81 (19), 69 (31), 57 (34), 41 (95). HRMS (EI) m/z: [M]+ calcd for C18H24O4, 304.1669; found, 304.1660.
(3aRS,4aSR,6aSR,9aSR,10aRS,12aRS)-3a,9a-Dimethyldodecahydro-1H,7H-cyclopenta[1,4]cyclobuta[1,2-b]cyclopenta[1,4]cyclobuta[1,2-f][1,5]dioxocine-1,7-dione (18)
According to general procedure 5, compound 18 was synthesized starting from 1f (61.7 mg, 405 μmol, 1.0 equiv) and thioxanthone 5 (8.50 mg, 40.0 μmol, 10 mol %) over 14 h. Purification by flash column chromatography (SiO2, P/Et2O = 3/1, KMnO4) afforded the product (11.1 mg, 36.5 μmol, 18%) as a colorless solid. Mp >220 °C. TLC (P/Et2O = 3/1): Rf = 0.30 [KMnO4]. 1H NMR (400 MHz, CDCl3, 300 K): δ 1.15 (s, 6H), 1.31 (dd, 2J = 12.9 Hz, 3J = 4.6 Hz, 2H), 1.58–1.70 (m, 2H), 1.72–1.89 (m, 4H), 2.27–2.47 (m, 4H), 2.67 (ddd, 2J = 17.8 Hz, 3J = 13.1, 8.8 Hz, 2H), 3.74 (dd, 2J = 12.8 Hz, 3J = 3.0 Hz, 2H), 4.46–4.53 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 22.7 (q), 28.9 (t), 34.0 (t), 35.8 (t), 36.0 (d), 44.5 (s), 66.8 (t), 81.3 (s), 220.2 (s). IR (ATR) ν̃: 2927 (m), 1735 (vs), 1456 (w), 1266 (w), 1114 (w), 1068 (s), 802 (w). MS (EI, 70 eV) m/z (%): 304 (33), 263 (25), 248 (53), 230 (20), 208 (49), 191 (15), 175 (10), 161 (13), 152 (72), 137 (100), 113 (54), 97 (74), 84 (67), 67 (63), 55 (85), 41 (95). HRMS (EI) m/z: [M]+ calcd for C18H24O4, 304.1669; found, 304.1674, [M]+ calcd for C1713C1H24O4, 305.1703; found, 305.1723.
(3aSR,3bSR,6aRS,6bRS)-3a,3b-Dimethyl-6a,6b-bis((2-methylallyl)oxy)octahydrocyclo-buta[1,2:3,4]di[5]annulene-1,6-dione (19)
According to general procedure 5, compound 19 was synthesized starting from 2f (66.9 mg, 402 μmol, 1.0 equiv) and thioxanthone 5 (8.50 mg, 40.0 μmol, 10 mol %) over 3 h. Purification by flash column chromatography (SiO2, P/Et2O = 9/1 → 3/1 → 1/1, KMnO4) afforded the product (11.3 mg, 34.0 μmol, 17%) as a colorless solid. Mp 50–65 °C. TLC (P/Et2O = 3/1): Rf = 0.44 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.26 (s, 6H), 1.66 (s, 6H), 1.69–1.78 (m, 2H), 2.21–2.42 (m, 4H), 2.63 (ddd, 2J = 17.6 Hz, 3J = 13.7, 9.4 Hz, 2H), 3.99 (d, 2J = 12.7 Hz, 2H), 4.10 (d, 2J = 12.7 Hz, 2H), 4.78 (virt. dt, 4J = 3.0 Hz, 2J ≅ 4J = 1.6 Hz, 2H), 4.83–4.94 (m, 2H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 19.1 (q), 19.6 (q), 32.7 (t), 37.2 (t), 44.4 (s), 70.3 (t), 85.8 (s), 110.5 (t), 142.3 (s), 214.0 (s). IR (ATR) ν̃: 2967 (m), 1749 (vs), 1450 (w), 1186 (w), 1097 (m), 1060 (w), 897 (w). MS (EI, 70 eV) m/z (%): 213 (13), 182 (25), 166 (65), 151 (44), 123 (23), 110 (41), 97 (23), 84 (46), 69 (27), 55 (100). HRMS (EI) m/z: [M]+ calcd for C20H28O4, 332.1982; found, 332.1998.
