Abstract
Ethylmalonic encephalopathy (EE) is a rare metabolic disorder caused by dysfunction of ETHE1 protein, a mitochondrial dioxygenase involved in hydrogen sulfide (H2S) detoxification. EE is usually a fatal disease with a severe clinical course mainly associated with developmental delay and regression, recurrent petechiae, orthostatic acrocyanosis, and chronic diarrhoea. Treatment includes antioxidants, antibiotics that lower H2S levels and antispastic medications, which are not curative. The mutations causing absence of the ETHE1 protein, as is the case for the described patient, usually entail a severe fatal phenotype. Although there are rare reported cases with mild clinical findings, the mechanism leading to these milder cases is also unclear. Here, we describe an 11-year-old boy with an ETHE1 gene mutation who has no neurocognitive impairment but chronic diarrhoea, which is controlled by oral medical treatment, and progressive spastic paraparesis that responded to Achilles tendon lengthening.
Keywords: ETHE1 gene, H2S, Mild course, Therapy response
Abbreviations: cIII, complex III; cIV, complex IV; CAT, cysteine aminotransferase; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; EE, ethylmalonic encephalopathy; EMA, ethylmalonic acid; GSH, glutathione; H2S, hydrogen sulfide; MTZ, metronidazole; NAC, N-acetylcysteine; 3-MST, 3-mercaptopyruvate sulfurtransferase; H2SO3, persulfide; SCAD, short-chain acyl-CoA dehydrogenase; SQR, sulfide quinone oxidoreductase; SDO, sulfur dioxygenase; SUOX, sulfite oxidase; TST, thiosulfate sulfur transferase; UQ, quinone
1. Introduction
Ethylmalonic encephalopathy (EE) (MIM #602473) is an autosomal recessive severe metabolic disorder of infancy affecting the brain, gastrointestinal tract, and peripheral vessels. The disorder is characterized by neurodevelopmental delay and regression, prominent pyramidal and extrapyramidal signs, seizures, recurrent petechiae, orthostatic acrocyanosis, and chronic diarrhoea [1]. Brain magnetic resonance imaging (MRI) shows widespread necrotic lesions in deep grey matter structures [2]. The onset and degree of severity of these symptoms vary from patient to patient, even with the same mutation, but mostly occur early in development [3].
EE is characterized by an unusual combination of biochemical findings, which include persistent lactic acidaemia, elevated concentrations of C4 and C5 plasma acylcarnitine species, markedly elevated urinary excretion of ethylmalonic acid (EMA), and elevated C4–6 acylglycines, notably isobutyrylglycine and 2-methylbutyrylglycine. In 2004, the EE locus was identified by autozygosity mapping in a region on chromosome 19, and the gene was identified and termed ETHE1, for Ethylmalonic Encephalopathy gene 1 [4]. ETHE1 is a 30-kDa polypeptide located in the mitochondrial matrix, which functions as a homodimeric, Fe-containing, sulfur dioxygenase (SDO) involved in the catabolic oxidation of hydrogen sulfide (H2S) to sulfate [5].
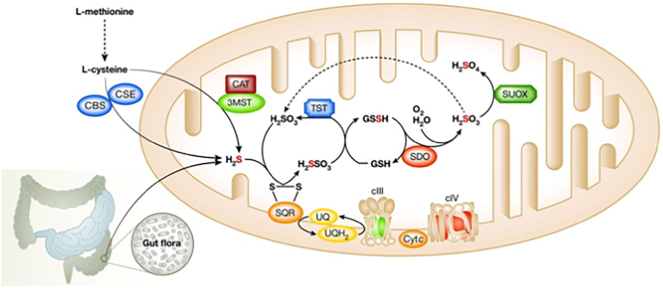
Low concentrations (< 125 μM) of H2S has many physiological functions in healthy humans, including signalling between neurons, cardioprotection, heart rate regulation, vasorelaxation, angiogenesis, and antioxidant, anti-inflammatory and cytoprotective effects in the epithelium. H2S is synthesized through cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) from cysteine, and a rather relevant amount is synthesized also by intestinal bacteria (Fig. 1). Although H2S acts in traces as a cytoprotective agent, at high concentrations, it is a powerful toxic agent that inhibits some important enzymes with antioxidative and energy-producing effects. Cytochrome c oxidase, carbonic anhydrase, and short-chain acylCoA dehydrogenase (SCAD) are the main enzymes which impairment causes clinical and laboratory findings during H2S intoxication [6].
