Abstract
Myocardial ischemia/reperfusion (I/R) injury is a common cardiovascular disease with high morbidity and mortality globally, which derives from acute myocardial infarction and coronary artery disease. Elabela has been proved to bind to apelin receptors in the heart. The present study aimed to investigate the protective effects of Elabela in myocardial I/R injury and illustrating the potential mechanisms. In this study, the rat I/R model was established in vivo. Following treatment with Elabela, the histopathological changes of heart tissue were evaluated by the hematoxylin and eosin- or Masson’s trichrome staining. Apoptosis of heart tissue was examined using TUNEL staining. The expression of type I or III collagen and apoptosis-associated proteins was measured using western blotting. Moreover, myocardial ultrastructure in myocardium was detected via electron microscopy analysis. H9c2 cells were treated with hypoxia/reoxygenation (H/R) to mimic the myocardial I/R injury in vitro. After treatment with Elabela or Elabela combined with LY294002, the levels of oxidative stress and apoptosis were examined. The results revealed that Elabela significantly improved the pathological changes of rat myocardial tissues induced by I/R. Additionally, Elabela treatment reduced cardiomyocyte I/R induced fibrosis and apoptosis as well as ameliorated mitochondrial dysfunction in animal and cells. Within inhibition of PI3K pathway, the protective effects of Elabela was reversed. Taken together, these findings demonstrated that Elabela could protect against fibrosis, apoptosis and oxidative stress via PI3K/ATK signaling pathway in cardiac ischemia reperfusion.
Keywords: Cardiac ischemia reperfusion, Elabela, apoptosis, fibrosis, mitochondrial function
Introduction
Myocardial ischemia (MI) results in severe mortality worldwide. Reperfusion is a standard strategy in the current MI therapy, however, injury induced by MI reperfusion (I/R) is emerged in approximately 30% of patients [1]. In most cases, the reasons for IR injury are induced by immune system and inflammatory cells are accumulated into the infarcted area under reperfusion [2]. Several mediators, including oxidative stress, intracellular Ca2+ overload, the rapid restoration of physiological PH at the time of reperfusion and inflammation, function in the myocardial I/R injury [3]. The cardiac apoptosis and inflammation have been recognized as hallmarks of myocardial I/R injury [4]. Elabela, also known as Toddler or Apela, is the second discovered endogenous ligand for apelin receptor (also known as APJ or APLNR) [5]. It is mostly expressed in embryos and can be expressed only in parts of adult tissues, including cardiac endothelium and blood vessels [6,7]. The Elabela/APJ signaling has been reported to have protective effects on cardiac diseases, such as heart failure, pulmonary arterial hypertension, and atherosclerosis [8-10]. APJ, a class A G-protein-coupled receptor (GPCR), is found closely similar to angiotensin II type 1 receptor but not binding to angiotensin II [11]. A previous investigation indicated that Elabela exerts the protective effect by RAS inactivation through inhibition of fork-head box M1 (FOXM1) transcription factor and angiotensin converting enzyme [12]. Recently, Yue Xi et al. found that Elabela protein alleviates the infarct heart dysfunction in myocardial infarction model, the apoptosis and heart fibrosis included [13]. However, whether Elabela has effects on myocardial I/R injury and the further mechanisms remains unknown.
Mitochondria is a major energy machine in cells to produce ATP and comprises 5 respiratory chain complexes. Besides, it participates in diversified metabolisms and signaling regulation [14]. Damaged mitochondria triggers cell or organ injury, along with low ATP level and high level of reactive oxygen species (ROS) [15]. Moreover, mitochondria dysfunction is associated with release of apoptotic factors such as Cyt-c activating cell apoptosis [16]. Hence, control of mitochondrial dysfunction is a potential mechanism in the progression of myocardial I/R injury. Both protection of mitochondrial integrity and reduction of oxidative stress play critical roles in avoiding myocardial I/R injury [17].
In the present study, we explored the effective and protective effects of Elabela on myocardial I/R injury in rats. We supposed that Elabela mitigated myocardial I/R injury by suppressing apoptosis and fibrosis. Also, amelioration of mitochondrial dysfunctions by Elabela might suggest its potential function during myocardial I/R injury.
Materials and methods
Animal experiments
Male Sprague-Dawley (SD) rats were anesthetized with intraperitoneal injection of pentobarbital sodium (70 mg/kg) and ventilated on a rodent respirator via a tracheostomy. We performed a midline sternotomy and placed a snare occlude around the left anterior descending coronary artery. Myocardial ischemia reperfusion was induced by tightening the snare for half an hour. After then, the snare was loosed. After reperfusion for 4 hours, the animal was sacrificed to collect blood samples for biochemistry measurement. The heart was used for pathological analysis and other experiments. Rats were received tail vein rejection of Elabela (0.7 mg/kg) at 5 min of reperfusion.
