Abstract
The efficacy and safety of intravenous thrombolysis (IVT) for acute ischemic stroke (AIS) in cancer patients remained uncertain due to low level evidence in the latest guideline for AIS. The aim of this study was to assess the efficacy and safety of IVT in cancer patients with stronger evidence. We searched Medline, Embase, CENTRAL and ClinicalTrials.gov until April 2020 for studies reporting outcomes of functional independence, hemorrhagic transformation (HT), symptomatic intracranial hemorrhage (SICH), major bleeding (MB), in-hospital mortality or 3-month mortality after IVT for AIS in cancer patients. For each outcome, the odds ratio between cancer and non-cancer patients, the risk difference between gastrointestinal and other malignancy, and the proportion in cancer patients were calculated. The meta-analysis showed no significant difference between cancer and non-cancer patients in favorable outcome, HT, SICH, MB, in-hospital mortality and 3-month mortality. Furthermore, there’s no significant difference between patients with gastrointestinal and other malignancy in favorable outcome, HT, SICH, MB and 3-month mortality. In race-based subgroup analysis, Asians implied greater likelihood of HT and SICH than non-Asians. Therefore, the study confirmed and strengthened the validity of the guideline with stronger evidence that cancer shouldn’t be an exclusion criterion of IVT. Inconsistent with the guideline, gastrointestinal malignancy may not remain an absolute contraindication of IVT while Asians implied increased HT and SICH, which needed further exploration.
Keywords: Acute ischemic stroke, intravenous thrombolysis, cancer, gastrointestinal malignancy, systemic review, meta-analysis
Introduction
It’s estimated that there would be 18.1 million new cancer cases and 9.6 million cancer deaths in 2018 worldwide [1]. A twofold increase to 3.0% was observed in the 6-month incidence of acute ischemic stroke (AIS) for cancer patients compared with the general population [2]. The independent association between incident cancer and subsequent strokes could partly be explained by cancer-mediated hypercoagulability, non-bacterial thrombotic endocarditis, direct tumor compression of blood vessels and radiotherapy related atherosclerosis [3,4]. Overlapped risk factors especially advanced age and smoking has furthermore led to the co-occurrence of cancer and AIS, which were the two leading causes of morbidity and mortality worldwide [5].
Intravenous thrombolysis (IVT) with alteplase (recombinant tissue plasminogen activator) is the only approved medical reperfusion treatment for AIS [6]. Previous randomized controlled trials showed good efficacy and safety of intravenous alteplase within 4.5 hours of stroke onset, including the National Institute of Neurological Disorders and Stroke (NINDS) trial [7] and the European Cooperative Acute Stroke Study (ECASS) III trial [8]. However, these authoritative trials failed to provide data on the efficacy and safety of IVT in cancer patients. Published studies on this issue were mainly limited to cohort studies and case series, which held different opinions on whether IVT could be used in cancer patients. In the latest guideline for the management of AIS, the American Heart Association and American Stroke Association (AHA/ASA) also stated that the efficacy and safety of IVT in patients with current malignancy weren’t well established [9]. But it still provided some instructions on this issue, whereby IVT was contraindicated in intra-axial intracranial neoplasms and gastrointestinal malignancy while recommended in extra-axial intracranial neoplasms and systemic malignancy with reasonable (> 6 months) life expectancy [9]. Noteworthy, these instructions were consensus of expert opinion based on clinical experience, which offered low level evidence. With longer survival owing to continued advances in cancer treatment, we needed to assess the efficacy and safety of IVT in cancer patients with higher level evidence.
Hence for the first time, we conducted a comprehensive meta-analysis with stronger evidence to evaluate the efficacy and safety of IVT in cancer patients via the following outcomes, including functional independence, hemorrhagic transformation (HT), symptomatic intracranial hemorrhage (SICH), major bleeding (MB), in-hospital mortality and 3-month mortality.
Materials and methods
The systematic review and meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10]. Ethical approval wasn’t needed because all data were based on previously published studies.
Literature search
The Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and the US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) were searched until April 2020. Various terms homologous with “stroke”, “cancer” and “thrombolysis” were applied to maximize the scope of the search. Detailed search terms and search procedures were described in Supplementary Search Strategy. Additional relevant studies were acquired by searching the reference lists of the included articles. No language restrictions were imposed.
Inclusion and exclusion criteria
The inclusion criteria for the meta-analysis were: (1) original articles or conference abstracts reporting randomized controlled trials, case-control studies, cohort studies, or case series; (2) studies with at least 5 cancer patients; (3) studies depicting IVT for AIS in cancer patients; (4) studies reporting the number, proportion or odds ratio of at least one of the following outcomes: functional independence, HT, SICH, MB, in-hospital mortality or 3-month mortality.
The exclusion criteria for the meta-analysis were: (1) articles reporting case reports, editorials, commentaries or reviews; (2) studies involving stroke mimic; (3) studies involving benign tumors were excluded if data of patients with malignancy couldn’t be extracted separately; (4) studies with intra-arterial thrombolysis or endovascular therapy were excluded if data of IVT couldn’t be extracted separately; (5) duplicate publications from the same study.
Data extraction and quality assessment
Data were independently extracted by 2 authors (SH and XL) and any disagreement was resolved by consensus. The following data from eligible studies were collected: first author, publication year, article type (article or abstract), country, study period, study design (retrospective cohort or case series), sample size, age, gender, baseline National Institutes of Health Stroke Scale (NIHSS), stroke onset to treatment (OTT) time, follow-up duration, outcomes (functional independence, HT, SICH, MB, in-hospital mortality or 3-month mortality) and matching factors (age, gender, baseline NIHSS, OTT time, anticoagulant use, atrial fibrillation). The outcomes were extracted as dichotomous data or adjusted odds ratio (OR) with 95% confidence interval (CI). The retrospective cohort studies provided data of cancer and non-cancer patients respectively, whereas case series only provided data of cancer patients. Besides, dichotomous data of outcomes were also extracted for gastrointestinal (GI) and other malignancy from involved studies.
