Abstract
Accumulating evidence demonstrated that circulating CD4+CXCR5+, follicular helper T (Tfh) and follicular regulatory T (Tfr) cells maintain immune homeostasis and humoral immune response and were involved in the pathogenesis of certain autoimmune/inflammatory diseases. The current study was aimed to investigate the correlation between frequencies of CD4+CXCR5+, Tfh and Tfr cells, Tfh/Tfr and the disease activity in chronic spontaneous urticaria (CSU). Frequencies of CD4+CXCR5+, Tfh and Tfh/Tfr, but not Tfr, in peripheral blood mononuclear cells (PBMCs) were elevated in patients with CSU as compared with healthy controls. No difference was observed in the frequency of these cells between different subgroups of patients based on autologous serum skin testing (ASST), skin prick testing (SPT) and the level of total IgE. The expression of CXCR5 in PBMCs was higher in CSU than in controls, both at mRNA and protein levels. Higher levels of plasma IL-4, IL-6 and total IgE were observed in CSU, with positive correlation between IL-4/IL-6 and total IgE. The IL-21 level was lower and negatively correlated with total IgE. Using receiver operating characteristic (ROC) curve, we found positive correlation between the urticaria activity score (UAS) and CD4+CXCR5+, Tfh, Tfh/Tfr and total IgE, respectively, with area under the curves (AUCs) all greater than 0.7 (P < 0.05). These results indicated that frequencies of circulating CD4+CXCR5+ cells, Tfh cells and Tfh/Tfr were abnormal and correlated positively with disease severity, suggesting the possible involvement of these cells in the immunopathogenesis of CSU.
Keywords: Chronic spontaneous urticaria, CD4+CXCR5+ cell, follicular helper T cell, follicular regulatory T cell, urticaria activity score
Introduction
Chronic spontaneous urticaria (CSU) is a common recurrent skin disease, with a great impact on the life quality and health [1]. According to autologous serum skin test (ASST) and skin prick test (SPT), CSU can be divided into several subtypes [2-4]. A variety of factors, including autoreactivity, infections, genetic factors, coagulation cascade and autoimmunity, can contribute to the pathogenesis of CSU [1], but the complexity of the disease remains elusive. Recent studies suggested the association with IgE to autoallergens and IgG autoantibodies to IgE or its receptor [5].
Follicular helper T (Tfh) cell is a subgroup of CD4+CXCR5+ (C-X-C chemokine receptor 5) cells that activates germinal center formation and provides help for B cells in producing autoantibodies and their differentiation into memory cells and long-lived plasma cells [6,7]. Follicular regulatory T (Tfr) cells are differentiated from T regulatory (Treg) cells that inhibit germinal center formation and B cell development [7]. Both Tfh and Tfr cells express CXCR5, but differ in that Tfr cells expresse CD25 and FoxP3 [8]. Increase or decrease of circulating CD4+CXCR5+, Tfh or Tfr cells was found to be associated with immune imbalance, contributing to the pathogenesis of certain diseases, such as atopic dermatitis [9], allergic asthma [10], psoriasis [11], bullous pemphigoid [12], systemic lupus erythematosus [13-15], primary sjogren,s syndrome [16], primary biliary cholangitis [17] and rthumatoid arthritis [18]. In addition, the complex cytokine signals network is essential for differentiation of Tfh cells and production of autoantibodies in B cells. Tfh cells produce cytokines, such as IL-4, IL-6 and IL-21, which help the class switching of B cells. For example, IL-6 could regulate the number of Tfh cells and the production of total IgE in sensitized mice [19], while IL-4 produced from Tfh cells could regulate the production of IgG1 and IgE in allergic immune mice [20]. Since IgE and IgE autoantibodies have been shown their roles in pathognesis of CSU, it is important to explore the changes of CD4+CXCR5+ subpopulations which maybe helpful to understand the class switching of B cells in CSU.
The current study was aimed to detect frequencies of CD4+CXCR5+, Tfh and Tfr cells and the Tfh/Tfr ratio in peripheral blood mononuclear cells (PBMCs) of patients with CSU as compared to normal healthy controls (HC), regarding their correlations to the disease activity. The expression levels of CXCR5 in PBMCs and the concentrations of inflammatory cytokines (IL-4, IL-6 and IL-21) and total IgE in the plasma of studied samples were also evaluated.