Fig. 1.
The pathways of H2S synthesis and removal (CBS: cystathionine β-synthase; CSE: cystathionine γ-lyase; CAT: cysteine aminotransferase; 3-MST: 3-mercaptopyruvate sulfurtransferase; SQR: sulfide quinone oxidoreductase; SDO/ETHE1: sulfur dioxygenase; SUOX: sulfite oxidase; TST: thiosulfate sulfur transferase; GSH: glutathione; UQ: quinone; cIII: complex III; cIV: complex IV).
A major mitochondrial enzymatic pathway is responsible for the removal of H2S from tissues. H2S is initially fixed by a membrane-bound sulfide quinone oxidoreductase (SQR). The electrons extracted from H2S enter the respiratory chain at the level of the quinone pool (UQH/UQ), are then transferred to complex III (cIII), and are finally fixed by cytochrome c oxidase (complex IV, cIV) to molecular oxygen with the formation of water. Sulfur dioxygenase (SDO/ETHE1), present as an Fe-binding dimer in the mitochondrial matrix, oxidizes the sulfur atom of H2S fixed in a persulfide moiety (H2SO3) to sulfite (SO32−) in a reaction that includes molecular oxygen and water (Fig. 1). Sulfite is further oxidized into sulfate (SO4) by sulfite oxidase [4]. These systems are likely to have an important physiological function in maintaining the low tissue concentrations of H2S. Impaired activity of ETHE1-SDO leads to the accumulation of H2S in critical tissues, which inhibits short-chain acyl-CoA dehydrogenase (SCAD), therefore accounting for EMA aciduria and high levels of C4 and C5 acylcarnitines; COX deficiency causes diffuse hypoxic tissue damage and SCAD deficiency causes elevated lactate and EMA in plasma, both resulting in severe oxidative stress and faulty bioenergetics [5]. H2S directly damages endothelial cells and alters the vessel tone determining vasodilation, thus accounting for the petechiae, orthostatic acrocyanosis and mucosal damage with chronic diarrhoea.
2. Case report
An 11-year-old boy was referred to the hospital with spastic paraparesis and intractable diarrhoea from 5 years of age. He was the first child of consanguineous parents with an unremarkable family history. He was born at term, following an uneventful pregnancy and delivery. His birth weight was 3250 g (50th–75th percentile), and his height was 53 cm (75th–90th percentile). He was breastfed until six months after birth. During weaning, he began complaining of nutrition therapy-resistant diarrhoea. Endoscopy and colonoscopy were performed considering food allergies or malabsorption syndromes. Both were normal. He began walking at 14 months of age. He was assessed at the orthopaedics unit because of frequent falls, and metabolic tests were suggested.
Upon physical examination, the only finding was spasticity in the lower limbs. The patient had normal neurodevelopmental progression. Plasma lactate was within normal levels. Metabolic tests showed slightly elevated C5-carnitine (0.39 μmol/L; n.v. 0.31–0.01) in plasma and elevated ethylmalonic acid (EMA) (46 mmol/mol creatinine; n.v. <8.4), isovaleryl glycine (19 mmol/mol creatinine; n.v. 0), and isobutyryl glycine (10 mmol/mol creatinine; n.v. <1,6). When mild clinical findings and these metabolites were evaluated together, they suggested SCAD deficiency. Brain MR imaging was performed to reconcile the unexpected lower limb spasticity with SCAD deficiency. Brain MRI and spectroscopy results were normal. The rest of the biochemical and metabolic work-up was unremarkable. EMG did not indicate muscle or nerve involvement. We repeated the analyses because the EMA excretion was rather low compared to canonical EE and obtained the same metabolic profile. For a conclusive diagnosis, mutation analysis revealed homozygosity for a c.3G > T (p.Met-1-Ile-) mutation in the ETHE1 gene. ETHE1 gene sequence analysis was done with MiSeq next generation sequencing (NGS) platform (Illumina, San Diego, CA, USA). Genomic DNA isolation was done according to the manufacturer's standard procedure with Anatolia magnetic bead kit (Anatolia, Turkey). Since this mutation is responsible for the severe phenotype, parental genetics and functional tests were performed to confirm the diagnosis. Western blot analysis of ETHE1 indicated that the protein was virtually absent in mutant skin fibroblasts (Fig. 2) showing only a very faint band. We can't exclude the presence of a shorter ETHE1 protein starting at a second methionine at position 28, which could not be visualised with the western blot conditions utilized.
