Abstract
Although the consequences of splenectomy are well understood in mice, much less is known about the immunologic changes that occur following splenectomy in humans. We sought to characterize the circulating immune cell populations of patients before and after elective splenectomy to determine if these changes are related to postsplenectomy survival outcomes. Retrospective clinical information was collected from 95 patients undergoing elective splenectomy compared with 91 patients undergoing pancreaticoduodenectomy (Whipple procedure). We further analyzed peripheral blood from five patients in the splenectomy group, collected before and after surgery, using single-cell cytometry by time-of-flight mass spectrometry. We compared pre- and postsplenectomy data to characterize both the major and minor immune cell populations in significantly greater detail. Compared with patients undergoing a Whipple procedure, splenectomized patients had significant and long-lasting elevated counts of lymphocytes, monocytes, and basophils. Cytometry by time-of-flight mass spectroscopy analysis demonstrated that the elevated lymphocytes primarily consisted of naive CD4+ T cells and a population of activated CD25+CD56+CD4+ T cells, whereas the elevated monocyte counts were mainly mature, activated monocytes. We also observed a significant increase in the expression of the chemokine receptors CCR6 and CCR4 on several cellular populations. Taken together, these data indicate that significant immunological changes take place following splenectomy. Whereas other groups have compared splenectomized patients to healthy controls, this study compared patients undergoing elective splenectomy to those undergoing a similar major abdominal surgery. Overall, we found that splenectomy results in significant long-lasting changes in circulating immune cell populations and function.
INTRODUCTION
Since Quittenbaum performed the first reported splenectomy in 1826, it has now become a well-known surgical procedure with ~27,000 splenectomies performed annually in the United States (1, 2). The indications for splenectomy include splenic rupture from trauma and a broad range of benign and malignant hematological, immunological, and oncological diseases (3, 4). Although solid primary tumors of the spleen are rare, because of the anatomical proximity to the pancreas, stomach, and colon, splenectomy is often performed during the resection of malignant pancreatic tail, gastric, and splenic flexure colon tumors to achieve complete oncologic resections (5-7).
Along with the complications associated with the surgical procedure itself, one of the main concerns of asplenia is the loss of important hematological and immunological functions (8, 9). The spleen accounts for 25% of the total lymphoid tissue mass and is separated by the perifollicular zone into two regions: the red and white pulp (10, 11). The macrophages in the red pulp filter blood and recycle iron from erythrocytes. The various immune cells in the white pulp, including B cells, T cells, NK cells, macrophages, and dendritic cells, are able to initiate and regulate innate and acquired immune responses (10, 12). It has been speculated that because of the altered hematological and immunological profiles postoperation, asplenic patients have an increased mortality risk regardless of the initial splenectomy indication, compared with the general population (13). Previous studies have shown that although the mortality risk substantially decreases after the first 90d, this risk remains elevated more than 1 y postoperation (13,14).
There are several possible explanations for the increased mortality risk of patients undergoing splenectomy. First, the spleen plays an essential role in the host defense against viruses, fungi, and, most importantly, encapsulated bacteria (10). Because of the severe impairment in their ability to recognize and clear these pathogens from the bloodstream, asplenic patients develop sepsis more easily, and infections are more likely to have a fulminant course (15). Although only occurring in a small fraction of patients, with a mortality rate of ~50%, overwhelming postsplenectomy infection is a potentially serious complication for asplenic patients (15). Furthermore, several vascular complications have been observed in asplenic patients due to activation of thrombocytes and hypercoagulability, including partial or total obstruction of arterial and venous blood vessels, pulmonary hypertension, and coronary artery disease (16-18). Finally, there is an association between splenectomy and cancer. Sun et al. (19) showed that after splenectomy, patients have a significantly higher risk of developing head and neck, gastrointestinal tract, and hematological malignancies. This finding is supported by a large study of American veterans, which also showed that splenectomy is associated with an increased risk of cancer (20).
Because the increased risk of death in asplenic patients is often immune related, we sought to determine how splenectomy alters peripheral immune cell populations in the years following splenectomy. Although several studies in animal models have examined the effects of splenectomy, detailed postsplenectomy peripheral immune cell changes have not been fully elucidated in humans. Therefore, we used both clinical laboratory testing and high-dimensional, single-cell mass cytometry (cytometry by time-of-flight mass spectrometry [CyTOF]) analysis to characterize the changes in frequency and phenotype of peripheral immune cells in the first years following splenectomy (21).
MATERIALS AND METHODS
Patient selection and information
A total of 95 patients who underwent an elective, standard-of-care splenectomy either in isolation or in conjunction with adjacent organ resection at the UCHealth University of Colorado Hospital (Aurora, CO) between 2011 and 2017 were identified. Reasons for these major abdominal surgeries included a broad range of benign and (pre)malignant indications. Patients who underwent surgery in an acute or trauma setting were excluded. Ninety-one patients who underwent a pancreaticoduodenectomy (Whipple procedure) without splenectomy at the same institution between 2011 and 2016 were selected as a comparator group. Retrospectively, patient characteristics (including age at surgery, sex, body mass index, indication for surgery, follow-up after surgery, and overall survival) were collected from their electronic medical record (Table I).
TABLE I.
Patient characteristics
| Splenectomy (n = 95) |
Nonsplenectomy (n = 91) |
|
|---|---|---|
| Age at surgery, y, median (range) | 58 (25-85) | 66 (19-88) |
| Sex, percent female (n) | 69% (66) | 47% (43) |
| BMI, median (range) | 25.2 (17.4-516) | 25.5 (16.7-38.0) |
| Follow up, y (range) | 2.67 (0.088-7.6) | 4.68 (0.16-7.10) |
| Deceased, percent (n) | 26% (25) | 45% (41) |
| Cancer, n | ||
| Adenocarcinoma: heada | 0 | 47 |
| Adenocarcinoma: tail | 22 | 0 |
| Neuroendocrine tumor: head | 1 | 12 |
| Neuroendocrine tumor: tail | 20 | 0 |
| Other tumorsb | 17 | 2 |
| Noncancer, n | ||
| Pancreatic cysts | 16 | 5 |
| Intraductal papillary mucinous neoplasm | 14 | 19 |
| Other pathologiesc | 5 | 6 |
| Node positive, percent (n) | 34% (32) | 36% (34) |
| Tumor, percent (n) | ||
| Locally advancedd | 19% (18) | 37% (34) |
| Distant metastasise | 20% (19) | 10% (9) |
Including adenocarcinomas of the extrahepatic bile duct, common bile duct, intrapancreatic bile duct, papilla of Vater, and duodenum.
Including melanoma, ovarian cancer, endometrium cancer, colon cancer, gastrointestinal stromal tumor, and solid pseudopapillary neoplasm.
