Abstract
Background
Due to its rarity, few studies have characterized the epidemiology of male breast cancer. The purpose of this study was to determine survival and risk factors for male breast cancer in a large U.S. population.
Methods
In this study, 19,795 male patients with breast cancer were identified from the National Cancer Database (2004–2014). Patient demographics, tumor characteristics and treatments were analyzed by using descriptive statistics. We used multivariate Cox regression and Kaplan Meier analysis.
Results
Over 10 years, the incidence of male breast cancer increased from 7.2% to 10.3%, while mortality decreased from 11% to 3.8%. Socioeconomic factors predicting mortality included income medium, and high vs low (HR = 0.78; 0.68), private vs no insurance (HR = 0.73) and the academic research facility vs community cancer center (HR = 0.79). Significant predictors of all-cause mortality included age (HR = 1.04), tumor size (HR = 1.01), hormone receptor expression (HR = 0.8) and cancer stage I vs II, III, and IV at the time of diagnosis (HR = 1.5, 2.7, 4.4, 9.9 respectively). Other predictors of mortality include surgery (HR = 0.4), chemotherapy (HR = 0.8), radiation (HR = 0.8), and hormonal therapy (HR-0.8).
Conclusions
Socioeconomic factors, cancer stage, tumor characteristics (size and grade), and high Charlson-Dayo score contributed to higher mortality among male patients diagnosed with breast cancer. Surgery was most effective, followed by radiation, chemotherapy, and hormonal therapy. Patients with positive ER or PR expression demonstrated better survival. Adjusting for socioeconomic factors, biomarker identification and timely, appropriately chosen treatment are likely to reduce the risk for mortality.
Keywords: Male breast, Survival, Epidemiology, Mortality, NCBD database
Highlights
-
1
Mortality was higher among patients with Low income and with no insurance.
-
2
Patients with positive ER or PR expression demonstrated better survival.
-
3
Surgery, chemotherapy, radiation, and hormonal therapy improved survival.
Introduction
Breast cancer is a common cancer in women but relatively rare in men, with male breast cancers accounting for less than 1% of all diagnosed cases [1]. According to Surveillance, Epidemiology, and End Results (SEER) data, male breast cancer incidence rose by 40% from 1975 to 2015, exceeding that of women by 25% [2]. The American Cancer Society estimated that in 2019, 2670 new cases of male breast cancer would be diagnosed in the United States, with 18% mortality [3].
Male breast cancer is not well understood. There is some evidence that male breast cancers are more likely to express the estrogen (ER) or androgen receptors than female and less likely to overexpress HER2 [4], [5]. However, studies to characterize male breast cancers or correlate prognostic factors with treatment outcomes are limited and, when available, underpowered [[5], [6], [7]]. Therefore, there is an urgent need to understand the risk factors associated with the disease. Here, we report the results of a retrospective study of NCDB data from 2004 to 2014. The purpose of this study was to determine risk and survival factors in men with breast cancer in a large U.S. population.
Methods
The data used in the study was derived from a de-identified National Cancer Database (NCDB) file. NCDB is an oncology outcomes database with more than 1500 U S. cancer programs accredited by the Commission on Cancer, a joint program of the American College of Surgeons and American Cancer Society.
The NCDB file consisted of 2,211,245 patients diagnosed with breast cancer from 2004 to 2014. Female patients (2,191,450; 99.1%) and any male patients for whom follow-up data or mortality status was missing were excluded from the analysis, leaving a study population of male breast cancer to be 19,795 (0.9%). All categorical and continuous variables were described using appropriate descriptive statistics, ie. frequency and percentage for categorical variables, mean with standard deviation for continuous numeric variables. For survival analysis, Kaplan Meier was used to show comparative survival between 2 or more groups. Multivariate Cox regression was used to determine hazard ratios (HR) predicting mortality. For all statistical tests, an alpha of 0.05 was used for statistical comparison, and statistical analysis was performed using SAS software (Version 9.4, SAS Institute Inc., Cary, NC). Information with relation to tumor recurrence and cause of the death was not available in this dataset. Therefore, only all-cause and not cancer-related mortality was analyzed. Detailed pathologic and treatment information and lifestyle/comorbidity information is not available through the database.
