Abstract
This study aimed to evaluate the efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy. In this multicentered, blinded, randomized, active-controlled, non-inferior phase III trial, 384 eligible patients who were about to undergo colonoscopy were randomized as a ratio of 1:1 into remimazolam and propofol group. Procedure success was assessed and defined as the completion of colonoscopy without administration of rescue sedative agent or more than 5 top-ups of trial drug in any 15 minute-period after initial administration of trial drug. Sedation quality was evaluated by Modified Observer’s Assessment of Alertness/Sedation score. Treatment-emergent adverse events were recorded. Procedure success rate was 96.91% (188/194) in remimazolam group and 100% (190/190) in propofol group, and the difference in rate was -3.09% with 95% confidence interval (CI) of -5.53%~-0.66%. Since the lower limit of 95% CI was greater than the non-inferiority margin of -8.00%, the efficacy of remimazolam tosylate was non-inferior to propofol. Besides, induction time of sedation was increased (P<0.001), while hypotension and respiratory depression was decreased in remimazolam group compared to propofol group; however, time to fully alert (P>0.05) or time to discharge (P>0.05) were unchanged. For safety assessment, total treatment-emergent adverse events were decreased in remimazolam group compared to propofol group (P<0.001); specifically, administration site pain (P<0.001), increased bilirubin (P=0.019), decreased respiratory rate (P<0.001) and decreased SpO2 (P<0.001) were less frequent in remimazolam group compared with propofol group. In conclusion, remimazolam tosylate is non-inferior in sedation efficacy while safer than propofol in patients undergoing colonoscopy.
Keywords: Remimazolam tosylate, propofol, colonoscopy, efficacy, treatment-emergent adverse events
Introduction
Colonoscopy is widely performed to diagnose or treat various colonic diseases, and its demand is increasing due to the raised prevalence of colonic diseases (such as colorectal cancer) and the growing life expectancy [1]. In order to provide patients with a relatively pleasant experience and to facilitate the endoscopists’ technical operation, colonoscopy is required to be conducted under sedation [2]. Traditionally, sedation for colonoscopy is induced by midazolam combined with opiates. However, midazolam has several drawbacks (such as the accumulation effect and the uncontrollable sedation time during repeated administration, etc.) [3]. In recent years, the use of propofol in colonoscopy is increasing, and the proportion of patients who received propofol has grown from one-third in 2009 to approximately one-half in 2014 in the United States [4]. Propofol offers deep sedation with quick onset and recovery, while there are still several side effects including cardiorespiratory depression and propofol infusion syndrome. Moreover, it should be avoided in patients with egg or soy allergy [5]. Considering the growing demand for colonoscopy and the defects of existing anesthetic, searching for novel effective agents with a more tolerable profile is urgent.
Remimazolam is a benzodiazepine acting on the γ-aminobutyric acid subtype A (GABAA) receptor, which has been reported to have quick onset and offset [6]. Moreover, remimazolam is characterized by the metabolism independent of organ function and first-order pharmacokinetics, which means it is unlikely to be accumulated [7]. Most importantly, it is shown that remimazolam has no cardiorespiratory depression effect [8]. According to the properties of remimazolam above, we hypothesized that remimazolam was an effective sedative agent with a relatively safer profile for patients undergoing colonoscopy. However, the efficacy and safety of remimazolam in Chinese patients undergoing colonoscopy were not clear. Therefore, we performed this randomized, active-controlled, blinded, non-inferior phase III trial at 20 centers, and aimed to explore the efficacy as well as safety profile of remimazolam in Chinese patients undergoing colonoscopy.
Methods
Study design
This was a multicenter, blinded, randomized, active-controlled, noninferior, phase III trial, assessing the efficacy and safety of remimazolam tosylate versus propofol in patients undergoing diagnostic or therapeutic colonoscopy. The trial was performed at 20 centers in China between June 2018 and September 2018, and a total of 388 patients were recruited. The patients were randomly allocated to remimazolam group (N=196) or propofol group (N=192) to receive remimazolam tosylate or propofol for sedation during diagnostic or therapeutic colonoscopy. The whole trial comprised of 3 stages: stage 1 (screening stage: 7 days before colonoscopy), stage 2 (colonoscopy stage: the day of colonoscopy), stage 3 (follow-up stage: 1 to 4 days post colonoscopy). The whole trial was conducted according to the Declaration of Helsinki and the International Conference on Harmonisation of Good Clinical Practice. All participating centers obtained approval from Institutional Review Board for participation, and all patients signed informed consents before initiation of the trial. And the trial was registered at ClinicalTrials.gov with registration number NCT03779061.
