Abstract
BACKGROUND
Intestinal mucosal barrier dysfunction plays an important role in the pathogenesis of ulcerative colitis (UC). Recent studies have revealed that impaired autophagy is associated with intestinal mucosal dysfunction in the mucosa of colitis mice. Resveratrol exerts anti-inflammatory functions by regulating autophagy.
AIM
To investigate the effect and mechanism of resveratrol on protecting the integrity of the intestinal mucosal barrier and anti-inflammation in dextran sulfate sodium (DSS)-induced ulcerative colitis mice.
METHODS
Male C57BL/6 mice were divided into four groups: negative control group, DSS model group, DSS + resveratrol group, and DSS + 5-aminosalicylic acid group. The severity of colitis was assessed by the disease activity index, serum inflammatory cytokines were detected by enzyme-linked immunosorbent assay. Colon tissues were stained with haematoxylin and eosin, and mucosal damage was evaluated by mean histological score. The expression of occludin and ZO-1 in colon tissue was evaluated using immunohistochemical analysis. In addition, the expression of autophagy-related genes was determined using reverse transcription-polymerase chain reaction and Western-blot, and morphology of autophagy was observed by transmission electron microscopy.
RESULTS
The resveratrol treatment group showed a 1.72-fold decrease in disease activity index scores and 1.42, 3.81, and 1.65-fold decrease in the production of the inflammatory cytokine tumor necrosis factor-α, interleukin-6 and interleukin-1β, respectively, in DSS-induced colitis mice compared with DSS group (P < 0.05). The expressions of the tight junction proteins occludin and ZO-1 in DSS model group were decreased, and were increased in resveratrol-treated colitis group. Resveratrol also increased the levels of LC3B (by 1.39-fold compared with DSS group) and Beclin-1 (by 1.49-fold compared with DSS group) (P < 0.05), as well as the number of autophagosomes, which implies that the resveratrol may alleviate intestinal mucosal barrier dysfunction in DSS-induced UC mice by enhancing autophagy.
CONCLUSION
Resveratrol treatment decreased the expression of inflammatory factors, increased the expression of tight junction proteins and alleviated UC intestinal mucosal barrier dysfunction; this effect may be achieved by enhancing autophagy in intestinal epithelial cells.
Keywords: Resveratrol, Ulcerative colitis, Autophagy, Intestinal mucosal barrier;Dextran sulfate sodium-induced colitis, Intestinal inflammation
Core tip: We established a chronic colitis model successfully via administration of dextran sulfate sodium (DSS), and we found that resveratrol ameliorates the production of the inflammatory cytokines in DSS-induced colitis mice. Meanwhile, resveratrol treatment alleviated intestinal mucosal barrier dysfunction in DSS-induced colitis and increased the expression of the tight junction proteins occludin and ZO-1. Further studies showed that resveratrol treatment increased the levels of LC3B and Beclin-1 in the colons of colitis mice, as well as the number of autophagosomes, which may via enhancing autophagy.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory condition involving the gastrointestinal tract, comprising ulcerative colitis (UC) and Crohn’s disease. UC is characterized by abdominal pain, diarrhoea, and haematochezia. The incidence of UC is rising with changes in dietary habits and the rhythm of life[1,2]. UC is often persistent or recurrent in patients and increases the risk of colorectal cancer[3]. Currently, drugs such as 5-aminosalicylic acid (5-ASA), corticosteroids and immunosuppressants are used, but the effect of these drugs is poor, and the long-term use of these drugs may cause severe adverse reactions, resulting in great psychological and economic burdens on the patients[4,5]. Therefore, effective and safe drugs against UC are urgently needed.
The initiating event and pathogenesis of UC currently remain elusive. Recent work has highlighted the importance of intestinal mucosal barrier dysfunction in disease pathophysiology[6]. Many studies have shown that the destruction of the intestinal mucosal barrier plays an important role in the deterioration of IBD, especially in UC patients[7-9]. Protecting the integrity of the intestinal mucosal barrier is thought to be a potential clinical treatment approach for UC.
Resveratrol (3,4′,5-Trihydroxy-trans-stilbene), a natural plant polyphenol found in grapes, is the principal biologically active component in red wine. Resveratrol has anti-inflammatory and immunoregulatory functions, with the advantages of low price and few side effects[10-12]. So far, very few clinical studies have indicated that resveratrol can alleviate clinical colitis activity and improve quality of life and in patients with active UC[13,14]. Although these limited studies show that resveratrol may ameliorate inflammation in UC, the mechanism remains unclear. Intestinal epithelial cells (IECs) are reported to act as the first line of defence in the intestinal mucosal barrier. The IECs tight junctions may play an important role in intestinal mucosal barrier. Occludin and ZO-1 are important tight junction proteins that play a significant role in maintaining the integrity of the intestinal mucosal barrier. Thus, we focused on whether resveratrol can alleviate intestinal mucosal barrier injury and inflammation. In addition, the mechanism underlying the anti-inflammatory effect of resveratrol in colitis was explored.
Autophagy is a cellular recycling process involving self-degradation and the reconstruction of damaged organelles and proteins[15]. Recent studies have shown that impaired autophagy is related to intestinal mucosal damage in mice with colitis, and stimulation of autophagy can prevent intestinal mucosal inflammation and ameliorates murine colitis in mice[16]. Furthermore, the regulatory effect of resveratrol on autophagy in inflammatory diseases has gradually attracted attention[17,18]. In the present study, we evaluated the effect of resveratrol on dextran sulfate sodium (DSS)-induced colitis in mice and explore the mechanism of resveratrol on protecting the integrity of the intestinal mucosal barrier and anti-inflammation.
