Abstract
Mycoplasma hyopneumoniae is the respiratory pathogen of porcine enzootic pneumonia, a chronic respiratory infectious disease that causes substantial pecuniary losses to pig husbandry worldwide. Commercial bacterins only provide incomplete protection and do not prevent the colonization and transmission of M. hyopneumoniae. Identification of new protective antigens is a key imperative for the development of more effective novel vaccine. The objective of this study was to evaluate antibody responses of 27 recombinant proteins in convalescent sera obtained from pigs that were naturally infected with M. hyopneumoniae. Fifteen proteins were identified as serological immunodominant antigens, while 3 proteins were not recognized by any convalescent serum. Moreover, Mhp462, a leucine aminopeptidase, was found to be a discriminative serological immunodominant antigen which reacted with convalescent sera but not with hyperimmune sera. The serological immunodominant proteins were antigenic and were expressed during infection; this suggests that these proteins (especially the discriminative one) are potential candidate antigens for the development of next generation vaccines against M. hyopneumoniae.
Keywords: Biotechnology, Microbiology, Infectious disease, Vaccination, Bacteria, Microbial biotechnology, Veterinary medicine, Mycoplasma hyopneumoniae, Serological immunodominant protein, Convalescent sera, Hyperimmune sera, ELISA
Biotechnology; Microbiology; Infectious disease; Vaccination; Bacteria; Microbial biotechnology; Veterinary medicine; Mycoplasma hyopneumoniae, Serological immunodominant protein, Convalescent sera, Hyperimmune sera, ELISA
1. Introduction
Mycoplasma hyopneumoniae is the respiratory pathogen of porcine enzootic pneumonia (PEP), a chronic infectious disease of pig, which results in decreased feed conversion, reduced daily weight gain, and significantly delayed time to market, and prevalent in almost all pig-rearing countries [1, 2]. More importantly, infected pigs are vulnerable to secondary infection by other pathogens, such as porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, swine influenza A virus, Pasteurella multocida, Actinobacillus pleuropneumoniae; these pathogens contribute to porcine respiratory disease complex (PRDC) [3, 4].
Several measures are implemented to control M. hyopneumoniae infection, including optimization of biosecurity precautions, use of antibacterial agents, and immunization [5]. Till date, vaccination has been the most commonly used preventive measure for control of M. hyopneumoniae because of several reasons. Firstly, vaccination reduces the clinical signs (such as non-productive cough, dyspnea and tachypnea) and minimizes macroscopic lung lesions and histopathological lesions. Secondly, the feed conversion rate was evidently improved in vaccinated groups compared to non-treatment pigs [6], even though the average daily weight gain was similar in the experimental and control groups [6, 7]. Thirdly, the colonization rate of M. hyopneumoniae in different parts of the respiratory tract of immunized pigs was significantly higher than that in non-vaccinated groups [7]. However, vaccination does not dramatically reduce the transmission of organism in the herds and between different groups [7, 8]. Several well-known proteins (including P97 and NrdF) have been used as antigens to test their protective roles in pigs after administration in a variety of forms and formulations. Nevertheless, only a few of the new vaccines have shown partial protection in vaccinated animals [9, 10, 11]. Therefore, exploration and identification of novel immunodominant proteins is a key imperative for the development of more efficient vaccines.
The genomes of several M. hyopneumoniae strains have been completely sequenced, annotated, and published and are available in the GenBank database. Five strains, 232, 7448, 168, 7422, and KM014 were characterized as pathogenic [12, 13, 14, 15, 16]. However, strain J is a non-pathogenic organism [13]. These data provide massive information for antigen screening. Previously, we have reported two methods for identification of immunodominant proteins of M. hyopneumoniae based on Mhp366 protein which reacts with IgG antibody obtained from pigs that are naturally infected with M. hyopneumoniae but is not recognized by hyperimmune sera. The first method was for screening the serological immunodominant protein antigens [17] while the second method was used to further identify the discriminative immunodominant proteins which can distinguish between naturally infected pathogen-stimulated convalescent sera and inactivated bacterin-induced hyperimmune sera [18]. In this study, subcellular localization of 27 proteins was predicted by bioinformatics tools. Then, we assessed the antigenicity and reactive properties of these proteins, which may be used as candidate antigens for new vaccine development or may serve as new targets for immunodiagnosis.
2. Materials and methods
2.1. Subcellular localization prediction of 27 M. hyopneumoniae proteins
Twenty-seven M. hyopneumoniae proteins, Mhp104, Mhp153, Mhp156, Mhp228, Mhp252, Mhp265, Mhp299, Mhp322, Mhp336, Mhp351, Mhp364, Mhp367, Mhp379, Mhp390, Mhp424, Mhp462, Mhp465, Mhp472, Mhp477, Mhp483, Mhp488, Mhp504, Mhp511, Mhp535, Mhp623, Mhp677 and Mhp682, according to 232 strain's naming pattern, were selected. The homologous proteins of the 27 selected proteins were searched through BLAST sequence alignment with the genomes of 7448, J, 168 and 7422 strains. Proteins existing in at least 3 genome-sequenced M. hyopneumoniae strains were designated as the common existing proteins, and only the common existing proteins were considered for prediction of subcellular localization.
Mycoplasmas stain as Gram-negative by Gram staining. However, these organisms have one lipid bilayer and their protein-secretion systems include secretion (Sec) and twin-arginine translocation (Tat) systems. Thus, we conducted the analysis using Gram-positive bacteria protein localization prediction.
Some online bioinformatics tools were used to predict the localization of 27 proteins. Firstly, Phobius [19] and TMHMM Server v. 2.0 [20] were developed to predict membrane proteins whose secondary structures contain alpha helices. The final localization information could also be acquired from the two online servers. Signal peptides associated with lipoproteins, with a characteristic N-terminal signal peptide that is cleaved by signal peptidase II (LspA), of the Sec translocator, were identified by LipoP 1.0 [21,22]. The signal peptides, present in substrates of the Tat exporter, which is responsible for the translocation of folded proteins, were recognized by TatP 1.0 [23]. The predictor SignalP 5.0 server [24] was used to detect the following: secretory signal peptides cleaved by signal peptidase I (LepB) and transported by the Sec translocon, signal peptides of lipoproteins cleaved by LspA and transported by the Sec translocon, and Tat signal peptides cleaved by LepB.
Manual curation was done after predicting workflow. Some membrane proteins and secretory proteins of M. hyopneumoniae have been identified by other studies [25, 26, 27, 28]. These data were considered in the final result. The localization of proteins in M. hyopneumoniae was mapped after workflow and comprehensive analysis.
2.2. Prokaryotic expression and purification of M. hyopneumoniae GST fusion proteins
Based on the genome sequence of strain 232, we synthesized 27 open reading frames (ORFs). The TGA codons encoding tryptophan in the original ORFs were modified to TGG (Sangon Biotech, China). Moreover, BamH I and SalI recognition sites were also added to the 5′ and 3′ end of the synthesized mhp156, mhp299 and mhp336 genes, respectively. While, BamH I and XhoI recognition sites were added to the 5′ and 3′ end of the other synthesized genes. Subsequently, the synthesized genes, except mhp488, which was ligated into expression vector pGEX-6P-2, were cloned into vector pGEX-6P-1 to generate recombinant plasmids. Recombinant plasmids were transformed into E. coli XL-1 Blue or E. coli BL21 (DE3) using the heat shock method. The identified colonies were cultured at 37 °C in Luria-Bertani broth with a final concentration of 100 μg/mL ampicillin and 1 mmol/L isopropyl-β-+;-thiogalactoside (IPTG) to induce the expression of recombinant proteins. Some important parameters, such as the gene length, mutant site, molecular weight of protein, recipient strain, and induction temperature are summarized in Table 1.