Material and methods
Participants and blood sampling
A total of 24 healthy controls (HC) and 63 patients diagnosed with CSU, matched in sex and age distribution, were recruited in the current study. The study was approved by the Ethics Committee of Southwest Hospital, Army Medical University, Chongqing, with informed consent from all the participants. Diagnosis of CSU was made and the disease severity (urticaria activity score, UAS) was measured at the time of blood sampling according to the EAACI/GA2LEN/EDF/WAO guideline [1]. PBMCs were prepared by density gradient centrifugation from peripheral blood and anticoagulated with EDTA-K2. Plasma samples were separated and stored at -80°C until used for ELISA. ASST and SPT were performed in patients with CSU as previously described [2,3]. The allergens of SPT included dust mites, house dust mites, cockroaches, mugwort pollen, fish (squid), shrimp, duck feather, silk, crab, egg, cow milk and peanut. Patients with CSU and SPT+ displaying one or more positive allergens were indicated as allergic sensitization.
Flow cytometry
For detection of Tfh and Tfr cells, human PBMCs were stained with FITC mouse anti-human CD4 (BD Biosciences, San Diego, CA, USA), CD185 (CXCR5)-APC human (Miltenyi Biotec, CA, USA), PE-CYTM7 mouse anti-human CD25 (BD Biosciences), and PE mouse anti-human CD127 (BD Biosciences). Cells were collected with FACSCantoTM II Flow Cytometer (BD Biosciences). Total CD4+CXCR5+ cells were gated by the forward/side scatter showing CD4/CXCR5 expression patterns. Circulating Tfh (CD4+CXCR5+CD25lowCD127high) and Tfr (CD4+CXCR5+CD25highCD127low) cells were gated based on expression levels of CD25 and CD127. Data were analyzed using FlowJo software (Stanford University, San Francisco, CA, USA).
Quantitative real-time PCR (qRT-PCR)
PBMCs were resuspended in RNAiso Plus (TaKaRa, Dalian, China) and total RNA was extracted according to instructions of manufacturer. For CXCR5 detection, cDNAs were synthesized using PrimeScriptTM RT reagent Kit (TaKaRa). qRT-PCR was performed with TB GerrnTM Premix Ex TaqTM II (TaKaRa) on a CFX ConnectTM Real-Time System (BIO-RAD, Singapore, USA). The reaction parameters were as follows: 95°C for 30 s, then 40 cycles of 95°C for 5 s and 60°C for 45 s. The expression levels were calibrated to the β-actin control and determined by the 2-ΔΔCt method. The primers used were as follows: CXCR5 forward, 5’-CAACAACTCCCTGCCACGTT-3’ and reverse, 5’-AGGAATCCCGCCACATGGTA-3’; β-actin forward, 5’-CTCTTCCAGCCTTCCTTCCT-3’ and reverse, 5’-AGCACTGTGTTGGCGTACAG-3’ (Sangon Biotech, Shanghai, China).
Western blotting
PBMCs were lysed in RIPA lysis buffer supplemented with phenylmethyl-sulfonyl fluoride protease inhibitor (Beyotime, Shanghai, China). Proteins were separated by electrophoresis in 4-20% SurePAGETM gel (GenScript, Nanjing, China) and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were incubated with primary antibodys against CXCR5 (Abcam, Cambridge, MA) or GAPDH (Bioss, Beijing, China), and then washed and incubated with the secondary antibody (Abclonal, Wuhan, China). Proteins were detected by scanning the membranes with ChemiDocTM Touch Imaging System (BIO-RAD), and band intensities were quantified using CFX Manager (BIO-RAD).
Enzyme-linked immunosorbent assays (ELISA)
Plasma levels of IL-4 (BD Biosciences), IL-6 (R&D Systems, Minneapolis, USA), IL-21 (Biolegend, USA) and total IgE (EUROIMMUN, Beijing, China) were measured using ELISAs according to instructions of the manufacturers.
Statistical analysis
Results were expressed as mean ± standard error of the mean (SEM). Two-tailed Student’s t-test was used to compare the two groups. Correlation analyses were conducted using Spearman’s rank test. Data were recorded and analyzed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA), and P < 0.05 was considered as statistically significant.
Results
A total of 63 patients with CSU were recruited. Among them, there were 15 ASST+ vs. 48 ASST-, 42 SPT+ vs. 21 SPT-. The control group included 23 HC. Their demographic and clinical characteristics were listed in Table 1. Significant differences were not observed in gender and age between CSU patients and HC.
Table 1.
Demographics and clinical characteristics of the patients with chronic spontaneous urticaria (CSU) and healthy controls (HC)
| HC (n = 24) | CSU (n = 63) | p value | |
|---|---|---|---|
| Gender (male/female) | 11/13 | 23/40 | 0.305 |
| Age (years), mean (range) | 36.6 (16-59) | 38.03 (13-70) | 0.802 |
| UAS, mean (range) | - | 4.95 (2-6) | - |
| ASST positive (%) | - | 15 (23.8%) | - |
| SPT positive (%) | - | 42 (66.7%) | - |
Note: CSU: chronic spontaneous urticaria; HC: healthy controls; UAS: urticaria activity score. ASST positive: positive reaction to autologous serum skin testing (ASST); SPT positive: positive reaction to skin prick testing (SPT). Significance between two groups was evaluated with a two-tailed Student’s t-test and indicated by p value (P < 0.05 as significant).
