Abstract
The cranial ossification sequence in Pleurodeles waltl is widely used in phylogenetic analyses of amphibian origin and evolution. However, the patterns published to date are far from completely resolved and contain certain discrepancies. Based on a large sample of P. waltl specimens ranging from early post‐hatching larvae to post‐metamorphic newts, we determined the most common cranial ossification sequence and revealed its intraspecific variations. Since thyroid hormones (THs) are involved in the mediation of skull development in salamanders, we studied the role of THs in the cranial development of P. waltl. The normal sequence and timing of bone appearance were compared with those in larvae reared under conditions of high (in 1 and 2 ng mL‐1 triiodothyronine) and low [in 0.02% thiourea (TU), which inhibits thyroid gland activity] TH levels. Metamorphosis was greatly accelerated in the TH‐treated larvae and was arrested in the TU‐treated larvae, which retained the larval pattern of the palate and rudimentary external gills even after 2 years of the experiment. Early‐appearing bones (the coronoid, vomer, palatine, dentary, squamosal, premaxilla, parasphenoid, pterygoid, prearticular, vomer, frontal, parietal, exoccipital, in this order) arise at the same stages and ages, and follow the same ossification sequence under different TH levels. The timing of the appearance of bones normally arising in the late larval and metamorphic periods (the quadratojugal, orbitosphenoid, prootic, maxilla, nasal, os thyroideum, prefrontal, quadrate, in this order) changes depending on the TH level. The maxilla and nasal display the most pronounced reaction to changes in the TH level: they appear precociously in TH‐treated animals, while their appearance is postponed and they remain rudimentary in TU‐treated animals. Because of different responses to THs, the order in which late‐arising bones appear changes depending on the TH level. Although bones appearing early in larval ontogeny (e.g. the premaxilla, vomer, squamosal, palatine) display no TH‐induced reaction when they start to develop, their further differentiation shows dependence on THs, and these bones become TH‐inducible closer to metamorphosis. These findings indicate that TH involvement in the mediation of cranial development changes from minimal (if at all) in its early stages to maximal during metamorphosis. It is likely that the appearance of bones early in development is mediated by factors other than THs. Their further development is accompanied by changes in the mechanisms mediating their morphological differentiation. That is, likely non‐hormonal mediation becomes replaced or/and complemented by hormonal mediation. The constituent parts of the same bone may exhibit differences in their reactions to changes in TH levels. Although in normal development, the overall cranial ossification sequence is constant, there was variation in the order in which late‐appearing bones was recorded. These observations suggest that this variation results from individual variability in the internal TH level. Comparison with other salamanders suggests that (a) the pattern of TH mediation described in P. waltl is common for cranial development of metamorphosing urodeles and (b) the same bone may differ in its TH dependence in different salamanders, e.g. there are interspecific variations in the degree of TH dependence of individual cranial bones.
Keywords: hormonal mediation, ontogeny, ossification sequence, Pleurodeles waltl, skeletogenesis, variability
Skull development and the role of thyroid hormone (TH) in its mediation were studied in Pleurodeles waltl (Urodela; Salamandridae). TH mediation, its changes within ontogeny, and its interplay with tissue inductive interactions are discussed and compared with those in other salamanders. Intraspecific variations in the sequence of cranial bones appearance revealed in P. waltl are proposed to be correlated with individual variations in the internal TH function.
1. INTRODUCTION
The Iberian newt, Pleurodeles waltl Michahelles, 1830 (Urodela, Salamandridae), an endemic species to the Iberian Peninsula and Morocco, is easily bred in the laboratory and can provide hundreds of eggs in one spawning. Thanks to these features, this salamander became a prime model for embryology and developmental and experimental biology. Now, this salamander is a model system in space biology (Gualandris‐Parisot et al. 2001; Frippiat, 2013, among others) and serves as a unique experimental model for regenerative biology (Elewa et al. 2017). For a long time, in the developmental and experimental investigations of P. waltl, the skull was studied as a model organ system. The development of chondrocranium was studied by Eyal‐Giladi and Zinberg (1964). The development of individual cranial cartilaginous elements was described by Regel (1964) and Lebedkina (1979, 2004). Corsin (1966b) in a narrative form and Lebedkina (1979, 2004) in detail described the normal cranial development of this species and provided appearance sequences of cranial bones. Information on the contribution of the neural crest to the development of the craniofacial skeleton was presented by Chibon (1964, 1966). The results of Corsin’s (1966a) experiments suggested the occurrence of inductive interrelations between the cranial cartilages and dermal bones. Medvedeva’s (1975, 1986) experiments revealed inductive interactions between the nasolacrimal duct and the prefrontal bone in the P. waltl skull. Cassin and Capuron (1977, 1979) revealed an epigenetic cascade of inductive interactions involved in the integration of cartilage and bone during P. waltl cranial development. As a result of this intense research, the skull of P. waltl (i.e. its morphology and ontogeny, and the regulatory mechanisms of its development) seems to be more highly studied than the skulls of other metamorphosing salamanders.
Regarding cranial ontogeny, however, the descriptions provided by Corsin and Lebedkina contain obvious discrepancies. The bone appearance sequences described by these authors differ in many aspects. Additionally, in both studies, the sequences are not completely resolved, as many bones are indicated to appear simultaneously, and some bones are omitted. In addition, both studies lack information about possible intraspecific variation in the sequence of bone appearance.
In recent years, sequences of bone appearance have received much attention in light of increasing interest in developmental sequences as a whole. The latter are regarded as (a) providing information on sequence heterochronies, which are considered a major mechanism of evolutionary changes (Smith, 2001; Maxwell and Harrison, 2009; McNamara, 2012; Keyte and Smith, 2014), and (b) containing a phylogenetic signal and being useful in phylogenetic reconstructions (Strauss, 1990; Jeffery et al. 2002; Maxwell and Harrison, 2009). In this regard, sequences of bone appearance have been analysed in different vertebrates, e.g. teleosts (Strauss, 1990; Mabee and Trendler, 1996), birds (Maxwell et al. 2010; Mitgutsch et al. 2011), and mammals (Goswami, 2007; Weisbecker et al. 2008).
These sequences are most often used, however, in studies concerning amphibians. Thus, they have been used, for example, as a possible source of information for understanding the origins of extant amphibians (Laurin et al. 2019, and references therein) and urodeles (Schoch and Carroll, 2003). Additionally, they have been used to reconstruct the evolution of the amphibian ontogeny (Schoch, 2002, 2006; Germain and Laurin, 2009). Data on ossification sequences are used to evaluate heterochronies (Yeh, 2002; Germain and Laurin, 2009; Weisbecker and Mitgutsch, 2010; Harrington et al. 2013, among others), which have been suggested to play a critical role in amphibian evolution (Gould, 1977; Raff and Wray, 1989; Reiss, 2002, among others).
Methods of such analyses are continuously improving (see Germain and Laurin, 2009), but for any method, the results of the analysis depend on the completeness of the ossification sequence (Harrington et al. 2013), which in turn depends on the sample size (Cubbage & Mabee 1996). Additionally, the results of these analyses can be affected by intraspecific variation of the ossification sequence (Mabee et al. 2000; Sheil et al. 2014).
Published bone appearance sequences of P. waltl (mainly that provided by Corsin and based on the examination of specimens from only five stages) are often considered typical for salamandrids and are used in current research on amphibian evolutionary biology (see Harrington et al. 2013; Laurin et al. 2019, among others). However, as these sequences are far from being completely resolved and lack any mention of possible individual variations, they should be used with caution.
Whereas the role of inductive tissue interactions in the mediation of skull development in P. waltl is quite well investigated, the involvement of hormonal factors remains poorly studied. Paradoxically, little is known about the role of thyroid hormones (THs) in the mediation of skull development in this newt. In the only study on this subject, artificially induced hypothyroidism was shown to retard and partially arrest metamorphic cranial remodelling, suggesting mediation by THs (Smirnov and Vasileva, 2001). This lack of attention is surprising given what is known about the TH mediation of the skull development of salamanders from different urodele families (Rose, 1995b; Smirnov and Vassilieva, 2003; Smirnov and Vassilieva, 2005; Smirnov et al. 2011). THs have been shown to play a major role in the regulation of skull development, especially metamorphic remodelling. The appearance of different bones and the sequence of their ossification have been revealed to depend on TH levels. It has been proposed that variations in thyroid activity account for urodele cranial diversification (Rose, 1996).
In the present study, we describe the pattern and timing of ossification of the skulls of P. waltl specimens ranging from newly hatched larvae to post‐metamorphic individuals. Based on the examination of an extensive developmental series, we provide a more complete and much more resolved sequence of cranial bone appearance than those described earlier. Additionally, intraspecific ossification variation, which has so far not been studied in P. waltl, is evaluated.
