Clinical decision-making regarding the use of medical therapies in preterm infants requires consideration of clinical benefits and adverse effects based on individualized risk-stratification. Therapies are implemented when the burden of the disease state and the certainty of the diagnosis exceed the burden of adverse effects from the intervention. Standardization of clinical decision-making with accurate, actionable risk-stratification tools has advanced care quality in neonates. For example, early-onset sepsis risk calculators that integrate multiple relevant clinical characteristics have reduced the use of antibiotics without increasing the risk of sepsis-related mortality.(1) However, if risk-stratification tools do not include factors such as race, sex, and socioeconomic status, which are known to contribute to differences in neonatal outcomes, they may be susceptible to hidden, intrinsic biases. In this commentary, we describe how omission of race-based risk from the bronchopulmonary dysplasia (BPD) risk estimator may lead to paradoxical under-prescription of postnatal corticosteroids in black preterm infants.
The Role of Late Postnatal Corticosteroids for the Prevention of BPD
The use of late postnatal corticosteroids, administered after 7 days of life, in the prevention of BPD in preterm infants remains controversial. Extensive literature documents the benefits and risks of postnatal corticosteroids, such as dexamethasone, which include reducing the risk of BPD by decreasing duration of invasive mechanical ventilation while potentially increasing the risk of cerebral palsy (CP).(2) More specifically, risk-weighted meta-regression analyses conducted by Doyle et al. in 2005 and 2014 demonstrate that the risk of death or CP decreases when postnatal corticosteroids are used in a targeted population of infants at high (≥60%) risk of moderate/severe BPD.(3,4)
Risk Stratification for BPD
Translation of this evidence into practice requires risk-stratification for identification of preterm infants most likely to benefit from corticosteroid therapy. The updated 2014 Doyle et al. meta-analysis suggests that the National Institute of Child and Health and Human Development Neonatal Research Network (NRN) BPD Outcome Estimator provides such a risk-stratification strategy.(4–6) The BPD Outcome Estimator is based on a cohort of 3,636 preterm infants cared for at 17 institutions across the United States between 2000 and 2004. Readily available demographic and clinical data, including gestational age, maternal self-reported race, and ventilator support, can be quickly transformed into individualized estimates of risk for mild, moderate, or severe BPD or death at six postnatal time points. Of note, this tool defines BPD by oxygen requirement at 36 weeks postmenstrual age, despite the limited correlation between this time point and long-term respiratory morbidity.(5,7) Despite this limitation, clinicians may be able to identify infants with a higher likelihood of benefit from postnatal corticosteroid therapy at specific postnatal assessment points using the BPD Outcome Estimator and the Doyle et al. threshold of ≥60% risk of moderate or severe BPD.(4,5,8)
The pitfall of competing outcomes
In developing institutional guidelines for infants at risk for BPD, we discovered limitations of the BPD outcome estimator when applied in specific clinical situations. For infants who require mechanical ventilation at 7 days of life, as fraction of inspired oxygen (FiO2) increases, risk of moderate and severe BPD decreases while risk of mortality increases (Figure 1). This paradox is explained by the early death of the sickest infants who would have otherwise gone on to develop moderate or severe BPD.(9) This finding highlights the competing roles of mortality and morbidity when utilizing risk-stratification tools in clinical decision-making. It is important to recognize that assessment of BPD risk can be confounded when there are stark differences in mortality risk between racial groups. These race-based disparities will lead to inconsistent prescription of late postnatal corticosteroids in clinical practice.