Results and discussion
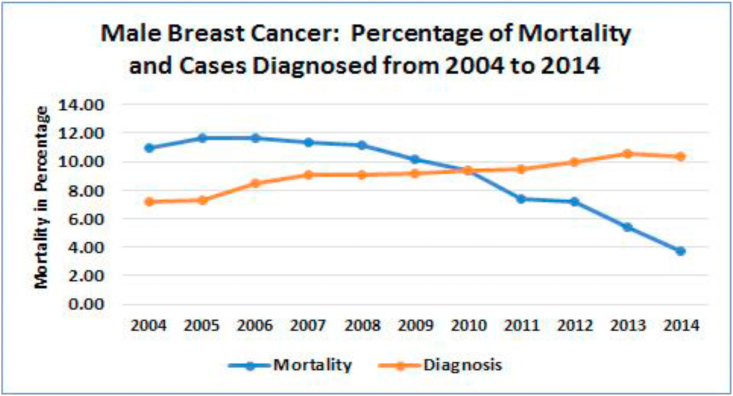
In general, male patients account for less than 1% of all patients with breast cancer [[8], [9], [10]], which is consistent with the SEER data base [4], suggesting that there is no bias in the selection process (19,795 or 0.9%). In the NCDB dataset, a little over seven percent (7.2%) of the cases were diagnosed in 2004 increasing steadily to reach 10.3% by 2014 (Fig. 1 orange line). The rate of all-cause mortality across the same time frame dropped from 11% in 2004 to 3.8% by 2014 (Fig. 1 blue line). However, overall mortality rate in same period remained significantly higher for men (27.2%) than women (17.4%) diagnosed with breast cancer (p < 0.001).
Fig. 1.
Percentage of the cases for male breast cancer per year out of total patients diagnosed with breast cancer from 2004 through 2014 (N = 19,795). Rates of diagnosis and mortality in male breast cancer. Orange line: Total cases of male breast cancer were graphed from 2004 to 2014. Blue line: Mortality rates for male breast cancer was calculated for the same time frame.
Patient demographics
Most of the cases self-identified as non-Hispanic white (75% as NHW), 12% as non-Hispanic black race (NHB), and 0.5% as Hispanic (Table 1 Patient Characteristics). The remaining 12% of patients were classified as Asian, Pacific Islander, American Indian, Aleutian or Eskimo. Mortality rates were highest in NHB (29%), then NHW (27%), Hispanic men (21%) and 25% among other races. The mean age was 64.6 ± 13.1 years. The distribution of mortality rates for median income, insurance status, location, health facility type, cancer stage and Charlson-Deyo score are all described in Table 1. Most patients lived in a metro area and had access to insurance.
Table 1.
Descriptive statistics for the incidences of male breast cancer from national cancer database.
| Patient characteristics | Total | Dead | Alive | P-Value | |||
|---|---|---|---|---|---|---|---|
| Age (Mean, SD) | 65 | 13.1 | 70 | 13.6 | 62 | 12.37 | <0.0001 |
| Race (N, %) | 0.0050 | ||||||
| Hispanic | 99 | 0.5 | 21 | 0.4 | 78 | 0.5 | |
| Non-Hispanic Black | 2387 | 12.1 | 687 | 12.8 | 1700 | 11.8 | |
| Non-Hispanic White | 14,408 | 74.8 | 4061 | 75.4 | 10,743 | 74.6 | |
| Other | 2505 | 12.7 | 620 | 11.5 | 1885 | 13.1 | |
| Median Income (N, %) | <0.0001 | ||||||
| < $37,999 | 3131 | 16.0 | 1015 | 19.2 | 2116 | 14.7 | |
| $38,000–47,999 | 4097 | 20.9 | 1231 | 23.3 | 2866 | 19.9 | |
| $48,000–67,999 | 5111 | 26.1 | 1366 | 25.9 | 3745 | 26.0 | |
| ≥ $68,000 | 7229 | 36.9 | 1670 | 31.6 | 5559 | 38.6 | |
| Location (N, %) | 0.0425 | ||||||
| Metro | 16,671 | 87.0 | 4436 | 86.0 | 12,235 | 87.4 | |
| Rural | 266 | 1.4 | 78 | 1.5 | 188 | 1.3 | |
| Urban | 2220 | 11.6 | 643 | 12.5 | 1577 | 11.3 | |
| Insurance (N, %) | |||||||
| Medicare/Medicaid | 10,272 | 51.9 | 3709 | 68.8 | 6563 | 45.6 | |
| Private | 8601 | 43.5 | 1417 | 26.3 | 7184 | 49.9 | |
| Non-insured/Unknown | 922 | 4.7 | 263 | 4.9 | 659 | 4.6 | |
| Facility (N, %) | <0.0001 | ||||||
| Community Cancer Program | 2374 | 12.4 | 691 | 13.1 | 1683 | 12.1 | |
| Comprehensive Comm Cancer Program | 9100 | 47.3 | 2597 | 49.1 | 6503 | 46.7 | |
| Academic/Research | 5571 | 29.0 | 1367 | 25.9 | 4204 | 30.2 | |
