Abstract
Lung cancer, a leading cause of cancer-related mortalities worldwide and non-small cell lung cancer (NSCLC) is the main subtype of lung cancer. As a first-line chemotherapeutic drug used for NSCLC, acquired resistance retarded the clinical application of cisplatin (DDP). We herein reported long non-coding RNA SNHG9 was over-expressed in NSCLC tissues and cell lines compared with normal lung tissues and cell line; Increased SNHG9 was also observed in DDP resistant NSCLC tissues and cell lines compared with their DDP sensitive counterparts. Elevated expression of SNHG9 was associated with lower overall survival (OS) rate in NSCLC patients. Besides, silence of SNHG9 suppressed DDP resistance of NSCLC cells. Furthermore, CAPRIN1 was positively regulated by SNHG9 and mediated the promoting role of SNHG9 in DDP resistance of NSCLC cells. SNHG9 could be used as a potential target for DDP resistant NSCLC therapy.
Keywords: SNHG9, CAPRIN1, cisplatin resistance, NSCLC
Introduction
Lung cancer is the third most prevalent cancer and a leading cause of cancer-related mortalities worldwide [1-3]. Lung cancer includes two main subtypes: small cell lung cancer and non-small cell lung cancer (NSCLC) which contains lung squamous cell carcinoma, lung adenocarcinoma and large cell lung carcinoma [1,3,4]. NSCLC accounts for more than 80% of all lung cancers [1,5]. Surgical resection, chemotherapy, radiotherapy and targeted therapy are considered as main treatments for NSCLC currently [1,6]. However, patients with NSCLC always suffer from recurrence, metastasis and treatment resistance with a 5-year survival rate no more than 20% [7,8]. Cisplatin (DDP), a first-line chemotherapeutic drug used for NSCLC, could successfully delay the progression of NSCLC [9,10]. However, acquired drug resistance of DDP in patients with NSCLC retarded the clinical application [9]. Multiple mechanisms have been reported to be involve in DDP resistance of human NSCLC, such as LncRNA MEG3/miR-21-5p/SOX7 pathway [9], LncRNA NNT-AS1/MAPK/Slug pathway [11], LncRNA OR3A4-CDK1 pathway [12], Annexin A2/JNK/p53 pathway [13] etc. The detailed and varied molecular mechanisms of DDP resistance in human NSCLC are desirable for further study.
Long non-coding RNA (LncRNA), commonly more than 200 nucleotides in size without protein-coding capacity, is one of important types of non-coding RNAs which plays pivotal roles in nearly all human physiological processes [14-16]. Recently, increasing literatures unveiled that LncRNAs could act as key tumor promoters or tumor suppressors in human cancers [17,18]. For example, LncRNA RHPN1-AS1 promoted EMT and progression of human breast cancer [19]; LncRNA CASC19 played important roles in progression and prognosis of human gastric cancer [20]; LncRNA HOXA-AS2 promoted malignant progression of human NSCLC via regulating microRNA-216a-5p [21] etc. Long non-coding RNAs could directly interact with microRNAs (miRNAs) or proteins and regulate the expression of target genes [22]. As reported previously, LncRNA SNHG9 was low expressed in human pancreatic cancer tissues and was significantly associated with the prognosis of pancreatic cancer patients [23]; SNHG9/miR-199a-5p/Wnt2 axis regulated cell growth and aerobic glycolysis in glioblastoma [24]. However, the role of LncRNA SNHG9 in human NSCLC remains unknown, and the related regulating mechanisms involved in SNHG9 pathway in NSCLC need to be further explored.
In the current study, we have examined that SNHG9 was over-expressed in human NSCLC tissues compared with normal lung tissues in both TCGA database and the fresh tissues collected from our home hospital. In both NSCLC tissues and cell lines, we observed the increased expression of SNHG9 in DDP resistant NSCLC compared with their DDP sensitive counterparts. High level of SNHG9 was associated with low overall survival (OS) rate in NSCLC patients. Furthermore, knockdown of SNHG9 suppressed cell proliferation and promoted cell apoptosis in DDP resistant NSCLC cells. Depletion of SNHG9 decreased the IC50 of DDP resistant NSCLC cells for DDP. Moreover, CAPRIN1 was determined to interact with SNHG9 directly and was positively regulated by SNHG9. CAPRIN1 mediated the promoting role of SNHG9 in DDP resistance of NSCLC cells. Therefore, LncRNA SNHG9 was oncogenic and contributed to DDP resistance in human NSCLC. SNHG9 based drugs could be potentially used for adjuvant therapy in DDP resistant NSCLC.
Materials and methods
Tissue samples from NSCLC patients
Fifty pairs of fresh NSCLC tissues and adjacent normal lung tissues were collected from NSCLC patients who underwent surgery in the department of thoracic surgery, the First Affiliated Hospital of Anhui Medical University in 2013-2015. Twenty-six of these NSCLC patients had not received DDP based chemotherapy and they were DDP sensitive. On the other hand, 24 NSCLC patients had received DDP therapy and had got DDP resistance. These patients were followed up for more than 40 months and the OS rates were documented. Local approval from the Institutional Review Boards of Anhui Medical University was got before we performed the work. All of the experiments were carried out according to The Code of Ethics of the World Medical Association (Declaration of Helsinki). Each patient enrolled had signed an informed consent.
Cell lines
Human normal lung cell line BEAS-2B, NSCLC cell lines SK-MES-1, H460, A549 and H1299 were all acquired from ATCC (the American Type Culture Collection, Rockville, MD). DDP resistant cell lines A549/DDP and H1299/DDP were induced in our present laboratory. Briefly, A549 and H1299 cells were treated continuously with DDP until significant DDP resistance was observed by assessing cell viability. All of these cells were cultured in RPMI-1640 medium (Thermo Fisher Scientifc, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientifc) under 5% CO2 and 37°C.