To evaluate THs involvement in the skull development of P. waltl, the normal timing and sequence of bone appearance were compared with those displayed by larvae reared in hypo‐ and hyperthyroid conditions. Then, data on TH mediation of skull development in P. waltl were compared with those for other urodeles studied in this regard.
By comparing the results of our experiment with those obtained by experimental embryologists and considering the tissue interactions thought to participate in the mediation of bone appearance, we analysed the interplay of non‐hormonal and hormonal factors in the regulation of P. waltl skull development. Additionally, a particular effort was made to try to correlate individual variations in the sequence of bone appearance with possible individual variations in thyroid activity.
2. MATERIALS AND METHODS
In the present study, we examined the timing and sequence of cranial bone appearance in P. waltl larvae reared under different TH levels: normal, high and low. The larvae used in this study were obtained from egg clutches derived from several successive laboratory crosses within one parental pair of adult P. waltl received from the Koltsov Institute of Developmental Biology (Moscow, Russia). The embryos and larvae from sibling cohorts were reared in (a) tap water (control), (b) 1 and 2 ng mL‐1 (approximately 1.54 and 3.07 nM) alkaline solutions of 3,31,5‐triiodothyronine (T3; Sigma Chemical Co.), and (c) 0.02% thiourea (TU; Solins, Russia), a chemical agent that represses the synthesis of endogenous THs (Brown, 1997), resulting in TH deficiency. The treatment of the embryos with TH and TU was started at 5 days post‐fertilization (dpf), ~ 1 week before hatching. In this study, we chose to use T3 rather than the usually used thyroxine (T4), because the latter is currently believed to be a prohormone and a reservoir for the more active T3 (Galton, 2017). The concentrations of T3 used in our experiment fall within the range of plasma TH levels found by Launay et al. (1998) during natural P. waltl metamorphosis. Similar concentrations were revealed to accelerate cranial development in different urodeles without evident teratogenic effects (Smirnov and Vassilieva, 2003; Smirnov and Vassilieva, 2005; Smirnov et al. 2011). Similarly, in our previous work (Smirnov and Vasileva, 2001), a 0.02% TU concentration did not cause teratogenic effects in P. waltl larvae, but it was enough to retard metamorphic remodelling. The newts from every experimental and control group were kept at the same density (120 L aquaria, each initially containing ~ 50 larvae) and temperature (18 °C) and under a natural light regime. One‐half of the rearing medium was replaced every day. The larvae were fed ad libitum with brine shrimp (Artemia salina) nauplii, followed by blood worms as they grew. The larvae were reared until the end of metamorphosis (normal and TH‐induced) and until the end of the experiment for the TU‐treated individuals. Additionally, several normally developing newts were reared until 10 months after the completion of metamorphosis. Among the TU‐treated individuals, 25 were > 100 dpf. The oldest TU‐treated individual was 783 days old. Mortality was low (~ 10%) and did not significantly differ in the larvae kept under different conditions. The larvae were sampled at regular intervals and fixed in 10% neutral buffered formalin. The larvae were staged according to the normal table for P. waltl development (Gallien and Durocher, 1957) and the snout–vent length (SVL) was measured with an electronic caliper to the nearest 0.1 mm. All the specimens were prepared as skeletal whole‐mounts. They were stained with Alizarin red to detect calcium deposits, cleared in KOH following the protocol described by Wassersug (1976), and analysed for skeleton development under a stereomicroscope (Olympus SZX7). The timing of ossification was recorded as the first observable sign of Alizarin red staining. The ossification sequences were determined according to the procedure described by Cubbage and Mabee (1996). A total of 323 laboratory‐reared specimens were examined (Table 1). The terminology used for the bones generally follows that advocated by Lebedkina (1979,2004).
Table 1.
Specimens of Pleurodeles waltl examined in the experimental study
| Stage | Controls | TU 0.02% | T3 1 ng mL‐1 | T3 2 ng mL‐1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dpf | SVL, mm | n | dpf | SVL, mm | n | dpf | SVL, mm | n | dpf | SVL, mm | n | |
| 34 | 10–17 | 5–7 | 5 | 17 | 6.5 | 1 | 15 | 6 | 1 | – | – | – |
| 35 | 12–13 | 5–7 | 2 | 14–18 | 5.5–6 | 2 | 15–17 | 6–7 | 3 | 10–12 | 5 | 3 |
| 36 | 13–19 | 5.5–8 | 11 | 14–19 | 6–7 | 3 | 14–15 | 7 | 2 | 14–20 | 5–6 | 3 |
| 37 | 15–20 | 6–7.5 | 10 | 17–20 | 7–7.5 | 3 | 16–20 | 7–7.5 | 5 | 16–17 | 6–7 | 3 |
| 38 | 18–21 | 6–8 | 9 | 19–21 | 7–8.5 | 2 | 19–20 | 8–8.5 | 2 | 18–21 | 6–7 | 3 |
| 39 | 19–28 | 7.5–10 | 6 | 21–23 | 7.5–8 | 2 | 22–24 | 7–8 | 2 | 20–24 | 7–8 | 5 |
| 40 | 22–23 | 7–10 | 5 | 20–23 | 7–8.5 | 3 | 22–23 | 7–8 | 2 | 20 | 8 | 1 |
| 41 | 22–26 | 8.5–10 | 8 | 21–24 | 8–8.5 | 3 | 22–29 | 8.5–9 | 6 | 23–26 | 8–9 | 3 |
| 42 | 23–29 | 9–10 | 6 | 23–29 | 8.5–9.5 | 6 | 23 | 9 | 1 | 26–28 | 8–10 | 3 |
| 43 | 28–30 | 11–12 | 3 | 29 | 10–11 | 3 | 29 | 11 | 1 | 30–39 | 10–12 | 4 |
| 44 | 30–34 | 9–11 | 4 | 30 | 11.5 | 1 | 30–31 | 11–16 | 4 | 30–33 | 11 | 2 |
| 45 | 32 | 10–14 | 2 | – | – | – | 37 | 12 | 1 | 33–34 | 11 | 2 |
| 46 | 32–35 | 10–11 | 5 | 35 | 10 | 1 | 32 | 12 | 2 | 32 | 12 | 1 |
| 47 | 32–36 | 10.5–13 | 4 | 34 | 10–11 | 2 | – | – | – | 32–35 | 11–12 | 2 |
| 48 | 34–37 | 11–15 | 10 | – | – | – | 35–38 | 11–16 | 4 | 37–38 | 11–12 | 2 |
| 49 | 39–43 | 11–13 | 4 | 34 | 12 | 1 | 39 | 11 | 1 | 44 | 11 | 1 |
| 50 | 37–39 | 11–13 | 5 | 35–38 | 12–15 | 2 | 39 | 11 | 1 | 38 | 14 | 1 |
| 51 | 37–41 | 12–16 | 4 | 41 | 14 | 1 | 39 | 14 | 1 | 40 | 13 | 1 |
| 52 | 38–41 | 13.5–19 | 12 | 38–47 | 12–18 | 5 | 41–44 | 11–14 | 4 | 45 | 15 | 1 |
| 53 | 39–47 | 13–20 | 12 | 41–47 | 17–21 | 3 | 44–49 | 13–16 | 3 | 52 | 13 | 1 |
| 54 | 43–54 | 19–24 | 11 | 49–783 | 16–80 | 26 | 47–49 | 18–19 | 2 | – | – | – |
| 55 | 61–81 | 28–36 | 9 | 51–86 | 19–33 | 10 | 50–79 | 18–24 | 6 | – | – | – |
| Total number | 147 | 80 | 54 | 42 | ||||||||
All the procedures were carried out in accordance with the Severtsov Institute's Animal Ethics Committee.
3. RESULTS
3.1. Larval development under experimental conditions
In the table for staging normal P. waltl development by Gallien and Durocher (1957), the stages of the larval period [stage (st.) 34–54] are based mainly on the degree of externally visible limb differentiation, whereas the stages of the metamorphic period (st. 55a, b, c) are defined on the basis of reductive changes in the external gills and caudal fins. Limb development in P. waltl was revealed to be TH‐independent; in contrast, the reduction in both gills and fins is strictly TH‐dependent (according to our observation).
