Abstract
Inflammatory bowel disease (IBD) incidence has been increasing steadily, most dramatically in the Western developed countries. Treatment often includes lifelong immunosuppressive therapy and surgery. There is a critical need to reduce the burden of IBD and to discover medical therapies with better efficacy and fewer potential side-effects. Repurposing of treatments originally studied in other diseases with similar pathogenesis is less costly and time intensive than de novo drug discovery. This study used a treatment repurposing methodology, the literature-related discovery and innovation (LRDI) text mining system, to identify potential treatments (developed for non-IBD diseases) with sufficient promise for extrapolation to treatment of IBD. By searching for desirable patterns of twenty key biomarkers relevant to IBD (e.g., inflammation, reactive oxygen species, autophagy, barrier function), the LRDI-based query retrieved approximately 9500 records from Medline. The most recent 350 records were further analyzed for proof-of-concept. Approximately 18% (64/350) met the criteria for discovery (not previously studied in IBD human or animal models) and relevance for application to IBD treatment. Many of the treatments were compounds derived from herbal remedies, and the majority of treatments were being studied in cancer, diabetes, and central nervous system disease, such as depression and dementia. As further validation of the search strategy, the query identified ten treatments that have just recently begun testing in IBD models in the last three years. Literature-related discovery and innovation text mining contains a unique search strategy with tremendous potential to identify treatments for repurposing. A more comprehensive query with additional key biomarkers would have retrieved many thousands more records, further increasing the yield of IBD treatment repurposing discovery.
Keywords: Treatment repurposing, Treatment repositioning, Inflammatory bowel disease, Literature-based discovery, Text mining, Crohn’s disease, Ulcerative colitis, Novel treatments
Core tip: A text-mining approach was used to identify treatments from non-inflammatory bowel disease (IBD) diseases that could be extrapolated to treat IBD. Sixty-four treatment concepts were identified in different phases of development, ranging from laboratory research to clinical application. Many more were possible with a longer and well-resourced study. Ten of the non-IBD concepts that were excluded from being classified as discovery would have been classified as discovery if the study had been conducted in 2016. Thus, this approach has the capability to identify/predict many new areas of research for treating IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal (GI) tract characterized by alternating phases of relapse and remission, clinically defined into two major subtypes: Crohn’s disease (CD) and ulcerative colitis (UC)[1]. Globally, the incidence of IBD is increasing, especially among newly industrialized countries where IBD was previously non-existent[2]. Childhood onset of IBD is also increasing in a similar pattern globally, suggesting evolving environmental risk factors[3].
In 2017, there were 6.8 million cases of IBD globally. The age-standardized prevalence rate increased from 79.5 per 100000 population in 1990 to 84.3 per 100000 population in 2017. At the regional level of global burden of disease (GBD), the highest age-standardized prevalence rate in 2017 occurred in North America (422.0 per 100000) and the lowest age-standardized prevalence rates were observed in the Caribbean (6.7 per 100000 population). High sociodemographic index (SDI) locations had the highest age-standardized prevalence rate, while low SDI regions had the lowest age-standardized prevalence rate[4].
The pathogenesis of IBD is multifactorial, which makes prevention and treatment of IBD challenging. Genetic predisposition contributes to dysregulation of both innate and adaptive immunity[5]. Environmental triggers (diet, infection, antibiotics, and toxin exposure) affect the intestinal microbiome and influence epigenetic changes that alter immune regulation[6]. Treatment, particularly in those with more aggressive disease, is generally lifelong, and often includes immunosuppressive therapy and surgery. There is a critical need to both (1) identify and eliminate contributing factors to disease to reduce the burden of IBD; and (2) discover novel medical therapies that provide better efficacy with fewer potential side-effects.
POTENTIAL IBD TREATMENT DISCOVERY
The standard approach to drug discovery is costly and time intensive. Repurposing treatments (1) already being developed; or (2) in clinical application for other conditions with overlapping pathogenesis is an attractive alternative to de novo drug discovery. This repurposing approach involves extrapolating a treatment developed for a non-IBD disease, such as rheumatoid arthritis, for use in IBD. Many of the upfront development costs can be avoided and some safety data already exists. However, given the vastness of the current published biomedical literature, quickly identifying potential novel treatments for IBD remains challenging.
A unique methodology to facilitate treatment repurposing has been developed by the first author using a literature-related discovery and innovation (LRDI) text-mining approach[7]. This approach involves matching patterns of biomarker changes (tests that measure a normal biologic, a pathologic process, a response to treatment, or predict a response[8]) in the disease-of-interest core literature with similar pattern changes in the remainder of the biomedical literature. The objective of this study is to use the LRDI approach to identify potential novel treatments for IBD that cannot be found in the IBD core literature.
However, the simultaneous removal of contributing factors to disease pathogenesis is equally important to treatment. A protocol (that includes identification and removal of contributing factors) to prevent and reverse chronic diseases is described in Appendix 1, along with examples of contributing factors associated with IBD. Contributing factors as used in the present study are, in theory, modifiable (reduced or eliminated through personal choice and/or government regulation), and can be broadly categorized as Lifestyle, Iatrogenic, Biotoxins, Occupational/Environmental, PsychoSocial/SocioEconomic. They include smoking, excessive alcohol, pesticides, wireless radiation, ionizing radiation, brominated flame retardants, etc[9].
METHODOLOGY
The specific details of LRDI-based treatment repurposing steps are contained in Appendix 2, as are the specific search terms used in the query. The query was executed April 19, 2020.
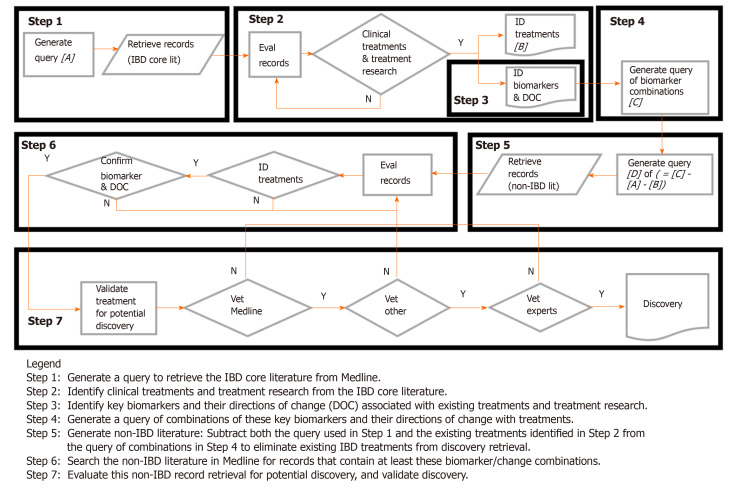
Figure 1 shows the flow diagram of the treatment discovery/repurposing process. Briefly, key biomarkers and their direction of change in the existing IBD literature were identified from examination of a large number of existing IBD treatments in all phases of development and clinical application (the reasons for identifying/using the large numbers of existing treatments are discussed in more detail in Appendix 3). These biomarkers and their desired treatment-derived directions of change are combined to form a query. The query was then used to search the non-IBD literature in Medline for potential treatments whose effects on biomarker changes were similar to those from existing IBD treatments in the core IBD literature (i.e., similar biomarker “signatures”). Those potential treatments from the non-IBD Medline literature that could not be found in the core IBD Medline literature were considered to be discovery.