Including immune thrombocytopenia, pancreatitis, pancreatolithiasis, benign vascular lesions, primary sclerosing cholangitis, and necrosis.
At the time of surgery.
Postsurgery.
For the high-dimensional immune cell profiling, five patients undergoing splenectomy were recruited to participate in this study: four females and one male, with a median age of 58 y (47–70y). The indications for surgery included various benign and malignant tumors, including a metastatic gastrointestinal stromal tumor, an adenocarcinoma of the pancreatic tail, a mucinous cystic neoplasm of the pancreatic body, a solid and pseudopapillary tumor of the pancreatic neck, and a stage IV high-grade serous ovarian carcinoma with a splenic infarction. Blood was collected from these patients prior to surgery and between 19 and 241 d postsurgery.
Written informed consent was obtained from all patients according to the Colorado Multiple Institutional Review Board (no. 11-1820).
Laboratory tests
Standard-of-care blood draws were used to obtain the grouped WBC count, RBC count, platelet count, and both the percentage and absolute number of neutrophils, lymphocytes, monocytes, eosinophils, basophils, and immature granulocytes at various time points. These time points include preoperation; 1–3 and 7–30 d postoperation; 1–2, 2–6, and 6–12 mo postoperation; and 1–2, 2–5, and >5 y postoperation (Supplemental Table I). Healthy normal blood count ranges were determined by the University of Colorado Hospital Clinical Laboratory.
Mass cytometry
For immune cell profiling using CyTOF, blood was collected in BD Vacutainer ACD tubes from five patients undergoing splenectomy. Prior to storage at −80°C, RBCs were lysed from whole blood using the BD Biosciences Lyse/Fix kit according to the manufacturer’s protocol. To decrease intrasample variability and allow analysis of cryosensitive granulocytic cells, all blood samples were prepared and cryopreserved immediately after collection. This precluded the use of a Live/Dead discriminator dye. Cells were thawed and stained individually with DNA-intercalating barcodes using the Fluidigm Barcoding Kit for simultaneous sample processing, as previously described (22). Cells were mixed and stained together for 30 min at room temperature with metal-conjugated cell surface Abs. The cells were then permeabilized using the BD Biosciences Transcription Factor Phospho Buffer Set according to the manufacturer’s protocol. Following permeabilization, the cells were stained with the intracellular Abs, followed by DNA intercalator to identify cellular events.
Mass cytometry run and sample normalization/debarcoding
Samples were collected on a Helios mass cytometer (Fluidigm). Samples were resuspended with equilibration beads to allow for signal normalization using the normalization software downloaded from the Nolan laboratory GitHub page (https://github.com/nolanlab) (23). Debarcoding software was used following normalization (https://github.com/nolanlab/single-cell-debarcoder). Normalized, debarcoded data were subjected to traditional Boolean gating in FlowJo, identifying nucleated events (191Ir+ 193Ir+). These events were then gated and exported for downstream analysis. Additional Boolean gating was later performed for CD45+ events (Fig. 3), CD4+CD3+CD19− (T cells, Fig. 4) events, or CD45+CD3− CD19− (myeloid cells, Fig. 5) events, with gating criteria identified within each figure.
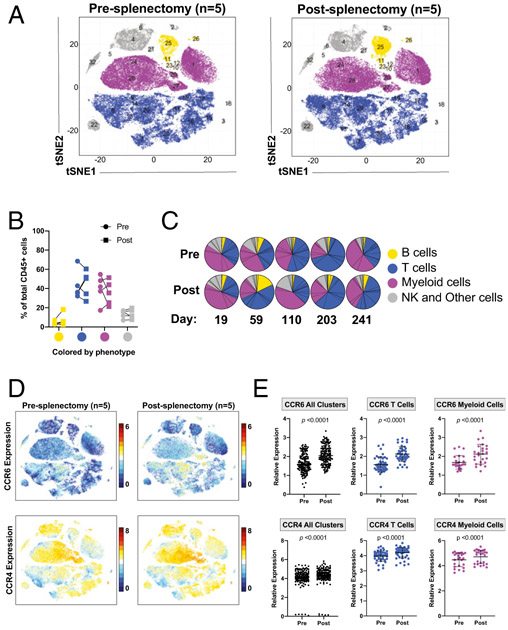
FIGURE 3. Splenectomy alters the expression of CCR4 and CCR6 on T cells and myeloid cells.
Mass cytometric analysis of lysed whole blood from patients before and after splenectomy. (C) The timing of the postsplenectomy blood draws from each patient. Files were normalized and gated on single CD45+ cells prior to analysis. (A) PhenoGraph analysis of CD45+ events comparing pre- and postsplenectomy samples from all five patients, colored by cellular phenotype (cell populations were identified based on the markers in Supplemental Table III). (B) The frequencies of PhenoGraph-defined clusters comparing the values of each cell type between pre- and postsplenectomy samples from each patient. (C) Comparison of the frequencies of PhenoGraph-defined clusters between pre- and postsplenectomy samples from each patient, including the number of days postsplenectomy the blood draw occurred. (D) The CD45+ events from pre- and postsplenectomy colored based on the expression of either CCR6 (top) or CCR4 (bottom). (E) Quantification of the mean metal intensity (relative expression level) of CCR6 (top) or CCR4 (bottom) compared between the designated cellular phenotypes and pre- and postsplenectomy samples compared across the designated cellular phenotypes.
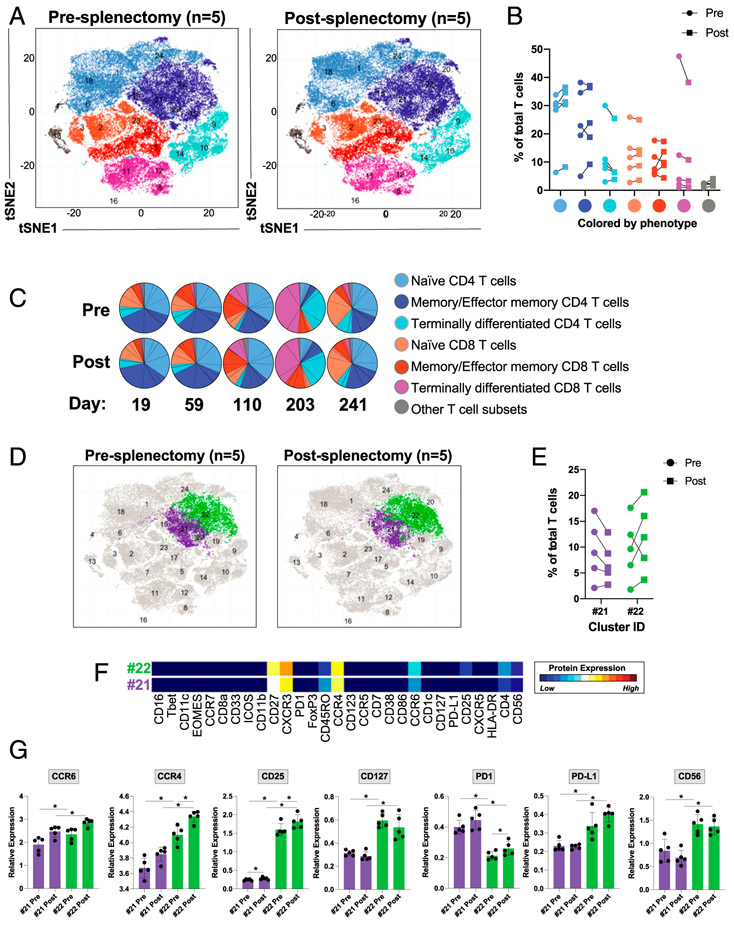
FIGURE 4. Splenectomy increases the frequency of naive CD4+ T cells, activates memory or effector memory CD4+ T cells, and increases the expression of CCR6 and CCR4 on these cells.