Consequently, no developmental differences were recorded in the early larval (st. 35–41) and midlarval (st. 42–52) ontogeny of newts kept under different hormonal regimes. That is, the newts attained the same stage at the same age. For example, st. 41 was attained at 22–29 dpf under all the TH regimes. Similarly, all the larvae needed 35–39 dpf to reach st. 50. In contrast, the timing of metamorphic changes varied greatly depending upon the experimental conditions. In normal development, external features indicating the onset of metamorphosis (a reduction in external gills and caudal fins) were revealed within ~ 60–70 dpf. In the TH‐treated larvae reared at 1 ng mL‐1, detectable signs of metamorphosis appeared at ~ 50 dpf. The newts kept in TU failed to metamorphose even after 2 years of the experiment. The oldest TU‐treated newts reached an age of 748 and 783 dpf (SVL 70 and 80 mm, respectively) while remaining at st. 54. Although the larval gills shrank in these individuals, they did not disappear completely. Additionally, the caudal fins, though greatly reduced, persisted. A 463‐day‐old TU‐treated female (SVL 78 mm) displayed large (1.9–2.3 mm in diameter), pigmented oocytes. The effect of TU was reversible, as shown by several TU‐treated newts that completed metamorphosis after exposure to TH.
3.2. Skeletal development under experimental conditions
Since cranial development in P. waltl has been described in detail by Lebedkina (1979, 2004), in the present paper, we omit developmental details and focus mainly on the timing and sequence of cranial bone appearance.
The bone composition of the larval and postmetamorphic P. waltl skulls is shown in Figure 1.
Figure 1.
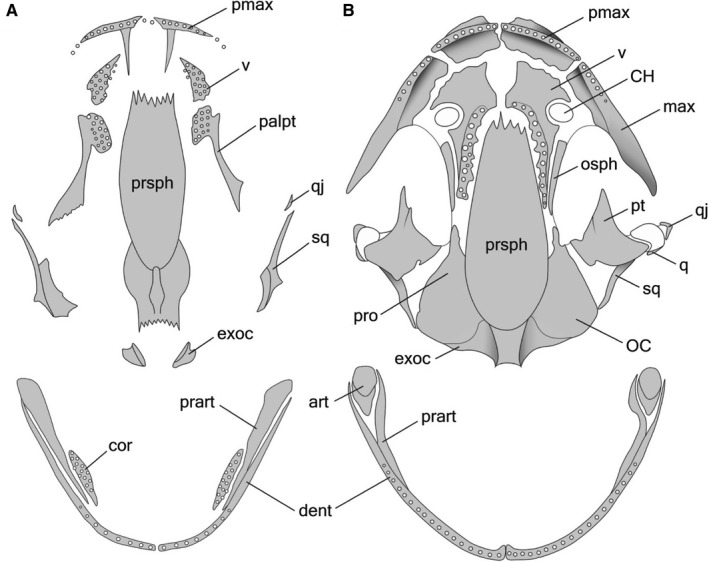
Skulls (ventral view) and lower jaws (dorsal view) of (A) larval and (B) adult Pleurodeles waltl. art, articular; CH, choana; cor, coronoid; dent, dentary; exoc, exoccipital; max, maxilla; OC, otic capsula; osph, orbitosphenoid; palpt, palatopterygoid; pmax, premaxilla; prart, prearticular; pro, prootic; prsph, parasphenoid; pt, pterygoid; q, quadrate; qj, quadratojugal; sq, squamosal; v, vomer.
In normally developing larvae (controls), no skull bones were revealed in the newly hatched (st. 34) larvae. The first cranial bones appear soon after hatching, in st. 35–36. During the early larval period (st. 35–41), 11 dermal bones appear: the coronoid, dentary, vomer, palatine, squamosal, premaxilla, parasphenoid, pterygoid, prearticular, frontal, and parietal, in that order (Tables 2 and 3). The first to arise are the paired coronoid and dentary on Meckel's cartilage and vomer and palatine in the palate. The appearance of these dentigerous bones is preceded by the appearance of the corresponding tooth buds associated with them. These bones are followed by (a) the squamosal, arising on the lateral side of the palatoquadrate, (b) the dentigerous premaxilla in the upper jaw, (c) the parasphenoid in the skull base, (d) the pterygoid, arising as a caudal outgrowth of the palatine, and (e) the prearticular on the medial side of Meckel's cartilage. Then, the frontal and parietal appear in the skull roof.
Table 2.
Sequence of cranial bones appearance in larval Pleurodeles waltl reared under different hormonal conditions
| Sequence position | Controls | T3 1 ng mL‐1 | T3 2 ng mL‐1 | TU 0.02% | |
|---|---|---|---|---|---|
| 1 | coronoid | coronoid | coronoid | coronoid | |
| 2 | vomer | vomer | vomer | vomer | |
| 3 | palatine | palatine | palatine | palatine | |
| 4 | dentary | dentary | dentary | dentary | |
| 5 | squamosal | squamosal | squamosal | squamosal | |
| 6 | premaxilla | premaxilla | premaxilla | premaxilla | |
| 7 | parasphenoid | parasphenoid | parasphenoid | parasphenoid | |
| 8 | pterygoid | pterygoid | pterygoid | pterygoid | |
| 9 | prearticular | prearticular | prearticular | prearticular | |
| 10 | frontal | frontal | frontal | frontal | |
| 11 | parietal | parietal | parietal | parietal | |
| 12 | exoccipital | maxilla | maxilla | exoccipital | |
| 13 | quadratojugal | exoccipital |
|
quadratojugal | |
| 14 | orbitosphenoid | quadratojugal | orbitosphenoid | ||
| 15 | prootic | orbitosphenoid | prootic | ||
| 16 | maxilla | nasal | nasal | quadrate | |
| 17 | nasal |
|
prefrontal | os thyroideum | |
| 18 | os thyroideum | prootic | maxilla | ||
| 19 | prefrontal | os thyroideum | prefrontal | ||
| 20 | quadrate | nasal |
Table 3.
Timing of cranial bones appearance and metamorphic palate remodelling in larval Pleurodeles waltl under different experimental conditions
| Bone | The earliest time of bone appearance and palate remodelling: age (dpf)/ stage | |||
|---|---|---|---|---|
| Controls | T3 1 ng mL‐1 | T3 2 ng mL‐1 | TU 0.02% | |
| coronoid | 14/ S. 36 | 15/ S. 35‐36 | 14/ S. 36 | 14/ S. 36 |
| vomer | 14/ S. 36 | 15/ S. 35‐36 | 14/ S. 36 | 15/ S. 36 |
| palatine | 14/ S. 36 | 15/ S. 35‐36 | 14/ S. 36 | 15/ S. 36 |
| dentary | 15/ S. 36 | 15/ S. 35‐36 | 14/ S. 36 | 15/ S. 36 |
| squamosal | 16/ S. 37 | 16/ S. 37 | 14/ S. 36 | 17/ S. 37 |
| premaxilla | 16/ S. 36 | 18/ S. 37 | 14/ S. 36 | 18/ S. 37 |
| parasphenoid | 16/ S. 36 | 18/ S. 37 | 16/ S. 37 | 17/ S. 37 |
| pterygoid | 16/ S. 36 | 18/ S. 37 | 16/ S. 37 | 18/ S. 37 |
| prearticular | 16/ S. 37 | 18/ S. 37 | 16/ S. 37 | 18/ S. 37 |
| frontal | 18/ S. 38 | 20/ S. 38 | 18/ S. 38 | 19/ S. 38 |
| parietal | 22/ S. 41 | 22/ S. 41 | 20/ S. 39 | 21/ S. 41 |
| exoccipital | 30/ S. 44 | 30/ S. 44 | 30/ S. 43 | 29/ S. 43 |
| quadratojugal | 37/ S. 50‐51 | 35/ S. 48 | 30/ S. 43 | 38/ S. 52 |
| orbitosphenoid | 43/ S. 54 | 39/ S. 49 | 30/ S. 43 | 41/ S. 53 |
| prootic | 45/ S. 54 | 39/ S. 49 | 30/ S. 43 | 41/ S. 53 |
| maxilla | 47/ S. 54 | 29/ S. 43 | 26/ S. 41 | 64/ S. 54 |
| nasal | 47/ S. 54 | 37/ S. 48 | 30/ S. 43 | 119/ S. 54 |
| os thyroideum | 51/ S. 54 | 39/ S. 51 | 32/ S. 46‐47 | 74/ S. 54 |
| prefrontal | 51/ S. 54 | 39/ S. 51 | – | 49/ S. 54 |
| quadrate | 51/ S. 54 | 39/ S. 51 | – | 53/ S. 55 |
| palatopterygoid desintegration | 70/ S. 55 | 35/ S. 47 | 30/ S. 43 | – |
| palatine complete resorption | 72/ S. 55 | 66/ S. 55 | 45/ S. 52 | – |
| coronoid complete resorption | 71/ S. 55 | 66/ S. 55 | 45/ S. 52 | – |
During further midlarval development (st. 42–53), the endochondral exoccipital appears near the upper‐posterior margin of the otic capsule, followed by the dermal quadratojugal, arising on the lateral surface of the palatoquadrate near the jaw joint (Figure 1A). Beginning in st. 42, in the coronoid, vomer and palatine portions of the palatopterygoid, bearing multiple rows of dentition (Figures 1A and 2A), the lateral margins show signs of resorption. The premaxillae, which fuse into a unipartite bone bearing two facial processes just after their appearance (st. 37–38), undergo separation beginning in st. 47, and from st. 50 on, all the individuals display a bipartite premaxilla (Figure 3).
