Abstract
Patients with schizophrenia have shown widespread white matter microstructural abnormalities and cognitive deficits, but the definitive relationship between white matter and cognitive performance remains unclear. In this study, we investigated the possible associations between white matter integrity and cognitive deficits in drug-naive first-episode schizophrenia (dn-FES) using diffusion tensor imaging (DTI). A total of 96 participants, including 46 dn-FES patients and 50 healthy individuals, underwent 3.0 T magnetic resonance diffusion-weighted imaging and cognitive assessments using the Chinese version of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB). Group differences were tested using tract-based spatial statistics (TBSS). Compared with the control group, the dn-FES group exhibited reduced white matter integrity, as indexed using fractional anisotropy (FA) metrics, in the right-hemispheric cluster comprising the posterior thalamic radiation, posterior corona radiata, superior longitudinal fasciculus, retrolenticular part of the internal capsule, tapetum, splenium of the corpus callosum, sagittal stratum, and inferior longitudinal fasciculus. We found that social cognitive deficit is significantly correlated with reduced FA in these white matter regions, except the sagittal stratum and inferior longitudinal fasciculus. Furthermore, we found that speed of processing is positively correlated with reduced FA in the right superior longitudinal fasciculus of dn-FES patients. In summary, white matter deficits were validated in dn-FES patients and could be associated with speed of processing and social cognition, providing clues about a neural basis of schizophrenia and a potential biomarker for clinical studies.
Keywords: Diffusion tensor imaging, fractional anisotropy, first episode schizophrenia, cognition, MATRICS consensus cognitive battery
Introduction
Schizophrenia is a complex, serious, and disabling mental disorder characterized by a broad array of symptoms including positive symptoms, negative symptoms, and cognitive deficits. The disconnection hypothesis recognizes schizophrenia as a disconnection illness caused by white matter tracts between multiple brain areas [1-5]. The white matter microstructure can be quantitatively assessed by diffusion tensor imaging (DTI) in vivo [6]. Currently, the most widely applied scalar measure is fractional anisotropy (FA) [7,8]. Recently, the field has largely focused on cerebral white matter connections in patients with schizophrenia [9-11]. These studies have revealed lower FA in the corpus callosum, thalamic radiation, corona radiata, longitudinal fasciculi, and other areas, as well as higher FA in the arcuate fasciculus [12,13] and caudate [14]. However, consensual white matter alterations have not yet been identified due to the heterogeneity in the anatomical scope of FA reduction reported across studies [15-18]. Sources of heterogeneity among these studies may be attributed to variations in medications, duration of illness, study samples, and other variable factors [19].
Cognitive deficits are a core symptom of schizophrenia [20,21] and have a substantial influence on patients’ psychosocial life. Assessed using the MATRICS Consensus Cognitive Battery (MCCB) [22], cognitive deficits have emerged as important targets of treatment-oriented research. Cumulative evidence has shown that patients with schizophrenia experience cognitive decline from before to after illness onset [23]. Recently, several studies have shown that white matter abnormalities are linked to cognitive performance [24-27]. However, these studies had small sample sizes or included only male patients, and their results were unclear. Further, only a limited number of studies have investigated the association between FA values and cognitive performance with all seven cognitive domains of the MCCB in the drug-naïve patients with first-episode schizophrenia. Recently, one study [25] used whole-brain tract-based spatial statistics (TBSS) [28] and the MCCB to reveal that mean FA values in the left superior longitudinal fasciculus show positive correlations with working memory and visual learning. However, the study found no significant correlation between FA alteration and the other cognitive domains: attention, speed of processing, verbal learning, reasoning/problem solving, and social cognition.
In the present study, we used the MCCB to assess cognition and TBSS to investigate white matter integrity in drug-naive first-episode schizophrenia (dn-FES) patients in order to address the variations in medications and minimize illness chronicity. Following the disconnection hypothesis [2], we expected widespread subtle white matter abnormalities characterized by lower FA in dn-FES patients than in healthy participants. In addition, we explored correlations between FA values and both cognitive performance and the five-factor model of the Positive and Negative Syndrome Scale (PANSS) [29]. We hypothesized that aberrant white matter fiber integrity is associated with levels of cognitive performance and clinical psychotic symptoms in patients with schizophrenia.
Materials and methods
Participants
Fifty-five never-medicated Chinese patients were recruited from the inpatient department or outpatient clinic of the Affiliated Brain Hospital of Nanjing Medical University, Jiangsu, China, from September 2015 to December 2019 (Table 1). They were diagnosed using the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 patient edition and determined to meet the criteria for schizophrenia according to two senior psychiatrists. Inclusion criteria for patients were as follows: Han ethnicity, right-handedness, age between 16 and 45, first episode illness, duration of untreated psychosis ≤ 24 months, and IQ greater than 70. Exclusion criteria included any diagnosis of physical or neurological illness, pregnancy, head injury, substance dependence, or contraindications for magnetic resonance imaging (MRI) scanning. Fifty-five healthy participants (control group) were recruited from the local area and matched with patients by age and gender (Table 1). Inclusion criteria for healthy participants were as follows: Han ethnicity, right-handedness, age between 16 and 45, no personal or family history of psychiatric or other inherited illness. Exclusion criteria were the same for healthy controls as those for the patients. After quality control procedures, 9 patients and 5 healthy participants were excluded, so the final analysis included 46 patients and 50 health participants.
Table 1.