RT-qPCR
RT-qPCR (RT-quantitative PCR) was performed to examine RNA levels essentially as described in previous studies [23,25]. In the present study, RNA levels of SNHG9 and CAPRIN1 were detected. GAPDH was detected as an internal control gene. The primers used were listed in Table 1.
Table 1.
Oligomers used in this study
| Name | Application | Sequence |
|---|---|---|
| qRT-SNHG9-F | qRT-PCR | GACTGCAGACCCCTAACCTT |
| qRT-SNHG9-R | qRT-PCR | ACCCGCATGCAGTGAGTTA |
| qRT-CAPRIN1-F | qRT-PCR | TCTCGGGGTGATCG ACAAGAA |
| qRT- CAPRIN1-R | qRT-PCR | CCCTTTGTTCATTCGTTCCTGG |
| qRT-GAPDH-F | qRT-PCR | TATGATGATATCAAGAGGGTAGT |
| qRT-GAPDH-R | qRT-PCR | TGTATCCAAACTCATTGTC ATAC |
| SNHG9-shRNA1-F | plasmid construction | ccggGCTGTCCCACGTCTTTCAAATggatccATTTGAAAGAC GTGGGACAGCtttttg |
| SNHG9-shRNA1-R | plasmid construction | aattcaaaaaGCTGTCCCACGTCTTTCAAATggatccATTT GAAAGACGTGGGACAGC |
| SNHG9-shRNA2-F | plasmid construction | ccggGCCGCGGCTCGGGAATCCACCggatccGGTGGATTCCCGAGCCGCGGCtttttg |
| SNHG9-shRNA2-R | plasmid construction | aattcaaaaaGCCGCGGCTCGGGAATCCACCggatccGGTGGATTCCCGAGCCGCGGC |
| SNHG9-clone-F | plasmid construction | ATTAGAATTC GCGGCCCGGGAATCTAC |
| SNHG9-clone-R | plasmid construction | ATTAGGATCCTTTTAGACAAAACAGCTTTATTT |
| CAPRIN1-clone-F | plasmid construction | ATTAGAATTC ATGCCCTCG GCCACCAGC |
| CAPRIN1-clone-R | plasmid construction | ATTAGGATCCTTAATTCACTTGCTGAGTG |
| SNHG9-DNA-1-sense | lncRNA pull down | (biotin)-AATCTACGTCACCCGAAAAGCGACTATAAA |
| SNHG9-DNA-1-antisense | lncRNA pull down | (biotin)-TTTATAGTCGCTTTTCGGGTGACGTAGATT |
| SNHG9-DNA-2-sense | lncRNA pull down | (biotin)-GGGAATCCACCCGAAGAGTGGCTATAAACG |
| SNHG9-DNA-2-antisense | lncRNA pull down | (biotin)-CGTTTATAGCCACTCTTCGGGTGGATTCCC |
| SNHG9-DNA-3-sense | lncRNA pull down | (biotin)-CGCCTGACACCGACGTCGCCAGGACCGCGG |
| SNHG9-DNA-3-antisense | lncRNA pull down | (biotin)-CCGCGGTCCTGGCGACGTCGGTGTCAGGCG |
Lentivirus production and stable cell line construction
shRNAs targeting SNGH9 were synthesized and cloned into pLKO.1 vector. The full length of SNGH9 DNA fragment and CDS of CAPRIN1 were cloned and inserted into pSin vector (pSin) according to the manufacturer’s instructions. All primers and shRNA sequences were listed in Table 1. Lentivirus production and transduction were performed as previously described [26,27]. Forty-eight hours after viral transduction, puromycin was used for selection of stably transduced cells.
Cell functional assays
In this study, MTT assay and cell colony formation assay were performed to evaluate cell proliferation essentially as described in previous studies [9,11]. Briefly, for MTT assay, 4×104 cells per well were seeded into 96-well cell plates, MTT evaluation was carried out after indicated time. For cell colony formation assay, 1000 cells per well were seeded into 6-well cell plates, cell colony formation was calculated after about two weeks.
IC50 analyzation was performed to evaluate cell sensitivity to DDP as described in former publications [11]. In brief, cells were treated with different dose of DDP for 48 h, cell viabilities were examined by MTT assay, and the IC50 value for DDP was analyzed according to the cell growth curves.
To evaluate cell apoptosis, caspase-3 activity assay and DNA fragmentation assay were carried out as described earlier [9,28,29]. For caspase-3 activity assay, the Caspase-3 Colorimetric Assay kit from Promega Corporation was used; for DNA fragmentation assay, cytoplasmic histone-associated DNA fragments were evaluated by a Nucleosome ELISA kit.
Biotin RNA pulldown assay
Biotin RNA pulldown assay was performed as previously described [30], to examine the interaction between CAPRIN1 protein and SNHG9. Briefly, sense or antisense biotin-labeled DNA oligomers targeting SNHG9 were incubated with lysates from A549/DDP and H1299/DDP cells. Streptavidin-coupled agarose beads (Invitrogen) were used to enrich the SNHG9 complex one hour after incubation. All steps were performed in RNase-free environment. The protein level of CAPRIN1 in the protein-RNA complex was next detected by western blot. Sequences of probes against SNHG9 were listed in Table 1.