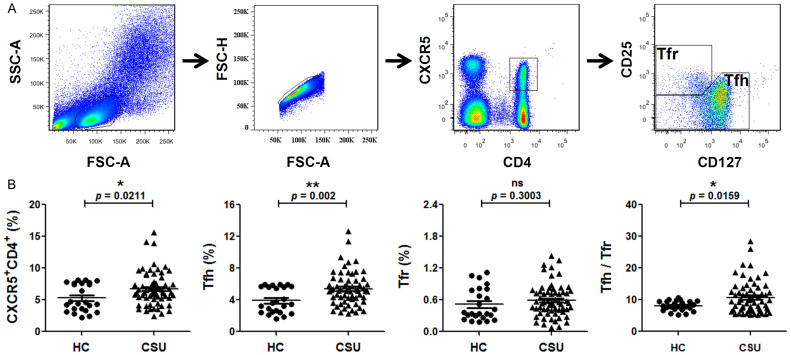
Increase in the frequencies of circulating CD4+CXCR5+ cells, Tfh cells and the Tfh/Tfr ratio in CSU
The specific gating strategy to identify Tfh and Tfr expressing CD4+CXCR5+ was shown in Figure 1A. The frequencies of circulating CD4+CXCR5+ cells, Tfh cells and the calculated Tfh/Tfr ratio were higher in CSU patients than in HC, without significant difference observed for the frequency of Tfr cells (Figure 1B).
Figure 1.
The status of circulating CD4+CXCR5+, Tfh, Tfr and Tfh/Tfr ratio in peripheral blood mononuclear cells (PBMCs) of patients with chronic spontaneous urticaria (CSU). A. Gating strategy to identify circulating Tfh (CD4+CXCR5+CD25lowCD127high) and Tfr (CD4+CXCR5+CD25highCD127low) cells among the CD4+CXCR5+ T cells in human peripheral blood. B. Percentage of circulating CD4+CXCR5+, Tfh and Tfr cells among PBMCs and the Tfh/Tfr in patients with CSU and healthy controls (HC). Each plot represented individual subject. Horizontal bars indicated the mean and error bars the SEM. Significance between two groups was evaluated with a two-tailed Student’s t-test and indicated by p value (*P < 0.05, **P < 0.01, ns P > 0.05).
Upregulation of CXCR5 expression in CSU
A significant upregulation of CXCR5 at the mRNA level (Figure 2A) as well as in the protein expression (Figure 2B and 2C) was observed in patients with CSU, as compared with HC.
Figure 2.
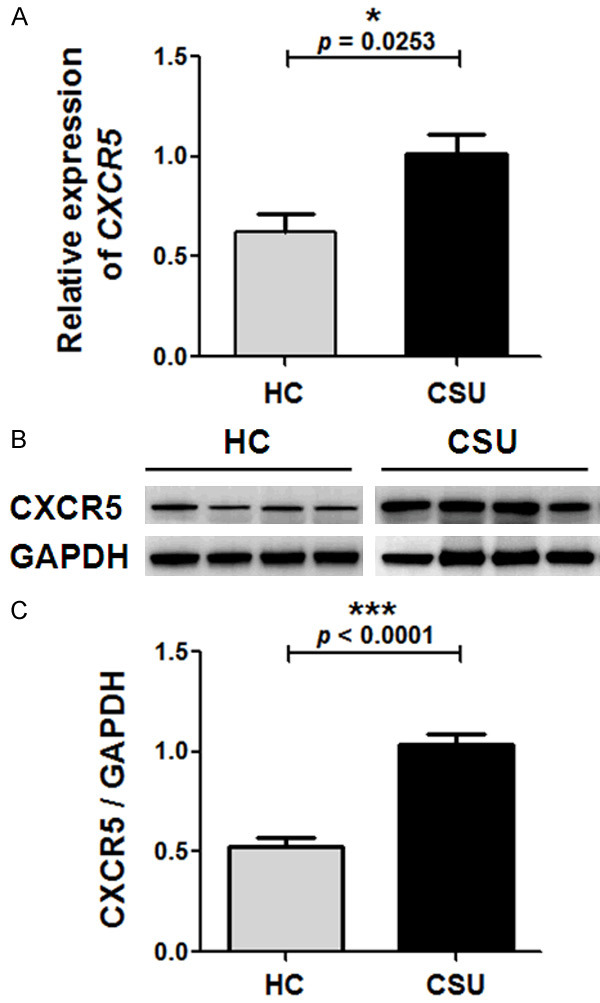
Relative expression of CXCR5 mRNA and protein levels in peripheral blood mononuclear cells (PBMCs) of patients with chronic spontaneous urticaria (CSU) and healthy controls (HC). A. The CXCR5 mRNA expression levels were determined by quantitative real-time (qRT)-PCR, with β-actin as an internal reference gene. B and C. The CXCR5 protein expression levels were determined by western blot, with GAPDH as the endogenous control. B. Representative data (n = 4) by the western blot analysis of total protein samples from PBMCs of CSU patients and HC. C. The CXCR5/GAPDH in PBMCs were calculated from the western blot results. Vertical bars represent SEM. Significance between two groups was evaluated with a two-tailed Student’s t-test and indicated by p value (*P < 0.05, ***P < 0.001).