(3aRS,4aSR,6aRS,9aSR,10aRS,12aSR)-3a,4a,9a,10a-Tetramethyldodecahydro-1H,7H-cyclopenta[1,4]cyclobuta[1,2-b]cyclopenta[1,4]cyclobuta[1,2-f][1,5]dioxocine-1,7-dione (20)
According to general procedure 5, compound 20 was synthesized starting from 2f (66.9 mg, 402 μmol, 1.0 equiv) and thioxanthone 5 (8.50 mg, 40.0 μmol, 10 mol %) over 3 h. Purification by flash column chromatography (SiO2, P/Et2O = 9/1 → 3/1 → 1/1, KMnO4) afforded the product (17.3 mg, 52.0 μmol, 26%) as a colorless solid. Mp 25–30 °C. TLC (P/Et2O = 3/1): Rf = 0.12 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 0.99 (s, 6H), 1.18 (s, 6H), 1.65 (dddd, 2J = 14.1 Hz, 3J = 9.2, 4.0 Hz, 4J = 0.9 Hz, 2H), 1.84 (d, 2J = 8.3 Hz, 2H), 2.27 (dt, 2J = 14.1 Hz, 3J = 8.7 Hz, 2H), 2.49–2.68 (m, 6H), 3.95 (d, 2J = 6.7 Hz, 2H), 4.11 (dd, 2J = 6.7 Hz, 4J = 1.7 Hz, 2H). 13C{1H} NMR (126 MHz, CDCl3, 300 K): δ 11.6 (q), 15.0 (q), 25.0 (t), 39.6 (t), 44.7 (t), 51.1 (s), 52.2 (s), 81.0 (t), 95.4 (s), 205.5 (s). IR (ATR) ν̃: 2957 (m), 1742 (vs), 1450 (w), 1043 (w), 976 (w), 902 (w). MS (EI, 70 eV) m/z (%): 151 (37), 137 (9), 123 (58), 110 (43), 95 (100), 81 (64), 67 (92), 55 (79), 41 (47). HRMS (EI) m/z: [M]+ calcd for C20H28O4, 332.1982; found, 332.1983.
(3aSR,3bSR,6aRS,6bRS)-3a,3b-Dimethyl-6a,6b-bis((3-methylbut-2-en-1-yl)oxy)octa-hydrocyclobuta[1,2:3,4]di[5]annulene-1,6-dione (21)
According to general procedure 5, compound 21 was synthesized starting from 3f (72.3 mg, 401 μmol, 1.0 equiv) and thioxanthone 5 (8.50 mg, 40.0 μmol, 10 mol %) over 4 h. Purification by flash column chromatography (SiO2, P/Et2O = 9/1 → 3/1 → 1/1, KMnO4) afforded the product (24.3 mg, 67.4 μmol, 34%) as a colorless solid. Mp 85–98 °C. TLC (P/Et2O = 3/1): Rf = 0.44 [KMnO4]. 1H NMR (500 MHz, CDCl3, 300 K): δ 1.24 (s, 6H), 1.58 (s, 6H), 1.64–1.72 (m, 8H), 2.23–2.39 (m, 4H), 2.62 (ddd, 2J = 17.6 Hz, 3J = 13.8, 9.3 Hz, 2H), 4.05 (dd, 2J = 11.6 Hz, 3J = 6.9 Hz, 2H), 4.14 (dd, 2J = 11.6 Hz, 3J = 6.5 Hz, 2H), 5.08–5.26 (m, 2H). 13C{1H} NMR (75 MHz, CDCl3, 300 K): δ 18.3 (q), 19.3 (q), 25.9 (q), 32.8 (t), 37.1 (t), 44.0 (s), 64.7 (t), 86.3 (s), 121.6 (d), 135.7 (s), 214.4 (s). IR (ATR) ν̃: 2967 (m), 1748 (vs), 1448 (w), 1381 (w), 1185 (w), 1090 (m), 1060 (w), 982 (w). MS (EI, 70 eV) m/z (%): 224 (13), 160 (14), 124 (11), 113 (43), 97 (21), 83 (29), 69 (100), 55 (39), 41 (64). HRMS (EI) m/z: [M]+ calcd for C22H32O4, 360.2295; found, 360.2296.
Acknowledgments
Financial support by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no 665951—ELICOS) is gratefully acknowledged. We thank Thomas Pickl for his help in the synthesis of starting materials and in the structure elucidation of a byproduct.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c01501.