MATERIALS AND METHODS
Chemicals and reagents
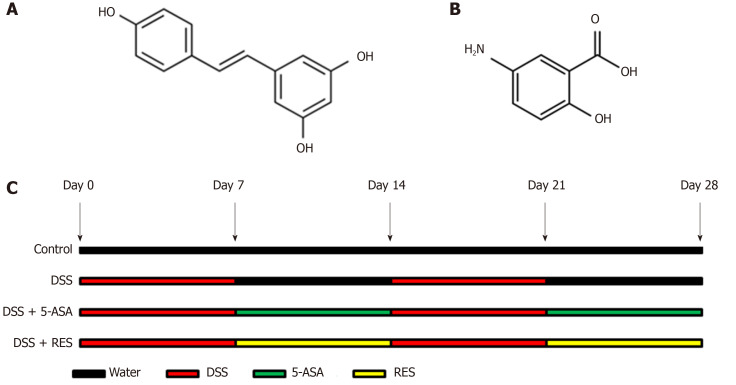
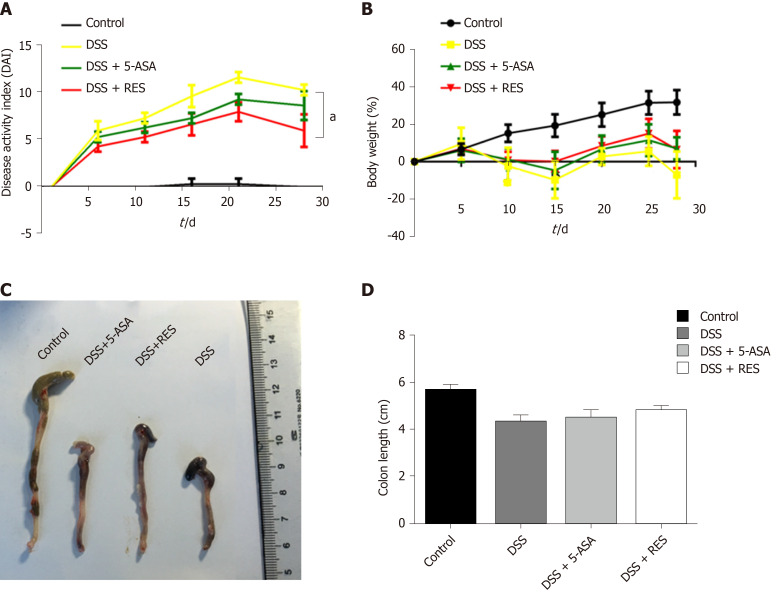
Resveratrol (3,4′,5-Trihydroxy-trans-stilbene; C14H12O3; Molecular Weight: 228.24 g/moL; Figure 1A) and 5-Aminosalicylic acid (5-ASA; C7H7NO3; Molecular Weight: 153.14 g/moL; Figure 1B) were purchased from Sigma Chemical (United States). The purity was checked via HPLC analysis and exceeded 99%. Dextran Sulfate Sodium (DSS, Molecular Weight: 36000-50000) was purchased from MP Biomedicals (United States).
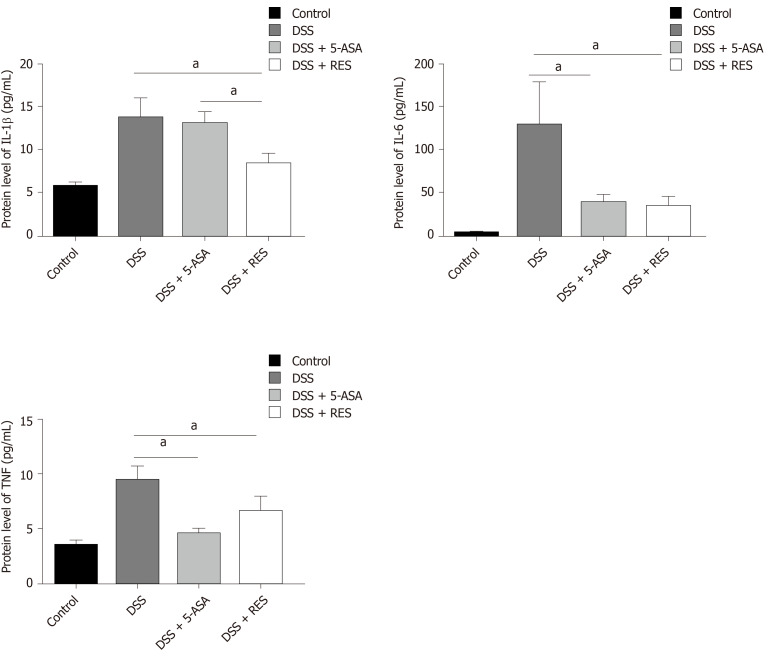
Figure 1.
Chemical structures of resveratrol and 5-aminosalicylic acid, and timeline of in vivo experiment. A: The chemical structures of resveratrol; B: The chemical structures of 5-aminosalicylic acid; and C: Timeline of in vivo experiment. Dextran sulfate sodium (DSS) group was induced by two cycles of intake of drinking water containing 3% DSS for 7 d and normal drinking water for 7 d. Other groups were also treated by two cycles, first received DSS by drinking water for 7 d, followed by treatment of 5-aminosalicylic acid or resveratrol by gavage for 7 d. DSS: Dextran sulfate sodium; 5-ASA: 5-aminosalicylic acid; RES: Resveratrol.
Animals
Male C57BL/6 mice aged 5 wk and weighing 17-19 g were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. Animals were housed in mouse cages at a controlled temperature (21 ± 2 °C) with a pre-set 12-h light/dark cycle. The mice were fed a standard laboratory diet and normal drinking water. The normal diet included crude proteins ≥ 20%, crude fat ≥ 6%, crude fibre ≤ 5%, crude ash ≤ 8%, lysine 1.32%, calcium 1%-1.8%, and phosphorus 0.6%-1.2%, respectively. The animals were acclimated to the experimental conditions for one week before the experiment. According to the guidelines for the care and use of laboratory animals, all the procedures for the treatment and execution of mice were approved by the Animal Care and Use Committee of Zhejiang Chinese Medical University.
Experimental design
DSS-induced colitis is a widely used model that morphologically and symptomatically resembles UC in humans. In this study, we established DSS-induced chronic colitis model to study UC. Mice were randomly divided into four experimental groups (n = 12 mice/group): Negative control group, DSS model group, resveratrol-treated group and 5-ASA positive-control group. Mice in the negative control group received normal drinking water for 28 d. In the DSS model group, chronic colitis was induced by two cycles of giving drinking water containing 3% DSS for 7 d and normal drinking water for 7 d. Resveratrol treatment group (DSS + RES) mice received two cycles of DSS by drinking water, and treatment of resveratrol 100 mg/kg per day by gavage for two cycles followed by DSS at the same time (Figure 1C). 5-ASA, the first-line therapy for UC, was used as a positive control to evaluate the efficacy of resveratrol. Mice in the positive-control group (DSS + 5-ASA) were treated with two cycles of DSS, and 5-ASA 200 mg/kg per day for 28 d at the same time. Body mass was detected every 5 d, and body mass loss was calculated as (detected body mass-initial mass) /initial mass. All animals were euthanized with carbon dioxide and sacrificed at humane end points.
