Abstract
Peripheral nerve injury is a common refractory disease in the clinic that often leads to dysfunction of movement and sensation. Different from other tissue injuries, peripheral nerve injury needs a longer time for regeneration. Therefore, effective drug therapy is needed to promote nerve regeneration in the treatment of peripheral nerve injury. Our preliminary studies have shown that continuous intramuscular injection of NP-1 promotes the regeneration of injured sciatic nerve in rats, but the mechanisms were still unknown. Schwann cells are very important cells in the formation of myelin sheath of peripheral nerves and participate in the repair and regeneration of peripheral nerve injury. To further investigate the effect of NP-1 on rat Schwann cells and the underlying mechanism, different concentrations of NP-1 were used to treat rat Schwann cell line RSC96. Light microscopy, CCK-8 assay, cell scratch assay, and special cell staining were performed to investigate RSC96 cell aging and apoptosis. mRNA and protein expression of NF-κB signaling pathway-related factors were determined using qPCR and immunohistochemistry respectively. Light microscopy, CCK-8 assay, cell scratch assay, and special cell staining showed NP-1 could improve the ability of proliferation, immigration of Schwann cells. QPCR and immunohistochemistry showed NP-1 influenced the expression of multiple factors associated with nerve regeneration which NF-κB signaling pathway played a key role. The results show that NP-1 promoted the proliferation and migration of RSC96 cells and inhibited cell aging and apoptosis possibly through the NF-κB signaling pathway. These findings provide a potential target for clinical treatment of peripheral neuropathy and experimental data support.
Keywords: Schwann cells, NP-1, NF-κB signaling pathway, proliferation, migration
Introduction
Peripheral nerve injury is a common complication of trauma, and can cause serious dysfunction, even limb disability [1]. Traction injury [2], crush injury [3], and infant shoulder dystocia [4] can cause peripheral nerve injuries. Nerve transplantation [5] and sutures [6] are often used to restore the continuity of the damaged nerve. This clinical repair process, depending on the location of the nerve injury, takes several months, usually, more than 10 months. However, in most patients, the clinical repair is unsatisfactory and nerve function is not completely restored. Therefore, the study of peripheral nerve injury has become an important area of research [7,8].
Schwann cells are special glial cells of the peripheral nervous system that surround the axons of neurons to form the myelin sheath [9]. They play important roles in the growth, development, maturation, function, and maintenance of peripheral nerves [10,11]. The number of Schwann cells present at the site of injury as well as their regeneration and proliferation abilities are the key factors responsible for the repair of a peripheral nerve injury [12]. Proteins [13], microRNAs [14], and long non-coding RNAs [15] affect the repair process of peripheral nerve injury by regulating the proliferation of Schwann cells. Moreover, the neurotrophic and nerve growth factors secreted by Schwann cells are involved in the repair of peripheral nerve injury [16,17]. Thus, the functional status of Schwann cells is very important for repair of injured peripheral nerves. The Schwann cell line, RSC96, was established through spontaneous transformation of primary cultures of rat Schwann cells that had been cultured for long durations. RSC96 cells have retained the characteristics of primary Schwann cells and can be sub-cultured several times, and therefore, are widely used in research involving peripheral nerves [18,19].
Neutrophil peptide 1 (NP-1), also known as α defensin 1, is a member of the α defensin family that is mainly secreted by neutrophils [20]. NP-1 is closely related to the repair of peripheral nerve injuries. Studies have shown that continuous injection of NP-1 is beneficial to the repair and regeneration of injured peripheral nerves in rats; moreover, it has a positive effect on the rate of regeneration after nerve injury and excitation and conduction of nerve impulses [21,22]. NP-1 can regulate synaptic transmission [23], affect neuronal function [24], and thereby exhibit a great potential for peripheral nerve repair. NF-κB signaling pathway is central to the responses of cells to inflammation, stress, and injury [25,26], and plays an important role in the repair of injured peripheral nerves [27,28]. It has been reported that NF-κB signaling pathway can regulate inflammation [29], proliferation [30], migration [31], aging, and apoptosis [32] of Schwann cells. However, few reports have described the effects of NP-1 on Schwann cell function. Moreover, whether the effects of NP-1 are mediated by the NF-κB signaling pathway has not been investigated in detail.
Herein, we investigated the effects of NP-1 on the repair of injured peripheral nerves and on Schwann cell proliferation, migration, aging, and apoptosis, as well as the underlying mechanism. Moreover, we investigated the underlying mechanism at the cellular level to provide additional experimental evidence for the use of NP-1 in the clinical treatment of peripheral nerve injury.
Methods and materials
Detection of RSC96 cell activity using CCK-8 assay
RSC96 cells (the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) (3000-4000 per well) were seeded in a 96-well plate. Cells were cultured for 12-24 h with culture medium supplemented with 2% fetal bovine serum (FBS) (Gibco, Life Technologies, Carlsbad, USA). Cells were then treated with different concentrations of NP-1 (0, 4, 8, 12, and 16 μg/mL) (Shanghai Qiangyao Biological Technology Co., Ltd., Shanghai, China) prepared in culture medium supplemented with 2% FBS. Cells in the negative control group were treated with 100 μL of culture medium supplemented with 2% FBS, but without NP-1. After treatment for 12, 24, 36, 48, and 60 h, cells in each well were treated with 10 μL of CCK-8 solution (Dongren Chemical Technology, Shanghai, China) at 37°C for 2 h. Optical density at 450 nm was measured using an ELISA reader (Bio-Rad, USA). Cell survival and inhibition rates were calculated according to the following formulas: cell survival rate = (As-Ab)/(Ac-Ab); cell inhibition rate = (Ac-As)/(Ac-Ab), where As represents the absorbance of experimental well (cell-containing culture medium + CCK-8 + different concentrations of NP-1), Ac represents the absorbance of control well (cell-containing culture medium + CCK-8), and Ab represents the absorbance of blank control well (CCK-8 + culture medium without cells + NP-1).