The study quality was assessed by two different tools. Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of cohort studies on the basis of cohort selection, comparability and outcomes [11]. It contained 8 items with a maximum of 9 points, and a score greater than 5 was regarded as high quality. The Institute of Health Economics (IHE) checklist was applied to appraise the quality of case series based on the objective, design, population, intervention and co-intervention, outcome measures, statistical analysis, results and conclusion, competing interests and sources of support. It included 20 items and the study with 14 or more yes responses was considered to be of acceptable quality [12].
Outcomes definition
The efficacy outcome was functional independence, which was defined as a modified Rankin Scale (mRS) score of 0-2 at 3 month. The safety outcomes included HT, SICH, MB, in-hospital mortality and 3-month mortality. HT was defined as any intracranial hemorrhage on the follow-up CT/MRI scan after IVT. SICH was defined as any intracranial hemorrhage which led to an increase of at least 4 points in NIHSS or death after IVT according to the ECASS III criteria [8]. MB was indicated as any hemorrhage which was life threatening or required blood transfusion. It included SICH and major local or systemic bleeding. In-hospital mortality and 3-month mortality were indicated as all-cause mortality recorded till hospital discharge and 3 month after IVT treatment, respectively.
Statistical analysis
STATA version 12.0 (Stata-Corp, College Station, TX, USA) was used for the statistical analyses. For the comparative analysis of outcomes between cancer and non-cancer patients, OR with 95% CI was calculated as the overall effect measure via the metan command. It’s either extracted directly from the study, or calculated from the dichotomous data in the 2*2 table by the Woolf method. If there’s one zero cell in the 2*2 table, 0.5 was added to each cell to calculate the OR. If there’re two zero cells, the study was discarded from the meta-analysis. For the comparative analysis of outcomes between patients with GI and other malignancy, risk difference (RD) with 95% CI was calculated as the overall effect measure. It equaled to the proportion of GI malignancy minus that of other malignancy. It’s calculated from the dichotomous data via the metan command. For the overall and race-based subgroup analysis of outcomes in cancer patients, proportion with 95% CI was calculated as the overall effect measure via the metaprop command. It’s calculated from the dichotomous data by Clopper-Pearson method and Freeman-Tukey double arcsine transformation [13,14]. All meta-analyses were conducted with the DerSimonian and Laird random effect model in case of clinical heterogeneity among studies [15]. A p-value of less than 0.05 was considered statistically significant. In race-based subgroup analyses, no overlapping in the 95% CI was considered as significant difference between Asian and non-Asian cancer patients.
Heterogeneity among studies was assessed by the I2 statistic and Q test, and it’s considered statistically significant when the p-value derived from Q test was < 0.1 [16]. The sensitivity analyses excluding conference abstracts were made due to absence of peer review. The publication bias was assessed by Egger test [17], and P < 0.05 was considered statistically significant.
Results
As shown in Figure 1, we collected 8328 studies from Medline, Embase, CENTRAL and ClinicalTrials.gov. After the review of the title and abstract, 31 potentially relevant studies were assessed for eligibility. We further excluded 2 studies whose involved tumors were mainly meningioma, 2 studies without definite diagnosis of AIS, 3 studies including intra-arterial thrombolysis or endovascular therapy, 4 studies without available data, 3 studies with sample size < 5, and 2 case reports. Finally, a total of 15 studies [18-32] were included in the meta-analysis. Detailed characteristics of included studies were demonstrated in Table 1. Of the 15 studies, there’re 11 articles [20,22-28,30-32] and 4 conference abstracts [18,19,21,29]. With respect to the study design, 11 studies [18-28] were retrospective cohort studies which made comparisons of outcomes after IVT between cancer and non-cancer patients, and 4 studies [29-32] were case series which only depicted outcomes in cancer patients. High quality of all cohort studies was observed with the NOS score higher than 5 (Supplementary Table 1), and good quality of 1 case series [32] was observed with 14 yes responses to the IHE checklist (Supplementary Table 2). The data of 34 cancer patients were extracted for meta-analysis from a total of 40 patients with neoplasms in 1 study [23].
Figure 1.
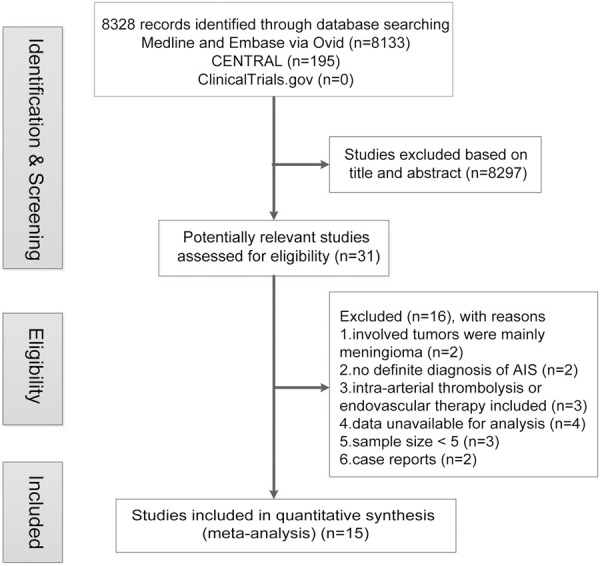
Flow chart of publication search and selection. Abbreviations: AIS, acute ischemic stroke.
Table 1.