Clinical evaluation of colitis
To assess the severity of colitis, the disease activity index (DAI) was calculated daily based on weight loss, stool consistency and rectal bleeding (Table 1)[19]. Stool consistency (0, normal; 2, loose stool; 4, watery diarrhea); bloody stools (0, normal; 2, slight bleeding; 4, gross bleeding); and body weight loss (0, < 2%; 1, decreased ≥ 2 - < 5%; 2, decreased ≥ 5 - < 10%; 3, decreased ≥ 10 - < 15%; 4, decreased ≥ 15). These values were assessed for each animal, and the sum of the 3 values constituted the DAI. For each parameter, the scores ranged from 0 to 4, resulting in a total DAI score ranging from 0 (unaffected) to 12 (severe colitis).
Table 1.
Assessment of the disease activity index
| Body mass loss (%) | Stool consistency | Bleeding | Score |
| < 2% | Normal | No bleeding | 0 |
| ≥ 2 - < 5% | - | - | 1 |
| ≥ 5 - < 10% | Loose stools | Slight bleeding | 2 |
| ≥ 10 - < 15% | - | - | 3 |
| ≥ 15 | Watery diarrhea | Gross bleeding | 4 |
Inflammatory cytokine assay
Mice were sacrificed, and blood samples (1.0 mL) were collected from the heart. The expression levels of tumour necrosis factor-α (TNF-α, MTA00B, R&D Systems Europe Ltd., Abingdon, England), interleukin (IL)-6 (M6000B, R&D Systems Europe Ltd., Abingdon, England ), and IL-1β (MLB00C, RD Systems Europe Ltd., Abingdon, England) in the plasma were detected using the enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions. The concentration of the cytokines was expressed as pg/mL per mg protein.
Histopathological analysis
Colon length can be used as an indirect marker of inflammation. Thus, when mice were sacrificed, the length of colons from the caecum to the anus was measured. Then, the colon was fixed in 10% formalin, dehydrated at gradient concentrations of ethanol, and embedded in paraffin. Tissue sections (8 µm) were prepared and stained with haematoxylin and eosin (HE), and mucosal damage was evaluated. To evaluate the colonic mucosal damage severity, 9 fields were randomly selected and observed under an inverted fluorescence microscope by two pathologists who were blinded to the experimental groups. Colonic mucosal damage was confirmed by the mean histological score (Table 2)[20]. Each histological score, such as inflammation (0-3), extent (0-3), regeneration (0-4) and crypt damage (0-4), was multiplied by the percentage of compromised tissue (1 point for 25%, 2 points for 26%-50%, 3 points for 51%-75%, and 4 points for 76%-100%). Therefore, the scores of inflammation and extent range from 0 to 12, and the scores of regeneration and crypt damage range from 0 to 16, which reached a maximum score of 56.
Table 2.
Histological scoring of colitis
| Features | Grade | Description |
| Inflammation | 0 | None |
| 1 | Slight | |
| 2 | Moderate | |
| 3 | Severe | |
| Extent | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Regeneration | 4 | No tissue repair |
| 3 | Surface epithelium not intact | |
| 2 | Regeneration with crypt depletion | |
| 1 | Almost complete regeneration | |
| 0 | Complete regeneration or normal tissue | |
| Crypt damage | 0 | None |
| 1 | Basal 1/3 damaged | |
| 2 | Basal 2/3 damaged | |
| 3 | Only surface epithelium intact | |
| 4 | Entire crypt and epithelium lost | |
| Percent involvement | 1 | 1%-25% |
| 2 | 26%-50% | |
| 3 | 51%-75% | |
| 4 | 76%-100% |
Immunohistochemical analysis
The expression of occludin and ZO-1 was detected by immunohistochemical analysis. Briefly, all blocks of colonic tissue were sectioned (3-5 µm). Then, sections were deparaffinized, rehydrated at graded ethanol concentrations and incubated with fresh 3% hydrogen peroxide for 10 min. After being rinsed with phosphate buffer saline (PBS), each tissue section was subjected to antigen retrieval by a suitable antigen retrieval method (boiling in 0.01 M citrate buffer). After endogenous peroxidases were blocked, sections were incubated with anti-occludin antibody (ab222691, 1:200 dilution in PBS, Abcam, United States) and a monoclonal ZO-1 antibody (ab221546, 1:300, Abcam, United States) overnight at 4°C. Subsequently, the sections were incubated for 20 min at room temperature with biotin-labelled secondary antibodies, stained with 3,3-diaminobenzidine, counterstained with haematoxylin, dehydrated and mounted. Primary antibody was replaced by PBS as the negative control. The following criterion was used for semi-quantitative staining: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow brown), and 3 (strong staining, brown). The proportion of stained tumor cells was scored according to the proportion of positively stained tumor cells as follows: 0 (< 5%); 1 (6-25%); 2 (26-50%); and 3 (> 51%). A composite score was obtained by the staining intensity score multiplied the proportion scores. The total score ≤ 4 was defined as low expression, and > 5 was regarded as high expression.
Western blotting analysis
Briefly, proteins were extracted using radioimmunoprecipitation assay buffer, separated by sodium dodecyl sulphate-polyacrylamide gel (5%-10%) electrophoresis, and then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, United States). These membranes were washed with PBS and blocked with Tris-buffered saline-Tween with 5% skim milk (232100, BD, Bioscience, United States). Primary β-actin (3700S, 1:1000, Cell Signal Technology, United States), LC3B (3868T, 1:1000, Cell Signal Technology, United States) and Beclin-1 (3495T, 1:1000, Cell Signal Technology, United States) antibodies were applied, followed by horseradish peroxidase-conjugated secondary antibodies. Then, an enhanced chemiluminescence detection system (Thermo Fisher Scientific, United States) was used to detect antibody binding. Meanwhile, β-actin was used as a negative control.