Demographic, cognitive and clinical characteristics of all subjects
| dn-FES (n = 46) | HC (n = 50) | statistic | ||
|---|---|---|---|---|
|
|
|
|
||
| Mean ± S.D. | Mean ± S.D. | t/t’/X2 | p | |
| Age (years) | 24.20 ± 7.85 | 26.06 ± 7.40 | t = -1.198 | 0.234 |
| Gender (male/female) | 32/14 | 26/24 | X2 = 3.09 | 0.079 |
| Handedness (right/left) | 46/0 | 50/0 | ||
| Years of education | 12.35 ± 2.51 | 14.5 ± 3.12 | t = -3.703 | 0.000 |
| DUP (months) | 9.67 ± 7.14 | NA | ||
| WAIS (IQ) | 102.76 ± 11.96 | 115.22 ± 8.70 | t = -5.868 | 0.000 |
| MCCB T-scores | ||||
| Speed of processing | 35.63 ± 12.09 | 51.12 ± 8.62 | t’ = -7.171 | 0.000 |
| Attention/vigilance | 36.91 ± 10.13 | 46.24 ± 6.21 | t’ = -5.386 | 0.000 |
| Working memory | 33.17 ± 10.64 | 44.32 ± 6.67 | t’ = -6.087 | 0.000 |
| Verbal learning | 36.02 ± 11.15 | 45.24 ± 10.61 | t = -4.150 | 0.000 |
| Visual learning | 40.33 ± 12.89 | 49.78 ± 8.44 | t’ = -4.212 | 0.000 |
| Reasoning/problem solving | 44.65 ± 11.69 | 52.52 ± 7.32 | t’ = -3.914 | 0.000 |
| Social cognition | 32.50 ± 9.71 | 37.90 ± 9.28 | t = -2.785 | 0.006 |
| MCCB overall composite | 28.93 ± 13.06 | 44.72 ± 7.94 | t’ = -7.081 | 0.000 |
| PANSS subscale score | ||||
| Positive factor | 16.20 ± 2.87 | NA | ||
| Negative factor | 18.04 ± 2.98 | NA | ||
| Excited factor | 10.57 ± 2.18 | NA | ||
| Depressed factor | 7.46 ± 2.04 | NA | ||
| Disorganized factor | 8.13 ± 2.21 | NA | ||
Abbreviations: dn-FES: drug-naïve first-episode schizophrenia; HC: healthy control; DUP: duration of untreated psychosis; WAIS: Wechsler Adult Intelligence Scale; IQ: intelligence quotient; MCCB: the MATRICS Consensus Cognitive Battery; PANSS: Positive and Negative Syndrome Scale; NA: not applicable.
The study was approved by the Medical Research Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University. All patients and healthy participants provided written informed consent before study participation. For those younger than 18, consent was provided by their parent or guardian.
Psychopathological assessment in patients
At both time points, psychiatric symptoms were assessed using the PANSS [30]. The five-factor model [31] was used, dividing the PANSS in the positive factor, negative factor, excited factor, disorganized factor, and depressed factor.
Intelligence quotient and cognitive assessment
The intelligence quotient (IQ) was estimated using the Chinese version of the Wechsler Adult Intelligence Scale-Revised, which includes the common sense, similarity, and picture completion tests, and block design subtests. Cognition was assessed with the Chinese version of the MCCB [32]. The MCCB provided the overall composite score and seven domains including speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning/problem solving, and social cognition [33]. The seven cognitive domain T-scores were corrected by age, gender, and years of education in the software and were matched with the MCCB.
MRI acquisition
All participants were asked to remain motionless and keep their eyes closed during scanning in a 3.0 T Siemens Verio magnetic resonance imaging scanner (Erlangen, Germany). Diffusion-weighted volumes were acquired using a pulsed gradient, echo planar imaging sequence: repetition time/echo time, 7900/97 ms; field of view, 23 × 23 cm2; matrix size, 122 × 122; voxel size, 1.9 × 1.9 × 2.3 mm3; slice thickness, 2.3 mm; 55 axial slices, no gap. Sixty-four diffusion weighted images (b-value = 1000 s/mm2) and one image with b = 0 (b0 image) were acquired. The total time of the diffusion tensor imaging sequence was approximately 9 minutes.
Data processing
The imaging data was processed using TBSS analysis in FMRIB Software Library 5.0.9 (FSL, http://www.fmrib.ox.ac.uk/fsl) [28]. First, the DICOM files were transformed into compressed NIFTI format using dcm2niigui in FSL. Next, it was corrected for eddy currents and head motion using the linear image registration tool (FLIRT v6.0) in FSL [34]. Then, the b0 image was skull stripped using the brain extraction tool (BET v2.1), and a brain mask was acquired. Finally, the FA images were generated through reconstruct diffusion tensors using FMRIB’s diffusion toolbox (FDT 3.0) in FSL. All above processing steps were the preprocessing stages.
For the next step, each FA image was aligned to 1 × 1 × 1 mm FMRIB58_FA standard space using FMRIB’s nonlinear image registration tool (FNIRT). The mean FA image and the mean FA skeleton were then created by averaging all the registered FA images. Next, the threshold was set at 0.2 to remove peripheral brain areas, followed by creation of a 4D file of all skeletonized images and a mean FA skeleton mask. Finally, all FA skeletonized images and the mean FA skeleton mask were inputted into voxel-wise statistical analyses.
Data analysis
Demographic, IQ, and cognitive characteristics were compared between patients and healthy participants using the chi-square and independent sample t-tests in the Statistical Package for the Social Sciences version (SPSS v.25.0). If the variance was not homogeneous, the t’-tests were used. The significant differences of FA data between the patient and control groups were detected by voxel-wise statistical analysis using randomize v2.9 in FSL. In the final statistical model, nonparametric permutation-based tests were applied with 5000 permutations, using age, gender, and years of education as covariates. The threshold for statistical significance was set at P < 0.05 with threshold-free cluster enhancement [35] and family-wise error correction for multiple comparisons using the null distribution of the maximal voxel-wise test statistic. The Johns Hopkins University (JHU) International Consortium for Brain Mapping DTI-81 white matter labels [36] were used to assign the white matter tract name with significant group differences; if the voxels did not match the labels, the JHU white matter tractography atlas was used to localize [28]. The mean FA value was extracted within each cluster mask with significant group differences. Pearson correlation analysis was used to further explore the correlation between the mean FA value of each cluster and both cognitive performance and clinical psychotic symptoms in the SPSS software.