1H and 13C{1H} NMR spectra for all compounds, configuration assignment by NOESY studies, luminescence spectrum of compound 1a, emission spectrum of the light source, and UV/Vis spectra of all photochemical substrates (PDF)
X-ray crystallographic data of compounds 18 (CIF)
X-ray crystallographic data of compounds 21 (CIF)
X-ray crystallographic data of compound S1 (CIF)
FAIR data, including the primary NMR FID files, for all compounds described in the Experimental Section (ZIP)
The authors declare no competing financial interest.
This paper was published on August 7, 2020. Due to production error, Scheme 4 contained incorrect information. The corrected version was reposted on August 14, 2020.
Supplementary Material
References
- General reviews:; a Poplata S.; Tröster A.; Zou Y.-Q.; Bach T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. 10.1021/acs.chemrev.5b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hoffmann N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]; c Fleming S. A.Photocycloaddition of Alkenes to Excited Alkenes. In Synthetic Organic Photochemistry, Molecular and Supramolecular Photochemistry; Griesbeck A. G., Mattay J., Eds.; Dekker: New York, 2005; Vol. 12, pp 141–160. [Google Scholar]
- Reviews with a focus on cyclic enones:; a Hehn J. P.; Müller C.; Bach T.. Formation of a Four-Membered Ring: From a Carbonyl-Conjugated Alkene. In Handbook of Synthetic Photochemistry; Albini A., Fagnoni M., Eds.; Wiley-VCH: Weinheim, 2010; pp 171–215. [Google Scholar]; b Margaretha P.Photocycloaddition of Cycloalk-2-enones to Alkenes. In Synthetic Organic Photochemistry, Molecular and Supramolecular Photochemistry; Griesbeck A. G., Mattay J., Eds.; Dekker: New York, 2005; Vol. 12, pp 211–237. [Google Scholar]; c Pete J.-P.Asymmetric Photoreactions of Conjugated Enones and Esters. Advances in Photochemistry; Wiley, 1996; Vol. 21, pp 135–216. [Google Scholar]; d Crimmins M. T.; Reinhold T. L. Enone Olefin [2 + 2] Photochemical Cycloadditions. Org. React. 1993, 44, 297–588. 10.1002/0471264180.or044.02. [DOI] [Google Scholar]; e Baldwin S. W. Synthetic Aspects of [2+2] Cycloadditions of α,β-Unsaturated Carbonyl Compounds. Org. Photochem. 1981, 5, 123–225. [Google Scholar]
- Reviews with a focus on intramolecular reactions:; a Crimmins M. T. Synthetic Applications of Intramolecular Enone-Olefin Photocycloadditions. Chem. Rev. 1988, 88, 1453–1473. 10.1021/cr00090a002. [DOI] [Google Scholar]; b Becker D.; Haddad N. Applications of Intramolecular (2+2)Photocycloadditions in Organic Synthesis. Org. Photochem. 1989, 10, 1–162. [Google Scholar]
- a Schuster D. I.; Lem G.; Kaprinidis N. A. New Insights into an Old Mechanism: [2 + 2] Photocycloaddition of Enones to Alkenes. Chem. Rev. 1993, 93, 3–22. 10.1021/cr00017a001. [DOI] [Google Scholar]; b Schuster D. I.Mechanistic Issues in [2 + 2]-Photocycloadditions of Cyclic Enones to Alkenes. In CRC Handbook of Photochemistry and Photobiology, 2nd ed.; Horspool W. M., Lenci F., Eds.; CRC Press: Boca Raton, 2004; pp 72/1–72/24. [Google Scholar]
- a Srinivasan R.; Carlough K. H. Mercury(3P1) photosensitized internal cycloaddition reactions in 1,4-, 1,5-, and 1,6-dienes. J. Am. Chem. Soc. 1967, 89, 4932–4936. 10.1021/ja00995a018. [DOI] [Google Scholar]; b Liu R. S. H.; Hammond G. S. Photosensitized Internal Addition of Dienes to Olefins. J. Am. Chem. Soc. 1967, 89, 4936–4944. 10.1021/ja00995a019. [DOI] [Google Scholar]; c Maradyn D. J.; Weedon A. C. Trapping of Triplet 1,4-Biradicals with Hydrogen Selenide in the Intramolecular Photochemical Cycloaddition Reaction of 3-(4′-Pentenyl)cycloalk-2-enones: Verification of the Rule of Five. J. Am. Chem. Soc. 1995, 117, 5359–5360. 10.1021/ja00124a019. [DOI] [Google Scholar]