Reverse transcription-polymerase chain reaction analysis
Total ribonucleic acid was extracted from tissue specimens using TRIzol reagent, and messenger ribonucleic acid (mRNA) was reverse transcribed to complementary deoxyribonucleic acid with a QuantiTect reverse transcription kit (205311, Qiagen, German) according to the manufacturer’s instructions. Quantitative polymerase chain reaction (qPCR) was performed using a SuperScript III Platinum SYBR green one-step qPCR kit (11732088, Thermo Fisher). The qPCR primer sequences were as follows: actin: 5’-CAT CCG TAA AGA CCT CTA TGC CAA C-3’ (forward) and 5’-ATG GAG CCA CCG ATC CAC A-3’ (reverse); Beclin: 5’-ATG GAG GGG TCT AAG GCG TC-3’ (forward) and 5’-TGG GCT GTG GTA AGT AAT GGA-3’ (reverse); LC3B: 5’-TCA AGT CCA ACT ACC GAG TCC-3’ (forward) and 5’-TCA GAG GTT TCC CAT CCA AG-3’ (reverse). Relative messenger ribonucleic acid expression levels were calculated based on the computerized tomography values and normalized using actin expression. All experiments were performed in triplicate on a Roche LightCycler 480 platform.
Transmission electron microscopy
First, colon tissues were fixed with glutaraldehyde and osmium teroxid, then dehydrated in ethanol, passed through propylene oxide, and embedded in Spurr resin. Fifty nanometres thick sections were cut by an ultramicrotome, and the sections were subsequently post-stained with 4% uranyl acetate for 10 min and Reynold's lead citrate for 1.5 min. The structure of the IECs and autolysosomes were observed by transmission electron microscopy (HITACHI H-7650).
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences software (version 13.0; Statistic Package for Social Science Inc., Chicago, IL, United States). Continuous variables were presented as the mean and standard deviation and analysed using one-way Anova or Kruskal-Wallis test. All values were expressed as the mean ± standard deviation. All statistical analyses were conducted with α = 0.05, and P < 0.05 was considered significant.
RESULTS
Clinical activity of DSS-induced colitis mice
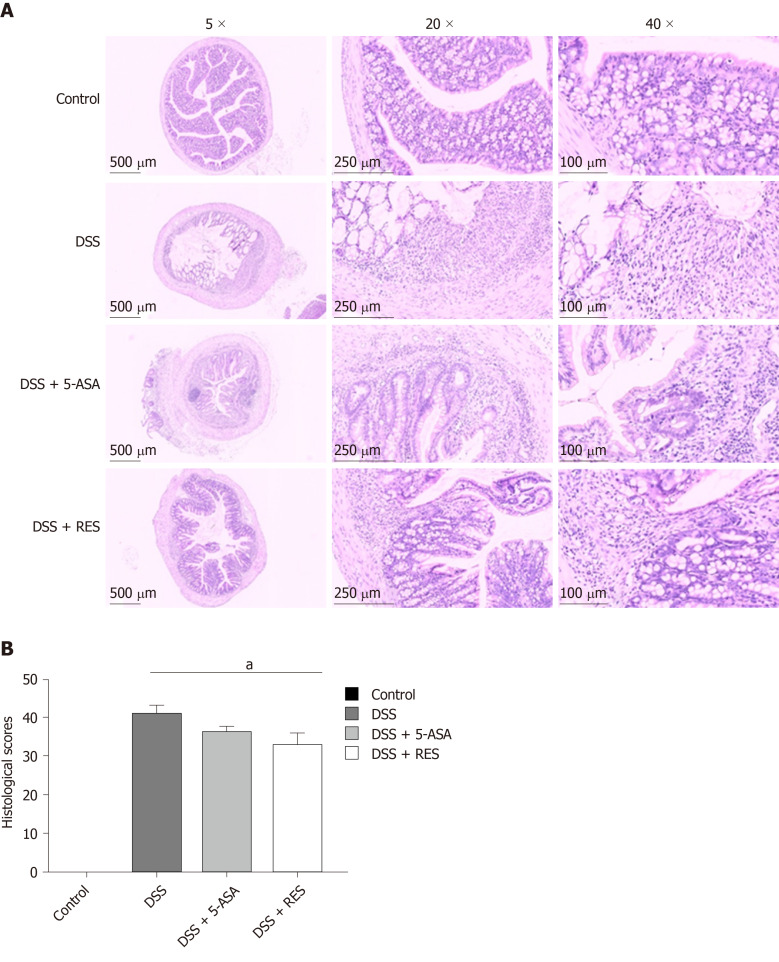
DSS-induced colitis was evaluated, and the clinical course was monitored and scored for the presence of bloody stool, watery diarrhoea and weight loss. During this experiment, three mice in the DSS group died, and one of the mice died on the 13th day, and the other two died on the 14th day; one mouse in the DSS + 5-ASA group died on the 13th day, and no mice in the DSS + RES group died. In comparison with those in the negative control group, mice in the DSS group showed progressively increased DAI score. However, the DSS + RES group showed 1.72-fold decrease in DAI score compared with the DSS group (P < 0.05, Figure 2A), which indicated that resveratrol may have a favourable effect on colitis.
Figure 2.
Clinical evaluation of dextran sulfate sodium-induced chronic colitis by disease activity index, body mass loss and colon length (n = 12 for the control group, n = 9 for disease activity index group, n = 11 for dextran sulfate sodium + 5-aminosalicylic acid group, n = 12 for dextran sulfate sodium + resveratrol treatment group). A: The disease activity index score was reduced in the resveratrol treated group compared with the dextran sulfate sodium group (aP < 0.05); B and C: Resveratrol treatment increased the body mass and colon length of dextran sulfate sodium-induced colitis mice. Body mass loss was calculated as (detected body mass - initial mass)/initial mass. DSS: Dextran sulfate sodium; 5-ASA: 5-aminosalicylic acid; RES: Resveratrol.
Effects of resveratrol on body mass and colon length in DSS-induced colitis mice
Body mass could be decreased by DSS-induced inflammation; therefore, we studied the effect of resveratrol on body mass in DSS-induced colitis mice. The body mass was measured every 5 d, and the results showed that DSS-treated mice exhibited a statistically significant decrease. Resveratrol and 5-ASA treatment increased the body mass of DSS-induced colitis mice, but the difference was not significant (Figure 2B). We also measured the colon length of DSS-induced colitis mice and found that the colon length of the resveratrol-treated group was longer than that of the DSS group, but the difference was also not significant (Figure 2C).