Results
Demographic, cognitive, and clinical characteristics
No significant group differences in age, gender, and handedness were found between patients with schizophrenia and healthy participants. However, years of education was lower in the patient group than in the control group. The cognitive domain T-scores are shown in Table 1. The dn-FES group had significantly lower scores in all seven cognitive domains than the control group. This was particularly true in two domains: speed of processing and working memory; the least impaired domain was social cognition (Table 1; Figure 1).
Figure 1.
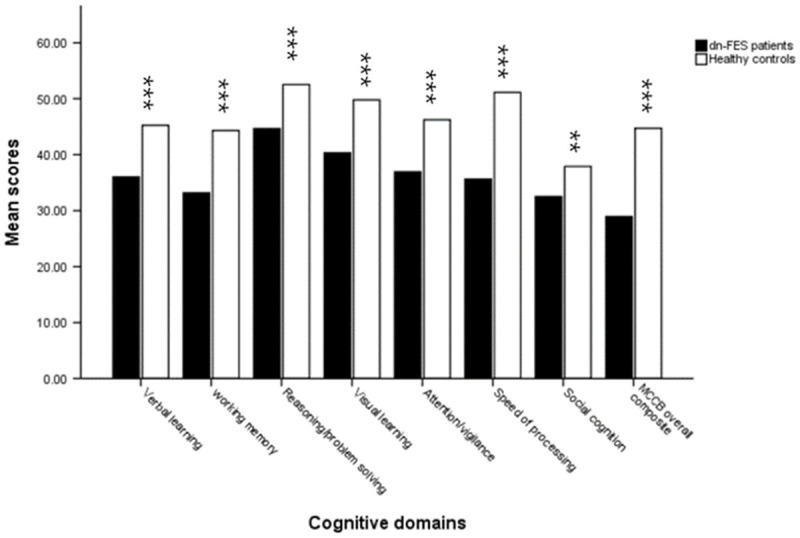
Seven cognitive domain scores in dn-FES patient group and healthy control group. Compared to healthy control group, the dn-FES patient group had significantly lower scores in all seven cognitive domains, particularly in two domains: speed of processing and working memory, while the least impairment domain was social cognition. **P < 0.01 compared with healthy controls at the same cognitive domain. ***P < 0.001 compared with healthy controls at the same cognitive domain.
Group differences in FA values
As shown in Figure 2, the patient group showed significantly lower FA than the control group, distributed over the posterior thalamic radiation, posterior corona radiata, superior longitudinal fasciculus, retrolenticular part of the internal capsule, tapetum, and splenium of corpus callosum of the right hemisphere (Cluster 6, Table 2). In addition, the dn-FES group exhibited lower FA in the right sagittal stratum (Cluster 5, Table 2), right superior longitudinal fasciculus (Cluster 4, Table 2), and right inferior longitudinal fasciculus (Cluster 3, Table 2). Lower FA values were also observed in splenium of corpus callosum, the posterior thalamic radiation, the posterior corona radiata (Cluster 2, Table 2) and the superior longitudinal fasciculus (Cluster 1, Table 2) of the right hemisphere. No significant cluster of FA was higher in patients than in healthy participants.
Figure 2.
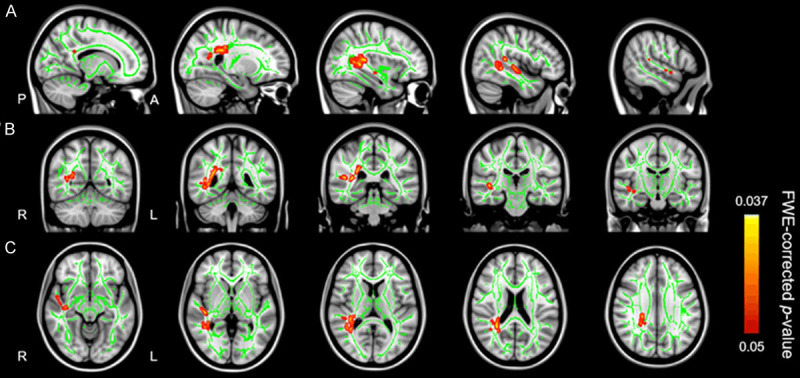
TBSS results in dn-FES patient group and healthy control group. The patient group showed significantly lower FA than the control group, distributed over the posterior thalamic radiation, posterior corona radiata, superior longitudinal fasciculus, retrolenticular part of the internal capsule, tapetum, sagittal stratum, the inferior longitudinal fasciculus and splenium of corpus callosum of the right hemisphere. No significant cluster of FA was higher in patients than in healthy participants. The green color shows the mean FA skeleton, the color scale red to yellow which expand by tbss_fill indicates significant FA reduction. Results are overlaid on (A) (sagittal slice, X = 15, 25, 35, 45, 55), (B) (coronal slice, Y = -55, -45, -35, -25, -15) and (C) (axial slices, Z = -10, 0, 10, 20, 30) from the Montreal Neurological Institute standard brain at a permutation-based threshold of P < 0.05 (FEW-corrected).
Table 2.