RNA immunoprecipitation (IP) assay
RNA immunoprecipitation (IP) assay was conducted to examine the interaction between CAPRIN1 protein and SNHG9 essentially as described in former studies [31]. The CAPRIN1 protein-SNHG9 RNA complex was captured by anti-CAPRIN1 antibody (Proteintech Group, Inc., Chicago, USA), and the captured SNHG9 RNA was examined by RT-qPCR. The anti-IgG antibody (Proteintech Group, Inc., Chicago, USA) was used as control.
Western blot
Protein levels of CAPRIN1 was detected by western blot, which was carried out according to former publications [25]. CAPRIN1 Rabbit Polyclonal antibody (1:1000, 15112-1-AP, Proteintech Group, Inc., Chicago, USA) and Actin Mouse Monoclonal antibody (1:10000, 66009-1-Ig, Proteintech Group, Inc., Chicago, USA) were used. Actin was detected as an internal control.
Statistical analyses
In this study, each experiment was repeated for 3 times at least, and the figures represented the average. For RT-qPCR and cell functional assays, we used unpaired two-tailed t test to perform statistical analyzation. Kaplan-Meier curves were made to analyze the OS difference in NSCLC patients and log-rank test was used to calculate statistical significance. It was statistically significant when P<0.05.
Results
SNHG9 was upregulated in NSCLC and associated with DDP-resistance and poor prognosis of NSCLC patients
To explore the expression pattern of SNHG9 in NSCLC. Firstly, the expression level of SNHG9 in 553 NSCLC tissues and 59 normal lung tissues was analyzed from The Cancer Genome Atlas (TCGA) database. As shown in Figure 1A, the expression level of SNHG9 was significantly higher in NSCLC tissues compared with normal lung tissues. Similarly, the expression level of SNHG9 was much higher in 50 NSCLC tissues compared with adjacent normal lung tissues (Figure 1B) collected from patients in the department of thoracic surgery, the First Affiliated Hospital of Anhui Medical University, which was determined by RT-qPCR. Among the 50 NSCLC patients, 26 of them were DDP sensitive and 24 of them were DDP resistant. As shown in Figure 1C, higher level of SNGH9 was observed in DDP resistant NSCLC tissues as compared with DDP sensitive NSCLC tissues. For further study, these 50 NSCLC patients were followed up for more than 40 months and the correlation of SNGH9 level with overall survival (OS) rates was analyzed. OS rates were significantly lower in NSCLC patients with high level of SNGH9 compared with patients with low level of SNGH9 (P=0.0086) (Figure 1D). Moreover, the expression level of SNGH9 in normal lung cell line BEAS-2B, NSCLC cell lines SK-MES-1, H460, A549, H1299 and DDP resistant NSCLC cell lines A549/DDP, H1299/DDP was also examined by RT-qPCR. Concordant with the tissue result, the expression level of SNGH9 was much higher in NSCLC cell lines compared with normal lung cell line BEAS-2B and was dramatically higher in DDP resistant NSCLC cell lines compared with DDP sensitive NSCLC cell lines (Figure 1E). Therefore, high level of SNGH9 was positively associated with DDP resistance and poor survival rates.
Figure 1.
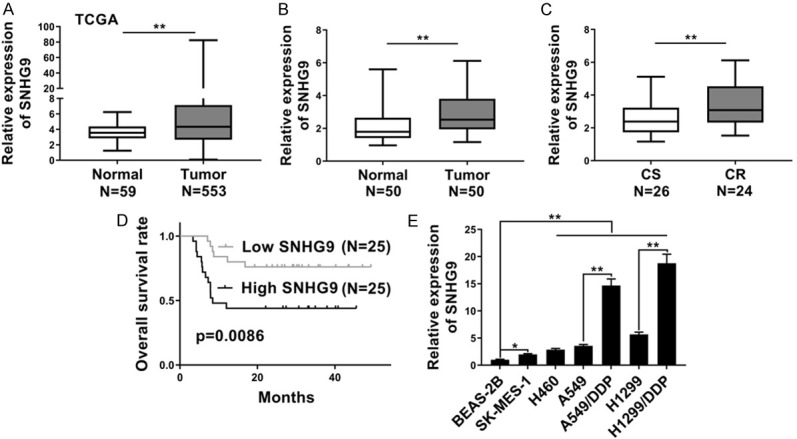
SNHG9 was upregulated in NSCLC and associated with DDP-resistance and poor prognosis of NSCLC patients. A. TCGA data analysis showed the expression level of SNHG9 was higher in 553 human NSCLC tissues compared with 59 normal lung tissues. B. RT-qPCR showed the expression level of SNHG9 was higher in 50 local NSCLC tissues compared with adjacent normal lung tissues. C. The expression level of SNHG9 in DDP sensitive (CS) were much lower than that in DDP resistant (CT) NSCLC tissues. D. Kaplan-Meier curves showed the different OS rates in high SNHG9 group and low SNHG9 group NSCLC patients. E. RT-qPCR showed the expression level of SNHG9 in BEAS-2B, SK-MES-1, H460, A549, A549/DDP, H1299 and H1299/DDP cells. GAPDH was detected as control for RT-qPCR. *P<0.05; **P<0.01.
