Abstract
Ontogenetic changes in the human masticatory complex suggest that bite force, a key measure of chewing performance, increases throughout growth and development. Current published bite force values for humans exist for molar and incisal biting, but few studies measure bite forces across all tooth types, or measure bite force potentials in subjects of different ages. In the absence of live data, models of bite force such as the Constrained Lever Model (CLM), are employed to predict bite force at different bite points for adults, but it is unclear whether such a model can accurately predict bite force potentials for juveniles or subadults. This study compares theoretically derived bite forces and live bite force data, and places these within an ontogenetic context in humans. Specifically, we test whether (1) patterns of maximum bite force increase along the tooth row throughout ontogeny, (2) bite force patterns estimated using the CLM match patterns observed from live bite force data, and (3) changes in bite forces along the tooth row and throughout ontogeny are associated with concomitant changes in adductor muscle leverage. Our findings show that maximum bite forces increase throughout ontogeny and change along the tooth row, with the highest forces occurring at the posterior dentition. These findings adhere to the expectations under the CLM and validate the model’s utility in predicting bite force values throughout development. Furthermore, adductor muscle leverage values reflect this pattern, with the greatest leverage values occurring at the posterior dentition throughout ontogeny. The CLM informs our study of mammalian chewing mechanics by providing a model of how morphological changes of the masticatory apparatus during ontogeny affect bite force distribution along the tooth row. Furthermore, the decreased bite force magnitudes observed in juveniles and subadults compared with adults suggest that differences in juvenile and subadult diets may partially be due to differences in bite force production potentials.
Keywords: bite force, constrained lever model, humans, jaw adductors, ontogeny
Using humans as a model, we measured how maximum bite forces changed across the tooth row throughout ontogeny. We found that the highest forces are produced by the posterior dentition, which supports the predictions under the Constrained Lever Model. By comparing live bite force data to predicted values from this model, we validated its utility to predict bite forces throughout growth and further contribute to our understanding of how the growing masticatory system produces increased bite force.
1. INTRODUCTION
Maximum bite force quantifies the potential force that can be exerted during a chewing bout and provides a gauge of jaw muscle activity and functioning (Koc et al., 2010). In addition, it is an important measure of masticatory performance (Helkimo et al., 1976; Bakke, 2006; Castelo et al., 2010; Habegger et al., 2012; de Abreu et al., 2014). Across mammals, maximum bite force varies in accordance with food material properties and location of the bite point, and is regulated by the muscular, mechanoreceptor, dental and skeletal systems (Raadsheer et al., 1999; Rentes et al., 2002; Woda et al., 2006; Shimada et al., 2012). Maximum bite potentials, therefore, limit the types of foods that can be consumed throughout an individual’s lifetime. During mammalian ontogeny, the masticatory system undergoes significant changes as teeth are shed and replaced (Smith et al., 1994), the shape of the dental row changes to accommodate the development and eruption of new teeth, and the masticatory muscles become more developed (Thompson et al., 2003); all of which contribute to the presumption that bite forces increase during ontogeny (Kamegai et al., 2005; Usui et al., 2007). The timing of these changes has important implications for the fitness of young individuals, as they must reconcile the demands of feeding throughout growth.
In primates, the emergence of the adult dentition, particularly the first permanent molars (M1s), marks key life‐history events (e.g. Smith, 1989; Kelley and Schwartz, 2010). Although variation exists among and between species (e.g. Godfrey et al., 2001; Machanda et al., 2015), M1 emergence typically signals the point at which juvenile primates are weaned and begin to consume adult foods. Concomitant increases in maximum bite force presumably accompany the emergence of adult teeth and the transition from a juvenile to adult diet, yet the magnitude and patterning of these force changes remain unclear. Given the constraints of collecting bite force data on individual teeth and throughout ontogeny across many mammalian taxa, models of bite force must be relied upon to predict bite force values in both living and extinct taxa. Humans are ideal subjects for collecting bite force data because they can render voluntary and precise bite force measures. Theoretical models and in vivo data on bite force measures in humans have found bite force varies throughout the dentition, with the highest magnitude forces occurring on the posterior dentition (molars) and decreasing as the bite point moves to the anterior teeth (Waltimo and Kӧnӧnen, 1993; 1994; Kikuchi et al., 1997; Tortopidis et al., 1998; Shinogaya et al., 2000; Ferrario et al., 2004).
Previous studies examining human bite force have focused on absolute maximum bite force production on the molars (Ingervall and Minder, 1997; Bakke, 2006; Castelo et al., 2010) and others have examined subject groups of limited age range (e.g. Helle et al., 1983; Mountain et al., 2011; Takaki et al., 2014) and often recorded values as a result of clenching the jaw (e.g. Tortopidis et al., 1998). To date, a comprehensive examination of how bite force changes both absolutely and along the tooth row during human ontogeny has not been undertaken. Although experimental data documenting live bite force values at different tooth types exist (e.g. molar region: Ringqvist, 1973a,b; Ferrario et al., 2004; Calderon et al., 2006; Takaki et al., 2014; Abe et al., 2017; the incisors: Ringqvist, 1973b; Dan et al., 2005), they are relatively limited across all tooth types. Additionally, although bite force values are known to increase from childhood to adulthood (Bakke et al., 1990; Kiliaridis et al., 1993; Ingervall and Minder, 1997), it is unclear whether the models used to predict bite force in adults are applicable to juveniles and subadults and whether the patterns of bite force magnitude are consistent across all age groups. Furthermore, it is unknown whether theoretically obtained values are valid in comparison with data collected on live human subjects. In the absence of live data collected at a specific tooth, bite force potentials can be predicted using a model that determines how the location of a bite point on a particular tooth affects the mechanical capability of the jaw and its resulting bite force.
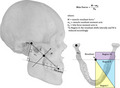
The Constrained Lever Model (CLM) developed by Greaves (1978) posits that forces applied to the mandible by the masticatory adductor muscles are resisted at three points: the bite point, the working‐side (w‐s) temporomandibular joint (TMJ), and the balancing‐side (b‐s) TMJ, making up the ‘triangle of support’ (Greaves, 1978). The adductor muscle resultant—composed of the masticatory adductor muscle vectors—must pass through the triangle of support for the TMJ to remain stable, forming the major muscle constraint of the system (Spencer and Demes, 1993). The model predicts that when the adductor muscle resultant falls within the triangle of support, loading will always be stable during mastication such that the TMJ will not be loaded with distractive forces. The components and construction of the TMJ do not lend themselves to resistance to high magnitude tensile forces (Greaves, 1978; Spencer and Demes, 1993; Spencer, 1995), thus the potential to generate relatively high magnitude bite forces will be compromised to avoid causing tensile loads at the joint, especially at posterior bite points.
The resultant lies in the midline when the w‐ and b‐s muscles are equally active (e.g. during anterior biting) and the TMJs can resist most of the load. As the bite point moves posteriorly, however, the triangle of support becomes smaller and, ultimately, the resultant falls outside of the triangle boundaries (Spencer, 1995; 1999; Lucas, 2012). To avoid loading the TMJ in tension and prevent TMJ distraction during posterior biting, the resultant is hypothesized to move away from the midline and towards the w‐s by reducing muscle force on the b‐s (Greaves, 1978). Although providing a safeguard for the TMJ, this lateral movement of the resultant, caused by the reduced forces on the b‐s, has the effect of incrementally reducing maximum bite forces as the bite point moves distally. Indeed, Hylander (1979) observed in his work with macaques that powerful isometric biting on the third molars (M3) led to an inability to control the placement of the muscle resultant and maintain it within the triangle of support, resulting in tension at the joint. Based on the anteroposterior position at which the resultant crosses the triangle of support and its mediolateral movements, the mandible has been divided into three regions (Figure 1). Region I contains bite points at which the muscle resultant stays in the midline. Region II contains bite points that cause the resultant to shift laterally in order to stay within the triangle of support. Region III does not contain teeth, as biting in this region would produce distractive forces at the TMJ.
Figure 1.