Table 1.
The information and parameters for the expression of 27 M. hyopneumoniae fusion proteins in this study.
| Gene | Gene size | Mutant site | Restriction sites | MWa of pure protein (kDa) | MW of GST fusion protein (kDa) | Vector | Recipient strain | Induction temperature (°C) |
|---|---|---|---|---|---|---|---|---|
| mhp104 | 984 bp | 199–201, 388–360, 628–630 | BamH Ⅰ, Xho Ⅰ | 37 | 63 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp153 | 1470 bp | 181–183, 481–483, 574–576, 1105–1107, 1405–1407, 1435–1437, 1459-1461 | BamH Ⅰ, Xho Ⅰ | 54 | 80 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp156 | 1062 bp | 133–135, 169–171, 193–195, 235–237, 256–258 | BamH Ⅰ, Sal Ⅰ | 41 | 67 | pGEX-6P-1 | XL-1 Blue | 16 |
| mhp228 | 561 bp | 394–396 | BamH Ⅰ, Xho Ⅰ | 21 | 47 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp252 | 1071 bp | 268–270 | BamH Ⅰ, Xho Ⅰ | 39 | 65 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp265 | 1125 bp | 304–306, 379–381, 586–588, 592–594, 625–627, 811–813, 889–891, 964–966 | BamH Ⅰ, Xho Ⅰ | 42 | 68 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp299 | 1383 bp | 139–141, 1096–1098, 1135–1137 | BamH Ⅰ, Sal Ⅰ | 51 | 77 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp322 | 1173 bp | 790–792 | BamH Ⅰ, Xho Ⅰ | 44 | 70 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp336 | 1473 bp | 82–84, 490–492, 925–927, 1159–1161, 1216–1218 | BamH Ⅰ, Sal I | 58 | 84 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp351 | 1461 bp | 268–270, 1114–1116 | BamH Ⅰ, Xho Ⅰ | 58 | 84 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp364 | 1599 bp | 676–678, 979–981, 1456–1458 | BamH Ⅰ, Xho Ⅰ | 61 | 87 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp367 | 1575 bp | 571–573, 736–738, 856–858, 868–870, 904–906, 1159–1161, 1207–1209 | BamH Ⅰ, Xho Ⅰ | 61 | 87 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp379 | 933 bp | 223–225, 310–312 | BamH Ⅰ, Xho Ⅰ | 36 | 62 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp390 | 1815 bp | 1066–1068, 1351–1353, 1576–1578 | BamH Ⅰ, Xho Ⅰ | 68 | 94 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp424 | 1149 bp | 10–12, 997–999 | BamH Ⅰ, Xho Ⅰ | 44 | 70 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp462 | 1380 bp | 895–897, 1193–1095, 1138–1140 | BamH Ⅰ, Xho Ⅰ | 51 | 77 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp465 | 942 bp | 319–321, 421–423, 745–747 | BamH Ⅰ, Xho Ⅰ | 37 | 63 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp472 | 729 bp | 19–21, 622–624 | BamH Ⅰ, Xho Ⅰ | 27 | 53 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp477 | 1539 bp | 1258–1260 | BamH Ⅰ, Xho Ⅰ | 58 | 84 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp483 | 804 bp | 319–321 | BamH Ⅰ, Xho Ⅰ | 31 | 57 | pGEX-6P-1 | BL21 (DE3) | 16 |
| mhp488 | 1215 bp | 430–432, 970–972 | BamH Ⅰ, Xho Ⅰ | 44 | 70 | pGEX-6P-2 | XL-1 Blue | 30 |
| mhp504 | 1848 bp | 580–582, 709–711, 1801–1803 | BamH Ⅰ, Xho Ⅰ | 66 | 92 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp511 | 1260 bp | 208–210, 301–303, 760-762 | BamH Ⅰ, Xho Ⅰ | 46 | 72 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp535 | 1677 bp | 388–390, 565–567, 928–930, 943–945, 1258–1260, 1270–1272, 1480–1482, 1489-1491 | BamH Ⅰ, Xho Ⅰ | 66 | 92 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp623 | 1356 bp | 19–21, 295–297, 412–414, 454–456, 520–522, 592–594, 736-738 | BamH Ⅰ, Xho Ⅰ | 50 | 76 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp677 | 1884 bp | 322–324, 553–555, 925-927 | BamH Ⅰ, Xho Ⅰ | 71 | 97 | pGEX-6P-1 | BL21 (DE3) | 30 |
| mhp682 | 1566 bp | 1045–1047, 1315–1317, 1348–1350, 1444-1446 | BamH Ⅰ, Xho Ⅰ | 60 | 86 | pGEX-6P-1 | BL21 (DE3) | 30 |
a MW, molecular weight.
The expressed bacteria were processed as described previously [18] and a small portion of each bacterium supernatant was purified with glutathione-conjugated agarose beads (GE Healthcare, Uppsala, Sweden). Crude purified proteins were cleaved off from the beads with PreScission protease. The purified fusion proteins and cleaved proteins were assessed by 12% sodium dodecyl sulfate-polyacrylamide gels electrophoresis (SDS-PAGE). E. coli BL21 (DE3) harboring empty pGEX-6P-1 and expressing GST served as controls, as reported previously [18].
2.3. Porcine serum samples
Three types of serum samples were used for the following assays. Seven negative sera were collected from a M. hyopneumoniae-free farm. Seven hyperimmune sera were obtained from 11-week-old pigs after their immunization with a commercial M. hyopneumoniae inactivated vaccine (MYPRAVAC SUIS, Laboratorios Hipra, La Selva, Spain) on the 7th day and 21st day after their birth in a M. hyopneumoniae-free farm. Eleven convalescent serum samples were acquired from naturally infected pigs aged 120–200 days with clinical signs or with a history of PEP. The presence of IgG antibodies against M. hyopneumoniae was confirmed in all serum samples using commercial ELISA kit (IDEXX laboratories, Westbrook, Maine, USA). Pathogens were also detected by nested PCR [30] conducted on bronchoalveolar lavage fluid (BALF) specimens obtained by fiberoptic bronchoscopy corresponding to the sera, as described in previous reports [31, 32]. Both negative sera and their corresponding BALF specimens were tested negative for M. hyopneumoniae. Hyperimmune sera were positive for IgG antibody; however, their corresponding BALF specimens were tested negative for nucleotide of M. hyopneumoniae. Both convalescent sera and their corresponding BALF specimens were positive by molecular biology and anti-M. hyopneumoniae IgG antibody detection. The experiment was performed as recommended in the Guide for the Care and Use of Laboratory Animals of the Ministry of Health, China. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Southwest University (Approval No: IACUC-20180702-10) and performed accordingly.