SNHG9 knockdown enhanced DDP sensitivity in DDP-resistant NSCLC
To figure out the specific functions of SNHG9 in DDP resistance of NSCLC. Firstly, two shRNAs (sh-SNHG9#1 and sh-SNHG9#2) against SNHG9 were stably transfected into DDP resistant cell lines A549/DDP and H1299/DDP to knockdown the endogenous SNHG9 (Figure 2A). Then functional assays were verified, as examined by MTT assay, cell viabilities of both A549/DDP and H1299/DDP cells dramatically decreased after transfected with sh-SNGH9#1 or sh-SNGH9#2 compared with sh-Ctrl over a period of 4 days with DDP treatment (Figure 2B, 2C). Concordantly, SNGH9 knockdown significantly decreased cell colony formation of A549/DDP and H1299/DDP cells with DDP treatment (Figure 2D). Moreover, in A549/DDP and H1299/DDP cells, inhibition of SNGH9 by sh-SNGH9#1 or sh-SNGH9#2 also dramatically decreased the IC50 for DDP (Figure 2E); increased cell apoptosis of A549/DDP and H1299/DDP cells after DDP treatment was also determined by caspase-3 activity (Figure 2F) and DNA fragmentation (Figure 2G). Taken together, knockdown of SNGH9 promoted DDP sensitivity in DDP resistant NSCLC cells.
Figure 2.
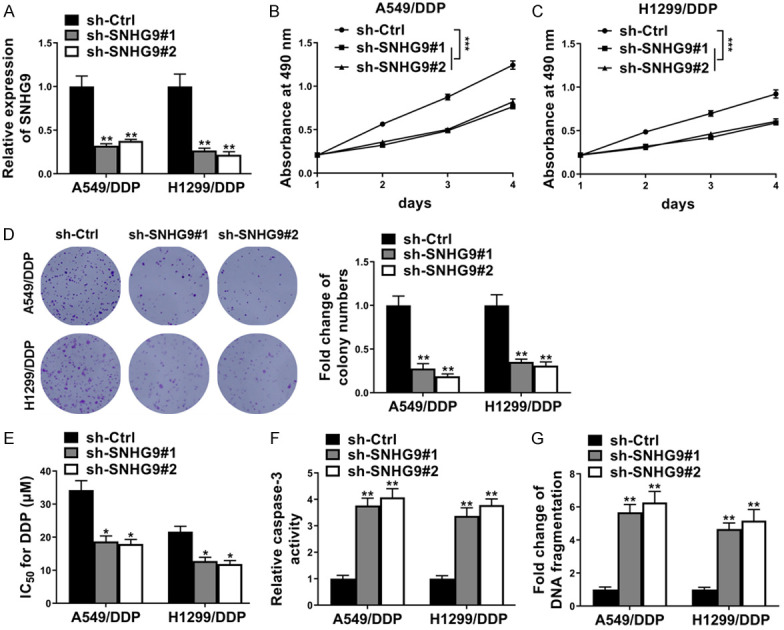
SNHG9 knockdown enhanced DDP sensitivity in DDP-resistant NSCLC cells. (A) RT-qPCR showed SNHG9 level in A549/DDP and H1299/DDP cells stably transfected sh-SNGH9#1/sh-SNGH9#2 or sh-Ctrl. GAPDH was detected as control. In A549/DDP and H1299/DDP cells with the treatment of DDP (5 μM), (B and C) MTT assay showed SNGH9 knockdown decreased cell viabilities during a period of 4 days; (D) cell colony formation assay showed SNGH9 knockdown decreased cell colony formation; (E) SNGH9 knockdown decreased IC50 for DDP; (F) caspase-3 activity assay and (G) DNA fragmentation assay showed SNGH9 knockdown increased cell apoptosis. *P<0.05; **P<0.01.
SNHG9 upregulated CAPRIN1 through direct interaction
For further study, potential targets that might be directly interacted with SNHG9 were searched for by using starBase v2.0 (http://starbase.sysu.edu.cn/). Several candidate proteins (including HuR, elF4AIII, DGCR8, FMRP, CAPRIN1 etc.) were predicted to be direct targets of SNHG9 (Figure 3A). Among these potential targets, CAPRIN1 was examined to be directly interacted with SNHG9 as CAPRIN1 could be pulled down by SNHG9-DNA-antisense biotin probe in both A549/DDP and H1299/DDP cells, which was determined by biotin RNA pulldown assay (Figure 3B-D). To confirm this result, RNA immunoprecipitation assay was carried out and SNHG9 could be enriched by CAPRIN1 antibody in both A549/DDP and H1299/DDP cells (Figure 3E-G). Moreover, the protein level of CAPRIN1 significantly decreased in both A549/DDP and H1299/DDP cells stably transfected with sh-SNGH9#1 or sh-SNGH9#2 compared with sh-Ctrl, as determined by western blot (Figure 3H). In addition, SNGH9 overexpression dramatically enhanced the protein level of CAPRIN1 as shown in Figure 3I. Therefore, SNHG9 directly interacted with CAPRIN1 protein and positively regulated the expression of CAPRIN1.
Figure 3.