- a Ikeda M.; Takahashi M.; Ohno K.; Tamura Y.; Kido M. Intramolecular photo(2+2)cycloaddition of 2-allyloxy- and 2-allylaminocyclohex-2-enones. Formation of 2-oxa- and 2-aza-bicyclo(2.1.1)hexane systems. Chem. Pharm. Bull. 1982, 30, 2269–2271. 10.1248/cpb.30.2269. [DOI] [Google Scholar]; b Ikeda M.; Takahashi M.; Uchino T.; Ohno K.; Tamura Y.; Kido M. Intramolecular [2 + 2] Photocycloaddition of 2-(Alkenyloxy)cyclohex-2-enones. J. Org. Chem. 1983, 48, 4241–4247. 10.1021/jo00171a017. [DOI] [Google Scholar]
- For some early examples, see:; a Lu Z.; Yoon T. P. Visible Light Photocatalysis of [2+2] Styrene Cycloadditions by Energy Transfer. Angew. Chem., Int. Ed. 2012, 51, 10329–10332. 10.1002/anie.201204835. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zou Y.-Q.; Duan S.-W.; Meng X.-G.; Hu X.-Q.; Gao S.; Chen J.-R.; Xiao W.-J. Visible light induced intermolecular [2+2]-cycloaddition reactions of 3-ylideneoxindoles through energy transfer pathway. Tetrahedron 2012, 68, 6914–6919. 10.1016/j.tet.2012.06.011. [DOI] [Google Scholar]; c Hurtley A. E.; Lu Z.; Yoon T. P. [2+2] Cycloaddition of 1,3-Dienes by Visible Light Photocatalysis. Angew. Chem., Int. Ed. 2014, 53, 8991–8994. 10.1002/anie.201405359. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Miller Z. D.; Lee B. J.; Yoon T. P. Enantioselective Crossed Photocycloadditions of Styrenic Olefins by Lewis Acid Catalyzed Triplet Sensitization. Angew. Chem., Int. Ed. 2017, 56, 11891–11895. 10.1002/anie.201706975. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Pagire S. K.; Hossain A.; Traub L.; Kerres S.; Reiser O. Photosensitised regioselective [2+2]-cycloaddition of cinnamates and related alkenes. Chem. Commun. 2017, 53, 12072–12075. 10.1039/c7cc06710k. [DOI] [PubMed] [Google Scholar]
- a Clay A.; Vallavoju N.; Krishnan R.; Ugrinov A.; Sivaguru J. Metal-Free Visible Light-Mediated Photocatalysis: Controlling Intramolecular [2 + 2] Photocycloaddition of Enones through Axial Chirality. J. Org. Chem. 2016, 81, 7191–7200. 10.1021/acs.joc.6b01066. [DOI] [PubMed] [Google Scholar]; b Elliott L. D.; Berry M.; Harji B.; Klauber D.; Leonard J.; Booker-Milburn K. I. A Small-Footprint, High-Capacity Flow Reactor for UV Photochemical Synthesis on the Kilogram Scale. Org. Process Res. Dev. 2016, 20, 1806–1811. 10.1021/acs.oprd.6b00277. [DOI] [Google Scholar]; c Zhao J.; Brosmer J. L.; Tang Q.; Yang Z.; Houk K. N.; Diaconescu P. L.; Kwon O. Intramolecular Crossed [2+2] Photocycloaddition through Visible Light-Induced Energy Transfer. J. Am. Chem. Soc. 2017, 139, 9807–9810. 10.1021/jacs.7b05277. [DOI] [PubMed] [Google Scholar]; d Williams J. D.; Nakano M.; Gérardy R.; Rincón J. A.; de Frutos Ó.; Mateos C.; Monbaliu J.-C. M.; Kappe C. O. Finding the Perfect Match: A Combined Computational and Experimental Study toward Efficient and Scalable Photosensitized [2 + 2] Cycloadditions in Flow. Org. Process Res. Dev. 2019, 23, 78–87. 