Fig. 2.
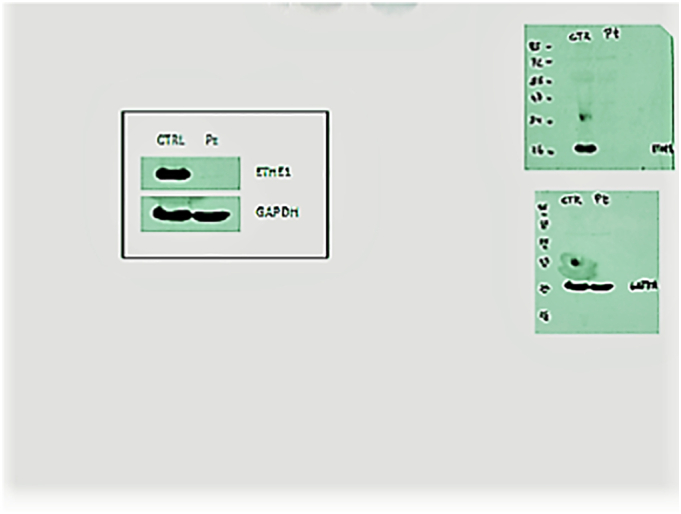
Virtual absence of ETHE1 protein in the case according to western blotting.
The patient received oral metronidazole (MTZ) and N-acetylcysteine (NAC) for 6 months. This regimen caused abdominal pain and discomfort. He was then given a supplementary probiotic agent (Saccharomyces boulardii CNCM I-745®) at the dosage 5 billion colony-forming units that limited the number of bowel discharges. To prevent oxidative damage due to H2S, coenzyme Q10 and riboflavine therapy were added to the therapeutic protocol. As a result of all these treatments, some clinical and biochemical benefits were observed as the EMA levels decreased. He never experienced a metabolic attack or encephalopathic coma (Table 1). Diarrhoea was under control, with marked amelioration of his quality of life, but spastic paraparesis progressively worsened. Lengthening of the Achilles tendons allowed him to walk without support, although muscle weakness persisted. Muscle strength was supported by regular physiotherapy, in addition to medical treatment. In the 2-year follow-up after the operation, rapid growth in height caused some deterioration in walking. However, this deterioration did not prevent him from walking without support. Currently, he has normal cognitive development with an IQ of 104, with good proficiency.
Table 1.
Clinical and laboratory findings for the case.
