Abstract
Objectives: To investigate the predictors for the occurrence of intracranial hemorrhage (ICH) after endovascular thrombectomy (EVT) therapy in acute ischemic stroke (AIS) patients. Methods: Patients with AIS who underwent EVT and bridging therapy were enrolled retrospectively. ICH was evaluated on follow-up noncontrast CT or MRI. Diffusion weighted imaging (DWI) volume, perfusion weighted imaging (PWI) volume, DWI-PWI mismatch (DPM) volume and other clinical data were collected for 135 AIS patients. Multivariate logistic regression analysis was used to predict ICH after therapy in AIS patients. Results: The DWI volume in patients undergoing EVT with ICH was significantly larger than that in patients without ICH (50.61±47.43 vs 26.65±29.51; t=-2.416, P=0.020). For patients treated with bridging therapy, patients with ICH had larger DWI volume (26.32±29.66 vs 13.04±20.14; t=-2.013, P=0.037) and PWI volume (174.21±75.12 vs 129.87±60.29; t=-2.618, P=0.011) than patients without ICH. More patients with ICH were attempted for >3 passes with retriever during EVT than patients without ICH (EVT: 51.72% vs 26.19%; χ2=5.131, P=0.028; bridging therapy: 48.15% vs 21.62%; χ2=4.982, P=0.033). Multivariable logistic regression analysis demonstrated that DWI volume (OR, 1.017 (95% CI, 1.002-1.033); P=0.022) and >3 passes with the retriever (OR, 0.327 (95% CI, 0.114-0.936); P=0.037) were independently associated with ICH after EVT in AIS patients. DWI volume (OR, 1.024 (95% CI, 1.011-1.048); P=0.046), PWI volume (OR, 1.010 (95% CI, 1.002-1.018); P=0.016) and >3 passes with the retriever (OR, 0.281 (95% CI, 0.089-0.887); P=0.030) were independently associated with ICH after bridging therapy in AIS patients. Conclusions: DWI volume, PWI volume and >3 passes with the retriever were able to predict the ICH in patients with AIS after EVT therapy.
Keywords: Stroke, diffusion weighted imaging, perfusion weighted imaging, intracranial hemorrhage
Introduction
Acute ischemic stroke (AIS) has the characteristics of high morbidity, high disability and high mortality. Intravenous thrombolysis (IVT) within 4.5 hours after symptom onset is an efficient treatment [1,2], and endovascular thrombectomy (EVT) is emerging as the first-line treatment for AIS patients with large vessel occlusion (LVO) [3-5]. Most patients received IVT prior to randomization to EVT (bridging therapy), which may improve the early relevant recanalization and collateral circulation. However, approximately 30%-40% of AIS patients experienced intracranial hemorrhage (ICH) after EVT therapy [6-8]. This serious complication was associated with poor outcome and increased the rate of morbidity and mortality [9]. The identification of certain predictors of ICH could affect periprocedural management, notably by anticipating needs for intensive care among patients at high risk of ICH.
AIS consists of an irreversibly affected ischemic core and a salvageable ischemic penumbra, and the target of AIS IVT/EVT therapy is to save all or part of the ischemic penumbra [10-13]. Both regions and their approximated extent can be identified by advanced magnetic resonance imaging (MRI) techniques. Diffusion perfusion mismatch (DPM), mismatch between diffusion weighted imaging (DWI) and perfusion weighted imaging (PWI) techniques after intermodality coregistration, is the most widely used technique for estimating the volume of penumbra in clinic. Previous studies found that certain parameters, such as age, systolic blood pressure, stroke onset to treatment time and blood glucose, could be predictors of ICH after IVT [14,15]. Other studies found that lower Alberta Stroke Program Early CT Score (ASPECTS) and adjectively intra-arterial thrombolysis were independent risk factors for ICH after EVT in acute stroke patients [9]. The influence of infarct volume and penumbra volume on ICH after EVT therapy is still limited. To provide a better understanding of the risk factors for ICH after EVT therapy in patients with AIS of the anterior circulation, we analyzed MRI data and clinical data of AIS patients treated with EVT therapy and bridging therapy to possibly identify clinically relevant predictors of ICH.
Therefore, in this study, we hypothesized that DWI, PWI and DPM volumes are associated with ICH and can predict ICH in AIS patients. This study aimed to investigate whether DWI, PWI and DPM volumes are associated with ICH in AIS patients treated with EVT therapy and to evaluate the risk factors for ICH in these patients.