10.1021/acs.oprd.8b00375. [DOI] [Google Scholar]; e Strieth-Kalthoff F.; Henkel C.; Teders M.; Kahnt A.; Knolle W.; Gómez-Suárez A.; Dirian K.; Alex W.; Bergander K.; Daniliuc C. G.; Abel B.; Guldi D. M.; Glorius F. Discovery of Unforeseen Energy-Transfer-Based Transformations Using a Combined Screening Approach. Chem 2019, 5, 2183–2194. 10.1016/j.chempr.2019.06.004. [DOI] [Google Scholar]; f Pecho F.; Zou Y.-Q.; Gramüller J.; Mori T.; Huber S. M.; Bauer A.; Gschwind R. M.; Bach T. A Thioxanthone Sensitizer with a Chiral Phosphoric Acid Binding Site: Properties and Applications in Visible Light-Mediated Cycloadditions. Chem.—Eur. J. 2020, 26, 5190–5194. 10.1002/chem.202000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For some early examples for hydrogen abstraction reactions, see:; a Corey E. J.; Bass J. D.; LeMahieu R.; Mitra R. B. A Study of the Photochemical Reactions of 2-Cyclohexenones with Substituted Olefins. J. Am. Chem. Soc. 1964, 86, 5570–5583. 10.1021/ja01078a034. [DOI] [Google Scholar]; b Hunter N. R.; MacAlpine G. A.; Liu H. J.; Valenta Z. Cyclobutanes in organic synthesis. Part I. Fragmentation to 1,4-dicarbonyl compounds. General considerations. Can. J. Chem. 1970, 48, 1436–1445. 10.1139/v70-235. [DOI] [Google Scholar]; c Greenlee M. L.; Fritzen E. L. Jr; Swenton J. S. A Unique Reversal of Regioselectivity in the Photoaddition of 2-Fluorocyclohexenone to Isobutylene. J. Org. Chem. 1978, 43, 4512–4515. 10.1021/jo00417a028. [DOI] [Google Scholar]; d Shih C.; Fritzen E. L.; Swenton J. S. Photocycloaddition Chemistry of 2-(Trimethylsilyl)cyclopentenone and 5-(Trimethylsilyl)uracil. The Utility of a Trimethylsilyl Group as a Removable Directing Group in Photochemistry. J. Org. Chem. 1980, 45, 4462–4471. 10.1021/jo01310a038. [DOI] [Google Scholar]; e Pirrung M. C.; Webster N. J. G. Cyclooctenone photocycloadditions. Tetrahedron Lett. 1986, 27, 3983–3986. 10.1016/s0040-4039(00)84890-x. [DOI] [Google Scholar]; f Pirrung M. C.; Webster N. J. G. Mechanism of Intramolecular Photocycloadditions of Cyclooctenones. J. Org. Chem. 1987, 52, 3603–3613. 10.1021/jo00392a020. [DOI] [Google Scholar]
- Rodríguez J. R.; Castedo L.; Mascareñas J. L. Synthesis of Eight- and Nine-Membered Carbocycles through a Ring-Closing Metathesis/Ring Fragmentation Strategy: A Rapid and Versatile Approach to Bicyclo[6.4.0]- and Bicyclo[7.4.0]alkene Ring Systems. Chem.—Eur. J. 2002, 8, 2923–2930. . [DOI] [PubMed] [Google Scholar]
- a Dauben W. G.; Ponaras A. A.; Chollet A. An Approach to Angularly Functionalized Methylhydrindan Systems. J. Org. Chem. 1980, 45, 4413–4417. 10.1021/jo01310a030. [DOI] [Google Scholar]; b Schick H.; Schwarz H.; Finger A.; Schwarz S. 2,2-Disubstituierte Cyclopentan-1,3-dione – 2: Untersuchung der Regioselektivität von Alkylierungsreaktionen an 2-Methyl-cyclopentan-1,3-dion. Tetrahedron 1982, 38, 1279–1283. 10.1016/0040-4020(82)85114-4. [DOI] [Google Scholar]; c Ponaras A. A. Axial allylation on the diosphenol claisen rearrangement. Tetrahedron Lett. 1983, 24, 3–6. 10.1016/s0040-4039(00)81311-8. [DOI] [Google Scholar]
- a Ponaras A. A. The diosphenol Claisen rearrangement. Tetrahedron Lett. 1980, 21, 4803–4806. 10.1016/0040-4039(80)80144-4. [DOI] [Google Scholar]; b Matoba K.; Karibe N.; Yamazaki T. Chemistry of 2-methoxy-2,5-cyclohexadienones. I. Photochemistry of 2-methoxy-4,4-dimethyl-2,5-cyclohexadienone. Chem. Pharm. Bull. 1982, 30, 3906–3911. 10.1248/cpb.30.3906. [DOI] [Google Scholar]; c Münster N.; Parker N. A.; van Dijk L.; Paton R. S.; Smith M. D. Visible Light Photocatalysis of 6π Heterocyclization. Angew. Chem., Int. Ed. 2017, 56, 9468–9472. 10.1002/anie.201705333. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Edtmüller V.; Pöthig A.; Bach T. Enantioselective photocyclisation reactions of 2-aryloxycyclohex-2-enones mediated by a chiral copper-bisoxazoline complex. Tetrahedron 2017, 73, 5038–5047. 10.1016/j.tet.2017.05.005. [DOI] [Google Scholar]