| 0 days | 1 month |
6 months |
1 year |
2 years |
3 years |
4 years |
|
|---|---|---|---|---|---|---|---|
| NAC-MTZ | NAC-MTZ | NAC-MTZ CoQ10-B2 | NAC-MTZ CoQ10-B2 | NAC-MTZ CoQ10-B2 | NAC-MTZ CoQ10-B2 operation | ||
| Clinic | |||||||
| Spastic paraparesis | ++ | ++ | ++ | ++ | +++ | +++ | + |
| Diarrhoea (times/day) | 15-20 | 15 | 10 | 4 | 4 | 3 | 2 |
| Laboratory | |||||||
| ethylmalonic acid (U)† | 46 | 36 | 10 | 23 | 31 | 28 | 19 |
| n.v. <8.4⁎ | |||||||
| isovaleryl glycine (U) | 19 | 16 | 16 | 3 | 1.5 | 2.4 | – |
| n.v. 0⁎ | |||||||
| isobutyryl glycine (U) | 10 | 8.4 | 6.6 | 4.1 | 2 | 3.5 | 0.12 |
| n.v.<1.6⁎ | |||||||
| methylsuccinic acid (U) | 1.6 | 0.4 | – | 0.8 | 1 | 0.16 | – |
| n.v. <4.4⁎ | |||||||
| 2-methylbutyrylglycine (U) | 0.8 | – | – | – | 0.4 | 0.21 | – |
| n.v. 0⁎ | |||||||
| C4-Carnitine (P)⁋ | 1.26 | 1.08 | 1.15 | 1.2 | 1.2 | 1.25 | 1 |
| n.v. 0-1.3⁎⁎ | |||||||
| C5-Carnitine (P) | 0.39 | 0.32 | 0.29 | 0.27 | 0.3 | 0.31 | 0.25 |
| n.v. 0.31- 0.01⁎⁎ | |||||||
| Lactate (P) | 14 | 15 | 12 | 13 | 15 | 17 | 11 |
| n.v. 4.5-19.8⁎⁎⁎ | |||||||
⁎mmol/mol creatinine; ⁎⁎μmol/L; ⁎⁎⁎mg/dl; ⁋ plasma; † urine
3. Discussion
EE is a metabolic disorder characterized by an unusual combination of specific and unique clinical phenotypes and biochemical features, leading to characteristic psychomotor retardation, chronic mucoid diarrhoea and relapsing petechiae with acrocyanosis. The biochemical profile includes lactic acidaemia, elevated plasma C4 and C5 acylcarnitines as well as C4–6 acylglycines and markedly increased urinary excretion of ethylmalonic acid. EE is caused by homozygous or compound heterozygous mutations in the ETHE1 gene, which is located on chromosome 19q13 and encodes a mitochondrial matrix sulfur dioxygenase. The association of ETHE1 gene mutations with the disease was reported by Tiranti et al. [4] in 2004. c.3G > T (p.Met-1-Ile-) mutation in the ETHE1 gene has also been identified by the tiranti as a severe mutation [5]. Since then, there has been an increase in the number of associated mutations and reported cases. No genotype-phenotype correlations have been shown with biallelic pathogenic variants to date. Clinical heterogeneity exists even in cases with the same mutation [7]. Despite the fact that the majority of typical cases have severe findings, some atypical individuals have also been reported [7,8]. It is also known that there are a few cases with mild atypical findings, whose diagnosis is often delayed until adolescence or adulthood [9].
Western blot analysis of the ETHE1 protein indicated that some of the missense mutations are associated with the presence of the protein, suggesting that the mutant amino acid impairs catalytic function [5]. Concentrations of EMA and other short-chain acylglycines were found to be lower in these mild cases than in severe cases, but it has been shown that metabolites increase rapidly in blood and urine during acute attacks. While it is understandable that there are mild clinical findings due to the presence of some detectable ETHE1 protein, it is difficult to explain the cases where the protein is absent, as in our case. The mutation in our case is a known loss-of-function change usually associated with a severe phenotype [4]. Accordingly, in our patient fibroblast cell line, the ETHE1 protein was undetectable by western blot analysis. Thus, additional genetic factors, including the activation of alternative pathways for the disposal of H2S, such as that involvement of cysteine dioxygenase, must play a compensatory role in this case. It is hard to explain why the clinical phenotype and biochemical findings are so mild when there is no synthesized ETHE1 protein. Although these three systems are said to have an equally important function in maintaining physiologically relevant tissue concentrations of H2S, the pathways may not be equally effective in each person. Interactions among antioxidant mechanisms have been reported in H2S detoxification [10].
Individual genetic differences, such as modifying genes or suppressor genes, may be other or additional influencing factors. The possibility of significant environmental factors or alternative non-ETHE1-related genetic factors influencing mutant gene expression must be considered. Another explanation is that the amount of ETHE1-protein produced can differ among tissues. The important difference in this case compared with other cases is that there was no pathology detected by brain MRI, whereas in other cases with milder symptoms, typical signs of brain disease were observed by MRI.