| Integrated Network Cancer Program | 2183 | 11.4 | 633 | 12.0 | 1550 | 11.1 | |
| Region (N, %) | 0.8670 | ||||||
| East | 9136 | 33.0 | 2519 | 47.6 | 6617 | 47.5 | |
| Central | 7524 | 54.0 | 2074 | 39.2 | 5450 | 39.1 | |
| West | 2256 | 13.0 | 695 | 13.1 | 1873 | 13.4 | |
| Charlson-Deyo Score (N, %) | <0.0001 | ||||||
| 0 | 15,797 | 79.8 | 3734 | 69.3 | 12,063 | 83.7 | |
| 1 | 3067 | 15.5 | 1139 | 21.1 | 1928 | 13.4 | |
| 2 | 712 | 3.6 | 377 | 7.0 | 335 | 2.3 | |
| ≥ 3 | 219 | 1.1 | 139 | 2.6 | 80 | 0.6 | |
| TUMOR CHARACTERISTICS | |||||||
| Tumor size (Mean, SD) | 42.76 | 135.7 | 49 | 137.5 | 40.4 | 134.9 | 0.0001 |
| Stage at Diagnosis (N, %) | <0.0001 | ||||||
| 0 | 1978 | 13.9 | 209 | 5.8 | 1769 | 16.8 | |
| I | 5224 | 36.8 | 843 | 23.2 | 4381 | 41.5 | |
| II | 4601 | 32.4 | 1248 | 34.4 | 3353 | 31.8 | |
| III | 1286 | 9.1 | 525 | 14.5 | 761 | 7.2 | |
| IV | 1095 | 7.7 | 808 | 22.2 | 287 | 2.7 | |
| Tumor Grade (N, %) | <0.001 | ||||||
| Undifferentiated | 130 | 0.7 | 51 | 1.1 | 79 | 0.6 | |
| Poorly differentiated | 5980 | 34.2 | 1975 | 41.7 | 4005 | 31.5 | |
| Moderately differentiated | 8632 | 49.4 | 2222 | 47.0 | 6410 | 50.4 | |
| Well differentiated | 2721 | 15.6 | 484 | 10.2 | 2237 | 17.6 | |
| Site of Tumor (N, %) | <0.0001 | ||||||
| Primary | 14,861 | 75.1 | 3598 | 66.8 | 11,263 | 78.2 | |
| Not primary | 4931 | 24.9 | 1789 | 33.2 | 3142 | 21.8 | |
| Invasiveness (N, %) | <0.0001 | ||||||
| Carcinoma in situ/in situ | 2544 | 12.8 | 278 | 5.2 | 2266 | 15.7 | |
| Invasive | 17,251 | 87.2 | 5111 | 94.8 | 12,140 | 84.3 | |
| Breast Location (N, %) | <0.0001 | ||||||
| Left | 10,365 | 52.4 | 2822 | 52.4 | 7543 | 52.4 | |
| Right | 9291 | 46.9 | 2481 | 46.0 | 6810 | 47.3 | |
| Bilateral/unknown | 139 | 0.7 | 86 | 1.6 | 53 | 0.4 | |
| Estrogen Receptor (ER) (N, %) | 0.0001 | ||||||
| Positive | 16,609 | 91.7 | 4403 | 90.4 | 12,206 | 92.2 | |
| Negative | 1504 | 8.3 | 467 | 9.6 | 1037 | 7.8 | |
| Progesterone Receptor (PR) (N, %) | <0.0001 | ||||||
| Positive | 14,792 | 82.9 | 3778 | 78.8 | 11,014 | 84.4 | |
| Negative | 3060 | 17.1 | 1017 | 21.2 | 2043 | 15.7 | |
| HER2a | 0.1669 | ||||||
| Positive Negative |
1001 6879 |
12.7 87.3 |
204 1276 |
13.8 86.2 |
797 5603 |
12.5 87.5 |
|
| TREATMENT MODALITIES (N, %) | |||||||
| Surgery | <0.0001 | ||||||
| Underwent surgery | 17,938 | 90.6 | 4372 | 81.2 | 13,566 | 94.2 | |
| No surgery | 1857 | 9.4 | 1017 | 18.9 | 840 | 5.8 | |
| Chemotherapy (N, %) | <0.0001 | ||||||
| Received chemotherapy | 6943 | 36.6 | 1764 | 33.9 | 5179 | 37.6 | |
| No chemotherapy | 12,048 | 63.4 | 3435 | 66.1 | 8613 | 62.5 | |
| Radiation (N, %) | <0.0001 | ||||||
| Received radiation | 6647 | 34.1 | 1622 | 30.5 | 5025 | 35.5 | |
| No radiation | 12,826 | 65.9 | 3695 | 69.5 | 9131 | 64.5 | |
| Hormonal Therapy (N, %) | 0.0191 | ||||||
| Received hormonal therapy | 5119 | 26.7 | 1340 | 25.5 | 3779 | 27.2 | |
| No hormonal therapy | 14,058 | 73.3 | 3920 | 74.5 | 10,138 | 72.9 | |
| Immunotherapy (N, %) | <0.0001 | ||||||
| Received immunotherapy | 216 | 1.1 | 30 | 0.6 | 186 | 1.3 | |
| No immunotherapy | 19,248 | 98.9 | 5274 | 99.4 | 13,974 | 98.7 | |
HER2 data was only available from 2010 through 2014. In the NCBD database HER2 was marked as “CS Site-Specific Factor 15: HER2: Summary Result of Testing”.