- Li X.; Jandl C.; Bach T. Visible-Light-Mediated Enantioselective Photoreactions of 3-Alkylquinolones with 4-O-Tethered Alkenes and Allenes. Org. Lett. 2020, 22, 3618–3622. 10.1021/acs.orglett.0c01065. [DOI] [PubMed] [Google Scholar]
- Iyer A.; Clay A.; Jockusch S.; Sivaguru J. Evaluating brominated thioxanthones as organo-photocatalysts. J. Phys. Org. Chem. 2017, 30, e3738 10.1002/poc.3738. [DOI] [Google Scholar]
- Singh A.; Teegardin K.; Kelly M.; Prasad K. S.; Krishnan S.; Weaver J. D. Facile Synthesis and Complete Characterization of Homoleptic and Heteroleptic Cyclometalated Iridium(III) Complexes for Photocatalysis. J. Organomet. Chem. 2015, 776, 51–59. 10.1016/j.jorganchem.2014.10.037. [DOI] [Google Scholar]
- a Juris A.; Balzani V.; Belser P.; von Zelewsky A. Characterization of the Excited State Properties of Some New Photosensitizers of the Ruthenium (Polypyridine) Family. Helv. Chim. Acta 1981, 64, 2175–2182. 10.1002/hlca.19810640723. [DOI] [Google Scholar]; b Kalyanasundaram K. Photophysics, photochemistry and solar energy conversion with tris(bipyridyl)ruthenium(II) and its analogues. Coord. Chem. Rev. 1982, 46, 159–244. 10.1016/0010-8545(82)85003-0. [DOI] [Google Scholar]
- a Angulo G.; Grilj J.; Vauthey E.; Serrano-Andrés L.; Rubio-Pons Ò.; Jacques P. Ultrafast Decay of the Excited Singlet States of Thioxanthone by Internal Conversion and Intersystem Crossing. ChemPhysChem 2010, 11, 480–488. 10.1002/cphc.200900654. [DOI] [PubMed] [Google Scholar]; b Mundt R.; Villnow T.; Ziegenbein C. T.; Gilch P.; Marian C.; Rai-Constapel V. Thioxanthone in apolar solvents: ultrafast internal conversion precedes fast intersystem crossing. Phys. Chem. Chem. Phys. 2016, 18, 6637–6647. 10.1039/c5cp06849e. [DOI] [PubMed] [Google Scholar]
- For recent reviews on sensitized photochemical reactions, see:; a Zhou Q.-Q.; Zou Y.-Q.; Lu L.-Q.; Xiao W.-J. Visible-Light-Induced Organic Photochemical Reactions through Energy-Transfer Pathways. Angew. Chem., Int. Ed. 2019, 58, 1586–1604. 10.1002/anie.201803102. [DOI] [PubMed] [Google Scholar]; b Strieth-Kalthoff F.; James M. J.; Teders M.; Pitzer L.; Glorius F. Energy Transfer Catalysis Mediated by Visible Light: Principles, Applications, Directions. Chem. Soc. Rev. 2018, 47, 7190–7202. 10.1039/c8cs00054a. [DOI] [PubMed] [Google Scholar]
- a House H. O.; Huber L. E.; Umen M. J. Empirical rules for estimating the reduction potential of.alpha.,.beta.-unsaturated carbonyl compounds. J. Am. Chem. Soc. 1972, 94, 8471–8475. 10.1021/ja00779a028. [DOI] [Google Scholar]; b Mandell L.; Heldrich F. J.; Day R. A. Jr The Electrochemical Reductive Coupling of Mono-Enol Ethers of β-Diketones. Synth. Commun. 1981, 11, 55–59. 10.1080/00397918108064282. [DOI] [Google Scholar]; c Harada J.; Sakakibara Y.; Kunai A.; Sasaki K. Electrochemical Carboxylation of α,β-Unsaturated Ketones with Carbon Dioxide. Bull. Chem. Soc. Jpn. 1984, 57, 611–612. 10.1246/bcsj.57.611. [DOI] [Google Scholar]