Regions of the mandible defined by the Constrained Lever Model (CLM; described in text) and measurements used to calculate maximum bite force using the CLM
The CLM was used by Spencer (1995) to predict bite forces in different regions of the jaws using the skulls of anthropoid primates. Under this model, the TMJ is vulnerable to distraction during biting on posterior teeth and therefore changes in the bite point and other masticatory system components are expected to occur (Spencer, 1995; 1999). Spencer’s comparative morphometric work suggests that anthropoid craniofacial form is constrained by the need to avoid TMJ distraction (Spencer, 1999) and that the bite points with highest force potentials will occur on the postcanine dentition where the mechanical advantage of the masticatory muscles is greatest. Based on Greaves’ CLM model (Greaves, 1978), bite force in adult humans is predicted to increase posteriorly until reaching M1, at which point bite forces should begin to decrease to M2 (and M3). This shift at the M1 occurs because the balancing side muscle force decreases (discussed in further detail in the Methods section), which divides the dentition into Region I and Region II. In support of these expectations, Spencer (1998) found that maximum bite force magnitudes were greatest at M1 and that relative b‐s masseter and anterior temporalis activity decreased along the molar row, indicating that all incisors, canines and premolars are positioned in Region I, whereas all molars are in Region II.
Although the CLM has been tested on adult humans, as described above, and applied to the masticatory morphology of extant and fossil anthropoids (e.g. Wright, 2005; Smith et al., 2015; Ledogar et al., 2016b), the ontogeny of bite force production and how well it is predicted by the CLM has received relatively little attention (but see Thompson et al., 2003). This study aims to fill this gap by addressing three specific goals
1. Determine the pattern of maximum bite force production across the tooth row throughout ontogeny. Several features of the immature masticatory system should influence bite force production. The dentition undergoes changes as deciduous teeth are shed and permanent ones are acquired. As the craniofacial complex undergoes growth and development (in both skeletal morphology and musculature), it is not likely to function at the same level as would be expected in adults. As individuals age, absolute bite forces are expected to increase at every bite point. Further, the pattern of bite force in juveniles and subadults is expected to be similar to that in adults: maximum bite forces should increase posteriorly in Region I and decrease posteriorly in Region II. How the location of the boundary between these two regions changes throughout ontogeny remains unknown, however.
2. Examine whether the bite force pattern estimated from skeletal samples using the CLM matches the pattern observed from live bite force data. To determine whether the CLM can be applied to the study of skeletal or fossil remains of juvenile and subadult primates, we aim to compare the pattern of bite forces determined based on the CLM to that derived from live data. Our objective is to compare the pattern of bite force production rather than absolute bite forces because estimates of the latter using both methods are not comparable in this study (i.e. we do not estimate muscle force for the skeletal sample).
3. Track any potential changes in adductor muscle leverage. This is done to determine whether any potential differences in bite force throughout ontogeny are influenced not only by the size of the muscles but also by potential changes in muscle leverage that may occur due to the shifting spatial configuration of the growing masticatory system.
2. METHODS
2.1. Study sample
Live bite force data were collected on 15 living human subjects (three male, 12 female) ranging from age 6.5 to age 29 years. These individuals were grouped as juveniles (6.5–11 years), subadults (18 years) and adults (ages 19–29 years) for the purposes of comparison and analysis. For the skeletal part of this research, data were collected from two cross‐sectional ontogenetic samples of human skulls. The majority of juveniles and subadults were from the Atkinson Collection, a collection of human skulls from Mexico, India, Europe, Peru, Asia and Australia/New Zealand amassed in the 1930s and housed at the Arthur A. Dugoni School of Dentistry at the University of the Pacific, San Francisco, CA, USA. The Robert J. Terry Anatomical Skeletal Collection of modern human skeletons was also used in this study. This cadaveric collection is housed at the National Museum of Natural History, Washington, DC, USA. For use in this study, the human skeletal sample was divided into four molar emergence categories (dp4‐emerged, n = 25; M1‐emerged, n = 21; M2‐emerged, n = 31; and M3‐emerged, n = 50). A molar was scored as emerged when the entire occlusal surface was above the alveolar plane. Individuals with craniofacial malformations, missing teeth with bone resorption, or tooth agenesis were excluded for the purposes of this study.
2.2. 3D landmark data collection
Following the methods of Spencer (1995) and Lucas (2012), data were collected on the spatial configuration of the masticatory system throughout ontogeny by digitizing 29 homologous landmarks (Table 1; Figure 2) characterizing masticatory configuration, including the position of teeth and the origins and insertions of the masticatory muscles. The data were obtained using a Microscribe G3X digitizer (Immersion Corp., San Jose, CA, USA). The coordinate data were used to calculate bite forces along the tooth row according to the CLM and muscle leverage at various bite points (see ‘Skeletal bite force and muscle leverage data collection’ section for more detail). All landmark data were analyzed using customized code written in R 3.0.2 (R Core Team, 2013) by H.G. (Appendix S2).
Table 1.
3D coordinates and marks collected for study
| Landmark # | Description |
|---|---|
| 1, 2 | Center of L and R articular eminence |
| 3 | Inferior edge of L zygomatic arch at the anterior‐most point of origin of the superficial masseter |
| 4 | L pterion |
| 5 | Center of medial surface of L lateral pterygoid plate |
| 6 | Hormion |
| 7 | Nasion |
| 8 | Alveolare |
| 9 | Most anterior projection of L ramus at coronoid process |
| 10 | Centroid of insertion of L superficial masseter on lateral ramus |
| 11 | Centroid of insertion of L medial pterygoid on medial angle of mandible |
| 12, 13 | Center of trigon basin of L and R M3 |
| 14, 15 | Center of trigon basin of L and R M2 |
| 16, 17 | Center of trigon basin of L and R M1 |
| 18, 19 | Center of trigon basin of L and R P4/dp4 |
| 20 | Center of trigon basin of L P3/dp3 |
| 21 | Tip of L maxillary C/dc |
| 22 | Center of incisal surface of L I2/di2 |
| 23 | Center of incisal surface of L I1/di1 |
| 24 | Distal to L P4/dp4 along alveolar border |
| 25 | Distal to L M1 along alveolar border |
| 26 | Distal to L M2 along alveolar border |
| 27 | Distal to L M3 along alveolar border |
| 28, 29 | L and R porion |
R, right; L, left. Lowercase tooth positions that begin with ‘d’ refer to deciduous dentition; uppercase tooth positions refer to permanent dentition; superscripts refer to maxillary dentition; subscripts refer to mandibular dentition.
Figure 2.
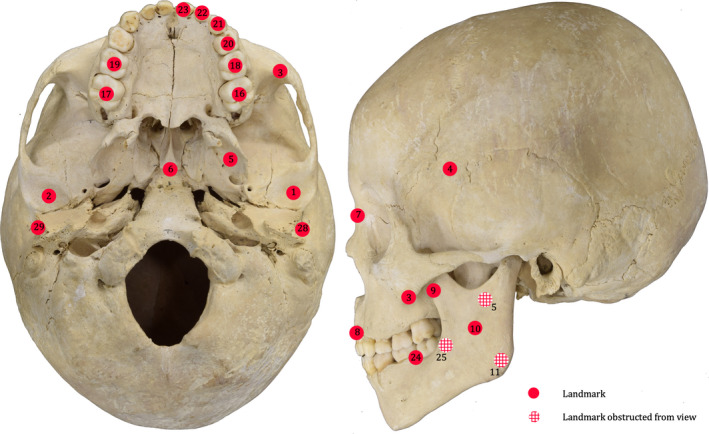
Landmarks used in this study. Landmark numbers correspond to those listed in Table 1. Landmarks 12–15 and 26–27 are not shown, as this juvenile individual does not possess M2s or M3s
2.3. Live bite force data collection
To participate in the study, living subjects were required to have good oral health and be free of permanent dental devices (e.g. braces, spacers, bridges). Arizona State University IRB approval was obtained for this study (IRB: STUDY00000067).