Tumor characteristics
Tumor data including size, stage at diagnosis, tumor grade, site of the tumor and invasiveness were collected (Table 1 Tumor Characteristics). Mean tumor size was 42.8 ± 135.7 mm, with most tumors falling into the moderately differentiated category (49.4%). Most tumors were primary (75.1%) lesions and invasive (87.2%). In addition, estrogen receptor (ER) and progesterone receptor (PR), HER2 status was reported, with most patients expressing ER and (or) PR (91.7% and 82.9%, respectively).
Treatments
Data was available for patients who received surgery, chemotherapy, radiation, hormonal therapy or immunotherapy (Table 1 Treatment Modalities). The vast majority of patients underwent surgery (90.6%) but did not receive chemotherapy (63.4%) or radiation (65.9%). The rate of patients which received hormonal therapy was 26.7% while the rate of patients who received immunotherapy was 1.1%.
The comparison of survival probability showed that overall survival probability was lower for male patients compared to female patients (log rank p < 0.001). The 5 and 10-year survival percentage was 85% and 71% for female patients and 75% and 56% for male patients, respectively. Similarly, in the matched cohort (13,011 male and 13,011 female) five-year and 10-year survival for female patients was 79% and 60% whereas in male patients it was 73% and 54% respectively (p < 0.001). Overall median survival for female was 13.2 years vs 11.4 years for male patients.
Multivariate Cox regression & Kaplan Meier analysis
Multivariate Cox regression was performed, and the data is shown in Table 2. In addition, Kaplan Meier survival probabilities were calculated. In our study, increasing age and tumor size were among the independent factors affecting mortality. Patients who were diagnosed at an older age had reduced survival (HR = 1.04, p < 0.0001) as well as those whose tumors were larger at diagnosis (HR = 1.01, p < 0.001). These findings are similar to those of previously reported, albeit smaller studies [11].
Table 2.
Multivariate Cox Regression: Predictors of mortality for male patients diagnosed with breast cancer.
| Parameter | Reference | Hazard Ratio | HR 95% Lower CI |
HR 95% Higher CI |
P Value |
|---|---|---|---|---|---|
| Patient Characteristics | |||||
| Age | 1.04 | 1.035 | 1.044 | <0.0001 | |
| Median Income | |||||
| $38,000-$47,999 | < $38.000 | 0.893 | 0.792 | 1.007 | 0.0644 |
| $48,000-$67,999 | < $38.000 | 0.778 | 0.691 | 0.876 | <0.0001 |
| ≥ $68,000 | < $38.000 | 0.682 | 0.609 | 0.765 | <0.0001 |
| Insurance | |||||
| Medicare/Medicaid | None | 0.936 | 0.762 | 1.151 | 0.5326 |
| Private | None | 0.733 | 0.594 | 0.905 | 0.0038 |
| Facility | |||||
| Academic/Research Program | Community Cancer | 0.791 | 0.692 | 0.904 | 0.0006 |
| Comprehensive Community | Community Cancer | 0.935 | 0.829 | 1.053 | 0.2685 |
| Integrated Network Cancer | Community Cancer | 0.919 | 0.785 | 1.076 | 0.2954 |
| Charlson-Deyo Score | |||||
| 1 | 0 | 1.582 | 1.438 | 1.74 | <0.0001 |
| 2 | 0 | 2.672 | 2.29 | 3.117 | <0.0001 |
| ≥3 | 0 | 2.833 | 2.258 | 3.554 | <0.0001 |
| TUMOR CHARACTERISTICS | |||||
| Tumor size | 1.001 | 1 | 1.001 | <0.001 | |
| Stage at Diagnosis | |||||
| I | Stage 0 | 1.54 | 1.214 | 1.953 | 0.0004 |
| II | Stage 0 | 2.734 | 2.16 | 3.46 | <0.0001 |
| III | Stage 0 | 4.481 | 3.479 | 5.773 | <0.0001 |
| IV | Stage 0 | 9.893 | 7.637 | 12.816 | <0.0001 |
| Tumor Grade | |||||
| Moderately differentiated | Undifferentiated | 0.398 | 0.254 | 0.625 | <0.0001 |
| Poorly differentiated | Undifferentiated | 0.539 | 0.343 | 0.846 | 0.0072 |
| Well differentiated | Undifferentiated | 0.341 | 0.214 | 0.543 | <0.0001 |
| Site of Tumor: Primary | Not primary | 0.816 | 0.75 | 0.888 | <0.0001 |
| Location | |||||
| Left | Bilateral | 1.041 | 0.43 | 2.519 | 0.9298 |
| Right | Bilateral | 1.183 | 0.489 | 2.865 | 0.7092 |
| Hormone Receptor Status | |||||
| ER positive | Negative | 0.822 | 0.69 | 0.978 | 0.0269 |
| PR positive | Negative | 0.821 | 0.725 | 0.929 | 0.0018 |
| Treatment | |||||
| Surgery | None | 0.415 | 0.363 | 0.474 | <0.0001 |
| Chemotherapy | None | 0.772 | 0.706 | 0.845 | <0.0001 |
| Radiation | None | 0.77 | 0.698 | 0.85 | <0.0001 |
| Hormonal therapy | None | 0.786 | 0.718 | 0.86 | <0.0001 |
Five and 10-year survival rates.
