Abstract
Moringa oleifera (MOI), an edible plant in the family of Moringaceae, has been used as food and medicine in many Asian countries. MOI exhibits neuroprotective, antioxidant, anti-inflammatory, and hypoglycemic functions. However, whether MOI seeds play a significant role in ischemic heart diseases has not been investigated. In this study, we found MOI seeds could improve the 28-day survival rate and the cardiac functions of myocardial infarction (MI) mice, with significantly increased ejection fraction and fractional shortening by day 28 post-MI. Correspondingly, the infarctional areas of heart were markedly decreased. Mechanistically, MOI seeds inhibited MI-induced apoptosis and repressed the degree of cardiac fibrosis. Further mechanistic studies indicated cardioprotective the effects of MOI seeds mainly via the suppression of oxidative and nitrosative stress. Taken together, our work suggested a beneficial role of MOI seeds in MI-induced myocardial damage and cardiac remodeling by suppressing cardiomyocyte apoptosis and reducing collagen production, highlighting a promising therapeutic strategy for MI.
Keywords: Moringa oleifera, myocardial infarction, cardiac remodeling, apoptosis, antioxidant
Introduction
Myocardial infarction (MI) is a matter of great concern, attributing to coronary artery obstruction and resulting in high morbidity and mortality worldwide. The pathology of MI injury is well characterized by cellular apoptosis [1] and oxidative stress [2]. During this process, disrupted redox homeostasis triggers inflammatory cascades and exacerbates the level of oxidative stress, subsequently facilitating excessive myocardial apoptosis [3]. The heart undergoes a train of repairing and wound healing responses following MI, referred to as cardiac remodeling. Myocardial infarct sizes and following cardiac remodeling directly affecting the structure and physiology of heart are the main determinants of long-term outcomes in MI patients [4]. In addition, redundant cardiac remodeling can develop into heart failure [5]. Therefore, alleviating infarct sizes and myocardial remodeling is significant to the precise treatment of MI and early prevention for heart failure. Although various therapeutic modalities have been proposed and tested to reduce MI injury [6-9], no effective therapies are available currently. Modern drugs are used in treating MI-related complications; however, they are mostly accompanied with adverse effects.
Traditional medicine, such as herbal plants and natural products, has gained more and more attention in recent years, especially because they are generally considered safe and sound. Moringa oleifera (MOI) known as drumstick, sahjana, and horseradish tree belongs to monogeneric family Moringaceae [10]. It is widely distributed in Asian countries and attributed with many pharmacological properties. All parts of MOI are valuable for useful food ingredients, drugs, and nutraceuticals [11]. Furthermore, MOI seeds are currently used as traditional medicines in tropical Africa, America, and Asia, and have been indisputably proven to benefit hypertension [12,13], myocardial damage [14], and ischemic stroke [15]. However, the therapeutic but empirical use of MOI seeds is generally not based on rational clinical or experimental data. In addition, the direct effect of MOI seeds on infarct sizes and cardiac remodeling following MI has not been established, and the underlying mechanism remains to be resolved.
Therefore, the aim of the current study was to evaluate the pharmacological efficacy of a diet containing MOI seeds to mitigate myocardial damage in mice with MI. It has been found that MOI seeds could inhibit cardiac remodeling, offering protection against MI by suppressing myocardial apoptosis, oxidative and nitrosative stress. Expectedly, the current study may provide a scientific rationale for the modulation of MOI seeds in the treatment of MI.
Materials and methods
Animals
Study protocols were reviewed and approved by the ethical committee of Shanghai Chest Hospital, Shanghai Jiao Tong University. All operating procedures of animal experiment are in accordance with the United States National Institutes of Health laboratory animal care and usage guidelines.
Eight-week-old wild-type C57/BL6 male mice (22-25 g) purchased from the Slac Laboratory (Shanghai, China) were used and housed at 25°C ± 5°C under a 12-h light/dark cycle with free access to food and water. The mice were randomly allocated to five groups composed of 20 animals each: WT-sham group, in which mice were fed with normal foods for 6 weeks; MOI-sham group, in which mice were fed with foods containing MOI seeds powder (600 mg/d or 900 mg/d) for 6 weeks; WT-MI group, in which mice were fed with normal foods for 2 weeks and received MI surgery on day 15, then fed with normal foods for 4 weeks; MOI 600-MI group, in which mice were fed with MOI seeds powder (600 mg/d) for 2 weeks and received MI surgery on day 15, then fed with MOI seeds powder (600 mg/d) for 4 weeks; and MOI 900-MI group, in which mice were fed with MOI seeds powder (900 mg/d) for 2 weeks and received MI surgery on day 15, then fed with MOI seeds powder (900 mg/d) for 4 weeks. The survival status of the five groups of mice was recorded.