2.4. Screening of serological immunodominant proteins
Screening of serological immunodominant proteins that reacted with convalescent sera was performed as previously published protocol [17], with minor modification. Bacterial lysates of recombinant bacteria and control bacterium were added to glutathione-coated 96-well microplates (ThermoFisher Scientific, Rockford, IL, USA) with a volume of 200 μL/well without dilution. After overnight incubation at 4 °C and five washes with PBS containing 0.05% Tween-20 (PBST), the plates were blocked at RT for 1 h by adding 200 μL PBS +10% NBS +2.5% skimmed milk. Following five washes with PBST, 100 μL convalescent serum or negative serum with blocking solution diluted at 1:500 was added and incubated at RT for 2 h. All serum samples were pre-absorbed with lysate of control bacterium to minimize the interference of cross-reactive antibodies. After five washes with PBST, the plates were conjugated with 100 μL HRP-labeled rabbit anti-pig IgG (Invitrogen, Rockford, IL, USA) diluted at 1:40000 at 37 °C for 1 h. The plates were washed as described above, and a colorimetric reaction was induced by the addition of chromogenic substrates, substrate A (100 mL H2O containing citric acid monohydrate 0.2078 g, anhydrous sodium acetate 2.72 g, 30% hydrogen peroxide 0.06 mL) and substrate B (100 mL H2O containing citric acid monohydrate 0.2078 g, EDTA·Na2 0.04 g, TMB·2HCl 0.0391 g, glycerol 10 mL) at RT for 10 min. Color development was terminated with 50 μL 2 M H2SO4, and the OD450 was recorded using a spectrophotometer (ThermoFisher Scientific, Vantaa, Finland). A total of 7 negative sera and 11 convalescent sera were used in the ELISA assay.
Seven negative sera were reacted with lysates of control bacterium and recombinant bacteria, respectively. The difference of OD450 from lysates of recombinant bacteria and control bacterium was calculated. The results are presented as average difference (X) and standard deviation (SD). Eleven convalescent sera were reacted with lysates of control bacterium and recombinant bacteria, respectively. Similarly, the difference of OD450 from lysates of control bacterium and recombinant bacteria was also calculated and named A. The cut-off value was calculated as X + 2SD. For the interpretation, the convalescent serum was classified as positive if A ≥ X + 2SD.
2.5. Identification of discriminative serological immunodominant proteins recognized by convalescent sera but not by hyperimmune sera
The identified serological immunodominant proteins were further used to identify discriminative serological immunodominant proteins that reacted with convalescent sera but not with hyperimmune sera. The main parameters were described as reported previously [18], with minor modification. Bacterial lysates of recombinant bacteria or control bacterium were diluted at 1:5. Convalescent serum or hyperimmune serum was diluted at 1:500 with blocking solution. All serum samples were also pre-absorbed with lysate of control bacterium. The discriminative immunodominant proteins were screened using the following steps.
The average difference of OD450 from 7 hyperimmune sera that reacted with lysates of control bacterium and recombinant bacteria, respectively, was named , and the standard deviation was named . Similarly, the difference of OD450 from 11 convalescent sera that reacted with lysates of recombinant bacteria and control bacterium, respectively, was also calculated and named . The cut-off value was calculated as . If , the convalescent serum was classified as positive.
3. Results
3.1. Sequence conservation and subcellular localization of selected proteins
Conservation analysis was carried out by BLAST sequence alignment of 27 proteins based on 5 fully genome sequenced and annotated M. hyopneumoniae strains (232, 7448, J, 168, and 7422). The results are shown in Table 2. Twenty-four proteins were found in all 5 strains. Mhp228 and Mhp367 existed in 4 strains. In addition, Mhp535 was conserved in 3 strains. The results showed that the 27 proteins were commonly present in the 5 genome annotated strains.
Table 2.
Conservation analysis of 27 proteins in five sequenced and annotated M. hyopneumoniae strains.
| 232 | 7448 | J | 168 | 7422 |
|---|---|---|---|---|
| Mhp104 | MHP7448_0276 | MHJ_0268 | MHP168_297 | MHL_2660 |
| Mhp153 | MHP7448_0225 | MHJ_0219 | MHP168_244 | MHL_2920 |
| Mhp156 | MHP7448_0223 | MHJ_0217 | MHP168_242 | MHL_3160 |
| Mhp228 | MHP7448_0154 | MHJ_0150 | MHP168_149 | |
| Mhp252 | MHP7448_0129 | MHJ_0125 | MHP168_174 | MHL_2988 |
| Mhp265 | MHP7448_0115 | MHJ_0111 | MHP168_186 | MHL_0518 |
| Mhp299 | MHP7448_0082 | MHJ_0078 | MHP168_085 | MHL_3365 |
| Mhp322 | MHP7448_0309 | MHJ_0301 | MHP168_325 | MHL_3066 |
| Mhp336 | MHP7448_0331 | MHJ_0314 | MHP168_347 | MHL_2646 |
| Mhp351 | MHP7448_0339 | MHJ_0331 | MHP168_362 | MHL_3367 |
| Mhp364 | MHP7448_0353 | MHJ_0348 | MHP168_378 | MHL_3041 |
| Mhp367 | MHP7448_0356 | MHJ_0351 | MHL_2647 | |
| Mhp379 | MHP7448_0368 | MHJ_0364 | MHP168_393 | MHL_3046 |
| Mhp390 | MHP7448_0378 | MHJ_0274 | MHP168_418 | MHL_2982 |
| Mhp424 | MHP7448_0408 | MHJ_0421 | MHP168_433 | MHL_2950 |
| Mhp462 | MHP7448_0464 | MHJ_0461 | MHP168_474 | MHL_3251 |
| Mhp465 | MHP7448_0467 | MHJ_0464 | MHP168_477 | MHL_2914 |
| Mhp472 | MHP7448_0474 | MHJ_0471 | MHP168_484 | MHL_3246 |
| Mhp477 | MHP7448_0479 | MHJ_0476 | MHP168_489 | MHL_1747 |
| Mhp483 | MHP7448_0485 | MHJ_0482 | MHP168_495 | MHL_3418 |
| Mhp488 | MHP7448_0490 | MHJ_0487 | MHP168_500 | MHL_1789 |
| Mhp504 | MHP7448_0507 | MHJ_0504 | MHP168_514 | MHL_1873 |
| Mhp511 | MHP7448_0513 | MHJ_0511 | MHP168_522 | MHL_3130 |
| Mhp535 | MHP7448_0290 | MHL_3044 | ||
| Mhp623 | MHP7448_0604 | MHJ_0606 | MHP168_614 | MHL_2997 |
| Mhp677 | MHP7448_0656 | MHJ_0656 | MHP168_668 | MHL_3107 |
| Mhp682 | MHP7448_0661 | MHJ_0661 | MHP168_673 | MHL_3047 |
The predicted bioinformatics results and the manually curated results are shown in Table 3. A predictive decision tree was generated according to computational tools used in this study. If the results from both Phobius and TMHMM Server v. 2.0 were identical, the predictive localization was determined. The predictive results from the two online tools showed that a small part of Mhp104 on the N terminal protruded out of the membrane while most of it was localized in the cytoplasm. Mhp483 was a multiple transmembrane protein, while 19 proteins (Mhp153, Mhp228, Mhp265, Mhp299, Mhp322, Mhp336, Mhp364, Mhp367, Mhp379, Mhp390, Mhp424, Mhp462, Mhp472, Mhp477, Mhp488, Mhp504, Mhp535, Mhp623 and Mhp677) were extracellular. Mhp351, Mhp465, Mhp511, and Mhp682 were predicted as localized on the membrane by Phobius and as extracellular by TMHMM Server v. 2.0. However, all these proteins were predicted to contain signal peptides by Phobius, LipoP 1.0, and/or SignalP 5.0. Moreover, the N terminals of these proteins after removal of the signal peptides were embedded in the membrane. Therefore, the 4 proteins might be localized on the surface of M. hyopneumoniae. Mhp156 was predicted to have hydrophobic region in the middle of the amino acid by Phobius. It was considered as membrane protein, although it was predicted to be extracellular by TMHMM Server v. 2.0. Mhp252 was predicted to be in cytoplasm by Phobius and secreted out of the organism by TMHMM Server v. 2.0; in addition, it did not contain signal peptide according to 4 signal peptide predictors. We presumed that Mhp252 was a cytoplasmic protein.