FA differences between 46 dn-FES patients and 50 healthy controls
| Cluster Index | Cluster voxels | p | MNI coordinates of peak voxel | Side | Anatomical region | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| X | Y | Z | |||||
| 6 | 852 | 0.037 | 32 | -48 | 17 | Right | Posterior thalamic radiation |
| Posterior corona radiata | |||||||
| Superior longitudinal fasciculus | |||||||
| Retrolenticular part of internal capsule | |||||||
| Tapetum | |||||||
| Splenium of corpus callosum | |||||||
| 5 | 236 | 0.046 | 42 | -23 | -3 | Right | Sagittal stratum |
| 4 | 54 | 0.046 | 46 | -35 | 14 | Right | Superior longitudinal fasciculus |
| 3 | 45 | 0.048 | 52 | -2 | -9 | Right | inferior longitudinal fasciculus |
| 2 | 44 | 0.049 | 28 | -59 | 14 | Right | Splenium of corpus callosum |
| Posterior thalamic radiation | |||||||
| Posterior corona radiata | |||||||
| 1 | 37 | 0.049 | 43 | -43 | 4 | Right | Superior longitudinal fasciculus |
Abbreviation: MNI: Montreal Neurological Institute. Notes: No regions of increased FA were found in patients vs control participants.
Correlation of FA values with cognitive domain scores and the PANSS subscale score
We calculated mean FA values within the clusters with significant group differences in FA. In the patient group, mean FA values within Cluster 6 (Figure 3B)-which consisted of the right posterior thalamic radiation, right posterior corona radiata, right superior longitudinal fasciculus, right retrolenticular part of the internal capsule, right tapetum, and splenium of the corpus callosum-were positively correlated with social cognition scores (r = 0.309, P = 0.037) (Figure 3A). In addition, mean FA values in Cluster 1 (Figures 4B and 5B), which consisted of the right superior longitudinal fasciculus, showed positive correlations with social cognition scores (r = 0.355, p = 0.015) and speed of processing (r = 0.352, P = 0.016) (Figures 4A, 5A). No significant correlations were found between FA values and the PANSS subscale score in the patient group. In addition, correlation analyses revealed there were no significant associations between the mean FA values and cognitive domain scores in the control group.
Figure 3.
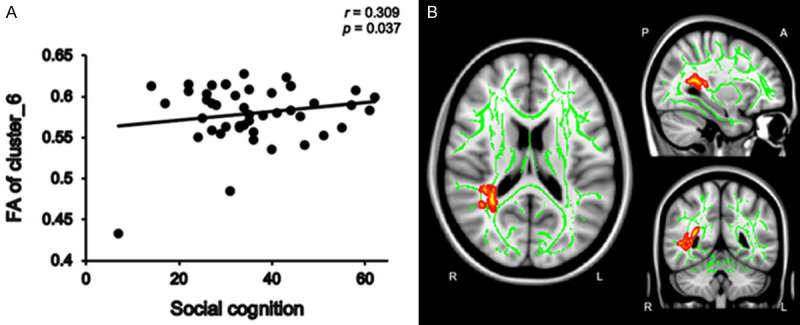
The positive correlation between social cognition and mean FA values within cluster 6 in dn-FES patient group. Mean FA values within Cluster 6 were positively correlated with social cognition scores (r = 0.309, P = 0.037) in the patient group (A). The green color shows the mean FA skeleton, the color scale red to yellow which expand by tbss_fill indicates cluster 6 (B), which was consisted of the right posterior thalamic radiation, right posterior corona radiata, right superior longitudinal fasciculus, right retrolenticular part of the internal capsule, right tapetum, and splenium of the corpus callosum.
Figure 4.
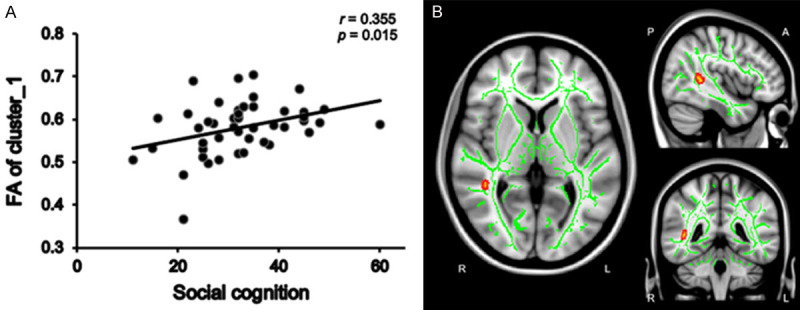
The positive correlation between social cognition and mean FA values within cluster 1 in dn-FES patient group. The mean FA values within cluster 1 were positively correlated with social cognition (r = 0.355, P = 0.015) in the patient group (A). The green color shows the mean FA skeleton, the color scale red to yellow which expand by tbss_fill indicates the right superior longitudinal fasciculus of cluster 1 (B).
Figure 5.
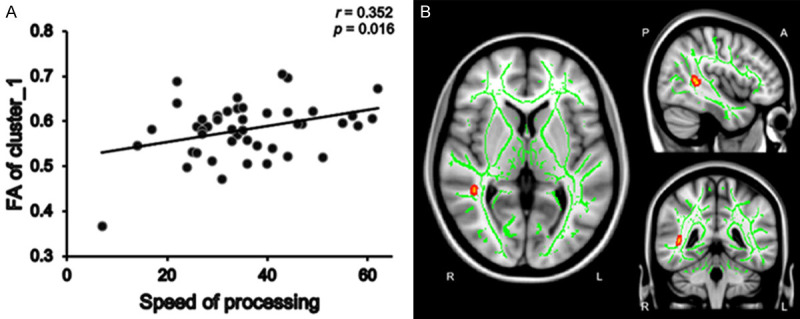
The positive correlation between speed of processing and mean FA values within cluster 1 in dn-FES patient group. The mean FA values within cluster 1 were positively correlated with speed of processing (r = 0.352, P = 0.016) in patient group (A). The green color shows the mean FA skeleton, the color scale red to yellow which expand by tbss_fill indicates the right superior longitudinal fasciculus of cluster 1 (B).