Mass cytometric analysis of lysed whole blood from patients before and after splenectomy. (C) The timing of the postsplenectomy blood draws from each patient. Files were normalized and gated on single CD45+CD3+CD19− cells prior to analysis. (A) PhenoGraph analysis of CD45+CD3+CD19− cells comparing pre- and postsplenectomy samples from all five patients, colored by cellular phenotype. (B) The frequencies of PhenoGraph-defined clusters comparing the values of each cell type between pre- and postsplenectomy samples from each patient (cell populations were identified based on the markers in Supplemental Table III). (C) Comparison of the frequencies of PhenoGraph-defined clusters between pre- and postsplenectomy samples from each patient, including the number of days postsplenectomy the blood draw occurred. (D and E) Identification of the two populations with the greatest degree of change between pre- and postsplenectomy. (F) Dendrogram heatmap generated in Cytofkit depicting the differences in the expression of the designated proteins between clusters 21 and 22. (G) Quantification of the mean metal intensity (relative expression level) of the designated proteins compared between clusters 21 and 22 from pre- and postsplenectomy samples. *p < 0.05.
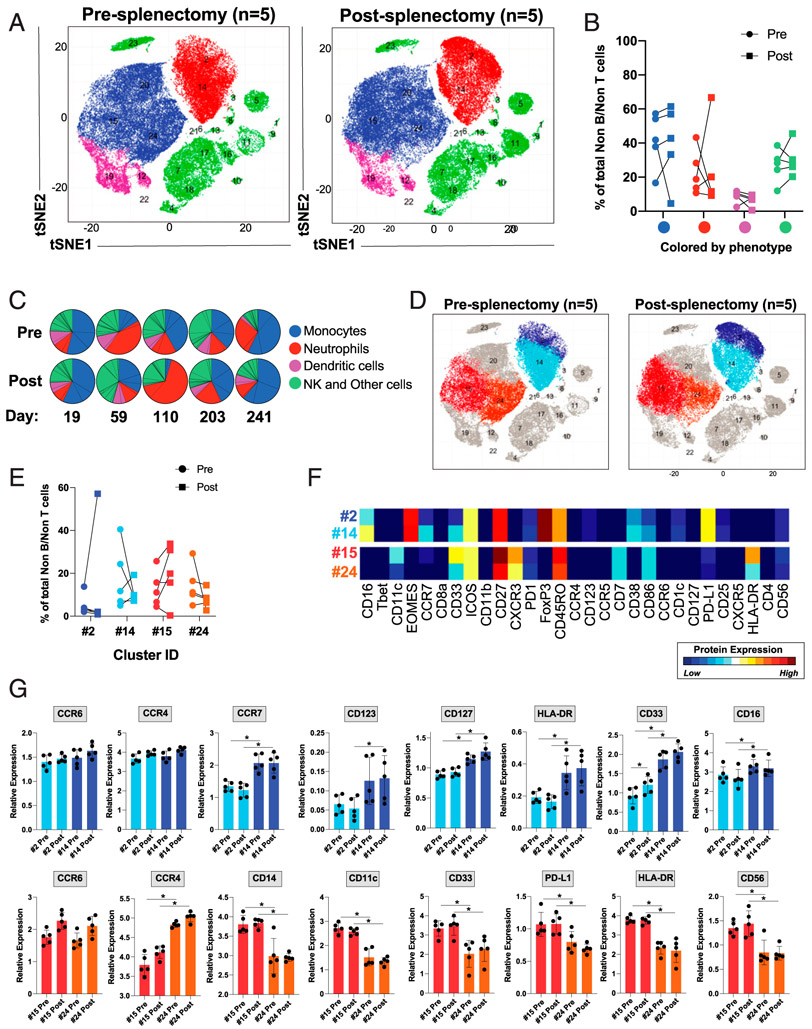
FIGURE 5. Splenectomy alters the frequency and activation of monocytes and neutrophils.
Mass cytometric analysis of lysed whole blood from patients before and after splenectomy. (C) The timing of the postsplenectomy blood draws from each patient. Files were normalized and gated on single CD45+CD3−CD19− cells prior to analysis. (A) PhenoGraph analysis focusing on CD45+CD3−CD19− myeloid cells, comparing pre- and postsplenectomy samples from all five patients, colored by cellular phenotype. (B) The frequencies of PhenoGraph-defined clusters comparing the values of each cell type between pre- and postsplenectomy samples from each patient. (C) Comparison of the frequencies of PhenoGraph-defined clusters between pre- and postsplenectomy samples from each patient, including the number of days postsplenectomy the blood draw occurred. (D and E) Identification of the populations with the greatest degree of change between pre- and postsplenectomy (cell populations were identified based on the markers in Supplemental Table III). (F) Dendrogram heatmap generated in Cytofkit depicting the differences in the expression of the designated proteins between clusters 2 and 14 or 15 and 24. (G) Quantification of the mean metal intensity (relative expression level) of the designated proteins compared between clusters 2 and 14 and between 15 and 24 from pre- and postsplenectomy samples. *p < 0.05.
PhenoGraph software downloads.
Version 1.1.453 of R Studio was downloaded from the official R Web site (http://www.r-project.org/). XQuartz v2.7.11 was downloaded (https://www.xquartz.org/). Release 3.7 of the Cytofkit package was downloaded from Bioconductor and opened using R Studio and XQuartz (http://Bioconductor.org/packages/release/bioc/html/cytofkit.html). Excel 16.15, FlowJo 10.5.3, GraphPad Prism 8.1.2, and Adobe Illustrator CC 22.1 were used to generate figures and graphs.
Manually gated nucleated events (191Ir+ 193Ir+) and further gated populations were selected, and relevant markers for clustering were chosen (note that markers used for gating imported populations were not used for clustering). PhenoGraph was run with the following settings: files were 1) merged using the merge method “min,” 2) transformed using the transformation method “cytofAsinh,” and 3) clustered using the “Rphenograph” method, coupled with the t-distributed stochastic neighbor embedding–visualization method. All other settings were automatically chosen, using default PhenoGraph settings.