Histopathological analysis
Hearts were soaked with formalin and embedded in paraffin for slicing. The hearts from rats were sliced into 5 μm-thick sections and stained with hematoxylin and eosin (H&E) or Masson’s Trichrome [18]. The morphology was observed with an inverted microscope.
Evaluation of apoptosis
The apoptosis was determined by TUNEL staining using TUNEL Apoptosis Detection Kit (Green Fluorescence, Abbkine, China). The embedded paraffin was immersed twice in xylene for 15 min. Subsequently, the rehydrate tissues were washed with 100% ethanol for 5 min twice, followed by gradient elution using 95, 70, 50% ethanol for 3 min. Prepare TdT labeling reaction buffer just before use following the manufacturer’s protocol. Add 50 μl reaction mixture to each sample and incubate them for 60 min at 37°C. 0.05 μg/ml DAPI in PBS was added for 10 min and next fluorescent microscopy was applied to observe the samples at 200 × magnification.
Transmission Electron Microscope (TEM)
The tissues were fixed by 4% glutaraldehyde, dehydrated by series of ethanol and embedded in Epon 812 as a previous investigation performed [19]. 2% uranyl acetate and lead citrate were applied to stain the ultramicrotomy (50-80 nm thick) sections. The image was captured by Hitachi Transmission Electron microscope (Hitachi, H-7500, Japan).
Immunohistochemical staining
Collected cardiac tissue specimens were fixed in 10% buffered formalin solution and then embedded in paraffin. Paraffin-embedded specimens (5 μm) were deparaffinized and rehydrated, followed by blocking with Endogenous Biotin Blocking kit (Beyotime Institute of Biotechnology). Subsequently, sections were incubated with primary antibodies. After incubation with horseradish peroxidase-secondary antibody, slides were stained with diaminobenzidine and counterstained with hematoxylin. Microscopic images were obtained by a phase-contrast microscope (Keyence, Osaka, Japan).
Cell culture
Rat H9c2 cells, a ventricular cardiomyocyte cell line, were purchased by Chinese Academy of Sciences, China. Cells were incubated with Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Thermo Fisher Scientific, USA) and 10% Fetal bovine serum (FBS; Gibco) at 37°C. H9c2 cells were treated with Hypoxia/reoxygenation (H/R) to mimic the myocardial I/R injury in vitro, which accorded with the previous investigation [20]. H9c2 cells were treated with 5 nM Elabela (GL Biochem, Shanghai, China) and 10 μM LY294002, alone or in combination.
Detection of mitochondrial function
Elabela in serum or cells was tested via an ELISA kit (Peninsula Laboratories International, USA, S-1506). ROS, GSH, SOD and MDA were respectively tested via kits bought from Beyotime (China, S0033, S0052, S0109 and S0131). Procedures followed operation introductions.
Western blotting
Cardiac tissues (n=3 in each group) was homogenized in RIPA lysis buffer (Thermo Fisher Scientific, USA). Cells were lysed by RIPA buffer, and protein concentrations were quantified by NanoDrop 2000/2000c (Thermo Fisher Scientific). Equal amounts of samples (50 μg) were separated by sodium dodecyl sulfate (SDS) polyacrylamide gel (8% and 15%) and subsequently transferred to nitrocellulose (NC) membranes (Bio-rad). The protocols were performed in accordance with the previous investigation [21]. Primary antibodies (Cell Signaling Technology, USA) were incubated overnight at 4°C. The secondary antibodies were incubated with membrane for 1 h at room temperature. Quantification of the protein bands were performed by Image J.
Statistical analysis
P value under 0.05 was thought as significantly different. All data were presented by mean ± standard deviation. Statistical analysis was performed using GraphPad Prism 6.0. Student’s t-test was used to compare the significant differences between the two groups. One way analysis of variance (ANOVA) followed by Tukey’s test was employed to compare multiple groups.