Cell cycle analysis by flow cytometry
RSC96 cells were seeded in a 12-well plate at a density of 3 × 105/well. Cells were cultured for 12-24 h with culture medium supplemented with 2% FBS. Cells were then treated for 36 h with different concentrations of NP-1 (0, 4, and 8 μg/mL) prepared in culture medium supplemented with 2% FBS. The following experimental steps were carried out according to the instructions of the biological cell cycle test kit: Cells from each well of the 12-well plate were transferred into a separate 1.5 mL Eppendorf tube (Eppendorf, Hamburg, Germany) and centrifuged at 2930 rcf for 5 min at 23°C-27°C. Supernatant was discarded, and cells were washed with phosphate-buffered saline (PBS) (Gibco, Life Technologies, Carlsbad, USA) and then centrifuged again at 2930 rcf for 5 min at 23°C-27°C. After discarding the supernatant, 1 mL DNA staining solution and 10 μL permeabilization solution were added to each tube. Subsequently, cells were suspended in working solution, shaken for 5-10 min, incubated for 30 min in the dark at room temperature, and then assessed by flow cytometry (Beckman Coulter, Brea, CA, USA).
Cell scratch assays
RSC96 cells were seeded in a 48-well plate with 2 × 105 per well and then incubated for 36 h in culture medium supplemented with 2% FBS. Cell monolayers were then scraped in horizontal direction to with create a “scratch” using a 200 μL pipet tip and washed with PBS. Subsequently, cells were treated with different concentrations of NP-1 (0, 4, and 8 μg/mL) prepared in culture medium supplemented with 2% FBS for 36 h. After treatment, cells were photographed using an optical microscope (Shanghai BIEM Optical Instrument Manufacturing Co., Ltd, Shanghai, China).
Detection of cell aging using β-galactosidase staining
RSC96 cells (3000-4000 per well) were seeded in a 96-well plate. Cells were cultured for 36 h with culture medium supplemented with 2% FBS for 12-24 h. Cells were then treated with different concentrations of NP-1 (0, 4, and 8 μg/mL) prepared in culture medium supplemented with 2% FBS. Subsequently, cells were stained with a β-galactosidase staining kit (Beyotime Biotechnology, Shanghai, China) and then photographed using an optical microscope (Olympus, Japan).
Detection of changes in cell nuclei using 4’,6-diamidino-2-phenylindole (DAPI) staining
RSC96 cells were cultured in an incubator with 1-3% CO2 at 37°C and seeded in a 96-well plate at approximately 3000-4000 cells/well. Cells were cultured for 12-24 h with culture medium supplemented with 2% FBS and then treated for 36 h with different concentrations of NP-1 (0, 4, and 8 μg/mL) prepared in culture medium supplemented with 2% FBS. Subsequently, cells were washed three times with PBS for 5 min each, fixed with 4% paraformaldehyde overnight, washed three times with PBS for 5 min each, stained with DAPI (Solarbio, Beijing, China) at room temperature for 10-15 min, washed three times with distilled water for 5 min each, and photographed using an optical microscope.
Detection of cell apoptosis using acridine orange (AO)/propidium iodide (PI) staining
RSC96 cells were seeded in a 48-well plate at approximately 2 × 104 cells/well. Cells were cultured for 12-24 h with culture medium supplemented with 2% FBS and then treated for 36 h with different concentrations of NP-1 (0, 4, and 8 μg/mL) prepared in culture medium supplemented with 2% FBS. Subsequent steps were performed according to the instructions provided by the AO/PI staining kits (BestBio, Shanghai, China). Finally, cell images were acquired at 488 nm laser excitation using fluorescence microscopy.
Detection of cell apoptosis using trypan blue staining
RSC96 cells were seeded in a 48-well plate at approximately 2 × 104 cells/well. Cells were cultured in medium supplemented with 2% FBS for 12-24 h and then treated for 36 h with different concentrations of NP-1 (0, 4, and 8 μg/mL) prepared in culture medium supplemented with 2% FBS. After washing with PBS, cells were stained with 0.4% trypan blue (Solarbio, Beijing, China), 100 μL/well, for 5 min at room temperature. Then, cells were washed with PBS and photographed using an optical microscope.
Quantitative fluorescence PCR
RSC96 cells were seeded in a 6-well plate at approximately 1.5 × 106 cells/well. Cells were cultured for 12-24 h with culture medium supplemented with 2% FBS and treated for 36 h with different concentrations of NP-1 (0, 4, and 8 μg/mL) prepared in culture medium supplemented with 2% FBS. Gene primers were designed and synthesized by BGI Tech (Shenzhen, Guangdong Province, China) and are shown in Table 1. Total RNA was extracted using the Trizol method and cDNA was synthesized using a kit (Abm Inc., Canada). Forty cycles of gene amplification were completed according to the annealing temperature of each target gene. The specificity of the amplification products was determined using the fusion curve, and data analysis was carried out using the 2-ΔΔCt method.
Table 1.
Sequences of primers used in PCR
| Gene | Sequences (5’-3’) |
|---|---|
| ICAM-1 | Forward: GATCATACGGGTTTGGGCTTCTCC |
| Reverse: GCCACTGCTCGTCCACATAGTATT | |
| IL-6 | Forward: AGTCACAGAAGGAGTGGCTAAGGA |
| Reverse: GCACACTAGGTTTGCCGAGTAGAC | |
| IL-1α | Forward: TCGTCCTAAGTCACTCGCATGG |
| Reverse: GGGCTGGTTCCACTAGGCTTTG | |
| p65 | Forward: AGGATTTACATCAAGCACCCAC |
| Reverse: TACGCCATCTTTCCTCCTGTTG | |
| iNOS | Forward: CACCTTTATGTTTGTGGCGATGT |
| Reverse: GAAGTAATCCTCAACCTGCTCCTC | |
| COX-2 | Forward: GTGGGTGCTTGGCTTGTGACTT |
| Reverse: TTTTGAAGAAGCCCACTGATACC | |
| AKT | Forward: CGCTTCTTTGCCAACATCG |
| Reverse: TCATCAAAATACCTGGTGTCGG | |
| JUN | Forward: GAACTCGGACCTTCTCACG |
| Reverse: GCAGCGTATTCTGGCTATG | |
| mTOR | Forward: TTTGGACGGTGTAGAACTTGGA |
| Reverse: CTTGTTTAAGGCTTCTGGTTTCAC | |
| GAPDH | Forward: ATCTGACATGCCGCCTGGAGAA |
| Reverse: ACAACCTGGTCCTCAGTGTAGCC |
Detection of the expression of related proteins using immunohistochemistry
RSC96 cells were seeded in a 96-well plate at a density of 1 × 104 cells/well. Cells were cultured for 12-24 h with culture medium supplemented with 2% FBS and then treated for 36 h with different concentrations of NP-1 (0, 4, and 8 μg/mL) prepared in culture medium supplemented with 2% FBS. Cells were then washed with PBS, and treated with H2O2 (Beijing Chemical Plant, Beijing, China) for 10-15 min in the dark. Then, cells were washed with PBS for 5 min, treated with 50 μL primary antibody working solution including NGF (1:100; Abcam, USA), iNOS (1:100; Abcam, USA), p65 (1:400; CST, USA), IL-1β (1:400; Bioss, Beijing, China), and NP-1 (1:100; ABclonal, Wuhan, Hubei Province, China) at 4°C overnight. After washing with PBS for 5 min, cells were treated with secondary antibody (GTVisionTM III anti mouse/rabbit immunohistochemistry test kit, Gene Tech, China) at 23°C-27°C for 30 min, washed again with PBS for 5 min, and then incubated with DAB working solution [solution B (DAB developer) and solution C (color buffer) was mixed at a volume ratio of 20 μL solution B to 1 mL solution C] for 3-10 min at room temperature. Subsequently, cells were washed with PBS for 5 min, stained with hematoxylin for 1 min, and washed with water. Finally, cells were photographed using an optical microscope. Under 400 × magnification, screenshots of the same size were randomly selected for each well. Total positive area was determined using Image Pro Plus v6.0 software (Media Cybernetics, Rockville, USA).