Discussion
In the present study, we used both a TBSS approach and the MCCB to explore the cognitive changes and the whole-brain white matter integrity alteration in dn-FES patients. We found that patients had significantly more cognitive deficits and lower FA than healthy participants. Furthermore, we identified significant correlations between cognitive domain scores and FA values in regions showing reduced white matter integrity in patients. Our findings revealed that social cognitive score is positively correlated with FA values in the posterior thalamic radiation, posterior corona radiata, superior longitudinal fasciculus, retrolenticular part of the internal capsule, tapetum of the right hemisphere, and splenium of the corpus callosum. The aberrant white matter integrity of right superior longitudinal fasciculus may be a potential neural basis of cognitive impairment in schizophrenia as evidenced by significant associations between its FA values and processing speed in our sample. However, no significant correlations were found between FA values and the PANSS subscale score in patients with schizophrenia in our study.
Cognitive deficits have been identified as a core feature of schizophrenia [21,37]. A meta-analysis found that schizophrenia has cognitive impairments, including speed of processing, working memory, verbal learning, reasoning/problem solving, visual learning, attention/vigilance, and social cognition, particularly in speed of processing and attention/vigilance [33]. Our study used the same instrument to demonstrate that dn-FES patients show severe and widespread cognitive deficits in all domains, particularly in speed of processing and working memory, with the least impairment shown in social cognition. However, we found that working memory, not attention/vigilance, was significantly lower in patients than in healthy participants. Similar to our study, one study found that cognitive deficits are present at the onset of schizophrenia but that the speed of processing and executive functions remain stable after ten years [38]. These concordant results may support that a subgroup of patients with schizophrenia would benefit from cognitive remediation.
Patients showed a significantly lower FA in the posterior thalamic radiation, posterior corona radiata, superior longitudinal fasciculus, retrolenticular part of the internal capsule, tapetum, sagittal stratum, and inferior longitudinal fasciculus of the right hemisphere, as well as the splenium of the corpus callosum. The results were largely consistent with previous DTI studies [9,15,39,40]. These findings support the disconnection hypothesis as the primary pathophysiology of schizophrenia, indicating disrupted white matter integrity between these brain areas are present even at the onset of schizophrenia. One study [15] evaluated DTI data from 1963 patients with schizophrenia and 2359 healthy participants and found that FA is lower globally across the whole-brain white matter skeleton; specifically, the anterior corona radiata, body, and genu of the corpus callosum showed the greatest effects. However, in our results, we found reduced FA across the posterior corona radiata and a portion of the splenium of the corpus callosum. To date, the most consistent research findings in the schizophrenia have included aberrant interhemispheric connectivity [15,41], and this was also demonstrated in our study. Notably, white matter changes are almost always observed in the right hemisphere; for example, one study also found more extensive disruption in the right hemisphere [42]. Although it is difficult to interpret, these results may suggest that a pathological disruption of white matter integrity in the right hemisphere is typical for first-episode schizophrenia.
Increasingly, studies have shown cognitive deficits are associated with white matter integrity, suggesting that disruption of white matter integrity leads to cognitive dysfunction [25,27,43,44]. One study found that deficits in working memory and visual learning are correlated with lower FA in the left longitudinal fasciculus [25]. However, we found that deficits in speed of processing is correlated with reduced FA value in the right superior longitudinal fasciculus. The precise functions of the superior longitudinal fasciculus are not fully understood. The superior longitudinal fasciculus is a major associative connection between the superior parietal, angular gyrus, and temporal and ipsilateral frontal lobes [45]. Recent findings [25,43] have indicated that the superior longitudinal fasciculus mediates the transmission of neurotransmitters, such as glutamate, the most common excitatory neurotransmitter [46], and may explain the lower speed of processing in schizophrenia patients. In addition, a new finding demonstrated that the interaction between glucose and FA in the longitudinal fasciculus is associated with the Trail Making Test Part A, which is part of speed of processing [47]. The relationship between the reduced FA in the longitudinal fasciculus and speed of processing may provide a biomarker for clinical studies of schizophrenia.
We also found that deficits in social cognition are associated with lower FA values in the posterior thalamic radiation, posterior corona radiata, superior longitudinal fasciculus, retrolenticular part of the internal capsule, and tapetum of the right hemisphere, as well as the splenium of the corpus callosum. The correlation between social cognition impairment and white matter integrity is similar to the findings of a previous study [48]. In addition, another recent study found that social cognition impairment is related to white matter disarray of the splenium, corpus callosum, forceps major, and inferior longitudinal fasciculus [9]. These fiber tracts could be partially included in the revised face perception circuitry, which is composed of the temporo-occipital regions and the frontal-limbic system [49]. Furthermore, Andreasen demonstrated the connection between the thalamus and the frontal cortex plays a vital role in social cognition in schizophrenia [50,51]. Therefore, it is plausible that the posterior thalamic radiation, posterior corona radiata, and superior longitudinal fasciculus (including the connection between the temporo-occipital region, frontal cortex, and thalamus) may be associated with social function in patients with schizophrenia. Another study [52] reported that motor dexterity is related to FA in the left frontal lobe, including the forceps minor, inferior fronto-occipital fasciculus, and anterior thalamic radiation. In addition, executive function impairment was associated with reduced FA values in the left and right anterior thalamic radiation, forceps minor, inferior fronto-occipital fasciculus, and left superior and inferior longitudinal fasciculus. However, we failed to find significant correlation between the aberrant white matter integrity and attention/vigilance, verbal learning, visual learning, reasoning, and problem solving or working memory in patients and controls. This may be because FA can be affected by multiple pathological processes, potentially leading to negative results.