MI surgery
MI procedures were performed utilizing a method described in a previous study [16]. In brief, mice were anesthetized with isoflurane inhalation. A small skin cut and a small hole in the fourth intercostal space were made over the left chest. With a mosquito clamp slightly open, the heart was gently and smoothly externalized through the hole. The left anterior descending branch of coronary artery was ligated at 2 mm above the left atrial appendage using a 6-0 silk suture. Then, the heart was timely put back into the intrathoracic space and the chest was closed layer by layer after the surgery with 5-0 silk suture. Sham-operated animals underwent similar surgical procedures, besides ligaturing the suture passed beneath the left anterior descending branch. Totally recovering from surgery, the mice were sent to standard animal housing conditions.
Echocardiography
In vivo cardiac function was examined by echocardiography (Vevo 770, Visual Sonic, Toronto, Canada) 4 weeks after surgery. Briefly, those mice were anesthetized and the chest hair was removed with topical depilation agent. Then, two-dimensional echocardiographic views of the ventricular short axis were gained at the level of papillary muscle tips. The values of left ventricular fractional shortening (LVFS, %) and left ventricular ejection fraction (LVEF, %) were calculated, and the heart rate was measured.
Histological examination
The hearts obtained from each group were fixed with 10% paraformaldehyde, embedded in paraffin, and sectioned serially (5 mm). Masson’s trichrome stain was performed for morphological examination, and Sirius red staining was used for quantification of collagen density. The infarct areas and left ventricular (LV) areas were measured using a quantitative digital image analysis system (Image-Pro Plus 6.0, Media Cybernetics Inc., Bethesda, MD) to determine infarct sizes. The ratio of infarct areas and LV areas was calculated and shown as percentage.
Western blot analysis
The myocardium tissue extracted from infarcted and non-infarcted regions of LV was lysed by cOmplete™ Lysis-M EDTA-free buffer (Roche 4719964001, Mannheim, Germany) supplemented with complete protease inhibitor cocktail (Roche) and PhosSTOP phosphatase inhibitor (Roche) for 20 minutes and centrifuged for 20 minutes (14,000 rpm, 4°C). The supernatant was collected and detected using the bicinchoninic acid (BCA; Pierce Chemical, Dallas, Texas, USA) to obtain the concentration of total protein. Twenty-five μg of lysate was resolved with 10% SDS-PAGE, and transferred onto NC membrane (Millipore, USA). The membrane was blocked with 5% milk in TBST for 1 hour, probed with primary antibody against Bax (CST 2772, 1:1000), Bcl2 (CST 2870, 1:1000), cytochrome c (CST 4272, 1:1000), gp91phox (abcam 129068, 1:1000), iNOS (abcam 3523, 1:1000), and GAPDH (CST, 1:1000) for overnight at 4°C, then washed 3 times, and followed by secondary antibody incubation for 1 hour at room temperature. The signals were detected with enhanced chemiluminescence (Share-Bio, Shanghai, China) and quantified with Gel-Pro Analyzer 4.0 software (Media Cybernetics, Silver Spring, MD).
Cardiac tissue NO levels
The end products of NO oxidation (nitrite/nitrate concentration reflecting synthesis of NO) were measured using griess reagent by spectrophotometry to detect cardiac nitrosative stress level.
Detection of cellular apoptosis in MI myocardium
Myocardial apoptosis was detected by TUNEL staining with In Situ Cell Death Detection Kit (Roche Diagnostics, Switzerland). Apoptotic nuclei were identified by green fluorescein staining and total nuclei were detected by DAPI.
Immunohistochemical study of gp91phox and iNOS expression
Immunohistochemical staining of cardiac tissue was performed with the standard method as depicted previously [17]. After being incubated with 3% hydrogen peroxide, the sections were probed with anti-gp91phox antibody (abcam 80508, 1:100) or anti-iNOS antibody (abcam 3523, 1:20) at 4°C overnight. Then they were incubated with horseradish peroxidase conjugated antibodies and detected. Finally, the percentage of positive staining was calculated.
Statistical analysis
All the data were shown as mean ± standard deviation and analyzed using SPSS 22.0 software (IBM, Armonk, NY, USA) or GraphPad Prism software version 5.0 (GraphPad, San Diego, CA). Two groups comparison was performed using t-test and more than two groups were compared by one-way analysis of variance (ANOVA). Kaplan-Meier curves were constructed to illustrate the survival rate in indicated groups following MI. Statistical significance was accepted when P < 0.05.