Statistical analysis
SPSS v20.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis and Image-pro plus v6.0 software was used for picture analysis. All measurement data are expressed as the mean ± SD. Comparisons between two groups were made with independent-sample t-tests and nonparametric tests. A level of P < 0.05 was considered statistically significant.
Results
Effects of NP-1 on RSC96 cell activity
RSC96 cells were treated with 0, 4, and 8 μg/mL NP-1 for 36 h and their viability was assessed by the CCK-8 assay. RSC96 cells treated with 4 μg/mL NP-1 showed the highest survival rate (i.e. the lowest cell inhibition rate) and significant cell activity followed by cells treated with 8 μg/mL NP-1 (Tables 2, 3).
Table 2.
RSC96 cell survival rates
| Treatment duration (h) | NP-1 concentration (μg/mL) | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 4 | 8 | 12 | 16 | |
| 12 | 1.00 | 0.95 | 1.10 | 1.04 | 1.12 |
| 24 | 1.00 | 1.28* | 1.12 | 1.30* | 1.21* |
| 36 | 1.00 | 1.35** | 1.31* | 1.26 | 1.28 |
| 48 | 1.00 | 1.04 | 1.14 | 1.09 | 1.18 |
| 60 | 1.00 | 1.01 | 0.99 | 1.13 | 1.07 |
*Absorbance value of other concentration vs. 0 μg/mL NP-1 at the same time point. SPSS v20.0 software was used for statistical analysis. Comparisons between two groups were made with independent-sample t-tests or nonparametric tests.
P < 0.05;
P < 0.05.
Table 3.
RSC96 cell inhibition rates
| Treatment duration (h) | NP-1 concentration (μg/mL) | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 4 | 8 | 12 | 16 | |
| 12 | 0.00 | 0.05 | -0.10 | -0.04 | -0.12 |
| 24 | 0.00 | -0.28* | -0.12 | -0.30* | -0.21* |
| 36 | 0.00 | -0.35** | -0.31* | -0.26 | -0.28 |
| 48 | 0.00 | -0.04 | -0.14 | -0.09 | -0.18 |
| 60 | 0.00 | -0.01 | 0.01 | -0.13 | -0.07 |
Absorbance value of other concentration vs. 0 μg/mL NP-1 at the same time point. SPSS v20.0 software was used for statistical analysis. Comparisons between two groups were made with independent-sample t-tests or nonparametric tests.
P < 0.05;
P < 0.05.
Effects of NP-1 on RSC96 cell cycle
We evaluated the effects of NP-1 on cell cycle of RSC96 cells. After treatment with 0, 4, and 8 μg/mL NP-1 for 36 h, there were 72 ± 2%, 68 ± 1%, and 69 ± 1% cells in G1 phase, respectively. The proportion of cells in phase G1 was significantly different between cells treated with 4 and 8 μg/mL NP-1 as compared with those in the control group (0 μg/mL NP-1; P = 0.049 and P = 0.048, respectively). After treatment with 0, 4, and 8 μg/mL NP-1 for 36 h, there were 16% ± 0.00, 18 ± 1%, and 19 ± 1% cells in S phase, respectively. The proportion of cells in phase S was significantly different between cells treated with 4 and 8 μg/mL NP-1 as compared with those in the control group (0 μg/mL NP-1; P = 0.030 and P = 0.009, respectively). After treatment with 0, 4, and 8 μg/mL NP-1 for 36 h, there were 12 ± 1%, 14 ± 1%, and 13 ± 1% cells in phase G2, respectively. However, there was no significant difference in the proportion of cells in phase G2 between cells treated with 4 and 8 μg/mL NP-1 as compared with those in the control group (0 μg/mL NP-1) (Figure 1).
Figure 1.

RSC96 cell cycle analysis using flow cytometry. Treatment with 0 μg/mL (A), 4 μg/mL (B), and 8 μg/mL (C) NP-1 for 36 h. Ratio of cells at phases G1 (D), S (E), and G2 (F). SPSS v20.0 software was used for statistical analysis. Comparisons between two groups were made with independent-sample t-tests or nonparametric tests. *P < 0.05, **P < 0.01.
Effects of NP-1 on RSC96 cell migration
To determine the effects of NP-1 on the migration of RSC96 cells, the cells were scratched and treated with 0, 4, and 8 μg/mL NP-1 for 36 h. At the end of treatment, the remaining area of scratch wound was 72751.67 ± 2468.89, 60589.67 ± 6728.84, and 68462.00 ± 8289.36, respectively. The cell scratch area remaining in the 4 μg/mL NP-1-treated group was significantly smaller than that in the control group (0 μg/mL NP-1) (P = 0.042). However, there was no significant difference in the cell scratch area remaining between 8 μg/mL NP-1-treated and control group (0 μg/mL NP-1) (Figure 2).
Figure 2.
Detection of RSC96 cell migration using cell scratch assays. Treatment with 0 μg/mL (A), 4 μg/mL (B), and 8 μg/mL (C) NP-1 for 36 h. (D) Remaining cell scratch area in each group. SPSS v20.0 software was used for statistical analysis. Image-pro plus v6.0 software was used for picture analysis. Comparisons between two groups were made with independent-sample t-tests or nonparametric tests. *P < 0.05.