Bite force data were collected from each participant with the use of a Tekscan© A201 FlexiForce sensor (Tekscan) and the flexicore software program (Tekscan). A pliable ‘puck’ was applied to either side of the sensor (Figure 3) to protect it against deformation caused by the cusps of the teeth during bite force trials. Each sensor was calibrated and conditioned before use in all trials following the manufacturer’s protocol. During bite force trials, subjects were seated upright in a chair with their head held in a neutral position. Each Tekscan FlexiForce sensor was placed in a hygienic plastic sleeve prior to being given to the subject. The subject was asked to position the padded sensor end on their right third premolar with the help of a hand mirror and the researcher. The subject was instructed to bite down, as hard as possible, onto the sensor for a period of about 5–7 s, during which the highest stable bite force reading was recorded. The subject then released their bite and was given several minutes to rest before repeating the same procedure on the next tooth. The tooth order was randomized to avoid any pattern due to moving the bite point back sequentially tooth by tooth. All tooth readings were taken on the right side, except in several child subjects who had missing or loose teeth on that side. In such cases, the left side was used. The data readings for the entire testing session were recorded in Newtons.
Figure 3.
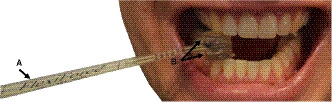
Bite force sensor and ‘puck’. (a) FlexiForce sensor used to measure live bite force values in human subjects, and (b) rubber ‘puck’ applied to each side of the sensor pad to protect it from deformation and damage during each trial
2.4. Skeletal bite force and muscle leverage data collection
Bite forces were estimated for each bite point using the CLM (Greaves, 1978). Methods for skeletal bite force estimation followed those of Lucas (2012) and are described in detail in Appendix S2. Bite force was measured as the product of muscle force and muscle resultant moment arm, divided by the bite force moment arm (Figure 1; Appendix S2). Muscle forces in Region II were adjusted by the extent of the lateral shift required for the resultant to stay in the triangle of support (see Appendix S2 for calculations). Maximum bite forces reported for skeletal material are listed in Newtons(N); however, because a constant (200 N) was used to represent muscle force, these values can only be compared within individuals, not between individuals, and are not comparable to the absolute bite force values reported for living subjects.
Using the same skeletal material, we collected additional data on muscle leverage at each bite point. Leverage was measured as the ratio of the muscle moment arm to the bite force moment arm. Leverage data were collected for the masseter, anterior temporalis and medial pterygoid muscles. Adductor muscle moment arms were measured as orthogonal distances from the TMJ to each muscle’s line of action (i.e. a line passing through the muscle’s origin and insertion sites (Figure 4, see Appendix S2 for calculations), in the sagittal plane. Bite point moment arms were measured as the distance from the TMJ to the bite point, in the occlusal plane (Figure 4, Appendix S2). Kruskal‐Wallis tests were performed at each bite point to examine variation in leverage across ontogeny (i.e. across molar‐emergence categories). The tests included post‐hoc pairwise comparisons between molar‐emergence categories. Additionally, one‐sided ttests were used to compare leverage at equivalent bite‐points as well as at the distal‐most bite point between molar‐emergence categories.
Figure 4.
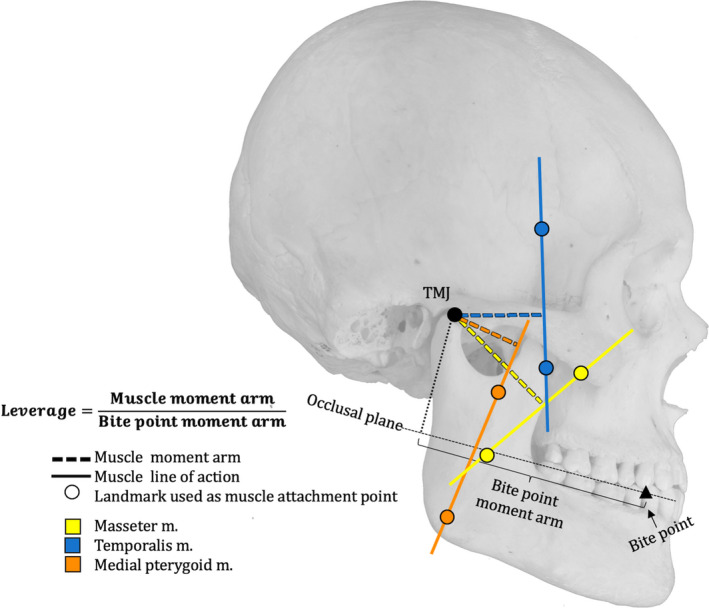
Measurements used to calculate leverage. See text for detail
3. RESULTS
Live bite forces measured from living subjects appear in Figure 5 and Table 2. The lowest bite forces were registered in the youngest individuals and the highest bite forces in the adults. The lowest bite force (5.4 N) occurred at the I2/di2 bite point in a 9‐year‐old individual. In all individuals, the lowest bite forces occurred at the anterior dentition (I1/di1, I2/di2, C/dc). The postcanine teeth yielded the highest bite forces in all individuals. The highest bite force (575.6 N) was recorded at the M1 bite point in a 20‐year‐old. In all but three adult individuals (i.e. 19–29 years of age), the highest bite forces were recorded at the M1 bite point.
Figure 5.
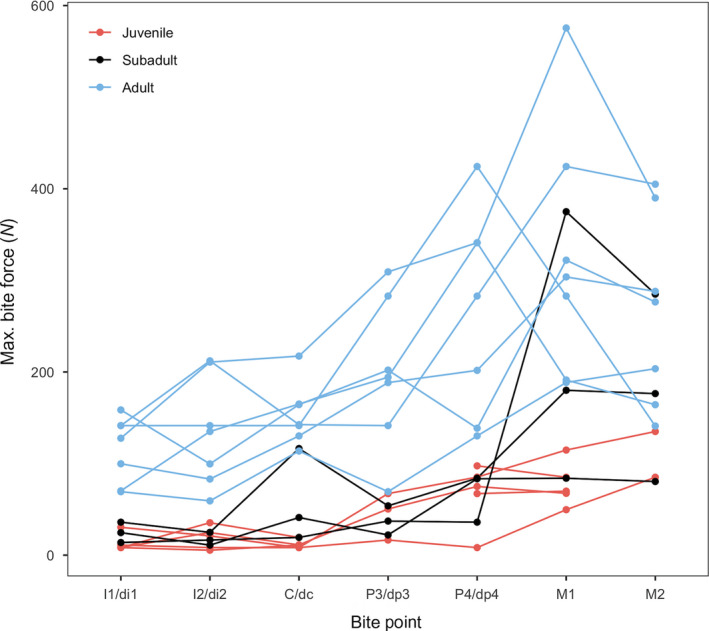
Maximum bite force data measured using a bite force sensor on human subjects. Tooth abbreviations as in Table 1
Table 2.
Live maximum bite force data measured in human subjects
| Maximum bite force (N) | |||||||
|---|---|---|---|---|---|---|---|
| Age (years) | I1/di1 | I2/di2 | C/dc | P3/dp3 | P4/dp4 | M1 | M2 |
| 6.5 | 8.3 | 35.4 | 19.4 | NA | 97.5 | 85.1 | NA |
| 9 | 8.3 | 5.4 | 11 | NA | 67.2 | 69.9 | NA |
| 10 | 8.4 | 24.6 | 11.3 | 50.4 | 75 | 67.5 | NA |
| 10 | 11 | 8.3 | 8.3 | 16.5 | 8.3 | 49.7 | 85.1 |
| 11 | 30.5 | 21 | 8.3 | 67.2 | 85.2 | 114.8 | 135 |
| 18 | 36 | 24.9 | 116.4 | 53.9 | 84 | 180 | 176.4 |
| 18 | 24.6 | 11 | 41.1 | 22.1 | 83.4 | 84 | 80.4 |
| 18 | 13.8 | 16.5 | 19.4 | 37.2 | 36 | 375 | 285 |
| 19 | 70.2 | 135 | 165 | 194.3 | 341.1 | 191.3 | 164.3 |
| 20 | 127.7 | 210.8 | 217.4 | 309.3 | 341.1 | 575.6 | 390 |
| 21 | 141.5 | 141.5 | 141.5 | 282.9 | 424.4 | 282.9 | 141 |
| 23 | 69.3 | 59.3 | 113.7 | 69.3 | 130.2 | 188.6 | 203.6 |
| 24 | 99.8 | 83.1 | 130.2 | 188.4 | 201.8 | 303.8 | 288 |
| 25 | 158.6 | 99.6 | 164.4 | 202.1 | 138.6 | 322.1 | 276.5 |
| 29 | 141.5 | 212.2 | 142.5 | 141.5 | 282.9 | 424.4 | 405 |
Tooth abbreviations as in Table 1.