Resveratrol ameliorates the production of inflammatory cytokines in DSS-induced colitis mice
The enzyme-linked immunosorbent assay results demonstrated that the protein expression levels of the inflammatory cytokines TNF-α, IL-6, and IL-1β were higher in the DSS-induced colitis group than in the control group (P < 0.05, Figure 3). Furthermore, the levels of TNF-α and IL-6 in the 5-ASA-treatment group were lower than those in the DSS-induced colitis group (P < 0.05). However, the levels of TNF-α, IL-6 and IL-1β showed 1.42, 3.81, and 1.65-fold decrease in the resveratrol group compared with the DSS group (P < 0.05), and level of IL-1β also showed a 1.57-fold decrease in the resveratrol group compared with that in the DSS + 5-ASA group (P < 0.05).
Figure 3.
Inflammatory cytokine expression in the dextran sulfate sodium-induced chronic colitis determined by enzyme-linked immunosorbent assay. The levels of interleukin-1β, interleukin-6 and tumor necrosis factor-α were decreased in the resveratrol treatment group compared with the dextran sulfate sodium group. aP < 0.05. TNF: Tumor necrosis factor; DSS: Dextran sulfate sodium; 5-ASA: 5-aminosalicylic acid; RES: Resveratrol.
Resveratrol alleviates intestinal mucosal barrier injury in DSS-induced colitis mice as determined by histopathology
We also assessed colon damage by histologic examination. DSS group showed significant colonic mucosal damages, crypt depletion, infiltration of inflammatory cells into the mucosa and submucosa, loss of epithelial barrier. The colon damage and intestinal inflammation in mice treated with resveratrol and 5-ASA were less severe than that in DSS-induced colitis mice (Figure 4A). Consistent with the histologic damage, the histological scores of colitis mice treated with resveratrol were significantly lower than those of DSS-induced colitis mice (P < 0.05, Figure 4B), while the histological scores of 5-ASA-treated colitis mice were not significantly different from those of mice in the DSS group. These results indicate that resveratrol may alleviate intestinal mucosal barrier dysfunction in DSS-induced colitis mice more effectively than 5-ASA.
Figure 4.
Histological staining showed colitis induced dysfunction. A: Dextran sulfate sodium group showed significant colonic mucosal damages, crypt depletion, infiltration of inflammatory cells into the mucosa and submucosa, loss of epithelial barrier. Resveratrol treatment group and 5-aminosalicylic acid could alleviate colitis-induced intestinal mucosal barrier dysfunction; and B: The histological score of resveratrol treatment group was lower than the dextran sulfate sodium group. aP < 0.05. DSS: Dextran sulfate sodium; 5-ASA: 5-aminosalicylic acid; RES: Resveratrol.
Resveratrol increases the production of ZO-1 and occludin in the colons of DSS-treated mice
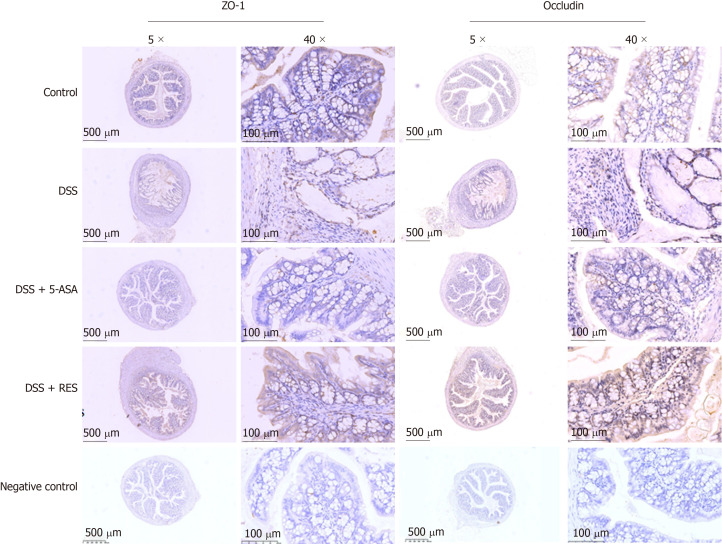
The tight junction proteins occludin and ZO-1 have been reported to play an important role in maintaining the integrity of the intestinal mucosal epithelial barrier. In the present study, we found that the expression levels of occludin and ZO-1 were higher in the resveratrol-treated DSS group than in the 5-ASA and DSS groups (Figure 5 and Table 3).
Figure 5.
Immunohistochemical staining of ZO-1 and occludin. Expressions of ZO-1 and occludin were higher in resveratrol treatment than in the dextran sulfate sodium group and 5-aminosalicylic acid treated group. Negative control: antibody was replaced by phosphate buffer saline. DSS: Dextran sulfate sodium; 5-ASA: 5-aminosalicylic acid; RES: Resveratrol.
Table 3.
ZO-1 and occludin expressions in colitis
| Group |
ZO-1 expression |
χ2/P value |
Occludin expression |
χ2/P value | ||
| Low (%) | High (%) | Low (%) | High (%) | |||
| Control | 6 (50.0%) | 6 (50.0%) | 8.812/0.029 | 7 (58.3%) | 5 (41.7%) | 10.987/0.010 |
| DSS | 7 (77.8%) | 2 (22.2%) | 8 (88.9%) | 1 (11.1%) | ||
| DSS + 5-ASA | 7 (63.6%) | 4 (36.4%) | 9 (81.8%) | 2 (18.2%) | ||
| DSS + RES | 2 (16.7%) | 10 (83.3%) | 3 (25.0%) | 9 (75.0%) | ||
DSS: Dextran sulfate sodium; 5-ASA: 5-aminosalicylic acid; RES: Resveratrol.