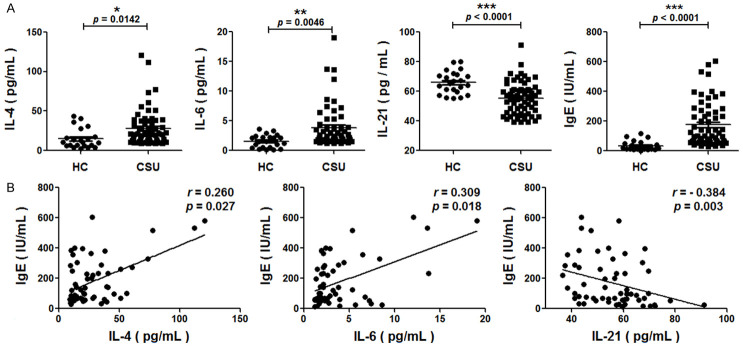
Correlation between the plasma levels of IL-4, IL-6, IL-21 and the total IgE in CSU
Significantly higher plasma levels of IL-4 and IL-6 but lower IL-21 were found in patients with CSU than in HC (Figure 3A). The levels of total IgE were significantly higher in patients with CSU (Figure 3A), and positively correlated with IL-4 and IL-6, but negatively with IL-21 (Figure 3B).
Figure 3.
Relationship between plasma cytokines and total IgE in patients with chronic spontaneous urticaria (CSU). A. Concentrations of IL-4, IL-6, IL-21 and total IgE in the plasma of CSU patients and healthy controls (HC). Differences between CSU and HC were evaluated with a two-tailed Student’s t-test. B. Correlation analysis between IL-4, IL-6, IL-21 and total IgE were conducted using Spearman’s rank test. Each plot represented one sample. P < 0.05 indicated significant difference (*P < 0.05, **P < 0.01, ***P < 0.001).
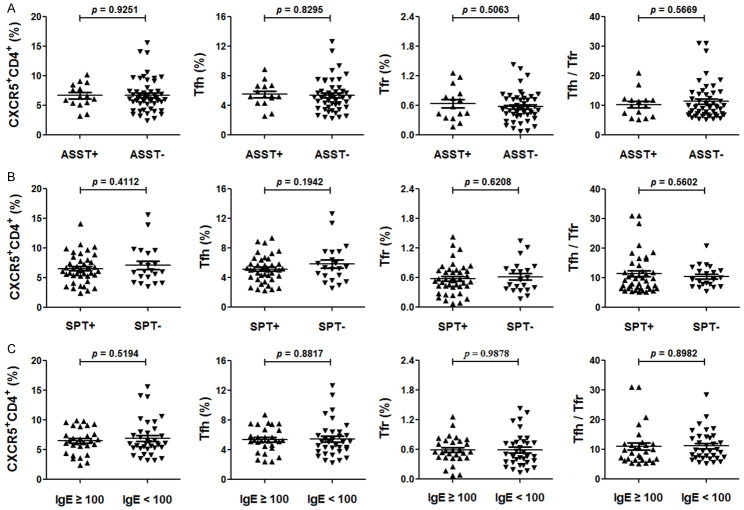
No significant difference of circulating CD4+CXCR5+ cells, Tfh cells, Tfr cells or the Tfh/Tfr ratio among the different subgroups
We divided CSU patients into different subgroups based on ASST, SPT or the levels of total IgE as before [2] to see possible differences. There are no significant differences in the frequencies of circulating CD4+CXCR5+ cells, Tfh cells, Tfr cells and the Tfh/Tfr in different subgroups (Figure 4). After further division of the patients into four groups, ASST+/SPT+, ASST+/SPT-, ASST-/SPT+ and ASST-/SPT-, we still failed to identify any difference among them (data not shown).
Figure 4.