Characteristics of included studies
| Author/Year | Type | Country/Study period | Study design | Cancer/Non-cancer or Cancer | Follow-up | Outcomes† | Matching factors‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Sample size | Age* | Male (%) | Baseline NIHSS* | OTT time* (min) | |||||||
| Schwarzbach/2012 [18] | Abstract | Germany/NA | RC | 18/27 | NA/NA | NA/NA | NA/NA | NA/NA | Discharge | 5 | d |
| Kolb/2013 [19] | Abstract | Israel/2008-2011 | RC | 9/107 | NA/NA | NA/NA | NA/NA | NA/NA | Discharge | 3 | NA |
| Murthy/2013 [20] | Article | USA/2009-2010 | RC | 614/21596 | NA/NA | NA/NA | NA/NA | NA/NA | Discharge | 2, 5 | a, b |
| Portilla/2014 [21] | Abstract | Spain/NA | RC | 23/134 | NA/NA | NA/NA | 7.7 (±7.1)/7.5 (±7.4) | < 270/NA | 3 months | 1, 2, 5, 6 | NA |
| Murthy/2015 [22] | Article | USA/2002-2011 | RC | 119/123667 | NA/NA | NA/NA | NA/NA | NA/NA | Discharge | 2, 5 | a, b, e |
| Sobolewski/2015 [23] | Article | Poland/2006-2013 | RC | 34/495 | 71.1 (±8.5)/70.7 (±12.1) | 53/53 | 13 [11-17]/11 [8-17] | < 270/< 270 | 3 months | 1, 3, 4, 6 | a, b, c, d, e, f |
| Geraldes/2017 [24] | Article | Portugal/2010-2015 | RC | 7/20 | 72.6 (±10.5)/NA | 86/NA | 13 (±6.2)/NA | < 180/NA | 3 months | 1, 2, 3, 4, 6 | a |
| Weeda/2019 [25] | Article | USA/2013-2014 | RC | 416/13577 | 75 [66-82]/71 [59-82] | 55/50 | NA/NA | NA/NA | Discharge | 2, 5 | a, f |
| Selvik/2018 [26] | Article | Norway/2006-2012 | RC | 5/261 | 68 (±8.5)/68.7 (±14.9) | 80/63 | 9 [8-14]/7 [4-15] | 73 [45-124]/90 [59-135] | Discharge | 2 | a, b, c, d, f |
| Owusu-Guha/2019 [27] | Article | USA/2013-2015 | RC | 11750/83375 | 74.2 (±32.5)/67.2 (±28.9) | 50/51 | NA/NA | NA/NA | Discharge | 5 | a, b, |
| Sallustio/2019 [28] | Article | Italy/2011-2018 | RC | 11/11 | NA/NA | NA/NA | NA/NA | NA/NA | 3 months | 1, 2, 3, 5, 6 | a, b, c, f |
| Lee/2011 [29] | Abstract | S.Korea/NA | CS | 24 | NA | NA | NA | NA | Discharge | 2 | - |
| Graber/2012 [30] | Article | USA/1999-2010 | CS | 6 | 72.8 (±7.7) | 33 | NA | < 180 | 3 months | 2, 3, 4, 6 | - |
| Cappellari/2013 [31] | Article | Italy/2004-2012 | CS | 11 | 72.5 (±9) | 64 | 10.5 (±5.9) | 170 [162-183] | 3 months | 1, 3, 4, 5, 6 | - |
| Nam/2017 [32] | Article | S.Korea/2010-2015 | CS | 12 | 69.3 (±8.5) | 67 | 12.2 (±7) | 60 [30-105] | 3 months | 1, 2, 5, 6 | - |
Mean (± SD) or median [interquartile range] reported. All known parameters without significant difference (P > 0.05).
Outcomes: 1, functional independence; 2, HT; 3, SICH; 4, MB; 5, in-hospital mortality; 6, 3-month mortality.
Matching factors (apply for RC studies only): a, age; b, gender; c, baseline NIHSS; d, OTT time; e, anticoagulant use; f, atrial fibrillation.
Abbreviations: NA, not available; USA, the United States of America; S.Korea, South Korea; RC, retrospective cohort; CS, case series; NIHSS, National Institutes of Health Stroke Scale; OTT, onset to treatment; HT, hemorrhagic transformation; SICH, symptomatic intracranial hemorrhage; MB, major bleeding; SD, standard deviation.
Comparative analyses of outcomes between cancer and non-cancer patients
A total of 11 retrospective cohort studies [18-28] were included for the comparisons between cancer and non-cancer patients. Among them, 5 studies [19,20,22,25,27] provided the adjusted OR. 1 study [22] only included malignant brain tumors while others included various cancers. For each study, there’s no significant difference between cancer and non-cancer patients in given information regarding age, sex, baseline NIHSS or OTT time (P > 0.05). As shown in Figure 2, there’s no significant difference between cancer and non-cancer patients in various outcomes, including functional independence (OR 1.00, 95% CI [0.51, 1.97]; I2 = 30.3%; 4 studies, 735 patients), HT (OR 1.40, 95% CI [0.93, 2.11]; I2 = 37.1%; 7 studies, 164061 patients), SICH (OR 2.12, 95% CI [0.33, 13.76]; I2 = 55.9%; 3 studies, 672 patients), MB (OR 1.09, 95% CI [0.21, 5.80]; I2 = 0.0%; 2 studies, 556 patients), in-hospital mortality (OR 1.30, 95% CI [0.93, 1.81]; I2 = 71.3%; 7 studies, 258938 patients), and 3-month mortality (OR 1.00, 95% CI [0.49, 2.03]; I2 = 0.0%; 4 studies, 735 patients). All analyses suggested no significant heterogeneity among studies (P > 0.1), except for in-hospital mortality (P = 0.002). The sensitivity analysis excluding the study of malignant brain tumors [22] still indicated no significant difference in HT (OR 1.20, 95% CI [0.80, 1.82]; I2 = 12.4%) and in-hospital mortality (OR 1.10, 95% CI [0.89, 1.35]; I2 = 32.3%) without significant heterogeneity (P > 0.1). All analyses indicated no significant publication bias by the Egger test (P > 0.05).
Figure 2.
