Abstract
This study was designed to assess the levels of human serum amyloid A (SAA) and C-reactive protein (CRP) in patients with coronavirus disease 2019 (COVID-19) to determine their prognostic value in predicting the severity of disease. Patients with COVID-19 who presented with acute respiratory distress syndrome (ARDS) shared distinct characteristics. For example, the patients were older, and had higher levels of inflammatory indicators [i.e., levels of CRP, SAA, procalcitonin (PCT), and interleukin-6; CRP-to-PCT ratio; SAA-to-CRP ratio; and neutrophil-to-lymphocyte ratio (NLR)], higher inflammatory cell counts (i.e., white blood cell count and neutrophil count), and lower lymphocyte counts compared with patients without ARDS. Patients without ARDS still exhibited mild illness and had elevated SAA levels but not CRP levels. In patients with elevated SAA and CRP levels, the NLR was statistically associated with disease severity. According to the receiver operating characteristic curve analysis, the combined predictive probability of CRP and SAA levels, along with white blood cell count, showed the highest area under the curve (AUC; 0.878), and was able to distinguish between patients with and without ARDS. The cut-off level for SAA to predict the severity of COVID-19 was 92.900, with a sensitivity of 95.8%, a specificity of 53.7%, and an AUC of 0.712. For patients with elevated levels of SAA but not CRP, a mild condition was predicted. For patients with elevated levels of both SAA and CRP, and a high NLR, a severe infection was predicted, requiring medical attention. Therefore, CRP and SAA levels demonstrate a prognostic value for predicting the severity of COVID-19.
Keywords: Coronavirus disease 2019 (COVID-19), CRP, human SAA, procalcitonin, neutrophil-to-lymphocyte ratio
Introduction
In December 2019, coronavirus disease 2019 (COVID-19) emerged in Wuhan, Hubei Province, China, as a result of an infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Owing to its extremely rapid rate of transmission [1], SARS-CoV-2 caused a large number of infections in a short period of time. COVID-19 remains a huge threat to the health of people worldwide, and many countries are facing a lack of medical resources [2]. Improving the utilization efficiency of medical resources has become a major focus globally. An accurate prediction of disease severity during the early stages of disease can help allocate hospital resources effectively and rationally. It is also essential to administer drug interventions during these early disease stages.
Both human SAA and CRP are acute reactive proteins that are elevated during the early stages of inflammation and infection. CRP is increased in cases of bacterial or mixed infections, but is only slightly, or not, increased in cases of viral infection. SAA is increased in both bacterial and viral infections [3], whereas procalcitonin (PCT) levels are often elevated in cases of bacterial infection. Mixed bacterial and viral infections may be responsible for the worsening of disease in patients with COVID-19. This study focused on the prognostic value of inflammatory markers such as SAA, CRP, and PCT in predicting COVID-19 severity. It also provides guidance on clinical decision-making during the treatment of patients with this disease.
Materials and methods
Subjects
In this study, 71 consecutively hospitalized patients were recruited from January 20 to February 29, 2020, at the First Affiliated Hospital of University of Science and Technology of China. The hospital, which is located in Hefei, Anhui Province, specializes in the treatment of patients with infectious disease. It was responsible for the unified treatments of all patients with COVID-19 assigned by the government in Hefei. All enrolled subjects were confirmed positive for SARS-CoV-2 by a viral nucleic acid test using a pharyngeal swab. This study gained approval by the hospital’s ethics committee, and informed consent was obtained from all enrolled patients. This study’s clinical registration number is ChiCTR2000032460. The inclusion criteria included a diagnosis of COVID-19 during hospitalization at the First Affiliated Hospital of University of Science and Technology of China and a confirmatory viral nucleic acid test for SARS-CoV-2. The exclusion criteria included refusal to be enrolled in the study and severe liver disease.