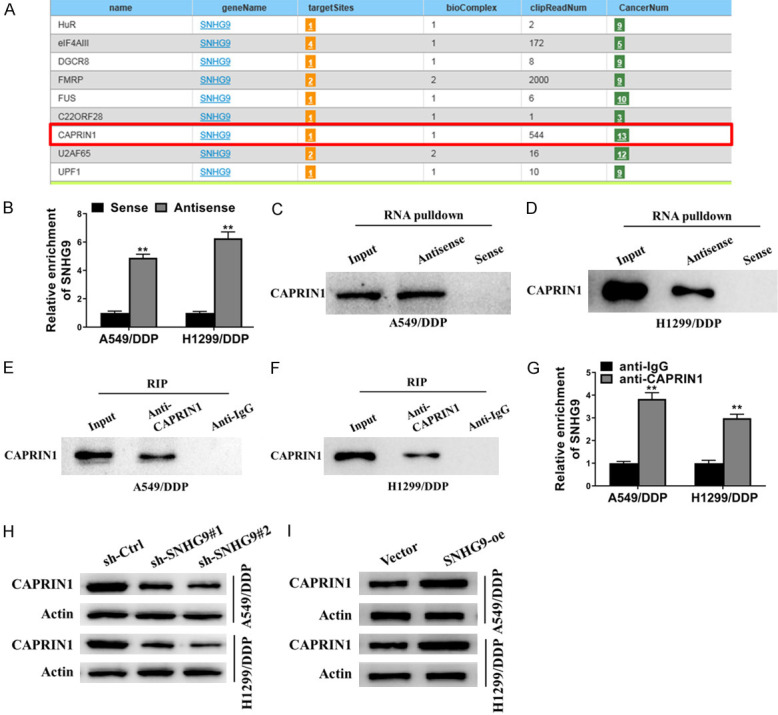
SNHG9 upregulated CAPRIN1 through direct interaction. A. StarBase v2.0 (http://starbase.sysu.edu.cn/) scanning found potential target proteins of SNHG9. B-D. Biotinylated RNA pulldown assay showed SNHG9-DNA-antisense biotin probes enriched more SNHG9 and CAPRIN1 protein than SNHG9-DNA-sense probes in A549/DDP and H1299/DDP cell lysates. E-G. RNA immunoprecipitation assay showed anti-CAPRIN1 antibody captured more CAPRIN1 protein and SNHG9 compared with control IgG in A549/DDP and H1299/DDP cell lysates. H. Western blot showed sh-SNGH9#1/sh-SNGH9#2 decreased the protein level of CAPRIN1 in A549/DDP and H1299/DDP cells. I. Western blot showed forced expression of SNGH9 (SNGH9 overexpressing plasmid (SNGH9-oe) transfection) increased the protein level of CAPRIN1 in A549/DDP and H1299/DDP cells. Actin was detected as control for western blot. *P<0.05; **P<0.01.
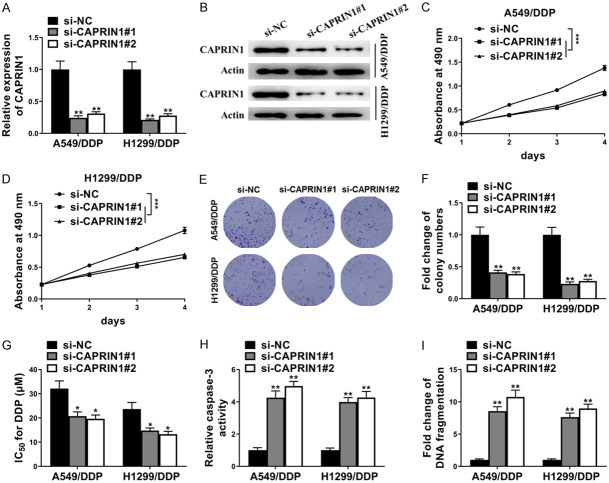
CAPRIN1 inhibition enhanced DDP sensitivity in DDP-resistant NSCLC
We next examined the role of CAPRIN1 in DDP resistance of NSCLC cells. As shown in Figure 4A and 4B, si-CAPRIN1#1 and si-CAPRIN1#2 dramatically decreased both RNA and protein levels of CAPRIN1 in A549/DDP and H1299/DDP cells. Cell viabilities of A549/DDP and H1299/DDP cells were significantly decreased after depletion of CAPRIN1 over a period of 4 days with DDP treatment (Figure 4C, 4D). Meanwhile, cell colony formation of A549/DDP and H1299/DDP cells with DDP treatment was significantly suppressed by si-CAPRIN1#1 or si-CAPRIN1#2 as shown in Figure 4E and 4F. The IC50 of A549/DDP and H1299/DDP cells for DDP was also decreased after transfection with si-CAPRIN1#1 or si-CAPRIN1#2 compared with control (Figure 4G). Concordantly, depletion of CAPRIN1 dramatically promoted cell apoptosis of A549/DDP and H1299/DDP cells after DDP treatment as determined by caspase-3 activity assay and DNA fragmentation assay (Figure 4H, 4I). As a result, CAPRIN1 inhibition enhanced DDP sensitivity in DDP-resistant NSCLC cells.
Figure 4.
CAPRIN1 inhibition enhanced DDP sensitivity in DDP-resistant NSCLC. In A549/DDP and H1299/DDP cells, (A) RT-qPCR showed si-CAPRIN1#1, si-CAPRIN1#2 transfection decreased CAPRIN1 mRNA level; (B) Western blot showed si-CAPRIN1#1, si-CAPRIN1#2 transfection decreased CAPRIN1 protein level; (C and D) MTT assay showed CAPRIN1 knockdown decreased cell viabilities during a period of 4 days with the treatment of DDP (5 μM); (E and F) Cell colony formation assay showed CAPRIN1 knockdown decreased cell colony formation with the treatment of DDP (5 μM); (G) CAPRIN1 knockdown decreased IC50 for DDP; (H) Caspase-3 activity assay and (I) DNA fragmentation assay showed SNGH9 knockdown increased cell apoptosis after the treatment of DDP (5 μM). *P<0.05; **P<0.01.