Treatment is mostly supportive, including antioxidants, antispastic medications, muscle relaxants, and antiepileptic drugs [11,12]. Among these drugs, NAC and metronidazole are prominent in treatment due to their H2S-reducing effects. NAC is a cell-permeable precursor of glutathione (GSH), an abundant compound in mitochondria, where it acts as one of the physiological acceptors of the sulfur atom of H2S via SQR [10,13,14]. Metronidazole is an antibiotic widely used to combat anaerobic infections by reducing the bacterial load in the large intestine, which is a major source of H2S [15]. The beneficial effects of both agents have been shown in most of the affected cases [10,14], but to date, curative treatment has not been achieved. Antioxidant cocktails have been tested, but their benefit is controversial [16] despite the observation that an untargeted metabolic approach on EE fibroblasts demonstrated an alteration of the redox state [17]. In our case, we observed laboratory and clinical benefits of such a cocktail when given in addition to NAC and metronidazole. Gene therapy and liver transplant, reported in some publications, are not routine treatments, and their effects encouraging, but the numbers of cases is insufficient to prove unequivocally their efficacy [18,19]. In addition to chronic treatments, cases that benefit from continuous dialysis renal replacement therapy (CRRT) in acute metabolic coma have been reported [9]. The Achilles tendon lengthening operation, performed on our patient to reduce the progressive lower limb spasticity that occurred despite medical treatments, improved his quality of life. This interventional method is suggested to improve the quality of life, especially in cases with good neurocognitive development.
4. Conclusion
Ethylmalonic encephalopathy can occasionally occur with a late onset and mild clinical symptoms, in addition to the severe clinical presentation that is more frequently observed. The metabolic profile may be subtle and thus create difficulty in the diagnosis of these mild cases. It can be argued that the response of these cases to antioxidant treatments is more obvious than that of more severe cases, and their prognosis is better. Our case allowed us to evaluate and discuss an unusually mild presentation of ethylmalonic encephalopathy, despite the absence of ETHE1 protein in functional tests.
Author contributions
ME: Data analysis and interpretation and planning and drafting of most of the article.
VT: Data analysis and interpretation and article contribution and revision.
MZ: Data analysis and interpretation and planning and drafting of the article.
Funding
This work did not receive any funding.
Patient consent
The patient and his parents provided informed consent for publication of this case report.
Ethical standards
The authors declare that the experiments complied with the current laws of Canada, the
country in which they were performed.
Declaration of Competing Interest
The authors have no competing interests to declare.
Contributor Information
Melike Ersoy, Email: zeynepcey@hotmail.com.
Valeria Tiranti, Email: Valeria.Tiranti@istituto-besta.it.
Massimo Zeviani, Email: massimo.zeviani@unipd.it.
References
- 1.Chen X., Han L., Yao H. Novel compound heterozygous variants of ETHE1 causing ethylmalonic encephalopathy in a Chinese patient: A case report. Front. Genet. 2020;11:341. doi: 10.3389/fgene.2020.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavasoli A.Z., Rostami P., Ashrafi M.R., Karımzadeh P. Neurological and vascular manifestations of ethylmalonic encephalopathy. Iran J. Child Neurol. 2017;11:57–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Pigeon N., Campeau P.M., Cyr D., Lemieux B., Clarke J.T.R. Clinical heterogeneity in ethylmalonic encephalopathy. J. Child Neurol. 2009;24:991–996. doi: 10.1177/0883073808331359. [DOI] [PubMed] [Google Scholar]