Data collection
We collected data on patient characteristics (e.g., gender, age, travel history to Wuhan, disease course, comorbidities, and symptoms) and inflammatory indicators (e.g., levels of CRP, SAA, and PCT; CRP-to-PCT ratio, SAA-to-PCT ratio; and SAA-to-CRP ratio) using the hospital’s electronic medical record system. The patients were divided into those with or without ARDS based on whether they developed ARDS during their hospitalization. The diagnostic criteria for ARDS were based on the Berlin definition [4]. The blood index was defined as the first serological test result within 24 hours of admission. Disease course was defined as the time from the onset of symptoms until examination by a doctor. Professional doctors (Meichao Chen and Yuanbo Wu) verified the data. Figure 1 shows a flow chart of the steps conducted in the study.
Figure 1.
Study flow chart. Study flow chart summarizing the research procedure performed. We recruited 71 hospitalized patients with COVID-19, who were then divided into two groups: with or without ARDS, according to the Berlin definition. Clinical data were analyzed and compared between the two groups, which allowed a determination of the prognostic value of CRP and SAA levels in predicting the severity of COVID-19.
Statistical analysis
Statistical software SPSS, version 20.0, was used to analyze the collected data. Continuous data and normally distributed data were expressed as the mean ± standard deviation using the Student’s t test for intergroup comparisons, whereas non-normally distributed data were expressed as the median (1/4, 3/4) using the Mann-Whitney U test. Categorical variables were expressed as counts (%) using the χ2 test for comparisons. Receiver operating characteristic (ROC) curves were drawn, cut-off values were determined, and the effects of inflammatory indicators on the severity of COVID-19 were analyzed. The cut-off values and their corresponding sensitivity and specificity were determined using the Youden index. P values less than 0.05 were considered statistically significant.
Results
General data
A total of 71 patients with COVID-19 were recruited, including 24 patients with ARDS and 47 patients without ARDS. Data were compared between the two groups in terms of general patient characteristics (e.g., gender, disease course, age), inflammatory indicators (e.g., levels of CRP, SAA, and PCT; CRP-to-PCT ratio; SAA-to-PCT ratio; and SAA-to-CRP ratio), and immune cell counts (e.g., white blood cells, neutrophils, monocytes, and lymphocytes).
Age, gender, and disease course were statistically different between the two groups. The age of patients with ARDS was generally higher than that of patients without ARDS (56.92 ± 15.87 years and 42.04 ± 15.96 years, respectively). Among patients with ARDS, 20 were male and 4 female. The disease course of patients with ARDS was longer than that of patients without ARDS (8.46 ± 3.83 and 5.54 ± 3.07, respectively). There was a statistically significant difference between the two groups in terms of the clinical symptom of chest tightness. Patients with ARDS tended to experience the feeling of chest tightness and had more significant comorbidities (cardiovascular diseases, cerebrovascular diseases, and other complications) than patients without ARDS. The baseline characteristics of hospitalized patients are shown in Table 1.
Table 1.
Baseline patient characteristics and laboratory results
| Total (n = 71) | Without ARDS (n = 47) | With ARDS (n = 24) | P value | ||
|---|---|---|---|---|---|
| Age, years ± SD | 47.07 ± 17.33 | 42.04 ± 15.96 | 56.92 ± 15.87 | 0.000 | |