CAPRIN1 rescued the deceased DDP-resistance due to SNHG9 knockdown in DDP-resistant NSCLC
To examine whether CAPRIN1 played a pivotal role in SNHG9-mediated decreased DDP resistance of NSCLC cells, A549/DDP cells was co-transfected with shSNHG9#1 or sh-Ctrl and CAPRIN1 overexpressing plasmid (designated as CAPRIN1-oe) or control plasmid (Vector), and then cell functional experiments were performed. As shown in Figure 5A, protein level of CAPRIN1 decreased after co-transfected with shRNA against SNHG9 and control plasmid (sh-SNHG9#1+Vector), and this decrease was rescued by CAPRIN1 overexpression (sh-SNHG9#1+CAPRIN1-oe) in A549/DDP cells. Concordant with former results, depletion of SNHG9 by sh-SNHG9#1 dramatically decreased cell viability (determined by MTT assay) (Figure 5B), cell colony formation (Figure 5C), IC50 for DDP (Figure 5D) in A549/DDP cells with the treatment of DDP. However, these decreases were abrogated by co-transfection with CAPRIN1-oe (Figure 5B-D). Moreover, knockdown of SNHG9 significantly enhanced cell apoptosis as determined by caspase-3 activity assay and DNA fragmentation assay in A549/DDP cells after the treatment of DDP; these changes were all abolished by co-transfection with CAPRIN1-oe (Figure 5E, 5F). Therefore, CAPRIN1 rescued the deceased DDP-resistance due to SNHG9 knockdown in DDP-resistant NSCLC cells and the role of SNHG9 in DDP resistance of NSCLC cells was mediated by CAPRIN1.
Figure 5.
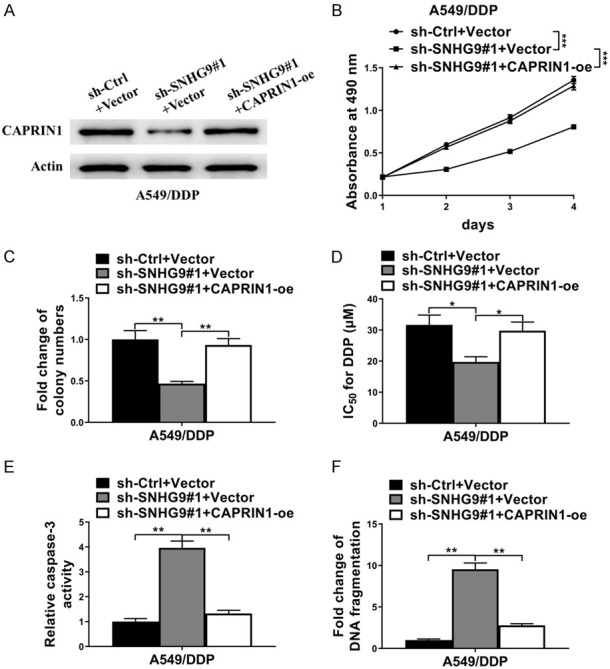
CAPRIN1 rescued the deceased DDP-resistance due to SNHG9 knockdown in DDP-resistant NSCLC. A549/DDP cells was co-transfected with sh-SNHG9#1/sh-Ctrl and CAPRIN1-oe/Vector. (A) Protein level of CAPRIN1 was examined by western blot. Actin was detected as control. (B) MTT assay, (C) Cell colony formation assay, (D) IC50 analyzation, (E) Caspase-3 activity assay and (F) DNA fragmentation assay showed SNGH9 knockdown decreased cell viabilities, cell colony formation, IC50 for DDP, increased cell apoptosis respectively, and forced expression of CAPRIN1 abolished these changes. *P<0.05; **P<0.01.
Discussion
In this study, we have documented that LncRNA SNHG9 was high expressed in human NSCLC tissues compared with normal lung tissues (including TCGA data and fresh tissues); expression level of SNHG9 increased significantly in DDP resistant NSCLC tissues compared with DDP sensitive NSCLC tissues; similar result was also found in induced DDP resistant cell lines A549/DDP and H1299/DDP compared with their respective parental cell lines. Moreover, SNHG9 was determined to be negatively correlated with OS rate in patients with NSCLC. shRNA mediated depletion of SNHG9 dramatically decreased cell viability, cell colony formation, IC50 for DDP, increased cell apoptosis in DDP resistant cells. In addition, SNHG9 was examined to directly interact with CAPRIN1 protein as determined by Biotin RNA pulldown assay and RNA-IP assay; the expression of CAPRIN1 was positively regulated by SNHG9. Furthermore, CAPRIN1 knockdown by siRNA concordantly decreased cell viability, cell colony formation, IC50 for DDP, increased cell apoptosis in DDP resistant cells A549/DDP and H1299/DDP. Rescue experiments showed forced expression of CAPRIN1 abolished the decreased cell proliferation, resistance to DDP and increased cell apoptosis induced by depletion of SNHG9 in A549/DDP cells. As reported in former studies, high level of SNHG9 was demonstrated to be associated with better prognosis in human pancreatic cancer patients [23]; SNHG9 promoted cell growth and aerobic glycolysis via miR-199a-5p/Wnt2 axis in glioblastoma [24]. We herein firstly reported the DDP resistance promoting role of SNHG9 in human NSCLC. The functions of SNHG9 were different among human pancreatic cancer, glioblastoma and NSCLC, which showed tissue specificity in different kinds of human cancers.
CAPRIN1 was examined to be the direct target of SNHG9 and SNHG9 positively regulated the expression of CAPRIN1. CAPRIN1 also acted as a promoting role in DDP resistance in DDP resistant NSCLC cells. As reported in previous studies, CAPRIN1 was an oncogene and promoted tumor growth and metastasis in human osteosarcoma [32]. CAPRIN1 was a novel target of miRNA-223 and promoted cell proliferation and invasion in human breast cancer cells [33]. In human hepatocellular carcinoma, CAPRIN1 was negatively regulated by miRNA-621 and promoted cell proliferation of hepatocellular carcinoma cells; abnormal high expression of CAPRIN1 was associated with poor prognosis in hepatocellular carcinoma patients [25,34]. Therefore, CAPRIN1 was a wide tumor promoter in various kinds of human cancers including NSCLC, and we herein firstly demonstrated the promoting role of CAPRIN1 in DDP resistance of human cancer.