Results
MOI seeds increased survival rate and protected cardiac function after MI
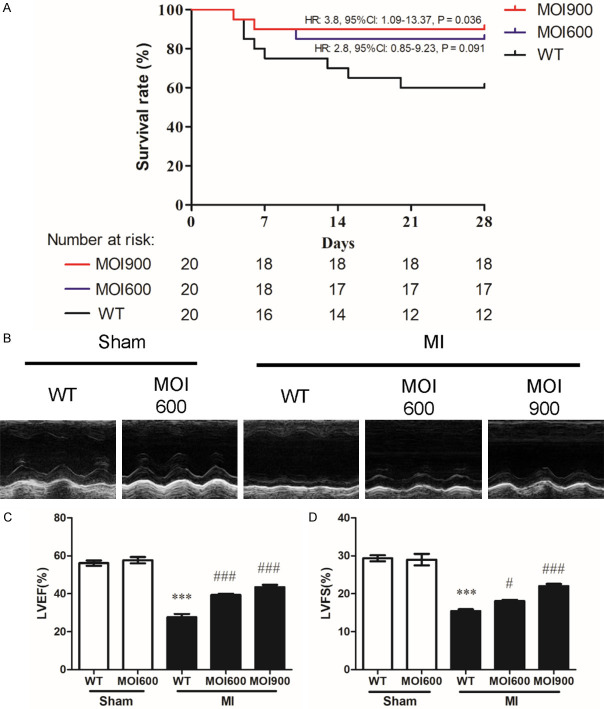
To elucidate the role of MOI seeds in MI, MOI seeds and normal foods orally administrated mice were established as MI model. Cumulative survival and echocardiograms were assessed. MOI 900-MI group mice had a higher survival rate than WT-MI group mice subjected to MI injury (HR: 3.8, 95% CI: 1.09-13.37, P = 0.036, Figure 1A). However, the survival rate of MOI 600-MI group mice had no difference with that of WT-MI group mice (HR: 2.8, 95% CI: 0.85-9.23, P = 0.091, Figure 1A). The representative M-mode echocardiograms indicated the abnormality of LV anterior wall movement was more serious in MI group compared to MOI 900-MI group and MOI 600-MI group (Figure 1B). Echocardiographic analysis suggested MOI seeds significantly alleviated contractile dysfunction, as demonstrated by increased values of LVEF (Figure 1C) and LVFS (Figure 1D). Taken together, these data indicate that MOI alleviated MI-induced mortality and cardiac dysfunction.
Figure 1.
MOI seeds improved the cumulative survival and cardiac function following MI. A. Survival curves of normal food (WT), MOI 600 mg/d (MOI 600), and MOI 900 mg/d (MOI 900) administered littermates after permanent coronary ligation surgery. n = 20 per group. B. Representative M-mode echocardiography images of mice in indicated groups. C, D. Quantitative analysis of LVEF and LVFS measured by echocardiography. n = 6 for each group, ***P < 0.001 vs. WT-sham, #P < 0.05 and ###P < 0.001 vs. WT-MI.
MOI seeds mitigated myocardial injury and cardiac remodeling induced by MI
To investigate the potential role of MOI seeds in MI-induced infarct sizes and cardiac fibrosis, pathological staining was examined. Macroscopically, at 4 weeks following MI, the mice in MOI 900-MI group and MOI 600-MI group had significantly smaller heart volume than mice in MI group (Figure 2A). The mice in MOI 900-MI group and MOI 600-MI group exhibited markedly thicker anterior wall of LV chamber and smaller volume of infarcted heart relative to that of mice in WT-MI group, reflecting mitigation of LV remodeling by MOI (Figure 2A). Besides, the mice in MOI 900-MI group and MOI 600-MI group showed a markedly smaller ratio of infarct areas/LV areas compared to the mice in MI group (Figure 2A, 2B), indicating MOI seeds significantly reduced MI-induced infarct sizes in accordance with significantly improved contractile dysfunction as measured by echocardiography compared to the control group. In addition, compared to WT-MI mice, MOI-treated MI mice had significant reductions in fibrotic scarring, as reflected by Masson’s trichrome staining, and collagen content, as determined by picrosirius red staining (P < 0.01, Figure 2A, 2C). These data suggest MOI alleviated MI-induced infarct sizes and cardiac remodeling.
Figure 2.