Bite force curves estimated using the CLM are presented in Figure 6. Although the absolute values of the bite force estimates should not be compared directly, an average bite force curve can be calculated that indicates the overall pattern of bite forces across the dental arcade (red bite force curves in Figure 6). Bite forces increased steadily from central incisors distally to the P3/dp3 bite point in all molar emergence categories. In the dp4‐emerged category, the highest bite force was achieved at the P3/dp3 bite point. In the M1‐ and M2‐emerged categories, the P4/dp4 bite point yielded the highest bite force, whereas in the M3‐emerged category, the M1 bite point yielded the highest bite force.
Figure 6.
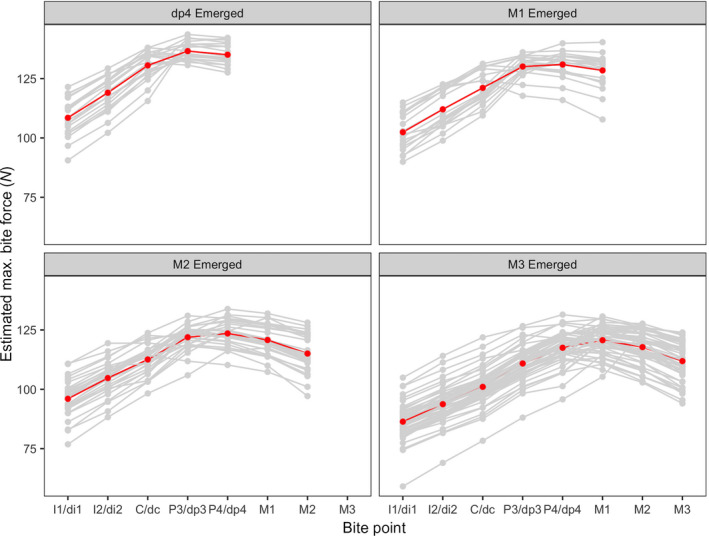
Estimated bite forces using the Constrained Lever Model for four molar emergence categories. Individual bite force curves are shown in gray. Red bite force curves represent the average curve calculated from all individuals in each molar emergence category. Tooth abbreviations as in Table 1
Muscle leverage results are illustrated in Figure 7. Across all molar emergence categories and all adductor muscles, muscle leverage generally increased posteriorly, particularly at postcanine bite points. Leverage was highest at the masseter m., followed by the anterior temporalis m., and the medial pterygoid m. Kruskal‐Wallis results revealed significant differences in leverage among molar‐emergence categories at anterior bite points (I1/di1, C/dc) for the masseter m. and at all bite points for the temporalis m.; no significant differences were found at any bite point for the medial pterygoid m. (Table 3). The ttest results for comparisons of leverage along the tooth row within molar emergence categories found significant differences among primarily posterior bite points (P3 to M3) in the masseter and temporalis m. In contrast, the muscle leverage between bite points of the medial pterygoid is not significant within the dp4 and M1 emergence stages. The only significant difference in medial pterygoid leverage occurred between posterior bite points in the M2‐emerged and M3‐emerged stages (Table 4).
Figure 7.
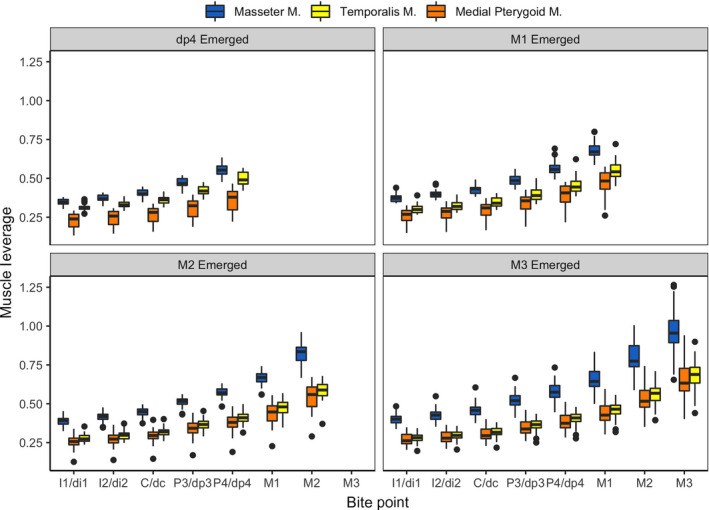
Muscle leverage, calculated as the ratio of muscle moment arm to bite point moment arm, at all bite points in four molar emergence categories. Tooth abbreviations as in Table 1
Table 3.
Kruskal‐Wallis tests for differences in muscle leverage among molar emergence categories with post hoc comparisons between molar emergence categories
| Bite Point | Masseter m. | Temporalis m. | Med. Pterygoid m. |
|---|---|---|---|
| I1/i1 | chi‐sq = 43.04 | chi‐sq = 27.45 | chi‐sq = 8.83 |
| p < .0001 | p < .001 | p = .0316 | |
| C/c | chi‐sq = 36.11 | chi‐sq = 33.70 | chi‐sq = 6.92 |
| p < .0001 | p < .001 | p = .0744 | |
| P4/dp4 | chi‐sq = 5.12 | chi‐sq = 51.69 | chi‐sq = 1.83 |
| p = .1634 | p < .001 | p = .6082 | |
| M1 | chi‐sq = 2.39 | chi‐sq = 29.79 | chi‐sq = 5.08 |
| p = .3026 | p < .001 | p = .0788 | |
| M2 | chi‐sq = 1.55 | chi‐sq = 4.53 | chi‐sq = 0.68 |
| p = .2136 | p = .0333 | p = .4089 |
| M1 em. | M2 em. | M3 em. | |
|---|---|---|---|
| Post‐hoc comparisons of masseter m. leverage at the I1/i1 bite point | |||
| dp4 em. | .0031 | <.0001 | <.0001 |
| M1 em. | ‐ | .1148 | .0013 |
| M2 em. | ‐ | ‐ | .0525 |
| Post‐hoc comparisons of masseter m. leverage at the C/c bite point | |||
| dp4 em. | .0052 | <.0001 | <.0001 |
| M1 em. | ‐ | .0545 | .0040 |
| M2 em. | ‐ | ‐ | .2417 |
| Post‐hoc comparisons of temporalis m. leverage at the I1/i1 bite point | |||
| dp4 em. | .2300 | <.0001 | .0001 |
| M1 em. | ‐ | .0053 | .0193 |
| M2 em. | ‐ | ‐ | .3952 |
| Post‐hoc comparisons of temporalis m. leverage at the C/c bite point | |||
| dp4 em. | .1605 | <.0001 | <.0001 |
| M1 em. | ‐ | .0033 | .0033 |
| M2 em. | ‐ | ‐ | .9419 |
| Post‐hoc comparisons of temporalis m. leverage at the P4/dp4 bite point | |||
| dp4 em. | .0060 | <.0001 | <.0001 |
| M1 em. | ‐ | .0025 | .0004 |
| M2 em. | ‐ | ‐ | .6725 |
| Post‐hoc comparisons of temporalis m. leverage at the M1 bite point | |||
|---|---|---|---|
| M2 em. | M3 em. | ||
| M1 em. | <.0001 | <.0001 | |
| M2 em. | ‐ | .1912 | |
| Post‐hoc comparisons of temporalis m. leverage at the M2 bite point | |||
|---|---|---|---|
| M3 em. | |||
| M2 em. | .0338 | ||
P‐values listed in bold indicate statisticallt significant values (alpha=0.05); em.,emerged.