Results
The levels of fibrosis and apoptosis of cardiomyocyte induced by I/R were associated with the low levels of Elabela
Hematoxylin and eosin (H&E) pathology detection exhibited the abnormity of myocardial structure including necrosis and infiltration of inflammatory cells (Figure 1A). Next, Masson’s staining indicated that interstitial fibrosis area in IRI group is more than that in the sham group (Figure 1B). Myocardial apoptosis was also induced in cardiomyocytes (Figure 1C). In addition, type I or III collagen, cleaved-caspase 3 and Cyt-c were were dramatically increased in the IRI group compared with the sham group (Figure 1D). Meanwhile, Elabela plasma levels was decreased in IRI group relative to the sham group (Figure 1E). Thus, these data revealed that Elabela might associate with IRI injury.
Figure 1.
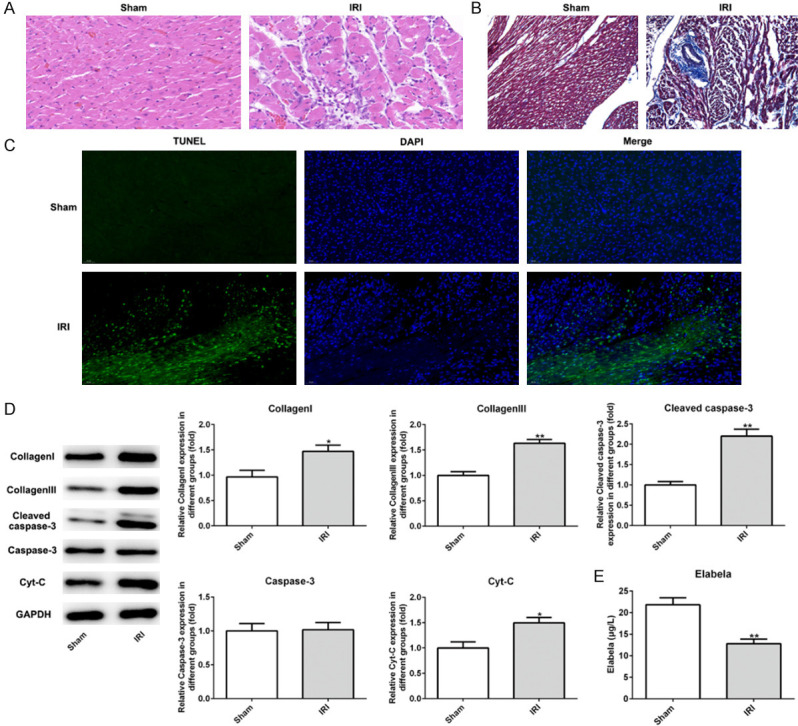
The level of Elabela was reduced after myocardial ischemia/reperfusion injury. A. Heart H&E staining from sham and ischemia reperfusion injury heart tissue. Scale bar: 20 μm. B. Myocardium underwent Masson’s trichrome staining. Red parts indexed cardiomyocytes and blue parts indexed fibrosis. Scale bar: 20 μm. C. TUNEL-positive (green) cardiomyocytes were evaluated by double staining with TUNEL (green) and 4’, 6’-diamidino-2-phenylindole (blue). The number of TUNEL-positive cardiomyocytes at infarct area after reperfusion was significantly increased compared to the sham group. Scale bar: 50 μm. D. Expression levels of Bcl-2, Bax, cleaved-caspase3, caspase-3 and Cyt-c tested by western blotting in vivo. E. Levels of Elabela in blood of rats. All results were obtained from at least three independent experiments. All numerical data were presented as the mean ± standard deviation. IRI, ischemia reperfusion injury. *P < 0.05, **P < 0.01 versus sham group.