Figure 2.
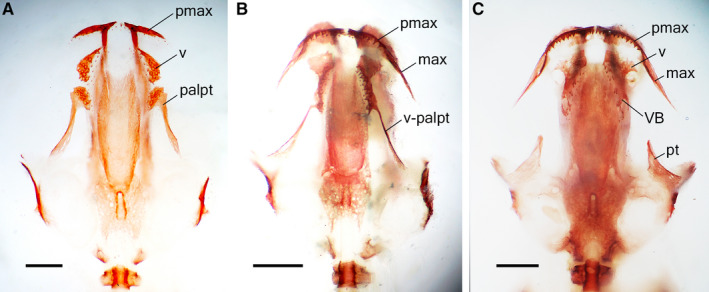
Ventral views of the skull in the same aged (41–44 days post‐fertilization) Alizarin‐stained larvae: (A) control larva at stage 52, snout–vent length (SVL) 18 mm – maxilla is missing, premaxilla lacks vomerine process, vomer and palatopterygoid, both bearing multi‐row dentition, are separate; (B) thyroid hormone (TH)‐treated (1 ng mL‐1) larva at stage 53, SVL 15.5 mm – premaxilla and maxilla are fused and bear vomerine processes which meet the vomerine edentate plate. Note the occurrence of the vomero‐palatopterygoid bearing one‐row dentition along its medial edge; (C) TH‐treated (2 ng mL‐1) larva at stage 52, SVL 15 mm – palatine is absent, vomer bears a small vomerine bar, pterygoid is enlarged and adult‐shaped. VB, vomerine bar; v‐palpt, vomero‐palatopterygoid; for other abbreviation definitions see Figure 1. Scale bars are 1 mm.
Figure 3.
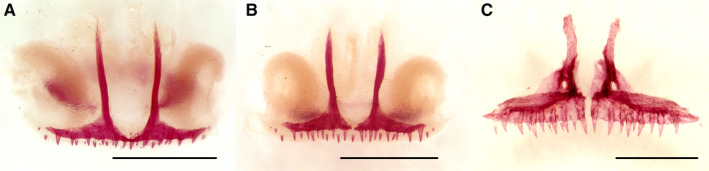
Premaxillae in Alizarin‐stained normally developing specimens of Pleurodeles waltl: (A) unipartite bone in larva at stage 47 [snout–vent length (SVL) 12.4 mm]; (B) start of separation at stage 48 (SVL 12.5 mm); (C) bipartite bones in metamorphosing animal at stage 55 (SVL 31.8 mm). Scale bars are 1 mm.
In late larval development (st. 54), two endochondral bones, the orbitosphenoid and, slightly later, the prootic, appear. They are followed by three dermal (the maxilla, nasal and prefrontal) and two endochondral (the os thyroideum and quadrate) bones, although at this stage, these bones are variably present in the specimens examined. Among 11 newts examined in st. 54, five had none of these late‐appearing bones, while two displayed the occurrence of a full set of these ossifications. In this set of bones, the maxilla and nasal are the first to appear. They are followed by the prefrontal, os thyroideum, and quadrate in a varying sequence. Whereas in the most common ossification sequence, the prefrontal was the 19th bone to appear (Table 3), in some specimens, it occupied the 18th position in the ossification sequence, appearing before the os thyroideum, which usually arises prior to the prefrontal. Moreover, in one newt, the prefrontal was the 17th bone to arise, as it ossified before the orbitosphenoid, which, in the most common ossification sequence, precedes the appearance of the prefrontal and occupies the 14th position. As a whole, a variation of one to three positions was observed in the bones appearing in the late larval period; thus, while the most common sequence is:
quadratojugal → orbitosphenoid →prootic → maxilla +nasal → os thyroideum → prefrontal, the following alternative sequences were also found:
quadratojugal → orbitosphenoid →prootic → maxilla +nasal → prefrontal →os thyroideum;
quadratojugal → prootic →maxilla + nasal →prefrontal → os thyroideum → orbitosphenoid.
The vomer and the palatine portion of the palatopterygoid retain multiple rows of dentition. The coronoid bears teeth in some individuals and is toothless in others. The frontal bone gives rise to an anterior extension, which expands over the roof of the nasal capsule. Additionally, the posterolateral edge of the frontal bone forms a process growing towards the squamosal. The upper portion of the squamosal, in turn, forms an anteriorly directed outgrowth extending towards the frontal. A band of connective tissue is stretched between these processes. The palatine process (pars palatina) of the premaxilla extends towards the vomer at this stage.
At metamorphosis (st. 55), the maxilla, nasal, prefrontal, quadrate, and os thyroideum occur in all individuals. The maxilla forms the facial and palatine processes, growing dorsally and towards the vomer, respectively. They are followed by a caudal process developing as a posterior extension of the dentigerous portion of the maxilla. In the palate, the strip of bone connecting the palatine and pterygoid portions of the palatopterygoid becomes greatly reduced. Then, the palatopterygoid disintegrates into separate palatine and pterygoid portions. For a short time, the palatine persists as a toothless rudimentary bone and then disappears. At the same time, in the lower jaw, the coronoid undergoes resorption, loses dentition, and disappears. The vomer develops outgrowths extending towards the palatine processes of the maxilla and premaxilla and a caudal process growing posteriorly along the lateral side of the parasphenoid (the vomerine bar). The multiple rows of vomerine dentition are replaced by one row of teeth situated along the medial side of the vomer and extending caudally along the vomerine bar (Figure 1B).
Furthermore, during postmetamorphic development, the endochondral articular appears in the lower jaw. Palatine processes from the maxilla and premaxilla meet lateral outgrowths (the 'edentate vomerine plate' according to Greven et al. 2015) from the vomer. The processes from the squamosal and frontal bones that are growing towards each other meet and complete the formation of the fronto‐squamosal arch. With age, in 6‐ to 8‐month‐old individuals, the dermal bones of the snout (maxilla, nasal, prefrontal and premaxilla) acquire ornamentations, and cranial crests develop.
3.2.1. Thyroid hormone‐treated larvae
In the larvae reared in a solution with 1 ng mL‐1 TH, all the bones that normally arise in the early larval period (~ 30 dpf, st. 35–41) appear in the same order (Table 2) as they do in natural development and almost at the same ages and stages as in the controls (Table 3, Figure 4). Of the bones arising in the midlarval period, the exoccipital appears as it does in natural development, whereas the quadratojugal arises somewhat precociously. Also precociously, the frontal bone gives rise to an anterior extension (st. 48) followed by a latero‐posterior process directed towards the squamosal (st. 51–52). Almost at the same time (st. 52), an anteriorly directed process growing from the upper portion of the squamosal towards the frontal bone appears.
Figure 4.
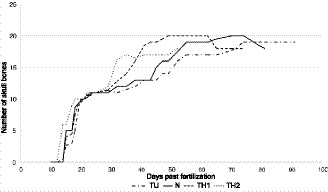
Dynamics of cranial development in Pleurodeles waltl individuals reared under different hormonal regimes: N, normally developing newts; thyroid hormone (TH)1, triiodothyronine (T3) 1 ng mL‐1‐treated newts; TH2, T3 2 ng mL‐1‐treated newts ; TU, thiourea 0.02%‐treated newts. Number of bones is given as a mean for all specimens at the same stage. Note the decrease of the number of cranial bones in the control and TH‐treated (1 ng mL‐1) animals after 70 and 60 days post‐fertilization, respectively, resulting from the metamorphic resorption of the coronoid and palatine bones.
Those bones that normally appear in the late larval period and metamorphosis arise precociously; by st. 52, they are present in all larvae. The degree of acceleration differs among these bones (Table 3), resulting in changes in the sequence of their appearance (Table 2); the most accelerated bone is the maxilla, which first appears as a toothless, thin strip of bone in st. 43. The maxilla and premaxilla grow towards one another and fuse in st. 45. Their further differentiation is also accelerated: the palatine processes, which extend towards the vomer, appear in the premaxilla and maxilla in st. 46 and st. 49–50, respectively. In st. 48, the maxillary facial process appears.