| Gender, n (%) | Male | 44 (61.97) | 24 (33.80) | 20 (28.17) | 0.008 |
| Female | 27 (38.03) | 23 (32.39) | 4 (5.63) | ||
| Wuhan travel exposure | 20 (28.17) | 16 (22.54) | 4 (5.63) | 0.124 | |
| Course of the disease, days ± SD | 6.53 ± 3.60 | 5.54 ± 3.07 | 8.46 ± 3.83 | 0.001 | |
| Symptoms, n | Fever | 61 | 41 | 20 | 0.655 |
| Cough | 44 | 30 | 14 | 0.652 | |
| Sputum | 8 | 5 | 3 | 0.814 | |
| Chest tightness | 12 | 5 | 7 | 0.049 | |
| Fatigue | 12 | 6 | 6 | 0.193 | |
| Muscle soreness | 8 | 3 | 5 | 0.069 | |
| Poor appetite | 4 | 2 | 2 | 0.481 | |
| Headache | 4 | 1 | 3 | 0.073 | |
| Comorbidity, n | Cardiovascular and cerebrovascular | 19 | 6 | 13 | 0.000 |
| Other | 12 | 4 | 8 | 0.008 | |
| CRP, mg/L (CI) | 17.4 (3.8-50.4) | 7.5 (1.8-27.2) | 43.4 (18.35-92.98) | 0.000 | |
| SAA, ng/mL ± SD | 129.94 ± 84.28 | 109.52 ± 85.63 | 169.92 ± 66.56 | 0.002 | |
| PCT, ng/mL (CI) | 0.15 (0.1-0.19) | 0.13 (0.1-0.17) | 0.18 (0.13-0.24) | 0.002 | |
| CPR (CI) | 81 (26.34-299.47) | 39 (12-132.57) | 261.34 (76.5-493.25) | 0.000 | |
| SPR ± SD | 838.87 ± 661.19 | 756.92 ± 682.85 | 978.86 ± 610.83 | 0.194 | |
| SCR (CI) | 6.90 (3.21-18) | 8.57 (3.95-21) | 3.85 (1.56-9.31) | 0.015 | |
| IL-6, pg/L (CI) | 6.21 (5.1-7.06) | 5.38 (4.94-6.32) | 7.15 (6.38-10.92) | 0.000 | |
| White blood cell count, × 109/L (CI) | 5.48 (4.18-6.8) | 5.26 (4.04-6.54) | 6.55 (4.93-7.46) | 0.007 | |
| Neutrophil count, × 109/L (CI) | 3.92 (2.48-5.75) | 3.49 (2.25-4.83) | 5.47 (4.01-6.54) | 0.003 | |
| Monocyte count, × 109/L ± SD | 0.42 ± 0.26 | 0.41 ± 0.23 | 0.43 ± 0.32 | 0.726 | |
| Lymphocyte count, × 109/L ± SD | 1.19 ± 0.83 | 1.37 ± 0.93 | 0.84 ± 0.46 | 0.011 | |
| NLR (CI) | 3.50 (2.10-9.83) | 2.76 (1.45-4.95) | 7.54 (4.08-12.18) | 0.001 | |
Abbreviations: SD = standard deviation, CI = confidence interval, ARDS = acute respiratory distress syndrome, CRP = C-reactive protein, SAA = serum amyloid A, PCT = procalcitonin, CPR = CRP-to-PCT ratio, SPR = SAA-to-PCT ratio, SCR = SAA-to-CRP ratio, IL = interleukin, NLR = neutrophil-to-lymphocyte ratio.
For the inflammatory indicators, levels of CRP, SAA, PCT, and interleukin (IL)-6; the CRP-to-PCT ratio; SAA-to-CRP ratio; and NLR were all statistically different between the two patient groups. Notably, the inflammatory indices of patients with ARDS were generally higher than those of patients without ARDS. In patients with ARDS, both the white blood cell and neutrophil counts were significantly higher than those of patients without ARDS, whereas lymphocyte counts in patients with ARDS were lower than those in patients without ARDS, as shown in Table 1.
Predictive value of inflammatory indicators
In both patient groups, ROC curves were drawn for levels of CRP, SAA, and PCT, in addition to the CRP-to-PCT ratio, the SAA-to-PCT ratio, and the SAA-to-CRP ratio. The AUCs and cut-off values were calculated according to their specificity and sensitivity as predictive factors. The combined predictive probability of CRP and SAA levels, and white blood cell counts had the highest AUC, which was 0.878 (95% CI (confidence interval) 0.788-0.968). This indicated the high prognosis value of the combined predictive factors. We also validated the combined predictive value using the net reclassification index (NRI): NRI = (new sensitivity - old sensitivity) + (new specificity - old specificity). The NRI (combined prediction - CRP level) was 9.4%, and the NRI (combined prediction - SAA level) was 15.7%, suggesting that the predictive power of the new model and the proportion of correct classifications were improved after the addition of new biomarkers.