Figure 1:
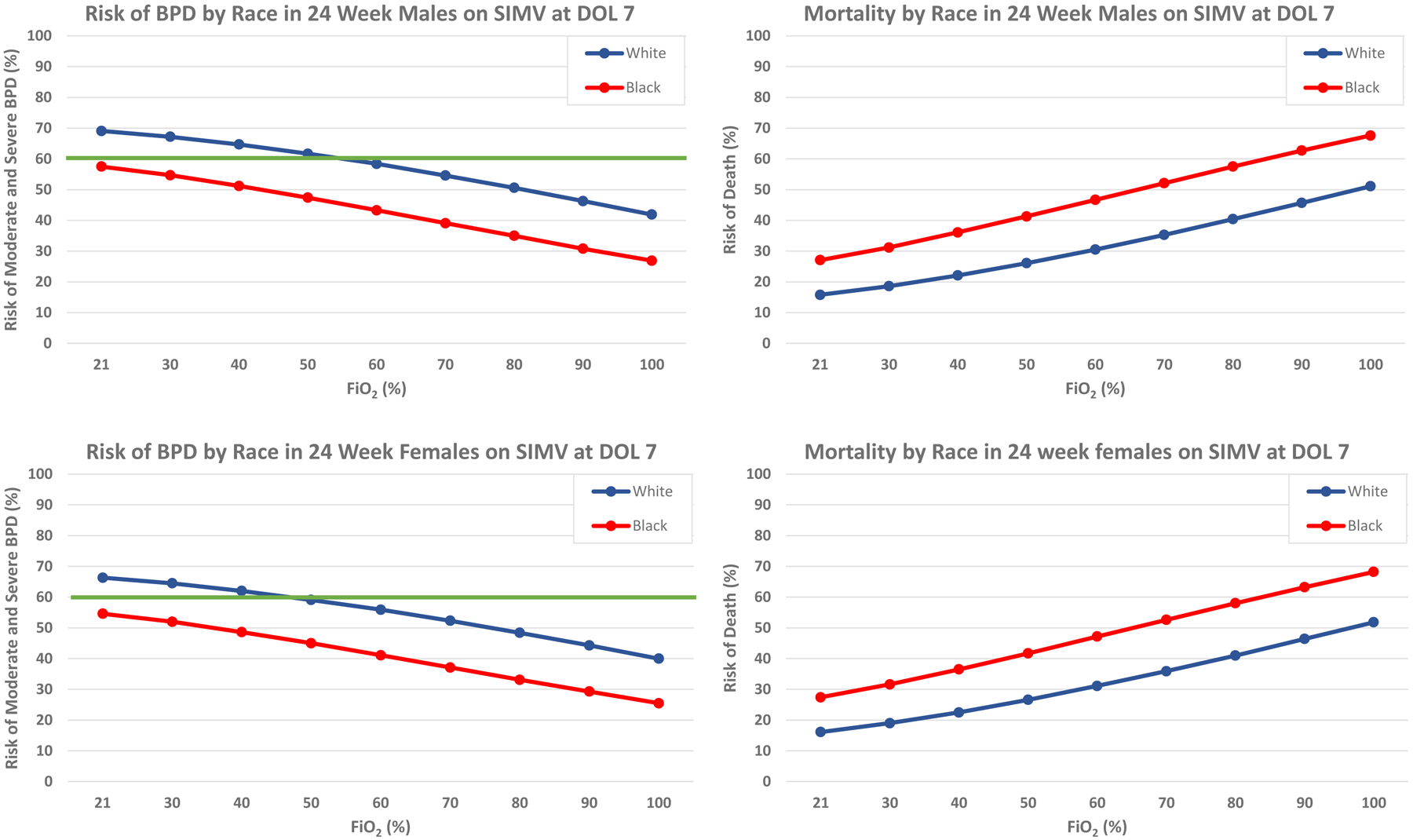
Illustrated above is the risk of moderate and severe BPD (left) and mortality (right) with increasing FiO2 requirement in 24 week male (top) and female (bottom) infants stratified by race. The threshold of 60% combined risk of moderate and severe BPD is indicated in bold.
The pitfall of disparate healthcare outcomes
To illustrate the impact of this disparity, we describe the following examples. A white infant born at 24 weeks gestation qualifies for corticosteroid therapy by exceeding the 60% moderate-severe BPD risk threshold at 7 days of age on synchronized intermittent mandatory ventilation (SIMV) at 0.21–0.50 FiO2 (Figure 1). In contrast, using the same calculator criteria, a 7-day old black infant born at the same gestational age, regardless of FiO2, would not qualify for corticosteroid therapy at the same threshold (Figure 1). The mortality projected by the BPD Outcome Estimator for black preterm infants is sufficiently high that in many cases the threshold risk of moderate-severe BPD is never reached, despite high severity of illness.