Elabela treatment alleviated cardiac fibrosis and apoptosis in cardiomyocyte I/R injury
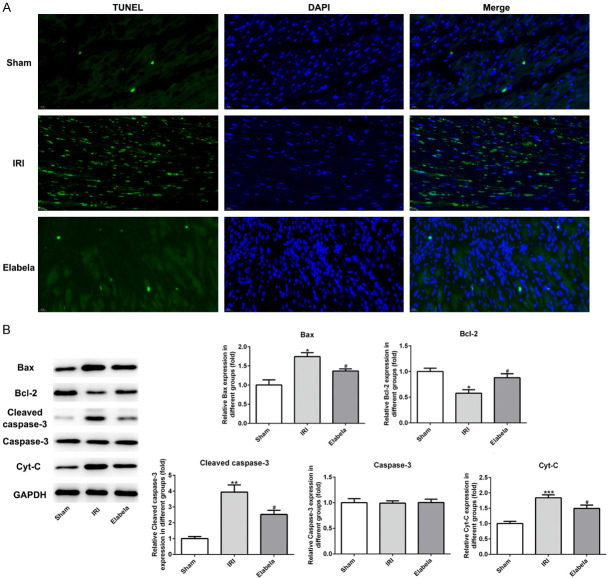
H&E and Masson’s staining as well as TUNEL assay were performed to evaluate whether post-treatment with Elabela affected cardiomyocyte I/R injury. Pathological analyses indicated that Elabela treatment reduced the infiltration of inflammatory cells and recovered the structure of cardiomyocytes (Figure 2A). As presented in Figure 2B and 2C, Elabela also alleviated the fibrosis of heart and lowered the protein level of type I/III collagen. In cardiac tissue from I/R injury rats subjected to 4 h I/R treatment, TUNEL assay manifested a dramatical upregulation in apoptotic cells as compared to the sham group, whereas Elabela-treated hearts showed less apoptosis (Figure 3A). Apoptosis in myocardium was further identified by analysis of caspase activation and apoptotic proteins. Elabela post-treatment in I/R rats inhibited caspase-3 activity and expression of Bax as compared with I/R injury group (Figure 3B). Conversely, the level of the anti-apoptotic protein, Bcl-2, was upregulated in Elabela-treated group (Figure 3B). Additionally, Cyt-c, mainly in the outer space of mitochondrial membrane and a caspase family activator, was prominently decreased by the treatment of Elabela in I/R injury group (Figure 3B).
Figure 2.
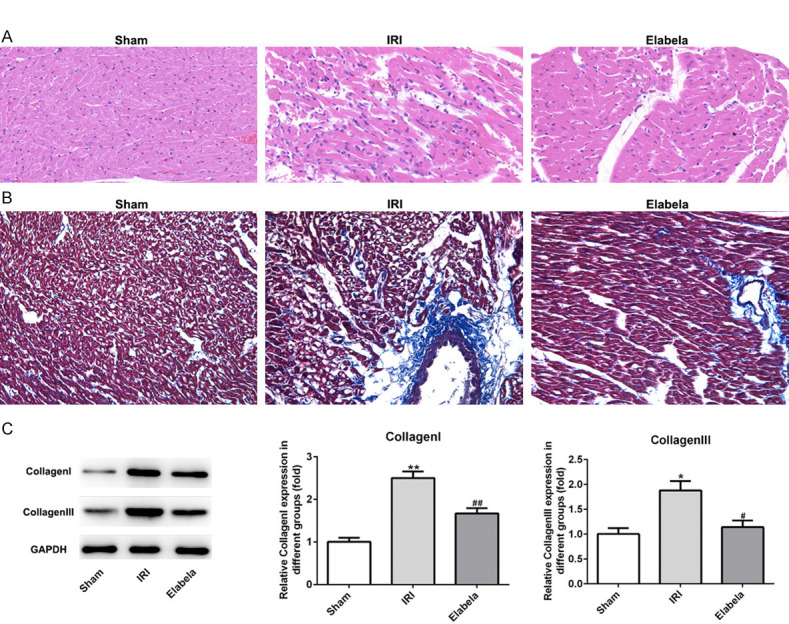
Elabela decreased cardiac fibrosis after myocardial ischemia/reperfusion injury. A. Heart H&E staining from sham and ischemia reperfusion injury heart tissue. Scale bar: 20 μm. B. Myocardium underwent Masson’s trichrome staining. Red parts indexed cardiomyocytes and blue parts indexed fibrosis. Scale bar: 20 μm. C. Expression levels of type I and type III collagen. IRI, ischemia reperfusion injury. All results were obtained from at least three independent experiments. All numerical data were presented as the mean ± standard deviation. *P < 0.05, **P < 0.01 versus sham group; #P < 0.05, ##P < 0.01 versus IRI group.
Figure 3.
Elabela alleviated apoptosis triggered by cardiac ischemia/reperfusion. A. TUNEL-positive (green) cardiomyocytes were evaluated by double staining with TUNEL (green) and 4’, 6’-diamidino-2-phenylindole (blue). Scale bar: 20 μm. B. Expression levels of Bcl-2, Bax, cleaved-caspase3, caspase-3 and Cyt-c were measured using western blotting. IRI, ischemia reperfusion injury. All results were obtained from at least three independent experiments. All numerical data were presented as the mean ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001 versus sham group; #P < 0.05 versus IRI group.