Recently, some cross-sectional studies examining the relationship between the integrity of white matter and clinical variables in schizophrenia have had inconsistent findings. One study reported that negative symptoms are negatively correlated with FA values in the forceps major, left superior longitudinal fasciculus, anterior thalamic radiation, and inferior fronto-occipital fasciculus [25]. Nevertheless, other studies reported that positive symptoms are negatively correlated with FA values in the left superior longitudinal fasciculus [53] but positively correlated with FA values in the left fronto-occipital fasciculus and left inferior longitudinal fasciculus [54]. Interestingly, a recent study reported that PANSS positive factor was negatively associated with reduced FA in the left inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and forceps major; moreover, PANSS negative factor was negatively related to FA in the left inferior longitudinal fasciculus [9]. Nevertheless, some studies failed to find correlations between the FA value with clinical symptoms [43,55-57]. We also failed to detect the correlation between reduced FA and positive factor, negative factor, excited factor, disorganized factor, or depressed factor in dn-FES patients. This may be because our study was of short duration and included dn-FES patients with mild to moderate symptoms and small variances in PANSS scores, potentially leading to negative results in correlational analyses between FA and the severity of clinical symptoms. In addition, the size of the sample and the heterogeneity of the patients’ characteristics may have also led to discrepancies.
Our study has some limitations. First, the years of education were not matched in the patient and control groups. However, the MCCB subtest scores were corrected by years of education so that the variable could be compared at the same level. Second, the cross-sectional study design had a limited capacity to demonstrate direct causal relationship between white matter deficits and cognitive deficits in patients with schizophrenia. Third, the size of the sample was relatively small. Ultimately, a longitudinal study with a larger sample size is warranted to reveal the relationship between white matter deficits and cognitive deficits in schizophrenia.
In conclusion, this study showed not only robust and widespread white matter changes across multiple regions but also cognitive deficits across all domains in patients with first-episode schizophrenia. In addition, our study suggests a correlational relationship exists between specific brain structures and cognitive deficits in schizophrenia, providing clues about the neural basis of the disease and a potential biomarker for clinical studies.
Acknowledgements
We would like to thanks for all of the patients and controls who made contributions to our study, and psychiatrists for their help in the recruitment and diagnosis of dn-FES patients and others participated in this study. This work was supported by the National Key Research and Development Program of China (2016YFC1306800).
Disclosure of conflict of interest
None.
References
- 1.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 2.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, Egan GF, Pantelis C. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanaan RA, Picchioni MM, McDonald C, Shergill SS, McGuire PK. White matter deficits in schizophrenia are global and don’t progress with age. Aust N Z J Psychiatry. 2017;51:1020–1031. doi: 10.1177/0004867417700729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 8.Kristensen TD, Mandl RCW, Raghava JM, Jessen K, Jepsen JRM, Fagerlund B, Glenthøj LB, Wenneberg C, Krakauer K, Pantelis C, Nordentoft M, Glenthøj BY, Ebdrup BH. Widespread higher fractional anisotropy associates to better cognitive functions in individuals at ultra-high risk for psychosis. Hum Brain Mapp. 2019;40:5185–5201. doi: 10.1002/hbm.24765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X, Sui Y, Yao J, Lv Y, Zhang X, Jin Z, Chen L, Zhang X. Reduced white matter integrity and facial emotion perception in never-medicated patients with first-episode schizophrenia: a diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:57–64. doi: 10.1016/j.pnpbp.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Reid MA, White DM, Kraguljac NV, Lahti AC. A combined diffusion tensor imaging and magnetic resonance spectroscopy study of patients with schizophrenia. Schizophr Res. 2016;170:341–350. doi: 10.1016/j.schres.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigucci S, Santi G, Corigliano V, Imola A, Rossi-Espagnet C, Mancinelli I, De Pisa E, Manfredi G, Bozzao A, Carducci F, Girardi P, Comparelli A. White matter microstructure in ultra-high risk and first episode schizophrenia: a prospective study. Psychiatry Res Neuroimaging. 2016;247:42–48. doi: 10.1016/j.pscychresns.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Rotarska-Jagiela A, Oertel-Knoechel V, DeMartino F, van de Ven V, Formisano E, Roebroeck A, Rami A, Schoenmeyer R, Haenschel C, Hendler T, Maurer K, Vogeley K, Linden DE. Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 2009;174:9–16. doi: 10.1016/j.pscychresns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Knöchel C, O’Dwyer L, Alves G, Reinke B, Magerkurth J, Rotarska-Jagiela A, Prvulovic D, Hampel H, Linden DE, Oertel-Knöchel V. Association between white matter fiber integrity and subclinical psychotic symptoms in schizophrenia patients and unaffected relatives. Schizophr Res. 2012;140:129–135. doi: 10.1016/j.schres.