Whereas the initial changes in the vomer, palatine and coronoid, i.e. the resorptions of their lateral edges, begin as in normal development in st. 42, the metamorphic remodelling of these bones is greatly accelerated. Moreover, the remodelling in the TH‐treated individuals demonstrates a striking departure from the natural pattern: beginning in st. 43, the vomer fuses with the palatopterygoid, forming a vomero‐palatopterygoid. Separation of the pterygoid from the vomero‐palatopterygoid may occur as early as st. 47, when the orbitosphenoid, nasal, prootic, prefrontal, quadrate, and os thyroideum, which normally arise prior to disintegration, are absent. However, in most individuals, the vomero‐palatopterygoid is retained until st. 55 (Figure 2B). In the vomer, in st. 48, the dentition is represented by one row of teeth. In the same stage, the vomer forms outgrowths extending towards the maxilla and premaxilla, and in st. 55, a caudal process extending along the lateral side of the parasphenoid and bearing a tooth row (the vomerine bar). The dentition may be lost from the coronoid as early as st. 53, although most individuals lose it during st. 55. At the same stage the coronoid bone completely disappears.
In the larvae reared in a solution with 2 ng mL‐1 TH, the bones that normally arise in the early larval period appear almost at the same ages and stages (Table 3, Figure 4) as those in the controls and follow the natural sequence of ossification (Table 2). However, the parietal appears somewhat precociously. Also precociously, in st. 48, the frontal bone gives rise to the anterior extension and the process towards the squamosal. In the same stage, the process growing from the upper portion of the squamosal towards the frontal appears.
Among the bones arising in the midlarval period, the exoccipital appears almost as in natural development, whereas the appearance of the quadratojugal is greatly accelerated. Most bones normally appearing in the late larval and metamorphic periods arise precociously (Tables 2 and 3). The development was most accelerated for the maxilla, which appears as early as st. 41. Just after its appearance, during the same stage, the maxilla fuses with the premaxilla. In st. 43, the maxillary facial process appears, followed by the palatine process, which arises in st. 47. The developmental transformations and metamorphic remodelling of the palate and coronoid are also accelerated. The lateral margins of the vomer and palatine portions of the palatopterygoid begin to resorb in st. 39 and those of the coronoid begin to resorb in st. 41.
In st. 39, the vomer fuses with the palatopterygoid. In st. 42, the vomer (coupled with the palatopterygoid) forms processes towards the premaxilla and maxilla. These processes almost reach the palatine processes from the premaxilla and maxilla in st. 47. In st. 46–47, the vomerine dentition becomes one‐rowed, and in st. 52, a caudal process extending along the lateral side of the parasphenoid appears. Disintegration of the palatopterygoid may occur as early as st. 43, before the appearance of six bones normally arising prior to this event (Tables 2 and 3). The timing of the splitting of the palatopterygoid is highly variable, and in some individuals, the vomero‐palatopterygoid remains intact until st. 51. However, beginning in st. 52, the vomero‐palatine is separated from the pterygoid in all the specimens. The palatine portion was not found in individuals in st. 52 (Figure 2C) but was recorded in a specimen in st. 53. Similarly, the coronoid may become toothless as early as st. 43, although in some individuals, rudimentary dentition persists until st. 48. By st. 51, the coronoid bone disappears completely. The quadrate bone and os thyroideum were not found in the examined specimens.
3.2.2. Thiourea‐treated larvae
In the TU‐treated larvae, all the bones that normally arise in the early larval and midlarval periods follow the natural ossification sequence (Table 2) and appear at almost the same ages and stages (Table 3, Figure 4) as those in the controls. The bones normally arising in the late larval period and during metamorphosis differ in their reaction to the TU treatment. The endochondral bones (the orbitosphenoid, prootic, os thyroideum and quadrate) appear at the same stages and ages as in the controls, whereas the dermal bones (the maxilla, nasal and prefrontal) are retarded in their appearance and are the last cranial ossifications to arise (Table 3). The first appearance of the maxilla is recorded at 64 dpf, although it is lacking in two older individuals (69 and 79 dpf). For a long time, the maxilla is represented only by the facial process; much later, the palatine process appears. Its earliest occurrence is recorded in the newt at 119 dpf, although the rudimentary palatine process is invariably present in all examined newts only beginning at 342 dpf. The maxillary dentigerous portion fails to appear. Although the maxillary dentigerous portion is absent, fully developed teeth are present in its site. These teeth, situated in connective tissue, are first recorded in a 74‐day‐old newt (see also Figure 6A). Their occurrence is highly variable; however, beginning at 342 dpf, teeth are present in all the examined TU‐treated newts. Moreover, two series of non‐ankylosed teeth were present in a 748‐day‐old newt.
Figure 6.
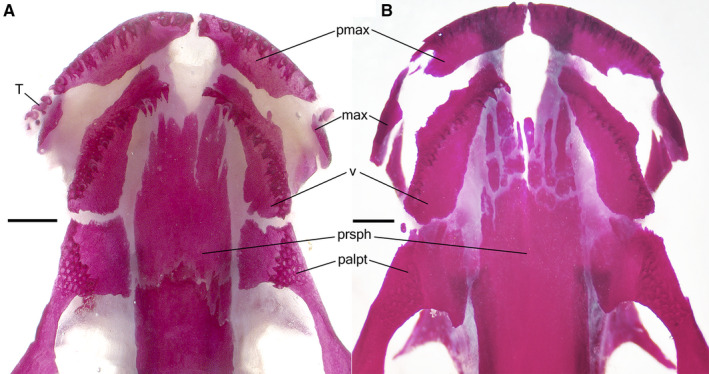
Ventral views of skull in the Alizarin‐stained 'early' (A) and 'late' (B) thiourea‐treated newts. (A) Stage 54, 157 days post‐fertilization (dpf), snout–vent length (SVL) 56 mm: palatine portion of the palatopterygoid and vomer (in its caudal region) bear multi‐row dentitions, toothless vomerine plate is missing. Note the occurrence of non‐ankylosed teeth near the rudimentary maxilla (left side). (B) Stage 54, 358 dpf, SVL 71 mm): vomerine dentition is one‐row, palatine teeth are missing, the palatine portion of the palatopterygoid bears resorption pits in sites of lost teeth. Note the occurrence of fragmented parasphenoid. T, teeth; for other abbreviation definitions see Figures 1 and 2. Scale bars are 1 mm.
The prefrontal, first appearing at 74 dpf, is variably present in older individuals, but after 95 dpf, it occurs in all newts, although it remains in a rudimentary state. The nasal is the last bone to arise, and it does not appear until 119 dpf, although even in older specimens (at 288, 344 and 350 dpf), it may be absent. If present, the nasal is highly variable in shape and size. Often, numerous ossifications (up to four) develop at the site of the nasal, as previously described (Smirnov et al. 2010). Even in the oldest newts, this bone remains rudimentary (Figure 5B).
Figure 5.
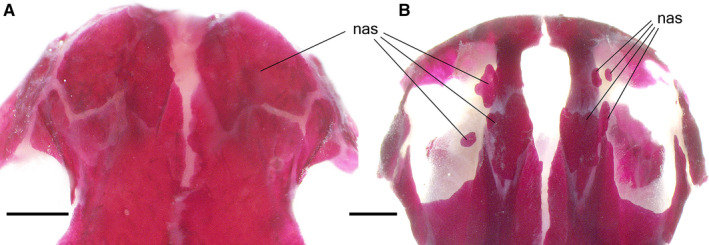
Dorsal views of the snout in the Alizarin‐stained Pleurodeles waltl showing normally developed nasal (A) in the control larva [stage 55b, 79 days post‐fertilization (dpf), snout–vent length (SVL) 36 mm] and numerous ossification centers of the nasal (B) in the thiourea‐treated larva (stage 54, 358 dpf, SVL 71 mm). nas, nasal. Scale bars are 1 mm.
In the vomer, the lateral margins begin to resorb in st. 39. Multiple rows of vomerine dentition remain in 95‐day‐old newt. In older individuals, beginning in a 118‐day‐old newt, the vomerine dentition is replaced by one tooth row situated along the lateral margin of the vomer. Vomerine processes extending towards the premaxilla and maxilla were first recorded in 82‐ and 420‐day‐old newts, respectively. They remain rudimentary even in the oldest newts. The caudal outgrowth (vomerine bar), which normally grows along the lateral side of the parasphenoid, fails to develop in TU‐treated newts (Figure 6).