Materials and methods
Subjects and clinical data
In this retrospective study, AIS patients who were admitted to the Nanjing First Hospital from January 2017 to June 2019 were evaluated. The eligibility criteria for the study were (1) a first-ever acute anterior circulation stroke within 24 h of symptom onset; (2) MRI examination with DWI and PWI before EVT therapy; (3) EVT therapy or bridging therapy; (4) follow-up MRI or CT within 24 h after therapy; and (5) a clinical follow-up with the modified Rankin scale (mRS) at 3 months. The exclusion criteria were as follows: (1) cerebral hemorrhage, tumor or trauma detected by the CT scanner; (2) any contraindication for MRI; (3) any missing mRS data at 3 months after stroke; (4) the refusal of EVT therapy; and (5) any MRI or DSA data that could not be evaluated due to a motion artifact. An emergency pathway was set up for all examinations and treatments. All patients were treated according to the guidelines for the management of AIS [16]. EVT was performed with a stent retriever (SR) (Solitaire, Covidien, Irvine) without or with a balloon guide catheter (BGC) (American, Gateway PTA Balloon Catheter, 2.0 mm × 9 mm). Successful recanalization was defined as a modified Thrombolysis in Cerebral Infarction (mTICI) score of 2b or 3 [17]. In this study, EVT therapy was defined as patients receiving EVT therapy alone, and bridging therapy was defined as patients receiving IVT and EVT therapy. All patients in this study provided written informed consent before examination.
The following clinical variables were collected: age; sex; time from onset to admission; time from onset to MRI; time from onset to IVT; time from onset to EVT; time from onset to recanalization; stroke severity assessed with the National Institutes of Health Stroke Scale (NIHSS) score on admission; and the history of hypertension (>140/90 mmHg), diabetes mellitus (fasting plasma glucose ≥126 mg/dL (7.00 mmol/L) or 2-h plasma glucose after a 75-g oral glucose tolerance test ≥200 mg/dL (11.1 mmol/L)), hyperlipidemia (blood serum total cholesterol >150 mg/dL (1.70 mmol/L)/triglycerides >220 mg/dL (5.72 mmol/L)/low density lipoprotein-cholesterol >140 mg/dL (3.64 mmol/L)), homocysteine (blood test level >15 μmol/L) and atrial fibrillation. The mRS score at 3 months was used to assess the functional outcome: a mRS score of 0~2 was considered a favorable functional outcome and a score of 3~6 was considered an unfavorable functional outcome.
MRI protocol
MRI at admission and follow-up MRI were performed on a 3.0 Tesla MRI scanner (Ingenia, Philips Medical Systems) with an 8-channel receiver array head coil. The MRI protocol included FLAIR (IR sequence, TR, 7000 ms; TE, 120 ms; acquisition matrix, 356*151; FOV, 230 mm*230 mm; FA, 90°; slices, 18; section thickness, 6 mm; and intersection gap, 1.3 mm), DWI (spin echo (SE) sequence, TR, 2501 ms; TE, 98 ms; acquisition matrix, 152*122; 3 directions; FOV, 230 mm*230 mm; FA, 90°; slices, 18; section thickness, 6 mm; and intersection gap, 1.3 mm, b values, 0 and 1000 s/mm2), 3D-MRA (fast field echo (FFE) sequence, TR, 4.9 ms; TE, 1.82 ms; acquisition matrix, 528*531; FOV, 330 mm*330 mm; section thickness, 1.2 mm) and DSC-PWI (repetition time ms/echo time ms, 2000/30; acquisition matrix, 96*93; 2 field of view; FOV, 224 mm*224 mm; FA, 90°; section thickness, 4 mm; and duration =88 s).
Image analysis
The PWI data were analyzed using a Philips advanced workstation. The arterial input function (AIF) was selected by manually identifying the M2 segment of the middle cerebral artery ipsilateral to the acute infarction. The cerebral blood volume (CBV) maps were generated after circular singular value decomposition of the concentration-time curve. DWI volume and PWI volume were measured by using ITK-SNAP software (http://www.itksnap.org/pmwiki/pmwiki.php) as follows: all DWI and CBV images of AIS patients were derived in “DICOM” format and then converted all DICOM format into “NII” format by using mricron software (https://www.nitrc.org/projects/mricron). The high-intensity signal area on DWI and the abnormal perfusion area on CBV were drawn using ITK-SNAP software, and then DWI volume and CBV volume were automatically calculated separately. CBV volume represented PWI volume in this study [18]. DPM volume was defined as PWI volume minus DWI volume. Routine follow-up brain imaging with noncontrast CT (NCCT) or cranial MRI was performed within 24 h after EVT therapy. HT was categorized according to the Heidelberg Bleeding Classification (HBC) [19] and the European Collaborative Acute Stroke Study (ECASS) classification [20]. ICH and contrast agent extravasation were distinguished by comparison with the previous CT or MRI scans, and the result was confirmed on CT or MRI scans at 3-7 days after therapy. ICH was also subcategorized into 2 types: symptomatic ICH (sICH) and asymptomatic ICH. sICH was defined as any hemorrhage on CT/MRI scan with a neurological deterioration ≥4 points on the NIHSS score from the baseline or the lowest value in the first seven days or any hemorrhage resulting in death according to the ECASS III research [21]. Two experienced neuroradiologists (LJ and MP), blinded to the clinical data, independently evaluated these images. In the case of a discrepant assessment results between the two readers, images were reviewed, and a consensus was established.
Statistical analysis
All statistical analyses were conducted using commercially available software (SPSS for Windows, version 19.0; SPSS). Continuous data are shown as the mean ± SD and were analyzed by using an independent-samples t-test or Fisher’s exact test, whereas categorical variables are presented as absolute and relative frequencies and were analyzed by using the chi-squared test. P<0.05 was considered to indicate statistical significance. Kappa values were used to determine interrater agreement in the evaluation of ICH. Multivariate logistic regression analysis of variables (P<0.05) was performed to predict the factors of ICH after therapy.