The AUC of CRP levels was 0.829 (95% CI 0.730-0.929); the sensitivity was 87.50% and the specificity was 68.30% for predicting disease severity when the cut-off level of CRP was 13.500 mg/L. The cut-off SAA level was set at 92.900 ng/mL, with a sensitivity of 95.8% and a specificity of 53.7% for predicting disease severity, and an AUC of 0.712 (95% CI 0.588-0.837), as shown in Figure 2 and Table 2.
Figure 2.
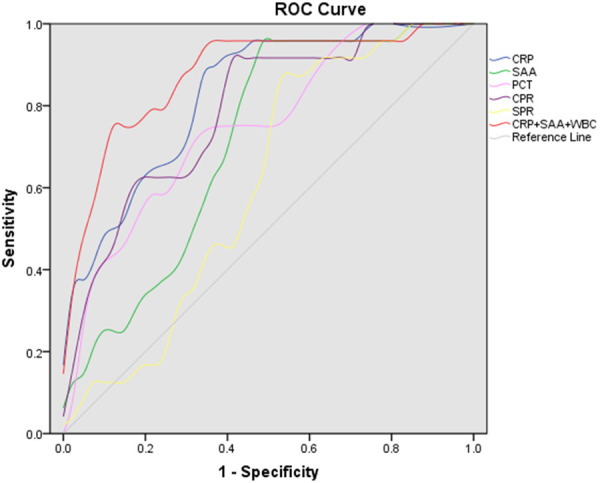
The ROC curves for inflammatory indicators to predict the severity of disease in hospitalized patients. ROC curves were drawn for the different inflammatory indicators. The predictive probability of CRP and SAA levels, and white blood cell counts were calculated using logistic regression, and the corresponding combined ROC was plotted.
Table 2.
AUCs and cut-off values for inflammatory indicators
| AUC (95% CI) | P value | Cut-off value | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| CRP, mg/L | 0.829 (0.730-0.929) | 0.000 | 13.500 | 0.875 | 0.683 |
| SAA, ng/ml | 0.712 (0.588-0.837) | 0.004 | 92.900 | 0.958 | 0.537 |
| PCT, ng/ml | 0.727 (0.594-0.859) | 0.002 | 0.155 | 0.708 | 0.683 |
| CPR | 0.788 (0.676-0.900) | 0.000 | 54.910 | 0.917 | 0.610 |
| SPR | 0.611 (0.474-0.747) | 0.138 | 504.980 | 0.875 | 0.488 |
| SCR | 0.677 (0.547-0.808) | 0.015 | 5.340 | 0.681 | 0.625 |
| CRP + SAA + WBC | 0.878 (0.788-0.968) | 0.000 | 0.421 | 0.750 | 0.902 |
Abbreviations: AUC = area under the curve, CI = confidence interval, CRP = C-reactive protein, SAA = serum amyloid A, PCT = procalcitonin, CPR = CRP-to-PCT ratio, SPR = SAA-to-PCT ratio, SCR = SAA-to-CRP ratio.
Combined prediction
Levels of both CRP and SAA were statistically significant between patients with and without ARDS. We assessed a combination of these levels to determine whether there was a predictive effect. To better explore the effects of this combination, patients were categorized into four groups, according to the levels of CRP and SAA: Group 1 - both CRP and SAA were elevated; Group 2 - CRP was elevated, but SAA was not; Group 3 - CRP was not elevated, but SAA was; and Group 4 - neither CRP nor SAA were elevated. No patients in Groups 3 and 4 had ARDS, as shown in Figure 3 and Table 3. In Group 1, there were 45 patients, including 24 with ARDS, whose NLR values were significantly higher than that of the 21 patients without ARDS (P = 0.036). In Group 3, patients exhibited only mild disease due to the absence of an obvious bacterial infection. When both CRP and SAA levels are increased and combined with a high NLR, a mixed bacterial/viral infection may be present, which is often severe. Therefore, the combination of CRP and SAA levels, along with the NLR, helped predict the severity of COVID-19 in this study.