Elabela ameliorated mitochondrial dysfunction during cardiomyocyte I/R injury
Due to the core role of mitochondria in the production of ROS and cell future, we examined the myocardial ultrastructure in myocardium. Electron microscopy analysis presented that I/R induced mitochondrial injury containing swelling, disorganization and reduction or vanish of the crista, which was reversed upon Elabela treatment (Figure 4A). Importantly, ROS production after 4 h of reperfusion was prominently augmented and Elabela treatment attenuated I/R-induced ROS level. Similar result was observed in MDA (Figure 4B and 4C). GSH and SOD, which possess anti-oxidative effects, as well as ATP were upregulated by Elabela treatment after reperfusion (Figure 4B and 4D).
Figure 4.
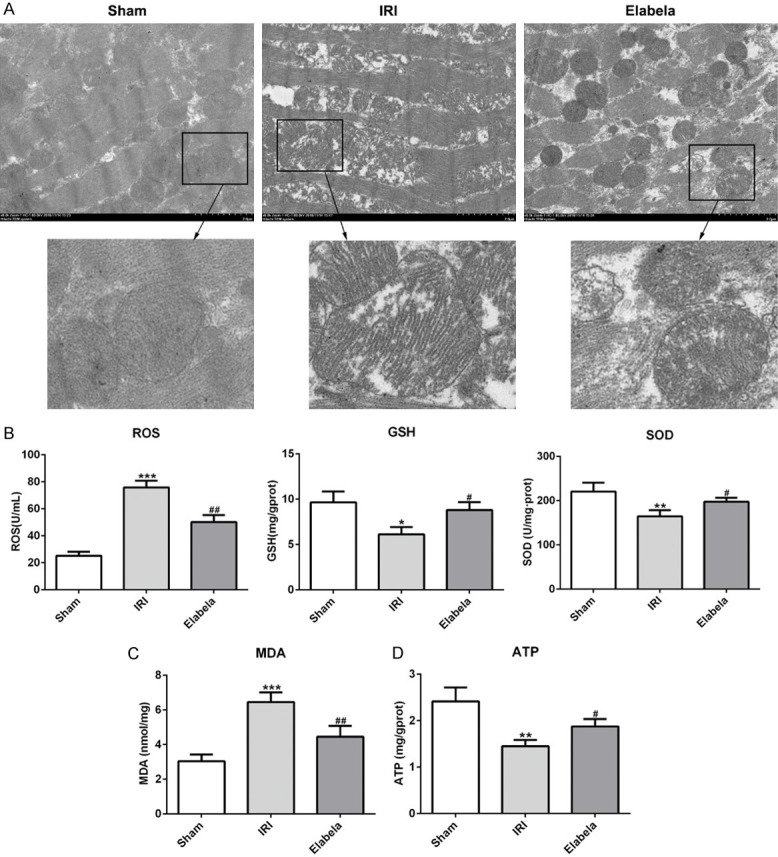
Elabela ameliorated mitochondrial dysfunction. (A) Transmission electron microscopic images from rat cardiac tissues. Scale bar: 2.0 μm. (B) Regular biomarkers including reactive oxygen species (ROS), glutathione (GSH), superoxide dismutase (SOD) and (C) malondialdehyde (MDA) were determined by commercial kit for oxidative stress. (D) Production of ATP was subjected by commercial kit. All results were obtained from at least three independent experiments. All numerical data were presented as the mean ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001 versus sham group; #P < 0.05, ##P < 0.01 versus IRI group.