Effects of NP-1 on RSC96 cell aging and apoptosis
The number of aging cells was the highest in the control group (0 μg/mL NP-1), which decreased after treatment with 4 and 8 μg/mL NP-1 for 36 h (Figure 3). Moreover, the number of broken nuclei, cells with red fluorescence, and cells stained with trypan blue were the highest in the control group cells (treated with 0 μg/mL NP-1), but these were decreased in cells treated with 4 and 8 μg/mL NP-1 (Figures 4, 5 and 6).
Figure 3.
Detection of cell aging using β-galactosidase staining (200 ×). Treatment with 0 μg/mL (A), 4 μg/mL (B), and 8 μg/mL (C) NP-1 for 36 h. Arrowheads indicate blue-stained aging cells.
Figure 4.
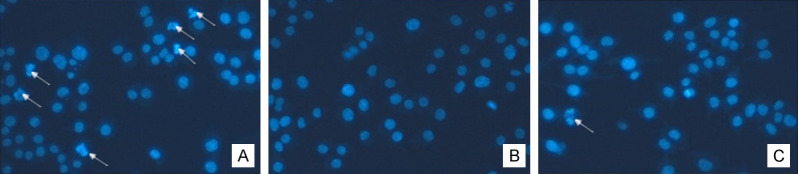
4’,6-Diamidino-2-phenylindole (DAPI) staining of nuclei (200 ×). Treatment with 0 μg/mL (A), 4 μg/mL (B), and 8 μg/mL (C) NP-1 for 36 h. Arrowheads indicate fragmented nuclei.
Figure 5.
Acridine orange (AO)/propidium iodide (PI) double staining (200 ×) of cells. Treatment with 0 μg/mL (A) 4 μg/mL (B), and 8 μg/mL (C) NP-1 for 36 h. The nuclei of normal cells exhibited yellow green fluorescence, and the nuclei of apoptotic cells exhibited red fluorescence. Arrowheads indicate cells with red fluorescence. There are too many fluorescent cells in A, and thus they are not marked with arrowheads.
Figure 6.
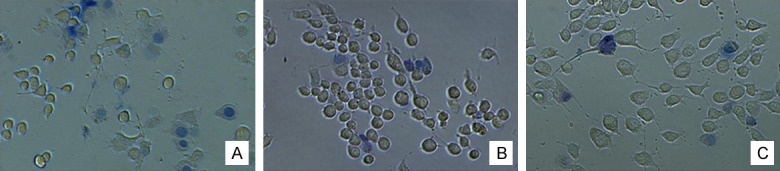
Trypan blue staining (200 ×) of cells. Treatment with 0 μg/mL (A), 4 μg/mL (B), and 8 μg/mL (C) NP-1 for 36 h. Blue cells are apoptotic cells.
Effect of NP-1 on mRNA expression of regulatory factors associated with NF-κB signaling pathway
The mRNA expression of nine factors involved in NF-κB signaling pathway (ICAM-1, IL-1α, IL-6, p65, iNOS, COX2, AKT, JUN, and mTOR) was determined by qPCR. The factors whose expression showed 15% change as compared to that in the control group are shown in Figure 7 and Table 4.
Figure 7.

mRNA expression of factors related to the NF-κB signaling pathway. Graphs correspond to 4 and 8 μg/mL NP-1-treated cells vs. 0 μg/mL NP-1-treated cells. *P < 0.05, **P < 0.01. SPSS v20.0 software was used for statistical analysis. Comparisons between two groups were made with independent-sample t-tests or nonparametric tests.
Table 4.
mRNA expression of genes related to the NF-κB signaling pathway
| Gene | NP-1 concentration | ||
|---|---|---|---|
|
| |||
| 0 μg/mL | 4 μg/mL | 8 μg/mL | |
| ICAM-1 | 1.03 ± 0.30 | 1.50 ± 0.25# | 1.67 ± 0.04# |
| IL-1α | 1.09 ± 0.50 | 1.22 ± 0.30 | 1.57 ± 0.15# |
| IL-6 | 1.02 ± 0.25 | 1.10 ± 0.07 | 1.34 ± 0.30# |
| p65 | 1.03 ± 0.28 | 1.19 ± 0.16# | 1.23 ± 0.14# |
| iNOS | 1.00 ± 0.11 | 1.18 ± 0.26# | 1.53 ± 0.31# |
| COX2 | 1.01 ± 0.17 | 1.26 ± 0.42# | 2.66 ± 0.49**,# |
| AKT | 1.20 ± 0.89 | 1.31 ± 0.17 | 1.21 ± 0.27 |
| JUN | 1.06 ± 0.43 | 1.54 ± 0.17# | 2.66 ± 0.38**,# |
| mTOR | 1.07 ± 0.43 | 1.03 ± 0.80 | 1.29 ± 0.87# |
*Treatment with 4 or 8 μg/mL NP-1 for 36 h vs. intervention with 0 μg/mL NP-1 for 36 h. SPSS v20.0 software was used for statistical analysis. Comparisons between two groups were made with independent-sample t-tests or nonparametric tests.
P < 0.01.
indicates the factor that changed over 15% in the 4 μg/mL NP-1-treated and 8 μg/mL NP-1-treated groups compared with the 0 μg/mL NP-1-treated group.
Effect of NP-1 on protein expression of factors related to the NF-κB signaling pathway
The effects of NP-1 on protein expression of four factors (p65, iNOS, IL-1β, and NGF) related to the NF-κB signaling pathway were evaluated. The protein expression of p65, iNOS, IL-1β, and NGF in cells treated with 4 and 8 μg/mL NP-1 for 36 h was significantly higher than that in the control group cells (0 μg/mL NP-1) (P < 0.05). There was no significant difference in protein expression between cells treated with NP-1 and those in the control group (0 μg/mL NP-1) (Figures 8, 9; Table 5).
Figure 8.

Images of immunohistochemistry analysis of cells (200 ×). (A) p65 protein, (B) iNOS protein, (C) IL-1β protein, (D) NGF protein, and (E) NP-1 protein. 1: 0 μg/mL NP-1-treated group; 2: 4 μg/mL NP-1-treated group; and 3: 8 μg/mL NP-1-treated group. Image-pro plus v6.0 software was used for picture analysis.
Figure 9.
Immunohistochemistry analysis of cells. SPSS v20.0 software was used for statistical analysis. Comparisons between two groups were made with independent-sample t-tests or nonparametric tests. *P < 0.05, **P < 0.01 for 4 μg/mL NP-1-treated and 8 μg/mL NP-1-treated groups vs. 0 μg/mL NP-1-treated group.
Table 5.