Table 4.
ttests comparing leverage along the tooth row within molar emergence categories
| Molar emergence category | Comparison | Masseter m. | Temporalis m. | Med. Pterygoid m. | |||
|---|---|---|---|---|---|---|---|
| t | p | t | p | t | p | ||
| dp4 emerged | P4/dp4 vs P3/dp3 | 8.47 | <.00001 | 6.96 | <.00001 | 2.89 | .00589 |
| P3/dp3 vs C/dc | 8.08 | <.00001 | 6.95 | <.00001 | 2.69 | .00983 | |
| C/dc vs I2/di2 | 4.48 | .00005 | 3.70 | .00056 | 1.45 | .15470 | |
| I2/di2 vs I1/di1 | 3.72 | .00053 | 2.98 | .00456 | 1.13 | .26440 | |
| M1 emerged | M1 vs P4/dp4 | 7.23 | <.00001 | 5.13 | .00001 | 3.29 | .00208 |
| P4/dp4 vs P3/dp3 | 5.52 | <.00001 | 3.82 | .00046 | 2.41 | .02065 | |
| P3/dp3 vs C/dc | 6.01 | <.00001 | 4.18 | .00016 | 2.28 | .02815 | |
| C/dc vs I2/di2 | 3.43 | .00137 | 2.45 | .01868 | 1.22 | .22950 | |
| I2/di2 vs I1/di1 | 2.90 | .00596 | 2.11 | .04098 | 1.09 | .28140 | |
| M2 emerged | M2 vs M1 | 10.73 | <.00001 | 8.08 | <.00001 | 5.26 | <.00001 |
| M1 vs P4/dp4 | 8.84 | <.00001 | 6.04 | <.00001 | 3.72 | .00044 | |
| P4/dp4 vs P3/dp3 | 6.63 | <.00001 | 4.48 | .00003 | 2.73 | .00842 | |
| P3/dp3 vs C/dc | 7.89 | <.00001 | 5.75 | <.00001 | 3.47 | .00098 | |
| C/dc vs I2/di2 | 3.95 | .00021 | 3.22 | .00209 | 1.89 | .06334 | |
| I2/di2 vs I1/di1 | 3.12 | .00278 | 2.64 | .01060 | 1.57 | .12150 | |
| M3 emerged | M3 vs M2 | 7.47 | <.00001 | 7.51 | <.00001 | 6.12 | <.00001 |
| M2 vs M1 | 8.20 | <.00001 | 7.87 | <.00001 | 6.30 | <.00001 | |
| M1 vs P4/dp4 | 6.18 | <.00001 | 5.71 | <.00001 | 4.45 | .00002 | |
| P4/dp4 vs P3/dp3 | 5.27 | <.00001 | 4.74 | .00001 | 3.59 | .00053 | |
| P3/dp3 vs C/dc | 7.37 | <.00001 | 6.37 | <.00001 | 4.68 | .00001 | |
| C/dc vs I2/di2 | 4.07 | .00010 | 3.42 | .00093 | 2.46 | .01552 | |
| I2/di2 vs I1/di1 | 3.44 | .00085 | 2.70 | .00822 | 1.99 | .04977 | |
p values listed in bold indicate significant tests after a Bonferroni correction (alpha = .00078). Tooth abbreviations as in Table 1.
4. DISCUSSION
Studying bite force has implications for understanding masticatory performance and the nature of food processing in various taxa, and broadly informs our understanding of feeding biomechanics in living and extinct taxa. Dietary diversity is directly related to the capacity for an individual to access and process food items efficiently throughout growth and development and also has the potential to exercise considerable selective pressure on the morphology of the masticatory system. Detailed analyses of the variation in bite forces along the tooth row and throughout growth provides a basis for understanding dietary diversity across primates as well as informs clinical dental practice from a broad evolutionary perspective.
4.1. Live bite forces
Under the CLM, bite force values are expected to increase as the bite point moves posteriorly, with the highest values occurring at the M1. While this model applies to adult masticatory configurations, this study tested whether these expectations were upheld throughout ontogeny. Among the adult sample, live bite force values were highest at M1 in all but three adults, which conforms to the expectation under the model. Among the adults whose highest values did not fall at M1, one subject (23 years old) bit hardest on M2, and two others (19 and 21 years old) rendered their highest bites on P4 (see Table 2). The M2 occurs in Region II and its close proximity to the M1 bite point may explain why the forces were higher at this point. High bite forces at the P4, which lies near the Region I/Region II border, may also reflect a similar circumstance. Idiosyncratic variation among the adult sample may explain this variation as well, given the differences in sex and facial architecture. Alternatively, the variation observed in these adults may simply result from the subject feeling more comfortable biting at the P4 relative to the M1, as the more anterior placement of the sensor is less likely to trigger a gag reflex or anxiety, which has been a behavior observed previously in studies of human bite force (Markland & Wennstrӧm, 1972; Wennstrӧm, 1971; 1972). All other adult subjects consistently rendered their highest bite forces at M1 relative to any other bite point, thus supporting the predictions of the model.
In the juveniles (ages 6.5–11 years) and subadults (age 18 years), bite forces increased posteriorly, with the highest bite forces generally occurring in the postcanine dentition as predicted by the model. In juveniles who had not yet erupted an M2, the highest values occurred at the P4/dp4 and then decreased at M1, whereas juveniles with erupted M2s had their highest values at M2. In some juveniles, bite forces increased to I2/di2, decreased at C/dc and then increased significantly at P4/dp4. This result is consistent with previous studies, which found significant inter‐ and intra‐individual variation in bite forces measured on the molars and incisors of children aged 3–6 years (Gavião et al., 2007; Mountain et al., 2011) and first molars of children aged 7–9 years (Maki et al., 2001). This variation may be attributable to several physiological factors, including masticatory muscle strength, sex, dental occlusion and dental condition (Maki et al., 2001; Mountain et al., 2011). The cooperation of juvenile participants, including their ability to render a maximum bite and sustain it for the recording, is also an important consideration for these types of studies. Adequate rest time between bite force intervals were used in this study to ensure juvenile participants were able to render accurate readings. Taken together, the overall pattern of bite force across the juvenile sample is akin to the pattern predicted by the model, suggesting that even in the relatively early stages of dental development, the posterior dentition generates the highest bite force potentials.
The subadult group rendered their highest bite forces at M1 consistently, with decreased bite forces at M2 and M3, and mirrored the pattern observed in the adult sample. The force ranges of bite force values for the subadult group compared with the adult group and juvenile group revealed that subadults possess a bite force pattern more similar to adults but generate bite force values lower in magnitude than adult values (Table 2). This suggests that although subadults possess a dental configuration more similar to adults, their bite forces are relatively lower at most bite points and more similar to values of relatively younger individuals. This pattern has been observed in previous work, where bite force was found to be significantly correlated with age in individuals ages 6–18 years (Pereira‐Cenci et al., 2007), with maximum forces increasing at older ages. Bite forces peak around age 20 and then decrease through adulthood (Bakke, 2006), a pattern also observed in this study among the adult sample. This suggests that optimum bite force values occur early in adulthood and then begin to decrease as the masticatory complex begins to senesce. Regardless of age, however, the patterning of bite force along the dental row remains consistent throughout growth and into adulthood and suggests that maintenance of this pattern is an important part of an individual’s masticatory performance.
Because an equal number of males and females could not be recruited for each age group, the effect of sex was not included as part of this study. Previous work on small‐range age cohorts of children have found significant differences in males vs. females at various ages. Maki et al. (2001) found that males and females rendered similar bite forces at ages 7 and 8, but that males showed significantly higher values than females by age 9. In contrast, Sonnesen and Bakke (2005) observed that males and females rendered similar forces (with males biting slightly harder on average) from ages 7 to 12, but females rendered higher values by age 13. In that study, the number of teeth erupted was the dominant factor affecting bite force in females, suggesting that differences in eruption timing between males and females affects observed bite forces (Sonnesen and Bakke, 2005). Adult males on average generate higher maximum bite forces than females (e.g. Ferrario et al., 2004; Bakke, 2006; Kim et al., 2006), resulting from a combination of relatively larger jaw size in men (Bakke et al., 1990; Miyaura et al., 1999; Hatch et al., 2001; Shinogaya et al., 2001), fiber type in the masseter muscle (Tuxen et al., 1999) and relatively greater muscle strength in men than women (Bakke et al., 1990; Kiliaridis et al., 1993; Waltimo and Kӧnӧnen, 1993). Regardless of sex differences, the expectation is that the patterning of forces would remain consistent, even when the magnitudes of the force at various bite points change.