Study population
Patients were eligible if they met all of following inclusion criteria: (1) scheduled to undergo a diagnostic or therapeutic colonoscopy; (2) aged 18~65 years; (3) American Society of Anesthesiologists (ASA) physical status classification system risk class I~II; (4) body mass index (BMI) 18~30 kg/m2; (5) patient was voluntary to participate in the trial and signed the informed consents, and willing to comply with study requirements. Patients were excluded if they had any of following conditions: (1) about to receive endotracheal intubation or laryngeal mask; (2) required complex endoscope procedures (such as cholangiopancreatography, endoscopic ultrasonography, endoscopic mucosal resection, endoscopic submucosal dissection, transoral endoscopic myotomy, etc.); (3) acute heart failure, unstable angina pectoris, myocardial infarction occurred within 6 months prior to screening, resting electrocardiograph (ECG) heart rate <50 beats/min, grade III atrioventricular block, severe arrhythmia, moderate to severe heart valve disease, QTc for male ≥450 ms, for female ≥470 ms; (4) patients with severe respiratory disease (obstructive sleep apnea syndrome, acute respiratory infection, acute onset of chronic obstructive pulmonary disease, uncontrolled asthma, etc.); (5) patients with psychiatric disorders (schizophrenia, mania, bipolar disorder, mental disorder, etc.), a long history of use of psychotropic drugs, and cognitive dysfunction; (6) difficult for respiratory management (grade IV Modified Mallampati Score); (7) anemia or thrombocytopenia: hemoglobin (Hb) <90 g/L, platelet (PLT) <80×109/L; (8) liver dysfunction (aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) ≥2.5 upper limit of normal (ULN), total bilirubin (TBIL) ≥1.5 ULN), or renal dysfunction (urea or urea nitrogen ≥1.5 ULN, serum creatinine >ULN); (9) history of drug abuse, and alcohol abuse within the 2 years before screening stage (alcohol abuse: average daily consumption of more than 2 single units of alcohol (1 unit =360 mL beer or 45 mL 40% liquor or 150 mL wine)); (10) uncontrolled blood pressure (in screening stage, sitting systolic blood pressure (SBP) ≥160 mmHg or ≤90 mmHg, and/or diastolic blood pressure (DBP) ≥100 mmHg); (11) use of benzodiazepines and/or opioids for nearly three months; (12) allergy or contraindication to benzodiazepines, opioids, propofol and their components; (13) pregnant or breastfeeding women, or family planning within 3 months (including men); (14) participated in any clinical trial within the last 3 months.
Randomization and grouping
Random allocation for enrolled patients was performed in a 1:1 ratio within 24 hours prior to the diagnostic or therapeutic colonoscopy. The central randomization method was used for grouping, with each center competing for admission, which was performed using Central Randomization System in School of Public Health, Nanjing Medical University. After screening each eligible subject, researchers at each center in this trial logged into the random system, filled in the screening data, obtained the information of the random number and distributed the corresponding study drugs according to the random number. All study drugs were packaged in similar packages, and patients were blind to the study drugs during the trial.