PhenoGraph-defined clusters were displayed on t-distributed stochastic neighbor embedding plots within the R package “Shiny”. Within the Shiny application, cluster color was altered or colored according to protein expression. Multiple .csv files were produced by the PhenoGraph analysis, including “cluster median data” and “cluster cell percentage,” which were used to determine cluster phenotype, distribution between conditions, and statistical significance between groups. These .csv files included mean metal intensity values for each cluster. The mean metal intensity was quantified in a similar manner to mean fluorescence intensity used in conventional flow cytometry to calculate the relative expression of each marker.
Statistical analysis
A two-tailed t test was used to compare patient characteristics between the two groups. An ordinary two-way ANOVA was used to assess the statistical significance between two groups across multiple time points. We corrected for multiple comparisons using the *Šidák method for the clinical data and Tukey method for CyTOF analysis. One-way ANOVA was used to assess the statistical significance between each time point and the preoperative value. We corrected for multiple comparisons using the Bonferroni method.
Statistical analysis was performed using GraphPad Prism version 8.1.2 for Mac (GraphPad) and IBM SPSS Statistics version 25.0.0.1. Ap value <0.05 was considered to be statistically significant.
RESULTS
To compare circulating immune cell populations before and after splenectomy, we compared clinical blood draws from 95 patients before and after a non-trauma-related, elective splenectomy across a broad range of time points: preoperation; 1–3 and 7–30 d postoperation; 1–2, 2–6, and 6–12 mo postoperation; and 1–2, 2–5, and >5 y postoperation. To control for the effect of a major abdominal surgery on circulating immune cells, we included a total of 91 patients who underwent a Whipple procedure (pancreaticoduodenectomy) at the same institution, during the same time period. We retrospectively obtained patient characteristics (Table I), overall survival, and the absolute number and percentage of the major populations of blood cells using standard clinical laboratory complete blood counts.
Patient characteristics
The median age was 58 y for the splenectomy group and 66 y for the nonsplenectomy group. Sixty-nine percent of the splenectomy group and 47% of the nonsplenectomy group were female. Median body mass index was 25.2 and 25.5, respectively. Median follow-up was 2.67 y for the splenectomy group, compared with 4.68 y for the nonsplenectomy group. Of the patients in the splenectomy group, 63% were diagnosed with a malignancy, and in the comparator nonsplenectomy group, 67% had a malignancy. At the time of surgery, 34% of the splenectomized patients had lymph node metastases, compared with 36% in the nonsplenectomized patients. During the follow-up period, 20% of the splenectomized patients and 10% of the nonsplenectomized patients developed distant metastatic disease (Table I).
Survival
Although there was not a statistically significant difference in survival, there was a trend toward improved overall survival in the splenectomy group compared with controls (p = 0.1846, Fig. 1A).
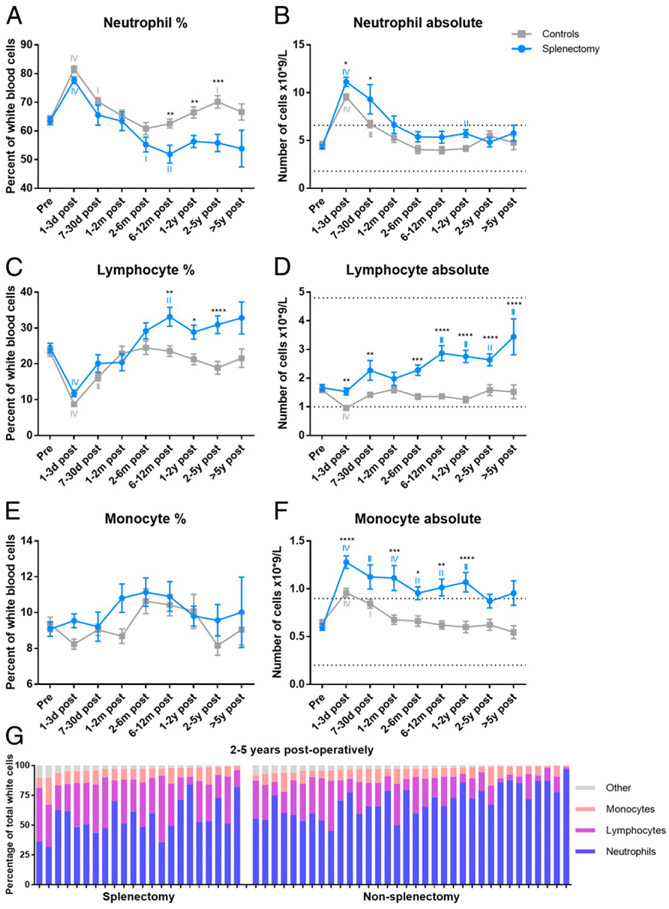
FIGURE 1. Survival and changes in major blood cell populations.
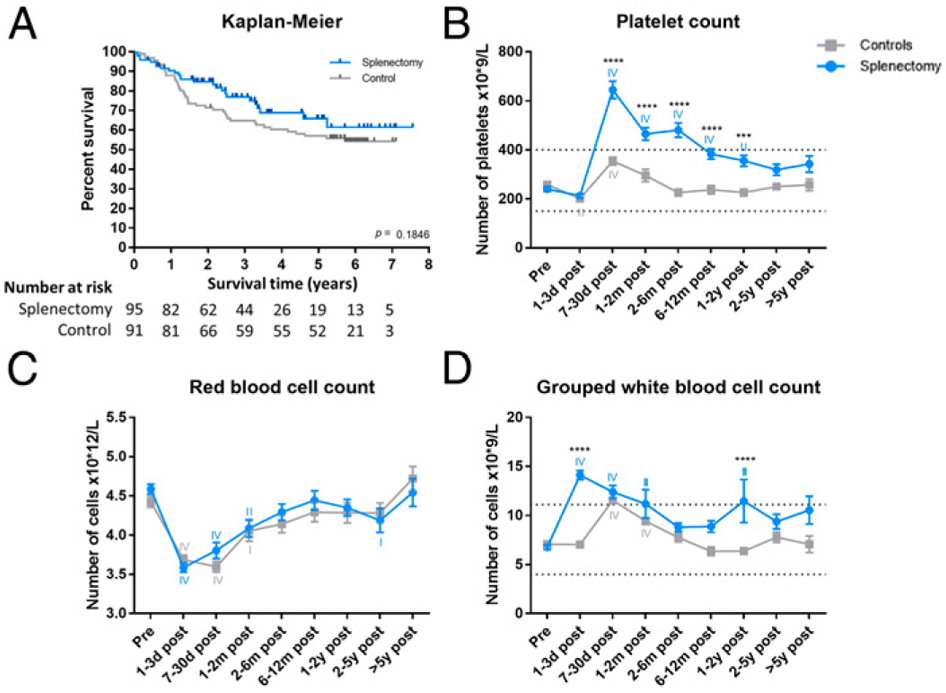
(A) Survival analysis comparing overall survival of splenectomized patients with the comparator surgery patients. The difference is not statistically significant (p = 0.1846). The absolute number of platelets (B), RBCs (C), and grouped WBCs (D) per time point. The blue line indicates the splenectomized patients, and the gray line indicates the control surgery patients. The asterisks (*) indicate statistical significance between the two groups. The Roman numerals indicate statistical significance between a certain time point and baseline. Dotted lines denote normal reference range. I indicates p ≤ 0.05, II indicates p ≤ 0.01, III indicates p ≤ 0.001, and IV indicates p ≤ 0.0001. ***p ≤ 0.001, ****p ≤ 0.0001.