Figure 3.
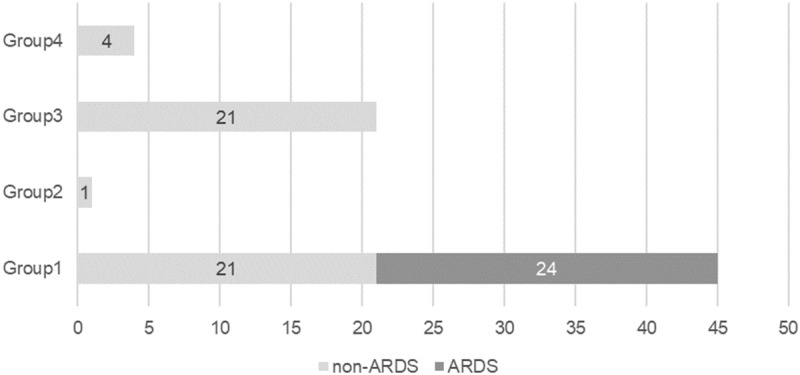
Combined prediction. Patients were divided into four groups according to their CRP and SAA levels, as defined in the text. No patients in Groups 2, 3, or 4 had ARDS. Group 1 was composed of 21 patients without ARDS and 24 patients with ARDS.
Table 3.
Combined prediction
| Total (n = 71) | Without ARDS (n = 47) | With ARDS (n = 24) | |
|---|---|---|---|
| Group 1 | 45 | 21 | 24 |
| Group 2 | 1 | 1 | 0 |
| Group 3 | 21 | 21 | 0 |
| Group 4 | 4 | 4 | 0 |
Discussion
Presently, SARS-CoV-2 is affecting people worldwide and challenging the medical systems of various countries. Because SARS-CoV-2 is highly infectious, hospitals are accepting a large number of patients in a short period of time and, as a result, many health systems are facing collapse [2]. Moreover, the existence of asymptomatic carriers increases the difficulty of controlling the current pandemic. This study focused on the value of inflammatory markers and immune cell counts to predict the severity of COVID-19.
SARS-CoV-2 infection causes changes in inflammatory markers and immune cells that are significantly different in patients with and without ARDS. Patients with ARDS had more complications and severe clinical manifestations. Furthermore, the virus enters the body through angiotensin-converting enzyme 2 (ACE2) receptors [5], whose expression is mainly concentrated on type II alveolar epithelial cells in the lungs [6]. ACE2 cellular expression in males appeared to be higher than that of females. Consistent with this finding, the number of male patients with ARDS was significantly higher than the number of female patients with ARDS in this study. However, this may be explained by the fact that individuals of Asian descent may have a higher proportion of cells expressing ACE2 compared with other individuals, and SARS-CoV-2 has different rates of infection among different races [7].
Viral infections can trigger inflammatory cytokine storms, which can result in worsening conditions or a poor prognosis in patients with COVID-19. In some severe cases, a cytokine storm, characterized by elevated levels of IL-1, IL-6, IL-12, and IFN-γ, was found [8]. A study of 99 COVID-19 patients in Wuhan Jinyintan Hospital showed that 52% of patients had elevated IL-6 levels [9]. In this study, levels of IL-6 were statistically different between patients with and without ARDS. However, levels of CRP and SAA, which are common inflammatory indicators, may be more conducive to universal screening. Increases in the levels of CRP, which is secreted by the liver, occur as a direct response to injury or infection. CRP activates the immune system, including the complement and mononuclear phagocytic system, resulting in clearance of viruses. During acute inflammation and infection, CRP levels can be correlated with disease severity [10], a finding also confirmed in this study. During the acute phase of disease, large amounts of cytokines (IL-1, IL-6, and TNF-α) stimulate the synthesis and release of SAA by liver cells. During viral infection, SAA levels are more sensitive than CRP [11], whereas CRP has greater specificity, for predicting disease severity. Lannergard [12] proposed that SAA is more sensitive than CRP for mild inflammatory lesions and can be used for viral infections, and in noninvasive and early invasive bacterial infections.