Table 3.
The predictive results of computational tools, comprehensive predictive localization and the manually curated localization.
| Protein name | Phobiusa | TMHMM Server v. 2.0 | LipoP 1.0b | TatP 1.0c | SignalP 5.0d | Comprehensive predictive localization | Reported localization | Manual curation |
|---|---|---|---|---|---|---|---|---|
| Mhp104 | Me | M | 1–22 | M | M | |||
| Mhp153 | Ef | E | E | M [25, 26] | M (Identified) | |||
| Mhp156 | M | E | M | M [25] | M (Identified) | |||
| Mhp228 | E | E | E | E [27] | E (Identified) | |||
| Mhp252 | Cytoplasm | E | Cytoplasm | M [33] | M (Identified) | |||
| Mhp265 | E | E | E | M [25] | M (Identified) | |||
| Mhp299 | E | E | E | M [25, 26] | M (Identified) | |||
| Mhp322 | E | E | E | M [25] | M (Identified) | |||
| Mhp336 | 1–25 (E) | E (surface) | 1–24 | 1–24 | E | E | ||
| Mhp351 | 1–33 (E) | M (surface) | 1–33 | M (surface) | M | |||
| Mhp364 | 1–30 (E) | E | 1–29 | 1–29 | E | M [25], E [27] | M/E (Identified) | |
| Mhp367 | 1–22 (E) | E | 1–21 | 1–21 | E | M [25] | M (Identified) | |
| Mhp379 | 1–27 (E) | E | 1–24 | 1–24 | E | M [25] | M (Identified) | |
| Mhp390 | 1–25 (E) | E | 1–23 | 1–23 | E | M [25] | M (Identified) | |
| Mhp424 | 1–28 (E) | E | 1–27 | 1–27 | E | E | ||
| Mhp462 | E | E | E | M [34] | M (Identified) | |||
| Mhp465 | 1–25 (E) | M (surface) | 1–23 | 1–23 | M (surface) | M [25] | M (Identified) | |
| Mhp472 | E | E | E | M [25] | M (Identified) | |||
| Mhp477 | E | E | E | M [25] | M (Identified) | |||
| Mhp483 | M (multiple transmembrane) | M (multiple transmembrane) | 1–39 | M (multiple transmembrane) | M (multiple transmembrane) | |||
| Mhp488 | E | E | E | M [25] | M (Identified) | |||
| Mhp504 | E | E | E | M [25, 26] | M (Identified) | |||
| Mhp511 | 1–29 (E) | M (surface) | 1–30 | 1–30 | M (surface) | M [25,26] | M (Identified) | |
| Mhp535 | 1–35 (E) | E | 1–34 | 1–34 | E | E | ||
| Mhp623 | 1–27 (E) | E | 1–23 | 1–28 | E | M [25, 26], E [27] | M/E (Identified) | |
| Mhp677 | 1–27 (E) | E | 1–29 | 1–29 | E | M [25, 26], E [27] | M/E (Identified) | |
| Mhp682 | 1–29 (E) | M (surface) | 1–25 | 1–25 | M (surface) | M [25], E [27] | M/E (Identified) |
a-d Position of the signal peptide in the protein.
e M: membrane.
f E: extracellular.
Sixteen proteins, including Mhp153 [25,26], Mhp156 [25], Mhp252 [28], Mhp265 [25], Mhp299 [25,26], Mhp322 [25], Mhp367 [25], Mhp379 [25], Mhp390 [25], Mhp462 [29], Mhp465 [25], Mhp472 [25], Mhp477 [25], Mhp488 [25], Mhp504 [25,26] and Mhp511 [25,26] have been identified as membrane proteins. Mhp228 has been reported as secreted protein [27]. Four proteins, Mhp364 [25,27], Mhp623 [25,26,27], Mhp677 [25,26,27] and Mhp682 [25,27] were localized on membrane or were secreted out by the pathogen.
Based on the results of literature search and bioinformatics localization prediction, we concluded that all proteins were either localized on the membrane or secreted out of the organism. The identified membrane proteins were Mhp153, Mhp156, Mhp252, Mhp265, Mhp299, Mhp322, Mhp367, Mhp379, Mhp390, Mhp462, Mhp465, Mhp472, Mhp477, Mhp488, Mhp504, and Mhp511; the definite secretory protein was Mhp228. Mhp364, Mhp623, Mhp677, and Mhp682 could be secreted out of organism as well as localized on the membrane. Mhp104, Mhp351 and Mhp483 were predictive membrane proteins, and Mhp336, Mhp424 and Mhp535 were calculated as extracellular.
3.2. Expression and purification of recombinant proteins
All 27 recombinant bacteria were induced to express target proteins which were fused with a GST protein. After crude purification of the bacterial lysates with glutathione-conjugated agarose beads, soluble fusion proteins were visualized by SDS-PAGE electrophoresis (Figure 1A). In most cases, a dominant band migrated at the expected location in the gel, except GST-Mhp156, GST-Mhp228 and GST-Mhp424, the molecular weights of which were smaller than that predicted. Fourteen proteins could be cleaved off at PreScission site from GST protein adhered to the glutathione immobilized on agarose bead and the molecular weights were as expected, except Mhp472. The actual molecular weight of Mhp472 was 35 kDa, which was larger than the 27 kDa theoretical molecular weight (Figure 1B). The other fusion proteins could not be cleaved off from GST at the PreScission site.
Figure 1.
Quality of GST-M. hyopneumoniae fusion proteins and crudely purified proteins cleaved off from the beads with PreScission protease. GST fusion proteins were precipitated from bacterial lysates using glutathione-agarose beads (A). Fourteen proteins could be cleaved off from the agarose bead-immunobilized GST fusion proteins with PreScission protease (B). The gel was stained with Coomassie blue dye. This method was routinely used to check the quality of each fusion protein. Shown on the gel are 27 GST fusion proteins expressed under a single induction condition as examples. The expected full-length fusion proteins are circled in white.