In the palatopterygoid, the lateral margin begins to resorb in st. 39. Multiple rows of dentition remain until 229 dpf, although they may be lost as early as 118 dpf. Beginning in 280‐day‐old newt, the palatopterygoid is a toothless bone, perforated by resorption pits in the sites of lost teeth. In the oldest TU‐treated newt (783 dpf), the resorption pits are absent. The palatine portion of the palatopterygoid expands anteriorly and medially towards the vomer and parasphenoid, respectively, contacting the vomer and parasphenoid in some individuals (as in the 229‐day‐old newt). No disintegration of the palatopterygoid was recorded in the TU‐treated newts (Figure 6).
In the coronoid, the lateral margin begins to resorb in st. 41. The multiple rows of dentition remain until they are replaced by two rows of teeth in the specimen 229 dpf. Beginning in the 280‐day‐old newt, dentition is represented by one tooth row. Initially, this row occupies the full length of the coronoid, although with age it becomes shorter and extends only to the middle of the coronoid (e.g. in a 463‐day‐old specimen) or even less (e.g. in a 658‐day‐old specimen). Although severely reduced, the coronoid bone remained in all the TU‐treated newts.
4. DISCUSSION
In general, our observations of cranial development in P. waltl coincide with the descriptions provided by Corsin (1966b) and Lebedkina (1979, 2004); however, some discrepancies should be mentioned. First, because of the limited sample size used in their studies, the sequence of bone appearance was not completely resolved, as many bones were recorded as appearing simultaneously. Second, according to Corsin, the quadrate precedes the appearance of at least four bones, including the maxilla, nasal and prefrontal; however, the bone indicated as the quadrate (figure 7, Corsin, 1966b) occupies the position which is characteristic of the dermal quadratojugal bone recorded in different urodeles (for details, refer to Rose, 2003). Furthermore, this separate ossification fuses with the endochondral quadrate. Lebedkina (1979, 2004) also recognized this bone as the quadratojugal, appearing in the midlarval period, whereas the endochondral quadrate arises in metamorphosis following the formation of the maxilla and nasal bone. Consequently, the true quadrate was omitted by Corsin. Third, neither Corsin nor Lebedkina mentioned the appearance of the os thyroideum, although this endochondral bone develops in P. waltl, as in other salamandrids studied in this respect (Reilly, 1987; Smirnov and Vassilieva, 2003; Vassilieva and Serbinova, 2013). Additionally, Corsin denies fusion of the premaxilla bones (although in Figures 1 and 3 of her paper, the premaxillae are represented as unipartite in st. 37 and st. 41, respectively). Our observations of the details of the premaxillae development (i.e. the appearance of two bones, their further fusion, and then their separation) coincide with those by Lebedkina (1979, 2004). To date, the separation of the unipartite larval premaxilla into paired adult bones has been recorded in some plethodontids (Alberch et al. 1986; Rose, 1995a). Finally, in the ossification sequence provided by Lebedkina, the orbitosphenoid is one of the last two bones to appear, arising after the maxilla, nasal and prefrontal. Although such a sequence of bone appearance was also recorded in our study, it does not seem to be common for P. waltl as, among 11 newts examined in st. 54, such late appearance of the orbitosphenoid was observed only once.
The comparison of skull development in P. waltl larvae reared under different hormonal levels revealed differences in the rate (Figure 4), timing and sequence (Table 2) of bone appearance in the normal, TH‐ and TU‐treated newts.
The bones that appear early arise at the same stages and ages under different hormonal levels, whereas the timing of the appearance of the bones that arise in the late larval and metamorphic periods changes dramatically depending on the TH level. These findings indicate that TH has less of an effect on the development of the cranial bones of P. waltl in the early larval stage (if at all) than in the late larval and metamorphic stages. Of the bones that appear in the late larval and metamorphic stages, the dermal bones are more TH‐responsive than the endochondral bones. The maximum responsiveness to and dependence on TH are displayed by the metamorphic coronoid resorption and palate remodelling, which are greatly accelerated in the TH‐treated newts and arrested in the TU‐treated newts. Such a peak in the degree of responsiveness to TH is consistent with the maximum level of TH activity in metamorphosing P. waltl individuals recorded by Launay et al. (1998).
Previously, comparable differential TH dependence in different cranial bones was recorded in similar experiments on Lissotriton vulgaris (Salamandridae), Ambystoma mexicanum (Ambystomatidae), and Salamandrella keyserlingii (Hynobiidae) (Smirnov and Vassilieva, 2003; Smirnov and Vassilieva, 2005; Smirnov et al. 2011). In these salamanders, the bones that ossify early in larval ontogeny are less responsive to TH than the bones that ossify closer to metamorphosis. According to Rose (1995b), cranial bones also differ in their TH dependence in Eurycea bislineata (Plethodontidae). Given that these salamanders belong to different urodele families, such ontogenetic dynamics of TH dependency are probably common for metamorphosing salamanders as a whole.
The comparison of the mediation of skull development by TH in P. waltl with that of another salamandrid, L. vulgaris, revealed that late‐appearing bones (e.g. the maxilla, nasal and prefrontal) show a similar degree of TH dependence. In contrast, in A. mexicanum and especially in S. keyserlingii, these bones are much less TH‐dependent. These findings indicate interspecific variations in the TH dependence of skull development in urodeles. Furthermore, they suggest that, in addition to changes in the level or rate of thyroid activity as proposed by Rose (1996), urodele evolution was accompanied by changes in TH dependence.
The present study revealed that certain bony elements display different reactions to TH changes depending on their stage of development. In the premaxilla, the dentigerous portion and facial process appear at the same stage and age under different hormonal conditions (in normal, high and low TH levels), whereas the palatine process appears precociously in the TH‐treated individuals and forms belatedly, if ever, in goitrogen‐treated individuals. In the frontal and squamosal, the main portions of these bones appear and develop similarly under all experimental conditions, whereas the development of their outgrowths, which extend towards each other to form the fronto‐squamosal arch, accelerates under high TH levels and decelerates under low TH levels. A similar phenomenon is displayed by the anterior extension of the frontal bone growing over the roof of the nasal capsule. Additionally, under different hormonal regimes, the appearance and early and midlarval differentiation of the vomer, coronoid and palatopterygoid proceed as in natural development, but their later metamorphic rearrangements proceed differently depending on hormonal level. Thus, in the vomer, the main bulk appears in st. 36 under all experimental conditions. In contrast, the appearance of the processes extending towards the premaxilla and maxilla is retarded in the TU‐treated newts and accelerated in the TH‐treated newts. The posterior extension of the vomer (the vomerine bar) develops precociously under high TH levels but fails to develop under hypothyroid conditions.
In sum, these observations indicate that (a) the constituent parts of certain bony elements exhibit differences in reaction to changes in TH levels and (b) although the bones that ossify early in larval ontogeny do not display a TH‐induced reaction when they start to appear, they become TH‐inducible closer to metamorphosis. These findings suggest that the early phases of their development are mediated by factors other than THs. Further development of these bones is accompanied by changes in mechanisms mediating their formation and morphological differentiation. That is, likely non‐hormonal mediation becomes replaced or/and complemented by hormonal mediation. In P. waltl, the involvement of non‐hormonal mediation is supported by the results of experimental embryology. Corsin’s (1966a) experiments showed that after the olfactory placode was extirpated, the facial process of the premaxilla failed to appear. Additionally, according to Cassin and Capuron (1979), in the cranial development of this newt, the vomer and palatine are induced by the trabecular cartilage, but the coronoid and the dentary by the Meckel's cartilage. Although early stages in the development of the vomer and palatine seem to be TH‐independent, the growth of these bones is greatly accelerated at high TH levels, resulting in their fusion into the vomero‐palatopterygoid. Similar fusion was also displayed by the premaxilla and maxilla in the TH‐treated newts. Previously, a vomero‐palatopterygoid was reported in the neotenic plethodontid salamander Eurycea rathbuni (Clemen et al. 2009) and in overwintered larvae of L. vulgaris (Greven et al. 2015). Interestingly, in both cases, metamorphosis was arrested, thus indicating TH dysfunction, which is in stark contrast with P. waltl, in which this bone results from hyperthyroidism.