Figure 1.
Literature-related discovery and innovation treatment repurposing flow diagram.
Once the biomarkers associated with IBD were identified, they were ranked by (1) prevalence in the literature; and (2) clinical relevance. From hundreds of potential biomarkers, the top twenty (and variants) were prioritized and, along with their treatment-derived directions of change, were used in the treatment discovery query. Biomarkers from multiple pathways known to be associated with the inflammatory/immune response in IBD (i.e., cytokines, reactive oxygen species, autophagy and barrier function markers) were selected. All combinations of two biomarkers were then used to define the pattern for matching (e.g., decrease CRP AND increase IL-10). Categorical phrases, such as “anti-inflammatory” were also used as a biomarker term. The resultant query was entered into the Web of Science search engine for Medline.
In a proof-of-concept model, the 350 most recent records from 9500 records retrieved were analyzed further to (1) demonstrate the potential power of the technique; and (2) provide useful results to the IBD community as well. These potential treatments were then validated independently to assess applicability to IBD, in order to ensure the treatment was not already being tested in IBD models.
RESULTS
The treatments identified in this study include both (1) potential novel IBD treatments (Table 1)[10-73]; and (2) examples of recently studied IBD treatments that would have also been identified as potential novel treatments had this study been performed in 2016 (Table 2)[74-95]. The latter reflect the predictive nature of the LRDI treatment repurposing technique.
Table 1.
Potential novel treatments
| Treatment | Biomarkers changed in desired direction | Ref. |
| Fluprostenol | iNOS, TNF-α, CD11c, IL-10, NF-kB, p65 | [10] |
| Liu Shen Wan | Anti-inflammatory, IL-1β, TNF-α, IFN-γ, IL-6, TLR4, NF-kB p65, p-IkBα | [11] |
| Erdosteine | Anti-oxidant, anti-inflammatory, IL-1β, COX-2, iNOS, P65, ADAMTS-5, MMP1, MMP3, MMP-13, MAPK, Wnt/β-catenin | [12] |
| 4-Octyl itaconate | Anti-inflammatory, TGF-β/Smad, NF-kB, ROS, autophagy | [13] |
| 2 ,3-dihydro-5,6-dimethoxy-1H-inden-1-one | ROS, LDH, MDA, TAC, anti-inflammatory | [14] |
| Neutrophilic granule protein | TNF-α, IL-1β, NF-kB, IL-10, anti-inflammatory | [15] |
| Dioscorea zingiberensis | BTB integrity, ZO-1, MDA, 8-OHdG, Nrf2, NOQ1, HO-1 | [16] |
| FCPR16 | TNF-α, Caspase-3, Caspase-8, NF-kB p65, iNOS, ROS, JNK | [17] |
| 7,8-dihydroxyflavone | GSH, nitrite, MDA, NF-kB, iNOS, caspase-3, Nrf2, HO-1, BDNF | [18] |
| X-inactive specific transcript | Anti-inflammatory, NF-kB, IL-6 | [19] |
| 3-[3-pyridinyl]-1-[4-pyridinyl]-2-propen-1-one | M1, autophagy, NF-kB, TNF-α, ICAM-1, VCAM-1 | [20] |
| NMDEA | IL-1β, IL-6, p65, iNOS | [21] |
| Cashew gum | MPO, TER, anti-inflammatory, barrier function | [22] |
| Ampelopsin | ROS, NOX2, NOX4, FN, Col IV, Nrf2, HO-1, NQO-1 | [23] |
| Esculentoside A | IL-1β, IL-6, IL-8, TNF-α, MMP -2, -3, -13, NF-kB, MAPK | [24] |
| Phyllanthus emblica | Antioxidant, GSH, SOD, MDA, inflammation | [25] |
| Anoectochilus roxburghii | Oxidative stress, SOD, GSH-PX, MDA, GPx-1, GPx-4 | [26] |
| Ivacaftor; Tezacaftor | IL-18, IL-1β, TNF, Caspase-1, IL-10, Anti-inflammatory | [27] |
| Floccularia luteovirens | SOD, GSH-Px, CAT, MDA, ROS, oxidative stress | [28] |
| Ishige okamurae; DPHC | ROS, elastase, MMPs, NF-kB, AP-1, MAPKs | [29] |
| Syzygium polyanthum (Wight) walp.; Bay leaf | CRP, MPO, anti-inflammatory | [30] |
| Empagliflozin | LDH, total leucocytic count, IL-6, TNF-α, TLR4, TGF-β1, oxidative stress, Nrf2/HO-1 | [31] |
| Isorhynchophylline | Antioxidant, TGF-β1, CTGF, 4-HNE, MDA, Nrf2, MAPK | [32] |
| Neoagarooligosaccharide | Nrf2, GSH, glutathione, ROS, inflammation, antioxidant | [33] |
| COMP-4; Muira puama | NO, antioxidative, apoptosis, HO-1, MPO, GSH/GSSG ratio | [34] |
| Quzhou fructus aurantii | Anti-inflammatory, MAPK, NF-kB, TNF, IL-6, IL-1β, IL-10 | [35] |
| Cerevisterol | MAPK, NF-kB, AP-1, Nrf2, HO-1, anti-inflammatory | [36] |
| Maslinic acid | HO-1, COX-2/PGE2, STAT-1, Nrf2, IL-1, iNOS, NF-kB | [37] |
| Scrophularia koraiensis nakai; Scrophulariaceae | Ig-E, anti-inflammatory, NF-kB, Nrf-2, HO-1 | [38] |
| Grateloupia lithophila | Blood glucose, TC, TGs, LDL, VLDL, HDL, SOD, GPx, MDA | [39] |
| Acetyl-l-carnitine | LDL, HDL, SOD, GSH-Px MDA, TNF-α, IL-1SS, iNOS, CRP | [40] |
| Methylseleninic acid | GPx, Nrf2, Socs3, p-JAK1, p-STAT3, NF-kB | [41] |
| Omentin-1 | Pro-inflammatory cytokines, NF-kB, Nrf2 | [42] |
| Dowijigi | NO, PGE2, TNF-α, IL-6, IL-1β, COX2, iNOS, NF-kB | [43] |
| AAL | NO, iNOS, TNF-α, IL-6, IL-1β, NF-kB | [44] |
| AXT and HupA | Oxidative stress, LDH, ROS, SOD, MDA | [45] |
| Continentalic acid (CNT) | GSH, GST, catalase, SOD, MDA, POD, MPO, NO, Nrf2, iNOS | [46] |
| 7-Methoxyflavanone (7MF) | IL-6, TNF-α, COX-2, iNOS, ICAM-1, MCP-1, TLR4, MyD88, p-JNK, p-ERK, Nrf2, NQO-1, Iba1 | [47] |