In general, the patterning of bite force according to the CLM is upheld through all age groups. Future work that encompasses ages within the age range of 12–17 years would be able to capture a critical growth period in which bite force magnitudes may differentiate between males and females.
4.2. Live bite force data vs. CLM
Prior to this study, no published work existed that directly compared bite force measures recorded from live human subjects with theoretical measures based on the CLM. Validating theoretical models with live data is key to ensuring the power and accuracy of the model’s predictions. Importantly, bite force curves generated from skeletally derived measures are able to capture overall patterns of force and general trends within the data; a caveat to this is that theoretical bite force values and live values are not directly comparable because predicted values are based only on simplified proxies for the masticatory system. When the patterns of theoretical bite force curves derived using the CLM are compared with live bite force values, similar patterns are observed. The congruity of the live bite force patterns compared with the theoretically derived curves confirms the CLM as an accurate model to predict bite forces along the tooth row. Furthermore, these findings support the extension of the model’s use beyond adults to include juveniles and subadults as well.
The variation in bite force magnitudes predicted by the model and observed in the live trials suggests that differences between juvenile and subadult diets may, in part, be due to the difference in potential bite force production. Furthermore, the utility of CLM extends to the study of hominin chewing mechanics by providing a model of how ontogenetic changes to masticatory morphology affect bite force production. This informs our understanding of how bite force potentials are patterned throughout growth and how they interface with changes in diet through time.
4.3. Muscle leverage
The jaw adductor muscles work together to power mastication and breakdown food through a series of coordinated actions. One goal of this study was to compare muscle leverage across bite points both within and between varying emergence stages to determine whether muscle leverage changes throughout growth. Changes in the position of a bite point affects the mechanical leverage, muscle stretch and gape required to process an item (Vinyard et al., 2008) and thus the associated bite force that can be produced will change as well. In this study, the muscle leverage for all jaw adductors increased as bite points moved posteriorly. This follows the expectation under a classic lever model, where moving the load closer to the fulcrum (i.e. TMJ) reduces the amount of muscular effort needed to power a given bite. The structural complexity of each adductor muscle also suggests that they can be recruited in many ways to produce submaximal bite forces during feeding (Hylander, 1979) and that such contributions likely vary throughout growth as the muscle develops and becomes better conditioned.
The leverage contribution of each muscle calculated in this study (masseter, temporalis m. and medial pterygoid m.) varied individually. The masseter m. consistently provided the greatest leverage compared with the other jaw adductors at each bite point and throughout each emergence stage. These results suggest that adductor muscle leverage parallels the portions of the tooth row where bite forces are also typically highest, and that this pattern remains consistent throughout ontogeny. At each emergence stage, the highest leverage values for all adductor muscles occur at the most posterior bite point present, which follows the prediction under a simple lever (i.e. the bite point that falls closest to the TMJ will exhibit the greatest mechanical leverage). Bite force values (both live and skeletally derived) confirm the expectation under the CLM that bite forces can increase posteriorly only up to a point, and thus are ‘constrained,’ as a matter of maintaining the muscle resultant within the triangle of support, and avoiding distractive forces at the TMJ during biting on the posterior teeth. Thus, the leverages calculated here simply reflect the relationship of moving a bite point closer to a muscle, which leads to greater mechanical advantage; however, the CLM accounts for how the complex architecture of the masticatory system has evolved a safety mechanism by which biting on posterior teeth will necessitate a decrease in the b‐s musculature to reduce the potential for distractive forces at the TMJ.
Significant variation in masseter muscle leverage between molar emergence stages was observed in anterior bite points (I1/di1 and C/dc) but not in posterior bite points (P4/dp4, M1, M2). However, significant differences in leverage between adjacent posterior bite points (e.g. P4/dp4, M1, M2) occurred across emergence stages in both the masseter and temporalis muscles. Unlike the posterior dentition, the anterior dentition lies in the same coronal plane. As a result, leverage values for the anterior dentition are not expected to differ significantly in this region. The leverage data presented here match what is expected based on the bite force data, where relative increases in muscle leverage mirror the increases in bite forces. Even at relatively young ages, the muscle leverage peak occurs at the P3/dp3 bite point.
In addition to factors of muscle strength and morphology, the size of the occlusal contact (primarily posterior tooth contact) is an important determinant of bite force potential (Bakke et al., 1990; Ingervall and Minder, 1997). Hidaka et al. (1999) noted that maximum bite force values increase from 30% to 100% when the contact area doubles. In children, the relatively smaller occlusal area of their teeth is expected to render smaller bite forces in comparison with adult counterparts. When the permanent set of teeth emerges, permanent premolars erupt in roughly the same area where the deciduous molars were present. In this study, it was clear that this deciduous molar region in children produced the highest bite force potentials. Children also are likely to experience their highest bite forces in this region because the rest of the deciduous dentition lack the requisite surface areas conducive to producing high forces. In adults, the permanent premolars and molars have larger surface areas available, which allow more possible places for high bite forces to be produced. As more teeth come into occlusion, they will also influence jaw movements during feeding and provide increased sensory feedback via the periodontal receptors.
5. CONCLUSION
Models of masticatory performance are routinely used in comparative analyses of bite force in modern humans as well as fossil hominins (e.g. O’Connor et al., 2005; Eng et al., 2013; Godinho et al., 2018). They find modern humans are relatively efficient at producing bite forces despite the reduction in the size of the feeding complex through evolution (e.g. Wroe et al., 2010; Ledogar et al., 2016). Indeed, the human adult masticatory complex is well attuned to processing a generalized diet and possesses key features (e.g. relatively thick tooth enamel and large tooth root surface areas) that make the dentition well suited to resist high loads (Kupczik and Dean, 2008; Olejniczak et al., 2008; Vogel et al., 2008). In combination with this tooth anatomy, human bite force on average achieves levels comparable to those of similarly sized extant hominids but with relatively lower recruitment from the jaw adductors (Wroe et al., 2010).
The shift to an adult diet can often include mechanically challenging items (i.e. foods that are highly tough and/or hard), which impose higher demands on oral processing through a combination of higher bite forces, greater muscle recruitment and longer processing times to break down a food item. In a broad evolutionary sense, the absolutely weaker bite forces generated in juvenile and subadults compared with adults illustrates a key difference in food processing potential. Indeed, the capacity to render weaker bite forces may restrict the types of foods an individual can consume and at least partially explains differences in diet among these groups. The juveniles and subadults in this study would have already shifted to an adult diet at this point in their development. While absolutely weaker bite force potentials presumably place younger individuals at a competitive disadvantage, bite force is not the sole indicator of the ability to process food items (Thompson et al., 2003). To circumvent constraints in force generation and still effectively fracture a food item, adjustments to other elements of the masticatory system (e.g. timing and succession of bites, placement of the food item on the tooth row or use of pre‐oral manipulation) can be employed.
Using humans as a model, we measured how maximum bite forces changed across the tooth row throughout ontogeny and found that the highest forces are produced by the posterior dentition (P4/dp4, M1 and M2), which followed the expectation of the CLM. Furthermore, the distribution of bite forces across the tooth row from live bite force data matched those derived from the CLM, which helps to validate the model for predictive purposes in adults and juveniles and supports the model as an accurate way to predict bite forces in humans throughout growth. Understanding how the growing masticatory system produces increased bite force can yield insight into ontogenetic shifts in diet, serves as a critical tool for reconstructing diet in extinct taxa, tracks important changes in force potential production through time, and informs clinical knowledge of dental ontogeny.