Changes in major blood cell populations
The blood values revealed broad, large-scale, and long-lasting alterations in several populations of circulating blood cells following both splenectomy and comparator Whipple procedures. These changes were significantly more pronounced and longer lasting in the splenectomized patients compared with controls. As previously observed, platelet count increased and remained elevated after splenectomy compared with control surgery, peaking 7–30 d postoperation (Fig. 1B) (24). RBC counts were significantly decreased up until 2–6 mo postoperation in both groups (Fig. 1C). Finally, we observed a significant increase in the absolute number of total white cells in patients immediately following splenectomy compared with control surgery (Fig. 1D).
Changes in individual WBC populations
We further analyzed both the percentage and absolute numbers of the individual populations of WBCs to determine which specific populations contribute to the increased WBC counts in splenectomized patients. After an initial increase in the percentage of neutrophils in both groups, we noted a decreased percentage in the splenectomy patients. In contrast with the comparator surgery patients who returned to baseline 2–6 mo postoperation, the percentage of neutrophils in the splenectomized patients dropped below baseline between 2–6 and 6–12 mo postoperation before returning to baseline after 1–2 y (Fig. 2A). When looking at the absolute neutrophil count, the splenectomized patients showed a higher number at 1–3 and 7–30 d postoperation; this was not statistically significantly different at later time points (Fig. 2B). As expected, the percentage of lymphocytes was significantly decreased immediately following surgery in both groups because of the major surgical stress (25). However, the percentage of lymphocytes was significantly elevated compared with baseline at 6–12 mo postoperation and remained higher than the control group until 2–5 y postoperation (Fig. 2C). Similarly, the absolute number of lymphocytes was significantly increased compared with the control group throughout the study (Fig. 2D). Although there were no differences in the percentages of monocytes between the two groups (Fig. 2E), the absolute number was significantly increased in the splenectomy group, both compared with baseline and the control group (Fig. 2F). We further analyzed the differences between the two groups at 2–5 y postoperation (the longest follow-up time with sufficient patients for analysis) to compare the major populations of total white cells in each patient at this time point to visualize the differences in cellular subsets (Fig. 2G).
FIGURE 2. Changes in individual WBC populations: neutrophils, lymphocytes, and monocytes.
The percentage (A) and absolute number (B) of neutrophils, the percentage (C) and absolute number (D) of lymphocytes, and the percentage (E) and absolute number (F) of monocytes per time point. The percentage of the major cellular subsets per patient at time point 2–5 y postoperation (G). The blue line indicates the splenectomized patients, and the gray line indicates the control surgery patients. The asterisks (*) indicate statistical significance between the two groups. The Roman numerals indicate statistical significance between a certain time point and baseline. Dotted lines denote normal reference range. I indicates p ≤ 0.05, II indicates p ≤ 0.01, III indicates p ≤ 0.001, and IV indicates p ≤ 0.0001. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Both groups demonstrated a steep decrease in percentage and absolute number of circulating eosinophils shortly after surgery, returning to baseline after 7–30 d postoperation (Supplemental Fig. 1A, 1B). Similarly, the percentage of basophils did not change between the two groups; however, the splenectomized patients exhibited increased absolute numbers of basophils compared with the control group starting at 1–2 mo postoperation (Supplemental Fig. 1C, 1D). Finally, we observed small differences in both the percentages and absolute numbers of the immature granulocytes (Supplemental Fig. 1E, 1F).
High-dimensional analysis of pre- and postsplenectomy blood samples
We have developed a 38-metal isotope-tagged mAb panel (Supplemental Table II) to identify the major circulating immune cell subsets and characterize their function. With this panel, we analyzed lysed whole-blood samples from five patients before and after splenectomy. We sought to visualize the potential relationships between cell populations without prior designation using the PhenoGraph clustering algorithm in Cytofkit (Fig. 3A) (26). Using this software, we identified the major populations of circulating immune cells: B cells, T cells, myeloid cells, and NK and other cell types (Fig. 3A-C, Supplemental Table III). Although we did not observe statistically significant changes in the frequency of any immune cell populations using the markers described, we did observe significant changes in the expression of several immunologically relevant markers across the various clusters.
CCR6 and CCR4 expression are increased after splenectomy
We observed that both C-C chemokine receptors type 6 and 4 (CCR6 and CCR4) were increased on CD45+ cells postsplenectomy across a broad variety of immune cell populations (Fig. 3D, 3E). We further noted that both CCR6 and CCR4 were specifically upregulated on T cells (CD45+CD3+CD19−) and myeloid cells (CD45+CD3−CD19−CD14/CD15/CD66b+) (Fig. 3E). Although B cells represent a significant proportion of the lymphocytes residing in the spleen (27), we did not note significant changes in this population in the peripheral blood, in either frequency or the expression of immunologically relevant cell surface marker expression following splenectomy.
Circulating T cells are more activated following splenectomy
To further characterize the differences in the T cell populations before and after splenectomy, we clustered and analyzed the CD45+CD3+CD19− T cells alone (Fig. 4A-C, Supplemental Table III). Although there were no statistically significant changes in the frequencies of any of these major groups of T cells, we did find increased naive CD4+ T cells and decreased terminally differentiated CD4+ T cells in four of the five patients following splenectomy (Fig. 4B, 4C) (28). Furthermore, we observed changes in two of the memory and effector memory CD4+ T cell populations in four of the five patients after splenectomy: a decrease in cluster 21 (more immature or less activated) and an increase in cluster 22 (more mature or activated) (Fig. 4D, 4E). This change in frequency was associated with an increased frequency of cells expressing higher levels of CCR6, CCR4, CD25, CD127, programed death-ligand 1 (PD-L1), and CD56, as well as decreased expression of programmed death receptor 1 (PD1) (Fig. 4F, 4G). Additionally, we noted that the expression of CCR4 and CD25 was increased on the cells in cluster 22 after splenectomy (Fig. 4G).