| Biseokeaniamide A | NO, iNOS, IL-1β, IkBα | [48] |
| Strigolactone GR24 | NF-kB, Nrf2, PPARγ, occludin | [49] |
| Timosaponin BII | MDA, GSH, PS, NLRP3, IL-1β, oxidative stress | [50] |
| Cycloastragenol (Y006) | BAX, COX2, GSK3β, TNF-α, IFN-γ, IL-17, IL-10, IL-4 | [51] |
| Ocellatin-K1(1-16); Ocellatin-K1(1-21) | Nitrite, MDA, SOD, GSH, ROS, NF-kB, oxidative stress | [52] |
| Leocarpinolide B (LB) | NO, PGE2, IL-6, TNF-α, MCP-1, COX-2, iNOS, NF-kB, ROS, HO-1, Nrf2 | [53] |
| Enteromorpha powder | GSH-Px, MDA, lipid peroxidation | [54] |
| Aminooxyacetic acid (AOAA) | ATP, IL-6, TNF-α, IL-10, NLRP3, caspase-1, IL-1β | [55] |
| Gastrodin | ROS, 8-OHDG, MDA, GSH-Px, SOD, Nrf2, HO-1, Bcl-2, Bax, caspase-3 | [56] |
| Cinnamtannin D1 (CTD-1) | IL-17, IL-6, IL-1β, TGF-β, IL-10, Th17, Treg, STAT5/Foxp3 | [57] |
| ent-Kaur-15-en-17-al-18-oic acid | ROS, MDA, GSH, SOD, NF-kB, bcl-2, p53, Bax, caspase-3 | [58] |
| Hederacoside-C (HDC) | IL-6, IL-1β, TNF-α, IL-10, TLR2, TLR4, MAPKs, NF-kB | [59] |
| PS-1145 dihydrochloride | IL-6, TNF-α, IL-1β, NF-kB, COX-2 | [60] |
| Cytokine-induced apoptosis inhibitor 1 (CIAPIN1) | ROS, MAPKs, NF-kB, Bax, caspase-3, COX-2, iNOS, IL-6, TNF-α | [61] |
| Ruscogenin | CRP, TNF-α, IL-6, IL-1β, ICAM-1, NF-kB, NOS-1 | [62] |
| Phascolosoma esculenta | IL-1β, TNF-α, IL-10, MDA, Nrf2, inflammation, oxidation | [63] |
| ALA/SFC | Anti-oxidation, RANKL, IL-6, Nrf2 | [64] |
| Xanthoplanine | Inflammatory cytokines, ROS, STAT5 | [65] |
| Lixisenatide | ROS, NADPH, NOX-1, TNF-α, IL-6, IL-1β, MMP -2 -9, TLR4, NF-kB | [66] |
| Germanium | MPO, TNF-α, IL-1β, IL-6, IL-10, NF-kB p65, p38, ERK, JNK | [67] |
| SDP | Permeability, ZO-1, E-cadherin, NFkB, Il-6, hydrogen peroxide, IL-10 | [68] |
| Amomum tsaoko | IL-6, VEGF, Nh-kB, P-STAT3 | [69] |
| Alkaline water | ROS, SOD-1, GSH, telomerase activity, telomeres length | [70] |
| Dihydrotestosterone | NO, PGE2, iNOS, COX-2, TNF-α, IL-1β, TLR4, NF-kB | [71] |
| Midazolam/Sufentanil | TNF-α, IL-1β, HMGB1, NF-kB, ROS, SOD, inflammatory | [72] |
| JNJ16259685 | Permeability, VASP, p-VASP, occludin, AQP | [73] |
Table 2.
Recently identified potential inflammatory bowel disease treatments.
| Treatment | Biomarkers changed in desired direction | Ref1 | Ref2 |
| Taraxasterol | ROS, MDA, Caspase-3, Bcl-2, Bax, Nrf2, HO-1, NQO-1, GPx-3 | [74] | [75] |
| Rhodiola rosea; Salidroside | IL-6, sIL-6R, IFN-gamma, IL-17A, IL-4, Th1 cells, Th17 cells, Treg cells, JAK1, JAK2, STAT3, RORgammat | [76-78] | [79] |
| VAS2870 | Nox2, ROS, Epithelium barrier integrity, Cell viability | [80] | [81] |
| Pinitol | Oxidative stress, ROS, TNF-alpha, IL-1beta, IL-6, NO, PGE2, iNOS, COX-2, IkappaBalpha, NF-kappaB, TREM2, Inflammation | [82] | [83] |
| TAK-242; Resatorvid | TLR4, Apoptosis, IL-1beta, Inflammation | [84] | [85] |
| Troxerutin | CK-mB, MDA, ROS, ATP | [86] | [87] |
| Vinpocetine | MAPK, NF-kB, MMP-9, AKT, ROS, Nrf2, HO-1, NQO-1, IL-1beta, TNF-alpha | [88] | [89] |
| Poria Cocos | ROS, MDA, SOD, LOX-1, Nrf2, HO-1, ERK, Oxidative stress | [90] | [91] |
| Carvacrol | Nrf2, ROS, MDA, SOD, Oxidative stress | [92] | [93] |
| Saururus Chinensis | NF-kB IL-6 IL-8 | [94] | [95] |
Table 1 contains the potential novel IBD treatments. The leftmost column contains the novel treatment and alternative name(s); the center column contains the biomarkers that were assessed in the reported study and that changed in the desired existing IBD treatment-derived directions; the rightmost column contains the reported study reference.
Table 2 shows examples of recent studies that would have been captured as novel treatments had the study/query been performed in 2016. These results reflect the predictive nature of the LRDI-based treatment repurposing methodology. The leftmost column contains the treatment and alternate name(s); the next column contains the biomarkers that were (1) assessed in the reported study; and that (2) changed in the desired treatment-derived direction; the final two columns reflect (1) the non-IBD application(s) of the treatment prior to 2016 (R1); and (2) the post-2016 evaluation of the potential treatment for IBD application (R2).
DISCUSSION OF RESULTS
The potential IBD treatments were derived from a wide swath of categories, including treatments for diabetes, cancer, and central nervous system disease, such as depression and dementia. Many of the compounds are derived from herbal remedies. Most are in the laboratory test phase, although many of these and their parent compounds have been in medical use for diseases other than the projected application represented by the paper cited. There is more emphasis on plant-based compounds and their derivatives than standard commercial synthetic drugs. This is to be expected, since the pharmaceutical companies who develop these drugs have substantial resources to devote towards searching for other applications for drugs under patent coverage with established manufacturing techniques.