- 4.Tiranti V., D’Adamo P., Briem E., Ferrari G., Mineri R., Lamantea E., Mandel H., Balestri P., Garcia-Silva M.T., Vollmer B., Rinaldo P., Hahn S.H., Leonard J., Rahman S., Dionisi-Vici C., Garavaglia B., Gasparini P., Zeviani M. Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am. J. Hum. Genet. 2004;74:239–252. doi: 10.1086/381653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiranti V. ETHE1 mutations are specific to ethylmalonic encephalopathy. J. Med. Genet. 2006;43:340–346. doi: 10.1136/jmg.2005.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Meo I., Lamperti C., Tiranti V. Mitochondrial diseases caused by toxic compound accumulation: from etiopathology to therapeutic approaches. EMBO Mol. Med. 2015;7:1257–1266. doi: 10.15252/emmm.201505040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Rocco M., Caruso U., Briem E., Rossi A., Allegri A.E.M., Buzzi D., Tiranti V. A case of ethylmalonic encephalopathy with atypical clinical and biochemical presentation. Mol. Genet. Metab. 2006;89:395–397. doi: 10.1016/j.ymgme.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Heberle L.C., Al Tawari A.A., Ramadan D.G., Ibrahim J.K. Ethylmalonic encephalopathy—report of two cases. Brain Dev. 2006;28:329–331. doi: 10.1016/j.braindev.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Kitzler T.M., Gupta I.R., Osterman B., Poulin C., Trakadis Y., Waters P.J., Buhas D.C. Acute and chronic management in an atypical case of ethylmalonic encephalopathy. JIMD Rep. 2018;45:57–63. doi: 10.1007/8904_2018_136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luna-Sánchez M., Hidalgo-Gutiérrez A., Hildebrandt T.M., Chaves-Serrano J., Barriocanal-Casado E., Santos-Fandila Á., Romero M., Sayed R.K.A., Duarte J., Prokisch H., Schuelke M., Distelmaier F., Escames G., Acuña-Castroviejo D., López L.C. CoQ deficiency causes disruption of mitochondrial sulfide oxidation, a new pathomechanism associated with this syndrome. EMBO Mol. Med. 2016;9:78–95. doi: 10.15252/emmm.201606345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viscomi C., Burlina A.B., Dweikat I., Savoiardo M., Lamperti C., Hildebrandt T., Tiranti V., Zeviani M. Combined treatment with oral metronidazole and N-acetylcysteine is effective in ethylmalonic encephalopathy. Nat. Med. 2010;16:869–871. doi: 10.1038/nm.2188. [DOI] [PubMed] [Google Scholar]
- 12.Mineri R., Rimoldi M., Burlina A.B., Koskull S., Perletti C., Heese B., von Dobeln U., Mereghetti P., Di Meo I., Invernizzi F., Zeviani M., Uziel G., Tiranti V. Identification of new mutations in the ETHE1 gene in a cohort of 14 patients presenting with ethylmalonic encephalopathy. J. Med. Genet. 2008;45:473–478. doi: 10.1136/jmg.2008.058271. [DOI] [PubMed] [Google Scholar]
- 13.Garone C., Viscomi C. Towards a therapy for mitochondrial disease: an update. Biochem. Soc. Trans. 2018;46:1247–1261. doi: 10.1042/bst20180134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiranti V., Zeviani M. Altered sulfide (H2S) metabolism in ethylmalonic encephalopathy. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 1999;43:1533–1541. doi: 10.1128/aac.43.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiner G., Barca E., Ziosi M., Emmanuele V., Xu Y., Hidalgo-Gutierrez A., Qiao C., Tadesse S., Area-Gomez E., Lopez L.C., Quinzii C.M. CoQ10 supplementation rescues nephrotic syndrome through normalization of H2S oxidation pathway. Biochim. Biophys. Acta Mol. basis Dis. 2018;1864:3708–3722. doi: 10.1016/j.bbadis.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahebekhtiari N., Nielsen C.B., Johannsen M., Palmfeldt J. Untargeted metabolomics analysis reveals a link between ETHE1-mediated disruptive redox state and altered metabolic regulation. J. Proteome Res. 2016 May 6;15(5):1630–1638. doi: 10.1021/acs.jproteome.6b00100. [DOI] [PubMed] [Google Scholar]
- 18.Di Meo I., Auricchio A., Lamperti C., Burlina A., Viscomi C., Zeviani M. Effective AAV-mediated gene therapy in a mouse model of ethylmalonic encephalopathy. EMBO Mol. Med. 2012;4:1008–1014. doi: 10.1002/emmm.201201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dionisi-Vici C., Diodato D., Torre G., Picca S., Pariante R., Giuseppe Picardo S., Di Meo I., Rizzo C., Tiranti V., Zeviani M., De Goyet J.D.V. Liver transplant in ethylmalonic encephalopathy: a new treatment for an otherwise fatal disease. Brain. 2016;139:1045–1051. doi: 10.1093/brain/aww013. [DOI] [PubMed] [Google Scholar]