Expression of proteins related to the NF-κB signaling pathway
| Protein | NP-1 concentration | ||
|---|---|---|---|
|
| |||
| 0 μg/mL | 4 μg/mL | 8 μg/mL | |
| P65 | 8319.00 ± 2330.62 | 16995.00 ± 3702.14* | 13736.33 ± 2074.40* |
| iNOS | 9170.00 ± 2087.41 | 19413.33 ± 946.34** | 15047.33 ± 507.01** |
| IL-1β | 5308.33 ± 754.91 | 9800.00 ± 981.08** | 10136.67 ± 1374.06** |
| NGF | 2372.33 ± 1349.73 | 9171.67 ± 1178.91** | 7288.00 ± 784.90** |
| NP-1 | 15952.00 ± 626.71 | 16381.33 ± 1788.95 | 15504.67 ± 1694.44 |
P < 0.05 for 4 μg/mL NP-1-treated and 8 μg/mL NP-1-treated groups vs. control (0 μg/mL NP-1) group.
P < 0.01 for 4 μg/mL NP-1-treated and 8 μg/mL NP-1-treated groups vs. control (0 μg/mL NP-1) group.
Discussion
Schwann cells are essential for the formation of myelin sheaths of peripheral nerves [33-36]. NP-1 is a small polypeptide involved in the repair of peripheral nerve injury. Our previous studies revealed that continuous injections of NP-1 into the gluteus maximus muscle promoted the repair and regeneration of injured rat sciatic nerve [29]. NP-1 has been reported to regulate the synaptic transmission of frog vestibular hair cells [23] and the function of primary neurons in the small intestine of guinea pigs [24], suggesting that NP-1 plays an important role in regulation of nerve cells. However, only a few studies have reported the effects of NP-1 on Schwann cells. Therefore, we aimed to investigate the effects of NP-1 on Schwann cells.
We first observed the specific morphology of Schwann cells (RSC96) on different days of culture. RSC96 cells showed fusiform, triangular, or polygonal morphology before reaching 100% confluence, after which, they clustered together exhibiting a morphology similar to “paving stones” with round or quasi round nuclei, fusiform, triangular, polygonal, or round cytoplasm.
Treatment with NP-1 (4 and 8 μg/mL) promoted the growth and migration of RSC96 cells, with the most obvious growth stimulation observed after 36 h. Therefore, the optimal concentration of NP-1 and duration of treatment for obtaining these effects were determined to be 4 and 8 μg/mL and 36 h, respectively. It has been reported that low concentrations of NP-1 promotes cell proliferation, whereas high concentrations of NP-1 exerts cytotoxic effects [37]. NP-1 stimulates cell proliferation, and p42/44 MAP kinase and Akt activation in a dose-dependent manner [38] and promotes cell migration through two-way activation of ERK1/2 [39]. Kang et al. [40] reported that folic acid treatment-mediated improvement in proliferation and migration of Schwann cells was helpful in the repair of peripheral nerve injury. The proliferation and migration of Schwann cells are also affected by small RNAs and nerve growth factors [41,42]. The results of the present study showed that low concentrations of NP-1 increased the proliferation and migration of Schwann cells, which affects the repair of injured peripheral nerves.
β-galactosidase, DAPI, AO/PI, and trypan blue staining results showed that treatment with 4 and 8 μg/mL NP-1 for 36 h inhibited RSC96 cell aging and apoptosis. A previous study reported that high concentrations of NP-1-3 (50 μg/mL) caused cell apoptosis through activation of caspase 3/7 [43]. However, aging and apoptosis of Schwann cells are not conducive to the repair of peripheral nerve injury [44,45]. In this study, we found that low concentrations of NP-1 (4 and 8 μg/mL) slowed down the aging and apoptosis of RSC96 cells. Therefore, we believe that low concentrations of NP-1 can slow down the aging and apoptosis of Schwann cells at the site of peripheral nerve injury, providing protection to sciatic nerve function. Moreover, as NP-1 promotes the proliferation and migration of Schwann cells, it can accelerate the repair of injured peripheral nerves as well as provide conducive environment for the recovery of nerves.
NF-κB is a family of transcription factors, including p65, RelB, c-Rel, p50, and p52. Several chemical mediators involved in various stages of inflammation and early stages of the immune response can be regulated by the NF-κB signaling pathway [46,47]. P65 has a transactivation domain that can activate target genes. The increased expression of p65 protein can initiate the activation of NF-κB signaling pathway. NF-κB and iNOS levels are increased in inflammatory diseases [48]. However, inhibition of the NF-κB signaling pathway results in downregulation of iNOS, COX-2, and IL-1β, indicating its anti-inflammatory role [49].
IL-1α, an upstream factor regulating NF-κB activity, is involved in cell aging [50] and inflammation [51]. Mei et al. [52] found that siRNA could inhibit cell proliferation and migration and decrease the expression of p65 and CCND1; moreover, the decrease in CCND1 expression was related to decrease in cell proliferation and increase in apoptosis [53]. Sahin et al. [54] found that tomato powder improves age-related inflammatory responses by inhibiting the expression of NF-κB and mTOR.
In this study, we determined the mRNA expression of mediators directly related to inflammation (ICAM-1, IL-1α, IL-6, p65, iNOS, and COX2) and cell function (AKT, JUN, and mTOR), all of which take part in NF-κB signaling pathway. We also determined the protein expression of five regulatory molecules: NGF, inflammation-related proteins (p65, iNOS, and IL-1β), and NP-1. We found that treatment with 4 and 8 μg/mL NP-1 for 36 h increased the protein expression of p65, iNOS, IL-1β, and NGF, as compared to that in the control group, whereas no obvious effects were observed on NP-1 protein expression. Moreover, 4 or 8 μg/mL NP-1 upregulated the mRNA expression of ICAM-1, IL-1α, IL-6, p65, iNOS, COX2, JUN, and mTOR, as compared to that in the control group. Low concentrations of NP-1 increased the expression of iNOS, IL-1β, COX2, and JUN by activating NF-κB signaling pathway; this also altered the secretion of inflammatory mediators and chemokines in RSC96 cells. We suggest that activation of the NF-κB signaling pathway resulted in the upregulation of iNOS, IL-1β, COX2, ICAM-1, IL-1α, and IL-6, which stimulated inflammation and promoted NGF secretion. NF-κB has been associated with the secretion of b-NGF in metabolic syndrome [55]. Moreover, NP-1 promoted cell proliferation and migration, and inhibited cell aging and apoptosis, via IL-1α, JUN, and mTOR modulation. Li et al. [56] reported that p65 knockout decreased the phosphorylation levels of c-jun and increased squamous apoptosis. Furthermore, mTOR activation was reported to promote cell proliferation [57], whereas mTOR inhibition was found to reduce cell migration ability [58]. Overall, these findings indicated that activation of NF-κB signaling pathway promoted the repair of injured peripheral nerves. Interestingly, NP-1 treatment did not produce significant changes in NP-1 protein expression in RSC96 cells, suggesting that exogenous NP-1 may not affect the levels of endogenous NP-1 in cells.