Author contributions
H.M.E. and H.G. conceived the project jointly, collected and analyzed the data, and wrote the paper.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Funding to H.M.E. and H.G. was provided by a Jumpstart grant from ASU’s GPSA. Funding to H.G. was also provided by NSF‐DDIG 1540338, Wenner‐Gren Foundation Doctoral Fieldwork Grant, Leakey Foundation Research Grant, James F. Nacey Fellowship, Elizabeth H. Harmon Research Endowment and Donald C. Johanson Paleoanthropological Research Endowment. We thank Lynn Lucas for access to her bite force calculations, Mark Spencer for helpful discussions on the development of this project, and Susanne Daly for help with Figure 3.
Edmonds H. M., and Glowacka H. (2020) The ontogeny of maximum bite force in humans. J. Anat.. 2020;237:529–542. 10.1111/joa.13218
References
- Abe, I. , Milczewski, M.S. and Souza, M.A. (2017) The force magnitude of a human bite measured at the molar intercuspidation using fiber Bragg gratings. Journal of Microwaves, Optoelectronics and Electromagnetic Applications, 16, 434–444. [Google Scholar]
- Bakke, M. (2006) Bite force and occlusion. Seminars in Orthodontics, 12, 120–126. [Google Scholar]
- Bakke, M. , Holm, B. , Jensen, B.L. et al (1990) Unilateral, isometric bite force in 8–68‐year‐old women and men related to occlusal factors. European Journal of Oral Sciences, 98, 149–158. [DOI] [PubMed] [Google Scholar]
- Calderon, P.D.S. , Kogawa, E.M. , Pereira, J.R. et al (2006) The influence of gender and bruxism on the human maximum bite force. Journal of Applied Oral Science, 14, 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo, P.M. , Pereira, L.J. , Bonjardin, L.R. et al (2010) Changes in bite force, masticatory muscle thickness, and facial morphology between primary and mixed dentition in preschool children with normal occlusion. Annals of Anatomy, 192, 23–26. [DOI] [PubMed] [Google Scholar]
- Dan, H. , Azuma, T. , Hayakawa, F. et al (2005) Measurement of bite force variables related to human discrimination of left‐right hardness differences of silicone rubber samples placed between the incisors. Archives of Oral Biology, 50, 517–526. [DOI] [PubMed] [Google Scholar]
- de Abreu, R.A.M. , Pereira, M.D. , Furtado, F. et al (2014) Masticatory efficiency and bite force in individuals with normal occlusion. Archives of Oral Biology, 59, 1065–1074. [DOI] [PubMed] [Google Scholar]
- Eng, C.M. , Lieberman, D.E. , Zink, K.D. et al (2013) Bite force and occlusal stress production in hominin evolution. American Journal of Physical Anthropology, 151, 544–557. [DOI] [PubMed] [Google Scholar]
- Ferrario, V.F. , Sforza, C. , Serrao, G. et al (2004) Single tooth bite forces in healthy young adults. Journal of Oral Rehabilitation, 31, 18–22. [DOI] [PubMed] [Google Scholar]
- Gavião, M.B. , Raymundo, V.G. and Rentes, A.M. (2007) Masticatory performance and bite force in children with primary dentition. Brazilian Oral Research, 21, 146–152. [DOI] [PubMed] [Google Scholar]
- Godfrey, L.R. , Samonds, K.E. , Jungers, W.L. et al (2001) Teeth, brains, and primate life histories. American Journal of Physical Anthropology, 114, 192–214. [DOI] [PubMed] [Google Scholar]
- Godinho, R.M. , Fitton, L.C. , Toro‐Ibacache, V., et al (2018) The biting performance of Homo sapiens and Homo heidelbergensis . Journal of Human Evolution, 118, 56–71. [DOI] [PubMed] [Google Scholar]
- Greaves, W.S. (1978) The jaw lever system in ungulates: a new model. Journal of Zoology, 184, 271–285. [Google Scholar]
- Habegger, M.L. , Motta, P.J. , Huber, D.R. et al (2012) Feeding biomechanics and theoretical calculations of bite force in bull sharks (Carcharhinus leucas) during ontogeny. Zoology, 115, 354–364. [DOI] [PubMed] [Google Scholar]
- Hatch, J.P. , Shinkai, R.S.A. , Sakai, S. et al (2001) Determinants of masticatory performance in dentate adults. Archives of Oral Biology, 46, 641–648. [DOI] [PubMed] [Google Scholar]
- Hidaka, O. , Iwasaki, M. , Saito, M. et al (1999) Influence of clenching intensity on bite force balance, occlusal contact area, and average bite pressure. Journal of Dental Research, 78, 1336–1344. [DOI] [PubMed] [Google Scholar]
- Helkimo, E. , Carlsson, G.E. and Helkimo, M. (1976) Bite force and state of dentition. Acta Odontologica Scandinavica, 35, 297–303. [DOI] [PubMed] [Google Scholar]
- Helle, A. , Tulensalo, T. and Ranta, R. (1983) Maximum bite force values of children in different age groups. Proceedings of the Finnish Dental Society, 79, 151–154. [PubMed] [Google Scholar]
- Hylander, W.L. (1979) Mandibular function in Galago crassicaudatus and Macaca fascicularis: An in vivo approach to stress analysis of the mandible. Journal of Morphology, 159, 253–296. [DOI] [PubMed] [Google Scholar]
- Ingervall, B. and Minder, C. (1997) Correlation between maximum bite force and facial morphology in children. Angle Orthodontist, 67, 415–422. [DOI] [PubMed] [Google Scholar]
- Kamegai, T. , Tatsuki, T. , Nagano, H. et al (2005) A determination of bite force in northern Japanese children. European Journal of Orthodontics, 27, 53–57. [DOI] [PubMed] [Google Scholar]
- Kelley, J. and Schwartz, G.T. (2010) Dental development and life history in living African and Asian apes. Proceedings of the National Academy of Sciences USA, 107, 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, M. , Korioth, T.W.P. and Hannam, A.G. (1997) The association among occlusal contacts, clenching effort, and bite force distribution in man. Journal of Dental Research, 76, 1316. [DOI] [PubMed] [Google Scholar]
- Kiliaridis, S. , Kjellberg, H. , Wenneberg, B. et al (1993) The relationship between maximal bite force, bite force endurance, and facial morphology during growth: A cross‐sectional study. Acta Odontologica Scandinavica, 51, 323–331. [DOI] [PubMed] [Google Scholar]
- Kim, K. , Choi, J. , Kim, S.T. et al (2006) Bite force, occlusal contact area and occlusal pressure of patients with temporomandibular joint internal derangement. Journal of Oral Medicine and Pain, 31, 265–274. [Google Scholar]
- Koc, D. , Dogan, A. and Bek, B. (2010) Bite force and influential factors on bite force measurements: a literature review. European Journal of Dentistry, 4, 223–232. [PMC free article] [PubMed] [Google Scholar]
- Kupczik, K. and Dean, M.C. (2008) Comparative observations on the tooth root morphology of Gigantopithecus blacki . Journal of Human Evolution, 54, 196–2004. [DOI] [PubMed] [Google Scholar]
- Ledogar, J.A. , Dechow, P.C. , Wang, Q. et al (2016a) Human feeding biomechanics: performance, variation, and functional constraints. PeerJ, 4, e2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledogar, J.A. , Smith, A.L. , Benazzi, S. et al (2016b) Mechanical evidence that Australopithecus sediba was limited in its ability to eat hard foods. Nature Communications, 7, 10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, L. (2012) Variation in dental morphology and bite force along the tooth row in anthropoids. PhD dissertation, Arizona State University.