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Faria AV, Crawford J, Ye C, Hsu J, Kenkare A, Schretlen D, Sawa A. Relationship between neuropsychological behavior and brain white matter in first-episode psychosis. Schizophr Res. 2019;208:49–54. doi: 10.1016/j.schres.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, Andreassen OA, Arango C, Banaj N, Bouix S, Bousman CA, Brouwer RM, Bruggemann J, Bustillo J, Cahn W, Calhoun V, Cannon D, Carr V, Catts S, Chen J, Chen JX, Chen X, Chiapponi C, Cho KK, Ciullo V, Corvin AS, Crespo-Facorro B, Cropley V, De Rossi P, Diaz-Caneja CM, Dickie EW, Ehrlich S, Fan FM, Faskowitz J, Fatouros-Bergman H, Flyckt L, Ford JM, Fouche JP, Fukunaga M, Gill M, Glahn DC, Gollub R, Goudzwaard ED, Guo H, Gur RE, Gur RC, Gurholt TP, Hashimoto R, Hatton SN, Henskens FA, Hibar DP, Hickie IB, Hong LE, Horacek J, Howells FM, Hulshoff Pol HE, Hyde CL, Isaev D, Jablensky A, Jansen PR, Janssen J, Jönsson EG, Jung LA, Kahn RS, Kikinis Z, Liu K, Klauser P, Knöchel C, Kubicki M, Lagopoulos J, Langen C, Lawrie S, Lenroot RK, Lim KO, Lopez-Jaramillo C, Lyall A, Magnotta V, Mandl RCW, Mathalon DH, McCarley RW, McCarthy-Jones S, McDonald C, McEwen S, McIntosh A, Melicher T, Mesholam-Gately RI, Michie PT, Mowry B, Mueller BA, Newell DT, O’Donnell P, Oertel-Knöchel V, Oestreich L, Paciga SA, Pantelis C, Pasternak O, Pearlson G, Pellicano GR, Pereira A, Pineda Zapata J, Piras F, Potkin SG, Preda A, Rasser PE, Roalf DR, Roiz R, Roos A, Rotenberg D, Satterthwaite TD, Savadjiev P, Schall U, Scott RJ, Seal ML, Seidman LJ, Shannon Weickert C, Whelan CD, Shenton ME, Kwon JS, Spalletta G, Spaniel F, Sprooten E, Stäblein M, Stein DJ, Sundram S, Tan Y, Tan S, Tang S, Temmingh HS, Westlye LT, Tønnesen S, Tordesillas-Gutierrez D, Doan NT, Vaidya J, van Haren NEM, Vargas CD, Vecchio D, Velakoulis D, Voineskos A, Voyvodic JQ, Wang Z, Wan P, Wei D, Weickert TW, Whalley H, White T, Whitford TJ, Wojcik JD, Xiang H, Xie Z, Yamamori H, Yang F, Yao N, Zhang G, Zhao J, van Erp TGM, Turner J, Thompson PM, Donohoe G. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23:1261–1269. doi: 10.1038/mp.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keymer-Gausset A, Alonso-Solís A, Corripio I, Sauras-Quetcuti RB, Pomarol-Clotet E, Canales-Rodriguez EJ, Grasa-Bello E, Álvarez E, Portella MJ. Gray and white matter changes and their relation to illness trajectory in first episode psychosis. Eur Neuropsychopharmacol. 2018;28:392–400. doi: 10.1016/j.euroneuro.2017.12.117. [DOI] [PubMed] [Google Scholar]
- 17.Tan AS, Chew QH, Sim K. Cerebral white matter changes in deficit and non-deficit subtypes of schizophrenia. J Neural Transm (Vienna) 2020;127:1073–1079. doi: 10.1007/s00702-020-02207-w. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Yang M, Du X, Liao W, Chen D, Fan F, Xiu M, Jia Q, Ning Y, Huang X, Wu F, Soares JC, Cao B, Wang L, Chen H. Glucose disturbances, cognitive deficits and white matter abnormalities in first-episode drug-naive schizophrenia. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0478-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2014;24:101–110. doi: 10.1111/j.1552-6569.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- 20.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 21.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 22.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Meier MH, Caspi A, Reichenberg A, Keefe RS, Fisher HL, Harrington H, Houts R, Poulton R, Moffitt TE. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry. 2014;171:91–101. doi: 10.1176/appi.ajp.2013.12111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hidese S, Ota M, Matsuo J, Ishida I, Hiraishi M, Teraishi T, Hattori K, Kunugi H. Association between the scores of the Japanese version of the brief assessment of cognition in schizophrenia and whole-brain structure in patients with chronic schizophrenia: a voxel-based morphometry and diffusion tensor imaging study. Psychiatry Clin Neurosci. 2017;71:826–835. doi: 10.1111/pcn.12560. [DOI] [PubMed] [Google Scholar]
- 25.Zeng B, Ardekani BA, Tang Y, Zhang T, Zhao S, Cui H, Fan X, Zhuo K, Li C, Xu Y, Goff DC, Wang J. Abnormal white matter microstructure in drug-naive first episode schizophrenia patients before and after eight weeks of antipsychotic treatment. Schizophr Res. 2016;172:1–8. doi: 10.1016/j.schres.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 26.Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seitz J, Zuo JX, Lyall AE, Makris N, Kikinis Z, Bouix S, Pasternak O, Fredman E, Duskin J, Goldstein JM, Petryshen TL, Mesholam-Gately RI, Wojcik J, McCarley RW, Seidman LJ, Shenton ME, Koerte IK, Kubicki M. Tractography analysis of 5 white matter bundles and their clinical and cognitive correlates in early-course schizophrenia. Schizophr Bull. 2016;42:762–771. doi: 10.1093/schbul/sbv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 29.van der Gaag M, Hoffman T, Remijsen M, Hijman R, de Haan L, van Meijel B, van Harten PN, Valmaggia L, de Hert M, Cuijpers A, Wiersma D. The five-factor model of the positive and negative syndrome scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006;85:280–287. doi: 10.1016/j.schres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 31.Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophr Res. 2012;137:246–250. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi C, Kang L, Yao S, Ma Y, Li T, Liang Y, Cheng Z, Xu Y, Shi J, Xu X, Zhang C, Franklin DR, Heaton RK, Jin H, Yu X. The MATRICS Consensus Cognitive Battery (MCCB): co-norming and standardization in China. Schizophr Res. 2015;169:109–115. doi: 10.1016/j.schres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Wang Y, Hu Y, Zhu Y, Zhang T, Wang J, Ma K, Shi C, Yu X, Li C. Meta-analysis of cognitive function in Chinese first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) profile of impairment. Gen Psychiatr. 2019;32:e100043. doi: 10.1136/gpsych-2018-100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 36.Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keefe RS. Should cognitive impairment be included in the diagnostic criteria for schizophrenia? World Psychiatry. 2008;7:22–28. doi: 10.1002/j.2051-5545.2008.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanelli J, Mollon J, Sandin S, Morgan C, Dazzan P, Pilecka I, Reis Marques T, David AS, Morgan K, Fearon P, Doody GA, Jones PB, Murray RM, Reichenberg A. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176:811–819. doi: 10.1176/appi.ajp.2019.18091088. [DOI] [PubMed] [Google Scholar]