Much of the research on plant-based treatments tends to be conducted by researchers indigenous to where these plants are most plentiful. They would not be expected to have the resources available to devote towards searching for the full spectrum of applications that the pharmaceutical companies have. Therefore, more targets of opportunity for treatment discovery exist in these non-mainline categories, as reflected in the results presented in this paper. It should be emphasized these drug/non-drug conclusions are based on results obtained using twenty selected biomarkers, out of a potential pool of hundreds of biomarkers. It is unknown whether the use of other available biomarkers for the discovery query would have generated novel treatments with a different drug/non-drug balance.
Approximately eighteen percent of the 350 records examined could be categorized as potential novel treatments for repurposing. Many of the records excluded from discovery consideration were done so on the basis of one or two prior recent records (published in 2017-2020) containing the same treatment applied to IBD (human or animal studies).
Thus, had this study been done four-five years ago, using the same query, those studies would have been identified as a potential novel treatment discovery. This is confirmation of the predictive power of the present technique for use as a repurposing approach.
CONCLUSION
Potential novel IBD treatments have been identified using a powerful text mining approach that identifies pattern changes of clinically relevant biomarkers being studied in non-IBD populations. Approximately 64 potential novel IBD treatments were identified in this proof-of-concept model, but many more were possible from an expanded study. Additionally, the predictive power of the approach for identifying future treatments that would be pursued through laboratory research was confirmed, showing the value of this approach for identifying and expanding relevant fields of research.
Footnotes
Conflict-of-interest statement: All authors have no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: May 5, 2020
First decision: May 15, 2020
Article in press: August 27, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gardlik R, Gazouli M, Sang LX S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
Contributor Information
Ronald Neil Kostoff, School of Public Policy, Georgia Institute of Technology, Gainesville, VA 20155, United States. ronald.kostoff@pubpolicy.gatech.edu.
Michael Brandon Briggs, Independent Consultant, Roscommon, MI 48653, United States.
Darla Roye Shores, The Hopkins Resource for Intestinal Vitality and Enhancement, the Johns Hopkins University School of Medicine, Baltimore, MD 21287, United States.
References
- 1.Ahluwalia B, Moraes L, Magnusson MK, Öhman L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. 2018;53:379–389. doi: 10.1080/00365521.2018.1447597. [DOI] [PubMed] [Google Scholar]
- 2.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 3.Sýkora J, PomahaÄová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24:2741–2763. doi: 10.3748/wjg.v24.i25.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DH, Cheon JH. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017;17:25–40. doi: 10.4110/in.2017.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogler G, Biedermann L, Scharl M. New insights into the pathophysiology of inflammatory bowel disease: microbiota, epigenetics and common signalling pathways. Swiss Med Wkly. 2018;148:w14599. doi: 10.4414/smw.2018.14599. [DOI] [PubMed] [Google Scholar]
- 7.Kostoff RN. Treatment Repurposing using Literature-related Discovery. J Scientometric Res. 2019;8:S74–S84. [Google Scholar]
- 8.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 9.Kostoff RN. Prevention and reversal of chronic disease: lessons learned. Georgia Institute of Technology. 2019. Available from: URL: http://hdl.handle.net/1853/62019. [Google Scholar]
- 10.Maehara T, Fujimori K. Contribution of FP receptors in M1 macrophage polarization via IL-10-regulated nuclear translocation of NF-κB p65. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158654. doi: 10.1016/j.bbalip.2020.158654. [DOI] [PubMed] [Google Scholar]
- 11.Ma Q, Huang W, Zhao J, Yang Z. Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-κB signaling pathway in vitro and in vivo. J Ethnopharmacol. 2020;252:112584. doi: 10.1016/j.jep.2020.112584. [DOI] [PubMed] [Google Scholar]
- 12.Xi Y, Huang X, Tan G, Chu X, Zhang R, Ma X, Ni B, You H. Protective effects of Erdosteine on interleukin-1β-stimulated inflammation via inhibiting the activation of MAPK, NF-κB, and Wnt/β-catenin signaling pathways in rat osteoarthritis. Eur J Pharmacol. 2020;873:172925. doi: 10.1016/j.ejphar.2020.172925. [DOI] [PubMed] [Google Scholar]
- 13.Tian F, Wang Z, He J, Zhang Z, Tan N. 4-Octyl itaconate protects against renal fibrosis via inhibiting TGF-β/Smad pathway, autophagy and reducing generation of reactive oxygen species. Eur J Pharmacol. 2020;873:172989. doi: 10.1016/j.ejphar.2020.172989. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarnia S, Teimuri-Mofrad R, Rashidi MR. Design, synthesis and biological evaluation of 2,3-dihydro-5,6-dimethoxy-1H-inden-1-one and piperazinium salt hybrid derivatives as hAChE and hBuChE enzyme inhibitors. Eur J Med Chem. 2020;191:112140. doi: 10.1016/j.ejmech.2020.112140. [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Tian LX, Tang X, Wang J, Tang WQ, Ma ZF, Chen T, Liang HP. Neutrophilic granule protein (NGP) attenuates lipopolysaccharide-induced inflammatory responses and enhances phagocytosis of bacteria by macrophages. Cytokine. 2020;128:155001. doi: 10.1016/j.cyto.2020.155001. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Xi Y, Zhang J, Tang J, Zhou X, Chen J, Nie C, Zhu Z, Ma B. Protective effect of Dioscorea zingiberensis ethanol extract on the disruption of blood-testes barrier in high-fat diet/streptozotocin-induced diabetic mice by upregulating ZO-1 and Nrf2. Andrologia. 2020;52:e13508. doi: 10.1111/and.13508. [DOI] [PubMed] [Google Scholar]