Study procedures and drug administration
All patients underwent bowel preparation in accordance with the center’s standard protocol within 24 hours before administration. Fentanyl citrate injection (Yichang Humanwell Pharmaceutical Co., LTD.) was administered to patients by intravenous injection before the assigned trial sedative medication (the dosage of fentanyl was shown in Supplementary Table 1). About 3±1 minutes later, an initial intravenous dose of remimazolam tosylate 5.0 mg (Jiangsu Hengrui Pharmaceutical Co. Ltd., China) for remimazolam group or propofol 1.5 mg/kg (AstraZeneca Pharmaceuticals Co. Ltd., China) for propofol group was administered to patients for the induction of sedation, and the colonoscopy was initiated when adequate sedation (Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score ≤3) was achieved. If patients did not achieve adequate sedation after the initial dose of remimazolam tosylate or propofol, they were given a maximum of 5 doses of remimazolam tosylate (2.5 mg per time in remimazolam group) or propofol (0.5 mg/kg per time in propofol group) in 15-min window. When the colonoscopy was initiated, sedation was maintained by injection of further top-up doses of remimazolam tosylate (2.5 mg per time) or propofol (0.5 mg/kg per time), and the top-up interval was required to be more than 1 minute. For the maintenance phase of sedation, adequate sedation was predefined as an MOAA/S score ≤3 in the two groups. If 5 doses (after the initial dose) within any 15-minute window were not sufficient to obtain or maintain adequate sedation, the patient was designated a treatment failure, and the rescue sedative medication (propofol or fentanyl) was administered to obtain or maintain adequate sedation for completion of colonoscopy.
Outcomes and definitions
The primary outcome was procedure success rate. The procedure success was defined as a composite measure (all three conditions must be met) including (1) completion of the procedure, (2) no requirement for rescue sedative medication, (3) no requirement for more than 5 top-ups of trial medication within any 15-minute period after initial administration of trial drugs. The secondary outcomes were: (1) the induction time of sedation: defined as the time interval from initial administration of trial drugs to first of MOAA/S score ≤3; (2) time to fully alert: defined as the time interval from the stop of trial drugs to the first of 3 consecutive MOAA/S scores of 5; (3) time to discharge: defined as the time interval from the stop of trial drugs to meeting discharge criteria (post-anesthesia discharge scoring system (PADSS) score ≥9, with 2 points in vital signs item); (4) hypotension: defined as the reduction of SBP ≥20% (compared to baseline SBP before sedation) or decreased to ≤80 mmHg in the duration from initial administration of trial drugs to fully alert; (5) hypotension requiring treatment: defined as the hypotension that needs treatment with vasopressor drugs in the duration from initial administration of trial drugs to fully alert; (6) respiratory depression: defined as respiratory rate <8 breaths/min and/or oxygen saturation <90% in the duration from initial administration of trial drugs to fully alert; (7) treatment-emergent adverse events (AEs) occurred during the whole trial period, which were defined as the AEs correlated with trial drugs. The severity of AEs was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0, where grade 1 to 4 events were classified as mild, moderate, severe, and life-threatening or disabling, respectively.
Sample size calculation
In this trial, the propofol was served as active control, and noninferiority test was performed on the primary outcome (procedure success rate) between two groups. The noninferiority margin was set as 8%. According to our previous phase III study (in patients undergoing gastroscopy) and other reported phase III study [9], the sample size calculation was based on the assumption: 93% procedural success rate in remimazolam group, and 93% procedural success rate in propofol group. Using a one-sided type I error rate of 0.025, a target power of 80%, and a 1:1 ratio, the required sample size was 160 in each group calculated by PASS V11.0 (NCSS, USA). While considering a 20% dropout, the sample size was increased to 192 in each group, resulting in a total sample size of 384.
Statistical analysis
Among initially recruited 388 patients, 4 patients were excluded from the full analysis set (FAS), per-protocol set (PPS), and safety set (SS), due to nonuse of any trial drugs. Therefore, 384 patients were included in FAS, PPS, and SS, and there was no difference in outcome analysis among the three sets. Categorical data and ranked data were described as number (percentage), and their comparisons between two groups were determined by the Chi-square test, Fisher’s exact test or Wilcoxon rank-sum test. Continuous data were described as mean and standard deviation (SD), and their comparisons between two groups were determined by Student’s t-test. Noninferiority analysis was conducted for the primary outcome (procedure success rate), with one-side type I error rate of 0.025 and the noninferiority margin of 8%. Noninferiority of remimazolam tosylate compared with propofol was concluded if the lower limit of the 95% confidence interval (CI) of the difference of procedure success rate was more than -8%. P value <0.05 was considered statistically significant. SAS 9.4 (SAS Institute Inc., USA) was used for statistical analysis.