Circulating monocytes, but not neutrophils, are activated following splenectomy
Similar to the T cells, we further analyzed the myeloid and NK cell populations by focusing on CD45+CD3−CD19− cells (Fig. 5A). In line with our findings in the larger clinical data set (Fig. 2A, 2F), we observed a decreased frequency of neutrophils (CD45+CD3−CD19−CD14−CD15+CD66b+) and increased frequency of monocytes (CD45+CD3−CD19−14+) in the majority of patients following splenectomy (Fig. 5B, 5C). We noted heterogeneous changes in frequency within the neutrophil clusters (clusters 2 and 14) (Fig. 5D, 5E). However, we did observe a decreased frequency of activated neutrophils (cluster 2, expressing higher levels of CCR7, CD123, CD127, HLA-DR, CD33, and CD16) in four of the five patients (Fig. 5D-G). In contrast with the majority of other circulating immune cell populations, we did not observe any changes in the expression of CCR6 or CCR4 in either population of neutrophils (Fig. 5F, 5G).
Within the monocyte clusters, we noted a trend toward a decreased frequency of immature monocytes (cluster 24) and increased frequency of mature monocytes (cluster 15) (Fig. 5D, 5E). The mature monocytes (cluster 15) expressed higher levels of CD14, CD11c, CD33, PD-L1, HLA-DR, and CD56 (Fig. 5F, 5G). There was a trend toward increased CCR6 but not CCR4 expression among both populations of monocytes following splenectomy, with higher CCR4 expression on the immature monocytes (cluster 24) (Fig. 5F, 5G).
DISCUSSION
Previous studies on the immunologic consequences of splenectomy have focused on changes shortly after splenectomy or have compared asplenic patients to a healthy control population (9, 14, 17, 20). Despite these studies, the human spleen, and its contributions to populations of circulating immune cells, remains understudied. In this study, we performed longitudinal clinical and mass cytometric analysis of circulating immune cells to address questions concerning alterations in human immune responses before and after splenectomy. In this study, to control for the effects of major abdominal surgery, we compared clinical blood counts and other patient characteristics between patients undergoing elective splenectomy (most often with a distal pancreatectomy or other concomitant organ removal) and those undergoing a Whipple procedure.
As has been previously reported (24), we observed a strikingly increased platelet count following splenectomy, which lasted up until 1–2 y postsurgery. This observation may be linked to the increased risk of thromboembolic and various other cardiovascular events in asplenic patients (29-31). Despite this, we did not observe an increased mortality in the splenectomized patients compared with those undergoing a Whipple procedure. Whereas previous studies have compared splenectomized patients to healthy controls, our control group mainly consisted of patients with a similar diagnosis to those undergoing splenectomy, in this case, pancreatic cancer (14, 20). Notably, cancer-specific survival may be the limiting factor determining the overall survival of both patient groups, considering that the majority of enrolled patients were diagnosed with a malignancy. However, differences in overall survival may become more evident during a longer follow-up.
In this study we found that, compared with control patients, patients undergoing splenectomy had increases in the absolute numbers of circulating lymphocytes, monocytes, and basophils. In contrast to these cell populations, we observed a decreased percentage of circulating neutrophils in splenectomized patients. This observation is likely the result of increased absolute numbers of monocytes and lymphocytes, as the absolute number of neutrophils remained unchanged. The increases in circulating lymphocytes, monocytes, and basophils were significant and long-lasting. We observed that the absolute number of lymphocytes and basophils was significantly elevated >5 y after splenectomy, whereas these cells rapidly returned to baseline levels in the control group. These results were mirrored in the monocyte population that was elevated for up to 2 y after splenectomy. As the largest secondary lymphoid organ, the spleen stores many types of immune cells (31). It is possible that the immune cells that are increased in the circulation of splenectomized patients would normally have been housed in the spleen but are now circulating, instead. Consistent with these findings, we noted a trend toward an increased frequency of naive and decreased frequency of terminally differentiated CD4+ T cells in the circulation following splenectomy.
We observed several changes in the frequency and level of activation and maturation of both T cells and myeloid cells following splenectomy. We found increased frequencies of activated mature CD4+ T cells and monocytes. For the CD4+ T cells, we noted increased expression of CD25 and an increased frequency of cells expressing the NK cell marker CD56. CD4+ T cells expressing CD56 are increased in frequency in patients infected with CMV and noted to be highly cytolytic (32, 33). Expansion of CD56+ CD4+ T cells may be indicative of reactivation of latent CMV associated with diminished IgM following splenectomy (32, 34, 35). CMV may be reactivated following splenectomy by the loss of IgM-producing memory B cells that normally reside in the spleen (35-37).
Additionally, we observed increased expression of several cell surface markers, including HLA-DR, CD14, CD33, and CD56, on monocytes that are associated with monocyte maturation and activation (38). These results, along with a shift in the frequency of immature populations toward mature populations, suggest that the monocytic populations that were increased in the clinical blood draws are more differentiated and mature than those from presplenectomy blood draws. We found smaller changes in the frequency and activation of B cells, CD8+ T cells, and neutrophils.
The most common statistically significant change observed across many of the cellular populations was increased expression of CCR6 and, to a lesser extent, CCR4. This change was more emphasized in the T cell and monocyte populations than in the neutrophils or B cells and may be a possible mechanism accounting for the differences in cellular frequencies observed in our clinical blood analysis. CCR4 and CCR6 expression are associated with Th2 and Th17 cell polarization (39-41). These cells produce cytokines and perform functions that are often associated with unfavorable outcomes in multiple types of cancer (39, 42, 43). In a large study of American military veterans, splenectomy was associated with an increased risk of several types of malignancies (20). While taking into account that the patients in our study have been previously diagnosed with a malignancy, one preliminary hypothesis that stems from this observation is that increased frequencies of CD4+ cells expressing CCR4 and CCR6 may prevent successful antitumor immunity in those not previously diagnosed with cancer. This could possibly account for the increased risk of postsplenectomy malignancies. However, the mechanism(s) that accounts for the increased expression of CCR6 and CCR4 was not analyzed as part of this study.
Patients undergoing elective splenectomy receive four prophylactic vaccines to protect against infections often observed in asplenic patients (44). These vaccines may play a role in the global immune perturbations we observed following splenectomy. Furthermore, these vaccines have been shown to increase the expression of CCR4 and CCR6 (32). However, these vaccines are generally given at least 2 wk prior to surgery (44), and with the presplenectomy blood draws occurring on the day of surgery, it is unlikely that the presplenectomy and postsplenectomy blood draws would differ drastically because of responses to these vaccines.