Although late‐appearing bones in P. waltl display evident TH‐dependence, it is likely that their development is additionally mediated by factors other than TH. For example, the prefrontal, revealed in this study to be TH‐inducible, was shown by Medvedeva's experiments to also depend on the nasolacrimal duct (Medvedeva, 1975, 1986). A similar phenomenon was recorded in S. keyserlingii (Hynobiidae): the late‐appearing lacrimal was revealed to depend on both the nasolacrimal duct (Medvedeva, 1975) and TH (Smirnov et al. 2011). Previously, Rose (2003) proposed that the ossification of the dermal bones in salamanders may depend on prior formation of the adjacent cartilage. Our experiments on L. vulgaris (Smirnov and Vassilieva, 2003) provided data suggesting the occurrence of such interplay between late‐appearing bones (the maxilla, nasal and prefrontal) and the underlying cartilaginous nasal capsule. Concerning the developmental interactions between bones and cartilage in P. waltl, relevant research is in progress in our laboratory.
The comparison of the skull development in newts reared under different TH levels revealed that the positions of early appearing bones in the sequence of ossification is constant, whereas the positions of late‐appearing bones vary depending on TH levels. For example, the parasphenoid is the 7th bone to develop under all the experimental conditions. In contrast, whereas in normal development the maxilla is the 16th–17th to appear, it occupies the 12th and 18th positions in the sequence of ossification in the TH‐ and TU‐treated individuals, respectively. Similarly, instead of the 16th–17th position in the normal ossification sequence, the nasal was the 15th and 12th to appear in the TH‐treated newts (reared in the 1 ng mL‐1 and 2 ng mL‐1 solutions, respectively) and the 20th to appear in the TU‐treated newts (Table 2).
Similar differences in the degree of variability are displayed by the bones in normally developing newts. The bones appearing in the early and midlarval periods did not vary in sequence position. In contrast, there were variations in the developmental ossification sequence among the late‐appearing bones.
Given that late‐appearing bones are TH‐dependent and changes in the TH level cause changes in the sequence of their appearance, variations in the natural order of their appearance may result from individual variability in internal TH levels. Such individual TH variability was previously recorded in midmetamorphic individuals of the plethodontid salamander E. bislineata (Alberch et al. 1986). Interestingly, it was in this salamander that Rose (1995a) revealed variations in the sequence of appearance of those bones (i.e. the late‐appearing bones), which were shown by him to be TH‐dependent. The positions of the same late‐appearing bones were shown by Alberch and Blanco (1996) to vary in the ossification sequence of Salamandra salamandra (Salamandridae). However, although interrelations between individual TH variability and variations in the ossification sequence seem highly likely, to elucidate the mechanisms responsible for individual variation in bone appearance in P. waltl and other salamanders, further studies are needed.
AUTHOR CONTRIBUTIONS
S.V. planned and designed the study, analysed the data, discussed the results, and wrote the manuscript. K.M. raised the animals, obtained and analysed the data, discussed the results. A.V. obtained and analysed the data, discussed the results, wrote the manuscript, and prepared figures and tables.
Acknowledgements
We gratefully acknowledge the technical assistance provided by Vitaly Trounov and Aleksei Tsessarsky. We also thank David Marjanović and an anonymous reviewer for helpful critical comments.
Smirnov SV, Merkulova KM, Vassilieva AB. Skull development in the Iberian newt, Pleurodeles waltl (Salamandridae: Caudata: Amphibia): timing, sequence, variations, and thyroid hormone mediation of bone appearance. J. Anat.. 2020;237:543–555. 10.1111/joa.13210
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alberch, P. and Blanco, M.J. (1996) Evolutionary patterns in ontogenetic transformation: From laws to regularities. International Journal of Developmental Biology, 40, 845–858. [PubMed] [Google Scholar]
- Alberch, P. , Gale, E.A. and Larsen, P.R. (1986) Plasma T4 and T3 levels in naturally metamorphosing Eurycea bislineata (Amphibia; Plethodontidae). General and Comparative Endocrinology, 61, 153–163. [DOI] [PubMed] [Google Scholar]
- Brown, D.D. (1997) The role of thyroid hormone in zebrafish and axolotl development. Proceedings of the National Academy of Sciences of the United States of America, 94, 13011–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassin, C. and Capuron, A. (1977) Evolution de la capacité morphogénétique de la région stomodéale chez l’embryon de Pleurodeles waltlii Michah. (Amphibien urodèle). Etude par transplantation intrablastocélienne et par culture in vitro. Wilhelm Roux’s Archvies, 181, 107–112. [DOI] [PubMed] [Google Scholar]
- Cassin, C. and Capuron, A. (1979) Buccal organogenesis in Pleurodeles waltlii Michah. (urodele amphibian), study by intrablastocelic transplantation and in vitro culture. Journal of Biological Buccale, 7, 61–76. [PubMed] [Google Scholar]
- Chibon, P. (1964) Analyse par la méthode de marque nucléaire à la thymidine tritiée des dérivés de la crête neurale cephalique chez l’Urodèle Pleurodeles waltlii . Comptes Rendus de l'Academie des Sciences. Serie III, Sciences de la Vie, 259, 3624–3627. [PubMed] [Google Scholar]
- Chibon, P. (1966) Analyse expérimentale de la régionalisation et des capacités morphogénétiques de la crète neurale chez l’Amphibien Urodèle Pleurodeles waltlii Michah. Mémoires de la Société zoologique de France, 36, 1–107. [Google Scholar]
- Clemen, G. , Sever, D. and Greven, H. (2009) Notes on the cranium of the paedomorphic Eurycea rathbuni (Stejneger, 1896) (Urodela: Plethodontidae) with special regard to the dentition. Vertebrate Zoology, 59, 157–168. [Google Scholar]
- Corsin, J. (1966a) Quelques problèmes de morphogenèse du crâne chez les urodèles. In Problèmes Actuels de Paléontologie (Evolution des Vertébrés) . Coll Int Cent Nat Rec Scient, 163, 295–299. [Google Scholar]
- Corsin, J. (1966b) The development of the osteocranium of Pleurodeles waltlii Michahelles. Journal of Morphology, 119, 209–216. [DOI] [PubMed] [Google Scholar]
- Cubbage, C.C. and Mabee, P.M. (1996) Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, Cyprinidae). J Morph, 229, 121–160. [DOI] [PubMed] [Google Scholar]
- Elewa, A. , Wang, H. , Talavera‐López, C. , Joven, A. , Brito, G. , Kumar, A. et al (2017) Reading and editing the Pleurodeles waltl genome reveals novel features of tetrapod regeneration. Nature Communications, 8, 2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal‐Giladi, H. and Zinberg, N. (1964) The development of chondrocranium in Pleurodeles waltlii . Journal of Morphology, 114, 527–548. [DOI] [PubMed] [Google Scholar]
- Frippiat, J.P. (2013) Contribution of the urodele amphibian Pleurodeles waltl to the analysis of spaceflight‐associated immune system deregulation. Molecular Immunology, 56, 434–441. [DOI] [PubMed] [Google Scholar]
- Gallien, I. and Durocher, M. (1957) Table chronologique du developpement chez Pleurodeles waltlii Michah. Bulletin Biologique de la France, 19, 97–114. [Google Scholar]
- Galton, V.A. (2017) The ups and downs of the thyroxine pro‐hormone hypothesis. Molecular and Cellular Endocrinology, 458, 105–111. [DOI] [PubMed] [Google Scholar]
- Germain, D. and Laurin, M. (2009) Evolution of ossification sequences in salamanders and urodele origins assessed through event‐pairing and new methods. Evolution & Development, 11, 170–190. [DOI] [PubMed] [Google Scholar]
- Goswami, A. (2007) Cranial modularity and sequence heterochrony in mammals. Evolution & Development, 9, 290–298. [DOI] [PubMed] [Google Scholar]
- Gould, S.J. (1977) Ontogeny and phylogeny. Cambridge, UK: Harvard University Press. [Google Scholar]
- Greven, H. , van de Kamp, T.h. , dos Santos, R.T. , Baumbach, T. and Clemen, G. (2015) The “tooth systems” of Lissotriton vulgaris (Amphibia: Urodela) with special regard to delayed metamorphosis. Vertebrate Zoology, 65, 81–99. [Google Scholar]