3.3. Identification of immunodominant antigens recognized by pig convalescent sera
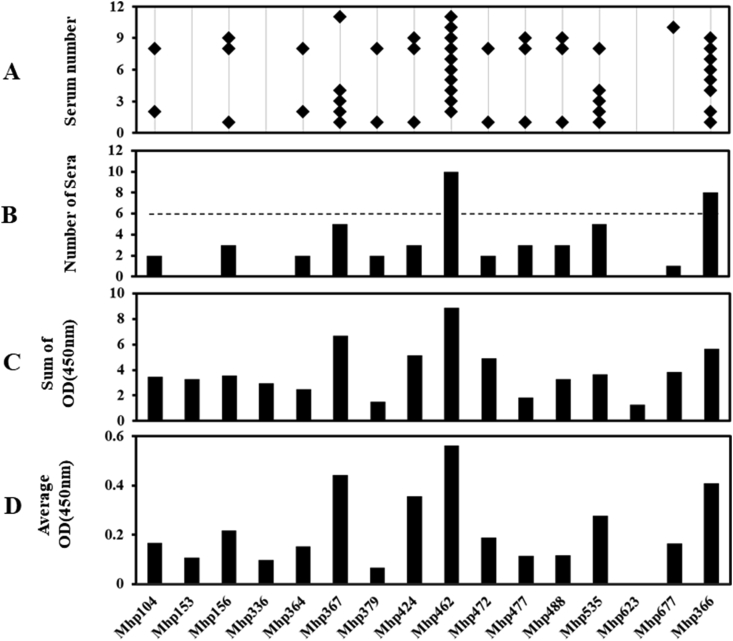
We analyzed the reactivity of 27 GST-M. hyopneumoniae fusion proteins in this study and the control antigen GST-Mhp366 with 11 convalescent sera. The number of fusion proteins recognized by each convalescent serum was recorded (Figure 2A). Sera 8/10, 1, 6/9/11, 2, 7, 5, 3/4 recognized 20, 17, 16, 13, 12, 8, and 7 out of 27 proteins, respectively. The above results indicated that each serum specimen exhibited a unique recognition form. However, several sera reacted with the same fusion proteins. We found that 24 of the 27 fusion proteins were positively recognized by one or more convalescent sera; this suggested that the 24 proteins were both expressed and exhibited immunogenicity during M. hyopneumoniae infection in pigs, although 3 unrecognized proteins have been identified in in vitro cultured organism [33]. The fusion proteins that reacted with convalescent antibodies with both positive results and high frequency (≥6 convalescent sera) were defined as immunodominant antigens [17] (Figure 2B). As the positive control, Mhp366 was recognized by 10 convalescent sera. A total of 15 proteins qualified the immunodominance requirements: Mhp367 and Mhp677 were identified by all tested convalescent sera, Mhp336 by 10 convalescent sera, Mhp364 by 9 convalescent sera, Mhp156, Mhp379, Mhp424, Mhp477, and Mhp535 by 8 convalescent sera, and Mhp104 and Mhp462 by 7 convalescent sera. Mhp153, Mhp472, Mhp488, and Mhp623 were confirmed by 6 convalescent sera. Other proteins were identified by less than 6 convalescent sera. For instance, none of the convalescent serum reacting with Mhp228, Mhp252, and Mhp465 was considered as positive. The antibody titers shown in Figure 2C (accumulative differences of OD450 value from convalescent and negative sera) and Figure 2D (average difference of OD450 value from convalescent and negative sera) were calculated. We found that the accumulative differences of OD450 and the average difference of OD450 were similar to the corresponding recognition frequency. The most immunodominant proteins, Mhp367 and Mhp677, and Mhp336 and Mhp366, recognized by 10 convalescent sera, had higher sum and average antibody titers. Surprisingly, Mhp511, which reacted with 5 convalescent sera, had the highest titers among all the tested proteins.
Figure 2.
Reactivity of porcine convalescent antibodies with 28 M. hyopneumoniae fusion proteins using the negative sera as background. (A) Eleven serum antibodies from pigs naturally infected with M. hyopneumoniae (numbers on y axis) reacted with 28 GST-M. hyopneumoniae fusion proteins using negative sera as background immobilized onto microplates (x axis) in an ELISA. Each positive reaction is marked with an asterisk. (B) Plot showing the number of sera that reacted with each fusion protein. (C) The accumulative differences of OD450 value from convalescent and negative sera to each fusion protein were added up. (D) Average differences of OD450 value were calculated.
3.4. Identification of discriminative serological immunodominant antigens recognized by pig convalescent sera but not by hyperimmune sera
We also identified discriminative serological immunodominant proteins that reacted strongly with sera from pigs infected with M. hyopneuomniae through respiratory tract but not those immunized with pathogen cultured in vitro. When the hyperimmune serum samples were used as control, a great variation in the antibody recognition frequency was also observed (Figure 3A). Sera 8, 1, 2/9, 4, 3, and 5/6/7/10/11 recognized 11, 9, 6, 4, 3, and 2 out of 16 fusion proteins, respectively. The criterion of reaction with ≥6 of the 11 convalescent but not hyperimmune serum samples was used to determine the discriminative serological immunodominant antigen (Figure 3B). Only Mhp462, which was recognized by 10 convalescent sera, was identified as discriminative serological immunodominant antigen. As the positive control, Mhp366 was recognized by 8 convalescent sera. Other 14 immunodominant proteins were recognized by less than 6 convalescent sera. Moreover, Mhp153, Mhp336, and Mhp623 could not be detected by any convalescent serum if hyperimmune sera were considered as the “negative sera”. As mentioned above, we also compared the antibody titers reactive to each fusion protein. The antibody titers calculated as the differences of OD450 value from convalescent serum and hyperimmune serum are shown in Figure 3C (accumulative differences of OD450 value from convalescent and hyperimmune sera) and Figure 3D (average differences of OD450 value from convalescent and hyperimmune sera). As the recognition frequency, Mhp462 displayed the highest antibody binding titers, when the titers of each individual antiserum binding to the fusion protein were added or averaged.
Figure 3.
Reactivity of porcine convalescent antibodies with 16 M. hyopneumoniae fusion proteins using the hyperimmune sera as background. (A) Eleven serum antibodies from pigs naturally infected with M. hyopneumoniae (numbers on y axis) reacted with 16 GST-M. hyopneumoniae fusion proteins using the hyperimmune sera as background immobilized onto microplates (x axis) in an ELISA. Each positive reaction is marked with an asterisk. (B) Plot showing the number of sera that reacted with each fusion protein. (C) The accumulative differences of OD450 value from convalescent and hyperimmune sera to each fusion protein were added up. (D) Average differences of OD450 value were calculated.
4. Discussion
Identification of immunoprotective antigens is a fundamental step in the development of improved vaccines against M. hyopneumoniae infection. Sequencing of genomes of several M. hyopneumoniae strains provided massive genomic and proteic information to screen novel immunodominant proteins by using method of reverse vaccinology. We selected 27 proteins to identify their immunodominance based on this method, which provided the opportunity to identify immunodominant proteins at the whole-genome scale which we had established previously [17, 18]. Firstly, we predicted and manually curated the subcellular localization of these proteins; we found that all proteins were membrane or extracellular. Therefore, they had the potential to be recognized by convalescent sera and considered as immunodominant. Secondly, we used pGEX vectors to construct recombinant plasmids and express proteins fused with extremely hydrophilic protein GST in soluble forms, although most proteins contain hydrophobic regions, even multiple transmembrane. Thirdly, 15 serological immunodominant proteins and 1 discriminative serological immunodominant protein Mhp462 were confirmed using Mhp366 as the positive control.
We selected 27 proteins to predict their subcellular localization and screen immunodominant antigens. Actually, we randomly chose more than 200 M. hyopneumoniae ORFs to predict their localization. Membrane proteins and secretory proteins, rather than cytoplasmic proteins, typically induce immunological reaction in the body; therefore, we chose the proteins that were preliminarily predicted as membrane and secretory proteins as the targets to carry out predicting workflow.