According to the most recent National Vital Statistics Report on Infant Mortality in the United States, the risk of mortality in black neonates is more than double that of their white counterparts.(10) Racial disparities in preterm birth are significant contributors to this discrepancy.(11) In the United States, the preterm birth rate is remarkably higher for black women, and prematurity-related mortality for black infants is 2- to 4-fold higher than that for white infants.(12,13) For this reason, racial disparities must be captured in calculators influencing clinical decision-making.
While much of the existing literature has cited a decreased risk of BPD in black infants, such studies fail to account for racial discrepancies in preterm birth and mortality.(14,15) In a recent editorial responding to PROP study outcomes in this journal, it was noted that cohorts with a gestational age-based selection criterion are vulnerable to inadvertent bias due to factors known to be associated with prematurity such as race, maternal health factors, and socio-economic status as well as unknown epigenetic and genetic factors.(14–17) In fact, in 2018 when Janevic et al. considered all possible infants utilizing the “fetus-at-risk” approach, black fetuses had a 4-fold higher risk of BPD than white fetuses, a difference not appreciated using a standard approach.(18,19) These data raise significant concern that racial disparities may create unrecognized bias for black infants in existing risk-stratification tools based upon observational cohorts. Adjustment for race-based differences in risk becomes particularly crucial for both routine clinical and clinical trial use of therapies like postnatal corticosteroids, where black infants are at a baseline disadvantage in both the competing outcomes of mortality and BPD.
Conclusions and Suggestions
As part of our efforts to standardize the use of postnatal corticosteroids for BPD prevention at our institution, we evaluated the utility of the BPD Outcome Estimator using the 60% moderate-severe BPD threshold suggested by Doyle. We found that this approach excludes infants with high oxygen requirements on invasive mechanical ventilation from qualifying for postnatal steroid therapy, and alarmingly excludes all mechanically-ventilated black infants regardless of their oxygen requirement. This finding appears to be the result of an imbalance in mortality in the black preterm population which is not captured by the calculator. This observation led us to consider the significance of racial differences in mortality estimates from the BPD Outcome Estimator. One of the most important strengths of the Estimator is also one of its most important caveats: a risk-stratification tool built with real data reflects real health disparities.
Racial disparities in both the incidence of prematurity and the impact on outcomes are under-represented in risk stratification. Decision-making tools should actively incorporate the effects of outcome disparities rather than passively permitting unrecognized influences. This strategy will ensure that neonates of all racial backgrounds have equal opportunity to benefit from potentially lifesaving or life-altering therapies, applied in a manner which takes their individual risk profile into account.
Until such tools exist, centers should examine their individual populations and adapt their risk-stratification approaches to optimize fit of their patients’ needs and outcomes, rather than utilizing a one-size-fits-all approach or relying solely on national data. Regular updates to the data underpinning risk stratification tools, as was recently done with the NRN Extremely Preterm Birth Outcomes Tool, could also enhance estimation accuracy.(20) An example of a widely-used clinical tool which has an adjustable risk profile is the Kaiser Permanente Neonatal Early-Onset Sepsis Calculator.(21,22) A similar approach, adjusting for race-based differences in risk to alter thresholds in existing risk-assessment tools, will better serve the unique risks of infants in different populations. Acknowledging that such disparities are present in existing clinical data and influence decision-making tools represents a significant step toward improving health equity for our patients.
Funding Sources:
This work was supported by the following grants:
1. NIH/NINDS K23 NS111086
Footnotes
Conflict of Interest Statement:
None of the authors has competing financial interests in relation to this work.