- a Dilling W. L.; Tabor T. E.; Boer F. P.; North P. P. Organic photochemistry. IX. Photocycloaddition of 2-cyclopentenone to cis-and trans-dichloroethylene. Evidence for initial attack at carbon-3 and rotational equilibration of the diradical intermediates. J. Am. Chem. Soc. 1970, 92, 1399–1400. 10.1021/ja00708a050. [DOI] [Google Scholar]; b Peet N. P.; Cargill R. L.; Bushey D. F. Synthesis and Acid-Catalyzed Rearrangements of Tricyclo[4.3.2.0]undecanones. J. Org. Chem. 1973, 38, 1218–1221. 10.1021/jo00946a034. [DOI] [Google Scholar]; c Becker D.; Nagler M.; Hirsh S.; Ramun J. Intramolecular [2 + 2] Photocycloadditions of E and Z Olefins to Cyclohex-2-enone. J. Chem. Soc., Chem. Commun. 1983, 371–373. 10.1039/c39830000371. [DOI] [Google Scholar]; d Becker D.; Nagler M.; Sahali Y.; Haddad N. Regiochemistry and stereochemistry of intramolecular [2+2] photocycloaddition of carbon-carbon double bonds to cyclohexenones. J. Org. Chem. 1991, 56, 4537–4543. 10.1021/jo00014a040. [DOI] [Google Scholar]; e Kelly J. F. D.; Kelly J. M.; McMurry T. B. H. Photochemistry of substituted cyclic enones. Part 12.1 Photocycloaddition of 3-phenylcyclopentenone and 3-phenylcyclohexenone to (E )- and (Z )-1-phenylpropene. J. Chem. Soc., Perkin Trans. 2 1999, 1933–1941. 10.1039/a902661d. [DOI] [Google Scholar]; f Brimioulle R.; Bauer A.; Bach T. Enantioselective Lewis Acid Catalysis in Intramolecular [2 + 2] Photocycloaddition Reactions: A Mechanistic Comparison between Representative Coumarin and Enone Substrates. J. Am. Chem. Soc. 2015, 137, 5170–5176. 10.1021/jacs.5b01740. [DOI] [PubMed] [Google Scholar]; g Poplata S.; Bauer A.; Storch G.; Bach T. Intramolecular [2+2] Photocycloaddition of Cyclic Enones: Selectivity Control by Lewis Acids and Mechanistic Implications. Chem.—Eur. J. 2019, 25, 8135–8148. 10.1002/chem.201901304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplata S.; Bach T. Enantioselective Intermolecular [2+2] Photocycloaddition Reaction of Cyclic Enones and Its Application in a Synthesis of (−)-Grandisol. J. Am. Chem. Soc. 2018, 140, 3228–3231. 10.1021/jacs.8b01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For related structures, see:Pete J.-P. Conformational effects and cyclodimer formation in intramolecular [2 + 2] photocycloadditions. Chem. Commun. 1998, 235–236. 10.1039/a707027f. [DOI] [Google Scholar]
- Examples:; a Eaton P. E. The Tricyclo[5.3.0.02,6]decane System. The Photodimers of Cyclopentenone. J. Am. Chem. Soc. 1962, 84, 2344–2348. 10.1021/ja00871a015. [DOI] [Google Scholar]; b Wagner P. J.; Bucheck D. J. Comparison of the Photodimerizations of 2-Cyclopentenone and of 2-Cyclohexenone in Acetonitrile. J. Am. Chem. Soc. 1969, 91, 5090–5097. 10.1021/ja01046a026. [DOI] [Google Scholar]; c Reinfried R.; Bellu D.; Schaffner K. Photochemical Reactions. Part 64 [1]. The Photochemistry of 3-Alkyl-cyclopent-2-en-ones. Helv. Chim. Acta 1971, 54, 1517–1531. 10.1002/hlca.19710540602. [DOI] [Google Scholar]; d Berenjian N.; Mayo P. D.; Sturgeon M.-E.; Sydnes L. K.; Weedon A. C. Biphasic photochemistry: micelle solutions as media for photochemical cycloadditions of enones. Can. J. Chem. 1982, 60, 425–436. 10.1139/v82-064. [DOI] [Google Scholar]; e Yang J.; Dewal M. B.; Profeta S.; Smith M. D.; Li Y.; Shimizu L. S. Origins of Selectivity for the [2+2] Cycloaddition of α,β-unsaturated Ketones within a Porous Self-assembled Organic Framework. J. Am. Chem. Soc. 2008, 130, 612–621. 10.1021/ja076001+. [DOI] [PubMed] [Google Scholar]