In our experiment, we used recombinant bacteria lysates instead of purified fusion proteins to coat 96-well microplates. It was suggested that purified proteins may be better than lysates during screening of immunodominant proteins. However, coating with lysates could achieve the same effect as purified proteins. Firstly, the pig sera were pre-absorbed with control bacterium, which reduced the non-specific antibodies. Secondly, theoretically, the glutathione-conjugated agarose beads can only bind to the GST fusion proteins, but not to other proteins. Therefore, the process of antigen coating also served as a process of protein purification. Thirdly, we calculated the difference of OD450 from lysates of recombinant bacteria and control bacterium, which further reduced the impact of non-specific reaction. Our previously established methods involving coating with bacterial lysates [17, 18] have been shown to be feasible for screening of immunodominant and discriminative immunodominant proteins of M. hyopneumoniae on a large scale.
Four immunodominant proteins, Mhp156, Mhp364, Mhp424, and Mhp488 have been tested for their antigenicity [5]. Mhp156 moderately reacted with convalescent sera and ELISA results indicated a low absorbance value of Mhp364. However, Mhp424 and Mhp488 were not recognized by convalescent sera. On Western blot analysis, the 4 proteins did not exhibit reaction with any of the positive sera. On literature search, we found that selected fragments but not whole genes of the 4 proteins were cloned into Champion pET200D/TOPO expression vector and expressed in E. coli [34, 35]. Therefore, the immunodominant fragments in the recombinant proteins might be deleted or be truncated from the whole length proteins. We used the full length genes to construct recombinant plasmids, which ensured that the immunodominant regions were not neglected [36]. That might be the reason why these 4 proteins showed high recognition frequency and were considered as immunodominant antigens in our experiment.
We identified 5 hypothetical proteins, including Mhp336, Mhp364, Mhp367, Mhp424, and Mhp535, which have not yet been characterized as immunodominant antigens. In our bioinformatics prediction and manual curation section, all of these were present or predicted to be on the surface of M. hyopneumoniae or (predicted to) be secreted out of organism. In the future, these membrane and/or secreted proteins should be further characterized for their potential critical roles, such as adherence to host cell, ion transport, signal transduction, and virulence.
Mhp104, a putative tRNA pseudouridine synthase B, was recognized by 7 convalescent sera and defined as serological immunodominant antigen. However, it has not been identified by any laboratory previously. This may be attributable to the lack of expression or very low expression of this protein by M. hyopneumoniae cultured in vitro. Another reason might be that Mhp104 is secreted out of organism immediately after its synthesis, which prevented its detection in M. hyopneumoniae. Therefore, the protein array assay helped identify novel immunodominant proteins. Moreover, in the current assay, each M. hyopneumoniae protein was arrayed onto microplates in soluble form, and the fusion protein antigens were efficiently recognized by convalescent antibodies. This method overcomes the drawback of assays based on the proteins obtained from cell free cultured M. hyopneumoniae in 2-dimensional gels, as it is liable to miss antigens that are only expressed after natural infection and denaturation of the antigens.
Some proteins were not recognized by convalescent sera or had low recognition frequencies in the assay, although bioinformatics prediction identified these as surface exposed or secreted proteins. The reasons might be as follows: These actually resided in the bacterial cytoplasm, although they were predicted to be membrane proteins or be secreted out of the organism. The second potential reason is that the proteins were not expressed or the expression level was too low to induce the production of antibodies. In addition, the proteins may not have been recognized by immune cells.
Although we identified 15 serological immunodominant antigens, only one discriminative serological immunodominant antigen, Mhp462, which reacted with convalescent sera but not hyperimmune sera, was identified. This characteristic was very similar to Mhp366; in a previous study, immunization of pigs with inactivated vaccine failed to induce IgG antibody against Mhp366 [37]. Mhp462, named MHJ_0461 in J strain, forms multimers comprising more than 10 subunits on the surface M. hyopneumoniae [32]. Two main complexes are approximately 500 kDa and 800 kDa naturally, although the monomer has a molecular weight of 51.4 kDa. Recombinant MHJ_0461 (rMHJ_0461) demonstrates greatest leucine aminopeptidase activity against leucine, alanine, phenylalanine, methionine, and positively charged arginine. Moreover, rMHJ_0461 binds heparin and plasminogen that lines the surface of eukaryotic cells [38, 39]. Plasminogen bound to rMHJ_0461 was readily converted to plasmin in the presence of tissue plasminogen activator. If its antibody can block the adhesion of Mhp462 to host epithelial cells, it will provide a good protection against M. hyopneumoniae.
Some proteins reacted with all convalescent sera, such as Mhp367 and Mhp677. The recognition frequencies of the two proteins were even more than the frequency of the control protein, Mhp366. Therefore, they have potential to be used as novel diagnostic antigens for the development of new ELISA kits. However, the exact time of generation of antibodies after M. hyopneumoniae infection or vaccination is not known. In previous studies, seroconversion occurred at 6 weeks [40] or was delayed up to 98 days [41] after vaccination. In farms with a history of PEP, sera positivity due to natural infection of M. hyopneumoniae was observed between the age of 8–24 weeks [42, 43]. Therefore, development of diagnostic methods for early detection of M. hyopneumoniae antibody is a key imperative. Evaluating the ability of serological immunodominant antigens for early detection of seroconversion induced by M. hyopneumoniae infection or by vaccination is important for the development of methods for early diagnosis.
We did not assess the antigenicity of immunodominant proteins by Western blot owing to the lack of availability of secondary antibody. Although several secondary antibodies purchased from different companies were tested, we always observed the same bands between different lanes loaded on different purified recombinant proteins.
Although we have determined 15 serological immunodominant proteins and 1 discriminative serological immunodominant protein, it is still not clear which fragments of these proteins are the main antigenic determinants. Precise delineation of the immunodominant regions of these proteins would necessitate further fragmentation of these proteins and determining the antigenicity of individual fragments by reacting them with different types of sera in the future.
In conclusion, we identified 15 serological immunodominant proteins recognized by convalescent sera and 1 discriminative serological immunodominant protein that reacted with convalescent sera but not with hyperimmune sera. The antigenicity of most of the immunodominant proteins has not been characterized. The immunodominant proteins are antigenic and expressed during infection, suggesting that these, especially the discriminative one, are potential vaccine candidate antigens against M. hyopneumoniae infection.
Declarations
Author contribution statement
Yaru Ning, Yaoqin Zhou: Conceived and designed the experiments; Performed the experiments.
Zhaodi Wang, Yukang Wen, Zuobo Xu, Yaqin Tian: Performed the experiments.
Mei Yang, Xudong Wang: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Yujiao Yang: Analyzed and interpreted the data.