- Machanda, Z. , Brazeau, N.F. , Bernard, A.B. et al (2015) Dental eruption in East African wild chimpanzees. Journal of Human Evolution, 82, 137–144. [DOI] [PubMed] [Google Scholar]
- Maki, K. , Nishioka, A.A. , Morimoto, M. et al (2001) A study on the measurement of occlusal force and masticatory efficiency in school age Japanese children. International Journal of Paediatric Dentistry, 11, 281–285. [DOI] [PubMed] [Google Scholar]
- Marklund, G. and Wennstrӧm, A . (1972) A pilot study concerning the relation between manifest anxiety and bite force. Svensk Tandläkarforbunds Tidningen 65, 107–110. [PubMed] [Google Scholar]
- Miyaura, K. , Matsuka, M. , Morita, M. et al (1999) Comparison of biting forces in different age and sex groups: A study of biting efficiency with mobile and non‐mobile teeth. Journal of Oral Rehabilitation, 26, 223–227. [DOI] [PubMed] [Google Scholar]
- Mountain, G. , Wood, D. and Toumba, J. (2011) Bite force measurement in children with primary dentition. International Journal of Paediatric Dentistry, 21, 112–118. [DOI] [PubMed] [Google Scholar]
- O’Connor, C.F. , Franciscus, R.G. and Holton, N.E. (2005) Bite force production capability and efficiency in neanderthals and modern humans. American Journal of Physical Anthropology, 127, 129–151. [DOI] [PubMed] [Google Scholar]
- Olejniczak, A.J. , Tafforeau, P. , Feeney, R.N.M. et al (2008) Three‐dimensional primate molar enamel thickness. Journal of Human Evolution, 54, 187–195. [DOI] [PubMed] [Google Scholar]
- Pereira‐Cenci, T. , Pereira, L.J. , Cenci, M.S. et al (2007) Maximal bite force and its association with temporomandibular disorders. Brazilian Dental Journal, 18, 65–68. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013) R. A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
- Raadsheer, M.C. , van Eijden, T.M.G.J. , van Ginkel, F.C. et al (1999) Contribution of jaw muscle size and craniofacial morphology to human bite force magnitude. Journal of Dental Research, 78, 31–42. [DOI] [PubMed] [Google Scholar]
- Rentes, A.M. , Gavião, M.B.D. and Amaral, J.R. (2002) Bite force determination in children with primary dentition. Journal of Oral Rehabilitation, 29, 1174–1180. [DOI] [PubMed] [Google Scholar]
- Ringqvist, M. (1973a) Fibre sizes of human masseter muscle in relation to bite force. Journal of the Neurological Sciences, 19, 297–305. [DOI] [PubMed] [Google Scholar]
- Ringqvist, M. (1973b) Isometric bite force and its relation to dimensions of the facial skeleton. Acta Odontologica Scandinavica, 31, 35–42. [DOI] [PubMed] [Google Scholar]
- Shimada, A. , Yamabe, Y. , Torisu, T. et al (2012) Measurement of dynamic bite force during mastication. Journal of Oral Rehabilitation, 39, 349–356. [DOI] [PubMed] [Google Scholar]
- Shinogaya, T. , Bakke, M. , Thomsen, C.E. et al (2000) Bite force and occlusal load in healthy young subjects – a methodological study. European Journal of Prosthodontics and Restorative Dentistry, 8, 11–15. [PubMed] [Google Scholar]
- Shinogaya, T. , Bakke, M. , Thomsen, C.E. et al (2001) Effects of ethnicity, gender and age on clenching force and load distribution. Clinical Oral Investigations, 5, 63–68. [DOI] [PubMed] [Google Scholar]
- Smith, B.H. (1989) Dental development as a measure of life history in primates. Evolution (NY), 43, 683–688. [DOI] [PubMed] [Google Scholar]
- Smith, B.H. , Crummett, T.L. and Brandt, K.L. (1994) Ages of eruption of primate teeth: A compendium for aging individuals and comparing life histories’. American Journal of Physical Anthropology, 37, 177–231. [Google Scholar]
- Smith, A.L. , Benazzi, S. , Ledogar, J.A. et al (2015) The feeding biomechanics and dietary ecology of Paranthropus boisei . Anatomical Record, 298, 145–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnesen, L. and Bakke, M. (2005) Molar bite force in relation to occlusion, craniofacial dimensions, and head posture in pre‐orthodontic children. European Journal of Orthodontics, 27, 58–63. [DOI] [PubMed] [Google Scholar]
- Spencer, M.A. (1995) Masticatory system configuration and diet in anthropoid primates. PhD dissertation, State University of New York at Stony Brook.
- Spencer, M.A. (1998) Force production in the primate masticatory system: electromyographic tests of biomechanical hypotheses. Journal of Human Evolution, 34, 25–54. [DOI] [PubMed] [Google Scholar]
- Spencer, M.A. (1999) Constraints on masticatory system evolution in anthropoid primates. American Journal of Physical Anthropology, 108, 483–506. [DOI] [PubMed] [Google Scholar]
- Spencer, M.A. and Demes, B. (1993) Biomechanical analysis of masticatory system configuration in Neandertals and Inuits. American Journal of Physical Anthropology, 91, 1–20. [DOI] [PubMed] [Google Scholar]
- Takaki, P. , Vieira, M. and Bommarito, S. (2014) Maximum bite force analysis in different age groups. International Archives of Otorhinolaryngology, 18, 272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, E.N. , Biknevicius, A.R. and German, R.Z. (2003) Ontogeny of feeding function in the gray short‐tailed opossum Monodelphis domestica: Empirical support for the constrained model of jaw biomechanics. Journal of Experimental Biology, 206, 923–932. [DOI] [PubMed] [Google Scholar]
- Tortopidis, D. , Lyons, M.F. , Baxendale, R.H. et al (1998) The variability of bite force measurement between sessions, in different positions within the dental arch. Journal of Oral Rehabilitation, 25, 681–686. [DOI] [PubMed] [Google Scholar]
- Tuxen, A. , Bakke, M. and Pinholt, E.M. (1999) Comparative data from young men and women on masseter muscle fibers, function and facial morphology. Archives of Oral Biology, 44, 509–518. [DOI] [PubMed] [Google Scholar]
- Usui, T. , Uematsu, S. and Kanegae, H. et al (2007) Change in maximum occlusal force in association with maxillofacial growth. Orthodontics and Craniofacial Research, 10, 226–234. [DOI] [PubMed] [Google Scholar]
- Vinyard, C.J. , Wall, C.E. , Williams, S.H. et al (2008) Patterns of variation across primates in jaw‐muscle electromyography during mastication. Integrative and Comparative Biology, 48, 294–311. [DOI] [PubMed] [Google Scholar]
- Vogel, E.R. , Van Woerden, J.T. , Lucas, P.W. et al (2008) Functional ecology and evolution of hominoid molar enamel thickness: Pan troglodytes schweinfurthii and Pongo pygmaeus wurmbii . Journal of Human Evolution, 55, 60–74. [DOI] [PubMed] [Google Scholar]
- Waltimo, A. and Kӧnӧnen, M.A. (1993) Novel bite force recorder and maximal isometric bite force values for healthy young adults. Scandinavian Journal of Dental Research, 101, 171. [DOI] [PubMed] [Google Scholar]
- Waltimo, A. and Kӧnӧnen, M.A. (1994) Bite force on single as opposed to all maxillary front teeth. Scandinavian Journal of Dental Research, 102, 372. [DOI] [PubMed] [Google Scholar]
- Wennstrӧm, A. (1971) Psychophysical investigation of bite force, parts I and II. Svensk Tandläkarforbunds Tidningen, 64(807–819), 821–827.
- Wennstrӧm, A. (1972) Psychophysical investigation of bite force. Darts III and IV. Svensk Tandläkarforbunds Tidningen, 65, 177–190.
- Woda, A. , Foster, K. , Mishellany, A. et al (2006) Adaptation of healthy mastication to factors pertaining to the individual or to the food. Physiology & Behavior, 89, 28–35. [DOI] [PubMed] [Google Scholar]
- Wright, B.W. (2005) Craniodental biomechanics and dietary toughness in the genus Cebus . Journal of Human Evolution, 48, 473–492. [DOI] [PubMed] [Google Scholar]
- Wroe, S. , Ferrara, T.L. , McHenry, C.R. et al (2010) The craniomandibular mechanics of being human. Proceedings of the Royal Society B, 277, 3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