Results
Among 219 randomized patients in the study, 135 patients (82 men and 53 women; mean age [years ± SD] 69.61±11.85; range, 58-81) were included. Eighty-four patients were excluded (28 patients without MRI at admission; 16 patients with severely artifacted DWI sequence; 22 patients with severely artifacted PWI sequence; 18 patients without mRS at 3 months). Figure 1 shows the flowchart of the study. In 135 patients, 71 patients (52.59%) received EVT therapy, and 29 patients (40.85%) had ICH after therapy. sICH was observed in 8 patients (8.45%); 64 patients (47.41%) received bridging therapy, and 27 patients (42.19%) had ICH after therapy, sICH was observed in 6 patients (9.38%). The interobserver agreement for ICH was k=0.96 (95% CI, 0.94-0.99). Bleeding classification specifics are shown in Table 1. Figure 2 shows that AIS patients after EVT therapy had ICH.
Figure 1.
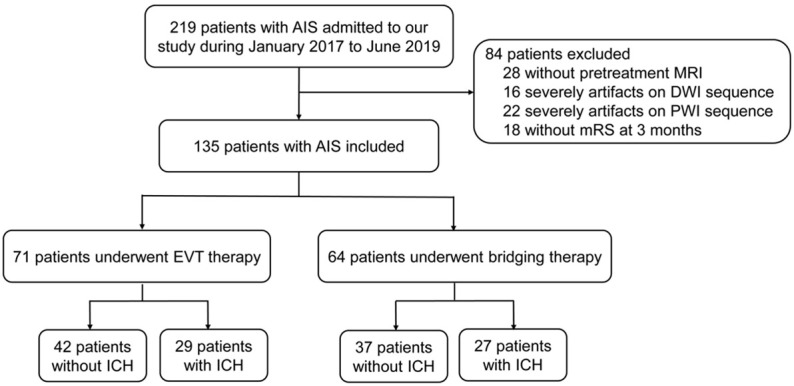
Flowchart of the study. Among 219 randomized patients in the study, 135 patients were included. In 135 patients, 71 patients received EVT therapy, 29 patients had ICH after therapy, 64 patients received bridging therapy, and 27 patients had ICH after therapy.
Table 1.
Overview of bleeding events, categorized with HBC and the ECASS classification, according to anatomical, descriptive, and clinical features
| HBC | ECASS | ICH after reperfusion therapy (n=56) | ICH after EVT therapy (n=29) | ICH after bridging therapy (n=27) |
|---|---|---|---|---|
| 1a | HI1 | 3 (5.36%) | 2 (6.90%) | 1 (3.70%) |
| 1b | HI2 | 5 (8.93%) | 2 (6.90%) | 3 (11.11%) |
| 1c | PH1 | 15 (26.79%) | 9 (31.03%) | 6 (22.22%) |
| 2 | PH2 | 18 (32.14%) | 7 (24.14%) | 11 (40.74%) |
| 3a | - | 1 (1.79%) | 1 (3.45%) | 0 |
| 3b | - | 0 | 0 | 0 |
| 3c | - | 13 (23.21%) | 8 (27.59%) | 5 (18.52%) |
| 3d | - | 1 (1.79%) | 0 | 1 (3.70%) |
Note: HBC: Heidelberg Bleeding classification; ECASS: European Collaborative Acute Stroke Study; ICH: intracranial hemorrhage; HI: hemorrhagic infarction; PH: parenchymatous hematoma; EVT: endovascular thrombectomy.
Figure 2.
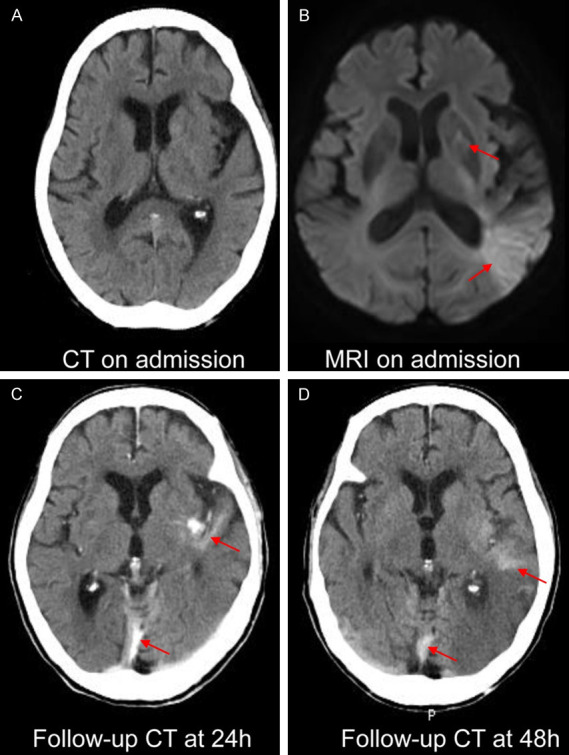
A case of ICH after EVT therapy in an AIS patients. The patient manifested drowsiness for four hours. CT on admission (A) showed no ICH; after IVT therapy, an MRI scan was performed, and acute stroke in the basal ganglia, temporal lobe and occipital lobe was found on MRI on admission (B). EVT therapy was performed immediately, follow-up CT at 24 h (C) showed ICH in the left temporal lobe and falx cerebri, and the ICH still existed on follow-up CT at 48 h (D).