A limitation of the present study is that we only investigated the effect of NP-1 on the NF-κB signaling pathway, but we did not investigate the effect of this pathway on NP-1, which will studied in the future.
In conclusion, we elucidated the possible mechanism underlying NP-1-induced changes in the function of RSC96 Schwann cells at the cellular level. Based on the results, we suggest that NP-1 promotes the proliferation and migration of RSC96 cells and inhibits their aging and apoptosis. These findings also indicate a possible mechanism of repair and regeneration of injured peripheral nerves and suggest that NP-1 can be a potential target for clinical treatment of peripheral nerve injury.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (grant numbers 31571236 and 81971177); the Key Laboratory of Trauma and Neural Regeneration (Peking University), Ministry of Education (grant number BMU2019XY007-01); the National Key Research and Development Program of China (grant number 2016YFC1101604); the Ministry of Education Innovation Program of China (grant number IRT_16R01); the Peking University People’s Hospital Research and Development Funds (grant number RDH2017-01, RDY2018-09); Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515110983 and No. 2019A1515011290); and Shenzhen “San-Ming” Project of Medicine (No. SZSM201612092).
Disclosure of conflict of interest
None.
References
- 1.Panagopoulos GN, Megaloikonomos PD, Mavrogenis AF. The present and future for peripheral nerve regeneration. Orthopedics. 2017;40:e141–e156. doi: 10.3928/01477447-20161019-01. [DOI] [PubMed] [Google Scholar]
- 2.Rasulic L, Savic A, Lepic M, Kovacevic V, Vitosevic F, Novakovic N, Mandic-Rajcevic S, Samardzic M. Viable C5 and C6 proximal stump use in reconstructive surgery of the adult brachial plexus traction injuries. Neurosurgery. 2020;86:400–409. doi: 10.1093/neuros/nyz179. [DOI] [PubMed] [Google Scholar]
- 3.Wong YR, Pang X, Lim ZY, Du H, Tay SC, McGrouther DA. Biomechanical evaluation of peripheral nerves after crush injuries. Heliyon. 2019;5:e01557. doi: 10.1016/j.heliyon.2019.e01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pondaag W, Malessy MJA. Outcome assessment for Brachial Plexus birth injury. Results from the iPluto world-wide consensus survey. J Orthop Res. 2018;36:2533–2541. doi: 10.1002/jor.23901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biro V. Applied methods instead of autologous nerve transplantation in the reconstruction of nerve injuries on the hand. Orv Hetil. 2017;158:1163–1167. doi: 10.1556/650.2017.30813. [DOI] [PubMed] [Google Scholar]
- 6.Leite APS, Pinto CG, Tibúrcio FC, Sartori AA, de Castro Rodrigues A, Barraviera B, Ferreira RS Junior, Filadelpho AL, Matheus SMM. Heterologous fibrin sealant potentiates axonal regeneration after peripheral nerve injury with reduction in the number of suture points. Injury. 2019;50:834–847. doi: 10.1016/j.injury.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeois M, Loisel F, Obert L, Pluvy I, Gindraux F. Can the amniotic membrane be used to treat peripheral nerve defects? A review of literature. Hand Surg Rehabil. 2019;38:223–232. doi: 10.1016/j.hansur.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Yu F, Yu YL, Niu SP, Zhang PX, Yin XF, Han N, Zhang YJ, Zhang DY, Kou YH, Jiang BG. Repair of long segmental ulnar nerve defects in rats by several different kinds of nerve transposition. Neural Regen Res. 2019;14:692–698. doi: 10.4103/1673-5374.247473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang M, Rao R, Wang J, Wang J, Xu L, Wu LM, Chan JR, Wang H, Lu QR. The TSC1-mTOR-PLK axis regulates the homeostatic switch from Schwann cell proliferation to myelination in a stage-specific manner. Glia. 2018;66:1947–1959. doi: 10.1002/glia.23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beirowski B, Wong KM, Babetto E, Milbrandt J. mTORC1 promotes proliferation of immature Schwann cells and myelin growth of differentiated Schwann cells. Proc Natl Acad Sci U S A. 2017;114:E4261–E4270. doi: 10.1073/pnas.1620761114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Maynard JC, Strickland A, Burlingame AL, Milbrandt J. Schwann cell O-GlcNAcylation promotes peripheral nerve remyelination via attenuation of the AP-1 transcription factor JUN. Proc Natl Acad Sci U S A. 2018;115:8019–8024. doi: 10.1073/pnas.1805538115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastien D, Lacroix S. Cytokine pathways regulating glial and leukocyte function after spinal cord and peripheral nerve injury. Exp Neurol. 2014;258:62–77. doi: 10.1016/j.expneurol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Han B, Zhao JY, Wang WT, Li ZW, He AP, Song XY. Cdc42 promotes schwann cell proliferation and migration through wnt/beta-catenin and p38 MAPK signaling pathway after sciatic nerve injury. Neurochem Res. 2017;42:1317–1324. doi: 10.1007/s11064-017-2175-2. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Chen C, Yi S, Wang S, Gong L, Liu J, Gu X, Zhao Q, Li S. miR-sc8 inhibits schwann cell proliferation and migration by targeting Egfr. PLoS One. 2015;10:e0145185. doi: 10.1371/journal.pone.0145185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan B, Shi ZJ, Yan JY, Li JH, Feng SQ. Long non-coding RNA NONMMUG014387 promotes Schwann cell proliferation after peripheral nerve injury. Neural Regen Res. 2017;12:2084–2091. doi: 10.4103/1673-5374.221168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Z, Li X, Li Q. Curcumin accelerates the repair of sciatic nerve injury in rats through reducing Schwann cells apoptosis and promoting myelinization. Biomed Pharmacother. 2017;92:1103–1110. doi: 10.1016/j.biopha.2017.05.099. [DOI] [PubMed] [Google Scholar]