- 39.Ruef A, Curtis L, Moy G, Bessero S, Badan Bâ M, Lazeyras F, Lövblad KO, Haller S, Malafosse A, Giannakopoulos P, Merlo M. Magnetic resonance imaging correlates of first-episode psychosis in young adult male patients: combined analysis of grey and white matter. J Psychiatry Neurosci. 2012;37:305–312. doi: 10.1503/jpn.110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tesli N, Westlye LT, Storvestre GB, Gurholt TP, Agartz I, Melle I, Andreassen OA, Haukvik UK. White matter microstructure in schizophrenia patients with a history of violence. Eur Arch Psychiatry Clin Neurosci. 2019 doi: 10.1007/s00406-019-00988-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Klauser P, Baker ST, Cropley VL, Bousman C, Fornito A, Cocchi L, Fullerton JM, Rasser P, Schall U, Henskens F, Michie PT, Loughland C, Catts SV, Mowry B, Weickert TW, Shannon Weickert C, Carr V, Lenroot R, Pantelis C, Zalesky A. White matter disruptions in schizophrenia are spatially widespread and topologically converge on brain network hubs. Schizophr Bull. 2017;43:425–435. doi: 10.1093/schbul/sbw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Lai Y, Wang X, Hao C, Chen L, Zhou Z, Yu X, Hong N. Reduced white matter integrity and cognitive deficit in never-medicated chronic schizophrenia: a diffusion tensor study using TBSS. Behav Brain Res. 2013;252:157–163. doi: 10.1016/j.bbr.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 44.Gas C, Canales-Rodríguez EJ, Radua J, Abasolo N, Cortés MJ, Salvadó E, Muntané G, Alemán-Gómez Y, Julià T, Marsal S, Sanjuan J, Guitart M, Costas J, Martorell L, Pomarol-Clotet E, Vilella E. Discoidin domain receptor 1 gene variants are associated with decreased white matter fractional anisotropy and decreased processing speed in schizophrenia. J Psychiatr Res. 2019;110:74–82. doi: 10.1016/j.jpsychires.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Bernal B, Altman N. The connectivity of the superior longitudinal fasciculus: a tractography DTI study. Magn Reson Imaging. 2010;28:217–225. doi: 10.1016/j.mri.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Guo H, Sun D, Duan J, Rao X, Xu F, Manyande A, Tang Y, Wang J, Wang F. Elevated glutamate, glutamine and GABA levels and reduced taurine level in a schizophrenia model using an in vitro proton nuclear magnetic resonance method. Am J Transl Res. 2019;11:5919–5931. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Yang M, Du X, Liao W, Chen D, Fan F, Xiu M, Jia Q, Ning Y, Huang X, Wu F, Soares JC, Cao B, Wang L, Chen H. Correction: glucose disturbances, cognitive deficits and white matter abnormalities in first-episode drug-naive schizophrenia. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0478-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Rigucci S, Rossi-Espagnet C, Ferracuti S, De Carolis A, Corigliano V, Carducci F, Mancinelli I, Cicone F, Tatarelli R, Bozzao A, Girardi P, Comparelli A. Anatomical substrates of cognitive and clinical dimensions in first episode schizophrenia. Acta Psychiatr Scand. 2013;128:261–270. doi: 10.1111/acps.12051. [DOI] [PubMed] [Google Scholar]
- 49.Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- 50.Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 51.Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry. 1997;42:27–33. doi: 10.1177/070674379704200104. [DOI] [PubMed] [Google Scholar]
- 52.Pérez-Iglesias R, Tordesillas-Gutiérrez D, McGuire PK, Barker GJ, Roiz-Santiañez R, Mata I, de Lucas EM, Rodríguez-Sánchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167:451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- 53.Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophr Res. 2008;98:157–162. doi: 10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheung V, Chiu CP, Law CW, Cheung C, Hui CL, Chan KK, Sham PC, Deng MY, Tai KS, Khong PL, McAlonan GM, Chua SE, Chen E. Positive symptoms and white matter microstructure in never-medicated first episode schizophrenia. Psychol Med. 2011;41:1709–1719. doi: 10.1017/S003329171000156X. [DOI] [PubMed] [Google Scholar]
- 55.Guo W, Liu F, Liu Z, Gao K, Xiao C, Chen H, Zhao J. Right lateralized white matter abnormalities in first-episode, drug-naive paranoid schizophrenia. Neurosci Lett. 2012;531:5–9. doi: 10.1016/j.neulet.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 56.Fitzsimmons J, Rosa P, Sydnor VJ, Reid BE, Makris N, Goldstein JM, Mesholam-Gately RI, Woodberry K, Wojcik J, McCarley RW, Seidman LJ, Shenton ME, Kubicki M. Cingulum bundle abnormalities and risk for schizophrenia. Schizophr Res. 2020;215:385–391. doi: 10.1016/j.schres.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Long Y, Ouyang X, Liu Z, Chen X, Hu X, Lee E, Chen EYH, Pu W, Shan B, Rohrbaugh RM. Associations among suicidal ideation, white matter integrity and cognitive deficit in first-episode schizophrenia. Front Psychiatry. 2018;9:391. doi: 10.3389/fpsyt.2018.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]