The clinical and demographic information is shown in Tables 2 and 3. For patients with EVT therapy, the DWI volume in patients with ICH was significantly larger than that in patients without ICH (50.61±47.43 vs 26.65±29.51; t=-2.416, P=0.020) (Figure 3A). PWI volume and DPM volume were no significant difference between the two groups (P=0.467; P=0.804). More patients with ICH were attempted for >3 passes with retriever during EVT than in patients without ICH (51.72% vs 26.19%; χ2=5.131, P=0.028) (Figure 3B). The successful reperfusion (mTICI 2b-3) ratio in patients with ICH was lower than that in patients without ICH (58.62% vs 64.29%), while there was no significant difference between the two groups (χ2=0.234; P=0.804). For patients treated with bridging therapy, patients with ICH had higher NIHSS scores on admission (13.15±5.25 vs 10.14±6.20; t=-1.413, P=0.045), larger DWI volume (26.32±29.66 vs 13.04±20.14; t=-2.013, P=0.037) and larger PWI volume (174.21±75.12 vs 129.87±60.29; t=-2.618, P=0.011) than patients without ICH (Figure 3A). More patients with ICH were attempted for >3 passes with retriever during EVT than in patients without ICH (48.15% vs 21.62%; χ2=4.982, P=0.033) (Figure 3B). The successful reperfusion (mTICI 2b-3) ratio in the two groups was also no significant difference (40.74% vs 64.86%; χ2=3.666, P=0.076). The functional outcome in patients without ICH was both better than that in patients with ICH in both therapies (1.90±1.87 vs 3.62±1.90; t=-3.766, P<0.001; 1.92±1.64 vs 2.85±2.01; t=-1.413, P=0.045) (Figure 4). For each therapy, there was no significant difference in sex, age, smoking, alcohol drinking, diabetes mellitus, hypertension, atrial fibrillation, hyperlipidemia and homocysteine between the two groups (P>0.05) (Tables 2 and 3).
Table 2.
Comparison of without ICH and with ICH in AIS patients after EVT therapy
| Without ICH (n=42) | With ICH (n=29) | t/χ2 | P | |
|---|---|---|---|---|
| Sex, male | 26 (61.90%) | 16 (55.17%) | 0.322 | 0.628 |
| Age, y | 70.24±12.09 | 69.90±10.85 | 0.840 | 0.903 |
| Time from onset to admission, h | 4.16±2.12 | 4.18±2.39 | -0.035 | 0.972 |
| Time from onset to first MRI, h | 5.70±1.56 | 5.60±2.08 | 0.237 | 0.823 |
| Time from onset to EVT, h | 6.80±1.38 | 6.95±1.81 | -0.407 | 0.700 |
| Time from onset to recanalization, h | 6.91±0.63 | 7.53±0.52 | 0.371 | 0.554 |
| Smoking | 9 (21.43%) | 8 (27.59%) | 0.357 | 0.582 |
| Alcohol drinking | 4 (9.52%) | 4 (13.79%) | 0.031 | 0.708 |
| Diabetes mellitus | 12 (28.57%) | 9 (31.03%) | 0.050 | 1.000 |
| Hypertension | 33 (78.57%) | 23 (79.31%) | 0.006 | 1.000 |
| Atrial fibrillation | 19 (45.24%) | 13 (44.83%) | 0.001 | 1.000 |
| Hyperlipidemia | 2 (4.76%) | 1 (3.45%) | 0.073 | 1.000 |
| Homocysteine | 1 (2.38%) | 2 (6.90%) | 0.109 | 0.563 |
| NIHSS on admission | 12.95±4.27 | 13.62±4.59 | -0.629 | 0.531 |
| >3 passes with retriever | 11 (26.19%) | 15 (51.72%) | 5.131 | 0.028* |
| Successful reperfusion (mTICI 2b-3) | 27 (64.29%) | 17 (58.62%) | 0.234 | 0.804 |
| DWI volume, mL | 26.65±29.51 | 50.61±47.43 | -2.416 | 0.020* |
| PWI volume, mL | 172.84±118.66 | 190.62±66.53 | -0.731 | 0.467 |
| DPM volume, mL | 146.19±121.80 | 140.01±65.61 | 0.252 | 0.804 |
| mRS at 3 months | 1.90±1.87 | 3.62±1.90 | -3.766 | <0.001* |
Note: AIS: acute ischemic stroke; ICH: intracranial hemorrhage; NIHSS: National Institutes of Health Stroke Scale; DWI: diffusion weighted imaging; PWI: perfusion weighted imaging; DPM: DWI-PWI mismatch; EVT: endovascular thrombectomy; mTICI: modified Thrombolysis in Cerebral Infarction; mRS: modified Rankin Scale;
P<0.05.
Table 3.