Honglei Ding: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the National Key Research and Development Program of China (No. 2017YFD0501703), Fundamental Research Funds for the Central Universities (No. XDJK2020B012, XDJK2020C020), Chongqing Special Project of Social Livelihood Science and Technology Innovation (No. cstc2019jscx-msxmX0402), and Beibei Application and Development Plan Project (No. 2018–28).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Maes D., Segales J., Meyns T., Sibila M., Pieters M., Haesebrouck F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 2008;126(4):297–309. doi: 10.1016/j.vetmic.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maes D., Sibila M., Kuhnert P., Segalés J., Haesebrouck F., Pieters M. Update on Mycoplasma hyopneumoniae infections in pigs: knowledge gaps for improved disease control. Transbound Emerg. Dis. 2018;S1:110–124. doi: 10.1111/tbed.12677. [DOI] [PubMed] [Google Scholar]
- 3.Chae C. Porcine respiratory disease complex: interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet. J. 2016;212:1–6. doi: 10.1016/j.tvjl.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Sunaga F., Tsuchiaka S., Kishimoto M., Aoki H., Kakinoki M., Kure K., Okumura H., Okumura M., Okumura A., Nagai M., Omatsu T., Mizutani T. Development of a one-run real-time PCR detection system for pathogens associated with porcine respiratory diseases. J. Vet. Med. Sci. 2020;82(2):217–223. doi: 10.1292/jvms.19-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simionatto S., Marchioro S.B., Galli V., Brum C.B., Klein C.S., Rebelatto R., Silva E.F., Borsuk S., Conceição F.R., Dellagostin O. Immunological characterization of Mycoplasma hyopneumoniae recombinant proteins. Comp. Immunol. Microbiol. Infect. Dis. 2012;35(2):209–216. doi: 10.1016/j.cimid.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Baccaro M.R., Hirose F., Umehara O., Gonçalves L.C., Doto D.S., Paixão R., Shinya L.T., Moreno A.M. Comparative efficacy of two single-dose bacterins in the control of Mycoplasma hyopneumoniae in swine raised under commercial conditions in Brazil. Vet. J. 2006;172(3):526–531. doi: 10.1016/j.tvjl.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Villarreal I., Meyns T., Dewulf J., Vranckx K., Calus D., Pasmans F., Haesebrouck F., Maes D. The effect of vaccination on the transmission of Mycoplasma hyopneumoniae in pigs under field conditions. Vet. J. 2011;188(1):48–52. doi: 10.1016/j.tvjl.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Meyns T., Dewulf J., de Kruif A., Calus D., Haesebrouck F., Maes D. Comparison of transmission of Mycoplasma hyopneumoniae in vaccinated and non-vaccinated populations. Vaccine. 2006;24(49-50):7081–7086. doi: 10.1016/j.vaccine.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Fagan P.K., Walker M., Chin J., Eamens G.J., Djordjevic S.P. Oral immunization of swine with attenuated Salmonella typhimurium aroA SL3261 expressing a recombinant antigen of Mycoplasma hyopneumoniae (NrdF) primes the immune system for a NrdF specific secretory IgA response in the lungs. Microb. Pathog. 2001;30(2):101–110. doi: 10.1006/mpat.2000.0412. [DOI] [PubMed] [Google Scholar]
- 10.Shimoji Y., Oishi E., Muneta Y., Nosaka H., Mori Y. Vaccine efficacy of the attenuated Erysipelothrix rhusiopathiae YS-19 expressing a recombinant protein of Mycoplasma hyopneumoniae P97 adhesin against mycoplasmal pneumonia of swine. Vaccine. 2003;21(5-6):532–537. doi: 10.1016/s0264-410x(02)00462-0. [DOI] [PubMed] [Google Scholar]
- 11.Okamba F.R., Arella M., Music N., Jia J.J., Gottschalk M., Gagnon C.A. Potential use of a recombinant replication-defective adenovirus vector carrying the C-terminal portion of the P97 adhesin protein as a vaccine against Mycoplasma hyopneumoniae in swine. Vaccine. 2010;28(30):4802–4809. doi: 10.1016/j.vaccine.2010.04.089. [DOI] [PubMed] [Google Scholar]
- 12.Minion F.C., Lefkowitz E.J., Madsen M.L., Cleary B.J., Swartzell S.M., Mahairas G.G. The genome sequence of Mycoplasma hyopneumoniae strain 232, the agent of swine mycoplasmosis. J. Bacteriol. 2004;6(21):7123–7133. doi: 10.1128/JB.186.21.7123-7133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasconcelos A.T., Ferreira H.B., Bizarro C.V., Bonatto S.L., Carvalho M.O., Pinto P.M., Almeida D.F., Almeida L.G., Almeida R., Alves-Filho L., Assunção E.N., Azevedo V.A., Bogo M.R., Brigido M.M., Brocchi M., Burity H.A., Camargo A.A., Camargo S.S., Carepo M.S., Carraro D.M., de Mattos Cascardo J.C., Castro L.A., Cavalcanti G., Chemale G., Collevatti R.G., Cunha C.W., Dallagiovanna B., Dambrós B.P., Dellagostin O.A., Falcão C., Fantinatti-Garboggini F., Felipe M.S., Fiorentin L., Franco G.R., Freitas N.S., Frías D., Grangeiro T.B., Grisard E.C., Guimarães C.T., Hungria M., Jardim S.N., Krieger M.A., Laurino J.P., Lima L.F., Lopes M.I., Loreto E.L., Madeira H.M., Manfio G.P., Maranhão A.Q., Martinkovics C.T., Medeiros S.R., Moreira M.A., Neiva M., Ramalho-Neto C.E., Nicolás M.F., Oliveira S.C., Paixão R.F., Pedrosa F.O., Pena S.D., Pereira M., Pereira-Ferrari L., Piffer I., Pinto L.S., Potrich D.P., Salim A.C., Santos F.R., Schmitt R., Schneider M.P., Schrank A., Schrank I.S., Schuck A.F., Seuanez H.N., Silva D.W., Silva R., Silva S.C., Soares C.M., Souza K.R., Souza R.C., Staats C.C., Steffens M.B., Teixeira S.M., Urmenyi T.P., Vainstein M.H., Zuccherato L.W., Simpson A.J., Zaha A. Swine and poultry pathogens: the complete genome sequences of two strains of Mycoplasma hyopneumoniae and a strain of Mycoplasma synoviae. J. Bacteriol. 2005;187(16):5568–5577. doi: 10.1128/JB.187.16.5568-5577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W., Feng Z., Fang L., Zhou Z., Li Q., Li S., Luo R., Wang L., Chen H., Shao G., Xiao S. Complete genome sequence of Mycoplasma hyopneumoniae strain 168. J. Bacteriol. 2011;193(4):1016–1017. doi: 10.1128/JB.01305-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siqueira F.M., Thompson C.E., Virginio V.G., Gonchoroski T., Reolon L., Almeida L.G., da Fonsêca M.M., de Souza R., Prosdocimi F., Schrank I.S., Ferreira H.B., de Vasconcelos A.T., Zaha A. New insights on the biology of swine respiratory tract mycoplasmas from a comparative genome analysis. BMC Genom. 2013;14:175. doi: 10.1186/1471-2164-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han J., Park B.S., Shin D.J., Song S.Y., Jeong Y.J., Lee N. Complete genome sequence of Mycoplasma hyopneumoniae strain KM014, a clinical isolate from South Korea. Genome Announc. 2017;5(38):e01012–e01017. doi: 10.1128/genomeA.01012-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., You F., Zhong J., Wang H., Ding H. Development of an ELISA for identification of immunodominant protein antigens of Mycoplasma hyopneumoniae. Sheng Wu Gong Cheng Xue Bao. 2018;34(1):44–53. doi: 10.13345/j.cjb.170220. [DOI] [PubMed] [Google Scholar]