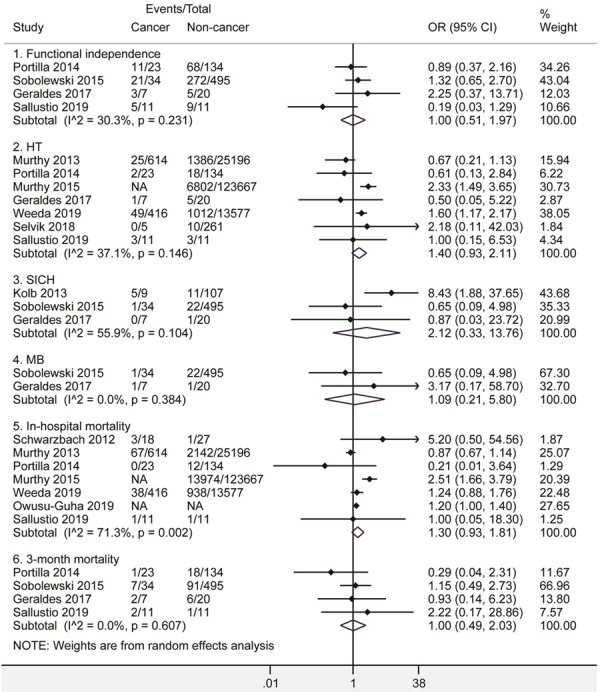
Forest plots of functional independence, HT, SICH, MB, in-hospital mortality and 3-month mortality after intravenous thrombolysis for acute ischemic stroke in cancer and non-cancer patients. The diamond indicated the estimated odds ratio (95% confidence interval) of cancer to non-cancer patients. The I2 statistic and p value showed on each figure was for heterogeneity test. Abbreviations: OR, odds ratio; HT, hemorrhagic transformation; SICH, symptomatic intracranial hemorrhage; MB, Major bleeding.
Furthermore, the sensitivity analysis excluding abstracts [18,19,21] also indicated no significant difference between patients with and without cancer (Supplementary Figure 1).
Comparative analyses of outcomes between patients with GI and other malignancy
6 studies [23,24,26,30-32] were included for the comparisons between patients with GI and other malignancy. The demographic characteristics of patients with GI and other malignancy were demonstrated in Table 2. For each study, there’s no significant difference between patients with GI and other malignancy in age, gender, baseline NIHSS, OTT time and cancer therapy (P>0.05, Table 2). As shown in Figure 3, there’s no significant difference between GI and other malignancy in various clinical outcomes, including functional independence (RD 8.63%, 95% CI [-30.43%, 47.69%]; I2 = 41.5%; 4 studies, 64 patients), HT (RD -4.56%, 95% CI [-39.75%, 30.63%]; I2 = 0.0%; 3 studies, 17 patients), SICH (RD 6.37%, 95% CI [-14.45%, 27.19%]; I2 = 0.0%; 5 studies, 62 patients), MB (RD 5.72%, 95% CI [-17.08%, 28.53%]; I2 = 0.0%; 4 studies, 57 patients), and 3-month mortality (RD -21%, 95% CI [-55.71%, 13.71%]; I2 = 53.9%; 5 studies, 69 patients). All analyses suggested no significant heterogeneity among studies (P > 0.1), except for 3-month mortality (P = 0.07). All analyses indicated no significant publication bias by the Egger test (P > 0.05).
Table 2.
Characteristics of patients with gastrointestinal and other malignancy
| Author/Year | SSa | Malea (%) | Agea,b | Baseline NIHSSa,b | OTT timea,b (min) | Cancer therapya (%) |
|---|---|---|---|---|---|---|
| Graber/2012 [30] | 1/4 | 0/25 | 68/66 [63-81] | NA/NA | < 180/< 180 | 100/25 |
| Cappellari/2013 [31] | 4/7 | 75/57 | 76 [63-84]/73 [59-84] | 14 [7-20]/7 [1-14] | 182 [100-225]/170 [122-270] | 75/43 |
| Sobolewski/2015 [23] | 3/31 | 33/55 | 69 [65-71]/72 [49-90] | 11 [11-14]/13 [6-26] | < 270/< 270 | NA/NA |
| Geraldes/2017 [24] | 1/6 | 100/83 | 76/75 [51-82] | 12/14 [6-22] | NA/NA | 100/67 |
| Nam/2017 [32] | 4/8 | 100/50 | 76 [51-82]/66 [56-76] | 7 [3-19]/14 [5-24] | < 120/< 120 | NA/NA |
| Selvik/2018 [26] | 3/2 | 67/100 | 77 [52-77]/65.5 [62-69] | 13 [9-15]/11 [8-14] | 75 [75-130]/165.5 [151-180] | 33/50 |
Gastrointestinal malignancy/other malignancy reported.
Median [range] reported.
Abbreviations: SS, sample size; NIHSS, National Institutes of Health Stroke Scale; OTT, onset to treatment; NA, not acquired.
Figure 3.
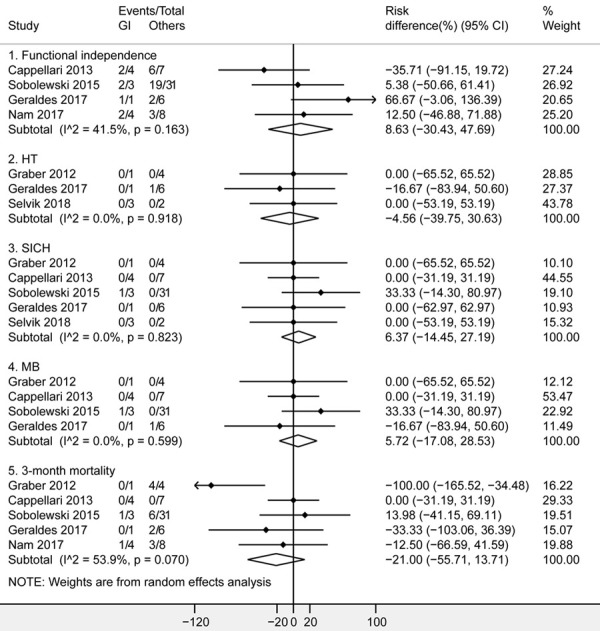
Forest plots of functional independence, HT, SICH, MB and 3-month mortality after intravenous thrombolysis for acute ischemic stroke in patients with GI and other malignancy. The diamond indicated the estimated risk difference (95% confidence interval) between GI and other malignancy. The I2 statistic and p value showed on each figure was for heterogeneity test. Abbreviations: GI, gastrointestinal; HT, hemorrhagic transformation; SICH, symptomatic intracranial hemorrhage; MB, Major bleeding.