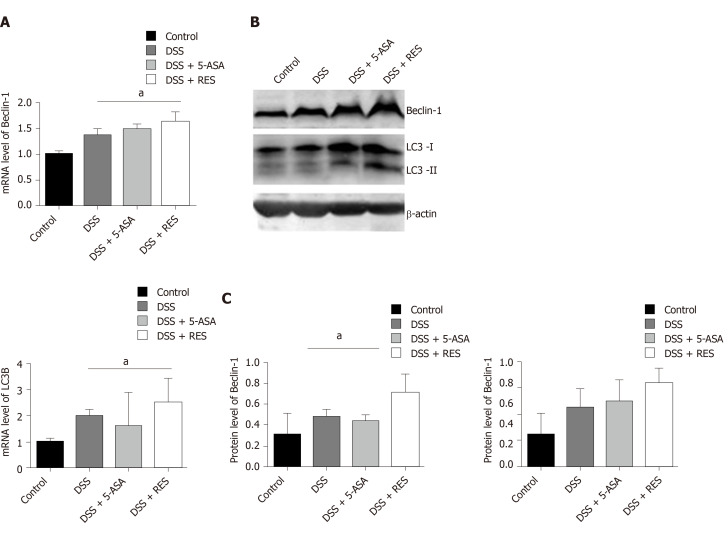
Resveratrol may reduce inflammation by the enhancement of autophagy in DSS-induced colitis
To explore whether the inhibition of colitis by resveratrol treatment is mediated by autophagy activation, we examined the mRNA and protein levels of LC3B and Beclin-1. As determined by real-time PCR, a substantial increase in the mRNA expression level of LC3B and Beclin-1 was observed in the DSS + RES group compared with the DSS group, suggesting that resveratrol increases the mRNA levels of LC3B and Beclin-1 in the colons of DSS-treated mice (P < 0.05, Figure 6A). Consistent with the mRNA level analysis, the western blot results also showed that resveratrol treatment induced significant increases in the LC3-II/I ratio and Beclin-1 level in DSS-induced colitis mice (P < 0.05, Figure 6B and C). However, the difference in the mRNA levels of LC3B and Beclin-1 was not significant between the 5-ASA-treated colitis group and the DSS group (P > 0.05).
Figure 6.
Expression levels of Beclin-1 and LC3B in dextran sulfate sodium induced chronic colitis. A: A substantial increase in the messenger ribonucleic acid expression level of LC3B and Beclin-1 was observed in the dextran sulfate sodium (DSS) + resveratrol treatment group compared with the DSS group (P < 0.05); B and C: The Western blotting showed that resveratrol treatment induced significant increases in the LC3-II/I ratio and Beclin-1 level in DSS-induced colitis mice; Mean grey level of Beclin-1 and LC3B protein level was increased in DSS + resveratrol treatment group. aP < 0.05. DSS: Dextran sulfate sodium; 5-ASA: 5-aminosalicylic acid; mRNA: Messenger ribonucleic acid; RES: Resveratrol.
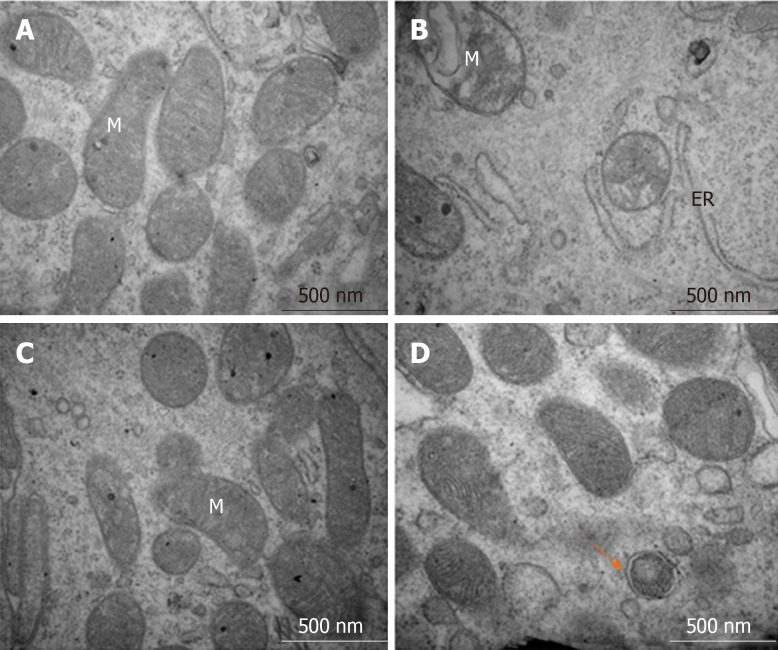
The transmission electron microscopy results showed that IECs in the control group had normal organelle structure with a normal endoplasmic reticulum and mitochondria (Figure 7A). However, in the DSS-induced colitis group, the organelle structure was markedly damaged, with swollen mitochondria, disrupted mitochondrial inner ridges and dilated rough endoplasmic reticulum (Figure 7B). Inflammation induced by the 5-ASA treatment and autophagosomes were rare (Figure 7C). Additionally, resveratrol administration increased the number of autophagosomes and improved the condition of the endoplasmic reticulum and mitochondria, which indicated that resveratrol promoted the progression of autophagy (Figure 7D).
Figure 7.
Structures of the intestinal epithelial cells and autolysosomes observed by transmission electron microscopy. A: Control group showed normal organelle structure; B: Dextran sulfate sodium induced colitis group showed mitochondrial swelling and ruptures of internal cristae, along with distention of the rough endoplasmic reticulum; C: 5-aminosalicylic acid group revealed that mitochondrial swelling was not obvious, and mitochondrial cristae showed blurred appearance; and D: Resveratrol treatment group showed that organelle structure was basically normal, and autophagosome could be observed. ER: Endoplasmic reticulum, M: Mitochondria.
DISCUSSION
UC is a recurrent chronic inflammation of the colon. DSS-induced colitis models, including acute and chronic colitis models, are most commonly used in UC. Acute colitis model is usually induced by administering DSS for 7 d. In this study, a chronic colitis model was successfully established by the administration of two cycles of DSS treatment. Each cycle involved the administration of 3% DSS for 7 d followed by normal drinking water for 7 d[21]. Mice in the chronic colitis group developed clinical symptoms, such as bloody stool and weight loss; the mice also showed pathological features similar to UC patients, such as shortened colon length, intestinal mucosa congestion, oedema and erosion, ulceration, crypt damage, and the infiltration of inflammatory cells into the mucosa and submucosa; the histological scores of colitis and serum inflammation indices (TNF-α, IL-6, IL-1β) in the DSS group increased. The chronic colitis model induced by administering repeated cycles of DSS more closely resembled human UC than the acute colitis model did.