- 17.Xiao J, Yao R, Xu B, Wen H, Zhong J, Li D, Zhou Z, Xu J, Wang H. Inhibition of PDE4 Attenuates TNF-α-Triggered Cell Death Through Suppressing NF-κB and JNK Activation in HT-22 Neuronal Cells. Cell Mol Neurobiol. 2020;40:421–435. doi: 10.1007/s10571-019-00745-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey SN, Kwatra M, Dwivedi DK, Choubey P, Lahkar M, Jangra A. 7,8-Dihydroxyflavone alleviated the high-fat diet and alcohol-induced memory impairment: behavioral, biochemical and molecular evidence. Psychopharmacology (Berl) 2020;237:1827–1840. doi: 10.1007/s00213-020-05502-2. [DOI] [PubMed] [Google Scholar]
- 19.Shenoda BB, Ramanathan S, Gupta R, Tian Y, Jean-Toussaint R, Alexander GM, Addya S, Somarowthu S, Sacan A, Ajit SK. Xist attenuates acute inflammatory response by female cells. Cell Mol Life Sci. 2020 doi: 10.1007/s00018-020-03500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrotta P, Van der Veken B, Van Der Veken P, Pintelon I, Roosens L, Adriaenssens E, Timmerman V, Guns PJ, De Meyer GRY, Martinet W. Partial Inhibition of Glycolysis Reduces Atherogenesis Independent of Intraplaque Neovascularization in Mice. Arterioscler Thromb Vasc Biol. 2020;40:1168–1181. doi: 10.1161/ATVBAHA.119.313692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An Q, Li C, Chen Y, Yang Y, Song R, Zhou L, Li J, Tong A, Luo Y. Scaffold hopping of agomelatine leads to enhanced antidepressant effects by modulation of gut microbiota and host immune responses. Pharmacol Biochem Behav. 2020;192:172910. doi: 10.1016/j.pbb.2020.172910. [DOI] [PubMed] [Google Scholar]
- 22.Figueiredo AA, Sales TMAL, Nicolau LAD, Nunes AAA, Costa-Filho HB, Moreira RLR, Nascimento RR, Sousa MKA, Silva LD, Carmo-Neto JP, Sidou FMNO, Paula SM, Medeiros JVR, Silva DA, Sifrim D, Souza MHLP. Laryngeal Mucosa Alterations in Mice Model of Gastroesophageal Reflux: Effects of Topical Protection. Laryngoscope. 2020 doi: 10.1002/lary.28597. [DOI] [PubMed] [Google Scholar]
- 23.Dong C, Wu G, Li H, Qiao Y, Gao S. Ampelopsin inhibits high glucose-induced extracellular matrix accumulation and oxidative stress in mesangial cells through activating the Nrf2/HO-1 pathway. Phytother Res. 2020 doi: 10.1002/ptr.6668. [DOI] [PubMed] [Google Scholar]
- 24.Shao Q, Xue S, Jiang Y, Lu H, Sang W, Wang C, Xue B, Liu Y, Zhu L, Ma J. Esculentoside A protects against osteoarthritis by ameliorating inflammation and repressing osteoclastogenesis. Int Immunopharmacol. 2020;82:106376. doi: 10.1016/j.intimp.2020.106376. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Zhang X, Chen R, Li Y, Miao J, Liu G, Lan Y, Chen Y, Cao Y. HPLC fingerprint analysis of Phyllanthus emblica ethanol extract and their antioxidant and anti-inflammatory properties. J Ethnopharmacol. 2020;254:112740. doi: 10.1016/j.jep.2020.112740. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Chen Q, Zhuang S, Wen Y, Cheng W, Zeng Z, Jiang T, Tang C. Effect of Anoectochilus roxburghii flavonoids extract on H2O2 - Induced oxidative stress in LO2 cells and D-gal induced aging mice model. J Ethnopharmacol. 2020;254:112670. doi: 10.1016/j.jep.2020.112670. [DOI] [PubMed] [Google Scholar]
- 27.Jarosz-Griffiths HH, Scambler T, Wong CH, Lara-Reyna S, Holbrook J, Martinon F, Savic S, Whitaker P, Etherington C, Spoletini G, Clifton I, Mehta A, McDermott MF, Peckham D. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. Elife. 2020;9 doi: 10.7554/eLife.54556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Jiao Y, Lu H, Shu X, Chen Q. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr Polym. 2020;229:115432. doi: 10.1016/j.carbpol.2019.115432. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Kim HS, Oh JY, Je JG, Jeon YJ, Ryu B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem Toxicol. 2020;136:110963. doi: 10.1016/j.fct.2019.110963. [DOI] [PubMed] [Google Scholar]
- 30.Hasan R, Lindarto D, Siregar GA, Mukhtar Z. The effect of bay leaf extract Syzygium polyanthum (Wight) Walp. on C-reactive protein (CRP) and myeloperoxidase (MPO) level in the heart of rat model of myocardial infarction. Med Glas (Zenica) 2020;17 doi: 10.17392/1068-20. [DOI] [PubMed] [Google Scholar]
- 31.Kabel AM, Estfanous RS, Alrobaian MM. Targeting oxidative stress, proinflammatory cytokines, apoptosis and toll like receptor 4 by empagliflozin to ameliorate bleomycin-induced lung fibrosis. Respir Physiol Neurobiol. 2020;273:103316. doi: 10.1016/j.resp.2019.103316. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Cui Y, Dai S, Deng W, Wang H, Qin W, Yang H, Liu H, Yue J, Wu D, Wang J, Guo H. Isorhynchophylline enhances Nrf2 and inhibits MAPK pathway in cardiac hypertrophy. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:203–212. doi: 10.1007/s00210-019-01716-0. [DOI] [PubMed] [Google Scholar]