Tumor stage carried the highest risk of mortality in this dataset, with HRs of 1.54, 2.734, 4.481, 9.893 (p < 0.001) for patients diagnosed with cancers at stages I, II, III and IV, respectively, compared to stage 0. Similarly, mortality increased with increasing Charlson-Deyo scores. HRs of 1.582 for a score of 1, 2.672 for score 2, and an HR of 2.833 for patients with scores of ≤3 (p < 0.001; Table 2).
On the other hand, primary cancers carried an HR of 0.816 (p < 0.0001) compared with breast cancers in a secondary site. Patients with poorly, moderately, or well-differentiated tumors also showed decreasing mortality compared with undifferentiated tumors (HR = 0.539, 0.398, 0.341, respectively; p < 0.001).
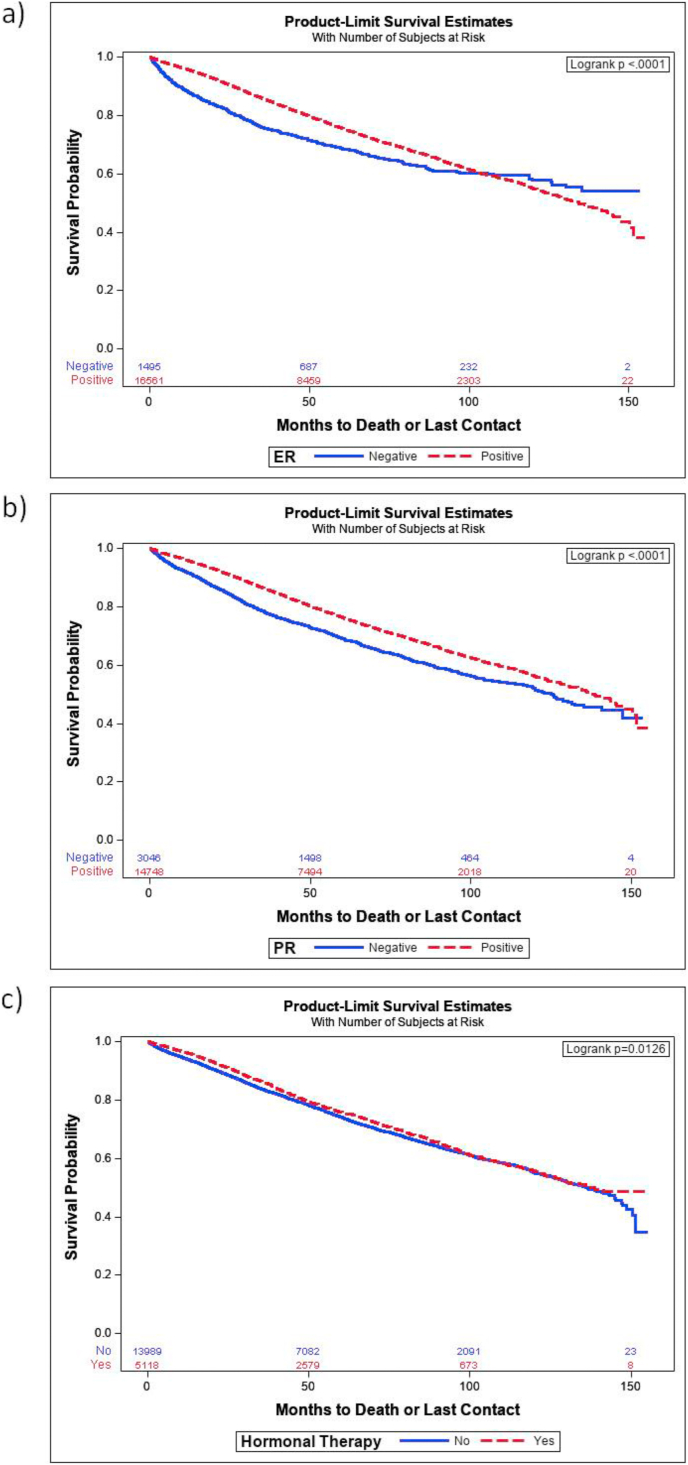
A higher proportion of ER and PR positivity was detected in male (91.8%, 83.2%) compared to female (81.2%, 70.7%) patients (p < 0.001) in this dataset and for male patients expression of either resulted in lower all-cause mortality than patients diagnosed with tumors which did not express ER or PR (HR = 0.82 for both, p < 0.05) (Table 2 Tumor Characteristics). Kaplan Meier survival curves demonstrated that patients with either ER (Fig. 3a) or PR expression (Fig. 3b) had higher probabilities of survival (p < 0.0001). Fig. 3a show the difference in survival probability of ER positive vs ER negative (p < 0.0001). Similarly, Fig. 3b shows the difference in survival probability of PR positive vs PR negative p < 0.0001). Fig. 3c shows the difference in survival of patients who received hormonal therapy vs who did not receive it (p = 0.0126).