- 17.Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou M, Hu M, He S, Li B, Liu C, Min J, Hong L. Effects of RSC96 schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med Sci Monit. 2018;24:7841–7849. doi: 10.12659/MSM.909509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YP, Xu P, Guo CX, Luo ZR, Zhu J, Mou FF, Cai H, Wang C, Ye XC, Shao SJ, Guo HD. miR-1b overexpression suppressed proliferation and migration of RSC96 and increased cell apoptosis. Neurosci Lett. 2018;687:137–145. doi: 10.1016/j.neulet.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Hazlett L, Wu M. Defensins in innate immunity. Cell Tissue Res. 2011;343:175–188. doi: 10.1007/s00441-010-1022-4. [DOI] [PubMed] [Google Scholar]
- 21.Xu C, Bai L, Chen Y, Fan C, Hu Z, Xu H, Jiang B. Effect of mutated defensin NP-1 on sciatic nerve regeneration after transection--a pivot study. Neurosci Lett. 2016;617:283–287. doi: 10.1016/j.neulet.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Nozdrachev AD, Kolosova LI, Moiseeva AB, Ryabchikova OV. The role of defensin NP-1 in restoring the functions of an injured nerve trunk. Neurosci Behav Physiol. 2006;36:313–315. doi: 10.1007/s11055-006-0018-8. [DOI] [PubMed] [Google Scholar]
- 23.Andrianov IuN, Nozdrachev AD, Ryzhova IV. Comparative analysis of the effect of endogenous antibiotic defensin NP-1 and aminoglycoside antibiotic gentamicin on synaptic transmission in receptors of the frog vestibular apparatus. Izv Akad Nauk Ser Biol. 2007:705–710. [PubMed] [Google Scholar]
- 24.Nozdrachev AD, Tolkunov YA, Zimina OA, Poliakov EL. Does defensin NP-1 influence the excitability of the primary afferent neurons of the guinea pig small intestine? Dokl Biol Sci. 2005;401:100–103. doi: 10.1007/s10630-005-0055-4. [DOI] [PubMed] [Google Scholar]
- 25.Curtis VF, Ehrentraut SF, Colgan SP. Actions of adenosine on cullin neddylation: implications for inflammatory responses. Comput Struct Biotechnol J. 2015;13:273–276. doi: 10.1016/j.csbj.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Ma G, Yang X. HDAC5 promotes mycoplasma pneumoniae-induced inflammation in macrophages through NF-kappaB activation. Life Sci. 2019;221:13–19. doi: 10.1016/j.lfs.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Komirishetty P, Areti A, Yerra VG, Ruby PK, Sharma SS, Gogoi R, Sistla R, Kumar A. PARP inhibition attenuates neuroinflammation and oxidative stress in chronic constriction injury induced peripheral neuropathy. Life Sci. 2016;150:50–60. doi: 10.1016/j.lfs.2016.02.085. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Sun Y, Cai M, Zhang H, Gao N, Huang H, Cui S, Yao D. Fas ligand gene (Faslg) plays an important role in nerve degeneration and regeneration after rat sciatic nerve injury. Front Mol Neurosci. 2018;11:210. doi: 10.3389/fnmol.2018.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang KJ, Zhang HL, Zhang XM, Zheng XY, Quezada HC, Zhang D, Zhu J. Apolipoprotein E isoform-specific effects on cytokine and nitric oxide production from mouse Schwann cells after inflammatory stimulation. Neurosci Lett. 2011;499:175–180. doi: 10.1016/j.neulet.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 30.Teare KA, Pearson RG, Shakesheff KM, Haycock JW. Alpha-MSH inhibits inflammatory signalling in Schwann cells. Neuroreport. 2004;15:493–498. doi: 10.1097/00001756-200403010-00022. [DOI] [PubMed] [Google Scholar]
- 31.Tang X, Wang Y, Zhou S, Qian T, Gu X. Signaling pathways regulating dose-dependent dual effects of TNF-alpha on primary cultured Schwann cells. Mol Cell Biochem. 2013;378:237–246. doi: 10.1007/s11010-013-1614-x. [DOI] [PubMed] [Google Scholar]
- 32.Xu S, Bao W, Men X, Liu Y, Sun J, Li J, Liu H, Cai H, Zhang W, Lou J, Peng L. Interleukin-10 protects schwann cells against advanced glycation end products-induced apoptosis via NF-kappaB suppression. Exp Clin Endocrinol Diabetes. 2020;128:89–96. doi: 10.1055/a-0826-4374. [DOI] [PubMed] [Google Scholar]
- 33.Poitelon Y, Lopez-Anido C, Catignas K, Berti C, Palmisano M, Williamson C, Ameroso D, Abiko K, Hwang Y, Gregorieff A, Wrana JL, Asmani M, Zhao R, Sim FJ, Wrabetz L, Svaren J, Feltri ML. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat Neurosci. 2016;19:879–887. doi: 10.1038/nn.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang YM, Chang HH, Tsai CC, Lin HJ, Ho TJ, Ye CX, Chiu PL, Chen YS, Chen RJ, Huang CY, Lin CC. Alpinia oxyphylla Miq. fruit extract activates IGFR-PI3K/Akt signaling to induce Schwann cell proliferation and sciatic nerve regeneration. BMC Complement Altern Med. 2017;17:184. doi: 10.1186/s12906-017-1695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Painter MW. Aging Schwann cells: mechanisms, implications, future directions. Curr Opin Neurobiol. 2017;47:203–208. doi: 10.1016/j.conb.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Lin M, Jiang M, Ding F, Cao Z. Syntaxin-4 and SNAP23 act as exocytic SNAREs to release NGF from cultured Schwann cells. Neurosci Lett. 2017;653:97–104. doi: 10.1016/j.neulet.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura M, Abiko Y, Kurashige Y, Takeshima M, Yamazaki M, Kusano K, Saitoh M, Nakashima K, Inoue T, Kaku T. Effect of defensin peptides on eukaryotic cells: primary epithelial cells, fibroblasts and squamous cell carcinoma cell lines. J Dermatol Sci. 2004;36:87–95. doi: 10.1016/j.jdermsci.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Raghunath M, Tan D, Lareu RR, Chen Z, Beuerman RW. Defensins HNP1 and HBD2 stimulation of wound-associated responses in human conjunctival fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:3811–3819. doi: 10.1167/iovs.05-1360. [DOI] [PubMed] [Google Scholar]