Results
Study flow
A total of 458 patients about to undergo diagnostic or therapeutic colonoscopy were screened for eligibility, and 70 of them were excluded (including 52 patients who either did not meet the inclusion criteria or met the exclusion criteria, and 18 patients who withdrew informed consents). The remaining 388 patients were randomized at a ratio of 1:1 into remimazolam group (N=196) and propofol group (N=192). In the remimazolam group, there were 194 patients who received allocated intervention but 2 patients did not receive any trial drugs (including 1 patient who had frequent ventricular premature beat and the other patient who had heart rate <50 beats/min after randomization). Finally, 194 patients in the remimazolam group were included in FAS/PPS/SS analyses. In the propofol group, there were 190 patients who received allocated intervention but 2 patients did not receive any trial drugs (including 1 patient who had fever and the other patient who had 223 mmHg of SBP and 104 mmHg of DBP on the day of colonoscopy). Finally, 190 patients in the propofol group were included in FAS/PPS/SS analyses. The detailed study flow was shown in Figure 1.
Figure 1.
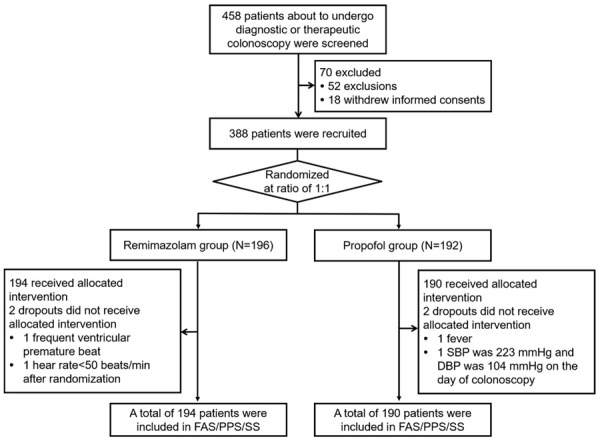
Study flow chart. SBP: systolic blood pressure; DBP: diastolic blood pressure; FAS: full analysis set; PPS: per-protocol set; SS: safety set.
Baseline characteristics
In the remimazolam group, the mean age of patients was 44.47±11.67 years, and there were 121 (62.37%) females as well as 73 (37.63%) males; 157 (80.93%) patients had ASA I and 37 (19.07%) patients had ASA II; 121 (62.37%) patients had grade I Modified Mallampati Score, 64 (32.99%) patients had grade II Modified Mallampati Score and 9 (4.64%) patients had grade III Modified Mallampati Score. In the propofol group, patients had a mean age of 44.43±11.37 years and there were 102 (53.68%) females as well as 88 (46.32%) males; 145 (76.32%) patients had ASA I and 45 (23.68%) patients had ASA II; 119 (62.63%) patients had grade I Modified Mallampati Score, 61 (32.11%) patients had grade II Modified Mallampati Score and 10 (5.26%) patients had grade III Modified Mallampati Score. Comparison analysis showed no difference in age (P=0.975), gender (P=0.085), height (P=0.395), weight (P=0.528), BMI (P=0.953), heart rate (P=0.384), SBP (P=0.106), DBP (P=0.645), respiratory rate (P=0.181), ASA score (P=0.270), Modified Mallampati Score (P=0.937), history of drink (P=0.327), or history of allergy (P=0.724) between the two groups (Table 1).
Table 1.
Baseline characteristics
| Items | Remimazolam (N=194) | Propofol (N=190) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 44.47±11.67 | 44.43±11.37 | 0.975 |
| Gender, No. (%) | 0.085 | ||
| Female | 121 (62.37) | 102 (53.68) | |
| Male | 73 (37.63) | 88 (46.32) | |
| Height (m), mean ± SD | 1.65±0.08 | 1.65±0.09 | 0.395 |
| Weight (kg), mean ± SD | 63.01±10.66 | 63.71±11.13 | 0.528 |
| BMI (Kg/m2), mean ± SD | 23.19±2.92 | 23.21±2.84 | 0.953 |
| Heart rate (beats/min), mean ± SD | 72.25±9.52 | 73.09±9.40 | 0.384 |
| SBP (mmHg), mean ± SD | 116.62±14.62 | 119.03±14.40 | 0.106 |
| DBP (mmHg), mean ± SD | 74.43±9.97 | 74.91±10.53 | 0.645 |
| Respiratory rate (breaths/min), mean ± SD | 17.31±2.30 | 17.61±2.10 | 0.181 |
| ASA score, No. (%) | 0.270 | ||
| I | 157 (80.93) | 145 (76.32) | |
| II | 37 (19.07) | 45 (23.68) | |
| Modified Mallampati Score, No. (%) | 0.937 | ||
| I | 121 (62.37) | 119 (62.63) | |
| II | 64 (32.99) | 61 (32.11) | |
| III | 9 (4.64) | 10 (5.26) | |
| History of drink, No. (%) | 0.327 | ||
| No | 157 (80.93) | 146 (76.84) | |
| Yes | 37 (19.07) | 44 (23.16) | |
| History of allergy, No. (%) | 0.724 | ||
| No | 165 (85.05) | 164 (86.32) | |
| Yes | 29 (14.95) | 26 (13.68) |
Comparison was determined by Student’s t test, Wilcoxon rank sum test, Chi-square test or Fisher’s exact test. SD, standard deviation; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ASA, American Society of Anesthesiologists Physical Status Classification System.