Fig. 3.
Survival probabilities for male breast cancer patients whose tumors expressed ER or PR and for those who received hormonal therapy. (a) Patients whose tumors were ER positive (red line) compared with those who tumors were ER negative (blue line). (b) Patients whose tumors expressed PR (red line) compared with those whose tumors were PR negative (blue line). Dx, diagnosis. (c) Patients who received hormonal therapy were compared with those who did not receive hormonal therapy. Dx: diagnosis. p-values are shown in the upper, right-hand corner of each graph.
When we stratified ER positive and negative by the presence of hormonal therapy. The results show that there are no survival differences with hormonal therapy for ER positive (p = 0.0826) as well as for ER negative (p = 0.3923). In a similar patient population from M.D. Anderson Cancer Center (Houston, TX), ER- and PR-positive tumors comprised 85% and 71% of male cases [12]. Despite the high proportion of ER- and PR-positive disease in male breast cancer patients, fewer men than women (ER: 31.5% vs 34.4%; p < 0.001; PR: 31.8% vs 34.8%, p < 0.001) received adjuvant endocrine therapy, suggesting that either there is poor compliance or male patients are not exposed to currently available treatments. Several studies have reported that males are also less likely to overexpress HER2 [13,14]. HER2 expression data from the NCDB was only available from 2010 through 2014 and in this study HER2 was used from breast cancer specific factor 15.
However, when this was analyzed, the results showed that HER2 expression on tumors was indeed associated with higher mortality. For example, there were 9.8% male and 13.3% female patients with HER2 positive expression and within this population mortality rate was 20.8% among male and 11.8% among female patients.
Previous studies using national databases showed that race, insurance, income, and facility type were all independent factors for mortality among male breast cancer patients [9,15]. In our study, using stepwise Cox regression, we found income, insurance and facility type to be independently associated with mortality. All-cause mortality was lower among patients in medium- ($48,000-$67,999) or high-income (>$68,000) groups as well as those with private insurance (HR = 0.733, p = 0.0038). Patients with Medicare or Medicaid insurance had an HR of 0.936, but this did not reach statistical significance (p = 0.5326). In addition, patients treated in academic/research facilities showed lower all-cause mortality compared with Community Cancer facilities (HR = 0.791, p = 0.006).