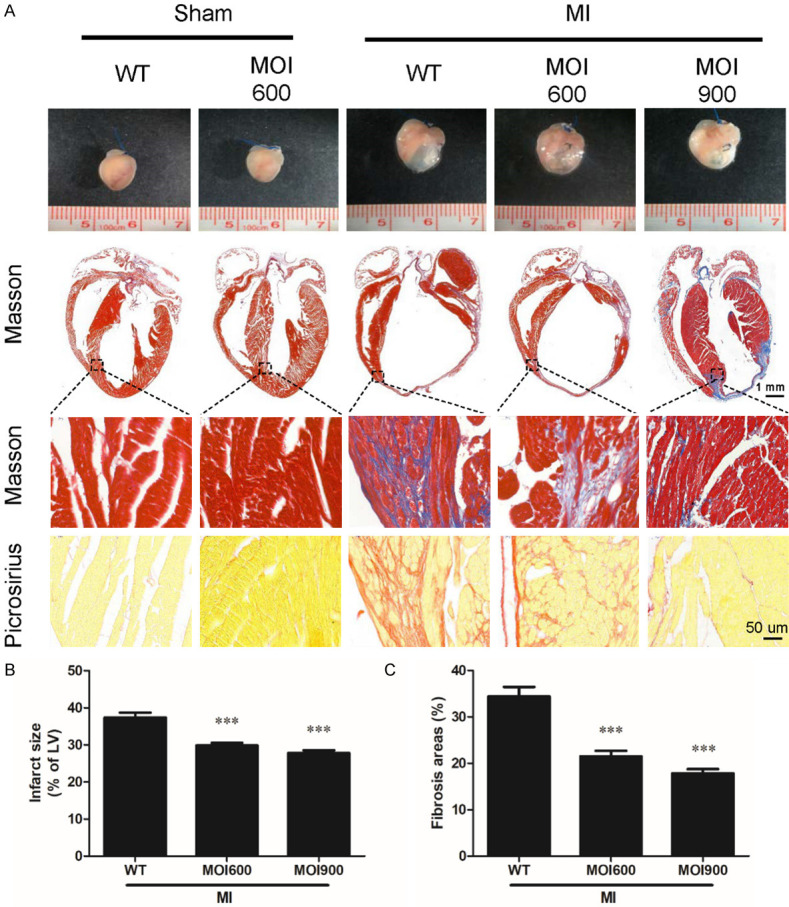
MOI seeds alleviated MI-induced infarct sizes and cardiac remodeling. A. Representative photographs of the whole heart, Masson’s trichrome and Sirius red staining in sections of hearts. The objective magnification is 1 × (upper panel, the scale bar was 1 mm), 400 × for Masson’s trichrome staining (middle panel), and 400 × for picrosirius red staining (bottom panel, the scale bar was 50 um). B. Myocardial infarct sizes were determined by Masson’s trichrome staining. n = 6 for each group, ***P < 0.001 vs. WT-MI. C. Quantifications of fibrosis areas in the myocardium stained by Sirius red staining. n = 6 for each group, ***P < 0.001 vs. WT-MI.
MOI seeds reduced MI-injury induced myocardial apoptosis
Myocardial apoptosis is an important contributor to MI-induced cardiac dysfunction and infarct sizes expansion [18]. Therefore, whether MOI affected MI-induced myocardial apoptosis was investigated. Compared to mice in WT-MI group, MOI seeds reduced MI-induced myocardial apoptosis, as evidenced by decreased TUNEL positive cells in the infarct border zone (Figure 3A, 3B). The antiapoptotic effect of MOI seeds was further addressed by measurement of Bax, Bcl2, and cytochrome-c (the key apoptosis markers) in non-infarct regions. Compared to sham operation, western blot analysis confirmed that MI significantly increased the expression of Bax and cytochrome-c, reduced the expression of Bcl2 (Figure 3C-G). While, the changes of Bax, Bcl2, and cytochrome-c were significantly reversed by MOI seeds (Figure 3C-G). Together, these results suggest that MOI seeds alleviated MI-induced myocardial apoptosis by attenuating mitochondrial-mediated apoptosis pathways.
Figure 3.
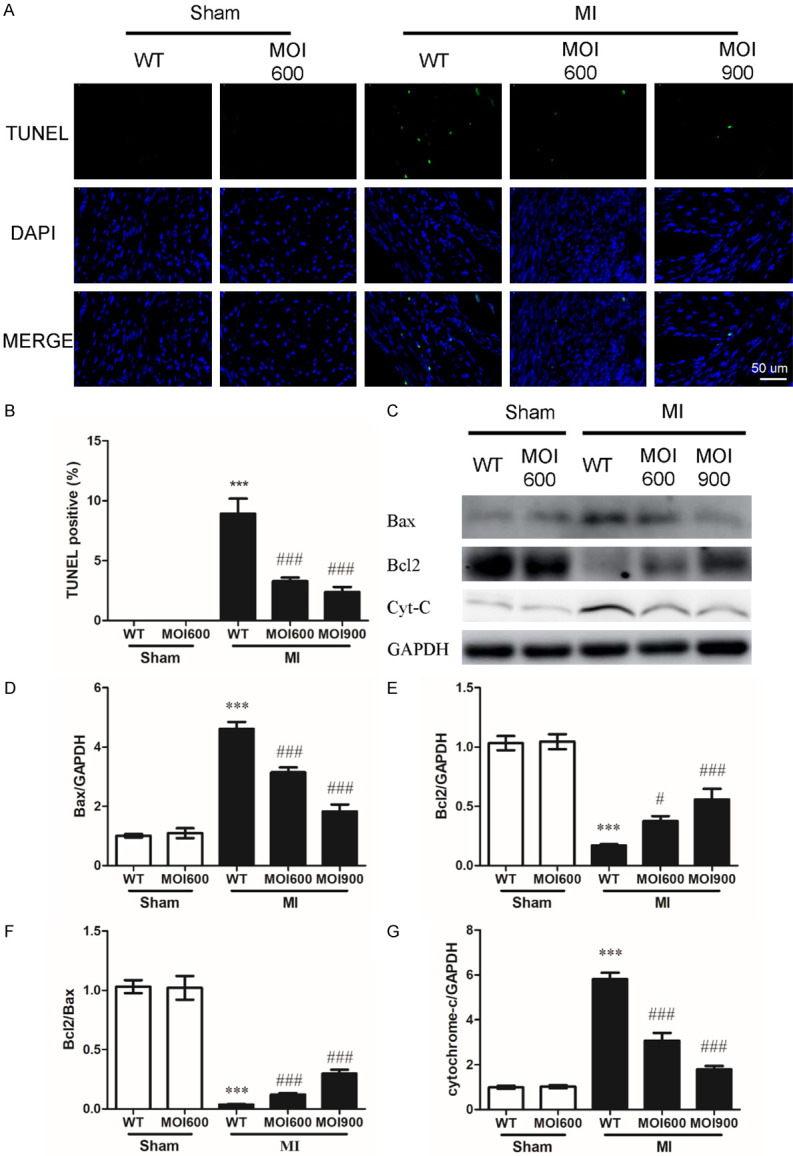
MOI seeds reduced MI-induced myocardial apoptosis by suppressing the mitochondrial-mediated apoptosis pathway. A. Myocardial apoptosis was determined by TUNEL labeling. TUNEL (green), apoptotic nuclei; DAPI (blue), total nuclei. The scale bar was 50 um. B. Quantification of apoptotic cells. n = 6 for each group, ***P < 0.001 vs. WT-sham and ###P < 0.001 vs. WT-MI. C. Western blot results showing expressions of Bax, Bcl2, and cytochrome-c (Cyt-C) in indicated groups. D-G. Quantification of the expression of Bax, Bcl2, Bcl2/Bax, and cytochrome-c. Data were normalized to GAPDH densitometry. n = 6 for each group, ***P < 0.001 vs. WT-sham, #P < 0.05 and ###P < 0.001 vs. WT-MI.