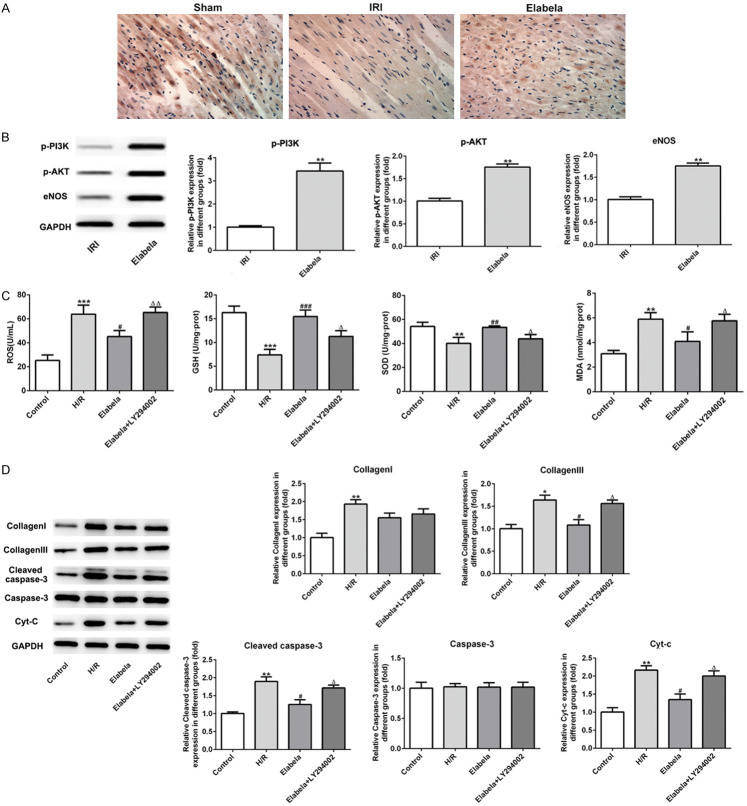
Elabela protected against fibrosis, apoptosis and oxidative stress via PI3K/ATK signaling pathway
As presented in Figure 5A, the expression of phosphorylation-AKT (p-AKT) in cardiac tissues was significantly reduced compared with the sham group, while Elabela intervention remarkably elevated the expression of p-AKT. Western blot analysis revealed that treatment with Elabela led to significant up-regulation of p-PI3K, p-Akt and eNOS expression (Figure 5B). To testify the mechanisms by which Elabela worked, LY294002, an inhibitor of p-PI3K, was applied in H/R-induced H9c2 cells. As shown in Figure 5C, LY294002 causing inactivation of PI3K/ATK signaling eliminated the effects of Elabela on antioxidation. Additionally, inhibition of PI3K also reversed the reduction of Collagen III, cleaved-Caspase-3 and Cyt-C expression caused by Elabela treatment (Figure 5D). These observations suggested that Elabela protects against fibrosis, apoptosis and oxidative stress via PI3K/ATK signaling pathway.
Figure 5.
Elabela modulated the apoptosis and fibrosis by activating PI3K/AKT signaling pathway. A. The expression of phosphorylation-AKT was detected using immunohistochemical staining. B. Expression levels of eNOS, phosphorylation PI3K and AKT were examined using western blot analysis in vivo. **P < 0.01 versus IRI group. H9c2 cells were subjected to high glucose and hypoxia/reperfusion treatment. C. Levels of ROS, GSH, SOD and MDA were detected by commercial kits. D. Levels of type I and type III collagen, cleaved-casepase3, caspase3 and Cyt-c were assessed using western blot analysis in high glucose and hypoxia/reperfusion (H/R)-treated H9c2 cells. All results were obtained from at least three independent experiments. All numerical data were presented as the mean ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group; #P < 0.05, ##P < 0.01, ###P < 0.001 versus H/R group; ΔP < 0.05 versus Elabela group.
Discussion
APJ, which includes seven transmembrane domains, has been confirmed to be mainly distributed in endothelial and smooth muscle cells and cardiomyocytes [22-24]. Activation of APJ by Apelin, a known ligand of APJ, has been reported to partake in the regulation of cardiac I/R injury [25,26]. Recently, Elabela, a new ligand for APJ, has been reported to be either similar or different to the signaling pathway and the biological effects in Apelin/APJ. Apelin-13 is crucially involved in the myocardial infarction mice model by reducing the myocardial apoptosis and cardiac fibrosis [26]. Most recently, Elabela was discovered to exert anti-heart failure effects by promoting proliferation and reducing apoptosis in myocardial ischemia models [13]. This is exemplified by the response to I/R injury. Our current investigation dedicated to exploring the effects of Elabela on cardiac I/R injury. Two weeks after Elabela treatment, the H&E staining firstly showed that Elabela could alleviate cardiac I/R injury. In addition, Elabela reduced the production of collagenous fiber which is closely related to cardiac fibrosis. Besides, Elabela attenuated myocardial I/R injury by reduction of apoptosis and amelioration of mitochondrial dysfunction. In consistent to our exploration, PI3K/AKT signaling was dramatically promoted by Elabela.
It has been proved that Elabela peptide treatment decreases the angiotensin-converting enzyme expression to reduce cardiac remodeling such as hypertrophy and fibrosis in Angiotensin II- induced cardiac models [12]. Cardiac fibrosis occurs under the accumulation of extracellular matrix (ECM) in most of the cardiac pathology [27]. Despite of what kind of reason for cardiac fibrosis, synthesis of type I and III collagen, the main components of ECM, are increased [28]. The current work proved that Elabela dramatically decreased the expression levels of type I and III collagen, which meant that the formation of fibrosis was inhibited. Under normal conditions, the cardiac fibroblasts maintain quiescent and do not proliferate dramatically, however, fibrosis may be activated by the cardiac injury and subsequent results in cardiac insufficiency [27]. Elabela ameliorated the abnormal cardiac function by reducing the cardiac fibrosis.