In this present study, we determined that SNHG9 directly interacted with CAPRIN1 protein. As known widely, LncRNAs could bind and sponge miRNAs, and the target genes of these miRNAs were in line indirectly regulated by LncRNAs [22,35]. As reported previously, CAPRIN1 was a direct target of miRNA-223 and miRNA-621, which negatively regulated the expression of CAPRIN1 [25,33]. In this article, we did not examine the interaction between LncRNA SNHG9 and miRNA-223 or miRNA-621. Moreover, there might be some other miRNAs that directly targeted CAPRIN1. As a result, besides direct interaction between SNHG9 and CAPRIN1, there might be some other indirect pathways involved in SNHG9/CAPRIN1 regulation. We will perform this exploration in future.
In summary, LncRNA SNHG9 promoted DDP resistance of human NSCLC cells. CAPRIN1 was positively regulated by SNHG9 and mediated the promoting role of SNHG9 in DDP resistance of NSCLC. The expression level of SNHG9 was negatively associated with OS rate in NSCLC patients. SNHG9 could be used as a potential target for DDP resistant NSCLC therapy.
Acknowledgements
This work was supported by the Natural Science Research Projects at Higher Institutions in Anhui Province (KJ2018ZD017).
Disclosure of conflict of interest
None.
References
- 1.Kang XW, Kong FW, Wu SJ, Liu QS, Yang C, Wu XM, Zhang W. microRNA-612 suppresses the malignant development of non-small-cell lung cancer by directly targeting bromodomain-containing protein 4. Onco Targets Ther. 2019;12:4167–4179. doi: 10.2147/OTT.S204004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.An YX, Shang YJ, Xu ZW, Zhang QC, Wang Z, Xuan WX, Zhang XJ. STAT3-induced long noncoding RNA LINC00668 promotes migration and invasion of non-small cell lung cancer via the miR-193a/KLF7 axis. Biomed Pharmacother. 2019;116:109023. doi: 10.1016/j.biopha.2019.109023. [DOI] [PubMed] [Google Scholar]
- 3.Di X, Jin X, Ma H, Wang RM, Cong S, Tan C, Liu JY, Zhao M, Li RW, Wang K. The oncogene IARS2 promotes non-small cell lung cancer tumorigenesis by activating the AKT/MTOR pathway. Front Oncol. 2019;9:393. doi: 10.3389/fonc.2019.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harada H, Murayama S. Proton beam therapy in non-small cell lung cancer: state of the art. Lung Cancer (Auckl) 2017;8:141–145. doi: 10.2147/LCTT.S117647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng FX, Wang XQ, Zheng WX, Zhao J. Long noncoding RNA HOXA-AS2 promotes cell migration and invasion via upregulating IGF-2 in non-small cell lung cancer as an oncogene. Eur Rev Med Pharmacol Sci. 2019;23:4793–4799. doi: 10.26355/eurrev_201906_18064. [DOI] [PubMed] [Google Scholar]
- 6.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D’Amico TA, Demmy TL, Ganti AK, Govindan R, Grannis FW Jr, Horn L, Jahan TM, Jahanzeb M, Kessinger A, Komaki R, Kong FM, Kris MG, Krug LM, Lennes IT, Loo BW Jr, Martins R, O’Malley J, Osarogiagbon RU, Otterson GA, Patel JD, Pinder-Schenck MC, Pisters KM, Reckamp K, Riely GJ, Rohren E, Swanson SJ, Wood DE, Yang SC, Hughes M, Gregory KM. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–1271. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 8.Stewart DJ. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer. Crit Rev Oncol Hematol. 2010;75:173–234. doi: 10.1016/j.critrevonc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P, Chen D, Ma HB, Li Y. LncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21-5p/SOX7 axis. Onco Targets Ther. 2017;10:5137–5149. doi: 10.2147/OTT.S146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pirker R. Adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. Transl Lung Cancer Res. 2014;3:305–310. doi: 10.3978/j.issn.2218-6751.2014.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Y, Dong ZY, Wang JY. LncRNA NNT-AS1 is a major mediator of cisplatin chemoresistance in non-small cell lung cancer through MAPK/Slug pathway. Eur Rev Med Pharmacol Sci. 2018;22:4879–4887. doi: 10.26355/eurrev_201808_15624. [DOI] [PubMed] [Google Scholar]
- 12.Shang J, Xu YD, Zhang YY, Li M. Long noncoding RNA OR3A4 promotes cisplatin resistance of non-small cell lung cancer by upregulating CDK1. Eur Rev Med Pharmacol Sci. 2019;23:4220–4225. doi: 10.26355/eurrev_201905_17926. [DOI] [PubMed] [Google Scholar]
- 13.Feng XM, Liu H, Zhang ZJ, Gu YX, Qiu HS, He ZM. Annexin A2 contributes to cisplatin resistance by activation of JNK-p53 pathway in non-small cell lung cancer cells. J Exp Clin Cancer Res. 2017;36:123. doi: 10.1186/s13046-017-0594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher CA, Kumar-Sinha C, Cao XH, Kalyana-Sundaram S, Han B, Jing XJ, Sam L, Barrette T, Palanisamy N, Chinnaiyan AM. Transcriptome sequencing to detect gene fusions in cancer. Nature. 2009;458:97–101. doi: 10.1038/nature07638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes JCR, Acuna SM, Aoki JI, Floeter-Winter LM, Muxel SM. Long non-coding RNAs in the regulation of gene expression: physiology and disease. Noncoding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YY, Chen XN, Sun H, Wang HT. Long non-coding RNAs in the regulation of skeletal myogenesis and muscle diseases. Cancer Lett. 2018;417:58–64. doi: 10.1016/j.canlet.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Xu S, Xia H, Gao Z, Huang R, Tang E, Jiang X. Long noncoding RNA FEZF1-AS1 in human cancers. Clin Chim Acta. 2019;497:20–26. doi: 10.1016/j.cca.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Al-Rugeebah A, Alanazi M, Parine NR. MEG3: an oncogenic long non-coding RNA in different cancers. Pathol Oncol Res. 2019;25:859–874. doi: 10.1007/s12253-019-00614-3. [DOI] [PubMed] [Google Scholar]