Comparison of without ICH and with ICH in AIS patients after Bridging therapy
| Without ICH (n=37) | With ICH (n=27) | t/χ2 | P | |
|---|---|---|---|---|
| Sex, male | 24 (64.86%) | 16 (59.26%) | 0.209 | 0.794 |
| Age, y | 66.19±13.48 | 71.85±7.22 | -2.165 | 0.052 |
| Time from onset to admission, h | 1.95±1.22 | 2.46±1.05 | -1.755 | 0.084 |
| Time from onset to first MRI, h | 4.33±1.10 | 4.61±0.87 | -1.089 | 0.280 |
| Time from onset to IVT, h | 2.86±1.19 | 3.31±0.96 | -1.619 | 0.111 |
| Time from onset to EVT, h | 5.96±0.89 | 6.20±0.64 | -1.267 | 0.233 |
| Time from onset to recanalization, h | 6.47±0.59 | 6.89±0.57 | -1.793 | 0.342 |
| Smoking | 10 (27.03%) | 3 (11.11%) | 2.443 | 0.207 |
| Alcohol drinking | 6 (16.22%) | 1 (3.70%) | 2.509 | 0.223 |
| Diabetes mellitus | 8 (21.62%) | 5 (18.52%) | 0.093 | 1.000 |
| Hypertension | 28 (75.68%) | 20 (74.07%) | 0.042 | 1.000 |
| Atrial fibrillation | 14 (37.84%) | 15 (55.56%) | 1.977 | 0.206 |
| Hyperlipidemia | 1 (2.70%) | 0 (0.00%) | 0.000 | 1.000 |
| Homocysteine | 1 (2.70%) | 0 (0.00%) | 0.000 | 1.000 |
| NIHSS on admission | 10.14±6.20 | 13.15±5.25 | -1.413 | 0.045* |
| >3 passes with retriever | 8 (21.62%) | 13 (48.15%) | 4.982 | 0.033* |
| Successful reperfusion (mTICI 2b-3) | 24 (64.86%) | 11 (40.74%) | 3.666 | 0.076 |
| DWI volume, mL | 13.04±20.14 | 26.32±29.66 | -2.013 | 0.037* |
| PWI volume, mL | 129.87±60.29 | 174.21±75.12 | -2.618 | 0.011* |
| DPM volume, mL | 116.83±58.89 | 147.89±67.55 | -2.165 | 0.055 |
| mRS at 3 months, mL | 1.92±1.64 | 2.85±2.01 | -1.413 | 0.045* |
Note: AIS: acute ischemic stroke; ICH: intracranial hemorrhage; NIHSS: National Institutes of Health Stroke Scale; DWI: diffusion weighted imaging; PWI: perfusion weighted imaging; DPM: DWI-PWI mismatch; mTICI: modified Thrombolysis in Cerebral Infarction; mRS: modified Rankin Scale;
P<0.05.
Figure 3.
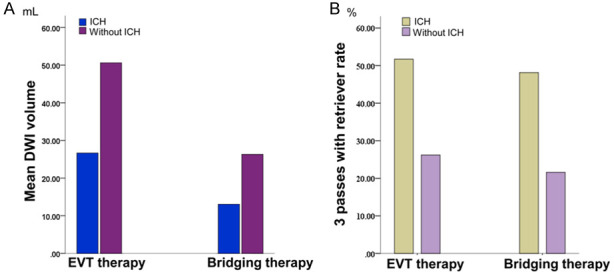
A. Mean DWI volume with ICH and without ICH after EVT therapy and bridging therapy in AIS patients. B. The rate of more than 3 passes with the retriever in AIS patients with ICH and those without ICH after EVT therapy and bridging therapy.
Figure 4.
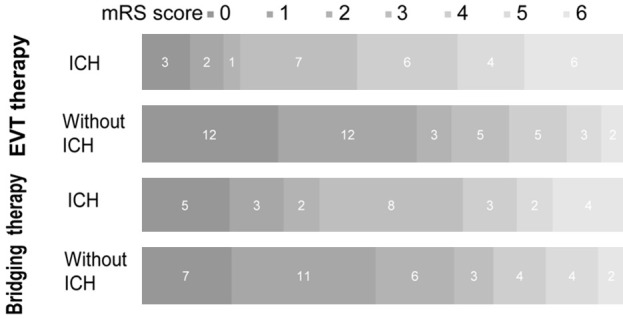
Distribution of mRS score at 3 months in patients with ICH and without ICH after EVT therapy and bridging therapy. There was a significant difference between patients with ICH and those without ICH in the overall distribution of mRS scores.
Multivariable logistic regression analysis demonstrated that DWI volume (OR, 1.017 (95% CI, 1.002-1.033); P=0.022) and >3 passes with the retriever (OR, 0.327 (95% CI, 0.114-0.936); P=0.037) were independently associated with ICH after EVT in AIS patients. DWI volume (OR, 1.024 (95% CI, 1.011-1.048); P=0.046), PWI volume (OR, 1.010 (95% CI, 1.002-1.018); P=0.016) and >3 passes with the retriever (OR, 0.281 (95% CI, 0.089-0.887); P=0.030) were independently associated with ICH after bridging therapy in AIS patients (Table 4).