Necrosis and apoptosis are persistently emerged in ischemia, while apoptosis is surged after reperfusion [29]. Hindering apoptosis has been successfully proved to reduce reperfusion produced damage in animal reperfusion injury models [29]. Elabela, in current study, presented its dramatically inhibitory effects on cardiac apoptosis due to cardiac I/R. Additionally, Elabela restored the mitochondrial function. During cardiac I/R injury, mitochondria is damaged, and thus low ATP production and high ROS generation are triggered [30]. In this investigation, Elabela dramatically decreased generation of ROS and enhanced the ATP production. Also, Elabela reduced the levels of anti-oxidant enzymatic SOD and non-enzymes GSH. The cardiac I/R injury commonly reduces the activation of Bcl-2, which blocks Cyt-c release [31], and promotes the pro-apoptotic Bax expression. Release of Cyt-c can activate caspase9 and caspase3 and thereby apoptosis is promoted [31]. Elabela promoted the Bcl-2 expression and simultaneously blocked the apoptosis. These above data indicated that Elabela may inhibit the mitochondrial apoptosis in cardiac I/R injury.
A previous study showed that Elabela activates the PI3K/AKT signaling in human embryonic stem cells [32], suggesting its protective effects through activation of PI3K/AKT signaling. In this work, Elabela treatment dramatically promoted the phosphorylation of PI3K and AKT, and the PI3K inhibitor, LY294002, reversed the effects of Elabela in I/R model on oxidative stress, fibrosis and apoptosis. However, only type III collagen was prominently reduced by LY294002. Thus, PI3K/AKT signaling partly showed the mediated roles in Elabela therapy in cardiac I/R injury.
Taken together, these findings for the first time demonstrated that Elabela could protect against fibrosis, apoptosis and oxidative stress via PI3K/ATK signaling pathway in cardiac ischemia reperfusion injury. All data founded in this investigation for Elabela deserves further exploration as a promising therapy strategy for cardiac protection from I/R injury.
Acknowledgements
The present study was supported by the National Nature Science Foundation of China (Grant number 81530058) and Military 12th Five-year Key Projects (Grant number BWS2J037).
Disclosure of conflict of interest
None.
References
- 1.Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y. Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res. 2018;64 doi: 10.1111/jpi.12471. [DOI] [PubMed] [Google Scholar]
- 2.Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GG, Possati G, Gaudino M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: a review of evidence. Eur J Cardiothorac Surg. 2004;25:304–311. doi: 10.1016/j.ejcts.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matute-Bello G, Martin TR. Science review: apoptosis in acute lung injury. Crit Care. 2003;7:355–358. doi: 10.1186/cc1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell. 2013;27:672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, Read C, Kuc RE, Buonincontri G, Southwood M, Torella R, Upton PD, Crosby A, Sawiak SJ, Carpenter TA, Glen RC, Morrell NW, Maguire JJ, Davenport AP. Elabela/toddler is an endogenous agonist of the apelin apj receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation. 2017;135:1160–1173. doi: 10.1161/CIRCULATIONAHA.116.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, Cao J, Li L, Chen L. Elabela, a new endogenous ligand of APJ, functions in embryos and adults organisms. Acta Biochim Biophys Sin (Shanghai) 2017;49:378–381. doi: 10.1093/abbs/gmx014. [DOI] [PubMed] [Google Scholar]
- 8.Sharma B, Ho L, Ford GH, Chen HI, Goldstone AB, Woo YJ, Quertermous T, Reversade B, Red-Horse K. Alternative progenitor cells compensate to rebuild the coronary vasculature in elabela- and apj-deficient hearts. Dev Cell. 2017;42:655–666. e653. doi: 10.1016/j.devcel.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He L, Chen L, Li L. The mechanosensitive APJ internalization via clathrin-mediated endocytosis: a new molecular mechanism of cardiac hypertrophy. Med Hypotheses. 2016;90:6–10. doi: 10.1016/j.mehy.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Murza A, Sainsily X, Coquerel D, Cote J, Marx P, Besserer-Offroy E, Longpre JM, Laine J, Reversade B, Salvail D, Leduc R, Dumaine R, Lesur O, Auger-Messier M, Sarret P, Marsault E. Discovery and structure-activity relationship of a bioactive fragment of ELABELA that modulates vascular and cardiac functions. J Med Chem. 2016;59:2962–2972. doi: 10.1021/acs.jmedchem.5b01549. [DOI] [PubMed] [Google Scholar]