- Gualandris‐Parisot, L. , Husson, D. , Foulquier, F. , Kan, P. , Davet, J. , Aimar, C. et al (2001) Pleurodeles waltl, amphibian, Urodele, is a suitable biological model for embryological and physiological space experiments on a vertebrate. Advances in Space Research, 28, 569–578. [DOI] [PubMed] [Google Scholar]
- Harrington, S.M. , Harrison, L.B. and Sheil, C.A. (2013) Ossification sequence heterochrony among amphibians. Evolution & Development, 15, 344–364. [DOI] [PubMed] [Google Scholar]
- Jeffery, J.E. , Richardson, M.K. , Coates, M.I. and Bininda‐Emonds, O.R.P. (2002) Analyzing developmental sequences within a phylogenetic framework. Systematic Biology, 51, 478–491. [DOI] [PubMed] [Google Scholar]
- Keyte, A.L. and Smith, K.K. (2014) Heterochrony and developmental timing mechanisms: changing ontogenies in evolution. Seminars in Cell & Developmental Biology, 34, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay, T. , Spenle, L. , Guyot‐Lenfant, M. , Gallien, C.L. and Chanoine, C. (1998) Analysis of muscle adaptations to terrestrial stepping in the Urodelan amphibian Pleurodeles waltlii . Pflügers Archiv, 436, 295–302. [DOI] [PubMed] [Google Scholar]
- Laurin, M. , Lapauze, O. and Marjanović, D. (2019) What do ossification sequences tell us about the origin of extant amphibians? bioRxiv 352609v4, peer‐reviewed by PCI Paleo. 10.1101/352609v4 [DOI] [Google Scholar]
- Lebedkina, N.S. (1979) Evolution of the amphibian skull. Moscow: Nauka; [in Russian]. [Google Scholar]
- Lebedkina, N.S. (2004) Evolution of the amphibian skull. Sofia: Pensoft. [Google Scholar]
- Mabee, P.M. , Olmstead, K.L. and Cubbage, C.C. (2000) An experimental study of intraspecific variation, developmental timing and heterochrony in fishes. Evolution, 54, 2091–2106. [DOI] [PubMed] [Google Scholar]
- Mabee, P.M. and Trendler, T.A. (1996) Development of the cranium and paired fins in Betta splendens (Teleostei: Percomorpha): intraspecific variation and interspecific comparisons. Journal of Morphology, 227, 249–287. [DOI] [PubMed] [Google Scholar]
- Maxwell, E.E. and Harrison, L.B. (2009) Methods for the analysis of developmental sequence data. Evolution & Development, 11, 109–119. [DOI] [PubMed] [Google Scholar]
- Maxwell, E.E. , Harrison, L.B. and Larsson, H.C.E. (2010) Assessing the phylogenetic utility of sequence heterochrony: evolution of avian ossification sequences as a case study. Zoology, 113, 57–66. [DOI] [PubMed] [Google Scholar]
- McNamara, K.J. (2012) Heterochrony: the Evolution of Development. Evolution: Education and Outreach, 5, 203–218. [Google Scholar]
- Medvedeva, I.M. (1975) The olfactory organ in amphibians and its phylogenetic significance. Leningrad: Nauka; [in Russian]. [Google Scholar]
- Medvedeva, I.M. (1986) On the origin of nasolacrimal duct in Tetrapoda In: Studies in herpetology. Third General Meeting of the Societas Europaea Herpetologica (ed Roček Z), pp. 37–40. Prague: Charles University. [Google Scholar]
- Mitgutsch, C. , Wimmer, C. , Sánchez‐Villagra, M.R. , Hahnloser, R. and Schneider, R.A. (2011) Timing of ossification in duck, quail, and zebra finch: intraspecific variation, heterochronies, and life history evolution. Zoological Science, 28, 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff, R.A. and Wray, G.A. (1989) Heterochrony: developmental mechanisms and evolutionary results. Journal of Evolutionary Biology, 2, 409–434. [Google Scholar]
- Regel, E.D. (1964) Homology of the antorbital processes in amphibians. Doklady Akademii Nauk SSSR, Biological Sciences Section, 154, 30–33. [Google Scholar]
- Reilly, S.M. (1987) Ontogeny of the hyobranchial apparatus in the salamanders Ambystoma talpoideum (Ambystomatidae) and Notophthalmus viridescens (Salamandridae): The ecological morphology of two neotenic strategies. Journal of Morphology, 191, 656–662. [DOI] [PubMed] [Google Scholar]
- Reiss, J.O. (2002) The phylogeny of amphibian metamorphosis. Zoology, 105, 85–96. [DOI] [PubMed] [Google Scholar]
- Rose, C.S. (1995a) Skeletal morphogenesis in the urodele skull: I. Postembryonic development in the Hemidactyliini (Amphibia: Plethodontidae). Journal of Morphology, 223, 125–148. [DOI] [PubMed] [Google Scholar]
- Rose, C.S. (1995b) Skeletal morphogenesis in the urodele skull: III. Effect of hormone dosage in TH‐induced remodeling. Journal of Morphology, 223, 243–261. [DOI] [PubMed] [Google Scholar]
- Rose, C.S. (1996) An endocrine–based model for developmental and morphogenetic diversification in metamorphic and paedomorphic urodeles. Journal of Zoology, 239, 253–284. [Google Scholar]
- Rose, C.S. (2003) The developmental morphology of salamander skulls In: Amphibian biology. Volume 5. Osteology (ed Heatwole, H.), pp. 1684–1781. Australia: Surrey Beatty and Sons. [Google Scholar]
- Schoch, R. (2002) The early formation of the skull in extant and Paleozoic amphibians. Paleobiology, 28, 278–296. [Google Scholar]
- Schoch, R. (2006) Skull ontogeny: developmental patterns of fishes conserved across major tetrapod clades. Evolution Development, 8, 524–536. [DOI] [PubMed] [Google Scholar]
- Schoch, R. and Carroll, R.L. (2003) Ontogenetic evidence for the Paleozoic ancestry of salamanders. Evolution & Development, 5, 314–324. [DOI] [PubMed] [Google Scholar]
- Sheil, C.A. , Jorgensen, M. , Tulenko, F. and Harrington, S. (2014) Variation in timing of ossification affects inferred heterochrony of cranial bones in Lissamphibia. Evolution & Development, 16, 292–305. [DOI] [PubMed] [Google Scholar]
- Smirnov, S.V. and Vasileva, A.B. (2001) The role of thyroid hormones in skull bone development in the ribbed newt, Pleurodeles waltl (Urodela, Salamandridae). Doklady Biological Sciences, 379, 396–398. [DOI] [PubMed] [Google Scholar]
- Smirnov, S.V. and Vassilieva, A.B. (2003) Skeletal and dental ontogeny in the smooth newt, Triturus vulgaris (Urodela: Salamandridae): role of thyroid hormone in its regulation. Russian Journal of Herpetology, 10, 93–110. [Google Scholar]
- Smirnov, S.V. and Vassilieva, A.B. (2005) Skull development in normal, TH‐exposed, and goitrogen‐treated axolotls, Ambystoma mexicanum . Russian Journal of Herpetology, 12, 113–126. [Google Scholar]
- Smirnov, S.V. , Vassilieva, A.B. and Merkulova, K.M. (2010) The nasal bone of the Iberian ribbed newt (Pleurodeles waltl; Salamandridae, Urodela): development and regulatory mechanism of its ontogeny. Doklady Biological Sciences, 431, 113–116. [DOI] [PubMed] [Google Scholar]
- Smirnov, S.V. , Vassilieva, A.B. and Merkulova, K.M. (2011) Thyroid hormone mediation in skull development of Siberian newt, Salamandrella keyserlingi (Urodela: Hynobiidae), with comparison to other species. Russian Journal of Herpetology, 18, 203–209. [Google Scholar]
- Smith, K.K. (2001) Heterochrony revisited: The evolution of developmental sequences. Biological Journal of the Linnean Society, 73, 169–186. [Google Scholar]
- Strauss, R.E. (1990) Heterochronic variation in the developmental timing of cranial ossifications in poeciliid fishes (Cyprinodontiformes). Evolution, 44, 1558–1567. [DOI] [PubMed] [Google Scholar]
- Vassilieva, A.B. and Serbinova, I.A. (2013) Bony skeleton in the Caucasian Salamander, Mertensiella caucasica (Urodela: Salamandridae): ontogeny and embryonization effect. Russian Journal of Herpetology, 20, 85–96. [Google Scholar]
- Wassersug, R.J. (1976) A procedure for differential staining of cartilage and bone in whole formalin‐fixed vertebrates. Stain Technology, 51, 131–134. [DOI] [PubMed] [Google Scholar]
- Weisbecker, V. and Mitgutsch, C. (2010) A large‐scale survey of heterochrony in anuran cranial ossification patterns. Journal of Zoological Systematics and Evolutionary Research, 48, 332–347. [Google Scholar]
- Weisbecker, V. , Goswami, A. , Wroe, S. and Sánchez‐Villagra, M.R. (2008) Ossification heterochrony in the therian postcranial skeleton and the marsupial‐placental dichotomy. Evolution, 62, 2027–2041. [DOI] [PubMed] [Google Scholar]
- Yeh, J. (2002) The evolution of development: two portraits of skull ossification in pipoid frogs. Evolution, 56, 2484–2498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