Table 4.
Multivariate logistic regression analysis (P<0.05) for predicting ICH in AIS patients after EVT therapy and bridging therapy
| OR | 95% CI | P | ||
|---|---|---|---|---|
| EVT therapy | DWI volume | 1.017 | 1.002-1.033 | 0.022* |
| >3 passes with retriever | 0.327 | 0.114-0.936 | 0.037* | |
| Bridging therapy | NIHSS at admission | 1.076 | 0.975-1.188 | 0.147 |
| DWI volume | 1.024 | 1.011-1.048 | 0.046* | |
| PWI volume | 1.010 | 1.002-1.018 | 0.016* | |
| >3 passes with retriever | 0.281 | 0.089-0.887 | 0.030* |
Note: AIS: acute ischemic stroke; ICH: intracranial hemorrhage; NIHSS: National Institutes of Health Stroke Scale; DWI: diffusion weighted imaging; PWI: perfusion weighted imaging; EVT: endovascular thrombectomy; OR: odds ratios; CI: confidence intervals.
P<0.05.
Discussion
The results of the present study suggested that after reperfusion therapy, patients with AIS had high rates of ICH (41.48%), which was associated with poor functional outcome. DWI volume and >3 passes with retriever were independently associated with ICH after EVT; DWI volume, PWI volume and >3 passes with the retriever were independently associated with ICH after bridging therapy.
In our study, we found that 41.48% of AIS patients experienced ICH after reperfusion therapy. Consistent with previous studies [8,22], we found that approximately 40.85% (29/71) of patients had ICH after EVT therapy, and 42.19% (27/64) of patients had ICH after bridging therapy. Katsanos et al [23] found that patients treated with bridging therapy and EVT did not differ in rates of symptomatic ICH. Pan et al [24] also demonstrated that the incidence of symptomatic ICH was no differences between the bridging strategies and EVT-alone treatments. Previous studies have suggested that bridging might demonstrate effectiveness that is similar to or better than that of EVT therapy [25-27], and a similar probability of symptomatic ICH [26]. In our study, we evaluated only the ICH rate in EVT and bridging therapy due to the small sample size, and we also found that the ICH rate was similar between EVT and bridging therapy. The mechanism of how bridging therapy leads to ICH remains unclear. IVT may have a positive impact on collateral and microvascular circulation. However, IVT might increase the risk of bleeding and also prevent the opportunity to use other endovascular thrombolytics in cases of remaining distal vessel occlusions.
The strengths of this study include that the risk factors for ICH in patients treated with EVT and bridging therapy were evaluated separately and the investigation of the prognostic value of quantified DWI, PWI and DPM volumes therapy in terms of ICH after reperfusion therapy. To minimize the effect of baseline characteristics imbalances due to selection bias, we adjusted for potential confounders, such as time from onset to admission, time from onset to EVT and time from onset to IVT. For patients with EVT therapy, patients with ICH had larger DWI volume lager than patients without ICH. For patients with bridging therapy, patients with ICH had lager DWI volume and PWI volume larger than patients without ICH. Logistic regression analysis demonstrated that DWI volume was independently associated with ICH after EVT in AIS patients. DWI volume and PWI volume were independently associated with ICH after bridging therapy in AIS patients. Neuberger et al [22] showed that patients with an infarct volume >1/3 of the affected territory were more prone to ICH after EVT. Kneihsl et al [28] identified large infarct size as a factor that was associated with ICH after EVT for acute anterior circulation stroke. When ischemic stroke occurs, regional blood flow increases, leading to increased permeability of the blood-brain barrier (BBB) which causes ICH after EVT [29]. In addition, rt-PA can exacerbate BBB disruption, such as the plasmin-mediated degradation of ECM proteins of the basal lamina, the activation of MMPs (especially MMP-9), and binding to low-density lipoprotein receptor-related protein 1, among others [30,31]. This may be the reason that PWI volume was independently associated with ICH after bridging therapy.
We also evaluated passes with retrievers and successful reperfusion (mTICI 2b-3) and found that more than 3 passes with the retriever were attempted in 34.81% (47/135) of patients in this study. The reason may be the higher proportion of intracranial atherosclerotic stenosis in Asian patients [32]. Atherosclerotic occlusion may be more difficult to recanalize due to severe stenosis. For patients with EVT and bridging therapy, more patients with ICH were attempted for >3 passes with the retriever during EVT than patients without ICH. In addition, >3 passes with the retriever were independently associated with ICH after EVT and bridging therapy in AIS patients. The ICH after EVT is roughly classified into 2 categories: ICH caused by reperfusion at the deep ischemic core and ICH following vessel or tissue injury directly caused by the EVT procedure and/or devices. While the mechanism of the former type is common to both IVT and EVT, the latter mechanism is inherent in EVT. Passes of thrombectomy devices may cause vascular injury and BBB disruption, both of which are associated with increased ICH [33]. In addition, patients receiving bridging therapy with ICH had significantly higher NIHSS scores on admission than patients without ICH. The meta-analysis of 55 studies demonstrated that ICH after IVT was associated with a higher stroke severity (higher NIHSS score) [34]. Our findings also confirmed that ICH caused any degree of clinical neurological worsening, which was associated with poor functional outcome [35].