The role of the spleen has been extensively studied in rodent models of cancer (45). Mouse models have shown that the spleen provides a specialized environment for the generation of immune tolerance to tumor Ags (46). Additionally, in mice, the timing of splenectomy can result in significantly altered outcomes (47). Significant differences exist between the structure and function of human and mouse spleen (48). Much less is known about the effects of splenectomy in human cancer patients (49). Although some studies have shown decreased overall survival in pancreatic and gastric cancer patients who undergo splenectomy versus those who do not, the mechanisms underlying the decreased overall survival have not been elucidated (50) and may be more related to the anatomic relationship of the tumor to the spleen than to splenic function. Analogous to the known increased risk of overwhelming postsplenectomy sepsis (51), splenectomy may perturb the antitumor immune responses required for the control or clearance of cancer cells in humans.
This study should be interpreted in light of its limitations. First, to control for the effects of surgery, we compared patients undergoing splenectomy to those undergoing a Whipple procedure. The vast majority of patients in both groups had been diagnosed with cancer, which may have altered the circulating immune cell populations reported in this study. Second, because of the heterogeneity of the patients selected for the CyTOF portion of this study, only broad postsplenectomy immunologic changes were observed. Furthermore, one of the patients in the CyTOF analysis portion presented with poor appetite and drainage from their surgical wound on the day of the postsurgical blood draw. This patient was subsequently diagnosed with pneumonia, a perigastric abscess, and a suspected Clostridium difficile infection 15 d after their postsplenectomy blood draw. This was evident in both the clinical blood draw and CyTOF analysis performed at this time point. Heterogeneity of malignancy and postsplenectomy infections are common clinical presentations for this patient population, and comparisons across these patients are representative of this complex population. Despite the variability in timing and heterogeneity of diagnoses, we observed consistent and statistically significant changes when comparing pre- and post-splenectomy blood draws across these patients. These data significantly expand our knowledge of how the human immune system is altered by splenectomy and may point to new avenues of research into why patients undergoing splenectomy for nonmalignant diagnoses are at a higher risk of death.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Human Immune Monitoring Shared Resource within the University of Colorado Human Immunology and Immunotherapy Initiative for expert assistance in analysis of the peripheral blood samples required to complete this study.
This work was supported by the University of Colorado Cancer Center Support Grant (P30CA046934), the Skin Diseases Research Cores Grant (P30AR057212), and the University of Colorado Academic Enrichment Fund.
Footnotes
The online version of this article contains supplemental material.
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. National hospital discharge survey. Available at: http://www.cdc.gov/nchs/nhds/nhds_tables.htm#detailed. Accessed September 12, 2019.
- 2.Neoptolemos JP 1994. Surgery of the spleen In Oxford Textbook of Surgery, Vol. 2 Clarke PJ and Malt RA, eds. Oxford University Press, New York, p. 2121–2130. [Google Scholar]
- 3.Katz SC, and Pachter HL. 2006. Indications for splenectomy. Am. Surg 72: 565–580. [PubMed] [Google Scholar]
- 4.Weledji EP 2014. Benefits and risks of splenectomy. Int. J. Surg 12: 113–119. [DOI] [PubMed] [Google Scholar]
- 5.Ferrone CR, Konstantinidis IT, Sahani DV, Wargo JA, Fernandez-del Castillo C, and Warshaw AL. 2011. Twenty-three years of the Warshaw operation for distal pancreatectomy with preservation of the spleen. Ann. Surg 253: 1136–1139. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Dang T, Meng X, Li K, Ren W, Ma X, Huang Y, Wu X, Han W, Zhang D, et al. 2019. Is concomitant splenectomy necessary in radical gastric cancer surgery? A systematic review and meta-analysis. Asia Pac. J. Clin. Oncol 15: e28–e35. [DOI] [PubMed] [Google Scholar]
- 7.Kim CW, Shin US, Yu CS, and Kim JC. 2010. Clinicopathologic characteristics, surgical treatment and outcomes for splenic flexure colon cancer. Cancer Res. Treat 42: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadili A, and de Gara C. 2008. Complications of splenectomy. Am. J. Med 121: 371–375. [DOI] [PubMed] [Google Scholar]
- 9.Rab MAE, Meerveld-Eggink A, van Velzen-Blad H, van Loon D, Rijkers GT, and de Weerdt O. 2018. Persistent changes in circulating white blood cell populations after splenectomy. Int. J. Hematol 107: 157–165. [DOI] [PubMed] [Google Scholar]
- 10.Bronte V, and Pittet MJ. 2013. The spleen in local and systemic regulation of immunity. Immunity 39: 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Krieken JH, and te Velde J. 1988. Normal histology of the human spleen. Am. J. Surg. Pathol 12: 777–785. [DOI] [PubMed] [Google Scholar]
- 12.Jordan KR, Kapoor P, Spongberg E, Tobin RP, Gao D, Borges VF, and McCarter MD. 2017. Immunosuppressive myeloid-derived suppressor cells are increased in splenocytes from cancer patients. Cancer Immunol. Immunother 66: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyaw MH, Holmes EM, Toolis F, Wayne B, Chalmers J, Jones IG, and Campbell H. 2006. Evaluation of severe infection and survival after splenectomy. Am. J. Med 119: 276.e1–276.e7. [DOI] [PubMed] [Google Scholar]
- 14.Yong M, Thomsen RW, Schoonen WM, Farkas DK, Riis A, Fryzek JP, and Sørensen HT. 2010. Mortality risk in splenectomised patients: a Danish population-based cohort study. Eur. J. Intern. Med 21: 12–16. [DOI] [PubMed] [Google Scholar]
- 15.Rubin LG, and Schaffner W. 2014. Clinical practice. Care of the asplenic patient. N. Engl. J. Med 371: 349–356. [DOI] [PubMed] [Google Scholar]
- 16.Crary SE, and Buchanan GR. 2009. Vascular complications after splenectomy for hematologic disorders. Blood 114: 2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinette CD, and Fraumeni JF Jr 1977. Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet 2: 127–129. [DOI] [PubMed] [Google Scholar]
- 18.Meera V, Jijina F, and Ghosh K. 2010. Pulmonary hypertension in patients with hematological disorders following splenectomy. Indian J. Hematol. Blood Transfus 26: 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun LM, Chen HJ, Jeng LB, Li TC, Wu SC, and Kao CH. 2015. Splenectomy and increased subsequent cancer risk: a nation-wide population-based cohort study. Am. J. Surg 210: 243–251. [DOI] [PubMed] [Google Scholar]