The status of circulating CD4+CXCR5+, Tfh, Tfr and Tfh/Tfr ratio in peripheral blood mononuclear cells (PBMCs) of different subgroups of patients with chronic spontaneous urticaria (CSU). Percentage of circulating CD4+CXCR5+, Tfh and Tfr cells in PBMCs and the Tfh/Tfr in ASST+ CSU and ASST- CSU patients (A), SPT+ CSU and SPT- CSU patients (B), the levels of total IgE ≥ 100 IU/ml CSU and IgE < 100 IU/ml CSU patients (C). Each plot represented individual subject. Horizontal bars indicated the mean and error bars the SEM. Significance between two groups was evaluated with a two-tailed Student’s t-test and indicated by p value (P < 0.05 indicated significant difference).
There was no significant difference in patients with CSU between different subgroups, regarding gender, age and the UAS (Table 2).
Table 2.
Comparison between different subgroups of patients with chronic spontaneous urticaria (CSU)
| ASST | p value | SPT | p value | total IgE (IU/ml) | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| positive | negative | positive | negative | ≥ 100 | < 100 | ||||
| Number | 15 | 48 | 42 | 21 | 29 | 34 | |||
| Female gender (%) | 12 (80%) | 28 (58.3%) | 0.132 | 24 (57.1%) | 16 (76.2%) | 0.143 | 19 (65.5%) | 21 (61.8%) | 0.762 |
| Age (years), mean (range) | 33.2 (13-52) | 39.5 (13-70) | 0.172 | 37.6 (13-70) | 39.5 (13-70) | 0.654 | 38.5 (13-64) | 38 (13-70) | 0.897 |
| UAS, mean (range) | 5.13 (2-6) | 4.9 (2-6) | 0.486 | 5.02 (2-6) | 4.82 (2-6) | 0.499 | 5.07 (2-6) | 4.85 (2-6) | 0.459 |
Note: CSU: chronic spontaneous urticaria; ASST: autologous serum skin testing; SPT: skin prick testing; UAS: urticaria activity score. Significance between two groups was evaluated with a two-tailed Student’s t-test and indicated by p value. p < 0.05 was indicated as significant.
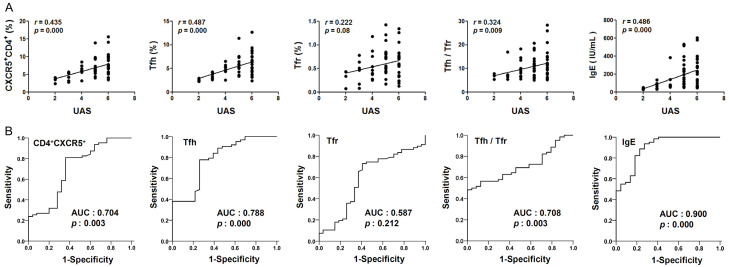
CD4+CXCR5+ cells, Tfh cells, the Tfh/Tfr ratio and total IgE correlated with CSU disease activity
There was a positive correlation between CD4+CXCR5+ cells, Tfh cells, the Tfh/Tfr ratio, total IgE and the urticaria activity (UAS), respectively. While no such correlation was observed for Tfr cells (Figure 5A). The ROC curve analysis showed that area under the curve (AUC) of Tfr cells was 0.587, there are no statistical difference (P > 0.05). The AUCs of CD4+CXCR5+, Tfh, Tfh/Tfr and total IgE were 0.704, 0.788, 0.708 and 0.900, respectively, with statistical significance (P < 0.05) (Figure 5B).
Figure 5.
Correlation between CD4+CXCR5+ cells, Tfh cells, the Tfh/Tfr ratio, total IgE in the peripheral blood of chronic spontaneous urticaria (CSU) and disease. A. Correlation between CD4+CXCR5+, Tfh, Tfr, Tfh/Tfr, total IgE and urticaria activity score (UAS) in patients with CSU. Each plot represented one sample. Correlation analysis were conducted using Spearman’s rank test. B. Receiver operating characteristic (ROC) curves for association of CSU disease (versus healthy controls) based on the CD4+CXCR5+, Tfh, Tfr, Tfh/Tfr and total IgE. Statistical results of area under the curve (AUC) and p value in ROC for association of CSU disease were shown in the graph. 0.5 < AUC < 1 and P < 0.05 indicated that they were associated with the disease activity.
Discussion
The pathogenic roles of CD4+CXCR5+, Tfh and Tfr cells in autoimmune and autoinflammatory diseases have received much attention in recent years. In children with atopic dermatitis, the percentages and absolute numbers of circulating Tfh-like cells (CD4+CXCR5+ICOS+PD-1+) were increased and their production of IL-21 cytokine positivily correlated with the disease severity index SCORAD [9]. In patients with systemic lupus erythematosus, the frequencies of Tfh and Tfr cells as well as the Tfh/Tfr ratio in peripheral blood were abnormal (increased or decresed) and correlated with the disease activity [13-15]. In bullous pemphigoid, the frequency of Tfh cells in peripheral blood of patients was increased and correlated with the production of anti-BP180-NC16A autoantibodies [12]. All these studies suggested the potential involvement of these cells in the pathogenesis of autoimmunity and disease.