Some limitations must be acknowledged. First, this study had a small sample size, and ICH was analyzed according to the ECSAA III classification in most studies [36]; however, due to the small sample size, ICH was not divided into subgroups for analysis in our study. We also did not analyze the MRI imaging differences between sICH and asymptomatic ICH due to the small sICH sample size. ICH types and sICH could drive changes in clinical management, so our further study will analyze different types of ICH and sICH by expanding the sample size. Second, due to ethical considerations, all MRI scans were performed after IVT in patients treated with bridging therapy, without ruling out the effect of IVT on the MRI. To reduce the influence of IVT on MRI images, we performed MRI scanning immediately after IVT through the emergency green channel. Future studies may have a larger sample size and analyze the factors of different types of ICH.
Conclusions
DWI volume, PWI volume and >3 passes with the retriever during EVT were associated with ICH in patients with AIS after reperfusion therapy. Assessments of DWI volume, PWI volume and >3 passes with the retriever during EVT might be useful for the prediction of ICH in AIS patients after reperfusion therapy.
Acknowledgements
This work was funded by the Jiangsu Provincial Special Program of Medical Science (BE2017614). LJ and LZ designed the experiment, collected the data, performed the analysis, and wrote the paper. HZ, WG, WY, JC, MP, and HC helped collect the data and perform the analysis. XY and YC contributed to the discussion and manuscript revision.
Disclosure of conflict of interest
None.
References
- 1.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong Q, Dong Y, Liu L, Xu A, Zhang Y, Zheng H, Wang Y. The Chinese Stroke Association scientific statement: intravenous thrombolysis in acute ischaemic stroke. Stroke Vasc Neurol. 2017;2:147–159. doi: 10.1136/svn-2017-000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derex L, Cho TH. Mechanical thrombectomy in acute ischemic stroke. Rev Neurol (Paris) 2017;173:106–113. doi: 10.1016/j.neurol.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 5.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, Guillemin F THRACE investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhao W, Che R, Shang S, Wu C, Li C, Wu L, Chen J, Duan J, Song H, Zhang H, Ling F, Wang Y, Liebeskind D, Feng W, Ji X. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. 2017;48:3289–3294. doi: 10.1161/STROKEAHA.117.019193. [DOI] [PubMed] [Google Scholar]
- 8.Hao Y, Yang D, Wang H, Zi W, Zhang M, Geng Y, Zhou Z, Wang W, Xu H, Tian X, Lv P, Liu Y, Xiong Y, Liu X, Xu G ACTUAL Investigators (Endovascular Treatment for Acute Anterior Circulation Ischemic Stroke Registry) Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke. 2017;48:1203–1209. doi: 10.1161/STROKEAHA.116.016368. [DOI] [PubMed] [Google Scholar]
- 9.Jiang F, Zhao W, Wu C, Zhang Z, Li C, Che R, Chen J, Hu W, Song H, Duan J, Ji X. Asymptomatic intracerebral hemorrhage may worsen clinical outcomes in acute ischemic stroke patients undergoing thrombectomy. J Stroke Cerebrovasc Dis. 2019;28:1752–1758. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Baron JC. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nat Rev Neurol. 2018;14:325–337. doi: 10.1038/s41582-018-0002-2. [DOI] [PubMed] [Google Scholar]
- 11.McCabe C, Arroja MM, Reid E, Macrae IM. Animal models of ischaemic stroke and characterisation of the ischaemic penumbra. Neuropharmacology. 2018;134:169–177. doi: 10.1016/j.neuropharm.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Fisher M, Bastan B. Identifying and utilizing the ischemic penumbra. Neurology. 2012;79(Suppl 1):S79–85. doi: 10.1212/WNL.0b013e3182695814. [DOI] [PubMed] [Google Scholar]
- 13.Catanese L, Tarsia J, Fisher M. Acute ischemic stroke therapy overview. Circ Res. 2017;120:541–558. doi: 10.1161/CIRCRESAHA.116.309278. [DOI] [PubMed] [Google Scholar]
- 14.Strbian D, Engelter S, Michel P, Meretoja A, Sekoranja L, Ahlhelm FJ, Mustanoja S, Kuzmanovic I, Sairanen T, Forss N, Cordier M, Lyrer P, Kaste M, Tatlisumak T. Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol. 2012;71:634–641. doi: 10.1002/ana.23546. [DOI] [PubMed] [Google Scholar]
- 15.Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D, Wahlgren N, Ahmed N SITS Investigators. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe implementation of treatments in stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012;43:1524–1531. doi: 10.1161/STROKEAHA.111.644815. [DOI] [PubMed] [Google Scholar]