Overall and race-based subgroup analyses of outcomes in cancer patients
A total of 13 studies [18-21,23-26,28-32] were included to pool the proportion of outcomes in cancer patients. As shown in Figure 4, cancer patients indicated a proportion of 54.31% for functional independence (95% CI [43.83%, 64.63%]; I2 = 0.0%; 6 studies, 98 patients), 13.74% for HT (95% CI [5.40%, 24.35%]; I2 = 86.4%; 9 studies, 1118 patients), 3.94% for SICH (95% CI [0%, 18.50%]; I2 = 65.5%; 6 studies, 78 patients), 1.31% for MB (95% CI [0%, 8.07%]; I2 = 0.0%; 4 studies, 58 patients), 7.84% for in-hospital mortality (95% CI [4.59%, 11.70%]; I2 = 42.4%; 7 studies, 1105 patients), and 18.26% for 3-month mortality (CI [5.89%, 34.29%]; I2 = 63.4%; 7 studies, 104 patients). Significant heterogeneity among studies were observed for HT (P = 0.0), SICH (P = 0.013) and 3-month mortality (P = 0.012). All analyses indicated no significant publication bias by the Egger test (P > 0.05).
Figure 4.
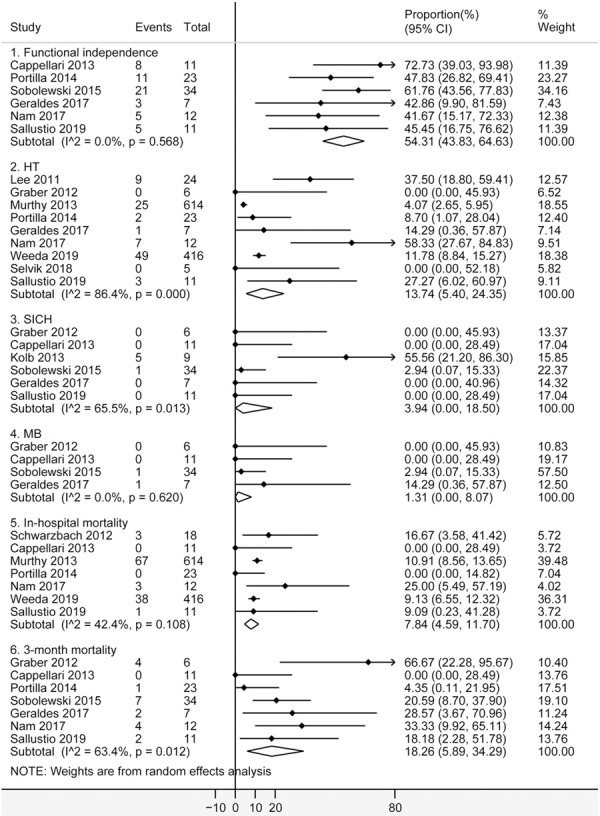
Forest plots of functional independence, HT, SICH, MB, in-hospital mortality and 3-month mortality after intravenous thrombolysis for acute ischemic stroke in cancer patients. The diamond indicated the estimated proportion (95% confidence interval) in cancer patients. The I2 statistic and p value showed on each figure was for heterogeneity test. Abbreviations: HT, hemorrhagic transformation; SICH, symptomatic intracranial hemorrhage; MB, Major bleeding.
Of the 13 included studies above, 3 studies [19,29,32] were conducted in Asia. Race-based subgroup analyses were made in Asian and non-Asian cancer patients respectively. As shown in Figure 5, Asians showed an increased proportion of HT as 44.27% (95% CI [27.88%, 61.26%]; 2 studies, 36 patients) compared with non-Asians (6.26%, 95% CI [1.20%, 13.63%]; I2 = 77.8%; 7 studies, 1082 patients), and SICH as 55.56% (95% CI [21.20%, 86.30%]; 1 studies, 9 patients) compared with non-Asians (0.27%, 95% CI [0%, 5.01%]; I2 = 0.0%; 5 studies, 69 patients). Heterogeneity among Asian studies couldn’t be assessed due to insufficient number of included studies. Significant heterogeneity among non-Asian studies were observed for HT (P = 0.0). All analyses indicated no significant publication bias by the Egger test (P > 0.05), except for SICH in non-Asians (P = 0.020).
Figure 5.
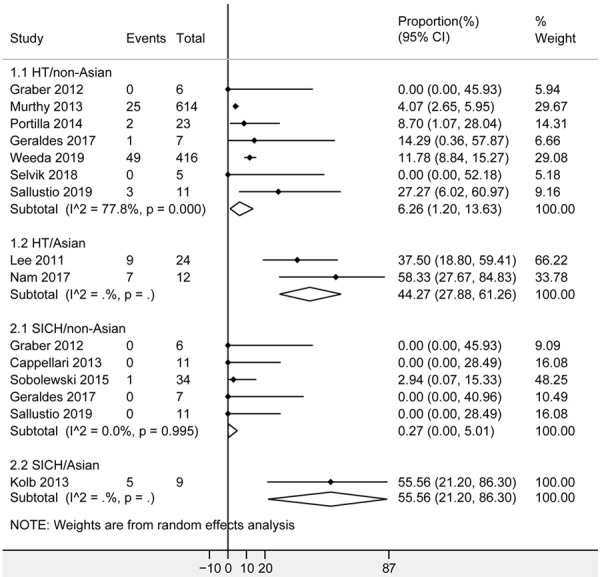
Forest plots of HT and SICH after intravenous thrombolysis for acute ischemic stroke in non-Asian and Asian patients with cancer. The diamond indicated the estimated proportion (95% confidence interval) in Asian or non-Asian cancer patients. The I2 statistic and p value showed on each figure was for heterogeneity test. Abbreviations: HT, hemorrhagic transformation; SICH, symptomatic intracranial hemorrhage.
Furthermore, the sensitivity analysis excluding abstracts [18,19,21,29] showed no significant changes in the proportion of outcomes in cancer patients (Supplementary Figure 2), and that Asians had greater likelihood of HT than non-Asians (Supplementary Figure 3).