MOI seeds reduced oxidative stress and nitrosative stress in MI
To assess the effect of MOI seeds on MI-induced oxidative stress, we directly assessed the production and the topographical distribution of gp91phox in infarct zone and non-infarct zone in mice from WT-MI group and MOI 900-MI group. Compared to WT-MI mice, mice from MOI 900-MI group displayed a marked decrease in gp91phox regardless of infarct zone or non-infarct zone (Figure 4A, 4B). These results are consistent with a systemic antioxidant effect of MOI treatment. Oxidative stress is generally accompanied by nitrosative stress. To assess the effect of MOI seeds on nitrosative stress, we thus analyze the expression of iNOS (a mediator of endoplasmic reticulum-stress apoptosis pathway) in myocardium from infarct zone and non-infarct zone by western blot. In line with the above findings, western blot analysis confirmed that MOI significantly reduced the expression of iNOS induced by MI (Figure 4A, 4C). Furthermore, immunostaining of gp91phox and iNOS indicated MOI seeds decreased the expression of gp91phox and iNOS (Figure 4D-G). Besides, NO level confirmed reduced cardiac nitrosative stress by MOI seeds in accordance with the above findings (Figure 4H).
Figure 4.
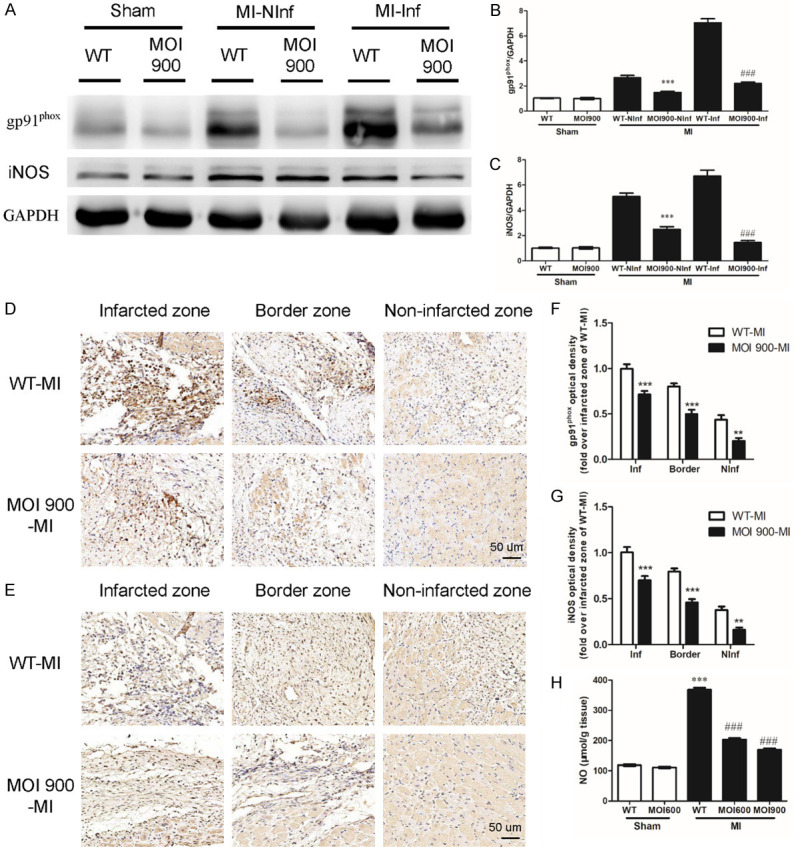
MOI seeds mitigated MI-induced oxidative stress and nitrosative stress in MI. (A) Western blot results showing expressions of gp91phox and iNOS from infarct zone (Inf) and non-infarct zone (NInf) in indicated groups. (B, C) Quantification of the expression of gp91phox and iNOS. Data were normalized to GAPDH densitometry. n = 6 for each group, ***P < 0.001 vs. WT-NInf and ###P < 0.001 vs. WT-Inf. Representative immunohistochemical images of gp91phox (D) and iNOS (E) indicated groups. The scale bar was 50 um. (F, G) Optical density of gp91phox and iNOS indicated groups (fold over infarcted zone of WT-MI). n = 6 for each group, **P < 0.01 and ***P < 0.001 vs. control group. (H) NO level. n = 6 for each group, ***P < 0.001 vs. WT-sham and ###P < 0.001 vs. WT-MI.