There are at least two distinct pathways, type I (IgE to autoallergens) and type II (IgG autoantibodies to IgE or its receptor) autoimmunity, have been proposed to contribute to the pathologic mechanisms of CSU [5]. Antibodies, which are produced by the associated B cells and helper T cells, may play an important role in the pathogenesis of urticaria [4,21]. Human studies have demonstrated a central role of CXCR5 in the migration of T and B cells into follicles, formation of germinal center, production of pathogenic autoantibodies, and governing the ability of Tfh cells to help B cells [22-24]. Our study provided the primary evidence of an upregulation of CXCR5 in PBMCs and the increased CD4+CXCR5+ T cells, Tfh cells and the Tfh/Tfr ratio in patients with CSU.
Studies reported that the production of IgE was mainly derived from two pathways, extrafollicular and germinal centers [25]. Early IgE antibody response arises from extrafollicular, whereas late IgE antibody response arises from germinal centers. IgE germinal center B cells mainly differentiate into short-lived IgE plasma cells, which produce IgE of high-mutation and mature affinity to bind antigens with higher affinity in the environment [25]. Most IgE cells are plasma cells and high affinity IgE is produced by the switching of IgG1 cells to IgE [26]. CD4+CXCR5+ cells and Tfh cells were found to regulate B cells to produce specific IgE and total IgE. In patients with asthma, the frequency of CD4+CXCR5+ cells in peripheral blood was increased and associated with the production of pathogenic total IgE antibody [10]. Tfh cells could regulate B cells to produce anti-OVA IgE and total IgE in allergic immune response to airborne allergens [27]. Our results that total IgE in plasma of patients with CSU was elevated and associated with UAS confirmed earlier reports [28,29]. Taken together, the formation of germinal centers and the increased frequencies of CD4+CXCR5+ cells and Tfh cells in peripheral blood may be involved in the production of total IgE and contributing to the pathogenesis in CSU patients.
Multiple factors, including cytokines, such as IL-4, IL-6, TGF-β, IL-12 and IL-21, are involved in the differentiation and function of Tfh cells [30]. In OVA plus alum-sensitized mice, anti-OVA and total IgE antibodies were induced and promoted by IL-4-secreting Tfh cells [20]. The specific block of IL-6R signaling on T cells can suppress the number of Tfh cells and the increase in the concentration of total IgE, Derp 1-specific IgE or IgG1 in B cells after sensitization in CD4Crexgp130-/- mice [19]. The IL-6/STAT3 signaling pathway has been shown to regulate the frequency of Tfh cells in the peripheral blood of patients with rheumatoid arthritis [18]. IL-21, a cytokine produced by Tfh cells, is essential for the immunomodulation of humoral and cellular immune responses. The role of IL-21 in the production of IgE remains controversial, regarding its promotion or inhibition of specific immune functions [31-34]. Our results indicated that the total IgE was related to IL-4, IL-6, and IL-21, which suggested that Tfh cells might promote the production of total IgE through IL-4 and IL-6, and induced the class switching of B cells in CSU patients, while IL-21 inhibited the production of IgE from B cells, as demonstrated in previous studies [4,21,35-38].
In the current study, the positive correlation between CD4+CXCR5+ cells, Tfh cells, Tfh/Tfr ratio and the UAS, and the higher AUCs of CD4+CXCR5+ and Tfh cells frequencies and Tfh/Tfr ratio indicated their potential use as serological markers in clinical diagnosis and follow-up of therapeutic response in patients with CSU.
There are some limitations in the current study. The lack of dynamic observations, such as the changes of the above parameters during the active versus and remission period of CSU, make it impossible to verify the time-course change of the immunological parameters. The relationship between the changes of the above parameters and the therapeutic efficacy is not clear. It remains to be confirmed whether increase of IL-4 and IL-6 and decrease of IL-21 can induce CD4+CXCR5+ cells and Tfh cells to help B cells to produce IgE in patients with CSU.
In conclusion, the positive correlation between CD4+CXCR5+ cells, Tfh cells and the UAS, and the higher AUCs of CD4+CXCR5+ and Tfh cells frequencies in CSU indicated their potential use as serological markers in clinical diagnosis and follow-up of therapeutic response in patients with CSU. Our results provided the primary evidence that the regulation of class switching of B cells may be associated with immunopathogenesis and disease severity of CSU.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81673059 and 81673068) and Southwest Hospital Innovation Team Training Program (SWH2018TD-02). We wish to thank Professor Bing Ni (Department of Pathophysiology, Army Medical University, China) for suggestions regarding experimental methods and research ideas.