Discussion
Up to date, there’re no randomized controlled trials about the efficacy and safety of IVT in cancer patients. Published studies on this issue were mainly limited to cohort studies and case series, which held different opinions on whether IVT could be used in cancer patients. Of the 11 cohort studies, 8 studies [18,20,21,23,24,26-28] favored IVT in cancer patients, 1 study [19] disfavored IVT in active cancer, 1 study [22] disfavored IVT in malignant brain tumors, and 1 study [25] failed to give the conclusion due to increased HT without increased in-hospital mortality in cancer patients. Although the latest AHA/ASA guideline [9] gave some instructions on this issue, they’re based on expert opinion with low level evidence. Therefore the efficacy and safety of IVT remained uncertain in cancer patients. Our meta-analysis favored IVT in cancer patients, because no significant difference was observed in the efficacy and safety of IVT between cancer and non-cancer patients. The conclusion was consistent with the AHA/ASA guideline that cancer shouldn’t be an exclusion criterion of IVT [9]. Hence, this study could guide clinical practice with stronger evidence based on meta-analysis. Noteworthy, this preliminary conclusion included various cancers as a whole and failed to provide the efficacy and safety of IVT in cancers of specific type, status, site, stage or treatment.
With respect to the cancer type, IVT was potentially harmful to patients with GI malignancy while beneficial to systemic malignancy with reasonable life expectancy according to the AHA/ASA guideline [9]. This aroused our interest in the efficacy and safety of IVT in patients with GI malignancy. It’s estimated that GI malignancy ranked top in the proportion of new cancer cases (15.7%) and cancer deaths (17.4%) in 2018 worldwide [1]. On the one hand, patients with GI cancers had increased risk of gastrointestinal bleeding (GIB) which varied from occult bleeding to massive hemorrhage [33]. A recent study also showed that GIB was associated with a 20-fold higher hazard of GI cancer diagnosis [34]. On the other hand, GIB was a common complication with an incidence of 0.1-8.0% during the acute phase of stroke [35]. The co-occurrence of GI malignancy and AIS might greatly increase the risk of GIB, which was significantly associated with poor clinical outcomes [36,37]. Therefore, the AHA/ASA guideline disapproved IVT in patients with GI malignancy or recent GIB [9]. Inconsistent with the guideline, one study [38] reported no significant difference in MB (OR 1.72, 95% CI [0.58-5.11]) and in-hospital mortality (OR 1.01, 95% CI [0.58-1.75]) between patients with and without GI malignancy or GIB after IVT. In our meta-analysis, all of the 6 included studies [23,24,26,30-32] reported zero incidence of GIB in AIS patients with GI malignancy after IVT, which was in line with the rare incidence of GIB in previous studies [36,37]. Furthermore, our meta-analysis revealed no significant difference in the efficacy and safety of IVT between patients with GI and other malignancy. The inconsistency might be attributed to insufficient sample size or prophylactic acid suppression therapies. However, it still provided the view that GI malignancy may not remain an absolute contraindication of IVT, especially with better cancer treatment and acid suppression treatment at present.
The AHA/ASA guideline recommended IVT in systemic malignancy with reasonable life expectancy of > 6 months [9]. One study [39] revealed that the presence of metastasis (hazard ratio 4.53, 95% CI [2.18, 9.42]) and the cancer type (hazard ratio: 2.07 and 2.389 for gastric/esophageal and pancreatic cancer, respectively) were significantly associated with 6-month mortality. Hence IVT might not be recommended in patients with metastasis or these cancers. Furthermore, another study [20] reported that solid malignancy (OR 3.02, 95% CI [1.37, 6.65]) and metastatic cancer (OR 10.41, 95% CI [4.53, 23.92]) had significantly higher in-hospital mortality than hematologic malignancy. As suggested in the guideline [9], IVT was potentially harmful to patients with intra-axial intracranial neoplasms while beneficial to extra-axial neoplasms. One study [22] showed that intra-axial neoplasms had higher mortality (OR: 2.51, 95% CI [1.20, 5.23]) than extra-axial neoplasms, which supported the guideline. As for the current malignancy which wasn’t well established [9], 4 studies [21,24,26,28] favored while 1 study [19] disfavored IVT in patients with current or active malignancy. We failed to make further meta-analysis in these specific populations due to lack of data, but these studies could work as reference in clinical practice. More detailed information should be added to future studies to assess the efficacy and safety of IVT in patients with specific cancer, such as the type, status (current, remote), site, stage or treatment of the cancer.
For the first time, our meta-analysis reported the pooled proportion of various outcomes after IVT for AIS in cancer patients. The pooled proportion of HT (13.74%, 95% CI [5.40%, 24.35%]), SICH (3.94%, 95% CI [0.0%, 18.50%]) and 3-month mortality (18.26%, 95% CI [5.89%, 34.29%]) were comparable to the randomized controlled NINDS trial (10.9%, 6.4% and 17%, respectively) [7]. However, the proportion of functional independence (54.31%, 95% CI [43.83%, 64.63%]) was slightly higher than the NINDS trial (42.6%) [7]. On the one hand, it could be explained by different definitions of functional independence which is indicated by mRS of 0-2 in this study while 0-1 in the NINDS trial. On the other hand, it may be due to the differences in stroke severity and time period (better stroke treatments at present). These results which were comparable to the on-license treated group in NINDS trial implied good efficacy and safety of IVT in cancer patients.
Of 15 million people annually having stroke worldwide, about 9 million were Asians [40]. Unique features of stroke in Asia included genetic disorders, effects of diet and lifestyle, and high prevalence of intracranial atherosclerosis and intracranial hemorrhage [40]. The Japanese Alteplase Clinical Trial [41] showed that various clinical outcomes with low-dose alteplase (0.6 mg/kg) in Asia were comparable to the outcomes with standard-dose alteplase (0.9 mg/kg) in North America and European Union. It suggested that Asians might have increased risk of intracranial hemorrhage at standard dose. In consistence with this, our meta-analysis showed that Asians implied greater likelihood of HT and SICH than non-Asians. Nevertheless, this conclusion was different from the ENCHANTED trial [42]. According to the ENCHANTED trial, low-dose alteplase didn’t show non-inferiority to standard-dose alteplase in mortality and disability (OR 1.09, 95% CI [0.95, 1.25]) in predominantly Asian patients [42], and no significant difference was observed between Asian and non-Asian cancer patients [43]. Therefore, low-dose alteplase wasn’t recommended in the latest AHA/ASA guideline [9]. In view of absence of detailed dose and huge discrepancy in the sample size between Asians and non-Asians, the ethnic difference which wasn’t biologically plausible might indicate a publication bias in our meta-analysis. The issue of whether Asia was a risk factor in the efficacy and safety of IVT still needed more data from Asian patients.