- 11.O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Sato C, Kadowaki A, Watanabe H, Ho L, Ishida J, Yamaguchi T, Kimura A, Fukamizu A, Penninger JM, Reversade B, Ito H, Imai Y, Kuba K. ELABELA-APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage. Cardiovasc Res. 2017;113:760–769. doi: 10.1093/cvr/cvx061. [DOI] [PubMed] [Google Scholar]
- 13.Xi Y, Yu D, Yang R, Zhao Q, Wang J, Zhang H, Qian K, Shi Z, Wang W, Brown R, Li Y, Tian Z, Gong DW. Recombinant Fc-Elabela fusion protein has extended plasma half-life andmitigates post-infarct heart dysfunction in rats. Int J Cardiol. 2019;292:180–187. doi: 10.1016/j.ijcard.2019.04.089. [DOI] [PubMed] [Google Scholar]
- 14.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Chen M, Jiang J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion. 2019;49:35–45. doi: 10.1016/j.mito.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Fang F, Gao Y, Tang G, Xu W, Wang Y, Kong R, Tuyihong A, Wang Z. ROS Induced by killerred targeting mitochondria (mtKR) enhances apoptosis caused by radiation via Cyt c/caspase-3 pathway. Oxid Med Cell Longev. 2019;2019:4528616. doi: 10.1155/2019/4528616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee FY, Shao PL, Wallace CG, Chua S, Sung PH, Ko SF, Chai HT, Chung SY, Chen KH, Lu HI, Chen YL, Huang TH, Sheu JJ, Yip HK. Combined therapy with SS31 and mitochondria mitigates myocardial ischemia-reperfusion injury in rats. Int J Mol Sci. 2018;19:2782. doi: 10.3390/ijms19092782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res. 2005;97:1156–63. doi: 10.1161/01.RES.0000190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK. Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid Redox Signal. 2011;14:2191–2200. doi: 10.1089/ars.2010.3486. [DOI] [PubMed] [Google Scholar]
- 20.Sulaiman D, Li J, Devarajan A, Cunningham CM, Li M, Fishbein GA, Fogelman AM, Eghbali M, Reddy ST. Paraoxonase 2 protects against acute myocardial ischemia-reperfusion injury by modulating mitochondrial function and oxidative stress via the PI3K/Akt/GSK-3beta RISK pathway. J Mol Cell Cardiol. 2019;129:154–164. doi: 10.1016/j.yjmcc.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG, Arad M, Hochhauser E. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1alpha’. Cardiovasc Diabetol. 2018;17:111. doi: 10.1186/s12933-018-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo X, Liu J, Zhou H, Chen L. Apelin/APJ system: a critical regulator of vascular smooth muscle cell. J Cell Physiol. 2018;233:5180–5188. doi: 10.1002/jcp.26339. [DOI] [PubMed] [Google Scholar]
- 23.Strohbach A, Pennewitz M, Glaubitz M, Palankar R, Gross S, Lorenz F, Materzok I, Rong A, Busch MC, Felix SB, Delcea M, Busch R. The apelin receptor influences biomechanical and morphological properties of endothelial cells. J Cell Physiol. 2018;233:6250–6261. doi: 10.1002/jcp.26496. [DOI] [PubMed] [Google Scholar]
- 24.Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, Woods CE, Purcell NH, Catalucci D, Akasaka T, Bueno OF, Vlasuk GP, Kaliman P, Bodmer R, Smith LH, Ashley E, Mercola M, Brown JH, Ruiz-Lozano P. APJ acts as a dual receptor in cardiac hypertrophy. Nature. 2012;488:394–398. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Lv SY, Lyu SK, Wu D, Chen Q. The protective effect of apelin on ischemia/reperfusion injury. Peptides. 2015;63:43–46. doi: 10.1016/j.peptides.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Zeng H, Chen JX. Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction. Am J Physiol Heart Circ Physiol. 2012;303:H605–618. doi: 10.1152/ajpheart.00366.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 29.Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho L, Tan SY, Wee S, Wu Y, Tan SJ, Ramakrishna NB, Chng SC, Nama S, Szczerbinska I, Chan YS, Avery S, Tsuneyoshi N, Ng HH, Gunaratne J, Dunn NR, Reversade B. ELABELA is an endogenous growth factor that sustains hESC self-renewal via the PI3K/AKT pathway. Cell Stem Cell. 2015;17:435–447. doi: 10.1016/j.stem.2015.08.010. [DOI] [PubMed] [Google Scholar]