Disclosure of conflict of interest
None.
References
- 1.Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, Church MK, Ensina LF, Gimenez-Arnau A, Godse K, Goncalo M, Grattan C, Hebert J, Hide M, Kaplan A, Kapp A, Abdul Latiff AH, Mathelier-Fusade P, Metz M, Nast A, Saini SS, Sanchez-Borges M, Schmid-Grendelmeier P, Simons FE, Staubach P, Sussman G, Toubi E, Vena GA, Wedi B, Zhu XJ, Maurer M. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868–887. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 2.Song Z, Zhai Z, Zhong H, Zhou Z, Chen W, Hao F. Evaluation of autologous serum skin test and skin prick test reactivity to house dust mite in patients with chronic spontaneous urticaria. PLoS One. 2013;8:e64142. doi: 10.1371/journal.pone.0064142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q, Zhai Z, Xu J, Chen W, Chen S, Zhong H, Huang X, Hao F, Song Z. Basophil CD63 expression in chronic spontaneous urticaria: correlation with allergic sensitization, serum autoreactivity and basophil reactivity. J Eur Acad Dermatol Venereol. 2017;31:463–468. doi: 10.1111/jdv.13912. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Zhong H, Chen WC, Zhai Z, Zhou Z, Song Z, Hao F. Different expression patterns of plasma Th1-, Th2-, Th17- and Th22-related cytokines correlate with serum autoreactivity and allergen sensitivity in chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2018;32:441–448. doi: 10.1111/jdv.14541. [DOI] [PubMed] [Google Scholar]
- 5.Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M. Autoimmune chronic spontaneous urticaria: what we know and what we do not know. J Allergy Clin Immunol. 2017;139:1772–1781. doi: 10.1016/j.jaci.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Y, Zou L, Liu YC. T follicular helper cells, T follicular regulatory cells and autoimmunity. Int Immunol. 2016;28:173–179. doi: 10.1093/intimm/dxv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabo K, Gaspar K, Dajnoki Z, Papp G, Fabos B, Szegedi A, Zeher M. Expansion of circulating follicular T helper cells associates with disease severity in childhood atopic dermatitis. Immunol Lett. 2017;189:101–108. doi: 10.1016/j.imlet.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Gong F, Zhu HY, Zhu J, Dong QJ, Huang X, Jiang DJ. Circulating CXCR5+CD4+ T cells participate in the IgE accumulation in allergic asthma. Immunol Lett. 2018;197:9–14. doi: 10.1016/j.imlet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Niu J, Song Z, Yang X, Zhai Z, Zhong H, Hao F. Increased circulating follicular helper T cells and activated B cells correlate with disease severity in patients with psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1791–1796. doi: 10.1111/jdv.13027. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Liu Z, Dang E, Jin L, He Z, Yang L, Shi X, Wang G. Follicular helper T Cells (Tfh) and IL-21 involvement in the pathogenesis of bullous pemphigoid. PLoS one. 2013;8:e68145. doi: 10.1371/journal.pone.0068145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Wang D, Song Y, Lu S, Zhao J, Wang H. Increased circulating CD4+CXCR5+FoxP3+ follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. Int Immunopharmacol. 2018;56:261–268. doi: 10.1016/j.intimp.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Xu B, Wang S, Zhou M, Huang Y, Fu R, Guo C, Chen J, Zhao J, Gaskin F, Fu SM, Yang N. The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clin Immunol. 2017;183:46–53. doi: 10.1016/j.clim.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yang J, Chu Y, Xue Y, Xuan D, Zheng S, Zou H. T follicular helper cells and regulatory B cells dynamics in systemic lupus erythematosus. PLoS One. 2014;9:e88441. doi: 10.1371/journal.pone.0088441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca VR, Romao VC, Agua-Doce A, Santos M, Lopez-Presa D, Ferreira AC, Fonseca JE, Graca L. The ratio of blood T follicular regulatory cells to t follicular helper cells marks ectopic lymphoid structure formation while activated follicular helper T cells indicate disease activity in primary sjogren’s syndrome. Arthritis Rheumatol. 2018;70:774–784. doi: 10.1002/art.40424. [DOI] [PubMed] [Google Scholar]