- A head-to-tail preference is normally expected although the selectivity is not pronounced:; a Hill E. A.; Theissen R. J.; Cannon C. E.; Miller R.; Guthrie R. B.; Chen A. T. Ring Cleavage Rearrangements of 2-Bicyclo[3.2.0]heptyl and Related Grignard Reagents. J. Org. Chem. 1976, 41, 1191–1199. 10.1021/jo00869a024. [DOI] [Google Scholar]; b Sydnes L. K.; Meling H. L.; Lundbäck K. M. O.; Pedersen E. B. Photochemical [2+2] Cycloadditions. II. Photochemical Addition of 3-Butyl-2-cyclopentenone to Some omega-Bromo-1-alkenes. Acta Chem. Scand., Ser. B 1987, 41b, 660–667. 10.3891/acta.chem.scand.41b-0660. [DOI] [Google Scholar]; c Rudolph A.; Weedon A. C. Radical clocks as probes of 1,4-biradical intermediates in the photochemical cycloaddition reactions of 2-cyclopentenone with alkenes. Can. J. Chem. 1990, 68, 1590–1597. 10.1139/v90-245. [DOI] [Google Scholar]; d Hastings D. J.; Weedon A. C. Origin of the Regioselectivity in the Photochemical Cycloaddition Reactions of Cyclic Enones with Alkenes: Chemical Trapping Evidence for the Structures, Mechanism or Formation and Fates of 1,4-Biradical Intermediates. J. Am. Chem. Soc. 1991, 113, 8525–8527. 10.1021/ja00022a052. [DOI] [Google Scholar]; e Griesbeck A. G.; Stadtmüller S.; Busse H.; Bringmann G.; Buddrus J. Stereoselektivität von Photocycloadditionen, 5[1] Photoreaktionen zwischen 2-Cyclopenten-1-on und Enolethern. Chem. Ber. 1992, 125, 933–940. 10.1002/cber.19921250427. [DOI] [Google Scholar]; f Andrew D.; Hastings D. J.; Weedon A. C. The Mechanism of the Photochemical Cycloaddition Reaction between 2-Cyclopentenone and Polar Alkenes: Trapping of Triplet 1,4-Biradical Intermediates with Hydrogen Selenide. J. Am. Chem. Soc. 1994, 116, 10870–10882. 10.1021/ja00103a003. [DOI] [Google Scholar]; g Sydnes L. K.; Van Ha D. Cyclobutanes and Cyclobutenes from Photochemical Cleavage of Some Bicyclo[3.2.0]heptan-2-ones. Aust. J. Chem. 2009, 62, 101–107. 10.1071/ch08391. [DOI] [Google Scholar]
- Ponaras A. A.; Meah M. Y. Synthesis of diosphenol ethers by means of alkoxytrimethylsilanes. Tetrahedron Lett. 1986, 27, 4953–4956. 10.1016/s0040-4039(00)85105-9. [DOI] [Google Scholar]
- Ponaras A. A. A New Variant of the Claisen Rearrangement Capable of Creating the Bond between Two Quaternary Centers. J. Org. Chem. 1983, 48, 3866–3868. 10.1021/jo00169a071. [DOI] [Google Scholar]
- Bergman R.; Magnusson G. Mechanism of Cyclopentene-1-carboxaldehyde Formation by Ring Contraction of 2,3-Epoxycyclohexanols. Improved Synthetic Procedures. J. Org. Chem. 1986, 51, 212–217. 10.1021/jo00352a015. [DOI] [Google Scholar]
- Kraft P.; Jordi S.; Denizot N.; Felker I. On the Dienone Motif of Musks: Synthesis and Olfactory Properties of Partially and Fully Hydrogenated Dienone Musks. Eur. J. Org. Chem. 2014, 554–563. 10.1002/ejoc.201301107. [DOI] [Google Scholar]
- Luengo J. I.; Koreeda M. Complete control of the rearrangement modes of enolates of.alpha.-allyloxy ketones: reversal from the [3,3]-Claisen to the [2,3]-Wittig pathway by the use of the metalated N,N-dimethylhydrazones. J. Org. Chem. 1989, 54, 5415–5417. 10.1021/jo00284a005. [DOI] [Google Scholar]
- Madelaine C.; Valerio V.; Maulide N. Unexpected Electrophilic Rearrangements of Amides: A Stereoselective Entry to Challenging Substituted Lactones. Angew. Chem., Int. Ed. 2010, 49, 1583–1586. 10.1002/anie.200906416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.