- 33.Yang JH, Na CS, Cho SS, Kim KM, Lee JH, Chen XQ, Ku SK, Cho IJ, Kim EJ, Lee JH, Ki SH. Hepatoprotective Effect of Neoagarooligosaccharide via Activation of Nrf2 and Enhanced Antioxidant Efficacy. Biol Pharm Bull. 2020;43:619–628. doi: 10.1248/bpb.b19-00697. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen S, Castellanos KA, Abraham A, Ferrini MG. Reduction of oxidative stress markers in the corpora cavernosa and media of penile dorsal artery in middle-aged rats treated with COMP-4. Int J Impot Res. 2020 doi: 10.1038/s41443-020-0233-9. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Chen J, Lin L, Pan G, Zhang S, Chen H, Zhang M, Xuan Y, Wang Y, You Z. Quzhou Fructus Aurantii Extract suppresses inflammation via regulation of MAPK, NF-κB, and AMPK signaling pathway. Sci Rep. 2020;10:1593. doi: 10.1038/s41598-020-58566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam MB, Chowdhury NS, Sohrab MH, Rana MS, Hasan CM, Lee SH. Cerevisterol Alleviates Inflammation via Suppression of MAPK/NF-κB/AP-1 and Activation of the Nrf2/HO-1 Signaling Cascade. Biomolecules. 2020;10 doi: 10.3390/biom10020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee W, Kim J, Park EK, Bae JS. Maslinic Acid Ameliorates Inflammation via the Downregulation of NF-κB and STAT-1. Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung TY, Lee AY, Song JH, Lee MY, Lim JO, Lee SJ, Ko JW, Shin NR, Kim JC, Shin IS, Kim JS. Scrophularia koraiensis Nakai Attenuates Allergic Airway Inflammation via Suppression of NF-κB and Enhancement of Nrf2/HO-1 Signaling. Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seedevi P, Ramu Ganesan A, Moovendhan M, Mohan K, Sivasankar P, Loganathan S, Vairamani S, Shanmugam A. Anti-diabetic activity of crude polysaccharide and rhamnose-enriched polysaccharide from G. lithophila on Streptozotocin (STZ)-induced in Wistar rats. Sci Rep. 2020;10:556. doi: 10.1038/s41598-020-57486-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Xu J, Zheng J, Zhang X, Shao J, Zhao L, Hao J. Anti-Inflammatory and Antioxidant Effects of Acetyl-L-Carnitine on Atherosclerotic Rats. Med Sci Monit. 2020;26:e920250. doi: 10.12659/MSM.920250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gawish RA, Fahmy HA, Abd El Fattah AI, Nada AS. The potential effect of methylseleninic acid (MSA) against γ-irradiation induced testicular damage in rats: Impact on JAK/STAT pathway. Arch Biochem Biophys. 2020;679:108205. doi: 10.1016/j.abb.2019.108205. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Gao Y, Lin F, Han K, Wang X. Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch Biochem Biophys. 2020;679:108187. doi: 10.1016/j.abb.2019.108187. [DOI] [PubMed] [Google Scholar]
- 43.Kim SM, Vetrivel P, Kim HH, Ha SE, Saralamma VVG, Kim GS. Artemisia iwayomogi (Dowijigi) inhibits lipopolysaccharide-induced inflammation in RAW264.7 macrophages by suppressing the NF-κB signaling pathway. Exp Ther Med. 2020;19:2161–2170. doi: 10.3892/etm.2020.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HJ, Joe HI, Zhang Z, Woo Lee S, Lee KY, Kook YB, An HJ. Anti-inflammatory effect of Acalypha australis L. via suppression of NF-κB signaling in LPS-stimulated RAW 264.7 macrophages and LPS-induced septic mice. Mol Immunol. 2020;119:123–131. doi: 10.1016/j.molimm.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Wei HM, Hu GY, Zhao J, Long LN, Li CJ, Zhao ZJ, Zeng HK, Nie H. Combining antioxidant astaxantin and cholinesterase inhibitor huperzine A boosts neuroprotection. Mol Med Rep. 2020;21:1043–1050. doi: 10.3892/mmr.2020.10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan AM, Khan AU, Ali H, Islam SU, Seo EK, Khan S. Continentalic acid exhibited nephroprotective activity against the LPS and E. coli-induced kidney injury through inhibition of the oxidative stress and inflammation. Int Immunopharmacol. 2020;80:106209. doi: 10.1016/j.intimp.2020.106209. [DOI] [PubMed] [Google Scholar]
- 47.Qu Z, Chen Y, Luo ZH, Shen XL, Hu YJ. 7-methoxyflavanone alleviates neuroinflammation in lipopolysaccharide-stimulated microglial cells by inhibiting TLR4/MyD88/MAPK signalling and activating the Nrf2/NQO-1 pathway. J Pharm Pharmacol. 2020;72:385–395. doi: 10.1111/jphp.13219. [DOI] [PubMed] [Google Scholar]
- 48.Ohno O, Terasaki T, Sano T, Hitomi Y, Miyamoto J, Matsuno K. Inhibitory effects of biseokeaniamide A against lipopolysaccharide-induced signal transduction. Bioorg Med Chem Lett. 2020;30:127069. doi: 10.1016/j.bmcl.2020.127069. [DOI] [PubMed] [Google Scholar]
- 49.Kurt B, Ozleyen A, Antika G, Yilmaz YB, Tumer TB. Multitarget Profiling of a Strigolactone Analogue for Early Events of Alzheimer's Disease: In Vitro Therapeutic Activities against Neuroinflammation. ACS Chem Neurosci. 2020;11:501–507. doi: 10.1021/acschemneuro.9b00694. [DOI] [PubMed] [Google Scholar]
- 50.Shi K, Zhu J, Chen D, Ren C, Guo M, Wang J, Wu X, Feng Y. Lipidomics Analysis of Timosaponin BII in INS-1 Cells Induced by Glycolipid Toxicity and Its Relationship with Inflammation. Chem Biodivers. 2020;17:e1900684. doi: 10.1002/cbdv.201900684. [DOI] [PubMed] [Google Scholar]
- 51.Ren YS, Li HH, Yao JC, Tan YJ, Pan LH, Peng T, Zhao LL, Zhang GM, Yue J, Hu XM, Liu Z, Li J. Application quantitative proteomics approach to identify differentially expressed proteins associated with cardiac protection mediated by cycloastragenol in acute myocardial infarction rats. J Proteomics. 2020;222:103691. doi: 10.1016/j.jprot.2020.103691. [DOI] [PubMed] [Google Scholar]
- 52.Sousa NA, Oliveira GAL, de Oliveira AP, Lopes ALF, Iles B, Nogueira KM, Araújo TSL, Souza LKM, Araújo AR, Ramos-Jesus J, Plácido A, Amaral C, Campelo YDM, Barbosa EA, Portugal CC, Socodato R, Lobo A, Relvas J, Bemquerer M, Eaton P, Leite JRSA, Medeiros JVR. Novel Ocellatin Peptides Mitigate LPS-induced ROS Formation and NF-kB Activation in Microglia and Hippocampal Neurons. Sci Rep. 2020;10:2696. doi: 10.1038/s41598-020-59665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linghu KG, Ma QS, Zhao GD, Xiong W, Lin L, Zhang QW, Bian Z, Wang Y, Yu H. Leocarpinolide B attenuates LPS-induced inflammation on RAW264.7 macrophages by mediating NF-κB and Nrf2 pathways. Eur J Pharmacol. 2020;868:172854. doi: 10.1016/j.ejphar.2019.172854. [DOI] [PubMed] [Google Scholar]