- 18.Ding H., Zhou Y., Wang H. Development of an indirect ELISA for detecting humoral immunodominant proteins of Mycoplasma hyopneumoniae which can discriminate between inactivated bacterin-induced hyperimmune sera and convalescent sera. BMC Vet. Res. 2019;15(1):327. doi: 10.1186/s12917-019-2077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Käll L., Krogh A., Sonnhammer E.L. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 2004;338(5):1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Krogh A., Larsson B., von Heijne G., Sonnhammer E.L. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. J. Mol. Biol. 2001;305(3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 21.Juncker A.S., Willenbrock H., von Heijne G., Brunak S., Nielsen H., Krogh A. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 2003;12(8):1652–1662. doi: 10.1110/ps.0303703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman O., Cummings S.P., Harrington D.J., Sutcliffe I.C. Methods for the bioinformatic identification of bacterial lipoproteins encoded in the genomes of Gram-positive bacteria. World J. Microbiol. Biotechnol. 2008;24(11):2377–2382. [Google Scholar]
- 23.Bendtsen J.D., Nielsen H., Widdick D., Palmer T., Brunak S. Prediction of twin-arginine signal peptides. BMC Bioinf. 2005;6:167. doi: 10.1186/1471-2105-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almagro Armenteros J.J., Tsirigos K.D., Sønderby C.K., Petersen T.N., Winther O., Brunak S., von Heijne G., Nielsen H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019;37(4):420–423. doi: 10.1038/s41587-019-0036-z. [DOI] [PubMed] [Google Scholar]
- 25.Reolon L.A., Martello C.L., Schrank I.S., Ferreira H.B. Survey of surface proteins from the pathogenic Mycoplasma hyopneumoniae strain 7448 using a biotin cell surface labeling approach. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0112596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tacchi J.L., Raymond B.B., Haynes P.A., Berry I.J., Widjaja M., Bogema D.R., Woolley L.K., Jenkins C., Minion F.C., Padula M.P., Djordjevic S.P. Post-translational processing targets functionally diverse proteins in Mycoplasma hyopneumoniae. Open Biol. 2016;6(2):150210. doi: 10.1098/rsob.150210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paes J.A., Lorenzatto K.R., de Moraes S.N., Moura H., Barr J.R., Ferreira H.B. Secretomes of Mycoplasma hyopneumoniae and Mycoplasma flocculare reveal differences associated to pathogenesis. J. Proteom. 2017;154:69–77. doi: 10.1016/j.jprot.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Robinson M.W., Buchtmann K.A., Jenkins C., Tacchi J.L., Raymond B.B., To J., Roy Chowdhury P., Woolley L.K., Labbate M., Turnbull L., Whitchurch C.B., Padula M.P., Djordjevic S.P. MHJ_0125 is an M42 glutamyl aminopeptidase that moonlights as a multifunctional adhesin on the surface of Mycoplasma hyopneumoniae. Open Biol. 2013;3(4):130017. doi: 10.1098/rsob.130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarocki V.M., Santos J., Tacchi J.L., Raymond B.B., Deutscher A.T., Jenkins C., Padula M.P., Djordjevic S.P. MHJ_0461 is a multifunctional leucine aminopeptidase on the surface of Mycoplasma hyopneumoniae. Open Biol. 2015;5(1):140175. doi: 10.1098/rsob.140175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Z.X., Shao G.Q., Liu M.J., Wang H.Y., Gan Y., Wu X.S. Development and validation of a SIgA-ELISA for the detection of Mycoplasma hyopneumoniae infection. Vet. Microbiol. 2010;143(2-4):410–416. doi: 10.1016/j.vetmic.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 31.Hensel A., Ganter M., Kipper S., Krehon S., Wittenbrink M.M., Petzoldt K. Prevalence of aerobic bacteria in bronchoalveolar lavage fluid from healthy pigs. Am. J. Vet. Res. 1994;55(12):1697–1702. [PubMed] [Google Scholar]
- 32.Ganter M., Hensel A. Cellular variables in bronchoalveolar lavage fluids (BALF) in selected healthy pigs. Res. Vet. Sci. 1997;63(3):215–217. doi: 10.1016/s0034-5288(97)90023-0. [DOI] [PubMed] [Google Scholar]
- 33.Pendarvis K., Padula M.P., Tacchi J.L., Petersen A.C., Djordjevic S.P., Burgess S.C., Minion F.C. Proteogenomic mapping of Mycoplasma hyopneumoniae virulent strain 232. BMC Genom. 2014;15:576. doi: 10.1186/1471-2164-15-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simionatto S., Marchioro S.B., Galli V., Hartwig D.D., Carlessi R.M., Munari F.M., Laurino J.P., Conceição F.R., Dellagostin O.A. Cloning and purification of recombinant proteins of Mycoplasma hyopneumoniae expressed in Escherichia coli. Protein Expr. Purif. 2010;69(2):132–136. doi: 10.1016/j.pep.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Simionatto S., Marchioro S.B., Galli V., Luerce T.D., Hartwig D.D., Moreira A.N., Dellagostin O.A. Efficient site-directed mutagenesis using an overlap extension-PCR method for expressing Mycoplasma hyopneumoniae genes in Escherichia coli. J. Microbiol. Methods. 2009;79(1):101–105. doi: 10.1016/j.mimet.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Wang J., Zhang Y., Yu P., Zhong G. Immunodominant regions of a Chlamydia trachomatis type III secretion effector protein, Tarp. Clin. Vaccine Immunol. 2010;17(9):1371–1376. doi: 10.1128/CVI.00218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meens J., Bolotin V., Frank R., Böhmer J., Gerlach G.F. Characterization of a highly immunogenic Mycoplasma hyopneumoniae lipoprotein Mhp366 identified by peptide-spot array. Vet. Microbiol. 2010;142(3-4):293–302. doi: 10.1016/j.vetmic.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Deutscher A.T., Jenkins C., Minion F.C., Seymour L.M., Padula M.P., Dixon N.E., Walker M.J., Djordjevic S.P. Repeat regions R1 and R2 in the P97 paralogue Mhp271 of Mycoplasma hyopneumoniae bind heparin, fibronectin and porcine cilia. Mol. Microbiol. 2010;78(2):444–458. doi: 10.1111/j.1365-2958.2010.07345.x. [DOI] [PubMed] [Google Scholar]
- 39.Seymour L.M., Deutscher A.T., Jenkins C., Kuit T.A., Falconer L., Minion F.C., Crossett B., Padula M., Dixon N.E., Djordjevic S.P., Walker M.J. A processed multidomain Mycoplasma hyopneumoniae adhesin binds fibronectin, plasminogen, and swine respiratory cilia. J. Biol. Chem. 2010;285(44):971–978. doi: 10.1074/jbc.M110.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sibila M., Nofrarías M., López-Soria S., Segalés J., Valero O., Espinal A., Calsamiglia M. Chronological study of Mycoplasma hyopneumoniae infection, seroconversion and associated lung lesions in vaccinated and non-vaccinated pigs. Vet. Microbiol. 2007;122(1-2):97–107. doi: 10.1016/j.vetmic.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Martelli P., Terreni M., Guazzetti S., Cavirani S. Antibody response to Mycoplasma hyopneumoniae infection in vaccinated pigs with or without maternal antibodies induced by sow vaccination. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2006;53(5):229–233. doi: 10.1111/j.1439-0450.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- 42.Djordjevic S.P., Eamens G.J., Romalis L.F., Saunders M.M. An improved enzyme linked immunosorbent assay (ELISA) for the detection of porcine serum antibodies against Mycoplasma hyopneumoniae. Vet. Microbiol. 1994;39(3-4):261–273. doi: 10.1016/0378-1135(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 43.Leon E.A., Madec F., Taylor N.M., Kobisch M. Seroepidemiology of Mycoplasma hyopneumoniae in pigs from farrow-to-finish farms. Vet. Microbiol. 2001;78(4):331–341. doi: 10.1016/s0378-1135(00)00303-5. [DOI] [PubMed] [Google Scholar]