Discussion
This study aimed to evaluate the therapeutic applicability of MOI seeds in ameliorating myocardial remodeling following MI. The results showed orally administrated MOI seeds restrained myocardial apoptosis, oxidative and nitrosative stresses, simultaneously decreased collagen deposition, and improved the survival rate and cardiac function of MI mice, providing evidence for the beneficial anti-apoptosis and antioxidant properties of MOI seeds in MI.
MOI is a well-known tree rich in proteins, essential amino acids, minerals, vitamin A, flavonoids, as well as isothiocyanates, and contains about 40 natural antioxidants [19], which is also a source of bioactive compounds, such as flavonoids and phenolic compounds. The extracts from MOI exhibit antioxidant, anti-inflammatory, neuroprotective, hypoglycemic, blood lipid-reducing, anti-cancer, and hepatoprotective functions [20]. Current and ongoing researches have revealed MOI is a significant plant with multifunctional applications in human nutrition, medicines, and products [21,22]. Every part of MOI has a rich profile of essential nutrients and bioactive compounds, and is used for cardioprotective agents, and lowering blood pressure and cholesterol [22].
Acute MI leads to the loss of viable myocardium, followed by adverse cardiac remodeling. Despite the success of therapies for acute MI, adverse cardiac remodeling and heart failure still develop [23]. With the growing prevalence of MI-induced heart failure, new medicines and therapies are needed, which can simultaneously prevent remodeling and protect cardiac function. Therefore, increasing attention has been devoted to MI-associated cardiac function, especially cardiac remodeling [24-26]. Emerging evidence highlights the importance of MOI seeds as protective roles in cardiovascular diseases, improving cardiovascular diastolic function, and reducing nighttime pulse rate without adjusting the circulatory strain in hypertensive rats [13]. In this study, MOI seeds restored the mechanical properties of the myocardium, markedly decreased infarct sizes and reduced the levels of fibrotic changes. The deposition of non-contractile scar tissue is essential to a successful healing process, however, excess fibrogenesis is a risk factor of adverse cardiac remodeling [27]. In addition, we have discovered a previously unrecognized function of MOI seeds that restored the cardiac function and improved the survival rate of MI-injured mice.
MI gives rise to rapid apoptotic and necrotic loss of cardiomyocytes within the ischemic regions, thus leading to the fibrosis and dysfunction of MI hearts. Preventing the apoptosis of cardiomyocytes can alleviate hypoxia and ischemia-induced injury [28,29]. Evidence showed MOI significantly reduced the myocardial damage and apoptosis in the isoproterenol-induced cardiotoxicity model of Wistar rats [30]. Bax, Bcl2, and cytochrome-c are all closely related to apoptosis. The present study demonstrates that MOI seeds reduce the expression of Bax, restore Bcl2, and simultaneously decrease the expression of cytochrome-c, implying antiapoptotic property. It is worth noting that MOI seeds reduce myocardial apoptosis to a greater extent, maybe due to its potent antioxidant activity, which is attributed to the presence of antioxidant compounds including Vitamin A, Vitamin C, tocopherols, and phenolic acids [22,31,32].
Oxidative stress post-MI due to excessive generation of oxygen-derived free radicals is involved in the pathophysiology of ischemic heart disease and considered to be a major factor in cardiac remodeling [33,34]. Oxygen radicals have deleterious effects on myocardium, leading to contractile dysfunction and structural damage [35,36]. MOI possesses a wide range of medicinal properties through mitigating oxidative stress by scavenging free radicals and enhancing neuroprotective roles [20]. The hydrocarbons of seed essential oil are demonstrated to have a radical scavenging effect [37]. Butanolic extract of MOI relieves oxidative stress in isoproterenol-induced rodents [30]. In addition, the use of MOI seeds in diet has an antioxidant function and can improve cardiovascular disorders with vascular oxidative stress, for example, hypertension [12]. Previous studies have also confirmed MOI can selectively inhibit the production of iNOS and significantly inhibit the secretion of inflammatory markers in lipopolysaccharide-induced RAW264.7 cells [38]. Consistent with those findings, our study observes a reduction of oxidative and nitrosative stress in myocardium from MOI 900-MI mice associated with a reduction of gp91phox and iNOS expression, thus revealing an antioxidant action of MOI seeds. Collectively, our study provides the clues that MOI seeds may represent a pathway mediating oxidative stress-related damage in myocardium.