Primary outcome
The procedure success rate was 96.91% (188/194, 95% CI: 94.47%-99.34%) in the remimazolam group and 100% (190/190, 95% CI: 100.00%-100.00%) in the propofol group. The difference in the procedure success rate between the remimazolam group and the propofol group was -3.09%, with 95% CI of -5.53%~-0.66%. Therefore, since the lower limit of the 95% CI of difference in procedure success rate did not cross the non-inferiority margin of -8.00%, remimazolam tosylate was considered non-inferior to propofol in sedative efficacy (Table 2).
Table 2.
Comparison of primary outcome between two groups
| Items | Remimazolam (N=194) | Propofol (N=190) |
|---|---|---|
| Procedure success, No. (%) | 188 (96.91) | 190 (100.00) |
| 95% Cl | (94.47, 99.34) | (100.00, 100.00) |
| Difference in rate | -3.09% | |
| 95% Cl | (-5.53%, -0.66%) | |
| Noninferiority margin | 8.00% |
CI, Confidence Interval.
Secondary outcomes
The induction time of sedation was increased (100.67±55.33 s vs. 75.00±33.91 s) (P<0.001); while the percentages of patients who had hypotension (46 (23.71%) vs. 97 (51.05%)) (P<0.001) and respiratory depression (6 (3.09%) vs. 32 (16.84%)) (P<0.001) were both decreased in the remimazolam group compared to the propofol group, respectively; however, no difference was found in time to fully alert (P=0.181), time to discharge (P=0.501) or hypotension requiring treatment (P=0.120) between the two groups (Table 3).
Table 3.
Comparison of secondary outcomes between two groups
| Items | Remimazolam (N=194) | Propofol (N=190) | P value |
|---|---|---|---|
| Induction time of sedation (s) | 100.67±55.33 | 75.00±33.91 | <0.001 |
| Time to fully alert (s) | 8.14±2.87 | 7.74±2.93 | 0.181 |
| Time to discharge (min) | 18.30±7.35 | 17.84±6.05 | 0.501 |
| Hypotension, No. (%) | 46 (23.71) | 97 (51.05) | <0.001 |
| Hypotension requiring treatment, No. (%) | 7 (3.61) | 14 (7.37) | 0.120 |
| Respiratory depression, No. (%) | 6 (3.09) | 32 (16.84) | <0.001 |
Comparison was determined by Student’s t test or Fisher’s exact test.
Safety assessment
In the remimazolam group, total treatment-emergent AEs occurred in 96 (49.48%) patients (including 85 (43.81%) patients had grade 1 AEs, 11 (5.67%) patients had grade 2 AEs, while no patient had grade 3 or grade 4 AEs); gait disorders (51 (26.29%)) and dizziness (46 (23.71%)) were the main contributors to total treatment-emergent AEs. In the propofol group, total treatment-emergent AEs occurred in 130 (68.42%) patients (including 98 (50.52%) patients had grade 1 AEs, 32 (16.49%) patients had grade 2 AEs while no patient had grade 3 or grade 4 AEs); gait disorders (63 (33.16%)) and dizziness (46 (24.21%)) were the main contributors to total treatment-emergent AEs. Compared to the propofol group, the remimazolam group had less total treatment-emergent AEs in general (P<0.001), and less administration site pain (P<0.001), less increased bilirubin (P=0.019), less decreased respiratory rate (P<0.001), and less decreased SpO2 (P<0.001) in specific. The detailed AEs were listed in Table 4.