- 19.Zheng SP, Lv PH, Su J, Miao KK, Xu H, Li MQ. Silencing of the long non-coding RNA RHPN1-AS1 suppresses the epithelial-to-mesenchymal transition and inhibits breast cancer progression. Am J Transl Res. 2019;11:3505–3517. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang WJ, Guo CA, Li R, Xu ZP, Yu JP, Ye Y, Zhao J, Wang J, Wang WA, Zhang A, Li HT, Wang C, Liu HB. Long non-coding RNA CASC19 is associated with the progression and prognosis of advanced gastric cancer. Aging (Albany NY) 2019;11:5829–5847. doi: 10.18632/aging.102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui TJ, Lin GS, Dai YM, Zheng JP, Chen Z, Chen Q, Zheng Y, Lin X. LncRNA HOXA-AS2 regulates microRNA-216a-5p to promote malignant progression of non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2019;23:264–273. doi: 10.26355/eurrev_201908_18656. [DOI] [PubMed] [Google Scholar]
- 22.Kong QL, Qiu M. Long noncoding RNA SNHG15 promotes human breast cancer proliferation, migration and invasion by sponging miR-211-3p. Biochem Biophys Res Commun. 2018;495:1594–1600. doi: 10.1016/j.bbrc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Zhang BG, Li CF, Sun ZX. Long non-coding RNA LINC00346, LINC00578, LINC00673, LINC00671, LINC00261, and SNHG9 are novel prognostic markers for pancreatic cancer. Am J Transl Res. 2018;10:2648–2658. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Qin DX, Jiang ZX, Zhang JN. SNHG9/miR-199a-5p/Wnt2 axis regulates cell growth and aerobic glycolysis in glioblastoma. J Neuropathol Exp Neurol. 2019;78:939–948. doi: 10.1093/jnen/nlz078. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, You W, Zhou HM, Chen ZQ, Han GY, Zuo XL, Zhang L, Wu JD, Wang XH. Downregulated miR-621 promotes cell proliferation via targeting CAPRIN1 in hepatocellular carcinoma. Am J Cancer Res. 2018;8:2116–2129. [PMC free article] [PubMed] [Google Scholar]
- 26.Sankaran VG, Menne TF, Scepanovic D, Vergilio JA, Ji P, Kim J, Thiru P, Orkin SH, Lander ES, Lodish HF. MicroRNA-15a and -16-1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13. Proc Natl Acad Sci U S A. 2011;108:1519–24. doi: 10.1073/pnas.1018384108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun LC, Song LB, Wan QF, Wu GW, Li XH, Wang YH, Wang J, Liu ZJ, Zhong XY, He XP, Shen SQ, Pan X, Li AL, Wang YL, Gao P, Tang HR, Zhang HF. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015;25:429–444. doi: 10.1038/cr.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang JM, Kamdar O, Le W, Rosen GD, Upadhyay D. Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am J Respir Cell Mol Biol. 2009;40:135–46. doi: 10.1165/rcmb.2007-0277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu YL, Kuo PL, Liu CF, Lin CC. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004;212:53–60. doi: 10.1016/j.canlet.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Cao L, Fan P, Mei Y, Wu M. LncRNA-MIF, a c-Myc-activated long non-coding RNA, suppresses glycolysis by promoting Fbxw7-mediated c-Myc degradation. EMBO Rep. 2016;17:1204–1220. doi: 10.15252/embr.201642067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang F, Zhang H, Mei Y, Wu M. Reciprocal regulation of HIF-1alpha and lincRNA-p21 modulates the Warburg effect. Mol Cell. 2014;53:88–100. doi: 10.1016/j.molcel.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Sabile AA, Arlt MJE, Muff R, Husmann K, Hess D, Bertz J, Langsam B, Aemisegger C, Ziegler U, Born W, Fuchs B. Caprin-1, a novel Cyr61-interacting protein, promotes osteosarcoma tumor growth and lung metastasis in mice. Biochim Biophys Acta. 2013;1832:1173–82. doi: 10.1016/j.bbadis.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Gong B, Hu HY, Chen J, Cao S, Yu J, Xue JX, Chen FH, Cai Y, He H, Zhang L. Caprin-1 is a novel microRNA-223 target for regulating the proliferation and invasion of human breast cancer cells. Biomed Pharmacother. 2013;67:629–36. doi: 10.1016/j.biopha.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Tan N, Dai L, Liu XJ, Pan GD, Chen H, Huang J, Xu Q. Upregulation of caprin1 expression is associated with poor prognosis in hepatocellular carcinoma. Pathol Res Pract. 2017;213:1563–1567. doi: 10.1016/j.prp.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Liu ZK, Wang YF, Wang L, Yao BW, Sun LK, Liu RK, Chen TX, Niu YS, Tu KS, Liu QG. Long non-coding RNA AGAP2-AS1, functioning as a competitive endogenous RNA, upregulates ANXA11 expression by sponging miR-16-5p and promotes proliferation and metastasis in hepatocellular carcinoma. Pathol Res Pract. 2017;213:1563–1567. [Google Scholar]