As we can observe, there’s substantial heterogeneity in the overall and race-based subgroup analysis of HT in cancer patients. Race might be the source of heterogeneity in HT, but it failed to compensate for the whole. Perhaps the heterogeneity was from discrepancies in study design, population and measuring methods, but the information was too little for further analysis.
Our meta-analysis had several limitations that should be considered when interpreting the results. Firstly, 4 conference abstracts were included, so we couldn’t obtain detailed information on the population. Secondly, most studies failed to provide data for all outcomes, which might cause selection bias. Thirdly, the 95% CI in some analyses were very broad, which indicated low statistical power. Fourthly, observational studies without randomization might cause biases that cancer patients in poor conditions were generally not considered for IVT while non-cancer patients would be considered less carefully.
In conclusion, according to this meta-analysis, cancer patients showed no significant difference from non-cancer patients in the efficacy and safety of IVT, which confirmed and strengthened the latest AHA/ASA guideline with stronger evidence. For cancer patients, there’s no significant difference in the efficacy and safety of IVT between gastrointestinal and other malignancy, and Asians showed greater likelihood of HT and SICH than non-Asians. Randomized controlled trials are needed to assess the efficacy and safety of IVT in cancers of specific type, status, site, stage or treatment.
Acknowledgements
SH and XL performed the study, analyzed the data and wrote the manuscript. LVT and YH conceived and designed the study, resolved discrepancies and edited the manuscript. The manuscript has been reviewed and approved by all authors. This study was supported by Novo Nordisc Haemophilia Research Fund 2016 (20160413), Wuhan Chen-Guang Project 2017 (2017050304010276), and the National Natural Science Foundation of China (grant 81500109).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, Panageas KS, DeAngelis LM. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MS, Panageas KS, DeAngelis LM. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291–300. doi: 10.1002/ana.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaorsky NG, Zhang Y, Tchelebi LT, Mackley HB, Chinchilli VM, Zacharia BE. Stroke among cancer patients. Nat Commun. 2019;10:5172. doi: 10.1038/s41467-019-13120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilbers J, Sondag L, Mulder S, Siegerink B, van Dijk EJ. Cancer prevalence higher in stroke patients than in the general population: the Dutch String-of-Pearls institute (PSI) stroke study. Eur J Neurol. 2020;27:85–91. doi: 10.1111/ene.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandrov AV, Köhrmann M, Soinne L, Tsivgoulis G, Barreto AD, Demchuk AM, Sharma VK, Mikulik R, Muir KW, Brandt G, Alleman J, Grotta JC, Levi CR, Molina CA, Saqqur M, Mavridis D, Psaltopoulou T, Vosko M, Fiebach JB, Mandava P, Kent TA, Alexandrov AW, Schellinger PD. Safety and efficacy of sonothrombolysis for acute ischaemic stroke: a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Neurol. 2019;18:338–347. doi: 10.1016/S1474-4422(19)30026-2. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 8.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 9.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed April 2020. [Google Scholar]
- 12.Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol. 2016;69:199–207. e2. doi: 10.1016/j.jclinepi.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stats. 1950;21:607–611. [Google Scholar]
- 14.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coory MD. Comment on: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39:932. doi: 10.1093/ije/dyp157. author reply 933. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarzbach CJ, Schaefer A, Hennerici MG, Fatar M. Systemic thrombolysis in cancer patients-is it safe and effective? Cerebrovasc Dis. 2012;33:64. [Google Scholar]
- 19.Kolb H, Bloch S, Borenstein N, Hallevi H. The risk of ICH in cancer patients treated with intravenous thrombolysis for acute ischemic stroke. Neurology. Conference: 65th American Academy of Neurology Annual Meeting. San Diego, CA United States. Conference Publication: 2013; 80: no pagination. [Google Scholar]
- 20.Murthy SB, Karanth S, Shah S, Shastri A, Rao CP, Bershad EM, Suarez JI. Thrombolysis for acute ischemic stroke in patients with cancer: a population study. Stroke. 2013;44:3573–3576. doi: 10.1161/STROKEAHA.113.003058. [DOI] [PubMed] [Google Scholar]
- 21.Portilla JC, Redondo I, Bragado I, Calle M, Falcon A, Serrano A, Fermin JA, Romero RM, Lopez F, Casado I. Intravenous thombolysis for acute ischemic stroke in patients with malignancy. Cerebrovasc Dis. 2014;37:655. [Google Scholar]
- 22.Murthy SB, Moradiya Y, Shah S, Shastri A, Bershad EM, Suarez JI. In-hospital outcomes of thrombolysis for acute ischemic stroke in patients with primary brain tumors. J Clin Neurosci. 2015;22:474–478. doi: 10.1016/j.jocn.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Sobolewski P, Brola W, Szczuchniak W, Fudala M, Sobota A. Safety of intravenous thrombolysis for acute ischaemic stroke including concomitant neoplastic disease sufferers - experience from Poland. Int J Clin Pract. 2015;69:666–673. doi: 10.1111/ijcp.12586. [DOI] [PubMed] [Google Scholar]
- 24.Geraldes T, Pereira L, Guarda C, Grunho M, Ribeiro AC, Coimbra J, Mendes I, Rodrigues M. Safety and outcome of rtPA in acute ischemic stroke in patients with active cancer: case-control study. Revista de Neurologia. 2017;65:13–18. [PubMed] [Google Scholar]