Table 4.
Comparison of treatment-emergent AEs occurred during the whole trial period between two groups
| SOC (PT) | Remimazolam (N=194) | Propofol (N=190) | P value* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Total | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
|
|
|
||||||||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Total treatment-emergent AEs | 96 (49.48) | 85 (43.81) | 11 (5.67) | 0 (0.00) | 0 (0.00) | 130 (68.42) | 98 (50.52) | 32 (16.49) | 0 (0.00) | 0 (0.00) | <0.001 |
| General disorders and administration site conditions | 52 (26.80) | 52 (26.80) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 63 (33.16) | 60 (30.93) | 3 (1.55) | 0 (0.00) | 0 (0.00) | 0.183 |
| Gait disorders | 51 (26.29) | 51 (26.29) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 51 (26.84) | 50 (25.77) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0.909 |
| Administration site pain | 1 (0.52) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 19 (10.00) | 17 (8.76) | 2 (1.03) | 0 (0.00) | 0 (0.00) | <0.001 |
| Nervous system disorders | 47 (24.23) | 45 (23.20) | 2 (1.03) | 0 (0.00) | 0 (0.00) | 46 (24.21) | 46 (24.21) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| Dizziness | 46 (23.71) | 44 (22.68) | 2 (1.03) | 0 (0.00) | 0 (0.00) | 46 (24.21) | 46 (24.21) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| Hypoesthesia | 1 (0.52) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| Vascular and lymphatic diseases | 16 (8.25) | 9 (4.64) | 7 (3.61) | 0 (0.00) | 0 (0.00) | 39 (20.53) | 24 (12.37) | 15 (7.73) | 0 (0.00) | 0 (0.00) | <0.001 |
| Bradypnea | 2 (1.03) | 2 (1.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (1.58) | 3 (1.55) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.683 |
| GI disorders | 6 (3.09) | 6 (3.09) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.53) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.123 |
| Nausea | 5 (2.58) | 5 (2.58) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.53) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.215 |
| Vomiting | 1 (0.52) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| Cardiac disorders | 5 (2.58) | 5 (2.58) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 9 (4.74) | 8 (4.12) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0.289 |
| Bradycardia | 2 (1.03) | 2 (1.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 7 (3.68) | 6 (3.09) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0.102 |
| Sinus bradycardia | 2 (1.03) | 2 (1.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (1.05) | 2 (1.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| First degree atrio-ventricular block | 1 (0.52) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| Investigations | 5 (2.58) | 4 (2.06) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 34 (17.89) | 24 (12.37) | 10 (5.15) | 0 (0.00) | 0 (0.00) | <0.001 |
| Bilirubin increased | 2 (1.03) | 2 (1.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 10 (5.26) | 6 (3.09) | 4 (2.06) | 0 (0.00) | 0 (0.00) | 0.019 |
| Unconjugated bilirubin increased | 2 (1.03) | 1 (0.52) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 7 (3.68) | 2 (1.03) | 5 (2.58) | 0 (0.00) | 0 (0.00) | 0.102 |
| ALT increased | 1 (0.52) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| AST increased | 1 (0.52) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| T-wave amplitude decreased | 1 (0.52) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 |
| Blood pressure decreased | 1 (0.52) | 1 (0.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (1.05) | 2 (1.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.620 |
| Respiratory rate decreased | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 13 (6.84) | 10 (5.15) | 3 (1.55) | 0 (0.00) | 0 (0.00) | <0.001 |
| SpO2 decreased | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 13 (6.84) | 10 (5.15) | 3 (1.55) | 0 (0.00) | 0 (0.00) | <0.001 |
Comparison of AE occurrence rate between two groups was determined by Chi-square test or Fisher’s exact test, and the P value indicating the difference of AE occurrence rate rather than the difference of AE severity.
AE, adverse events; SOC, System Organ Classification; PT, preferred term; GI, gastrointestinal; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; SpO2, oxygen saturation.