- 16.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 17.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, Mocco J, Wardlaw JM, Barnwell SL, Jovin TG, Linfante I, Siddiqui AH, Alexander MJ, Hirsch JA, Wintermark M, Albers G, Woo HH, Heck DV, Lev M, Aviv R, Hacke W, Warach S, Broderick J, Derdeyn CP, Furlan A, Nogueira RG, Yavagal DR, Goyal M, Demchuk AM, Bendszus M, Liebeskind DS Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell BC, Christensen S, Parsons MW, Churilov L, Desmond PM, Barber PA, Butcher KS, Levi CR, De Silva DA, Lansberg MG, Mlynash M, Olivot JM, Straka M, Bammer R, Albers GW, Donnan GA, Davis SM EPITHET and DEFUSE Investigators. Advanced imaging improves prediction of hemorrhage after stroke thrombolysis. Ann Neurol. 2013;73:510–519. doi: 10.1002/ana.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, San Roman L, Saver JL, Strbian D, Whiteley W, Hacke W. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 21.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 22.Neuberger U, Kickingereder P, Schonenberger S, Schieber S, Ringleb PA, Bendszus M, Pfaff J, Mohlenbruch MA. Risk factors of intracranial hemorrhage after mechanical thrombectomy of anterior circulation ischemic stroke. Neuroradiology. 2019;61:461–469. doi: 10.1007/s00234-019-02180-6. [DOI] [PubMed] [Google Scholar]
- 23.Katsanos AH, Malhotra K, Goyal N, Arthur A, Schellinger PD, Kohrmann M, Krogias C, Turc G, Magoufis G, Leys D, Ahmed N, Khatri P, Goyal M, Alexandrov AV, Tsivgoulis G. Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann Neurol. 2019;86:395–406. doi: 10.1002/ana.25544. [DOI] [PubMed] [Google Scholar]
- 24.Pan X, Liu G, Wu B, Liu X, Fang Y. Comparative efficacy and safety of bridging strategies with direct mechanical thrombectomy in large vessel occlusion: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14956. doi: 10.1097/MD.0000000000014956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber R, Nordmeyer H, Hadisurya J, Heddier M, Stauder M, Stracke P, Berger K, Chapot R. Comparison of outcome and interventional complication rate in patients with acute stroke treated with mechanical thrombectomy with and without bridging thrombolysis. J Neurointerv Surg. 2017;9:229–233. doi: 10.1136/neurintsurg-2015-012236. [DOI] [PubMed] [Google Scholar]
- 26.Mistry EA, Mistry AM, Nakawah MO, Chitale RV, James RF, Volpi JJ, Fusco MR. Mechanical thrombectomy outcomes with and without intravenous thrombolysis in stroke patients: a meta-analysis. Stroke. 2017;48:2450–2456. doi: 10.1161/STROKEAHA.117.017320. [DOI] [PubMed] [Google Scholar]
- 27.Tsivgoulis G, Zand R, Katsanos AH, Goyal N, Uchino K, Chang J, Dardiotis E, Putaala J, Alexandrov AW, Malkoff MD, Alexandrov AV. Safety of intravenous thrombolysis in stroke mimics: prospective 5-year study and comprehensive meta-analysis. Stroke. 2015;46:1281–1287. doi: 10.1161/STROKEAHA.115.009012. [DOI] [PubMed] [Google Scholar]
- 28.Kneihsl M, Niederkorn K, Deutschmann H, Enzinger C, Poltrum B, Fischer R, Thaler D, Hermetter C, Wunsch G, Fazekas F, Gattringer T. Increased middle cerebral artery mean blood flow velocity index after stroke thrombectomy indicates increased risk for intracranial hemorrhage. J Neurointerv Surg. 2018;10:882–887. doi: 10.1136/neurintsurg-2017-013617. [DOI] [PubMed] [Google Scholar]
- 29.Cartmell SCD, Ball RL, Kaimal R, Telischak NA, Marks MP, Do HM, Dodd RL, Albers GW, Lansberg MG, Heit JJ. Early cerebral vein after endovascular ischemic stroke treatment predicts symptomatic reperfusion hemorrhage. Stroke. 2018;49:1741–1746. doi: 10.1161/STROKEAHA.118.021402. [DOI] [PubMed] [Google Scholar]
- 30.Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(Suppl 1):S52–7. doi: 10.1212/WNL.0b013e3182697e70. [DOI] [PubMed] [Google Scholar]
- 31.Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7:243–253. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 33.Desilles JP, Rouchaud A, Labreuche J, Meseguer E, Laissy JP, Serfaty JM, Lapergue B, Klein IF, Guidoux C, Cabrejo L, Sirimarco G, Lavallee PC, Schouman-Claeys E, Amarenco P, Mazighi M. Blood-brain barrier disruption is associated with increased mortality after endovascular therapy. Neurology. 2013;80:844–851. doi: 10.1212/WNL.0b013e31828406de. [DOI] [PubMed] [Google Scholar]
- 34.Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke. 2012;43:2904–2909. doi: 10.1161/STROKEAHA.112.665331. [DOI] [PubMed] [Google Scholar]
- 35.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 36.Neuberger U, Mohlenbruch MA, Herweh C, Ulfert C, Bendszus M, Pfaff J. Classification of bleeding events: comparison of ECASS III (European Cooperative Acute Stroke Study) and the new Heidelberg bleeding classification. Stroke. 2017;48:1983–1985. doi: 10.1161/STROKEAHA.117.016735. [DOI] [PubMed] [Google Scholar]