References
- 1.Kuzniewicz MW, Puopolo KM, Fischer A, Walsh EM, Li S, Newman TB, et al. A Quantitative, Risk-Based Approach to the Management of Neonatal Early-Onset Sepsis. JAMA Pediatr. 2017. April 1;171:365–71. [DOI] [PubMed] [Google Scholar]
- 2.Doyle L, Cheong J, Ehrenkranz R, Halliday H. Late (> 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev [Internet]. 2017; Available from: 10.1002/14651858.CD001145.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of Postnatal Systemic Corticosteroids on Mortality and Cerebral Palsy in Preterm Infants: Effect Modification by Risk for Chronic Lung Disease. Pediatrics. 2005. March 1;115:655. [DOI] [PubMed] [Google Scholar]
- 4.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An Update on the Impact of Postnatal Systemic Corticosteroids on Mortality and Cerebral Palsy in Preterm Infants: Effect Modification by Risk of Bronchopulmonary Dysplasia. J Pediatr. 2014. December 1;165:1258–60. [DOI] [PubMed] [Google Scholar]
- 5.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011. June 15;183:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NICHD Neonatal Research Network. Neonatal BPD Outcome Estimator [Internet]. [cited 2019 Dec 11]. Available from: https://neonatal.rti.org/index.cfm?fuseaction=BPDCalculator.start
- 7.Steinhorn R, Davis JM, Göpel W, Jobe A, Abman S, Laughon M, et al. Chronic Pulmonary Insufficiency of Prematurity: Developing Optimal Endpoints for Drug Development. J Pediatr. 2017. December 1;191:15–21.e1. [DOI] [PubMed] [Google Scholar]
- 8.Isayama T, Lee SK, Yang J, Lee D, Daspal S, Dunn M, et al. Revisiting the Definition of Bronchopulmonary Dysplasia: Effect of Changing Panoply of Respiratory Support for Preterm Neonates. JAMA Pediatr. 2017. March 1;171(3):271–9. [DOI] [PubMed] [Google Scholar]
- 9.Jobe Alan H., Bancalari Eduardo. Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2001. June 1;163:1723–9. [DOI] [PubMed] [Google Scholar]
- 10.Ely Danielle M., Driscoll Anne K.. Infant Mortality in the United States, 2017: Data From the Period Linked Birth/Infant Death File. Natl Vital Stat Rep. 2019. August 1;68:20. [PubMed] [Google Scholar]
- 11.Manuck TA. Racial and ethnic differences in preterm birth: A complex, multifactorial problem. Curr Preterm Birth Prev Strateg Part 2. 2017. December 1;41:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.March of Dimes. Premature Birth Report Card [Internet]. 2019. [cited 2019 Nov 20]. Available from: https://www.marchofdimes.org/mission/prematurity-reportcard-tv.aspx
- 13.Riddell CA, Harper S, Kaufman JS. Trends in Differences in US Mortality Rates Between Black and White Infants. JAMA Pediatr. 2017. September 1;171:911–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burris HH, Hwang SS, Collins JW, Kirpalani H, Wright CJ. Re-conceptualizing Associations between Race and Morbidities of Extreme Prematurity. J Pediatr. 2019. April 1;207:10–14.e1. [DOI] [PubMed] [Google Scholar]
- 15.Ryan RM, Feng R, Bazacliu C, Ferkol TW, Ren CL, Mariani TJ, et al. Black Race Is Associated with a Lower Risk of Bronchopulmonary Dysplasia. J Pediatr. 2019. April 1;207:130–135.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017. August 1;217:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Askie LM, Davies LC, Schreiber MD, Hibbs AM, Ballard PL, Ballard RA. Race Effects of Inhaled Nitric Oxide in Preterm Infants: An Individual Participant Data Meta-Analysis. J Pediatr. 2018. February 1;193:34–39.e2. [DOI] [PubMed] [Google Scholar]
- 18.Janevic T, Zeitlin J, Auger N, Egorova NN, Hebert P, Balbierz A, et al. Association of Race/Ethnicity With Very Preterm Neonatal Morbidities. JAMA Pediatr. 2018. November;172:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph KS, Kramer MS. The fetuses-at-risk approach: survival analysis from a fetal perspective. Acta Obstet Gynecol Scand. 2018. Apr 1;97:454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rysavy MA, Horbar JD, Bell EF, Li L, Greenberg LT, Tyson JE, et al. Assessment of an Updated Neonatal Research Network Extremely Preterm Birth Outcome Model in the Vermont Oxford Network. JAMA Pediatr. 2020. March 2;e196294–e196294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puopolo KM, Draper D, Wi S, Newman TB, Zupancic J, Lieberman E, et al. Estimating the Probability of Neonatal Early-Onset Infection on the Basis of Maternal Risk Factors. Pediatrics. 2011. November 1;128:e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escobar GJ, Puopolo KM, Wi S, Turk BJ, Kuzniewicz MW, Walsh EM, et al. Stratification of Risk of Early-Onset Sepsis in Newborns ≥34 Weeks’ Gestation. Pediatrics. 2014. January 1;133:30. [DOI] [PMC free article] [PubMed] [Google Scholar]