Discussion
This study was designed to evaluate the sedative efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy, and we found that: (1) the sedative efficacy of remimazolam tosylate was non-inferior to propofol in patients undergoing colonoscopy; (2) remimazolam tosylate presented a relatively longer induction time of sedation, and similar recovery time compared to propofol; (3) remimazolam tosylate presented higher safety profile compared to propofol.
Current sedation strategies for patients undergoing colonoscopy include the administration of midazolam or propofol. Although these two commonly used sedative agents present acceptable sedative effect and safety, several drawbacks limit their application [5]. For example, midazolam has a relatively long onset time as well as recovery time, which might cause overdosage and uncontrollable sedation time; meanwhile, propofol has cardiorespiratory depression effect and accumulation effect due to its non-linear pharmacokinetics, and should be avoided in patients with egg and/or soy allergy [1]. Therefore, searching for additional sedative agents with equivalent efficacy and a higher safety profile is needed. Remimazolam was first synthesized and reported in 2007, which shows high affinity to the GABAA receptor and is able to be metabolized into low affinity (300 times lower) carboxylic acid metabolite [6]. Previous in vivo studies in sheep reveal that 0.37-2.21 mg/kg of remimazolam is able to induce sedation for 9-25 minutes without cardiorespiratory depression effect; also, the pharmacokinetic and pharmacodynamic analysis shows remimazolam has a quick clearance and stable half-life of 10.9 minutes (non-compartmental analysis) [10,11]. Further clinical phase III trials display that remimazolam has quicker onset and recovery than midazolam in patients undergoing colonoscopy and bronchoscopy [12,13]. Notably, remimazolam presents high safety profile [14]. Therefore, remimazolam tosylate might be a promising sedative agent for patients undergoing colonoscopy.
Owning to that remimazolam has a quick onset time and swift recovery time, as well as less cardiorespiratory depression effect and other AEs [10,14], we hypothesized that remimazolam tosylate might be a proper sedative agent in patients undergoing colonoscopy. However, previous phase III clinical trial evaluating the efficacy and safety of remimazolam was performed in the U.S., and the sedative effect and safety profile of remimazolam tosylate in Chinese patients were unclear. Therefore, we performed this study and observed that most of the patients receiving remimazolam tosylate successfully finished the procedure of colonoscopy without administration of rescue sedative agent or more than 5 top-ups of remimazolam tosylate in any 15 minute-period after initial administration of remimazolam tosylate (96.91%, 188/194), which was in line with the previous data that the sedation success rate of remimazolam in patients undergoing colonoscopy is 94% (278/296) [9]. Furthermore, the non-inferiority analysis revealed that the sedative efficacy of remimazolam tosylate was non-inferior to propofol. Possible explanations could be that: remimazolam tosylate might present similar high affinity to the GABAA receptor as propofol; therefore, its sedative efficacy was non-inferior to propofol in this study.
Regarding the onset and recovery time of remimazolam, one previous phase III clinical trial displays that remimazolam presents a shorter induction time of sedation as well as recovery time compared to midazolam in patients undergoing bronchoscopy [13]. In this study, we found that the onset time of remimazolam tosylate was decreased compared to propofol, while remimazolam tosylate had a fast recovery similar to that of propofol, which could be because: (1) Remimazolam tosylate might present slower distribution than propofol, thus it has slower onset; (2) Remimazolam tosylate is metabolized rapidly into non-active metabolite by non-specific esterase in the tissues according to previous study [7], therefore it presented quick recovery in the present study.
As to the safety profile of remimazolam, previous studies reveal that remimazolam is a well-tolerated sedative agent with fewer AEs including hypotension, respiratory depression, administration site pain, etc. in patients undergoing endoscopy [12,13]. In our study, we found that: (1) Patients who received remimazolam tosylate had less hypotension and respiratory depression. (2) Patients who received remimazolam tosylate had fewer treatment-emergent AEs in general, and less administration site pain, increased bilirubin, decreased respiratory rate, and decreased SpO2 in specific compared to patients who received propofol. Possible explanation for our data might be that: the cardiorespiratory suppress effect of propofol is caused by its effect on the central chemoreceptor sensitivity [15], while few studies report similar effects of remimazolam tosylate. Therefore, remimazolam tosylate presented a high safety profile in our study. (3) The treatment-emergent AEs were of grade I and grade II in both of the two groups, therefore, remimazolam tosylate and propofol were well tolerable to some extent in patients undergoing colonoscopy. (4) The AEs mainly included gait disorders as well as dizziness in both of the remimazolam group and the propofol group. While one previous phase III clinical trial reveals that the AEs of remimazolam in colonoscopy are mainly hypotension, hypertension and bradycardia [9]. The difference between the previous study and our study might be caused by the difference in the definition of AEs as well as individual differences.