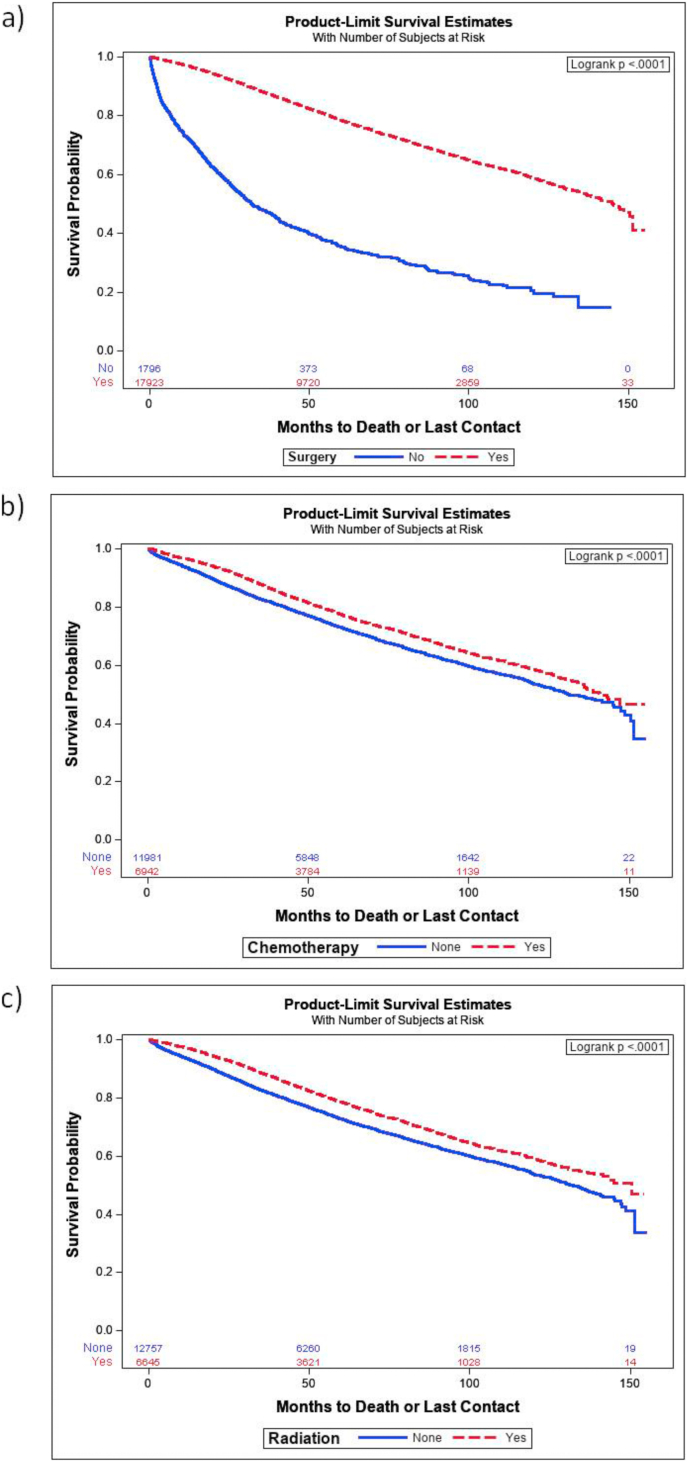
Previous studies have reported improved survival in male patients with breast cancer who receive surgery, chemotherapy, radiation and hormonal therapy [16,17]. We investigated survival rates using multivariate Cox regression and Kaplan Meier survival probabilities. All-cause mortality was lower for patients who underwent surgery, with an HR of 0.415 (p < 0.0001) and there was a significant increase in Kaplan Meier survival (p < 0.001, Fig. 2a). Chemotherapy and radiation treatments reduced all-cause mortality, with HRs of 0.77 and 0.79, respectively (p < 0.0001). Kaplan Meier survival curves showed that both chemotherapy and radiation treatment increased survivals up to 150 months. In our study population, radiation was part of the treatment for approximately 65% of the cases and was found to be an independent predictor for survival, thus radiation remains an important treatment strategy for male breast cancer [18].
Fig. 2.
Kaplan Meier survival probability of male breast cancer patients who received either surgery, chemotherapy or radiation following initial diagnosis. The numbers of patients in each category are shown on the bottom of each graph. (a) Survival probabilities for patients who underwent surgery were calculated. The solid blue line is survival of patients who did not receive surgery and the red, dashed line is of the patients who underwent surgery. (b) Survival curves for patients who received chemotherapy treatment (red line) vs. those who did not receive chemotherapy (blue line). (c) Survival curves for patients who received radiation treatment (red line) comparing those who did not (blue line). Dx: diagnosis. p-values are shown in the upper, right-hand corner of each graph.
Discussion
The number of diagnosed cases of male breast cancer increased over the past 2 decades, which highlights the need for raising awareness of this disease in the community. Diagnosis of biomarkers such as ER, PR, and HER2 through early screening could guide clinicians for better prognosis and outcomes. In fact, some studies have suggested that more male breast cancer patients are diagnosed with advanced disease compared to women due to lack of awareness of screening for breast cancer in men [12,19]. In addition, there is a lack of randomized trials aimed at patients with male breast cancer. Though male breast cancer is different than female breast cancer, some of the molecular markers are present in both sexes. Large multicenter trials are required to understand the disease and determine the most effective therapies. Additional information related to treatment, biological attributes, and lifestyle (e.g., smoking, drinking, body mass index) could be assessed to develop treatment tailored for men with breast cancer. Early detection along with a comprehensive treatment strategy consisting of surgery, hormonal therapy or combination therapies would improve survival of male patients with breast cancer.
Conclusions
The data from present study suggests disparity among male and female patients. Male breast cancer is relatively rare but had lower survival and higher mortality. While the rate of mortality has been falling, there is still more work to be done to adequately provide early screening and treatment to improve prognosis. Socioeconomic status may be one of the reasons that men with breast cancer do less well than women. However, even after matching for the income level, insurance status and for facility type we found that survival was better for female compared to male patients. However, there is also a possibility of late detection of biomarkers for male patients compared to female patients.