- 39.Aarbiou J, Verhoosel RM, Van Wetering S, De Boer WI, Van Krieken JH, Litvinov SV, Rabe KF, Hiemstra PS. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am J Respir Cell Mol Biol. 2004;30:193–201. doi: 10.1165/rcmb.2002-0267OC. [DOI] [PubMed] [Google Scholar]
- 40.Kang WB, Chen YJ, Lu DY, Yan JZ. Folic acid contributes to peripheral nerve injury repair by promoting Schwann cell proliferation, migration, and secretion of nerve growth factor. Neural Regen Res. 2019;14:132–139. doi: 10.4103/1673-5374.243718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu QY, Miao Y, Wang XH, Wang P, Cheng ZC, Qian TM. Increased levels of miR-3099 induced by peripheral nerve injury promote Schwann cell proliferation and migration. Neural Regen Res. 2019;14:525–531. doi: 10.4103/1673-5374.245478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi S, Yuan Y, Chen Q, Wang X, Gong L, Liu J, Gu X, Li S. Regulation of Schwann cell proliferation and migration by miR-1 targeting brain-derived neurotrophic factor after peripheral nerve injury. Sci Rep. 2016;6:29121. doi: 10.1038/srep29121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aarbiou J, Tjabringa GS, Verhoosel RM, Ninaber DK, White SR, Peltenburg LT, Rabe KF, Hiemstra PS. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm Res. 2006;55:119–127. doi: 10.1007/s00011-005-0062-9. [DOI] [PubMed] [Google Scholar]
- 44.Scheib JL, Hoke A. An attenuated immune response by Schwann cells and macrophages inhibits nerve regeneration in aged rats. Neurobiol Aging. 2016;45:1–9. doi: 10.1016/j.neurobiolaging.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Pan H, Ding Y, Yan N, Nie Y, Li M, Tong L. Trehalose prevents sciatic nerve damage to and apoptosis of Schwann cells of streptozotocin-induced diabetic C57BL/6J mice. Biomed Pharmacother. 2018;105:907–914. doi: 10.1016/j.biopha.2018.06.069. [DOI] [PubMed] [Google Scholar]
- 46.Shokri S, Mahmoudvand S, Taherkhani R, Farshadpour F, Jalalian FA. Complexity on modulation of NF-kappaB pathways by hepatitis B and C: a double-edged sword in hepatocarcinogenesis. J Cell Physiol. 2019 doi: 10.1002/jcp.28249. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Kermarrec L, Eissa N, Wang H, Kapoor K, Diarra A, Gounni AS, Bernstein CN, Ghia JE. Semaphorin-3E attenuates intestinal inflammation through the regulation of the communication between splenic CD11C(+) and CD4(+) CD25(-) T-cells. Br J Pharmacol. 2019;176:1235–1250. doi: 10.1111/bph.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostojic M, Soljic V, Vukojevic K, Dapic T. Immunohistochemical characterization of early and advanced knee osteoarthritis by NF-kappaB and iNOS expression. J Orthop Res. 2017;35:1990–1997. doi: 10.1002/jor.23504. [DOI] [PubMed] [Google Scholar]
- 49.Song C, Hong YH, Park JG, Kim HG, Jeong D, Oh J, Sung GH, Hossain MA, Taamalli A, Kim JH, Kim JH, Cho JY. Suppression of Src and Syk in the NF-kappaB signaling pathway by Olea europaea methanol extract is leading to its anti-inflammatory effects. J Ethnopharmacol. 2019;235:38–46. doi: 10.1016/j.jep.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 50.Su Y, Xu C, Sun Z, Liang Y, Li G, Tong T, Chen J. S100A13 promotes senescence-associated secretory phenotype and cellular senescence via modulation of non-classical secretion of IL-1alpha. Aging (Albany NY) 2019;11:549–572. doi: 10.18632/aging.101760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki H, Bando K, Tada H, Kiyama T, Oizumi T, Funayama H, Sugawara S, Takahashi T, Endo Y. Augmentation of lipopolysaccharide-induced production of IL-1alpha and IL-1beta in mice given intravenous zoledronate (a nitrogen-containing bisphosphonate) and its prevention by clodronate (a non-nitrogen-containing bisphosphonate) Biol Pharm Bull. 2019;42:164–172. doi: 10.1248/bpb.b18-00408. [DOI] [PubMed] [Google Scholar]
- 52.Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J, Shi ZZ. miR-145-5p suppresses tumor cell migration, invasion and epithelial to mesenchymal transition by regulating the Sp1/NF-kappaB signaling pathway in esophageal squamous cell carcinoma. Int J Mol Sci. 2017;18:1833. doi: 10.3390/ijms18091833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dai J, Wei RJ, Li R, Feng JB, Yu YL, Liu PS. A study of CCND1 with epithelial ovarian cancer cell proliferation and apoptosis. Eur Rev Med Pharmacol Sci. 2016;20:4230–4235. [PubMed] [Google Scholar]
- 54.Sahin K, Orhan C, Tuzcu M, Tastan H, Bilir B, Sahin N, Oner DA, Kucuk O. Tomato powder modulates NF-kappaB, mTOR, and Nrf2 pathways during aging in healthy rats. J Aging Res. 2019;2019:1643243. doi: 10.1155/2019/1643243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Listwak SJ, Rathore P, Herkenham M. Minimal NF-kappaB activity in neurons. Neuroscience. 2013;250:282–299. doi: 10.1016/j.neuroscience.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Liu Y, Duan P, Yu R, Gu Z, Li L, Liu Z, Su L. NFkappaB regulates HSF1 and cJun activation in heat stressinduced intestinal epithelial cell apoptosis. Mol Med Rep. 2018;17:3388–3396. doi: 10.3892/mmr.2017.8199. [DOI] [PubMed] [Google Scholar]
- 57.Stamateris RE, Sharma RB, Kong Y, Ebrahimpour P, Panday D, Ranganath P, Zou B, Levitt H, Parambil NA, O’Donnell CP, Garcia-Ocana A, Alonso LC. Glucose induces mouse beta-cell proliferation via IRS2, MTOR, and cyclin D2 but not the insulin receptor. Diabetes. 2016;65:981–995. doi: 10.2337/db15-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang T, Ji D, Wang P, Liang D, Jin L, Shi H, Liu X, Meng Q, Yu R, Gao S. The atypical protein kinase RIOK3 contributes to glioma cell proliferation/survival, migration/invasion and the AKT/mTOR signaling pathway. Cancer Lett. 2018;415:151–163. doi: 10.1016/j.canlet.2017.12.010. [DOI] [PubMed] [Google Scholar]