In recent years, the role of intestinal mucosal barrier dysfunction in the pathogenesis of UC has attracted wide attention. IECs are the first-line defence in the intestinal mucosal barrier[22]. The IECs tight junctions are key epithelial intracellular junctions[23,24]. Occludin and ZO-1 are important tight junction proteins that play a significant role in maintaining the integrity of the intestinal mucosal barrier. The integrity of the intestinal mucosal barrier prevents and defends against the invasion of inflammatory factors and bacteria and further mitigates intestinal inflammation. Resveratrol is a well-known phytophenol with pleiotropic properties and has anti-oxidative and anti-inflammatory activity. Recently, resveratrol has also been shown to alleviate intestinal inflammation[25-27]. Consistent with these studies, our results also showed that resveratrol can reduce intestinal inflammation and alleviate intestinal mucosal barrier dysfunction in DSS-induced colitis mice. Resveratrol significantly increased levels of tight junction proteins (occludin and ZO-1) and reduced histological colonic mucosa injury scores, even than 5-ASA did. Resveratrol also decreased serum inflammation indices (TNF-α, IL-6, and IL-1β), although there was no significant difference between resveratrol and 5-ASA treatment. These results showed that both resveratrol and 5-ASA alleviated intestinal inflammation, and resveratrol performed better at maintaining the integrity of the intestinal mucosal epithelial barrier than 5-ASA.
Autophagy is a cellular self-protective mechanism stimulated by both internal and external adverse environmental factors. In this process, degenerated proteins, aged and damaged organelles, even pathogenic microorganisms, are recycled via autophagic degradation to maintain cellular metabolic balance and homeostasis[15,28,29]. Autophagy can be activated when an organ is stimulated by adverse factors. Saito M and others found a reduced LC3-II/I ratio, expression of adhesion proteins and IECs adhesion, and a damaged mucosal epithelial barrier when IECs were exposed to TNF-α and treated with autophagy inhibitors[30]. In this study, we found that resveratrol alleviated the intestinal mucosal barrier dysfunction and reduced the intestinal inflammatory response by enhancing autophagy in experimental chronic colitis(two cycles of DSS treatment for 28 d), as the expression of LC3B and Beclin-1, and the number of autophagosomes in resveratrol-treated group were increased when compared to that in the chronic colitis model group and the negative control group.
A previous study conducted by Zhang et al[31] reported that DSS-inducing increased the autophagy in acute colitis models(DSS treatment for 7 d), and curcumin and resveratrol could protect against colitis by reducing autophagy rather than enhancing autophagy[31]. We speculated that this inconsistency may be caused by the different colitis models used in the two studies. The acute colitis model resembles the early stage of human UC, while the chronic colitis model resembles the advanced stage of UC. Our previous study showed that autophagy was dynamic in the progression of colitis. It was activated in IECs by inflammatory factors in the early stage of a rat model of sepsis-induced acute colitis,and decreased as the disease progressed, thereby reducing the removal of oxidative stress products and the damaged organelles and other substances in cells, which lead to the destruction of the intestinal mucosal barrier and the aggravation of intestinal inflammation[32]. We believe that the chronic colitis mouse model is a better tool to explore the pathogenesis and drug effects of ulcerative colitis.
However, there are still many limitations in the present study. The detailed autophagy involved in resveratrol-induced protection of intestinal mucosal barrier was unclear as we did not study the effect of autophagy inhibitors on colitis treated by resveratrol. On the other hand, we only investigated the effect of resveratrol on animals, therefore, further research is still needed for clinical application.
ARTICLE HIGHLIGHTS
Research background
Intestinal mucosal barrier disorder plays a very important role in the pathogenesis of ulcerative colitis (UC). Recent studies have revealed that impaired autophagy is associated with intestinal mucosal dysfunction in the mucosa of colitis mice.
Research motivation
Resveratrol can regulate autophagy in the treatment of a few inflammatory diseases. Recently, few studies have indicated that resveratrol can alleviate clinical colitis activity in patients with active UC, while the mechanism for its anti-inflammatory effect remains elusive.
Research objectives
The aim of the study was to explore the effect and mechanism of resveratrol on protecting the integrity of the intestinal mucosal barrier and anti-inflammation in dextran sulfate sodium (DSS)-induced ulcerative colitis.
Research methods
DSS-induced ulcerative colitis was induced by DSS, then the disease activity index was used to assess the severity of colitis. Inflammatory cytokines were detected by enzyme-linked immunosorbent assay. Tissue sections were stained with haematoxylin and eosin, and mucosal damage was evaluated by mean histological score. The expression of occludin and ZO-1 was detected by immunohistochemical analysis. Reverse transcription-polymerase chain reaction and Western-blot were used to analyze autophagy-related gene expression, and morphology of autophagy was observed by transmission electron microscopy.
Research results
The resveratrol treatment group showed a 1.72-fold decrease in disease activity index scores and 1.42-, 3.81-, and 1.65-fold decrease in the production of the inflammatory cytokines tumor necrosis factor-α, interleukin-6 and interleukin-1β, respectively, in DSS-induced colitis mice compared with DSS group (P < 0.05). The expression of the tight junction proteins occludin and ZO-1 in DSS model group was reduced, while in resveratrol-treated colitis group was increased. Resveratrol also increased the levels of LC3B (1.39-fold compared with DSS group) and Beclin-1 (1.49-fold compared with DSS group) (P < 0.05), as well as the number of autophagosomes, which implies that the resveratrol may alleviate intestinal mucosal barrier dysfunction in DSS-induced UC mice by enhancing autophagy.
Research conclusions
Resveratrol treatment reduces the expression of inflammatory factors, increases the expression of tight junction proteins and alleviates UC intestinal mucosal barrier dysfunction; this effect is achieved via the regulation of autophagy in intestinal epithelial cells.
Research perspectives
This work suggests that resveratrol may be useful as a new approach to treat UC by enhancing autophagy. Further study of the functionary mechanism will help us to understand the role of resveratrol in colitis and provide a theoretical basis for future clinical application.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Institutional Review Board of Zhejiang Chinese Medical University.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Ethics Committee of Zhejiang Chinese Medical University.
Conflict-of-interest statement: No potential conflicts of interest exist.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: May 17, 2020
First decision: June 18, 2020
Article in press: August 12, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gregorio BM, Hu J, Katada, K, Li CY S-Editor: Zhang L L-Editor: MedE-Ma JY P-Editor: Ma YJ
Contributor Information
Hang-Hai Pan, Department of Gastroenterology, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou 310014, Zhejiang Province, China.
Xin-Xin Zhou, Department of Gastroenterology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Ying-Yu Ma, Key Laboratory of Gastroenterology of Zhejiang Province, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou 310014, Zhejiang Province, China. myy011525@163.com.
Wen-Sheng Pan, Department of Gastroenterology, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou 310014, Zhejiang Province, China.
Fei Zhao, Department of Gastroenterology, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou 310014, Zhejiang Province, China.