- 54.Ma WQ, Cheng HZ, Zhao DH, Yang J, Wang SB, Wu HZ, Lu MY, Xu L, Liu GJ. Effects of dietary Enteromorpha powder supplementation on productive performance, egg quality, and antioxidant performance during the late laying period in Zi geese. Poult Sci. 2020;99:1062–1068. doi: 10.1016/j.psj.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao P, Zhou W, Zhang Y, Li J, Zhao Y, Pan L, Shen Z, Chen W, Hui J. Aminooxyacetic acid attenuates post-infarct cardiac dysfunction by balancing macrophage polarization through modulating macrophage metabolism in mice. J Cell Mol Med. 2020;24:2593–2609. doi: 10.1111/jcmm.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu XC, Wu CZ, Hu XF, Wang TL, Jin XP, Ke SF, Wang E, Wu G. Gastrodin Attenuates Neuronal Apoptosis and Neurological Deficits after Experimental Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis. 2020;29:104483. doi: 10.1016/j.jstrokecerebrovasdis.2019.104483. [DOI] [PubMed] [Google Scholar]
- 57.Shi C, Zhang H, Wang X, Jin B, Jia Q, Li Y, Yang Y. Cinnamtannin D1 attenuates autoimmune arthritis by regulating the balance of Th17 and treg cells through inhibition of aryl hydrocarbon receptor expression. Pharmacol Res. 2020;151:104513. doi: 10.1016/j.phrs.2019.104513. [DOI] [PubMed] [Google Scholar]
- 58.Zhang C, Zhao X, Lin S, Liu F, Ma J, Han Z, Jia F, Xie W, Zhang Q, Li X. Neuroprotective Effect of ent-Kaur-15-en-17-al-18-oic Acid on Amyloid Beta Peptide-Induced Oxidative Apoptosis in Alzheimer's Disease. Molecules. 2019;25 doi: 10.3390/molecules25010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akhtar M, Shaukat A, Zahoor A, Chen Y, Wang Y, Yang M, Umar T, Guo M, Deng G. Hederacoside-C Inhibition of Staphylococcus aureus-Induced Mastitis via TLR2 & TLR4 and Their Downstream Signaling NF-κB and MAPKs Pathways In Vivo and In Vitro. Inflammation. 2020;43:579–594. doi: 10.1007/s10753-019-01139-2. [DOI] [PubMed] [Google Scholar]
- 60.Cernackova A, Mikova L, Horvathova L, Tillinger A, Mravec B. Cachexia induced by Yoshida ascites hepatoma in Wistar rats is not associated with inflammatory response in the spleen or brain. J Neuroimmunol. 2019;337:577068. doi: 10.1016/j.jneuroim.2019.577068. [DOI] [PubMed] [Google Scholar]
- 61.Yeo HJ, Shin MJ, You JH, Kim JS, Kim MY, Kim DW, Kim DS, Eum WS, Choi SY. Transduced Tat-CIAPIN1 reduces the inflammatory response on LPS- and TPA-induced damages. BMB Rep. 2019;52:695–699. doi: 10.5483/BMBRep.2019.52.12.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ercan G, Ilbar Tartar R, Solmaz A, Gulcicek OB, Karagulle OO, Meric S, Cayoren H, Kusaslan R, Kemik A, Gokceoglu Kayali D, Cetinel S, Celik A. Examination of protective and therapeutic effects of ruscogenin on cerulein-induced experimental acute pancreatitis in rats. Ann Surg Treat Res. 2019;97:271–281. doi: 10.4174/astr.2019.97.6.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Z, Pan Y, Chen J, Zhang H, Wei H, Wu Z, Liu L. Anti-inflammatory, anti-oxidative stress effect of Phascolosoma esculenta oligosaccharides on Escherichia coli-induced sepsis mice. Food Sci Biotechnol. 2019;28:1871–1879. doi: 10.1007/s10068-019-00620-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narimiya T, Kanzaki H, Yamaguchi Y, Wada S, Katsumata Y, Tanaka K, Tomonari H. Nrf2 activation in osteoblasts suppresses osteoclastogenesis via inhibiting IL-6 expression. Bone Rep. 2019;11:100228. doi: 10.1016/j.bonr.2019.100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi X, Pan S, Li Y, Ma W, Wang H, Xu C, Li L. Xanthoplanine attenuates macrophage polarization towards M1 and inflammation response via disruption of CrkL-STAT5 complex. Arch Biochem Biophys. 2020;683:108325. doi: 10.1016/j.abb.2020.108325. [DOI] [PubMed] [Google Scholar]
- 66.Li P, Liu Q, Zhang T, Guo W, Qiao W, Deng M. Protective Effects of Lixisenatide against Lipopolysaccharide-Induced Inflammation Response in MAC-T Bovine Mammary Epithelial Cells: A Therapeutic Implication in Mastitis. Chem Res Toxicol. 2020;33:982–987. doi: 10.1021/acs.chemrestox.9b00524. [DOI] [PubMed] [Google Scholar]
- 67.Wang YS, Teng GQ, Zhou H, Dong CL. Germanium Reduces Inflammatory Damage in Mammary Glands During Lipopolysaccharide-Induced Mastitis in Mice. Biol Trace Elem Res. 2020 doi: 10.1007/s12011-020-02106-x. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Just A, Miró L, Pérez-Bosque A, Amat C, Polo J, Pallàs M, Griñán-Ferré C, Moretó M. Dietary Spray-Dried Porcine Plasma Prevents Cognitive Decline in Senescent Mice and Reduces Neuroinflammation and Oxidative Stress. J Nutr. 2020;150:303–311. doi: 10.1093/jn/nxz239. [DOI] [PubMed] [Google Scholar]
- 69.Chen C, You F, Wu F, Luo Y, Zheng G, Xu H, Liu Y. Antiangiogenesis Efficacy of Ethanol Extract from Amomum tsaoko in Ovarian Cancer through Inducing ER Stress to Suppress p-STAT3/NF-kB/IL-6 and VEGF Loop. Evid Based Complement Alternat Med. 2020;2020:2390125. doi: 10.1155/2020/2390125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Logozzi M, Mizzoni D, Di Raimo R, Andreotti M, Macchia D, Spada M, Fais S. In vivo antiaging effects of alkaline water supplementation. J Enzyme Inhib Med Chem. 2020;35:657–664. doi: 10.1080/14756366.2020.1733547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang L, Tong Y, Chen PF, Miao S, Zhou RY. Neuroprotection of dihydrotestosterone via suppression of the toll-like receptor 4/nuclear factor-kappa B signaling pathway in high glucose-induced BV-2 microglia inflammatory responses. Neuroreport. 2020;31:139–147. doi: 10.1097/WNR.0000000000001385. [DOI] [PubMed] [Google Scholar]
- 72.Zhou H, Zhu ZH, Liu Y, Liu YY. Effects of midazolam combined with sufentanil on injury and expression of HMGB1 and NF-κB in rats with pancreatitis. Eur Rev Med Pharmacol Sci. 2020;24:2102–2109. doi: 10.26355/eurrev_202002_20390. [DOI] [PubMed] [Google Scholar]
- 73.Zhang C, Jiang M, Wang WQ, Zhao SJ, Yin YX, Mi QJ, Yang MF, Song YQ, Sun BL, Zhang ZY. Selective mGluR1 Negative Allosteric Modulator Reduces Blood-Brain Barrier Permeability and Cerebral Edema After Experimental Subarachnoid Hemorrhage. Transl Stroke Res. 2020;11:799–811. doi: 10.1007/s12975-019-00758-z. [DOI] [PubMed] [Google Scholar]