- 25.Weeda ER, Bohm N. Association between comorbid cancer and outcomes among admissions for acute ischemic stroke receiving systemic thrombolysis. Int J Stroke. 2019;14:48–52. doi: 10.1177/1747493018778135. [DOI] [PubMed] [Google Scholar]
- 26.Selvik HA, Naess H, Kvistad CE. Intravenous Thrombolysis in ischemic stroke patients with active cancer. Front Neurol. 2018;9:811. doi: 10.3389/fneur.2018.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owusu-Guha J, Guha A, Miller PE, Pawar S, Dey AK, Ahmad T, Attar H, Awan FT, Mitchell D, Desai NR, Addison D. Contemporary utilization patterns and outcomes of throm-bolytic administration for ischemic stroke among patients with cancer. Int J Stroke. 2019:1747493019895709. doi: 10.1177/1747493019895709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallustio F, Mascolo AP, Marrama F, Koch G, Alemseged F, Davoli A, Da Ros V, Morosetti D, Konda D, Diomedi M. Safety and efficacy of reperfusion therapies for acute ischemic stroke patients with active malignancy. J Stroke Cerebrovasc Dis. 2019;28:2287–2291. doi: 10.1016/j.jstrokecerebrovasdis.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Lee KY, Kim YD, Oh SH, Ahn SH, Kim SH, Han SW. Increased hemorrhagic risk of thrombolysis in acute ischemic stroke patient with malignancy. Stroke. 2011;42:e227. [Google Scholar]
- 30.Graber JJ, Nayak L, Deangelis LM. Use of recombinant tissue plasminogen activator in cancer patients with acute stroke. J Neurooncol. 2012;107:571–573. doi: 10.1007/s11060-011-0780-5. [DOI] [PubMed] [Google Scholar]
- 31.Cappellari M, Carletti M, Micheletti N, Tomelleri G, Ajena D, Moretto G, Bovi P. Intravenous alteplase for acute ischemic stroke in patients with current malignant neoplasm. J Neurol Sci. 2013;325:100–102. doi: 10.1016/j.jns.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Nam KW, Kim CK, Kim TJ, An SJ, Oh K, Ko SB, Yoon BW. Intravenous thrombolysis in acute ischemic stroke with active cancer. Biomed Res Int. 2017;2017:4635829. doi: 10.1155/2017/4635829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schatz RA, Rockey DC. Gastrointestinal bleeding due to gastrointestinal tract malignancy: natural history, management, and outcomes. Dig Dis Sci. 2016;62:491–501. doi: 10.1007/s10620-016-4368-y. [DOI] [PubMed] [Google Scholar]
- 34.Eikelboom JW, Connolly SJ, Bosch J, Shestakovska O, Aboyans V, Alings M, Anand SS, Avezum A, Berkowitz SD, Bhatt DL, Cook-Bruns N, Felix C, Fox KAA, Hart RG, Maggioni AP, Moayyedi P, O’Donnell M, Rydén L, Verhamme P, Widimsky P, Zhu J, Yusuf S. Bleeding and new cancer diagnosis in patients with atherosclerosis. Circulation. 2019;140:1451–1459. doi: 10.1161/CIRCULATIONAHA.119.041949. [DOI] [PubMed] [Google Scholar]
- 35.Chou YF, Weng WC, Huang WY. Association between gastrointestinal bleeding and 3-year mortality in patients with acute, first-ever ischemic stroke. J Clin Neurosci. 2017;44:289–293. doi: 10.1016/j.jocn.2017.06.068. [DOI] [PubMed] [Google Scholar]
- 36.Rumalla K, Mittal MK. Gastrointestinal bleeding in acute ischemic stroke: a population-based analysis of hospitalizations in the United States. J Stroke Cerebrovasc Dis. 2016;25:1728–1735. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 37.Ogata T, Kamouchi M, Matsuo R, Hata J, Kuroda J, Ago T, Sugimori H, Inoue T, Kitazono T. Gastrointestinal bleeding in acute ischemic stroke: recent trends from the fukuoka stroke registry. Cerebrovasc Dis Extra. 2014;4:156–64. doi: 10.1159/000365245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inohara T, Liang L, Kosinski AS, Smith EE, Schwamm LH, Hernandez AF, Bhatt DL, Fonarow GC, Peterson ED, Xian Y. Thrombolytic therapy in older acute ischemic stroke patients with gastrointestinal malignancy or recent bleeding. Eur Stroke J. 2020;5:47–55. doi: 10.1177/2396987319871784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoo J, Nam HS, Kim YD, Lee HS, Heo JH. Short-Term outcome of ischemic stroke patients with systemic malignancy. Stroke. 2019;50:507–511. doi: 10.1161/STROKEAHA.118.023044. [DOI] [PubMed] [Google Scholar]
- 40.Toyoda K, Koga M, Hayakawa M, Yamagami H. Acute reperfusion therapy and stroke care in Asia after successful endovascular trials. Stroke. 2015;46:1474–1481. doi: 10.1161/STROKEAHA.115.008781. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, Shinohara Y Japan Alteplase Clinical Trial (J-ACT) Group. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT) Stroke. 2006;37:1810–1815. doi: 10.1161/01.STR.0000227191.01792.e3. [DOI] [PubMed] [Google Scholar]
- 42.Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, Broderick JP, Chen X, Chen G, Sharma VK, Kim JS, Thang NH, Cao Y, Parsons MW, Levi C, Huang Y, Olavarría VV, Demchuk AM, Bath PM, Donnan GA, Martins S, Pontes-Neto OM, Silva F, Ricci S, Roffe C, Pandian J, Billot L, Woodward M, Li Q, Wang X, Wang J, Chalmers J ENCHANTED Investigators and Coordinators. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016;374:2313–2323. doi: 10.1056/NEJMoa1515510. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Robinson TG, Lee TH, Li Q, Arima H, Bath PM, Billot L, Broderick J, Demchuk AM, Donnan G, Kim JS, Lavados P, Lindley RI, Martins SO, Olavarria VV, Pandian JD, Parsons MW, Pontes-Neto OM, Ricci S, Sharma VK, Thang NH, Wang JG, Woodward M, Anderson CS, Chalmers J. Low-dose vs standard-dose alteplase for patients with acute ischemic stroke. JAMA Neurol. 2017;74:1328. doi: 10.1001/jamaneurol.2017.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