In conclusion, orally administrated MOI seeds have anti-apoptosis and antioxidant effect on cardiac function in a mouse model of MI, probably own to original composition and the synergistic action of multiple antioxidant compounds. Taken together, we scientifically provide the first direct evidence that the empirical use of MOI seeds pivotally relieves MI injury via ameliorating myocardial apoptosis, oxidative and nitrosative stress, which suggests MOI seeds may hold therapeutic promise for constraining MI injury and other cardiovascular disorders.
Acknowledgements
The study was supported by grants from the National Natural Science Foundation of China (81830010, 81330006, 81770428, 91539106) and the Shanghai Committee of Science and Technology, China (18411950400, 19JC1415702).
Disclosure of conflict of interest
None.
References
- 1.Wu P, Du Y, Xu Z, Zhang S, Liu J, Aa N, Yang Z. Protective effects of sodium tanshinone IIA sulfonate on cardiac function after myocardial infarction in mice. Am J Transl Res. 2019;11:351–360. [PMC free article] [PubMed] [Google Scholar]
- 2.Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 3.Neri M, Fineschi V, Di Paolo M, Pomara C, Riezzo I, Turillazzi E, Cerretani D. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol. 2015;13:26–36. doi: 10.2174/15701611113119990003. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse remodeling and reverse remodeling after myocardial infarction. Curr Cardiol Rep. 2017;19:71. doi: 10.1007/s11886-017-0876-4. [DOI] [PubMed] [Google Scholar]
- 6.Tian XQ, Yang YJ, Li Q, Xu J, Huang PS, Xiong YY, Li XD, Jin C, Qi K, Jiang LP, Chen GH, Qian L, Liu J, Geng YJ. Combined therapy with atorvastatin and atorvastatin-pretreated mesenchymal stem cells enhances cardiac performance after acute myocardial infarction by activating SDF-1/CXCR4 axis. Am J Transl Res. 2019;11:4214–4231. [PMC free article] [PubMed] [Google Scholar]
- 7.Wu WQ, Peng S, Song ZY, Lin S. Collagen biomaterial for the treatment of myocardial infarction: an update on cardiac tissue engineering and myocardial regeneration. Drug Deliv Transl Res. 2019;9:920–934. doi: 10.1007/s13346-019-00627-0. [DOI] [PubMed] [Google Scholar]
- 8.Davidson SM, Ferdinandy P, Andreadou I, Botker HE, Heusch G, Ibanez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D, Action CC. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol. 2019;73:89–99. doi: 10.1016/j.jacc.2018.09.086. [DOI] [PubMed] [Google Scholar]
- 9.Ji Q, Zhao Y, Yuan A, Pu J, He B. Deficiency of liver-X-receptor-alpha reduces glucose uptake and worsens post-myocardial infarction remodeling. Biochem Biophys Res Commun. 2017;488:489–495. doi: 10.1016/j.bbrc.2017.05.072. [DOI] [PubMed] [Google Scholar]
- 10.Marrufo T, Nazzaro F, Mancini E, Fratianni F, Coppola R, De Martino L, Agostinho AB, De Feo V. Chemical composition and biological activity of the essential oil from leaves of Moringa oleifera Lam. cultivated in Mozambique. Molecules. 2013;18:10989–11000. doi: 10.3390/molecules180910989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saini RK, Sivanesan I, Keum YS. Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016;6:203. doi: 10.1007/s13205-016-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randriamboavonjy JI, Rio M, Pacaud P, Loirand G, Tesse A. Moringa oleifera seeds attenuate vascular oxidative and nitrosative stresses in spontaneously hypertensive rats. Oxid Med Cell Longev. 2017;2017:4129459. doi: 10.1155/2017/4129459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randriamboavonjy JI, Loirand G, Vaillant N, Lauzier B, Derbre S, Michalet S, Pacaud P, Tesse A. Cardiac protective effects of Moringa oleifera seeds in spontaneous hypertensive rats. Am J Hypertens. 2016;29:873–881. doi: 10.1093/ajh/hpw001. [DOI] [PubMed] [Google Scholar]
- 14.Nandave M, Ojha SK, Joshi S, Kumari S, Arya DS. Moringa oleifera leaf extract prevents isoproterenol-induced myocardial damage in rats: evidence for an antioxidant, antiperoxidative, and cardioprotective intervention. J Med Food. 2009;12:47–55. doi: 10.1089/jmf.2007.0563. [DOI] [PubMed] [Google Scholar]