This study examined immune cell counts in patients with and without ARDS. There were significant differences in the counts of white blood cells, neutrophils, and lymphocytes. Moreover, the NLR significantly reflected the inflammatory state of the body. Francisco [13] proposed that both the NLR and LCR (lymphocyte-to-CRP ratio) helped predict the clinical severity of COVID-19 in a meta-analysis; these results suggested that an elevated NLR and a low LCR reflect an aggravated inflammatory process and might indicate a poor prognosis. A study by Du et al. [14] on the clinical characteristics of 85 fatal COVID-19 cases in Wuhan, China, showed that levels of CRP and PCT were significantly increased in these patients [14]. Li et al. [15] found that neutrophils, as well as levels of SAA, PCT, and CRP, were all increased compared with the initial analysis of deaths from COVID-19 in Wuhan. The levels of CRP significantly increase during bacterial and mixed infections, but not during viral infections. This differs from SAA levels, which become elevated in both bacterial and viral infections. Severe cases of disease may be associated with a mix of viral and bacterial infections. Therefore, using a combination of CRP and SAA levels, and NLR may better predict the severity of disease. The combined predictive probability of CRP and SAA levels, and white blood cell count had the highest AUC. In patients with elevated CRP and SAA levels, the NLR was statistically different, which is consistent with our other findings. In this study, we better defined the relationship between inflammatory indicators and mixed infection. Additionally, we also calculated the CPR, SPR, and SCR. Although the CPR and SCR were statistically significant, their predictive value was not as effective as using the combined prediction.
There are a few limitations in this study. The relationship between CRP and SAA levels, and the dynamic changes in immune cell counts, warrants further investigation. Furthermore, the 71 patients with COVID-19 included in this study were all from a single medical center. Our study could have been improved by including patients from other cities in China or countries to construct a more accurate predictive model.
Our results suggest that CRP, SAA, and PCT levels can be used to determine disease severity. The combined predictive probability of CRP and SAA levels, and white blood cell count greatly improve the sensitivity and specificity of predicting disease severity. For patients with elevated SAA levels but not elevated CRP levels, mild illness is predicted. In stark contrast, patients with elevated CRP and SAA levels, combined with a high NLR, may indicate a combined bacterial infection, which may be severe and require more medical attention.
Disclosure of conflict of interest
None.
References
- 1.Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brunink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 2.Maxmen A. How poorer countries are scrambling to prevent a coronavirus disaster. Nature. 2020;580:173–174. doi: 10.1038/d41586-020-00983-9. [DOI] [PubMed] [Google Scholar]
- 3.Frame NM, Kumanan M, Wales TE, Bandara A, Fandrich M, Straub JE, Engen JR, Gursky O. Structural basis for lipid binding and function by an evolutionarily conserved protein, serum amyloid A. J Mol Biol. 2020;432:1978–1995. doi: 10.1016/j.jmb.2020.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronson JK, Ferner RE. Drugs and the renin-angiotensin system in covid-19. BMJ. 2020;369:m1313. doi: 10.1136/bmj.m1313. [DOI] [PubMed] [Google Scholar]
- 7.Chowell G, Mizumoto K. The COVID-19 pandemic in the USA: what might we expect? Lancet. 2020;395:1093–1094. doi: 10.1016/S0140-6736(20)30743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhang J, Sheng H, Li H, Wang R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv Clin Chem. 2019;90:25–80. doi: 10.1016/bs.acc.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Lannergard A, Larsson A, Kragsbjerg P, Friman G. Correlations between serum amyloid A protein and C-reactive protein in infectious diseases. Scand J Clin Lab Invest. 2003;63:267–272. [PubMed] [Google Scholar]
- 13.Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): ameta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan: aretrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Wang L, Yan S, Yang F, Xiang L, Zhu J, Shen B, Gong Z. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]