- 20.Kristinsson SY, Gridley G, Hoover RN, Check D, and Landgren O. 2014. Long-term risks after splenectomy among 8,149 cancer-free American veterans: a cohort study with up to 27 years follow-up. Haematologica 99: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newell EW, Sigal N, Bendall SC, Nolan GP, and Davis MM. 2012. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. [Published erratum appears in 2013 Immunity 38: 198–199.] Immunity 36: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimball AK, Oko LM, Kaspar RE, van Dyk LF, and Clambey ET. 2019. High-dimensional characterization of IL-10 production and IL-10-dependent regulation during primary gamma-herpesvirus infection. Immunohorizons 3: 94–109. [DOI] [PubMed] [Google Scholar]
- 23.Kimball AK, Oko LM, Bullock BL, Nemenoff RA, van Dyk LF, and Clambey ET. 2018. A beginner’s guide to analyzing and visualizing mass cytometry data. J. Immunol 200: 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bordage G, Carlin B, and Mazmanian PE, American College of Chest Physicians Health and Science Policy Committee. 2009. Continuing medical education effect on physician knowledge: effectiveness of continuing medical education: American college of chest physicians evidence-based educational guidelines. Chest 135(3 Suppl.): 29S–36S. [DOI] [PubMed] [Google Scholar]
- 25.Kirov SM, Shepherd JJ, and Donald KD. 1979. Intraoperative and postoperative changes in peripheral white blood cell counts: the contribution of stress. Aust. N. Z. J. Surg 49: 738–742. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Lau MC, Wong MT, Newell EW, Poidinger M, and Chen J. 2016. Cytofkit: a bioconductor package for an integrated mass cytometry data analysis pipeline. PLOS Comput. Biol 12: e1005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis SM, Williams A, and Eisenbarth SC. 2019. Structure and function of the immune system in the spleen. Sci. Immunol 4: eaau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahnke YD, Brodie TM, Sallusto F, Roederer M, and Lugli E. 2013. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur. J. Immunol 43: 2797–2809. [DOI] [PubMed] [Google Scholar]
- 29.Lee DH, Barmparas G, Fierro N, Sun BJ, Ashrafian S, Li T, and Ley EJ. 2015. Splenectomy is associated with a higher risk for venous thromboembolism: a prospective cohort study. Int. J. Surg 24(Pt A): 27–32. [DOI] [PubMed] [Google Scholar]
- 30.Kimmig LM, and Palevsky HI. 2016. Review of the association between splenectomy and chronic thromboembolic pulmonary hypertension. Ann. Am. Thorac. Soc 13: 945–954. [DOI] [PubMed] [Google Scholar]
- 31.Pommerening MJ, Rahbar E, Minei K, Holcomb JB, Wade CE, Schreiber MA, Cohen MJ, Underwood SJ, Nelson M, and Cotton MA. 2015. Splenectomy is associated with hypercoagulable thrombelastography values and increased risk of thromboembolism. Surgery 158: 618–626. [DOI] [PubMed] [Google Scholar]
- 32.Chanouzas D, Sagmeister M, Faustini S, Nightingale P, Richter A, Ferro CJ, Morgan MD, Moss P, and Harper L. 2019. Subclinical reactivation of cytomegalovirus drives CD4+CD28null T-cell expansion and impaired immune response to pneumococcal vaccination in antineutrophil cytoplasmic antibody-associated vasculitis. J. Infect. Dis 219: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almehmadi M, Flanagan BF, Khan N, Alomar S, and Christmas SE. 2014. Increased numbers and functional activity of CD56+ T cells in healthy cytomegalovirus positive subjects. Immunology 142: 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, et al. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 104: 3647–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, et al. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med 197: 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assy N, Gefen H, Schlesinger S, and Karim W. 2007. Reactivation versus primary CMV infection after splenectomy in immunocompetent patients. Dig. Dis. Sci 52: 3477–3479. [DOI] [PubMed] [Google Scholar]
- 37.Wasserstrom H, Bussel J, Lim LC, and Cunningham-Rundles C. 2008. Memory B cells and pneumococcal antibody after splenectomy. J. Immunol 181: 3684–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sconocchia G, Keyvanfar K, El Ouriaghli F, Grube M, Rezvani K, Fujiwara H, McCoy JP Jr., Hensel N, and Barrett AJ. 2005. Phenotype and function of a CD56+ peripheral blood monocyte. Leukemia 19: 69–76. [DOI] [PubMed] [Google Scholar]
- 39.Nandi B, Pai C, Huang Q, Prabhala RH, Munshi NC, and Gold JS. 2014. CCR6, the sole receptor for the chemokine CCL20, promotes spontaneous intestinal tumorigenesis. PLoS One 9: e97566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh SP, Zhang HH, Foley JF, Hedrick MN, and Farber JM. 2008. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J. Immunol 180: 214–221. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, Toyoda M, Seki T, Morohashi M, Matsushima K, and Miyawaki T. 2000. Differential expression of the chemokine receptors by the Th1-and Th2-type effector populations within circulating CD4+ T cells. J. Leukoc. Biol 68: 568–574. [PubMed] [Google Scholar]
- 42.Kobayashi M, Kobayashi H, Pollard RB, and Suzuki F. 1998. A pathogenic role of Th2 cells and their cytokine products on the pulmonary metastasis of murine B16 melanoma. J. Immunol 160: 5869–5873. [PubMed] [Google Scholar]
- 43.Chen C, and Gao FH. 2019. Th17 cells paradoxical roles in melanoma and potential application in immunotherapy. Front. Immunol 10: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonanni P, Grazzini M, Niccolai G, Paolini D, Varone O, Bartoloni A, Bartalesi F, Santini MG, Baretti S, Bonito C, et al. 2017. Recommended vaccinations for asplenic and hyposplenic adult patients. Hum. Vaccin. Immunother 13: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higashijima J, Shimada M, Chikakiyo M, Miyatani T, Yoshikawa K, Nishioka M, Iwata T, and Kurita N. 2009. Effect of splenectomy on antitumor immune system in mice. Anticancer Res. 29: 385–393. [PubMed] [Google Scholar]
- 46.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, and Bronte V. 2012. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2: 628–639. [DOI] [PubMed] [Google Scholar]
- 47.Levy L, Mishalian I, Bayuch R, Zolotarov L, Michaeli J, and Fridlender ZG. 2015. Splenectomy inhibits non-small cell lung cancer growth by modulating anti-tumor adaptive and innate immune response. OncoImmunology 4: e998469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steiniger BS 2015. Human spleen microanatomy: why mice do not suffice. Immunology 145: 334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mebius RE, and Kraal G. 2005. Structure and function of the spleen. Nat. Rev. Immunol 5: 606–616. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz RE, Harrison LE, Conlon KC, Klimstra DS, and Brennan MF. 1999. The impact of splenectomy on outcomes after resection of pancreatic adenocarcinoma. J. Am. Coll. Surg 188: 516–521. [DOI] [PubMed] [Google Scholar]
- 51.Hansen K, and Singer DB. 2001. Asplenic-hyposplenic overwhelming sepsis: postsplenectomy sepsis revisited. Pediatr. Dev. Pathol 4: 105–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.