Although we had found a lot of interesting results, there were several limitations in this study. Firstly, the enrolled patients in this trial were mainly middle-aged but fewer elderly, and the safety and efficacy of remimazolam tosylate in the aged should be investigated further. Secondly, because propofol is more commonly used for colonoscopy than midazolam in China recently, we did not set a midazolam arm in this study. Finally, all the centers were top-ranked hospitals in this multiple-centered trial, and the results of this study might be different in lower-level hospitals due to technique differences.
To be conclusive, remimazolam tosylate is non-inferior in sedative efficacy and more tolerable than propofol in patients undergoing colonoscopy, which could be a relatively ideal sedative agent for colonoscopy.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Tetzlaff JE. Practical considerations in the management of sedation for colonoscopy. Curr Opin Anaesthesiol. 2016;29:512–518. doi: 10.1097/ACO.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 2.Lin OS. Sedation for routine gastrointestinal endoscopic procedures: a review on efficacy, safety, efficiency, cost and satisfaction. Intest Res. 2017;15:456–466. doi: 10.5217/ir.2017.15.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triantafillidis JK, Merikas E, Nikolakis D, Papalois AE. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol. 2013;19:463–481. doi: 10.3748/wjg.v19.i4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Predmore Z, Nie X, Main R, Mattke S, Liu H. Anesthesia service use during outpatient gastroenterology procedures continued to increase from 2010 to 2013 and potentially discretionary spending remained high. Am J Gastroenterol. 2017;112:297–302. doi: 10.1038/ajg.2016.266. [DOI] [PubMed] [Google Scholar]
- 5.Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36:1021–1027. doi: 10.1002/phar.1806. [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, Wiard RP, Feldman PL, Collins H, Waszczak BL, Tilbrook GS. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60–66. doi: 10.1097/01.anes.0000267503.85085.c0. [DOI] [PubMed] [Google Scholar]
- 7.Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115:274–283. doi: 10.1213/ANE.0b013e31823f0c28. [DOI] [PubMed] [Google Scholar]
- 8.Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115:284–296. doi: 10.1213/ANE.0b013e318241f68a. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, Barish CF, Pruitt R, Cash BD, Quirk D, Tiongco F, Sullivan S, Bernstein D. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88:427–43. e426. doi: 10.1016/j.gie.2018.04.2351. [DOI] [PubMed] [Google Scholar]
- 10.Upton R, Martinez A, Grant C. A dose escalation study in sheep of the effects of the benzodiazepine CNS 7056 on sedation, the EEG and the respiratory and cardiovascular systems. Br J Pharmacol. 2008;155:52–61. doi: 10.1038/bjp.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upton RN, Somogyi AA, Martinez AM, Colvill J, Grant C. Pharmacokinetics and pharmacodynamics of the short-acting sedative CNS 7056 in sheep. Br J Anaesth. 2010;105:798–809. doi: 10.1093/bja/aeq260. [DOI] [PubMed] [Google Scholar]
- 12.Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, Wahidi M, Shojaee S, Tanner NT, Callahan SP, Feldman G, Lorch DG Jr, Ndukwu I, Pritchett MA, Silvestri GA Investigators PAION. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155:137–146. doi: 10.1016/j.chest.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Pambianco DJ, Borkett KM, Riff DS, Winkle PJ, Schwartz HI, Melson TI, Wilhelm-Ogunbiyi K. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83:984–992. doi: 10.1016/j.gie.2015.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Borkett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, Wilhelm-Ogunbiyi K. A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120:771–780. doi: 10.1213/ANE.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 15.Sahinovic MM, Struys M, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57:1539–1558. doi: 10.1007/s40262-018-0672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