- 15.Kirisattayakul W, Wattanathorn J, Tong-Un T, Muchimapura S, Wannanon P, Jittiwat J. Cerebroprotective effect of Moringa oleifera against focal ischemic stroke induced by middle cerebral artery occlusion. Oxid Med Cell Longev. 2013;2013:951415. doi: 10.1155/2013/951415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu L, Li Z, Dong X, Xue X, Liu Y, Xu S, Zhang J, Han J, Yang Y, Wang H. Polydatin protects diabetic heart against ischemia-reperfusion injury via notch1/hes1-mediated activation of pten/akt signaling. Oxid Med Cell Longev. 2018;2018:2750695. doi: 10.1155/2018/2750695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omodanisi EI, Aboua YG, Oguntibeju OO. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules. 2017;22:439. doi: 10.3390/molecules22040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kou X, Li B, Olayanju JB, Drake JM, Chen N. Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients. 2018;10:343. doi: 10.3390/nu10030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falowo AB, Mukumbo FE, Idamokoro EM, Lorenzo JM, Afolayan AJ, Muchenje V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: a review. Food Res Int. 2018;106:317–334. doi: 10.1016/j.foodres.2017.12.079. [DOI] [PubMed] [Google Scholar]
- 22.Dhakad AK, Ikram M, Sharma S, Khan S, Pandey VV, Singh A. Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother Res. 2019;33:2870–2903. doi: 10.1002/ptr.6475. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 24.Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res. 2018;123:107–128. doi: 10.1161/CIRCRESAHA.118.312017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Du Y, Shi K, Yang Y, Yang Z. Resveratrol improves cardiac function by promoting M2-like polarization of macrophages in mice with myocardial infarction. Am J Transl Res. 2019;11:5212–5226. [PMC free article] [PubMed] [Google Scholar]
- 26.Sheu JJ, Lee MS, Wallace CG, Chen KH, Sung PH, Chua S, Lee FY, Chung SY, Chen YL, Li YC, Yip HK. Therapeutic effects of adipose derived fresh stromal vascular fraction-containing stem cells versus cultured adipose derived mesenchymal stem cells on rescuing heart function in rat after acute myocardial infarction. Am J Transl Res. 2019;11:67–86. [PMC free article] [PubMed] [Google Scholar]
- 27.Frangogiannis NG. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Wu T, Wu D, Wu Q, Zou B, Huang X, Cheng X, Wu Y, Hong K, Li P, Yang R, Li Y, Cheng Y. Knockdown of long non-coding RNA-ZFAS1 protects cardiomyocytes against acute myocardial infarction via anti-apoptosis by regulating miR-150/CRP. J Cell Biochem. 2017;118:3281–3289. doi: 10.1002/jcb.25979. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Huang X, Hu F, Fu X, Jiang Z, Chen K. LncRNA ANRIL knockdown relieves myocardial cell apoptosis in acute myocardial infarction by regulating IL-33/ST2. Cell Cycle. 2019;18:3393–3403. doi: 10.1080/15384101.2019.1678965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panda S. Butanolic fraction of Moringa oleifera Lam. (Moringaceae) attenuates isoprotrenol-induced cardiac necrosis and oxidative stress in rats: an EPR study. EXCLI J. 2015;14:64–74. doi: 10.17179/excli2014-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh RSG, Negi PS, Radha C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J Funct Foods. 2013;5:1883–1891. [Google Scholar]
- 32.Saa RW, Fombang EN, Ndjantou EB, Njintang NY. Treatments and uses of Moringa oleifera seeds in human nutrition: a review. Food Sci Nutr. 2019;7:1911–1919. doi: 10.1002/fsn3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rababa’h AM, Guillory AN, Mustafa R, Hijjawi T. Oxidative stress and cardiac remodeling: an updated edge. Curr Cardiol Rev. 2018;14:53–59. doi: 10.2174/1573403X14666180111145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou L, Guo J, Xu F, Weng X, Yue W, Ge J. Cardiomyocyte dimethylarginine dimethylaminohydrolase1 attenuates left-ventricular remodeling after acute myocardial infarction: involvement in oxidative stress and apoptosis. Basic Res Cardiol. 2018;113:28. doi: 10.1007/s00395-018-0685-y. [DOI] [PubMed] [Google Scholar]
- 35.Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019;307:41–48. doi: 10.1016/j.toxlet.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Lefer DJ, Granger DN. Oxidative stress and cardiac disease. Am J Med. 2000;109:315–323. doi: 10.1016/s0002-9343(00)00467-8. [DOI] [PubMed] [Google Scholar]
- 37.Yassa N, Masoomi F, Rankouhi SER, Hadjiakhoondi A. Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascena from Iran, Population of Guilan. DARU. 2009;17:175–180. [Google Scholar]
- 38.Arulselvan P, Tan WS, Gothai S, Muniandy K, Fakurazi S, Esa NM, Alarfaj AA, Kumar SS. Anti-inflammatory potential of ethyl acetate fraction of Moringa oleifera in downregulating the NF-kappaB signaling pathway in lipopolysaccharide-stimulated macrophages. Molecules. 2016;21:1452. doi: 10.3390/molecules21111452. [DOI] [PMC free article] [PubMed] [Google Scholar]