Mo-Sang Yu, Department of Gastroenterology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Jing-Quan Liu, Critical Care Unit, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou 310014, Zhejiang Province, China.
Data sharing statement
No additional data are available.
References
- 1.Sairenji T, Collins KL, Evans DV. An Update on Inflammatory Bowel Disease. Prim Care. 2017;44:673–692. doi: 10.1016/j.pop.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Yu YR, Rodriguez JR. Clinical presentation of Crohn's, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26:349–355. doi: 10.1053/j.sempedsurg.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 4.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Planella E, Mañosa M, Van Domselaar M, Gordillo J, Zabana Y, Cabré E, López San Román A, Domènech E. Long-term outcome of ulcerative colitis in patients who achieve clinical remission with a first course of corticosteroids. Dig Liver Dis. 2012;44:206–210. doi: 10.1016/j.dld.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Vindigni SM, Zisman TL, Suskind DL, Damman CJ. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Therap Adv Gastroenterol. 2016;9:606–625. doi: 10.1177/1756283X16644242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis. 2014;32:475–483. doi: 10.1159/000358156. [DOI] [PubMed] [Google Scholar]
- 8.Keane TJ, Dziki J, Sobieski E, Smoulder A, Castleton A, Turner N, White LJ, Badylak SF. Restoring Mucosal Barrier Function and Modifying Macrophage Phenotype with an Extracellular Matrix Hydrogel: Potential Therapy for Ulcerative Colitis. J Crohns Colitis. 2017;11:360–368. doi: 10.1093/ecco-jcc/jjw149. [DOI] [PubMed] [Google Scholar]
- 9.Sina C, Kemper C, Derer S. The intestinal complement system in inflammatory bowel disease: Shaping intestinal barrier function. Semin Immunol. 2018;37:66–73. doi: 10.1016/j.smim.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Javid AZ, Hormoznejad R, Yousefimanesh HA, Haghighi-Zadeh MH, Zakerkish M. Impact of resveratrol supplementation on inflammatory, antioxidant, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Diabetes Metab Syndr. 2019;13:2769–2774. doi: 10.1016/j.dsx.2019.07.042. [DOI] [PubMed] [Google Scholar]
- 11.De Oliveira MTP, de Sá Coutinho D, Tenório de Souza É, Stanisçuaski Guterres S, Pohlmann AR, Silva PMR, Martins MA, Bernardi A. Orally delivered resveratrol-loaded lipid-core nanocapsules ameliorate LPS-induced acute lung injury via the ERK and PI3K/Akt pathways. Int J Nanomedicine. 2019;14:5215–5228. doi: 10.2147/IJN.S200666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann-Franco DC, Esteves B, Lacerda LM, Souza IO, Santos JAD, Pinto NCC, Scio E, da Silva AD, Macedo GC. In vitro and in vivo anti-inflammatory properties of imine resveratrol analogues. Bioorg Med Chem. 2018;26:4898–4906. doi: 10.1016/j.bmc.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch Med Res. 2015;46:280–285. doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Samsamikor M, Daryani NE, Asl PR, Hekmatdoost A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch Med Res. 2016;47:304–309. doi: 10.1016/j.arcmed.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macias-Ceja DC, Cosín-Roger J, Ortiz-Masiá D, Salvador P, Hernández C, Esplugues JV, Calatayud S, Barrachina MD. Stimulation of autophagy prevents intestinal mucosal inflammation and ameliorates murine colitis. Br J Pharmacol. 2017;174:2501–2511. doi: 10.1111/bph.13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji G, Wang Y, Deng Y, Li X, Jiang Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health Dis. 2015;14:134. doi: 10.1186/s12944-015-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen ML, Yi L, Jin X, Liang XY, Zhou Y, Zhang T, Xie Q, Zhou X, Chang H, Fu YJ, Zhu JD, Zhang QY, Mi MT. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy. 2013;9:2033–2045. doi: 10.4161/auto.26336. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Zhou Z, Yang Y, Chen N, Xiang H. Therapeutic effect of imiquimod on dextran sulfate sodium-induced ulcerative colitis in mice. PLoS One. 2017;12:e0186138. doi: 10.1371/journal.pone.0186138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunes NS, Kim S, Sundby M, Chandran P, Burks SR, Paz AH, Frank JA. Temporal clinical, proteomic, histological and cellular immune responses of dextran sulfate sodium-induced acute colitis. World J Gastroenterol. 2018;24:4341–4355. doi: 10.3748/wjg.v24.i38.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017;23:6016–6029. doi: 10.3748/wjg.v23.i33.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 23.Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol (1985) 2016;120:692–701. doi: 10.1152/japplphysiol.00536.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capaldo CT, Powell DN, Kalman D. Layered defense: how mucus and tight junctions seal the intestinal barrier. J Mol Med (Berl) 2017;95:927–934. doi: 10.1007/s00109-017-1557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A, Poudyal D, Nagarkatti M, Nagarkatti PS, Singh UP, Hofseth LJ. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res (Phila) 2010;3:549–559. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao J, Wei C, Wang JY, Zhang R, Li YX, Wang LS. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J Gastroenterol. 2015;21:6572–6581. doi: 10.3748/wjg.v21.i21.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdin AA. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol. 2013;718:145–153. doi: 10.1016/j.ejphar.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lassen KG, Xavier RJ. Mechanisms and function of autophagy in intestinal disease. Autophagy. 2018;14:216–220. doi: 10.1080/15548627.2017.1389358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito M, Katsuno T, Nakagawa T, Sato T, Noguchi Y, Sazuka S, Saito K, Arai M, Yokote K, Yokosuka O. Intestinal epithelial cells with impaired autophagy lose their adhesive capacity in the presence of TNF-α. Dig Dis Sci. 2012;57:2022–2030. doi: 10.1007/s10620-012-2133-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Xue H, Zhao G, Qiao C, Sun X, Pang C, Zhang D. Curcumin and resveratrol suppress dextran sulfate sodium-induced colitis in mice. Mol Med Rep. 2019;19:3053–3060. doi: 10.3892/mmr.2019.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan SX, Shi B, Lou XL, Liu JQ, Ma GG, Liang DY, Ma S. Ghrelin protects small intestinal epithelium against sepsis-induced injury by enhancing the autophagy of intestinal epithelial cells. Biomed Pharmacother. 2016;83:1315–1320. doi: 10.1016/j.biopha.2016.08.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.