- 17.Zheng J, Wang T, Zhang L, Cui L. Dysregulation of circulating Tfr/Tfh ratio in primary biliary cholangitis. Scand J Immunol. 2017;86:452–461. doi: 10.1111/sji.12616. [DOI] [PubMed] [Google Scholar]
- 18.Niu Q, Huang ZC, Wu XJ, Jin YX, An YF, Li YM, Xu H, Yang B, Wang LL. Enhanced IL-6/phosphorylated STAT3 signaling is related to the imbalance of circulating T follicular helper/T follicular regulatory cells in patients with rheumatoid arthritis. Arthritis Res Ther. 2018;20:200. doi: 10.1186/s13075-018-1690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble A, Zhao J. Follicular helper T cells are responsible for IgE responses to Der p 1 following house dust mite sensitization in mice. Clin Exp Allergy. 2016;46:1075–1082. doi: 10.1111/cea.12750. [DOI] [PubMed] [Google Scholar]
- 20.Harada Y, Tanaka S, Motomura Y, Harada Y, Ohno S, Ohno S, Yanagi Y, Inoue H, Kubo M. The 3’ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Huilan Z, Runxiang L, Bihua L, Qing G. Role of the subgroups of T, B, natural killer lymphocyte and serum levels of interleukin-15, interleukin-21 and immunoglobulin E in the pathogenesis of urticaria. J Dermatol. 2010;37:441–447. doi: 10.1111/j.1346-8138.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 22.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 23.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol. 2014;14:247–259. doi: 10.1038/nri3632. [DOI] [PubMed] [Google Scholar]
- 26.He JS, Subramaniam S, Narang V, Srinivasan K, Saunders SP, Carbajo D, Wen-Shan T, Hidayah Hamadee N, Lum J, Lee A, Chen J, Poidinger M, Zolezzi F, Lafaille JJ, Curotto de Lafaille MA. IgG1 memory B cells keep the memory of IgE responses. Nat Commun. 2017;8:641. doi: 10.1038/s41467-017-00723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol. 2017;139:300–313. doi: 10.1016/j.jaci.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altrichter S, Hawro T, Liedtke M, Holtappels G, Bachert C, Skov PS, Maurer M. In chronic spontaneous urticaria, IgE against staphylococcal enterotoxins is common and functional. Allergy. 2018;73:1497–1504. doi: 10.1111/all.13381. [DOI] [PubMed] [Google Scholar]
- 29.Kessel A, Helou W, Bamberger E, Sabo E, Nusem D, Panassof J, Toubi E. Elevated serum total IgE--a potential marker for severe chronic urticaria. Int Arch Allergy Immunol. 2010;153:288–293. doi: 10.1159/000314370. [DOI] [PubMed] [Google Scholar]
- 30.Yan L, de Leur K, Hendriks RW, van der Laan LJW, Shi Y, Wang L, Baan CC. T follicular helper cells as a new target for immunosuppressive therapies. Front Immunol. 2017;8:1510. doi: 10.3389/fimmu.2017.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fina D, Fantini MC, Pallone F, Monteleone G. Role of interleukin-21 in inflammation and allergy. Inflamm Allergy Drug Targets. 2007;6:63–68. doi: 10.2174/187152807780077246. [DOI] [PubMed] [Google Scholar]
- 32.Wood N, Bourque K, Donaldson DD, Collins M, Vercelli D, Goldman SJ, Kasaian MT. IL-21 effects on human IgE production in response to IL-4 or IL-13. Cell Immunol. 2004;231:133–145. doi: 10.1016/j.cellimm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster DC, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 34.Brandt K, Singh PB, Bulfone-Paus S, Ruckert R. Interleukin-21: a new modulator of immunity, infection, and cancer. Cytokine Growth Factor Rev. 2007;18:223–232. doi: 10.1016/j.cytogfr.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002;109:694–700. doi: 10.1067/mai.2002.123236. [DOI] [PubMed] [Google Scholar]
- 36.Maged Amin M, Rushdy M. Hyperlipidemia in association with pro-inflammatory cytokines among chronic spontaneous urticaria: case-control study. Eur Ann Allergy Clin Immunol. 2018;50:254–261. doi: 10.23822/EurAnnACI.1764-1489.68. [DOI] [PubMed] [Google Scholar]
- 37.Ferrer M, Luquin E, Sanchez-Ibarrola A, Moreno C, Sanz ML, Kaplan AP. Secretion of cytokines, histamine and leukotrienes in chronic urticaria. Int Arch Allergy Immunol. 2002;129:254–260. doi: 10.1159/000066772. [DOI] [PubMed] [Google Scholar]
- 38.Papadopoulos J, Karpouzis A, Tentes J, Kouskoukis C. Assessment of Interleukins IL-4, IL-6, IL-8, IL-10 in Acute Urticaria. J Clin Med Res. 2014;6:133–137. doi: 10.14740/jocmr1645w. [DOI] [PMC free article] [PubMed] [Google Scholar]