- 74.Che L, Li Y, Song R, Qin C, Hao W, Wang B, Yang L, Peng P, Xu F. Anti-inflammatory and anti-apoptosis activity of taraxasterol in ulcerative colitis in vitro and in vivo. Exp Ther Med. 2019;18:1745–1751. doi: 10.3892/etm.2019.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piao T, Ma Z, Li X, Liu J. Taraxasterol inhibits IL-1β-induced inflammatory response in human osteoarthritic chondrocytes. Eur J Pharmacol. 2015;756:38–42. doi: 10.1016/j.ejphar.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Cai J, Fan P, Zhang N, Cao Y. The Abilities of Salidroside on Ameliorating Inflammation, Skewing the Imbalanced Nucleotide Oligomerization Domain-Like Receptor Family Pyrin Domain Containing 3/Autophagy, and Maintaining Intestinal Barrier Are Profitable in Colitis. Front Pharmacol. 2019;10:1385. doi: 10.3389/fphar.2019.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H, Shen L, Lv T, Wang R, Zhang N, Peng H, Diao W. Salidroside attenuates dextran sulfate sodium-induced colitis in mice via SIRT1/FoxOs signaling pathway. Eur J Pharmacol. 2019;861:172591. doi: 10.1016/j.ejphar.2019.172591. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Pan Y, Cao Y, Zhou W, Lu J. Salidroside regulates the expressions of IL-6 and defensins in LPS-activated intestinal epithelial cells through NF-κB/MAPK and STAT3 pathways. Iran J Basic Med Sci. 2019;22:31–37. doi: 10.22038/ijbms.2018.26994.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim SH, Hyun SH, Choung SY. Antioxidative effects of Cinnamomi cassiae and Rhodiola rosea extracts in liver of diabetic mice. Biofactors. 2006;26:209–219. doi: 10.1002/biof.5520260306. [DOI] [PubMed] [Google Scholar]
- 80.Yu T, Wan P, Zhu XD, Ren YP, Wang C, Yan RW, Guo Y, Bai AP. Inhibition of NADPH oxidase activities ameliorates DSS-induced colitis. Biochem Pharmacol. 2018;158:126–133. doi: 10.1016/j.bcp.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Frasier CR, Moukdar F, Patel HD, Sloan RC, Stewart LM, Alleman RJ, La Favor JD, Brown DA. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc Res. 2013;98:47–55. doi: 10.1093/cvr/cvt009. [DOI] [PubMed] [Google Scholar]
- 82.Lin Y, Zheng X, Chen J, Luo D, Xie J, Su Z, Huang X, Yi X, Wei L, Cai J, Sun Z. Protective Effect of Bruguiera gymnorrhiza (L.) Lam. Fruit on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice: Role of Keap1/Nrf2 Pathway and Gut Microbiota. Front Pharmacol. 2019;10:1602. doi: 10.3389/fphar.2019.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y, Park CM, Cho CW, Song YS. Protective effect of pinitol against D-galactosamine-induced hepatotoxicity in rats fed on a high-fat diet. Biosci Biotechnol Biochem. 2008;72:1657–1666. doi: 10.1271/bbb.70473. [DOI] [PubMed] [Google Scholar]
- 84.Gao XJ, Li T, Wei B, Yan ZX, Yan R. [Regulatory mechanisms of gut microbiota on intestinal CYP3A and P-glycoprotein in rats with dextran sulfate sodium-induced colitis] Yao Xue Xue Bao. 2017;52:34–43. [PubMed] [Google Scholar]
- 85.Rice TW, Wheeler AP, Bernard GR, Vincent JL, Angus DC, Aikawa N, Demeyer I, Sainati S, Amlot N, Cao C, Ii M, Matsuda H, Mouri K, Cohen J. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 86.Corsale I, Carrieri P, Martellucci J, Piccolomini A, Verre L, Rigutini M, Panicucci S. Flavonoid mixture (diosmin, troxerutin, rutin, hesperidin, quercetin) in the treatment of I-III degree hemorroidal disease: a double-blind multicenter prospective comparative study. Int J Colorectal Dis. 2018;33:1595–1600. doi: 10.1007/s00384-018-3102-y. [DOI] [PubMed] [Google Scholar]
- 87.Fan SH, Zhang ZF, Zheng YL, Lu J, Wu DM, Shan Q, Hu B, Wang YY. Troxerutin protects the mouse kidney from d-galactose-caused injury through anti-inflammation and anti-oxidation. Int Immunopharmacol. 2009;9:91–96. doi: 10.1016/j.intimp.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 88.Colombo BB, Fattori V, Guazelli CFS, Zaninelli TH, Carvalho TT, Ferraz CR, Bussmann AJC, Ruiz-Miyazawa KW, Baracat MM, Casagrande R, Verri WA., Jr Vinpocetine Ameliorates Acetic Acid-Induced Colitis by Inhibiting NF-κB Activation in Mice. Inflammation. 2018;41:1276–1289. doi: 10.1007/s10753-018-0776-9. [DOI] [PubMed] [Google Scholar]
- 89.Imamoto T, Tanabe M, Shimamoto N, Kawazoe K, Hirata M. Cerebral circulatory and cardiac effects of vinpocetine and its metabolite, apovincaminic acid, in anesthetized dogs. Arzneimittelforschung. 1984;34:161–169. [PubMed] [Google Scholar]
- 90.Liu X, Yu X, Xu X, Zhang X, Zhang X. The protective effects of Poria cocos-derived polysaccharide CMP33 against IBD in mice and its molecular mechanism. Food Funct. 2018;9:5936–5949. doi: 10.1039/c8fo01604f. [DOI] [PubMed] [Google Scholar]
- 91.Wang Q, Chen S, Han L, Lian M, Wen Z, Jiayinaguli T, Liu L, Sun R, Cao Y. Antioxidant activity of carboxymethyl (1→3)-β-d-glucan (from the sclerotium of Poria cocos) sulfate (in vitro) Int J Biol Macromol. 2014;69:229–235. doi: 10.1016/j.ijbiomac.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 92.Nowotarska SW, Nowotarski K, Grant IR, Elliott CT, Friedman M, Situ C. Mechanisms of Antimicrobial Action of Cinnamon and Oregano Oils, Cinnamaldehyde, Carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map) Foods. 2017;6 doi: 10.3390/foods6090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jayakumar S, Madankumar A, Asokkumar S, Raghunandhakumar S, Gokula dhas K, Kamaraj S, Divya MG, Devaki T. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem. 2012;360:51–60. doi: 10.1007/s11010-011-1043-7. [DOI] [PubMed] [Google Scholar]
- 94.Xiao J, Wang J, Chen Y, Zhou Z, Gao C, Guo Z. Sauchinone ameliorates intestinal inflammation and promotes Th17 cell production of IL-10 via Blimp-1. Biochem Biophys Res Commun. 2020;522:435–441. doi: 10.1016/j.bbrc.2019.11.122. [DOI] [PubMed] [Google Scholar]
- 95.Lee WS, Baek YI, Kim JR, Cho KH, Sok DE, Jeong TS. Antioxidant activities of a new lignan and a neolignan from Saururus chinensis. Bioorg Med Chem Lett. 2004;14:5623–5628. doi: 10.1016/j.bmcl.2004.08.054. [DOI] [PubMed] [Google Scholar]