In this study we identified factors for both risk and survival for male breast cancer patients. Most patients underwent surgery positively impacted survival, and mortality is reduced in men who received chemotherapy, radiation, or hormonal therapy, compared to men who did not receive either of these treatments. All-cause mortality was significantly lower in men whose tumors expressed ER or PR, but these men often did not receive treatment beyond surgery, even though hormonal therapy for treatment demonstrated a reduced probability of mortality. Further studies need to be done to determine specific reasons for the disparity in care.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Advocate Aurora Healthcare Foundation partially funded this project.
Declaration of competing interest
None.
Acknowledgement
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB files. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. The authors are indebted to Dr Jennifer Jacob for her editorial assistance and for formatting the final manuscript.
References
- 1.Miao H., Verkooijen H.M., Chia K.S. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29(33):4381–4386. doi: 10.1200/JCO.2011.36.8902. [DOI] [PubMed] [Google Scholar]
- 2.Noone A.M., Howlader N., Krapcho M. SEER web site. National Cancer Institute; Bethesda, MD: April 2018. SEER cancer statistics review; pp. 1975–2015.https://seercancergov/csr/1975_2015/ Based on November 2017 SEER data submission. [Google Scholar]
- 3.DeSantis C.E., Ma J., Gaudet M.M. Breast cancer statistics. CA A Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. 2019. [DOI] [PubMed] [Google Scholar]
- 4.Giordano S.H. Breast cancer in men. N Engl J Med. 2018;378(24):2311–2320. doi: 10.1056/NEJMra1707939. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F., Bartlett J.M.S., Slaets L. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer program. Ann Oncol. 2018;29(2):405–417. doi: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu N., Johnson K.J., Ma C.X. Male breast cancer: an updated surveillance, epidemiology, and End results data analysis. Clin Breast Canc. 2018;18(5):e997–e1002. doi: 10.1016/j.clbc.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Abdelwahab Yousef A.J. Male breast cancer: epidemiology and risk factors. Semin Oncol. 2017;44(4):267–272. doi: 10.1053/j.seminoncol.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA A Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. 2018. [DOI] [PubMed] [Google Scholar]
- 9.Wang F., Shu X., Meszoely I. Overall mortality after diagnosis of breast cancer in men vs women. JAMA Oncol. 2019;5(11):1589–1596. doi: 10.1001/jamaoncol.2019.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fentiman I.S. Male breast cancer is not congruent with the female disease. Crit Rev Oncol Hematol. 2016;101:119–124. doi: 10.1016/j.critrevonc.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Wan B.A., Ganesh V., Zhang L. Treatment outcomes in male breast cancer: a retrospective analysis of 161 patients. Clin Oncol. 2018;30(6):354–365. doi: 10.1016/j.clon.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Giordano S.H., Perkins G.H., Broglio K. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005;104(11):2359–2364. doi: 10.1002/cncr.21526. [DOI] [PubMed] [Google Scholar]
- 13.Nofal M.N., Yousef A.J. The diagnosis of male breast cancer. Neth J Med. 2019;77(10):356–359. [PubMed] [Google Scholar]
- 14.Abdeljaoued S., Lhem B., Nasri M. Prognostic implications of the intrinsic molecular subtypes in male breast cancer. J BUON. 2017;22(2):377–382. [PubMed] [Google Scholar]
- 15.Restrepo D.J., Boczar D., Huayllani M.T. Survival disparities in male patients with breast cancer. Anticancer Res. 2019;39(10):5669–5674. doi: 10.21873/anticanres.13764. [DOI] [PubMed] [Google Scholar]
- 16.Atalay C., Kanlioz M., Altinok M. Prognostic factors affecting survival in male breast cancer. J Exp Clin Canc Res. 2003;22(1):29–33. [PubMed] [Google Scholar]
- 17.Leone J.P., Zwenger A.O., Iturbe J. Prognostic factors in male breast cancer: a population-based study. Breast Canc Res Treat. 2016;156(3):539–548. doi: 10.1007/s10549-016-3768-1. [DOI] [PubMed] [Google Scholar]
- 18.Rogowski P., Schonecker S., Pazos M. Pattern of care of adjuvant radiotherapy in male breast cancer patients in clinical practice: an observational study. Strahlenther Onkol. 2019;195(4):289–296. doi: 10.1007/s00066-018-1337-8. [DOI] [PubMed] [Google Scholar]
- 19.Giordano S.H. A review of the diagnosis and management of male breast cancer. Oncol. 2005;10(7):471–479. doi: 10.1634/theoncologist.10-7-471. [DOI] [PubMed] [Google Scholar]